Abstract
Objective
To identify the association between brain vascular changes and cortical volumes on MRI and late-onset epilepsy.
Methods
In 1993–1995, 1,920 participants (median age 62.7, 59.9% female) in the community-based Atherosclerosis Risk in Communities (ARIC) Study underwent MRI, and white matter hyperintensities were measured. In addition, in 2011–2013, 1,964 ARIC participants (median age 72.4, 61.1% female) underwent MRI, and cortical volumes and white matter hyperintensities were measured. We identified cases of late-onset epilepsy (starting at age 60 or later) from ARIC hospitalization records and Medicare claims data. Using the 1993–1995 MRI, we evaluated the association between white matter hyperintensities and subsequent epilepsy using survival analysis. We used the 2011–2013 MRI to conduct cross-sectional logistic regression to examine the association of cortical volumes and white matter hyperintensities with late-onset epilepsy. All models were adjusted for demographics, hypertension, diabetes, smoking, and APOE ε4 allele status.
Results
Ninety-seven ARIC participants developed epilepsy after having an MRI in 1993–1995 (incidence 3.34 per 1,000 person-years). The degree of white matter hyperintensities measured at ages 49–72 years was associated with the risk of late-onset epilepsy (hazard ratio 1.27 per age-adjusted SD, 95% confidence interval [CI] 1.06–1.54). Lower cortical volume scores were associated cross-sectionally with higher odds of late-onset epilepsy (odds ratio 1.87, 95% CI 1.16–3.02) per age-adjusted SD.
Conclusions
This study demonstrates associations between earlier-life white matter hyperintensities on MRI and later-life incident epilepsy, and between cortical volumes measured later in life and late-onset epilepsy. These findings may help illuminate the causes of late-onset epilepsy.
New-onset epilepsy is more common in older age than at any other time of life,1 with annual incidence estimates as high as 2.5 per 1,000.2,3 While some of these cases are attributed to stroke or dementia,2,4–6 a large proportion have no clear etiology and are presumed to be due to occult cerebrovascular disease.7 Case-control studies have shown that white matter T2 hyperintensities (a marker for small vessel cerebrovascular disease) are more prevalent in patients with late-onset epilepsy (LOE) than in age-matched controls.8,9 The volume of cortical structures is also of interest as a possible marker for cerebrovascular disease and neurodegeneration.10 One small case-control study found lower cortical gray matter volumes in patients with seizures (mainly in the temporal lobes).9 A separate case-control study found more medial temporal lobe atrophy in older adults with epilepsy than cognitively normal controls.11
To our knowledge, there have been no longitudinal studies to date showing a relationship between earlier white matter changes and later epilepsy. Furthermore, evaluation of white matter and cortical volumes in LOE has been taken from case-control studies matched on age, without adjustment for hypertension, smoking, or other vascular risk factors that are known to be highly associated with white matter hyperintensities (WMHs) and markers of cerebrovascular disease12 and that we previously demonstrated are associated with LOE.13
The goals of this study were to determine the longitudinal association of white matter disease with the development of epilepsy in older adults, and to determine the cross-sectional association of brain cortical volumes with LOE, adjusting for demographic factors, APOE ε4 genotype, hypertension, diabetes, and smoking.
Methods
Participant inclusion
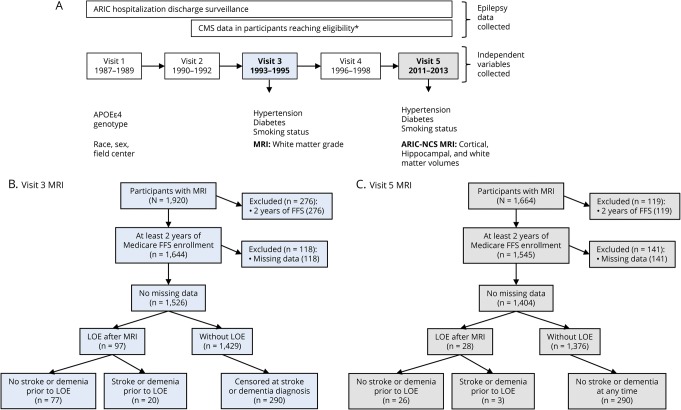
Men and women between 45 and 64 years of age were recruited to the Atherosclerosis Risk in Communities (ARIC) cohort from 1987 to 1989 from 4 US communities, using probability sampling (n = 15,792).14 Participants were recruited from Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; Jackson, Mississippi; and Washington County, Maryland. Participants were examined in-person at 6 visits between 1987 and 2017, contacted yearly by telephone (with semiannual calls starting in 2012), and followed continuously with hospital discharge surveillance. Visit 1 occurred between 1987 and 1989, visit 2 between 1990 and 1992, visit 3 between 1993 and 1995, visit 4 between 1996 and 1998, and visit 5 between 2011 and 2013. A subset of participants participated in the ARIC Neurocognitive Study (ARIC-NCS) at visit 5 (having had prior MRI or low cognitive scores, or chosen as part of an age-stratified random sample). Participants had sampling weights assigned based on inverse sampling fractions and the probability of completing the examination.15
Standard protocol approvals, registrations, and patient consents
The institutional review boards at all participating institutions approved the study, and all participants provided written informed consent at each study visit.
Outcome ascertainment
Cases of late-onset epilepsy were defined as participants with at least 2 ICD-9 codes from any setting for epilepsy, seizures, or convulsions (345.* or 780.39) in ARIC hospitalization records or in Medicare claims data (outpatient, inpatient, and carrier claims files). We used diagnosis code data from Centers for Medicare & Medicaid Services (CMS) Medicare fee-for-service (FFS) data for 1991–2013. ARIC participant data were linked to CMS data using birth date, sex, and social security number,16 and linkage was established for 14,899 participants.
To qualify as incident LOE, the first seizure-related code had to occur after at least 2 years of data without a seizure-related code, and had to occur at age 60 or later. In primary analysis, we included only participants with at least 2 years of Medicare FFS enrollment.
We excluded participants with a claims-identified first seizure before age 60 (n = 64), and with multiple sclerosis, brain tumor, brain surgery, or brain radiation identified from ARIC data (n = 95). Due to small numbers, we excluded participants of races other than black or white (n = 48), and black participants from Minnesota and Maryland (n = 55). Participants who did not give permission for DNA research were also excluded (n = 46). From longitudinal analysis of WMHs, we excluded participants with a first seizure prior to the date of the visit 3 MRI (n = 13).
Covariates
For this analysis, covariates were ascertained from information collected at the third ARIC visit (for longitudinal analysis of WMHs) or fifth ARIC visit (for cross-sectional analysis of cortical volumes and WMHs). Sitting blood pressure was measured 3 times, and the 2nd and 3rd values averaged; hypertension was defined as systolic blood pressure mean ≥140 mm Hg, diastolic blood pressure mean ≥90 mm Hg, or use of an antihypertensive medication. Diabetes was defined as fasting blood glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, use of diabetic medications or insulin, or self-report of physician-diagnosed diabetes. Participants self-identified as never, former, or current smokers. APOE ε4 genotype was previously ascertained, and participants classified into 0 or 1–2 ε4 alleles (TaqMan assay; Applied Biosystems, Foster City, CA).17 Participant field center and race (self-reported) were used to generate a combined center–race variable, as is standard in ARIC analyses.18,19
Prevalent stroke information on all ARIC participants is collected at visit 1 by participant report, and incident stroke information is collected from hospitalization records and adjudicated by physician reviewers and computer algorithm during study follow-up.20 Dementia diagnosis was made at visit 5 by expert panel, using neurocognitive assessments, surveillance data, informant interviews, and telephone interviews.21
Imaging variables
Visit 3 MRI was performed between 1993 and 1995, with a 1.5T MRI obtained on 1920 ARIC participants. A white matter grade (developed for the Cardiovascular Health Study [CHS]22) of 0–9 was assigned to each MRI, using evaluation of subcortical and periventricular WMHs by expert radiologists18 (with interrater agreement within 1 grade of 92%).12 Because of high collinearity of age at MRI with WMH score, white matter grade was transformed to an age-adjusted z score.
The ARIC-NCS MRI was performed between 2011 and 2013, with a 3T MRI obtained on 1,964 participants including all those who had MRI at visit 4, those with cognitive decline or low cognitive scores, and an age-stratified random sample of cognitively normal individuals.15 Cortical and hippocampal volumes were obtained using Freesurfer software (version 5.1),23 and the total cortical volume, hippocampal volume, and volume of each cortical region (frontal, temporal, parietal, occipital) calculated as a proportion of total intracranial volume and transformed to an age-adjusted z score, which was used to compare the strength of association with LOE. The volume of WMHs was measured using an algorithm developed at the Mayo Clinic,24 and calculated as a percentage of total white matter volume, which was also transformed to an age-adjusted z score.
Data analysis
Statistical analysis was performed with Stata 15.0 software (StataCorp, College Station, TX) and a 2-sided p value of 0.05 was considered significant. We used a t test or χ2 test as appropriate to compare participants with and without LOE.
To analyze the longitudinal association of WMHs with later LOE, we used survival analysis with a Cox proportional hazards model to estimate the hazard ratio (HR) of developing LOE, using the visit 3 date as the origin time, and the date of the first seizure code as the event time failure. Participants were censored at date of death or last ARIC or CMS contact. White matter CHS grade was included as an age-adjusted z score. Participants with a first seizure prior to visit 3 MRI were excluded from this analysis. As the majority of cases of LOE developed after visit 3 MRI, we used longitudinal analysis to assess the relationship between visit 3 WMHs and LOE.
To analyze the cross-sectional associations of cortical, hippocampal, and WMH volumes with LOE, we used multivariable logistic regression models incorporating sampling weights, with LOE as the dependent variable. Volumes were adjusted for total intracranial volume and converted to an age-adjusted z score. Models were adjusted for field center, education level, hypertension, diabetes, smoking status, and APOE ε4 status. As few cases of LOE developed after visit 5 MRI based on available hospitalization and CMS data, we used cross-sectional analysis to assess the relationships between visit 5 regional volumes and LOE.
Participants with missing variables at analysis baseline were excluded from primary analyses. We performed sensitivity analyses using multiple imputation for missing baseline data (hypertension, diabetes, smoking status, APOE ε4 genotype). We also performed a sensitivity analysis using participants' 67th birthdays as the origin, the age at which participants who had Medicare coverage starting at age 65 and epilepsy codes only from CMS data (rather than ARIC hospitalization data) could be eligible for the diagnosis of LOE.
Data availability
The full data are not publicly available because of participant consent and privacy. A public-use ARIC dataset is available through BioLINCC. Additional data are available to researchers who submit a manuscript proposal to the ARIC publications committee.
Results
Visit 3 MRI
Of the 1,920 participants with MRI at visit 3, 1,644 had at least 2 years of Medicare CMS enrollment (at any time). Of those, 1,526 had no missing baseline data and were included in primary analysis, and 97 developed LOE after the MRI was performed (incidence 3.34 per 1,000 person-years, 95% confidence interval [CI] 2.74–4.08; figure, B).
Figure. Atherosclerosis Risk in Communities (ARIC) study design, data collection, and visits 3 and 5 MRI participant inclusion.
Flowcharts show (A) data collection and MRI measurements in the ARIC cohort, (B) participant inclusion/exclusion for analysis of visit 3 MRI, and (C) participant inclusion/exclusion for analysis of visit 5 MRI. *Participants are eligible for Medicare coverage after reaching age 65, or in cases of end-stage renal disease, amyotrophic lateral sclerosis, or disability. ARIC-NSC = ARIC Neurocognitive Study; CMS = Centers for Medicare & Medicaid Services; FFS = fee-for-service; LOE = late-onset epilepsy.
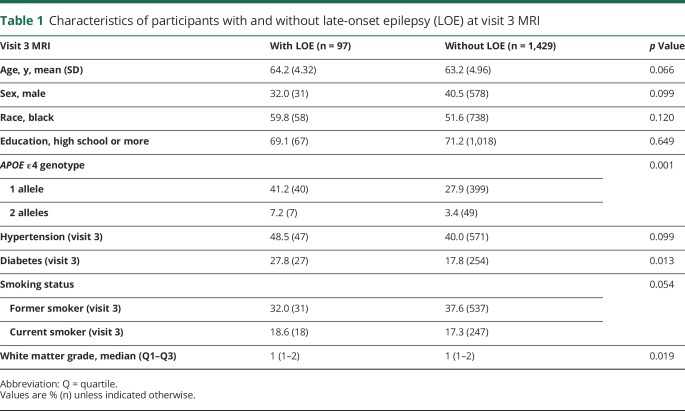
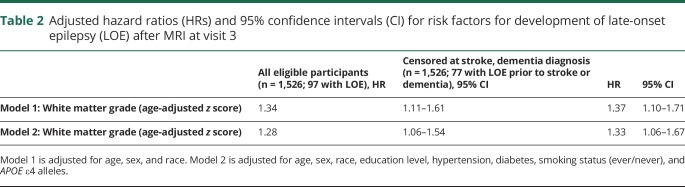
Participants with LOE had significantly higher white matter CHS grades than did participants without LOE (table 1). After adjusting for demographics, hypertension, diabetes, APOE ε4 genotype, and smoking, white matter grade at visit 3 was associated with later development of LOE (HR 1.27, 95% CI 1.06–1.54 per age-adjusted z score; table 2).
Table 1.
Characteristics of participants with and without late-onset epilepsy (LOE) at visit 3 MRI
Table 2.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for risk factors for development of late-onset epilepsy (LOE) after MRI at visit 3
A total of 252 participants who had MRI at visit 3 were later diagnosed with dementia, and 69 were later diagnosed with stroke (including 13 with both stroke and dementia; figure, B). Of these, 20 later developed LOE. When these participants were censored at the time of stroke or dementia diagnosis and after adjusting for demographics, hypertension, diabetes, APOE ε4 genotype, and smoking status, the association between white matter grade at visit 3 LOE persisted (HR 1.34, 95% CI 1.07–1.67).
Visit 5 MRI
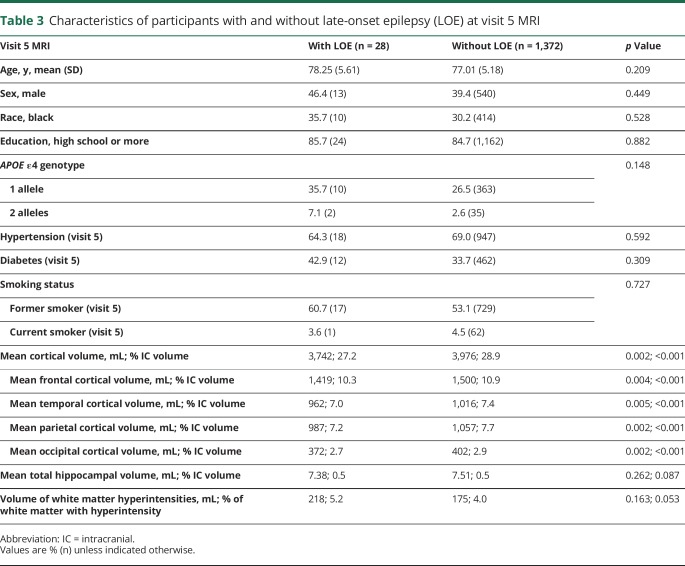
Of the 1,664 participants with MRI during the ARIC-NCS study at visit 5, 1,545 had at least 2 years of Medicare FFS enrollment. Of these, 1,404 had no missing baseline data and were included in primary analysis, and 28 had a diagnosis of LOE (figure, C). Participants with LOE had lower cortical volumes as a proportion of total intracranial volume (total cortical volume, and in each region) than did participants without LOE (table 3). After adjusting for demographics, hypertension, diabetes, smoking, and APOE ε4 genotype, lower global cortical volume was associated with LOE (adjusted odds ratio [OR] 1.87, 95% CI 1.16–3.02 per age-adjusted z score; table 4).
Table 3.
Characteristics of participants with and without late-onset epilepsy (LOE) at visit 5 MRI
Table 4.
Odds ratios (ORs) for late-onset epilepsy and total and regional cortical volumes at visit 5 MRI (n = 1,404)
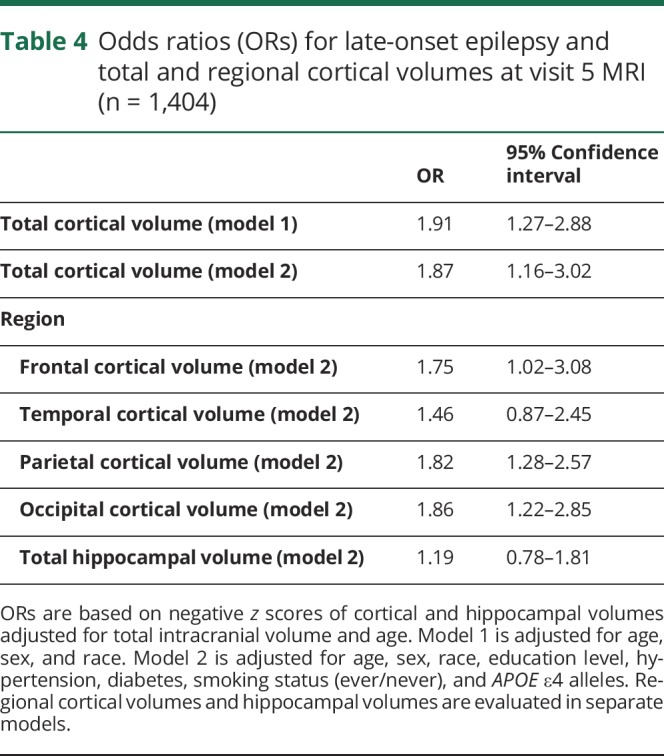
Total hippocampal volume was not associated with LOE (adjusted OR 1.19, 0.78–1.81). Volume of WMHs from ARIC-NCS MRI was also not associated with LOE (adjusted OR 1.33, 0.85–2.08).
A comparison of ORs for each region of cortex when analyzed separately found that the individual volumes of the frontal, parietal, and occipital regions were associated with LOE in separate models (table 4).
A total of 120 participants who had MRI at visit 5 were diagnosed with stroke or dementia at any time. Of these, 3 had stroke diagnosed prior to development of LOE (none had dementia prior to LOE; figure, C). When only participants without stroke or dementia by the time of MRI were analyzed, the association between lower total cortical volume and LOE persisted (adjusted OR 1.96, 1.24–3.09).
Sensitivity analyses
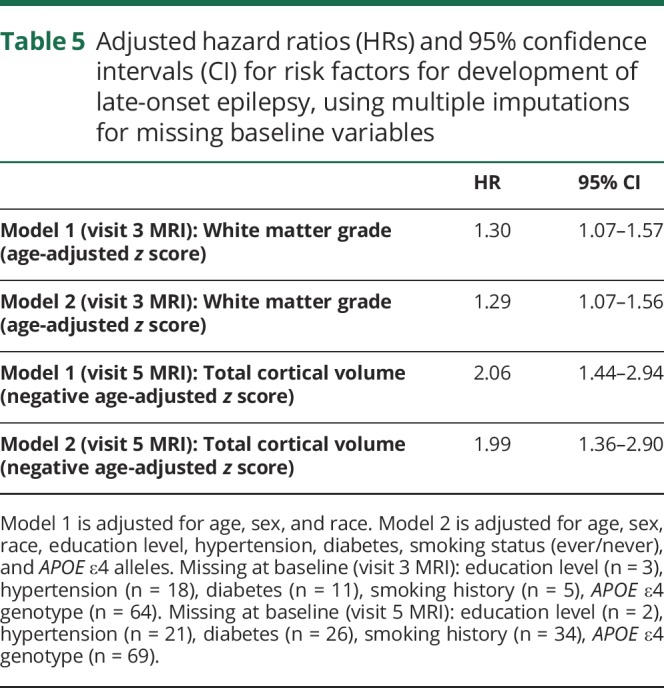
Use of multiple imputation for participants missing data (hypertension, diabetes, education level, smoking status, APOE ε4 genotype) did not substantially alter the results (table 5).
Table 5.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for risk factors for development of late-onset epilepsy, using multiple imputations for missing baseline variables
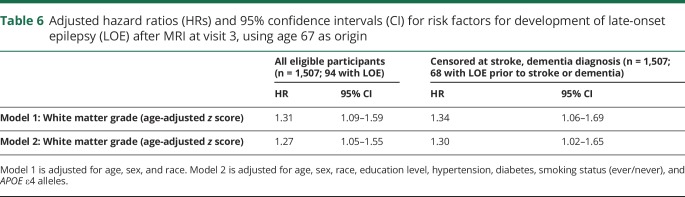
A model using participants' 67th birthday as the origin time for longitudinal analysis of LOE development after visit 3 MRI yielded similar results (table 6).
Table 6.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for risk factors for development of late-onset epilepsy (LOE) after MRI at visit 3, using age 67 as origin
Discussion
We found an association between higher degree of WMHs on MRI and increased likelihood of later development of LOE, which persisted after adjusting for demographics and vascular risk factors, as well as after censoring at time of stroke or dementia. These results are in agreement with existing case-control studies that have demonstrated increased white matter disease in age-matched participants with LOE,8 and furthermore demonstrate a longitudinal relationship, as we included only participants with a first seizure after the date of the MRI for this analysis. WMHs are known to be associated with hypertension,25 infarcts,15 and cortical microinfarcts26 and seem to be a marker for overall brain vascular health,25 thus in this study may be a marker for cortical disease as well. This would explain the association between white matter disease and seizures, which originate in gray matter rather than white matter.
We also found an association between lower total cortical volumes and LOE, which similarly persisted after adjusting for demographics and vascular risk factors. This finding is in agreement with prior literature, as one small case-control study of 16 patients with LOE and controls matched for age previously suggested lower cortical volumes in patients with LOE than controls.9 As very few participants developed LOE after cortical volumes were measured at the ARIC-NCS MRI, no temporal relationship between cortical volumes and LOE was possible to observe at this time. We previously demonstrated that the APOE ε4 genotype is associated with LOE,1 which held true in this study as well. APOE ε genotype is also known to be associated with cortical volume, with lower hippocampal and cortical volumes associated with the APOE ε4 genotype.11,12 In this study, the association of LOE with cortical volume persisted after adjusting for APOE ε4 genotype.
We did not find an association between hippocampal volume and LOE. This was somewhat unexpected, as decreased hippocampal volume is associated with some types of Alzheimer disease,27 which is associated with an increased risk of seizures28 in the elderly. However, some studies suggest that seizures occur relatively late in the Alzheimer disease course,29,30 and these participants may not have been included in the visit 5 MRI. WMH burden is known to be associated with cortical atrophy in the ARIC cohort, with a higher white matter score associated with lower cortical volume.15,31 This was the case for our analysis as well, with higher white matter grade at visit 3 associated with lower total cortical volume at visit 5, p = 0.013.
Interestingly, the cross-sectional association between WMH volume at visit 5 and LOE was not significant in multivariable analysis. WMHs are increasingly prevalent with increasing age, and are present in over 90% of the general population by age 80.32 No association was found between ARIC-NCS MRI white matter disease (at which participants were age 67–90) and LOE. This suggests that earlier white matter disease (as identified at visit 3, at which participants were age 49–72) is indicative of the microvascular disease pathology that leads to LOE, which is cumulative or progressive rather than immediately present when white matter disease develops.
A previous comparison of poststroke epilepsy to leukoaraiosis-associated epilepsy found a higher proportion of the leukoaraiosis-associated epilepsy patients with temporal lobe epilepsy than frontal lobe epilepsy, while the opposite was true in poststroke epilepsy.33 We found that low cortical volumes in the frontal, parietal, and occipital regions as well as global cortical volume were associated with LOE; however, whether low regional cortical volumes correspond to the seizure focus in participants with LOE remains to be determined in further studies.
We previously found the incidence of LOE in ARIC participants to be 3.3 per 1,000 person-years,13 somewhat higher than other recently reported incidences of 2.4–2.5 per 1,000 person-years2,3; this may be due in part to the relatively high proportion of black participants (25% of ARIC participants) as the rate of LOE is known to be higher in black than in white individuals.2,3,13
The strengths of this study are the longitudinal nature of comparison of white matter scores and later epilepsy, large size, and prospective nature. These strengths allow us to include and adjust for vascular and lifestyle risk factors in our models, which is of vital importance given the high association of hypertension and smoking with the imaging measures under study. Limitations include the small number of cases of LOE with MRI at visit 5, and the reliance on hospitalization discharge and CMS claims data and resulting imperfection of case identification; this reliance may also limit the generalizability to persons who do not use Medicare FFS. Cortical and regional volumes from visit 3 MRI are not available, which precludes longitudinal analysis of association of cortical volumes with later epilepsy. Numbers were insufficient to allow examination of association of epilepsy with specific patterns of WMHs or infarcts. In addition, there were only 8 participants with LOE and both visit 3 MRI and ARIC-NCS MRI, which prevented analysis of the effects of white matter progression over time. The lower rate of repeat MRI (at the ARIC-NCS MRI after visit 3 MRI) in participants with LOE compared to without LOE may be due to higher rates of missed visits and deaths in participants with LOE. The reasons for this remain to be explored, but may include increased medical comorbidities in participants with LOE.
Our findings suggest the role of occult cerebrovascular disease in LOE (as both white matter disease and cortical volumes are associated with cerebrovascular disease), even in the absence of stroke or dementia.7 However, besides cerebrovascular disease, cortical volume loss is associated with aging,34 impaired glucose metabolism,35 and neurodegenerative disease including Alzheimer disease,36 which may also contribute to LOE. We adjusted for hypertension, diabetes, smoking, and APOE ε4 genotype in our models, and excluded participants with known dementia, to attempt to address these considerations. The degree of these structural findings adds additional risk of LOE beyond the known risk factors of hypertension, diabetes, smoking, and APOE ε4,13 which were included in our models. This may imply that there is heterogeneity in susceptibility to the effects of these vascular and lifestyle risk factors; further studies to develop prediction models to identify patients at risk for LOE should incorporate imaging findings as well as medical and social risk factors.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their contributions.
Glossary
- ARIC
Atherosclerosis Risk in Communities
- ARIC-NCS
Atherosclerosis Risk in Communities Neurocognitive Study
- CHS
Cardiovascular Health Study
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- FFS
fee-for-service
- HR
hazard ratio
- ICD-9
International Classification of Diseases–9
- LOE
late-onset epilepsy
- OR
odds ratio
- WMH
white matter hyperintensity
Appendix. Authors
Study funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part by federal funds from the National Heart, Lung, and Blood Institute, NIH, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. Neurocognitive data are collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917, with previous brain MRI examinations funded by R01-HL70825.
Disclosure
E. Johnson reports no disclosures. G. Krauss is a consultant and investigator for Eisai Laboratory and SK Bios and an investigator for UCB Pharma. A. Lee reports no disclosures. A. Schneider is supported by the NIH/NINDS via an administrative supplement to award R25NS065729. A. Kucharska-Newton and J. Huang report no disclosures. C. Jack receives research funding from the NIH and the Alexander Family Alzheimer Disease Research Professorship of the Mayo Foundation Family. R. Gottesman serves as Associate Editor for Neurology® and receives research support from the NIH. Go to Neurology.org/N for full disclosures.
References
- 1.Cloyd J, Hauser W, Towne A, et al. Epidemiological and medical aspects of epilepsy in the elderly. Epilepsy Res 2006;68(suppl 1):S39–S48. [DOI] [PubMed] [Google Scholar]
- 2.Choi H, Pack A, Elkind MSV, Longstreth WT, Ton TGN, Onchiri F. Predictors of incident epilepsy in older adults: the Cardiovascular Health Study. Neurology 2017;88:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older U.S. Medicare beneficiaries. Neurology 2012;78:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia 2006;47:867–872. [DOI] [PubMed] [Google Scholar]
- 5.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993;34:453–468. [DOI] [PubMed] [Google Scholar]
- 6.Rowan AJ, Ramsay RE, Collins JF, et al. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology 2005;64:1868–1873. [DOI] [PubMed] [Google Scholar]
- 7.Gibson LM, Hanby MF, Al-Bachari SM, Parkes LM, Allan SM, Emsley HC. Late-onset epilepsy and occult cerebrovascular disease. J Cereb Blood Flow Metab 2014;34:564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell H, Hanby M, Parkes LM, Gibson LM, Coutinho C, Emsley HCA. Prevalence and subtypes of radiological cerebrovascular disease in late-onset isolated seizures and epilepsy. Clin Neurol Neurosurg 2013;115:591–596. [DOI] [PubMed] [Google Scholar]
- 9.Hanby MF, Al-Bachari S, Makin F, Vidyasagar R, Parkes LM, Emsley HCA. Structural and physiological MRI correlates of occult cerebrovascular disease in late-onset epilepsy. Neuroimage Clin 2015;9:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitkunan A, Lanfranconi S, Charlton R, Barrick T, Markus H. Brain atrophy and cerebral small vessel disease. Stroke 2011;42:133–138. [DOI] [PubMed] [Google Scholar]
- 11.Lapointe E, Deacon C, Royer-Perron L, Cunnane S, Castellano CA, Bocti C. Temporal lobe atrophy may be underrecognized in older patients with new-onset epilepsy. Can J Neurol Sci 2016;43:731–734. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman RF, Coresh J, Catellier DJ, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson EL, Krauss GL, Lee AK, et al. Association between midlife risk factors and late-onset epilepsy. JAMA Neurol 2018;75:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The ARIC Investigators. The Atherosclerosis Risk in Communities study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities-Neurocognitive Study. Stroke 2015;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucharska-Newton AM, Heiss G, Ni H, et al. Identification of Heart failure events in Medicare claims: the Atherosclerosis Risk in Communities (ARIC) study. J Card Fail 2016;22:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC-PET amyloid imaging study: brain amyloid differences by age, race, sex, and APOE. Neurology 2016;87:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power MC, Deal JA, Sharrett AR, et al. Smoking and white matter hyperintensity progression: the ARIC-MRI study. Neurology 2015;84:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottesman RF, Schneider ALC, Albert M, et al. Midlife hypertension and 20-year cognitive change. JAMA Neurol 2014;71:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones SA, Gottesman RF, Shahar E, Wruck L, Rosamond WD. Validity of hospital discharge diagnosis codes for stroke. Stroke 2014;45:3219–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lonstreth W, Manolio T, Arnold A, et al. Clinical correlates of white matter findings on cranial imaging of 3301 elderly people. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B. FreeSurfer. Neuroimage 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR, O'Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging 2001;14:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moroni F, Ammirati E, Rocca MA, Filippi M, Magnoni M, Camici PG. Cardiovascular disease and brain health: focus on white matter hyperintensities. IJC Hear Vasc 2018;19:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilal S, Sikking E, Shaik MA, et al. Cortical cerebral microinfarcts on 3T MRI: a novel marker of cerebrovascular disease. Neurology 2016;87:1583–1590. [DOI] [PubMed] [Google Scholar]
- 27.ten Kate M, Barkhof F, Boccardi M, et al. Clinical validity of medial temporal atrophy as a biomarker for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging 2017;52:167–182. [DOI] [PubMed] [Google Scholar]
- 28.Irizarry MC, Jin S, He F, et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol 2012;69:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez MF, Catanzaro P, Doss RC, Arguello R. Seizures in Alzheimer’s disease: clinicopathologic study. J Geriatr Psychiatr Neurol 2014;7:230–233. [DOI] [PubMed] [Google Scholar]
- 30.Romanelli MF, Morris JC, Ashkin K, Coben LA. Advanced Alzheimer's disease is a risk factor for late-onset seizures. Arch Neurol 1990;47:847–850. [DOI] [PubMed] [Google Scholar]
- 31.Mosley TH, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning the Atherosclerosis Risk in Communities study. Neurology 2005;64:2056–2062. [DOI] [PubMed] [Google Scholar]
- 32.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study: The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasparini S, Ferlazzo E, Beghi E, et al. Epilepsy associated with leukoaraiosis mainly affects temporal lobe: a casual or causal relationship? Epilepsy Res 2015;109:1–8. [DOI] [PubMed] [Google Scholar]
- 34.Raji CA, Lopez OL, Kuller LH, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging 2012;33:834.e7–834.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw ME, Nettersheim J, Sachdev PS, Anstey KJ, Cherbuin N. Higher fasting plasma glucose is associated with increased cortical thinning over 12 years: the PATH through life study. Brain Topogr 2017;30:408–416. [DOI] [PubMed] [Google Scholar]
- 36.Dickerson BC, Wolk DA. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 2012;78:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full data are not publicly available because of participant consent and privacy. A public-use ARIC dataset is available through BioLINCC. Additional data are available to researchers who submit a manuscript proposal to the ARIC publications committee.