Abstract
Objective
This study explores the use of quantitative data on strength and fatigability of orofacial muscles in patients with facioscapulohumeral muscular dystrophy (FSHD) and assesses the frequency of swallowing and communication difficulties and their relationship to orofacial muscle involvement.
Methods
We included 43 patients with FSHD and 35 healthy controls and used the Iowa Oral Performance Instrument (IOPI) to obtain quantitative measurements of strength and endurance of lip compression, cheek (buccodental) compression, and tongue elevation. For the assessment of swallowing and communication difficulties, we used the dysphagia-specific quality of life (SWAL-QOL) and Communicative Participation Item Bank questionnaires.
Results
Cheek compression strength was reduced in patients with FSHD compared to healthy controls. Dysphagia and difficulty with verbal communication were reported by 25% and 35% of patients, respectively, and correlated to cheek compression strength and endurance and to anterior tongue elevation endurance. Prolonged cheek compression or anterior tongue elevation endurance (decreased fatigability) made swallowing or speech problems less likely to occur.
Conclusion
Cheek compression strength is the most sensitive IOPI measure for orofacial weakness in FSHD. Orofacial weakness contributes to dysphagia and speech difficulties in FSHD, which are both common, though generally mild. Higher endurance of orofacial muscles was associated with a lower chance of dysphagia or speech problems. More research is required for further refinement of the pattern of facial muscle involvement in FSHD and to provide new insights for improvement of speech and language therapy.
Facioscapulohumeral muscular dystrophy (FSHD) is a progressive inherited muscle disorder. One of the first and most characteristic symptoms of FSHD is weakness of the facial muscles, which is often asymmetrical and varies from minimal weakness that is barely notable to marked paresis of mimetic muscles.1 The circular muscles around the eyes and mouth (orbicularis oculi and orbicularis oris) and the muscle that raises the corners of the mouth (zygomaticus major) are frequently affected.1,2 Weakness of the facial muscles limits facial expression and is identified by patients as a disabling symptom of FSHD that affects their lives.3,4 Despite the frequency and relevance of facial weakness in FSHD, there are few studies on the severity, progression, and functional and emotional consequences of facial weakness.
While studies report mild to moderate dysphagia or involvement of the tongue muscle in a subgroup of patients with FSHD,5–8 to our knowledge, studies assessing the consequences of facial weakness on speech or communication have not been reported in FSHD. Here we performed a cross-sectional 2-site study to obtain quantitative data on strength and fatigability of orofacial muscles in FSHD. In addition, we assess the frequency of self-reported swallowing and communication difficulties, and explore how these relate to orofacial muscle involvement.
Methods
Participants
Patients with genetically confirmed FSHD aged 18 years and older were recruited in 2016–2017 at the Kansas University Medical Center (Kansas City) and at the University of Utah Hospital (Salt Lake City). Healthy controls were recruited in 2017 at the University of Utah Hospital.
Standard protocol approvals, registrations, and patient consents
The study was approved by the human subjects committee. Written informed consent was obtained from all participants.
Disease severity rating
All patients with FSHD underwent a standardized physical examination with a physician rating the severity of their FSHD symptoms. The FSHD clinical score rates muscle involvement in the face, shoulders and arms, core, and legs, and provides a combined score that ranges from 0 (unaffected) to 15 (severely affected).9
The physician also graded facial functioning for all patients with FSHD (facial function score). The ability to furrow the brow, close the eyes, and protrude the lips were each scored bilaterally, and the ability to puff out the cheeks was scored overall on a scale from 0 to 2 (0 = normal; 1 = partial weakness; 2 = cannot perform). The total score ranges from 0 to 14, in which higher scores represent higher disease severity.
Quantitative measurements of orofacial muscles
Quantitative strength measurements of orofacial muscles were obtained with the Iowa Oral Performance Instrument (IOPI) (IOPI Medical, Redmond, WA), a handheld device that attaches via tubing to an air-filled bulb to measure pressure.10 Measurements of strength and endurance for motions of lip compression, cheek compression, and anterior and posterior tongue elevation were performed based on standardized procedures as described by Clark and Solomon10 with the following modification: lip compression measurements were performed by having the participants squeeze their lips with the bulb positioned between the lips at midline (without tongue depressors). Cheek (buccodental) compression measurements were obtained with the bulb placed inside the cheek next to the corner of the mouth and along the occlusal surface of the teeth and having the participants purse their lips, subsequently squeezing the bulb against the teeth. For tongue elevation measurements, participants were instructed to elevate their tongue against the palate, with the bulb positioned on the anterior or posterior tongue. For strength measurements, participants were asked to exert maximum pressure on the bulb and peak strength was recorded in kPa. Three trials were recorded with 10 seconds of rest in between. For endurance measurements, an indicator of muscle fatigability, participants were asked to maintain 50% of their maximum pressure for as many seconds as possible and timing ceased when participants demonstrated a decrease from target pressure that was sustained for longer than 1 second. Two trials were performed with a rest period of 2 minutes in between each trial. For analyses of both strength and endurance, the maximum score out of the different trials was used. The IOPI was calibrated monthly according to the manufacturer's description.
Swallowing questionnaire
All patients with FSHD completed the dysphagia-specific quality of life (SWAL-QOL) questionnaire to capture symptoms related to dysphagia and to assess how they perceive swallowing problems affecting day-to-day quality of life.11 This questionnaire contains 44 items with 5 response options, divided into 10 domains (burden, eating duration, eating desire, food selection, communication, fear of choking, mental health, social, sleep, and fatigue). Both the total score and the scores for the domains are scored as a percentage of the maximum achievable score, in which higher scores indicate less difficulty. Normative data were taken from the study by Ginocchio et al.12
Communication questionnaire
The Communicative Participation Item Bank (CPIB), a patient-reported instrument, was used to measure communicative participation in the patients with FSHD.13 The total score from 10 items ranges from 0 to 30, in which higher scores are more favorable. As the CPIB is a Rasch-built scale, raw ordinal summary scores (0–30) are converted to an interval scale in which scores are expressed as logits. The logits range from −2.58 to 2.10 logits, with 0 logits representing the mean for the calibration sample.13 High, positive scores are preferable. This questionnaire was not filled out by the healthy controls.
Statistical analyses
Statistical analyses were performed using SPSS version 22 (SPSS Inc., Chicago, IL). Descriptive statistics were calculated for all measures and are presented as mean, SD, and range unless stated otherwise. To compare strength measurements between patients with FSHD and healthy controls, we performed independent-samples t tests. For correlations of strength measurements with other outcome measures, the Pearson correlation coefficient was used. For the correlation of strength measurements to the facial function score (an ordinal scale), we used a Spearman rho analysis. For correlations of endurance measurements, we used a Spearman rho analysis, because of the non-normal distribution. For the assessment of same day reproducibility (test-retest reliability) of the quantitative strength and endurance measurements, the intraclass coefficient (ICC) was used. p Values of <0.05 were considered statistically significant. Because of the exploratory nature of the study, we did not adjust for multiple testing when testing subcategories of the questionnaires.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Participants
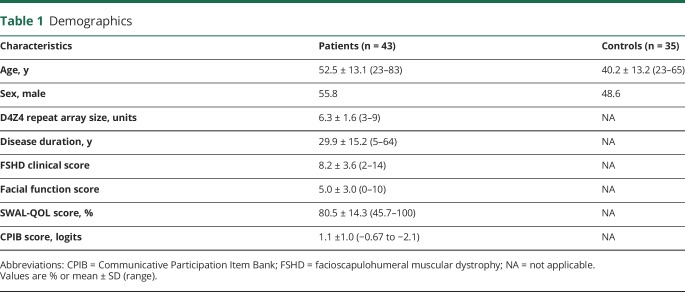
We included 43 patients with FSHD comprising the entire clinical spectrum and 35 healthy controls. Demographics are presented in table 1. The controls were younger than the patients (40.2 vs 52.5 years, p = 0.000).
Table 1.
Demographics
Quantitative measurements of orofacial muscles
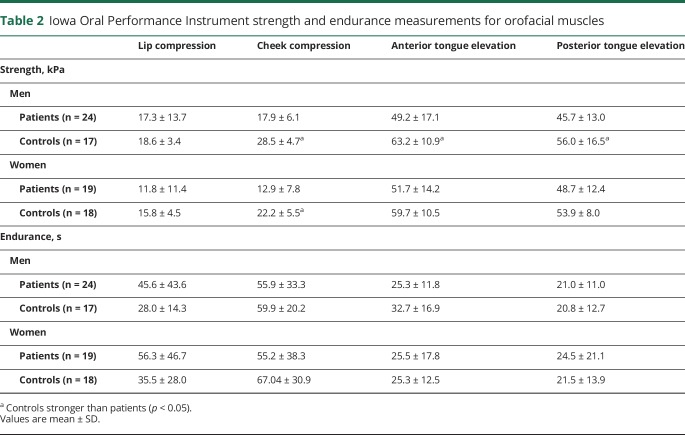
Results of the quantitative strength and endurance measurements are given in table 2. Cheek compression strength was reduced in patients with FSHD compared to healthy controls. Both anterior and posterior tongue elevation strength was reduced in male participants only. Cheek compression strength was the only quantitative measure that correlated to disease duration and overall disease severity (r = −0.494, p = 0.001 and r = −0.454, p = 0.003, respectively). In addition, it was the only quantitative measure that correlated to the physician-reported facial function score (ρ = −0.489, p < 0.001). Test-retest reliability between the different trials in patients was good to excellent for all quantitative measurements with ICCs of 0.947, 0.972, 0.951, and 0.945 for lip compression, cheek compression, anterior tongue, and posterior tongue strength, respectively, and 0.955, 0.923, 0.853, and 0.888 for endurance measures, respectively. The lower ICCs for the tongue elevation endurance measurements were probably due to fatiguing, although the differences between the trials were nonsignificant. Strength and endurance measurements did not correlate to each other, except for lip compression measurements (ρ = 0.375, p = 0.032). IOPI measurements did not correlate to the D4Z4 repeat array size. However, the various repeat array sizes were unevenly distributed, with approximately half of the patients having 7 D4Z4 repeat units.
Table 2.
Iowa Oral Performance Instrument strength and endurance measurements for orofacial muscles
Swallowing
On the SWAL-QOL questionnaire, only one patient achieved the maximum total score, while scores of less than 75% of the maximum score were obtained by 11 (25.6%) patients. The SWAL-QOL scores were lower in patients with FSHD compared to normative values from the literature on all 10 domains (range of differences 6%–36%, all p < 0.001).12 The largest differences between patients and normative values were found in the domains on fatigue and sleep (scores of 43% and 56%, respectively; normal values 79% and 81%). Of the domains related to swallowing, the largest differences were found in eating duration and fear of choking (scores of 82.4% and 86.8%, respectively; normative values 98% and 99%). Although patients reported a fear of choking, only 2 (4.7%) patients reported actually choking often when eating foods and 3 (7.0%) when taking liquids.
Communication
On the CPIB, 18/43 (42%) of the patients had a maximum score, indicating no communication difficulties. Scores of less than 75% of the maximum score were obtained by 15 (34.9%) of the patients. Items that patients with FSHD scored the lowest on were “say something quickly” and “getting your turn in a fast-moving conversation,” both with 22/43 patients (46.5%) reporting difficulty.
Relation of orofacial muscle involvement to swallowing and communication
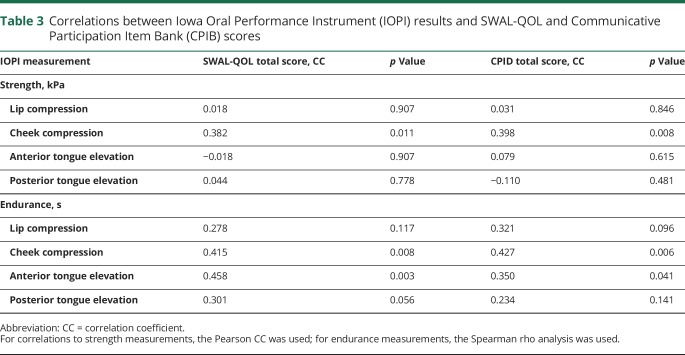
The SWAL-QOL scores decreased with lower cheek compression strength (r = 0.382, p = 0.011) (table 3). The correlation of the total SWAL-QOL score to cheek compression strength was driven by correlations on 4 domains: eating duration, eating desire, communication, and fatigue (range r 0.378–0.400, all p < 0.05). No correlations were found between SWAL-QOL scores and lip compression or tongue elevation strength.
Table 3.
Correlations between Iowa Oral Performance Instrument (IOPI) results and SWAL-QOL and Communicative Participation Item Bank (CPIB) scores
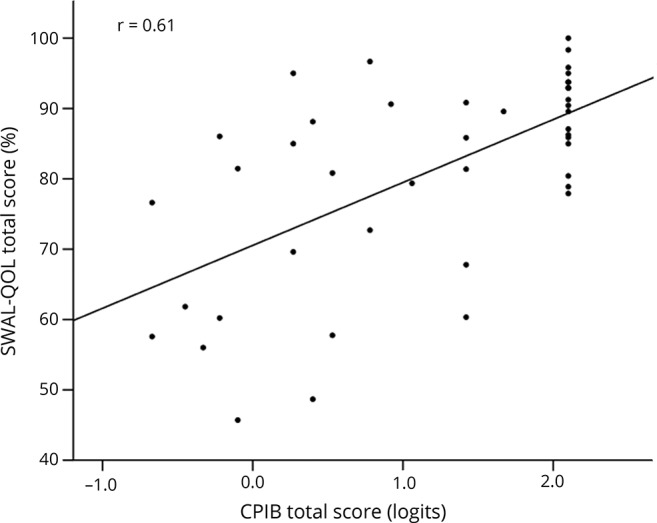
Higher total SWAL-QOL scores correlated to longer cheek compression endurance and anterior tongue elevation endurance (ρ = 0.415, p = 0.008 and ρ = 0.458, p = 0.004, respectively), but not lip compression and posterior tongue elevation endurance. Both correlations were mainly driven by patients who demonstrated extended lingual endurance (>40 seconds) and all of these patients, save one patient, self-reported little to no dysphagia on the SWAL-QOL. The one patient who did report dysphagia (SWAL-QOL score of 68%) demonstrated long cheek compression endurance of 126 seconds, but had the lowest cheek compression strength of all participants (2.5 kPa). The CPIB total score decreased with lower cheek compression strength (r = 0.398, p < 0.01). No correlations were found between CPIB total scores and lip compression and tongue elevation strength. Correlations were found between CPIB scores and cheek compression endurance and anterior tongue elevation endurance, but not lip compression and posterior tongue elevation endurance. Again, there was a subset of patients with long endurance (>75 seconds for cheek compression endurance and >40 seconds for anterior tongue elevation endurance), who all performed well on the CPIB (score > 1.4 logits), except for one patient with very low cheek compression strength (6.0 kPa). There was a partial overlap in patients who reported swallowing problems and communication difficulty. Higher SWAL-QOL scores correlated moderately to higher CPIB scores (r = 0.61; p = 0.000) (figure).
Figure. Correlation between SWAL-QOL and Communicative Participation Item Bank (CPIB) scores.
Discussion
This study reports a systematic approach to assess strength and fatigability of orofacial muscles in FSHD. To our knowledge, this is this the first report of quantitative strength measurements on orofacial muscles in patients with FSHD. We show that cheek compression strength is reduced compared to healthy controls. Cheek compression is a motion that is caused by the contraction of various facial muscles, including the circumferential muscle complex that surrounds the mouth, in particular the orbicularis oris. The orbicularis oris muscle is known to be frequently affected in FSHD.1 Lip compression strength was not reduced compared to healthy controls even though that is also a motion driven by the orbicularis oris. This could suggest that weakness of the lips and mouth is not simply caused by weakness of the orbicularis oris, but in fact is caused by a more complex interplay with other facial muscles like the buccinators or risorius muscles. Indeed, the orbicularis oris is not a simple circular sphincter muscle, but is formed by muscle fibers running in different directions and originating from various facial muscles including the buccinator muscles. This hypothesis is also supported by the reduced cheek compression strength in patients with FSHD. Additional research is required to study involvement of various facial muscles in more detail.
Twenty-five percent of the patients in our study had low scores on the SWAL-QOL questionnaire indicating swallowing difficulties. In the literature, the reported prevalence of dysphagia in FSHD ranges from 2% to 25%.5–8 One case has been reported of a patient with infantile FSHD who required gastrostomy because of swallowing difficulties.14 Scores on the SWAL-QOL domains of fatigue and sleep were the lowest scores, but these findings might be unrelated to swallowing problems and more related to overall disease severity. However, also on all domains regarding more specific swallowing difficulties, patients with FSHD scored lower compared to the normative values. In concordance with 2 previous studies, our study shows that swallowing problems in FSHD occur, but are generally mild.5,6
Low scores on the CPIB questionnaire indicating communication difficulties were found in approximately 35% of the patients in this study. This is in line with a previous study reporting 35% of patients with FSHD having difficulty pronouncing words.7 Possibly, our study underestimates the prevalence of communication difficulties, because the questions asked were nearly all focused on verbal communication, i.e., speech. Though speech can be affected due to orofacial weakness, the lack of facial expression may hinder social communication more than problems with speech. In addition, emotional consequences of facial weakness were not studied, though they may be very relevant to patients.
Cheek compression strength and endurance and anterior tongue endurance correlated to both dysphagia and communication difficulties. A previous study suggested that dysphagia in FSHD is caused by weakness of the lingual and orofacial muscles and showed that in some patients oral and pharyngeal transport of food is delayed.6 The results of our study support this hypothesis, although the only moderately strong correlations indicate that additional unidentified factors must be involved in swallowing and communication difficulties in FSHD. This is also in line with a previous study showing modest correlations between SWAL-QOL results and videofluoroscopy findings in patients with oropharyngeal dysphagia.15 Whether the tongue muscles are involved in FSHD remains uncertain. Multiple studies report small series of patients with FSHD with tongue atrophy or weakness, although the prevalence of tongue involvement seems much lower than the prevalence of dysphagia.6,8,16 In our study, dysphagia and communication difficulties were not related to weakness of the tongue muscles, but instead correlations were driven by a beneficial effect of an extended tongue elevation endurance. One could hypothesize that the longer endurance is in fact a compensatory mechanism to prevent dysphagia and communication difficulties, especially since endurance of the tongue muscles can be improved by training.17 If this is the case, improvement through training of the endurance of facial muscles could potentially be used by speech therapists to teach patients a compensatory strategy.
The most important limitation of this study was the age difference between patients with FSHD and healthy controls for the quantitative strength measurements. Since younger individuals are expected to have higher strength and endurance, this would potentially lead to an overestimation of the difference. For tongue elevation measurements, multiple studies have shown that in group comparisons the oldest participants (aged 60 years and older) have reduced strength.10,18–20 As the patients were older compared to the controls, an effect of age on the differences found in tongue elevation strength in male patients cannot be ruled out. For tongue elevation endurance and for lip and cheek compression strength, there is no association with age in the general population.10,19 The lip compression strength in healthy controls in this study was much lower than normative values that have been previously reported.10 We repeated the measurements in multiple healthy controls and found consistent values. Therefore, it is currently unclear what is causing the differences between the 2 healthy control populations. Test-retest reliability was good to excellent for all IOPI measurements, similar to reliability reported in the literature.21–23
In our study, we used normative values from the literature for the SWAL-QOL, which were collected in an Italian cohort. Although cultural differences cannot be ruled out completely, we chose to use these normative data as the Italian study included a large cohort of healthy volunteers. A limited number of orofacial movements was assessed in this study. To increase knowledge on facial weakness and further refinement of the pattern of involvement, muscles in the upper part of the face should be studied as well. In addition, longitudinal studies could provide valuable information on disease progression over time, and on the correlation between changes in orofacial strength and endurance and changes in dysphagia or communication difficulties.
Cheek compression strength is decreased in patients with FSHD and correlates to dysphagia and communication difficulties. Decreased fatigability of cheek compression and tongue elevation make dysphagia or speech problems unlikely to occur. Additional research is required to confirm and extend these findings, which may be of relevance to gain insights into the clinical phenotype of FSHD, counseling, and potentially treatment of patients with FSHD regarding swallowing and speech.
Acknowledgment
The authors thank the patients with FSHD and family members who were the impetus for the study.
Glossary
- CPIB
Communicative Participation Item Bank
- FSHD
facioscapulohumeral muscular dystrophy
- ICC
intraclass coefficient
- IOPI
Iowa Oral Performance Instrument
Appendix. Authors
Study funding
This study was supported by a CTSA Multi-Institute Pilot grant. Additional funding was provided in part by a CTSA grant from NCATS awarded to the University of Utah and to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (#UL1TR000001). J.M.S.: work on this project was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center (#KL2TR000119). K.M.: supported by a grant from the Dr. Jan Meerwaldt Foundation and the Prinses Beatrix Spierfonds (W.OR12-22).
Disclosure
K. Mul receives support from the Dr. Jan Meerwaldt Foundation and the Prinses Beatrix Spierfonds and a grant from the FSHD Stichting. K. Berggren and M. Sills report no disclosures relevant to the manuscript. B. van Engelen receives grants from Prinses Beatrix Spierfonds, Association Francaise contre les Myopathies, Stichting Spieren voor Spieren, FSHD Stichting, and NWO Dutch Organisation for scientific research. N. Johnson reports serving as a consultant or on advisory boards for the following companies: AMO Pharma, FLEX Pharma, AveXis, Cytokinetics, and Vertex. He has received research support from NINDS (R01NS104010, 1K23NS091511-01), the FDA (R01FD006071), the Myotonic Dystrophy Foundation, and the Muscular Dystrophy Association. J. Statland worked as a consultant or was on Ad Board for Acceleron, Fulcrum, Expansion, Regeneron, Strongbridge, PTC, and Sarepta. Go to Neurology.org/N for full disclosures.
References
- 1.Mul K, Lassche S, Voermans NC, Padberg GW, Horlings CG, van Engelen BG. What's in a name? The clinical features of facioscapulohumeral muscular dystrophy. Pract Neurol 2016;16:201–207. [DOI] [PubMed] [Google Scholar]
- 2.Padberg GW. Facioscapulohumeral Disease. Leiden: University of Leiden; 1982. [Google Scholar]
- 3.Johnson NE, Quinn C, Eastwood E, Tawil R, Heatwole CR. Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve 2012;46:951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker M, Schipper K, Geurts A, Abma T. It's not just physical: a qualitative study regarding the illness experiences of people with facioscapulohumeral muscular dystrophy. Disabil Rehabil 2016;39:978–986. [DOI] [PubMed] [Google Scholar]
- 5.Stübgen JP. Facioscapulohumeral muscular dystrophy: a radiologic and manometric study of the pharynx and esophagus. Dysphagia 2008;23:341–347. [DOI] [PubMed] [Google Scholar]
- 6.Wohlgemuth M, de Swart BJ, Kalf JG, Joosten FB, Van der Vliet AM, Padberg GW. Dysphagia in facioscapulohumeral muscular dystrophy. Neurology 2006;66:1926–1928. [DOI] [PubMed] [Google Scholar]
- 7.Statland JM, Tawil R. Risk of functional impairment in facioscapulohumeral muscular dystrophy. Muscle & nerve 2014;49:520–527. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka G, Goto K, Matsumura T, et al. Tongue atrophy in facioscapulohumeral muscular dystrophy. Neurology 2001;57:733–735. [DOI] [PubMed] [Google Scholar]
- 9.Lamperti C, Fabbri G, Vercelli L, et al. A standardized clinical evaluation of patients affected by facioscapulohumeral muscular dystrophy: the FSHD clinical score. Muscle Nerve 2010;42:213–217. [DOI] [PubMed] [Google Scholar]
- 10.Clark HM, Solomon NP. Age and sex differences in orofacial strength. Dysphagia 2012;27:2–9. [DOI] [PubMed] [Google Scholar]
- 11.McHorney CA, Robbins J, Lomax K, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III: documentation of reliability and validity. Dysphagia 2002;17:97–114. [DOI] [PubMed] [Google Scholar]
- 12.Ginocchio D, Alfonsi E, Mozzanica F, et al. Cross-cultural adaptation and validation of the Italian version of SWAL-QOL. Dysphagia 2016;31:626–634. [DOI] [PubMed] [Google Scholar]
- 13.Baylor C, Yorkston K, Eadie T, Kim J, Chung H, Amtmann D. The Communicative Participation Item Bank (CPIB): item bank calibration and development of a disorder-generic short form. J Speech Lang Hear Res 2013;56:1190–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TH, Lai YH, Lee PL, et al. Infantile facioscapulohumeral muscular dystrophy revisited: expansion of clinical phenotypes in patients with a very short EcoRI fragment. Neuromuscul Disord 2013;23:298–305. [DOI] [PubMed] [Google Scholar]
- 15.McHorney CA, Martin-Harris B, Robbins J, Rosenbek J. Clinical validity of the SWAL-QOL and SWAL-CARE outcome tools with respect to bolus flow measures. Dysphagia 2006;21:141–148. [DOI] [PubMed] [Google Scholar]
- 16.Korf BR, Bresnan MJ, Shapiro F, Sotrel A, Abroms IF. Facioscapulohumeral dystrophy presenting in infancy with facial diplegia and sensorineural deafness. Ann Neurol 1985;17:513–516. [DOI] [PubMed] [Google Scholar]
- 17.Oh JC. Effects of tongue strength training and detraining on tongue pressures in healthy adults. Dysphagia 2015;30:315–320. [DOI] [PubMed] [Google Scholar]
- 18.Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A Biol Sci Med Sci 1996;51:M247–M250. [DOI] [PubMed] [Google Scholar]
- 19.Youmans SR, Youmans GL, Stierwalt JA. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia 2009;24:57–65. [DOI] [PubMed] [Google Scholar]
- 20.Vanderwegen J, Guns C, Van Nuffelen G, Elen R, De Bodt M. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy Belgian adults. Dysphagia 2013;28:159–166. [DOI] [PubMed] [Google Scholar]
- 21.Adams V, Mathisen B, Baines S, Lazarus C, Callister R. A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument (IOPI). Dysphagia 2013;28:350–369. [DOI] [PubMed] [Google Scholar]
- 22.Adams V, Mathisen B, Baines S, Lazarus C, Callister R. Reliability of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument with healthy adults. Dysphagia 2014;29:83–95. [DOI] [PubMed] [Google Scholar]
- 23.Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech Lang Pathol 2003;12:40–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.