Abstract
Objective
To describe the histopathologic features of a case of facial-onset sensory and motor neuronopathy (FOSMN).
Methods
We describe a postmortem examination performed on a 54-year-old man with FOSMN associated with personality change.
Results
Postmortem examination revealed TAR DNA-binding protein (TDP) 43 proteinopathy with widespread distribution. TDP43 pathology was seen in the neurons and glial cells and was most pronounced in the subthalamic nucleus followed by the spinal cord, including dorsal root ganglia, brainstem, and other deep cerebral nuclei. In the medial temporal lobe, neocortex and subcortical hemispheric white matter TDP43 pathologic inclusions were very rare. In contrast to TDP43 pathologies associated with typical amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD)–TDP, in this case, there were more frequent TDP43-positive oligodendroglial, coiled body–like cytoplasmic inclusions than neuronal inclusions. Neuronal cytoplasmic TDP43 inclusions with globular and skein-like morphology were seen in both anterior horn cells and dorsal root ganglia. No β-amyloid, α-synuclein, or significant hyperphosphorylated tau pathology was seen.
Conclusion
This case provides further evidence that FOSMN is a neurodegenerative disease characterized by TDP43 pathology. Despite minimal cortical TDP43 pathology, the clinical features of the behavioral variant of FTD in this patient suggest that FOSMN may fall within or overlap with the FTD-ALS spectrum.
Facial-onset sensory and motor neuronopathy (FOSMN) is a rare clinical syndrome characterized by asymmetric facial numbness or paresthesia, bulbar palsy, and facial weakness, which may progress to the upper limbs.1 The FOSMN syndrome was first described in 2006, and since then, >40 cases have been described.2,3 Onset is typically in the fifth to seventh decade (but has been reported in patients as young as 7 years of age4), and the rate of progression can vary from months to decades.
The pathogenesis of FOSMN remains controversial. Initial reports described the presence of anti-ganglioside antibodies and response to immunotherapy,5 whereas others have described a progressive and terminal decline, resistant to immunotherapy and suggestive of bulbar-onset amyotrophic lateral sclerosis (ALS).3,6–8 Two of 3 postmortem studies of patients with FOSMN have revealed the presence of TAR DNA-binding protein (TDP) 43 inclusions in the brainstem nuclei and cervical motor neurons.3,7,8 In this article, we describe a patient with FOSMN and behavioral change in whom postmortem examination revealed frequent pathologic TDP43 inclusions in the deep cerebral nuclei, brainstem, and spinal cord and rarely in the medial temporal lobe, frontal cortex, and dorsal root ganglia (DRG).
Methods
Formalin-fixed, paraffin-embedded brain and spinal cord tissue was available from selected regions (table 1). The paraffin sections were cut at 5 μm, mounted on glass slides, and stained with routine hematoxylin and eosin. Representative 5-μm sections were immunostained for TDP43, β-amyloid, α-synuclein, hyperphosphorylated tau, p62, and CD68 with the following antibodies: TDP43 (2E2-D3, 1:3,000, Abcam, Cambridge, UK), β-amyloid (6F3D, 1:50, DAKO, Glostrup, Denmark), α-synuclein (KM51, 1:50, Leica/Novocastra, Buffalo Grove, IL), AT8, (MN1020, 1:100, Invitrogen, Carlsbad, CA), p62 (3/P62LCK Ligand, 1:100, BD Transduction, East Rutherford, NJ), and CD68 (PG-M1, 1:100, DAKO), respectively. Immunostaining was performed on either a BondMax autostainer (Leica Microsystems, Wetzlar, Germany) or a Roche (Basel, Switzerland) Ventana Discovery automated staining platform following the manufacturer's guidelines, using biotinylated secondary antibodies and a horseradish peroxidase–conjugated streptavidin complex and diaminobenzidine as a chromogen. All immunostainings were carried with appropriate controls. Gliosis, microglial activity, and the density of TDP43 pathologic inclusions were scored semiquantitatively.
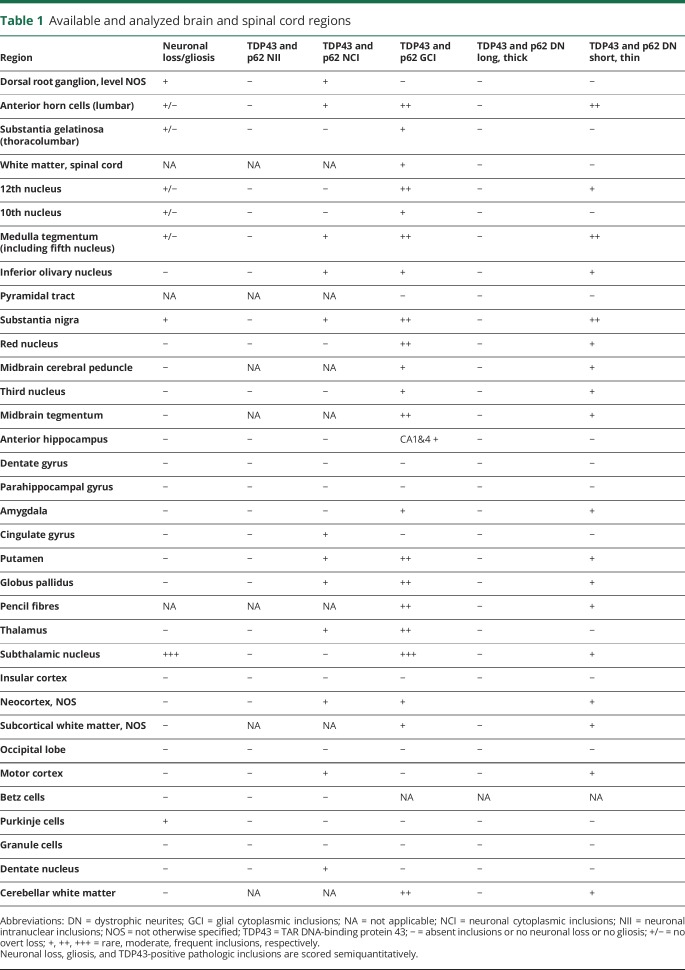
Table 1.
Available and analyzed brain and spinal cord regions
Data availability statement
All data relevant to this study are contained within the manuscript.
Results
A 54-year-old right-handed man presented with symptoms of facial numbness, dysarthria, and dysphagia and inappropriate behavior. Nine years previously, after a dental extraction, he developed numbness of the left upper lip that over 5 years spread to involve his tongue and left cheek and was associated with dysphagia and nasal regurgitation. A collateral history obtained from his wife reported that since the onset of the dysphagia, the patient's behavior had changed and he had difficulty maintaining his train of thought. There was no family history of neurodegenerative disease.
A Mini-Mental State Examination was normal; however, he was noted to have an inappropriate jocular manner. Clinical examination revealed a cachectic man. There was diminished light touch sensation in all territories of the trigeminal nerve and diminished pinprick sensation in the left mandibular territory. The corneal reflexes were absent. There was bilateral facial myokymia. The gag reflex was absent, and there was weakness of neck flexion, along with wasting and fasciculation of the tongue. In the upper limbs, there was wasting and weakness of the deltoids. There was no wasting, weakness, or sensory disturbance of the lower limbs. The knee and ankle reflexes were preserved, and the plantar responses were absent.
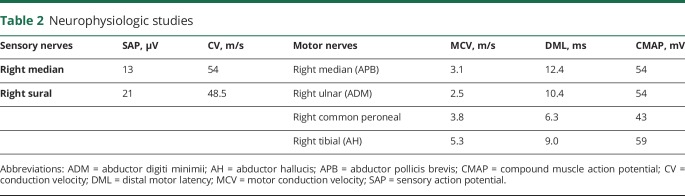
Nerve conduction studies performed 5 years after the onset of his symptoms revealed normal sensory and motor action potentials and conduction velocities in the upper and lower limbs (table 2). EMG demonstrated large individual motor units firing at high rates in a grossly reduced interference pattern, indicative of chronic denervation, in the sternocleidomastoid and orbicularis oculi. There was no evidence of acute denervation. Blink reflexes were absent bilaterally.
Table 2.
Neurophysiologic studies
MRI scans of the brain and spine were normal. Fluorodeoxyglucose PET CT scan revealed an enlarged jugular lymph node that was subsequently biopsied and showed nonspecific reactive lymphoid hyperplasia. Duodenal biopsies were negative for Whipple disease. A lip biopsy was unremarkable. CSF examination was normal, including oligoclonal bands and Whipple PCR. Genetic testing for Kennedy disease, dentatorubral-pallidoluysian atrophy, and spinocerebellar ataxia types 1, 2, 3, 6, and 7 was negative. Routine blood tests were all normal.
Formal neuropsychometry demonstrated a verbal IQ of 115 and performance IQ of 114, with average subtest scores except for arithmetic and picture completion, which were superior. Recognition memory tests were almost flawless (words 48 of 50, faces 50 of 50). Although he performed adequately on tests sensitive to frontal lobe dysfunction, he was noted to be inappropriately jocular during testing.
The patient was diagnosed with a progressive brainstem syndrome. In hindsight, the clinical syndrome is FOSMN, but this diagnosis had not been described at the time the patient presented. Because an inflammatory brainstem disorder remained a possibility, the patient was treated with a 3-month course of oral steroids, but his condition continued to deteriorate, and he was readmitted to hospital with an aspiration pneumonia. A percutaneous gastrostomy tube was inserted, but against medical advice, he continued to eat and drink, leading to a final episode of aspiration pneumonia, of which he died, 9 years after the onset of his symptoms.
Postmortem examination
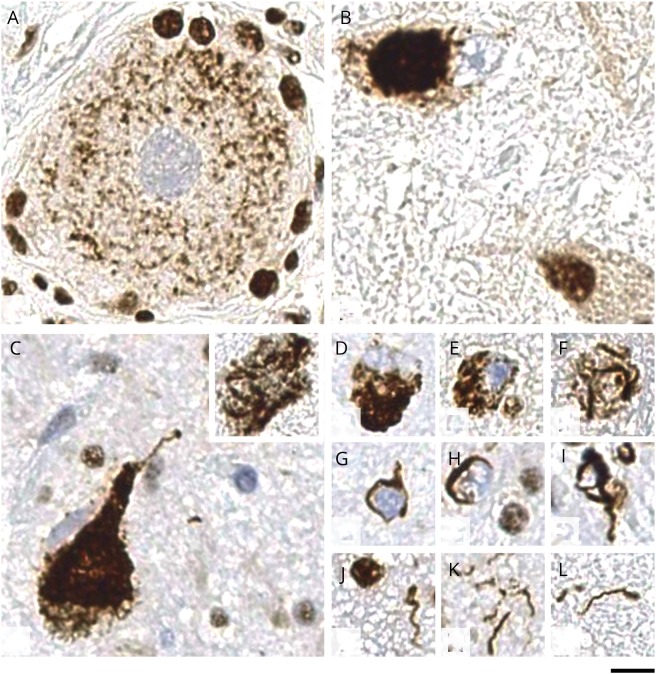
Pathologic TDP43-positive inclusions were seen in the form of neuronal cytoplasmic inclusions (NCIs), oligodendroglial, coiled body–like cytoplasmic inclusions (GCIs), and dystrophic neurites (figure). GCIs and dystrophic neurites were more frequent than NCIs. TDP43 pathology was most prominent in the subthalamic nucleus, followed by the thalamus, putamen, and globus pallidus (other deep cerebral nuclei were not available for the assessment), brainstem, and spinal cord (table 1). NCIs with globular and skein-shaped morphology were seen in both motor (anterior horn cells) and sensory (dorsal root ganglion) neurons. In the neocortex, subcortical hemispheric white matter, anterior hippocampus, amygdala, and cingulate gyrus, TDP43 pathology was present but very rare. In the cerebellum, there were GCIs in the white matter and rare NCIs in the dentate nucleus but none in the cerebellar cortex (table 1). TDP43-positive inclusions also showed immunoreactivity for p62. There were no diagnostically specific p62 immunoreactive inclusions in the cerebellum or hippocampal dentate gyrus to suggest C9ORF72 pathology or neuronal intranuclear inclusions characteristic of VCP or p62 mutations. Microglial activity, highlighted with CD68 immunostaining, was most prominent in the subthalamic nucleus with comparably mild activation elsewhere, including the corticospinal tracts and anterior and posterior nerve roots in the spinal cord. No β-amyloid, α-synuclein, or significant hyperphosphorylated tau pathology was seen.
Figure. TDP43 proteinopathy in FOSMN.
(A) Pathologic thread-like deposits in a neuron within dorsal root ganglion. (B) Globular cytoplasmic inclusion in the lumbar anterior horn motor neuron. (C) Large globular and skein-like (inset) cytoplasmic inclusions in pigmented neurons of the substantia nigra. (D–F) Various morphologies of neuronal cytoplasmic inclusions. (G– I) glial cytoplasmic inclusions, all of which show similar, coiled body–like morphology. (J–L) Appearances of dystrophic neurites, all of which are short and curved with no evidence of long neurites. All sections are immunostained with nonphosphorylated TAR DNA-binding protein 43 (TDP43) antibody, which detects normal nuclear TDP43 labeling and shows absent nuclear labeling in cells where there is TDP43 mislocalization from the nucleus to cytoplasm or cell processes. Scale bar: 10 μm in panels A–L. FOSMN = facial-onset sensory and motor neuronopathy.
Discussion
In this report, we describe a case of FOSMN with personality change and a postmortem examination characterized by TDP43-positive inclusions in the cerebral cortex, brainstem, and spinal cord motor neurons and DRGs. Our findings corroborate the findings of 2 of 3 previous postmortem examinations of patients with FOSMN in which TDP43 inclusions were identified in the brainstem.3,7,8 This report provides further evidence that FOSMN may be considered a forme fruste of bulbar-onset ALS, a notion further supported by the behavioral change and TDP43 inclusions in the frontal cortex. We were unable to measure β-amyloid42 and tau in the CSF because this assay was not available 15 year ago at the time of the patient's illness. It should also be noted that although the presence of TDP43 inclusions in the neocortex was unequivocal, they were infrequent.
The clinical syndrome of FOSMN was first described in 2006, several years after our patient’s death.2 Further histologic analysis was prompted by reports of TDP43 inclusions in postmortem tissue of patients with FOSMN.8 At the time of our patient's illness, mutations in C9ORF72, FUS, and TARDBP had not yet been discovered to cause ALS.
This report also describes the identification of TDP43 inclusions in the DRG of a patient with a sensory neuronopathy. This is an intriguing observation, and it is tempting to speculate that a neurodegenerative pathology, characterized by TDP43 inclusion in the DRG, may underlie a proportion of patchy asymmetric sensory neuronopathies that follow a progressive course and are resistant to immunosuppressive therapy.9 In further support of the pathogenicity of the TDP43 inclusions in the DRG is the recent report of a patient with an asymmetric patchy and progressive sensory and motor neuronopathy in whom a p.Arg382Pro missense mutation in TARDBP was discovered.10 This mutation is not present in the Exac database and has previously been described in patients with ALS.11 An additional distinctive feature seen in this patient and reported in the literature8 is the presence of widespread glial TDP43 pathology. TDP43-positive glial inclusions are also observed in both ALS and frontotemporal dementia–TDP, but to a much lesser extent, suggesting that there might be differences in molecular pathogenesis between FOSMN and ALS–frontotemporal dementia spectrum.
We describe TDP43 proteinopathy in a patient with FOSMN and personality change. The presence of TDP43 proteinopathy in the brain, spinal cord, and DRG and the discovery of a pathogenic TDP-43 mutation in a patient with an asymmetric sensory and motor neuronopathy indicate that some sensory neuronopathies have a degenerative pathogenesis.
Glossary
- ALS
amyotrophic lateral sclerosis
- DRG
dorsal root ganglia
- FOSMN
facial-onset sensory and motor neuronopathy
- GCI
oligodendroglial coiled body–like cytoplasmic inclusion
- NCI
neuronal cytoplasmic inclusion
- TDP
TAR DNA-binding protein
Appendix. Authors
Study funding
A.M.R. is funded by a Wellcome Trust Postdoctoral Fellowship for Clinicians (110043/Z/15/Z). M.M.R. is grateful to the Medical Research Council (MRC) for an MRC Centre grant (G0601943) and to the National Institute of Neurological Disorders and Stroke (NINDS) and Office of Rare Diseases (ORDR) (U54NS065712) for their support. The Inherited Neuropathy Consortium (U54NS065712) is part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the ORDR, NCATS, funded through a collaboration between NCATS and the NINDS. This research was also supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Disclosure
A. Rossor has received support from Alnylam UK Ltd to attend scientific meetings and an honorarium for speaking at a sponsored symposium. Z. Jaunmuktane, M. Rossor, G. Hoti, and M. Reilly report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Broad R, Leigh PN. Recognising facial onset sensory motor neuronopathy syndrome: insight from six new cases. Pract Neurol 2015;15:293–297. [DOI] [PubMed] [Google Scholar]
- 2.Vucic S, Tian D, Chong PST, Cudkowicz ME, Hedley-Whyte ET, Cros D. Facial onset sensory and motor neuronopathy (FOSMN syndrome): a novel syndrome in neurology. Brain 2006;129:3384–3390. [DOI] [PubMed] [Google Scholar]
- 3.Ziso B, Williams TL, Walters RJL, et al. Facial onset sensory and motor neuronopathy: further evidence for a TDP-43 proteinopathy. Case Rep Neurol 2015;7:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karakis I, Vucic S, Srinivasan J. Facial onset sensory and motor neuronopathy (FOSMN) of childhood onset. Muscle Nerve 2014;50:614–615. [DOI] [PubMed] [Google Scholar]
- 5.Knopp M, Vaghela NN, Shanmugam SV, Rajabally YA. Facial onset sensory motor neuronopathy: an immunoglobulin-responsive case. J Clin Neuromuscul Dis 2013;14:176–179. [DOI] [PubMed] [Google Scholar]
- 6.Dalla Bella E, Rigamonti A, Mantero V, et al. Heterozygous D90A-SOD1 mutation in a patient with facial onset sensory motor neuronopathy (FOSMN) syndrome: a bridge to amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2014;85:1009–1011. [DOI] [PubMed] [Google Scholar]
- 7.Vucic S, Stein TD, Hedley-Whyte ET, et al. FOSMN syndrome: novel insight into disease pathophysiology. Neurology 2012;79:73–79. [DOI] [PubMed] [Google Scholar]
- 8.Sonoda K, Sasaki K, Tateishi T, et al. TAR DNA-binding protein 43 pathology in a case clinically diagnosed with facial-onset sensory and motor neuronopathy syndrome: an autopsied case report and a review of the literature. J Neurol Sci 2013;332:148–153. [DOI] [PubMed] [Google Scholar]
- 9.Marquez-Infante C, Murphy SM, Mathew L, et al. Asymmetric sensory ganglionopathy: a case series. Muscle Nerve 2013;48:145–150. [DOI] [PubMed] [Google Scholar]
- 10.Camdessanché JP, Belzil VV, Jousserand G, et al. Sensory and motor neuronopathy in a patient with the A382P TDP-43 mutation. Orphanet J Rare Dis 2011;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daoud H, Valdmanis PN, Kabashi E, et al. Contribution of TARDBP mutations to sporadic amyotrophic lateral sclerosis. J Med Genet 2009;46:112–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to this study are contained within the manuscript.