Abstract
Nerve regeneration after delayed nerve repair is often unsuccessful. Indeed, the expression of genes associated with regeneration, including neurotrophic and gliotrophic factors, is drastically reduced in the distal stump of chronically transected nerves; moreover, Schwann cells undergo atrophy, losing their ability to sustain regeneration. In the present study, to provide a three-dimensional environment and trophic factors supporting Schwann cell activity and axon re-growth, we combined the use of an effective conduit (a chitosan tube) with a promising intraluminal structure (fresh longitudinal skeletal muscle fibers). This enriched conduit was used to repair a 10-mm rat median nerve gap after 3-month delay and functional and morphometrical analyses were performed 4 months after nerve reconstruction. Our data show that the enriched chitosan conduit is as effective as the hollow chitosan conduit in promoting nerve regeneration, and its efficacy is not statistically different from the autograft, considered the “gold standard” technique for nerve reconstruction. Since hollow tubes not always lead to good results after long defects (> 20 mm), we believe that the conduit enriched with fresh muscle fibers could be a promising strategy to repair longer gaps, as muscle fibers create a favorable three-dimensional environment and release trophic factors. All procedures were approved by the Bioethical Committee of the University of Torino and by the Italian Ministry of Health (approval number: 864/2016/PR) on September 14, 2016.
Keywords: tubulization, Schwann cells, scaffold, tissue engineering, nerve reconstruction, morphometrical analyses, grasping test, secondary repair, median nerve, nerve regeneration
Chinese Library Classification No. 459.9; R361; R741
Introduction
Peripheral nerve regeneration is affected by different factors, including the time span occurring between the injury and the repair (Sunderland, 1990; Hoke, 2006). Indeed, in polytraumatic patients nerve reconstruction might be delayed in time up to several weeks to allow the resolution of concomitant more severe injuries (Evriviades et al., 2011; Moore et al., 2015).
The consequence of a secondary surgery is the progressive degeneration of the distal nerve stump that leads to target organ (skeletal muscle and sense organs) denervation with a final unsatisfactory functional recovery, also after a successful microsurgical nerve repair (Sulaiman and Gordon, 2013). This poor clinical outcome can eventually result in life-long paralysis with motor and sensory deficits that compromise patients’ quality of life.
Currently, the gold standard technique used to bridge large peripheral nerve defects is the autologous nerve graft (autograft). However, this procedure has some limitations including additional surgery need, donor side morbidity and limited donor nerve availability (Hallgren et al., 2013; Faroni et al., 2015). These limitations inspired researchers to develop alternative techniques to repair large nerve defects.
In the last years, artificial peripheral nerve guidance conduits made by non-nervous materials, either of biological or synthetic origin, have been widely investigated. Among these, muscle-in-vein combined conduits have been employed as alternative to autograft for injured nerve repair, leading to good functional regeneration (Battiston et al., 2000a, b; Marcoccio and Vigasio, 2010; Manoli et al., 2014). The combination of a structure that prevents muscle fiber dispersion (the vein) with a structure that forestalls vein collapse and creates a favorable environment for Schwann cell migration and axonal regrowth (the muscular tissue) is the basis of this technique success (Raimondo et al., 2005).
In recent studies, a chitosan nerve guidance conduit gave promising preclinical results in both standard and critical length nerve reconstruction (Haastert-Talini et al., 2013; Meyer et al., 2016; Bak et al., 2017; Stenberg et al., 2017) and is now available for peripheral nerve gap reconstruction in human patients (Reaxon® Nerve Guides; Medovent GmbH, Germany).
We recently evaluated peripheral nerve regeneration after primary nerve reconstruction using a chitosan nerve conduit filled with fresh skeletal muscle fibers (muscle-in-tube, MIT). We demonstrated that, although MIT performance 12 weeks after surgery was comparable to hollow chitosan conduit in terms of functional recovery and quantitative morphometry, in vitro experiments and in vivo short-term analyses demonstrated that fresh skeletal muscle can be a source of important gliotrophic factors, absent in the hollow tube (Ronchi et al., 2018).
The aim of this study was to evaluate whether MIT can support nerve regeneration after a delayed surgical peripheral nerve reconstruction (“secondary repair”).
Materials and Methods
Animals and surgery
Fifteen female adult Wistar rats were used (ENVIGO, Bresso, Italy; body weight 200–250 g, approximately 2 months old). Animals were housed in a room with controlled temperature and humidity, with light/dark cycles of 12/12 hours, fed ad libitum with food and water.
Every attempt was made to reduce animal suffering. All procedures were approved by the Bioethical Committee of the University of Torino and by the Italian Ministry of Health (approval number: 864/2016/PR) on September 14, 2016. Moreover, these procedures are in agreement with the National Institutes of Health guidelines (NIH Publication No. 85-23, revised 1996), the Italian Law for Care and Use of Experimental Animals (DL26/14), and the European Communities Council Directive (2010/63/EU).
During surgical procedures, animals were placed under general anesthesia induced by intramuscular injection of Tiletamine and Zolazepam (Zoletil, Carros, France; 3 mg/kg) and were located in supine position.
Using an incision from the nipple to the elbow crease, the left median nerve was exposed, isolated and transected about in the middle of the exposed part; the proximal nerve stump was buried in the pectoral muscle to prevent regeneration; the distal stump was blocked through stitches to adjacent innervated muscles and allowed to degenerate for 3 months.
Three months after median nerve denervation, median nerves were repaired according to the following experimental groups (Figure 1):
Figure 1.
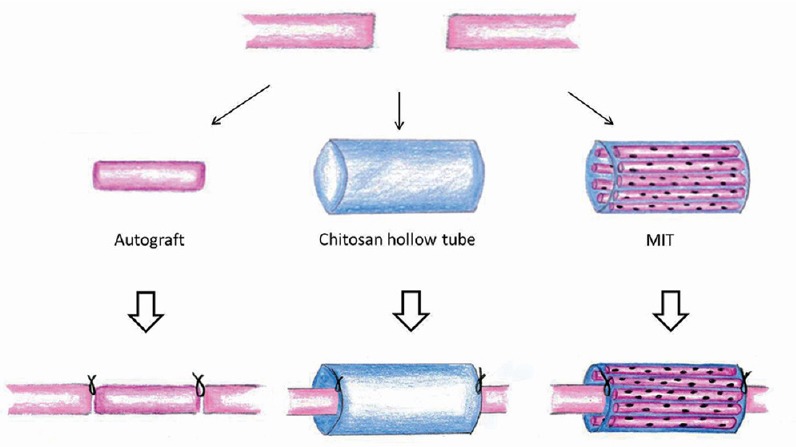
Diagram describing the three experimental groups of nerve repair.
The median nerve was transected and after 3 months of degeneration was repaired with autograft, chitosan hollow tube or muscle-in-tube (MIT). The distal part of the regenerated nerves was withdrawn 4 months after the reconstruction and analyzed.
- Chitosan-based hollow tube: an 8 mm nerve segment was removed and a 10-mm long chitosan tube was used to bridge the nerve defect by inserting 1 mm of the two nerve ends inside the conduit. The nerve guide was sutured at each end.
- Chitosan-based tube filled with fresh skeletal muscle –MIT: an 8 mm nerve segment was removed and a 10-mm long chitosan tube enriched with a longitudinal piece of the pectoralis major muscle (withdrawn from the same animal) was used to bridge the nerve defect by inserting 1 mm of the two nerve ends inside the conduit.
Medical grade chitosan for chitosan nerve guides manufacturing was supplied by Altakitin SA (Lisbon, Portugal). Chitosan-based tubes (Reaxon® Nerve Guide) were supplied by Medovent GmbH (Mainz, Germany).
- Autograft: an 10 mm median nerve segment was cut out, reversed (distal-proximal) and sutured to the nerve ends of the same animal.
To prevent interferences during the grasping test, the right median nerve of each animal was transected at the middle third of the brachium and its proximal stump was sutured to the pectoralis major muscle to avoid spontaneous reinnervation.
All animals were sacrificed after 4 months from the surgery by an overdose of Tiletamine and Zolazepam (40 mg/kg; Zoletil) and a segment of 5 mm of the regenerated distal nerve stump was harvested and analyzed for stereological and morphometrical analysis.
Grasping test
The strength of the flexor digitorum profundus muscle and flexor digitorum sublimis muscle, which are both innervated by the median nerve in rats, was assessed by the grasping test. The grasping test was therefore performed to estimate functional recovery after nerve reconstruction. The analysis was carried out before animal sacrifice (4 months after nerve repair) following the same procedure previously described (Papalia et al., 2003; Ronchi et al., 2009). Rats were acclimatized before the testing. Each animal was tested three times and the average value was recorded.
High resolution light microscopy and electron microscopy analysis
Regenerated median nerve distal portions, harvested 4 months after repair, were processed for resin embedding as previously described and semi-thin sections (2.5 µm thick) were cut and stained with 1% toluidine blue for light microscopy analysis (Ronchi et al., 2017). Ultra-thin sections (70 nm thick) were cut with the same ultramicrotome used for semi-thin sections. Sections were analyzed using a JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan) equipped with a Mega-View-III digital camera and a Soft-Imaging-System (SIS, Münster, Germany) for image acquisition.
Morphometrical analysis
The quantification of myelinated nerve fibers was performed on the toluidine blue-stained semi-thin sections. A systematic random sampling protocol earlier described was performed (Geuna, 2000; Geuna et al., 2000) with a magnification of 100×. In each sampling field, a 2-D dissector procedure was carried out. Total number of myelinated fibers, fiber and axon diameter, myelin thickness, g-ratio and fiber distribution were then estimated. IM50 image manager system (Leica Microsystems) was used for these analysis.
Statistical analysis
For statistical analysis IBM SPSS Statistics 25.0 software (IBM, Armonk, NY, USA) was used. Data were expressed as the mean + standard deviation (SD). Data sets were processed by one-way analysis of variance (ANOVA) with Bonferroni correction.
Results
To evaluate the efficacy of chitosan tubes enriched with fresh skeletal muscle fibers (MIT) in promoting regeneration after delayed nerve repair, median nerves were repaired 3 months after injury and regenerated nerves were analyzed 4 months after repair. Three experimental groups were analyzed: autograft (positive control), hollow chitosan tube (not enriched control), MIT. Macroscopic images of the regenerated nerves can be appreciated in Figure 2. Inside the hollow tube (Figure 2B), the regenerated nerve segment is clearly visible (it is notable that it is thinner than proximal and distal nerve stumps); MIT conduit is still full of muscle tissue that has not degenerated yet, therefore the regenerated fibers are not visible (Figure 2C).
Figure 2.
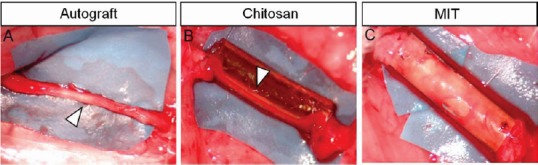
Macroscopic images of regenerated nerves 4 months after median nerve repair.
(A) Autograft; (B) nerve repaired with hollow chitosan conduit; (C) nerve repaired with MIT. White arrowheads indicate the regenerated nerves; inside the hollow tube the regenerated nerve is clearly visible, while inside MIT the regenerated nerve is not observable, because the conduit is still full of muscle tissue. MIT: Muscle-in-tube.
Morphological evaluation
Morphological evaluation carried out on toluidine blue-stained semi-thin sections obtained distally to the graft reveals that regenerated nerves in the three experimental groups exhibit many regrowing myelinated fibers organized in microfascicles Figure 3A, E, and I). Electron microscopy images allow better appreciating the ultrastructure of myelinated fibers with well-defined axoplasm and well-organized myelin sheaths; moreover, unmyelinated fibers can also be detected (Figure 3B–D, F–H, and J–L).
Figure 3.
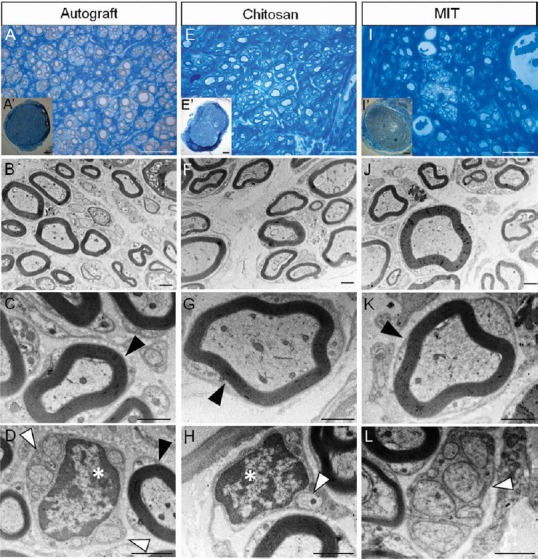
Morphology of regenerated nerve fibers 4 months after median nerve repair.
Representative high and low magnification light microscopy images of toluidine blue-stained semi-thin cross sections of nerve repaired with autograft (A and A’), nerve repaired with hollow chitosan conduit (E and E’) and nerve repaired with MIT (I and I’). Scale bars: 20 µm in A, E, I; 100 µm in A’, E’, I’. Representative high magnification transmission electron microscopy images of ultrathin cross sections of nerve repaired with autograft (B–D), with hollow chitosan conduit (F–H) and with MIT (J–L). Scale bars: 1 µm in B–D, F–H, and J–L. Black arrowheads: myelinated fibers with well-defined axoplasm and well-organized myelin sheaths; white arrowheads: unmyelinated fibers; white asterisks: Schwann cell nuclei. All experimental groups display regenerated fibers with many regrowing myelinated and unmyelinated fibers. MIT: Muscle-in-tube.
Functional recovery analysis
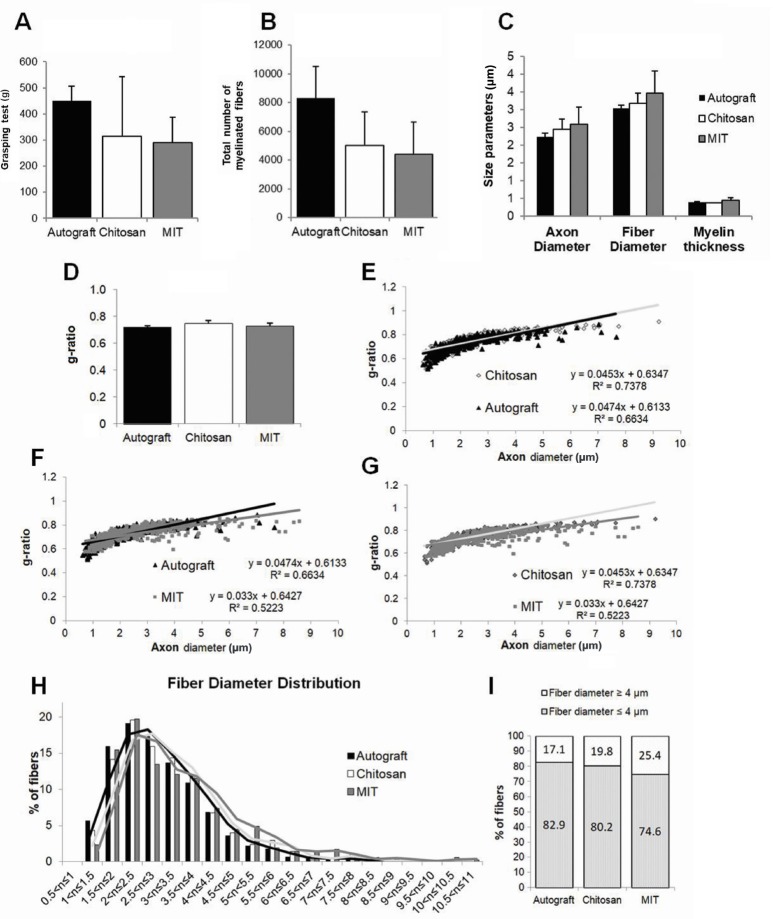
Figure 4A reports the results of the functional recovery 4 months after nerve repair. The function of finger flexor muscles, innervated by the median nerve, was recovered in the three experimental groups with no significant differences among them.
Figure 4.
Functional and stereological assessment of regenerated nerve fibers 4 months after nerve repair.
(A) Graphic reporting the results of the functional recovery, assessed by the grasping test; each animal was tested three times and the average value was recorded. Results of quantitative analysis, obtained through stereological methods, of (B) total number of myelinated nerve fibers; (C) parameters related to the size: axon diameter, fiber diameter and myelin thickness; and (D) g-ratio (fiber diameter/axon diameter). (A–D) No differences between the different groups were detected. Data are expressed as mean ± SD. (E–G) Scatter plots displaying the g-ratio of individual myelinated axons as a function of the respective axon diameter. (E) Autograft versus hollow chitosan conduit; (F) autograft versus MIT; (G) hollow chitosan conduit versus MIT. The regression line for the MIT group has a lower slope in comparison with the other experimental groups. (H) Histograms showing the percentage of the nerve fiber diameter distribution in the three experimental groups. All experimental groups exhibit a unimodal distribution of fiber diameter. (I) Histograms showing the percentage of fibers with diameters ≥ 4 µm. MIT: Muscle-in-tube.
Morphometrical analysis
Quantitative analysis on semi-thin sections shows that the total number of myelinated fibers, obtained with stereological methods, is not statistically different among groups (autograft group has the highest number of myelinated fibers, as expected, but this value is not statistically different compared to both the hollow chitosan and the MIT groups) (Figure 4B).
Regarding size parameters (axon and fiber size, myelin thickness and g-ratio) (Figure 4C and D), no significant differences can be appreciated among groups.
The analysis of individual fiber g-ratio/axon diameter correlation (Figure 4E–G) shows that the MIT group displays a regression line with a lower slope compared with the other two groups (autograft and hollow chitosan groups have overlapping regression lines).
Finally, fiber diameter frequency distribution shows that the three experimental groups exhibited a fiber diameter unimodal distribution. In particular, all these groups show a peak at 2–2.5 µm (Figure 4H), but the MIT group has a higher percentage of bigger fibers (> 4 µm diameter) compared with the other two groups (Figure 4I).
Discussion
Traumatic lesions of peripheral nerves are of clinical importance owing to their high incidence, resulting as a consequence of motorcycle or car accidents, domestic falls, work accidents or after surgeries (iatrogenic nerve injuries) (Jones et al., 2016). The peripheral nerve regenerative ability has been recognized for more than a century; however, clinical and experimental evidences show that such regeneration is often unsatisfactory, especially following severe lesions: approximately 50% of surgical cases achieves normal to good function (Pfister et al., 2011).
Although primary repair (within 1 week) is the optimal approach for peripheral nerve injuries, secondary (delayed) repair is indicated when an immediate repair is not possible due to other concomitant and complicating injuries (Griffin et al., 2014) and, unfortunately, the longer is the delay, the worsen is the prognosis. Indeed, nerve regeneration after delayed nerve repair is often incomplete, due to many factors, including the inability of the target organ to recover from atrophy, the inability of injured axons to completely regenerate and the inability of Schwann cells to sustain regeneration (Grinsell and Keating, 2014).
After injury, chronically denervated distal nerve stump undergoes several changes, both morphological and molecular. From a molecular point of view, the regeneration-associated genes (α1-tubulin, actin and growth associated protein 43), the ciliary neurotrophic factor, the transcription factor c‐Jun, Neuregulin1, S100 and glial fibrillary acidic protein are drastically reduced (Sendtner et al., 1992; Gordon and Tetzlaff, 2015; Jessen and Mirsky, 2016; Ronchi et al., 2017). On the contrary, the neurotrophic factors brain-derived neurotrophic factor, glial cell-derived neurotrophic factor and the p75 receptor are up-regulated (Michalski et al., 2008; Ronchi et al., 2017). Moreover, after long-term denervation Schwann cells undergo atrophy, and endoneurial tubes (the basal laminae of Schwann cells) progressively shrink and decrease in diameter (Hoke, 2011).
Several studies aimed to find new strategies to improve nerve regeneration after delayed nerve repair. Recently, chitosan conduits (regular or corrugated, hollow or filled with a longitudinal z-folded chitosan film) were used to bridge a 15-mm nerve gap after a 45-day delayed repair (Stenberg et al., 2017; Stößel et al., 2018), demonstrating their efficacy, although less effective in comparison to immediate repair (Meyer et al., 2016).
In the present study, the chitosan conduit was enriched with fresh muscle fibers (MIT) because it has been shown in vitro and in vivo that muscle cells produce and release gliotrophic factors important to support axon re-growth and Schwann cell activity (Montoya et al., 2009; Ronchi et al., 2018).
In a previous study we demonstrated that MIT efficiently supports peripheral nerve regeneration when used for a primary repair (Ronchi et al., 2018); therefore, the aim of this study was to evaluate the efficacy of this enriched conduit to reconstruct a 10-mm nerve gap after 3 months of denervation.
This study results show that MIT is as effective as hollow chitosan conduit in promoting nerve regeneration after delayed 10-mm nerve gap reconstruction. Indeed, functional and quantitative morphological data do not show statistic differences between these two experimental groups and autograft, which is the “gold standard” technique for nerve reconstruction (autograft shows more regenerated fibers and better performance in functional test, but these values are not statistically significant compared to both MIT and hollow chitosan conduit).
Therefore, data about hollow chitosan conduit confirm the results obtained in other previous studies (Stenberg et al., 2017; Stößel et al., 2018), while enrichment with fresh nerve fibers does not seem to further improve its effectiveness; nevertheless, a slightly higher percentage of myelinated nerve fibers with a diameter bigger than 4 µm can be observed.
The reasons for the positive results obtained with MIT –but not better than those obtained with hollow chitosan tube –may be different: (i) the release of factors by fresh muscle fibers occurs within the first few days; probably the muscle has a short-time beneficial effect that is lost over time and after 4 months is no longer perceptible; (ii) the hollow conduit, as previously extensively demonstrated (Haastert-Talini et al., 2013; Gonzalez-Perez et al., 2015; Meyer et al., 2016; Shapira et al., 2016; Bak et al., 2017), is very effective in promoting nerve regeneration; for this reason, the different strategies to further enrich it, to date, did not further improve its effectiveness, especially for short nerve gaps: the use of fresh muscle fibers as a filler could probably give better results when used to repair larger gaps (> 20 mm). Indeed, hollow tubes not always lead to good results after long defects and conduit enrichment might create a favorable environment for nerve re-growth. In particular, muscle fibers create a favorable three-dimensional environment provided by their basal lamina useful for Schwann cell migration and axonal regrowth.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Financial support: This study was supported by the European Community’s Seventh Framework Programme (FP7-HEALTH-2011), No. 278612 (to SG); and by Compagnia di San Paolo, No. D86D15000100005; InTheCure project (to SR). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: All procedures were approved by the Bioethical Committee of the University of Torino and by the Italian Ministry of Health (approval number: 864/2016/PR) on September 14, 2016. Moreover, these procedures are in agreement with the National Institutes of Health guidelines (NIH Publication No. 85-23, revised 1996), the Italian Law for Care and Use of Experimental Animals (DL26/14), and the European Communities Council Directive (2010/63/EU).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the European Community’s Seventh Framework Programme (FP7-HEALTH-2011), No. 278612 (to SG); and by Compagnia di San Paolo, No. D86D15000100005; InTheCure project (to SR).
C-Editor: Zhao M, Li CH; T-Editor: Liu XL
References
- 1.Bak M, Gutkowska ON, Wagner E, Gosk J. The role of chitin and chitosan in peripheral nerve reconstruction. Polim Med. 2017;47:43–47. doi: 10.17219/pim/75653. [DOI] [PubMed] [Google Scholar]
- 2.Battiston B, Tos P, Cushway TR, Geuna S. Nerve repair by means of vein filled with muscle grafts I. Clinical results. Microsurgery. 2000a;20:32–36. doi: 10.1002/(sici)1098-2752(2000)20:1<32::aid-micr6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Battiston B, Tos P, Geuna S, Giacobini-Robecchi MG, Guglielmone R. Nerve repair by means of vein filled with muscle grafts. II. Morphological analysis of regeneration. Microsurgery. 2000b;20:37–41. doi: 10.1002/(sici)1098-2752(2000)20:1<37::aid-micr7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Evriviades D, Jeffery S, Cubison T, Lawton G, Gill M, Mortiboy D. Shaping the military wound: issues surrounding the reconstruction of injured servicemen at the Royal Centre for Defence Medicine. Philos Trans R Soc Lond B Biol Sci. 2011;366:219–230. doi: 10.1098/rstb.2010.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. 2015;82-83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Geuna S. Appreciating the difference between design-based and model-based sampling strategies in quantitative morphology of the nervous system. J Comp Neurol. 2000;427:333–339. [PubMed] [Google Scholar]
- 7.Geuna S, Tos P, Battiston B, Guglielmone R. Verification of the two-dimensional disector, a method for the unbiased estimation of density and number of myelinated nerve fibers in peripheral nerves. Ann Anat. 2000;182:23–34. doi: 10.1016/S0940-9602(00)80117-X. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Perez F, Cobianchi S, Geuna S, Barwig C, Freier T, Udina E, Navarro X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery. 2015;35:300–308. doi: 10.1002/micr.22362. [DOI] [PubMed] [Google Scholar]
- 9.Gordon T, Tetzlaff W. Regeneration-associated genes decline in chronically injured rat sciatic motoneurons. Eur J Neurosci. 2015;42:2783–2791. doi: 10.1111/ejn.13070. [DOI] [PubMed] [Google Scholar]
- 10.Griffin MF, Malahias M, Hindocha S, Khan WS. Peripheral nerve injury: principles for repair and regeneration. Open Orthop J. 2014;8:199–203. doi: 10.2174/1874325001408010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haastert-Talini K, Geuna S, Dahlin LB, Meyer C, Stenberg L, Freier T, Heimann C, Barwig C, Pinto LF, Raimondo S, Gambarotta G, Samy SR, Sousa N, Salgado AJ, Ratzka A, Wrobel S, Grothe C. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials. 2013;34:9886–9904. doi: 10.1016/j.biomaterials.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 13.Hallgren A, Bjorkman A, Chemnitz A, Dahlin LB. Subjective outcome related to donor site morbidity after sural nerve graft harvesting: a survey in 41 patients. BMC Surg. 2013;13:39. doi: 10.1186/1471-2482-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoke A. Mechanisms of Disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 15.Hoke A. A (heat) shock to the system promotes peripheral nerve regeneration. J Clin Invest. 2011;121:4231–4234. doi: 10.1172/JCI59320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, Eisenberg HM, Jia X. Advances and future applications of augmented peripheral nerve regeneration. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091494. pii: E1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manoli T, Schulz L, Stahl S, Jaminet P, Schaller HE. Evaluation of sensory recovery after reconstruction of digital nerves of the hand using muscle-in-vein conduits in comparison to nerve suture or nerve autografting. Microsurgery. 2014;34:608–615. doi: 10.1002/micr.22302. [DOI] [PubMed] [Google Scholar]
- 19.Marcoccio I, Vigasio A. Muscle-in-vein nerve guide for secondary reconstruction in digital nerve lesions. J Hand Surg Am. 2010;35:1418–1426. doi: 10.1016/j.jhsa.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Meyer C, Stenberg L, Gonzalez-Perez F, Wrobel S, Ronchi G, Udina E, Suganuma S, Geuna S, Navarro X, Dahlin LB, Grothe C, Haastert-Talini K. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials. 2016;76:33–51. doi: 10.1016/j.biomaterials.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 21.Michalski B, Bain JR, Fahnestock M. Long-term changes in neurotrophic factor expression in distal nerve stump following denervation and reinnervation with motor or sensory nerve. J Neurochem. 2008;105:1244–1252. doi: 10.1111/j.1471-4159.2008.05224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montoya GJ, Sutachan JJ, Chan WS, Sideris A, Blanck TJ, Recio-Pinto E. Muscle-conditioned media and cAMP promote survival and neurite outgrowth of adult spinal cord motor neurons. Exp Neurol. 2009;220:303–315. doi: 10.1016/j.expneurol.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Moore AM, Wagner IJ, Fox IK. Principles of nerve repair in complex wounds of the upper extremity. Semin Plast Surg. 2015;29:40–47. doi: 10.1055/s-0035-1544169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papalia I, Geuna S, Tos PL, Boux E, Battiston B, Stagno D’Alcontres F. Morphologic and functional study of rat median nerve repair by terminolateral neurorrhaphy of the ulnar nerve. J Reconstr Microsurg. 2003;19:257–264. doi: 10.1055/s-2003-40582. [DOI] [PubMed] [Google Scholar]
- 25.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 26.Raimondo S, Nicolino S, Tos P, Battiston B, Giacobini-Robecchi MG, Perroteau I, Geuna S. Schwann cell behavior after nerve repair by means of tissue-engineered muscle-vein combined guides. J Comp Neurol. 2005;489:249–259. doi: 10.1002/cne.20625. [DOI] [PubMed] [Google Scholar]
- 27.Ronchi G, Nicolino S, Raimondo S, Tos P, Battiston B, Papalia I, Varejao AS, Giacobini-Robecchi MG, Perroteau I, Geuna S. Functional and morphological assessment of a standardized crush injury of the rat median nerve. J Neurosci Methods. 2009;179:51–57. doi: 10.1016/j.jneumeth.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Ronchi G, Cillino M, Gambarotta G, Fornasari BE, Raimondo S, Pugliese P, Tos P, Cordova A, Moschella F, Geuna S. Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J Neurosurg. 2017;127:843–856. doi: 10.3171/2016.9.JNS16140. [DOI] [PubMed] [Google Scholar]
- 29.Ronchi G, Fornasari BE, Crosio A, Budau CA, Tos P, Perroteau I, Battiston B, Geuna S, Raimondo S, Gambarotta G. Chitosan Tubes Enriched with Fresh Skeletal Muscle Fibers for Primary Nerve Repair. Biomed Res Int. 2018;2018:9175248. doi: 10.1155/2018/9175248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sendtner M, Stockli KA, Thoenen H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol. 1992;118:139–148. doi: 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapira Y, Tolmasov M, Nissan M, Reider E, Koren A, Biron T, Bitan Y, Livnat M, Ronchi G, Geuna S, Rochkind S. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery. 2016;36:664–671. doi: 10.1002/micr.22418. [DOI] [PubMed] [Google Scholar]
- 32.Stenberg L, Stossel M, Ronchi G, Geuna S, Yin Y, Mommert S, Martensson L, Metzen J, Grothe C, Dahlin LB, Haastert-Talini K. Regeneration of long-distance peripheral nerve defects after delayed reconstruction in healthy and diabetic rats is supported by immunomodulatory chitosan nerve guides. BMC Neurosci. 2017;18:53. doi: 10.1186/s12868-017-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stößel M, Wildhagen VM, Helmecke O, Metzen J, Pfund CB, Freier T, Haastert-Talini K. Comparative Evaluation of Chitosan Nerve Guides with Regular or Increased Bendability for Acute and Delayed Peripheral Nerve Repair: A Comprehensive Comparison with Autologous Nerve Grafts and Muscle-in-Vein Grafts. Anat Rec (Hoboken) 2018;301:1697–1713. doi: 10.1002/ar.23847. [DOI] [PubMed] [Google Scholar]
- 34.Sulaiman W, Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J. 2013;13:100–108. [PMC free article] [PubMed] [Google Scholar]
- 35.Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13:771–784. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]