Keywords: nerve regeneration, subarachnoid hemorrhage, Wnt/Frizzled signaling pathway, early brain injury, nuclear factor-κB, M2 type microglia, peroxisome proliferator-activated receptor-γ, inflammatory cytokines, neural regeneration
Abstract
The Wnt/Frizzled signaling pathway participates in many inflammation-linked diseases. However, the inflammatory response mediated by the Wnt/Frizzled signaling pathway in experimental subarachnoid hemorrhage has not been thoroughly investigated. Consequently, in this study, we examined the potential role of the Wnt/Frizzled signaling pathway in early brain injury in rat models of subarachnoid hemorrhage. Simultaneously, possible neuroprotective mechanisms were also investigated. Experimental subarachnoid hemorrhage rat models were induced by injecting autologous blood into the prechiasmatic cistern. Experiment 1 was designed to examine expression of the Wnt/Frizzled signaling pathway in early brain injury induced by subarachnoid hemorrhage. In total, 42 adult rats were divided into sham (injection of equivalent volume of saline), 6-, 12-, 24-, 48-, 72-hour, and 1-week subarachnoid hemorrhage groups. Experiment 2 was designed to examine neuroprotective mechanisms of the Wnt/Frizzled signaling pathway in early brain injury induced by subarachnoid hemorrhage. Rats were treated with recombinant human Wnt1 (rhwnt1), small interfering Wnt1 (siwnt1) RNA, and monoclonal antibody of Frizzled1 (anti-Frizzled1) at 48 hours after subarachnoid hemorrhage. Expression levels of Wnt1, Frizzled1, β-catenin, peroxisome proliferator-activated receptor-γ, CD36, and active nuclear factor-κB were examined by western blot assay and immunofluorescence staining. Microglia type conversion and inflammatory cytokine levels in brain tissue were examined by immunofluorescence staining and enzyme-linked immunosorbent assay. Our results show that compared with the sham group, expression levels of Wnt1, Frizzled1, and β-catenin were low and reduced to a minimum at 48 hours, gradually returning to baseline at 1 week after subarachnoid hemorrhage. rhwnt1 treatment markedly increased Wnt1 expression and alleviated subarachnoid hemorrhage-induced early brain injury (within 72 hours), including cortical cell apoptosis, brain edema, and neurobehavioral deficits, accompanied by increasing protein levels of β-catenin, CD36, and peroxisome proliferator-activated receptor-γ and decreasing protein levels of nuclear factor-κB. Of note, rhwnt1 promoted M2-type microglia conversion and inhibited release of inflammatory cytokines (interleukin-1β, interleukin-6, and tumor necrosis factor-α). In contrast, siwnt1 RNA and anti-Frizzled1 treatment both resulted in an opposite effect. In conclusion, the Wnt/Frizzled1 signaling pathway may participate in subarachnoid hemorrhage-induced early brain injury via inhibiting the inflammatory response, including regulating microglia type conversion and decreasing inflammatory cytokine release. The study was approved by the Animal Ethics Committee of Anhui Medical University and First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China (approval No. LLSC-20180202) in May 2017.
Chinese Library Classification No. R446; R364; R741
Introduction
Subarachnoid hemorrhage (SAH) is a familiar but severe cerebrovascular disease, resulting in neurological disorder and high mortality and disability rates (Miller et al., 2014; Okada and Suzuki, 2017). Recently, there have been significant improvements in minimally invasive and intervention therapy, but the outcome of patients who suffer a SAH still appears poor (Li et al., 2018). SAH-induced early brain injury is recognized as an early and acute cerebral vascular event that occurs within the first 72 hours, and is already regarded as a significant cause of poor prognosis (Yuksel et al., 2012; Nishikawa and Suzuki, 2017). Currently, multiple pathological processes have been shown to aggravate early brain injury, including increased intracranial pressure, blood-brain barrier destruction, decrease of cerebral blood flow, inflammatory injury, and neuronal apoptosis (Yan et al., 2017; Conzen et al., 2018; Han et al., 2018). Further, the acute inflammatory response after SAH has gained increasing attention in recent years. Undoubtedly, a severe inflammatory reaction aggravates neural cell apoptosis and brain edema, and destroys the blood-brain barrier.
Immunoinflammatory responses play a critical role in early brain injury and cerebral vasospasm after SAH. After aneurysm rupture, erythrocytes, leukocytes, mononuclear macrophages, and plasma proteins are released into the subarachnoid space and infiltrate around the parenchyma. Subsequently, these infiltrated inflammatory cells activate microglia and release a large number of inflammatory cytokines (Huang et al., 2017; Shi et al., 2017; Zheng and Wong, 2017; Savarraj et al., 2018). Previous studies have shown that inflammatory factors, such as interleukin (IL)-6, IL-1β, and tumor necrosis factor alpha (TNF-α), undergo an obvious increase in the acute stage of SAH. Accordingly, interventions of IL-1 and TNF-α and their receptors can effectively improve early brain injury (Ye et al., 2016; Fan et al., 2017). Multiple signaling pathways, including Ras-MAPK-nuclear factor (NF)-κB, JAK/STAT, and TLR4/NF-κB, also participate in the release of inflammatory cytokines (Wang et al., 2014; Feng et al., 2016; Li et al., 2018). Therefore, using drug interventions to improve outcome at key points of these inflammation-associated signaling pathways has become a hot topic. Specifically, inflammation and relevant signaling pathways are important factors that lead to aggravated early brain injury after SAH. It is well known that M1-type microglia play a major role in promoting the inflammatory reaction, and can promote release of inflammatory mediators, such as TNF-α, IL-1β, and IL-6, and induce cell apoptosis, neurotoxicity, and secondary injury (Ueba et al., 2018; Zhang et al., 2018). In contrast, M2-type microglia can exert opposite and favorable effects, promoting release of anti-inflammatory mediators, and ultimately, repair of damaged arteries and tissue (Yang et al., 2018). CD36 is a member of the class B scavenger receptor family that is expressed in microglia and participates in phagocytosis. Moreover, CD36 is regulated by numerous drugs and agonists (Fang et al., 2014; Kim et al., 2017). Consequently, microglia-type and CD36 expression is recognized as a strong connection with inflammation in many diseases (Hashemi-Monfared et al., 2018). The Wnt/Frizzled signaling pathway was named because Wnt proteins are the starting signal. It is considered the most classical pathway and therefore intensely investigated. At present, research related to the Wnt/Frizzled signaling pathway has mainly focused on tumor proliferation. Hence, molecular inhibitors for specific targets of signal transduction pathways have been tested and already become anti-neoplastic drugs (Amado et al., 2014; Silva-García et al., 2014). In addition, the Wnt/Frizzled pathway is involved in a variety of related inflammatory diseases that regulate airway inflammation induced by smoke via peroxisome proliferator-activated receptor (PPAR)-δ/p38 pathways. Simultaneously, the Wnt/Frizzled pathway is suppressed, accompanied by down-regulation of PPARγ levels and up-regulation of inflammatory factors in lung tissue or bronchial epithelial cells of patients with chronic obstructive pulmonary disease caused by smoke inhalation (Kwak et al., 2015). In acute pneumonia, activation of the Wnt/Frizzled signaling pathway can reduce inflammatory damage by reducing neutrophil number and macrophage adhesion (Guo et al., 2015; Zu et al., 2016). In recent research, the Wnt/Frizzled signaling pathway was also shown to regulate proliferation and differentiation of immune cells, while a Wnt antagonist increased inflammation by promoting T cell conversion into Th2 cells. Simultaneously, Wnt3a could suppress the inflammatory response induced by pseudomonas aeruginosa, and thereby enhance macrophage phagocytosis of bacteria (Chae et al., 2016; Chen et al., 2016). These findings show that the Wnt/Frizzled pathway may modulate macrophage/microglia activation.
Lately, involvement of the Wnt/Frizzled signaling pathway in central nervous system diseases has gained increasing attention. In a cerebral ischemic model, Wnt/Frizzled pathway activation reduced infarction hemorrhage (Wang et al., 2016) and improved the microenvironment after ischemia coordinated by hypoxia-inducible factor-1α channels and vascular endothelial growth factor (Wu et al., 2016). In the acute phase of intracerebral hemorrhage, the Wnt/Frizzled pathway altered proliferation and apoptosis of neural cells and improved secondary brain injury (Zhou et al., 2014). Nevertheless, function of the Wnt/Frizzled signaling pathway in early brain injury after SAH has not yet been investigated, especially with regard to classification of microglia activation, inflammatory cytokine release, and potential mechanisms. Thus, we aimed to investigate the role of the Wnt/Frizzled signaling pathway and its underlying neuroprotective mechanisms in early brain injury after SAH in rat models, and here show involvement of the inflammatory response in this process.
Materials and Methods
Ethics and animals
A total of 150 adult male Sprague-Dawley rats aged 8 weeks were provided by the Experimental Animal Center of Anhui Medical University, China (production license No. SCXK [Wan] 2017-001; user license No. SYXK [Wan] 2017-006).
Overall, 114 rats were used to successfully establish SAH models. The average weight of healthy rats was 300–350 g. The body temperature of experimental rats was sustained at 37°C, and rats were separately fed in a constant temperature and humidity environment. All animal experiments were approved and supervised by the Animal Ethics Committee of Anhui Medical University and the First Affiliated Hospital of University of Science and Technology of China (approval No. LLSC-20180202) in May 2017. All staff abided strictly by the regulations of the National Institutes of Health concerning feeding and use of experimental animals.
SAH model induction
The prechiasmatic cistern injection method (Wang et al., 2015) was chosen to induce the SAH model. First, intraperitoneal anesthesia was accomplished using urethane (1000 mg/kg). Autologous and arterial blood was extracted from femoral arteries that were separated from femoral veins using an operating microscope. Second, a specific stereotaxic instrument was used to fix the rat’s head, and a stereotaxic needle with a rounded tip and side aperture orientated towards the prechiasmatic cistern was prepared beforehand (Wang et al., 2015). The insertion position was 7.5 mm anterior to bregma in the midline, with an insertion direction along an angle of 45° in the coronal plane. The side aperture of the needle was facing the right side when the bone hole was completed. The puncture depth was approximately 10–12 mm from the skull bone surface, and the needle tip reached the optic chiasm cistern. Finally, 0.3 mL autologous and arterial blood was slowly injected into the prechiasmatic cistern over 20 seconds using an aseptic syringe pump. Correspondingly, 0.3 mL saline was injected into the prechiasmatic cistern in sham groups. Cardiac compression was performed immediately once apnea occurred after injection of blood. Most rats returned to normal respiration soon after. Vital signs of rats were observed for 45 minutes following injection of blood, and then the rats were returned to their cages at a constant temperature and humidity. Moderate normal saline was injected into subcutaneous tissue immediately to maintain homeostasis of the internal environment of experimental rats. Meanwhile, vital signs were monitored continuously and kept stable. SAH models were successfully established when 0.3 mL autologous blood was steadily injected into the prechiasmatic cistern, with brain tissue obtained at the appointed time. No rats died (0/18 rats) in the sham group. In contrast, the mortality rate of experimental rats was 27.3% (36/132 rats) after induction of SAH.
Experimental design and intervention
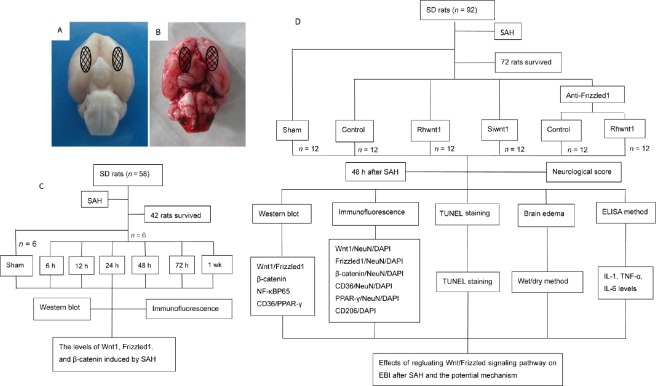
Experiment 1 was designed to confirm involvement of the Wnt/Frizzled signaling pathway in early brain injury after SAH. Accordingly, Wnt1, Frizzled1, and β-catenin levels were examined by western blot assay and immunofluorescence assay at different stages. In total, 42 adult male Sprague-Dawley rats were randomly divided into seven groups (n = 6): sham, 6-hour SAH, 12-hour SAH, 24-hour SAH, 48-hour SAH, 72-hour SAH, and 1-week SAH group. Subtemporal brain tissue was always covered by a blood clot in this experiment. Hence, brain tissue below the blood clot was obtained. Schematic diagram of the analyzed areas is shown in Figure 1A and B. Rats were killed by intraperitoneal anesthesia with urethane (1000 mg/kg). Brain tissue was obtained from SAH rats at different time points for western blot assay and immunofluorescence assay. The whole experimental flow graph is shown in Figure 1C.
Figure 1.
Schematic of areas taken for analysis and experimental design.
(A) Sham group and (B) SAH group. (C) Experiment 1 was designed to determine involvement of the Wnt/Frizzled signaling pathway in EBI under SAH condition. (D) Experiment 2 was designed to determine involvement of neuroprotection through the Wnt/Frizzled signaling pathway via suppression of the inflammatory response. SAH: Subarachnoid hemorrhage; EBI: early brain injury; h: hours; wk: week; IL: interleukin; TNF: tumor necrosis factor; SD: Sprague-Dawley; ELISA: enzyme-linked immunosorbent assay; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Experiment 2 was designed to investigate involvement of the inflammatory response including microglia somatotypes and inflammatory cytokines aggravated by early brain injury. Additionally, neuronal cells apoptosis, brain water content, and neurological behavior deficits after SAH were examined. Interventions used recombinant human Wnt1 (rhwnt1), small interfering Wnt1 RNA (siwnt1RNA), and monoclonal antibody of Frizzled1 (anti-Frizzled1). In total, 72 adult male rats were randomly divided into six groups (n = 12), including sham, SAH + control (saline), SAH + rhwnt1, SAH + siwnt1, SAH + anti-Frizzled1 + control, and SAH + anti-Frizzled1 + rhwnt1. Rats received intracerebroventricular injection of rhwnt1 and siwnt1RNA at 6 hours before they were killed. At 48 hours, neurological behavior was examined in all experimental rats. Brain tissue from the temporal lobe of six rats was cut into slices and used for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining and immunofluorescence. The remaining six rats were exsanguinated and brain samples collected for western blot assay, inflammatory cytokine level quantification, and brain edema evaluation. The whole experimental flow graph is shown in Figure 1D.
Drug treatment
Following establishment of SAH models, rhwnt1 (ab84080; Abcam Ltd., New Territories, Hong Kong, China) was dissolved and diluted in normal saline to obtain an final concentration of 1 ng/20 μL (Silva et al., 2017). Next, rhwnt1 (1 ng) was injected into the lateral cerebral ventricle using a Hamilton microsyringe guided by a stereotaxic instrument. The same volume of saline was injected into the lateral cerebral ventricle in the SAH + control group. Siwnt1RNA and control siRNA (Guangzhou RiboBio Co., Ltd., Guangzhou, China) were dissolved in RNase-free H2O to a final concentration of 1000 pmol/10 μL. Subsequently, 1000 pmol siwnt1RNA or control siRNA was diluted with transfection reagent (Entransterin vivo; Engreen, Beijing, China) and mixed evenly by shaking gently (Han et al., 2015). Following anesthesia, the mixture was intracerebroventricularly injected using a Hamilton microsyringe guided by a stereotaxic instrument. The stereotaxic coordinates were: 1.5 mm posterior, 1.0 mm lateral, and 3.2 mm below the horizontal plane of bregma (Dang et al., 2015). Anti-Frizzled1 (sc-398082; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), a monoclonal antibody of Frizzled1, served as a Frizzled1 suppressant. Once SAH models were established, the rats received anti-Frizzled1 (3 mg/kg) by intravenous tail injection.
Western blot assay
In experiment 1, brain tissue samples were obtained at 6, 12, 24, 48, and 72 hours and 1 week after SAH. In experiment 2, rhwnt1, siwnt1 RNA, and anti-Frizzled1 interventions were applied once SAH models were established. Brain tissue samples were all obtained at 48 hours in the different groups. Brain tissue samples were cut into small pieces using a brain chisel, and ground in a mixture of lysis buffer and phenylmethylsulphonyl fluoride (P0013; Beyotime, Shanghai, China). Protein concentrations of brain samples were measured using the Enhanced BCA Protein Assay Kit (P0009; Beyotime). First, equivalent volumes of protein samples (20 μg/lane) were heated for 5 minutes at 95°C, loaded on 12% sodium dodecyl sulphate-polyacrylamide gels, separated by electrophoresis apparatus, and electrophoretically transferred to polyvinylidene difluoride membrane (IPVH00010; Millipore, Billerica, MA, USA). Membranes were blocked with 5% bovine serum albumin for 1 hour at room temperature. Meanwhile, primary antibodies against Wnt1 (rabbit polyclonal antibody, ab85060; Abcam), Frizzled1 (goat polyclonal antibody sc-30428; Santa Cruz Biotechnology Inc.), β-catenin (rabbit monoclonal antibody, ab32572; Abcam), CD36 (rabbit polyclonal antibody, ab64014; Abcam), PPAR-γ (rabbit polyclonal antibody, ab209350; Abcam), and NF-κBp65 (rabbit polyclonal antibody, ab16502; Abcam) were diluted (1:1000), added, mixed, and incubated with membranes overnight at 4°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab9485; Abcam) was used as a loading control. Membranes were washed three times and further incubated with polyclonal donkey anti-goat IgG-horseradish peroxidase (rabbit polyclonal antibody, sc-2020; 1:5000; Santa Cruz Biotechnology Inc.) or goat anti-rabbit IgG-horseradish peroxidase (sc-2004; 1:5000; Santa Cruz Biotechnology Inc.), and conjugated secondary antibodies for 2 hours at room temperature. Finally, band signals were developed after adding enhanced chemiluminescence western reagent (P0018AM; Beyotime) and exposure to X-ray film (FF057; Beyotime). Films were scanned into images using an Epson Perfection 2480 scanner (Seiko Corp., Nagano, Japan). Related proteins were quantified with ImageJ program 1.8.0 version (NIH, Bethesda, MD, USA).
Immunofluorescence assay
Brain tissue samples were obtained at corresponding time points after SAH. Interventions of rhwnt1, siwnt1 RNA, and anti-Frizzled1 were applied once SAH models were established. Expression of Wnt1, Frizzled1, β-catenin, and CD36 in neurons was measured by immunofluorescent double staining. CD206-positive cells were identified as M2-type microglia. First, brain samples were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm sections. Primary antibodies (1:100) of Wnt1 (rabbit polyclonal antibody, ab85060; Abcam), Frizzled1 (goat polyclonal antibody, sc-30428; Santa Cruz Biotechnology Inc.), β-catenin (rabbit monoclonal antibody, ab32572; Abcam), CD206 (rabbit polyclonal antibody, ab64693; Abcam), and CD36 (rabbit polyclonal antibody, ab64014; Abcam) were added and incubated for 12 hours at 4°C. Sections were washed three times, and then the corresponding secondary antibodies (1:500 dilution) were added and incubated for 1 hour. Finally, sections were covered with anti-fading mounting medium containing 4′,6-diamino-2-phenyl indole (DAPI) (C1002; Beyotime) and observed using a fluorescence microscope (BX50/BX-FLA/DP70; Olympus, Tokyo, Japan). Fluorescence intensity attached to different groups was analyzed using ImageJ program 1.8.0 version and normalized to fluorescence intensity of the sham group.
Enzyme linked immunosorbent assay assay of inflammatory cytokines
Brain tissue samples were obtained at corresponding time points after SAH. Moreover, rhwnt1, siwnt1 RNA, and anti-Frizzled1 interventions were applied once SAH models were established. Levels of IL-1β, IL-6, and TNF-α in brain tissue were measured using specific enzyme linked immunosorbent assay kits for rats (PI303, PI328, PT516; Beyotime), in accordance with the manufacturer’s instructions. Briefly, equal quantities of brain tissue from different groups were cut and ground to generate protein samples. Standard samples were diluted to different concentrations and target samples added into corresponding wells of 100 μL. Reaction wells were sealed with transparent film, and incubated at room temperature for 120 minutes. Next, prepared biotinylated antibody was added into the reaction well, and incubated for 1 hour at room temperature. Horseradish peroxidase was added to detect streptavidin, and incubated for 20 minutes at room temperature. Finally, 3,3′,5,5′-tetramethylbenzidine solution was added and incubated for 20 minutes at room temperature. After stop solution was added, absorbance values were measured at 450 nm immediately using a spectrophotometer (FilterMax F5, Molecular Devices, San Francisco, CA, USA). Values were expressed as pg/mL.
TUNEL staining
Brain tissue samples were obtained at corresponding time points after SAH. Interventions of rhwnt1, siwnt1 RNA, and anti-Frizzled1 were applied once SAH models were established. Cortical cell apoptosis was evaluated by TUNEL staining, which was performed in accordance with the manufacturer’s introductions (C1086; Beyotime). Briefly, brain tissue was fixed, embedded, sliced, heated, and then dewaxed. Following dewaxing, sections were incubated with proteinase K for 15 minutes at room temperature. Next, sections were washed three times with phosphate buffered saline and then incubated with TUNEL detection liquid at 37°C for 1 hour. Sections were again washed three times. DAPI mounting medium and anti-fluorescence quenching were added and sections covered with coverslips. Finally, sections were observed using a fluorescence microscope (BX50/BX-FLA/DP70; Olympus). To measure the degree of cortical cell apoptosis, the apoptotic index was defined as the percentage of TUNEL-positive cells in each section.
Brain water content assessment
Brain tissue samples were obtained at corresponding time points after SAH. Moreover, interventions of rhwnt1, siwnt1 RNA, and anti-Frizzled1 were applied once SAH models were established. The wet/dry method was used to assess the brain water content. Following brain tissue removal and collection, brain samples were weighed immediately (wet weight), dried in an oven at 100°C for 48 hours, and then weighed again (dry weight). The brain edema index was calculated according to the formula: (wet weight − dry weight/wet weight) × 100%.
Neurological impairment
In all rats at 48 hours after intervention, activity, appetite, and neurological defects were assessed using a published and recognized scoring system to detect neurological damage (Table 1) (Yamaguchi et al., 2004; Li et al., 2018). Higher scores indicate more severe neurological impairments.
Table 1.
Behavior and activity scores
| Category | Behavior | Score |
|---|---|---|
| Appetite | Finished meal | 0 |
| Left meal unfinished | 1 | |
| Scarcely ate | 2 | |
| Activity | Walk and reach at least three corners of the cage | 0 |
| Walk with some stimulations | 1 | |
| Almost always lying down | 2 | |
| Deficits | No deficits | 0 |
| Unstable walk | 1 | |
| Impossible to walk | 2 |
Statistical analysis
All data are shown as the mean ± SEM. SPSS 11.5 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were analyzed by one-way analysis of variance followed by Scheffé F test for post hoc analysis. Statistical significance was set to α = 0.05.
Results
Decreased expression levels of Wnt1, Frizzled1, and β-catenin after SAH
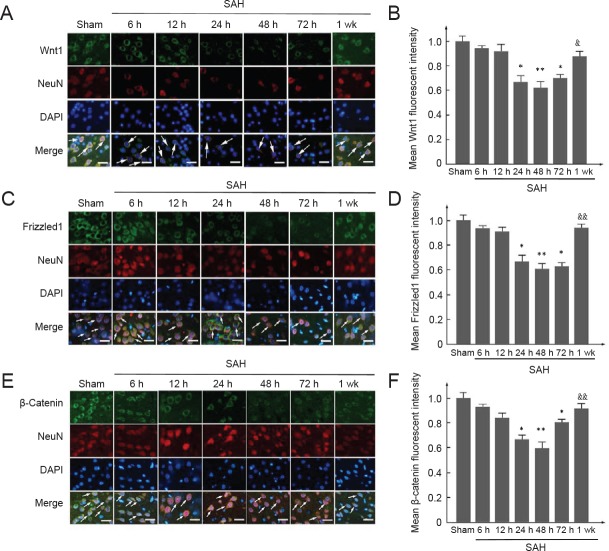
To investigate expression of Wnt1, Frizzled1, and β-catenin during early brain injury after SAH, western blot assay of brain tissue was performed (Figure 2A–D). Protein levels of Wnt1 (12 hours), Frizzled1 (24 hours), and β-catenin (24 hours) were all lower compared with the sham group, being lowest at 48 hours after SAH (P < 0.05 or P < 0.01), and then gradually increasing at 72 hours (P < 0.05 or P < 0.01). Nonetheless, at 1 week after SAH, Wnt1, anti-Frizzled1, and β-catenin protein levels were still visibly lower than the sham group. Double immunofluorescence staining using Wnt1, Frizzled1, and β-catenin antibodies with a neuronal marker (NeuN) was performed (Figure 3A–F). Consistently, the results showed a similar trend as the western blot assay: expression of Wnt1, Frizzled1, and β-catenin attached to the cytoplasm of neuronal cells reached a lowest point at 48 hours. The fluorescent intensity of Wnt1, Frizzled1, and β-catenin returned to normal levels at one week. These results suggest that the Wnt/Frizzled signaling pathway may participate in the pathological process during early brain injury, and is apparently suppressed after SAH. Further, 48 hours after SAH might be the most appropriate time point for intervention in experiment 2.
Figure 2.
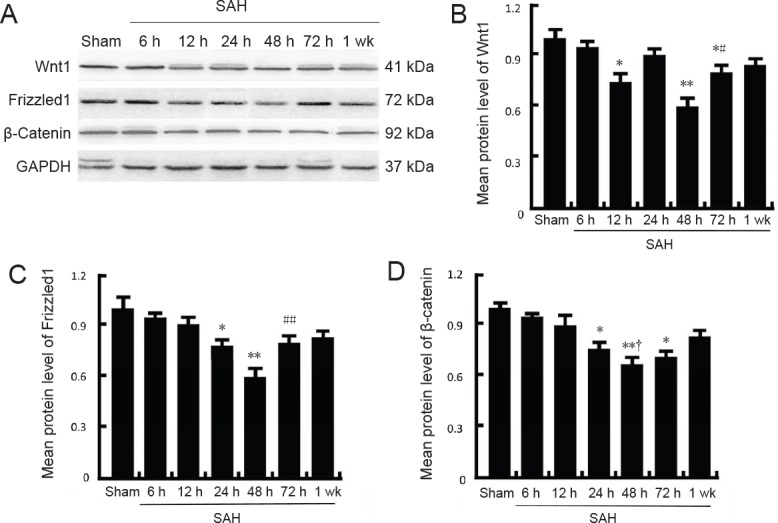
Changes in protein levels of Wnt1, Frizzled1, and β-catenin measured by western blot assay after SAH.
(A) Representative bands detected from western blots of Wnt1 (41 kDa), Frizzled1 (72 kDa), and β-catenin (92 kDa) expression in the sham group, 6 h SAH group, 12 h SAH group, 24 h SAH group, 48 h SAH group, 72 h SAH group, and 1 wk SAH group. (B–D) Mean protein levels of Wnt1, Frizzled1, and β-catenin in the sham group were used as the standard. Data are shown as the mean ± SEM (n = 6; one-way analysis of variance followed by Scheffé F post hoc test). *P < 0.05, **P < 0.01, vs. sham groups; #P < 0.05, ##P < 0.01, vs. 24 h and 48 h SAH groups. †P < 0.05, vs. 24 h SAH group. SAH: Subarachnoid hemorrhage; h: hours; wk: week.
Figure 3.
Double immunofluorescence of Wnt1, Frizzled1, and β-catenin in neurons after experimental SAH.
(A, C, E) Double immunofluorescence analysis of antibodies for Wnt1, Frizzled1, and β-catenin (green) and neuronal marker (NeuN; red). Nuclei were stained with DAPI (blue). Arrows indicate Wnt1, Frizzled1, and β-catenin immunoreactive neurons. Scale bars: 32 μm. (B, D, F) Mean fluorescent intensity in the sham group was used as the standard. Data are shown as the mean ± SEM (n = 6; one-way analysis of variance followed by Scheffé F post hoc test). *P < 0.05, **P < 0.01, vs. sham group; &P < 0.05, &&P < 0.01, vs. 24 h SAH group, 48 h group, and 72 h SAH group. SAH: Subarachnoid hemorrhage; DAPI: 4′,6-diamidino-2-phenylindole; h: hours; wk: week.
Rhwnt1 treatment increases and siwnt1 treatment decreases Wnt1 protein levels, while anti-Frizzled1 treatment decreases Frizzled1 protein levels in brain tissue under SAH conditions
Western blot assay (Figure 4A–D) and immunofluorescent staining (Figure 4E–H) both showed significantly lower Wnt1 protein levels at 48 hours after SAH compared with the sham group (P < 0.05). Wnt1 protein levels were significantly increased by rhwnt1 intervention and significantly decreased by siwnt1 RNA intervention (P < 0.01). Further, western blot assay and immunofluorescent staining results also indicated down-regulated Frizzled1 protein levels in the SAH + control group (P < 0.01), which were significantly further reduced by anti-Frizzled1 treatment (P < 0.01).
Figure 4.
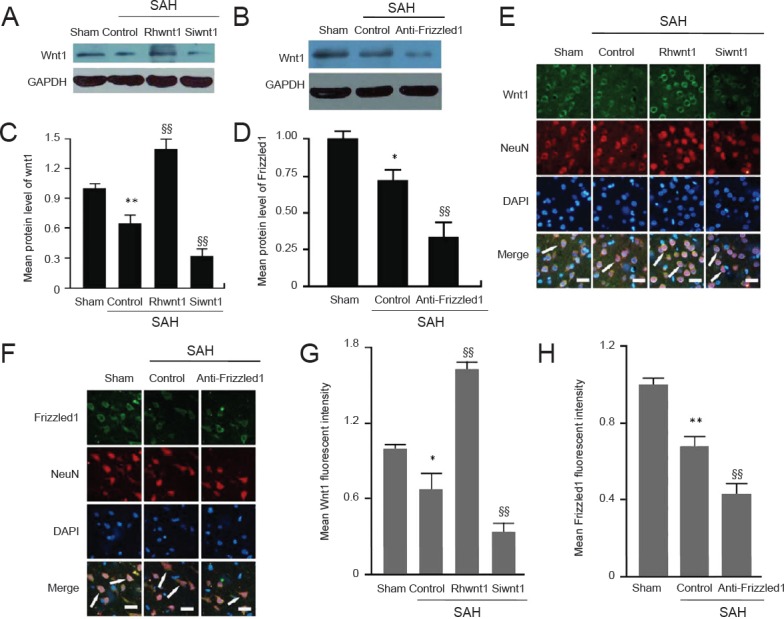
Changes in Wnt1 and Frizzled1 expression altered by rhwnt1, siwnt1, and anti-Frizzled1 treatment after experimental SAH.
(A, B) Representative bands detected from western blots of Wnt1 (41 kDa) expression in sham, SAH + control, SAH + rhwnt1, and SAH + siwnt1 groups, and Frizzled1 (92 kDa) expression in sham, SAH + control, and SAH + anti-Frizzled1 groups. (C, D) Mean protein levels of wnt1 and Frizzled1 in the sham group were used as the standard. (E, F) Double immunofluorescence analysis by fluorescence microscopy with antibodies for Wnt1 and Frizzled1 (green) and neuronal marker (NeuN; red). Nuclei were fluorescently labeled with DAPI (blue). Arrows indicate Wnt1 and Frizzled1 immunoreactive cells in neurons. Scale bars: 32 μm. (G, H) Mean fluorescent intensity in the sham group was used as the standard. Data are shown as the mean ± SEM (n = 6; one-way analysis of variance followed by Scheffé F post hoc test). *P < 0.05, **P < 0.01, vs. sham group; §§P < 0.01, vs. SAH + control group. SAH: Subarachnoid hemorrhage; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Rhwnt1 treatment decreases, while siwnt1RNA and anti-Frizzled treatment increases SAH-induced cortical cell apoptosis
TUNEL staining was performed to examine the effect of the rhwnt1, siwnt1, and anti-Frizzled1 interventions on cortical cell apoptosis in the brain at 48 hours after SAH (Figure 5A and B). Significant increase of TUNEL-immunoreactive cells was observed in the SAH + control group compared with the sham group (P < 0.01). The percentage of TUNEL-immunoreactive cells was decreased by rhwnt1 treatment (P < 0.01) and increased by siwnt1 and anti-Frizzled1 treatment (P < 0.05). Simultaneously, the pro-apoptotic effect of anti-Frizzled1 was partially neutralized by rhwnt1 (P < 0.05).
Figure 5.
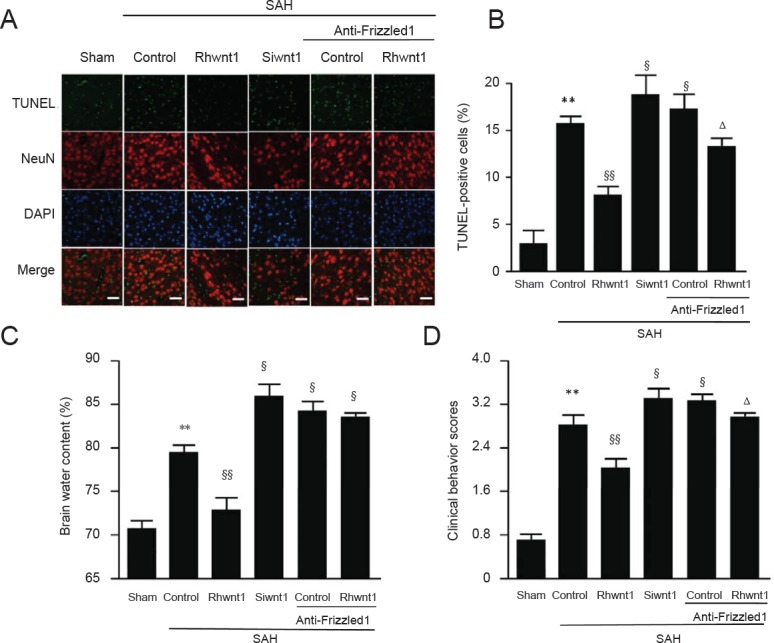
Effect of rhwnt1, siwnt1, and anti-Frizzled1 treatment on cortical cell apoptosis and brain edema after experimental SAH.
(A) Immunofluorescence of TUNEL (green) and DAPI (blue) staining. Arrows indicate apoptotic cells in brain tissue. Scale bars: 32 μm. (B) Variation of apoptotic cell percentage in different groups. (C) Variation of brain water content percentage in different groups. Data are shown as the mean ± SEM (n = 6; one-way analysis of variance followed by Scheffé F post hoc test). **P < 0.01, vs. sham group; §P < 0.05, §§P < 0.01, vs. SAH + control group; ΔP < 0.05, vs. SAH anti-Frizzled1 control group. SAH: Subarachnoid hemorrhage; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; DAPI: 4′,6-diamidino-2-phenylindole.
Rhwnt1 treatment decreases, while siwnt1RNA and anti-Frizzled1 treatment increases SAH-induced brain edema and neurological impairment
First, brain edema index was significantly higher in the SAH + control group than the sham group (P < 0.01) (Figure 5C). In contrast, after rhwnt1 treatment, brain edema index was significantly less pronounced (P < 0.01). Mean brain edema index was higher in rats after siwnt1RNA and anti-Frizzled1 treatment than in the SAH + control group (P < 0.05). Simultaneously, brain water content was significantly lower in the SAH + rhwnt1 group than in the SAH + control group (P < 0.01). However, there were no significant variations between the SAH + anti-Frizzled + control group and SAH + anti-Frizzled1 + rhwnt1 group. Rats with SAH showed severe neurological behavioral defects compared with the sham group (2.87 ± 0.32 points vs. 0.67 ± 0.15 points; P < 0.01; Figure 5D). These neurological behavioral defects were significantly less pronounced after rhwnt1 treatment (2.03 ± 0.58 points vs. 2.87± 0.32 points; P < 0.01). After siwnt1RNA (3.35 ± 0.29 points vs. 2.87 ± 0.32 points) and anti-Frizzled1 (3.41 ± 0.47 points vs. 2.87 ± 0.32 points) treatment, neurological behavioral defects were significantly more pronounced compared with the SAH + control group (P < 0.05). Moreover, remarkable variation in neurological behavioral defects was detected in the SAH + anti-Frizzled1 + rhwnt1 group compared with the SAH + anti-Frizzled + control group (3.03 ± 0.38 points vs. 3.41 ± 0.47 points; P < 0.05).
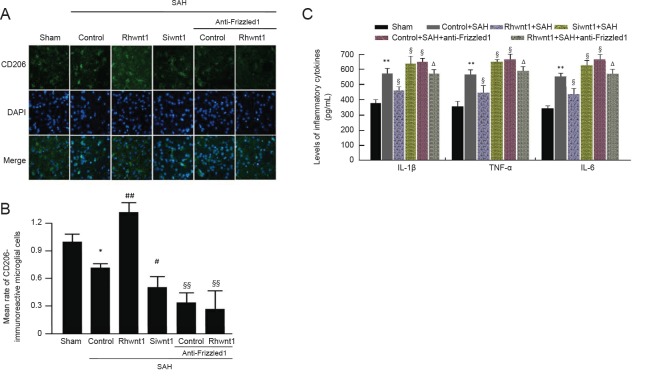
Rhwnt1 promotes M2 microglia conversion and inhibits inflammatory cytokine release, while siwnt1 and anti- Frizzled1 have opposite effects after experimental SAH
The effect of rhwnt1, siwnt1, and anti-Frizzled1 interventions on microglia type in the brain at 48 hours after SAH was investigated by immunofluorescence staining with CD206 (Figure 6A and B). Significant reduction of CD206-immunoreactive cells was observed in the SAH + control group compared with the sham group (P < 0.05). The percentage of CD206-immunoreactive cells was increased by rhwnt1 treatment and decreased by siwnt1RNA and anti-Frizzled1 treatment (P < 0.05 or P < 0.01). However, no remarkable difference in CD206-immunoreactive cells was detected between the SAH + anti-Frizzled1 control group and SAH + anti-Frizzled1 + rhwnt1 group. In the SAH + control group, average levels of IL-1β, IL-6, and TNF-α in brain tissue were significantly increased compared with the sham group (P < 0.01; Figure 6C). Rhwnt1 treatment abolished this increase (P < 0.05), while siwnt1RNA and anti-Frizzled1 treatment intensified the increase (P < 0.05). The proinflammatory effect of anti-Frizzled1 was partially, but significantly, neutralized by rhwnt1 treatment (P < 0.05).
Figure 6.
Effect of rhwnt1, siwnt1, and anti-Frizzled1 treatment on microglia conversion into M2-type and inflammatory cytokine release after experimental SAH.
(A) Double immunofluorescence analysis by fluorescence microscopy (original magnification, 100×) with CD206 antibody (green) and nuclei fluorescently stained with DAPI (blue). Arrows indicate CD206-immunoreactive cells. (B) Mean rate of CD206-immunoreactive microglial cells in the sham group was used as the standard. (C) Levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) measued by ELISA method. Data are shown as the mean ± SEM (n = 6; one-way analysis of variance followed by Scheffé F post hoc test). *P < 0.05, **P < 0.01, vs. sham group; §P < 0.05, vs. SAH + control group; ΔP < 0.05, vs. SAH + anti-Frizzled1 control group. SAH: Subarachnoid hemorrhage; DAPI: 4′,6-diamidino-2-phenylindole; IL: interleukin; TNF: tumor necrosis factor; ELISA: enzyme linked immunosorbent assay.
Rhwnt1 treatment increases β-catenin, PPAR-γ, and CD36 expression and decreases NF-κBp65 expression, while siwnt1 and anti-Frizzled1 treatment exert opposite effects after experimental SAH
Expression of NF-κBp65, β-catenin, CD36, and PPAR-γ was examined to determine the mechanism of microglia type conversion by western blot assay (Figure 7A–E) and immunofluorescent double labeling (Figure 8A–D). Evident reduction in protein levels of β-catenin, CD36, and PPAR-γ was found in the SAH + control group (P < 0.01). Rhwnt1 treatment resulted in significantly higher protein levels of β-catenin, CD36, and PPAR-γ compared with the SAH + control group (P < 0.01). However, β-catenin, CD36, and PPAR-γ expression was less after siwnt1RNA and anti-Frizzled1 intervention compared with the SAH + control group (P < 0.05, P < 0.01). These results also showed that protein levels of β-catenin, CD36, and PPAR-γ were lower after anti-Frizzled1 treatment compared with the siwnt1RNA group (P < 0.05 or P < 0.01). In contrast, a significant increase in protein levels of NF-κBp65 was observed in the SAH + control group (P < 0.05), while rhwnt1 treatment significantly down-regulated NF-κBp65 protein levels (P < 0.01). NF-κBp65 was significantly higher in the SAH + anti-Frizzled1 control group than the SAH + control group (P < 0.05).
Figure 7.
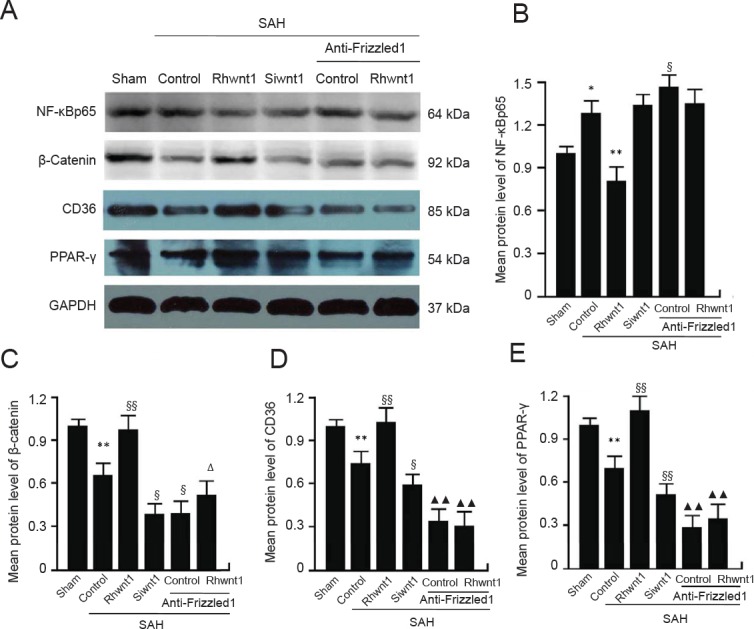
Changes in NF-κBp65, β-catenin, CD36, and PPAR-γ following rhwnt1, siwnt1, and anti-Frizzled1 intervention after experimental SAH.
(A) Representative bands detected from western blots of NF-κBp65 (64 kDa), β-catenin (92 kDa), CD36 (85 kDa), and PPAR-γ (54 kDa) expression in sham, SAH + control, SAH + rhwnt1, SAH + siwnt1, SAH + anti-Frizzled1 + control, and SAH + anti-Frizzled1 + rhwnt1 groups. (B–E) Mean protein levels of NF-κBp65, β-catenin, CD36, and PPAR-γ in the sham group were used as the standard. Data are shown as the mean ± SEM (n = 6; one-way analysis of variance followed by Scheffé F post hoc test). *P < 0.05, **P < 0.01, vs. sham group; §P < 0.05, §§P < 0.01, vs. SAH + control group; ΔP < 0.05, vs. SAH + anti-Frizzled1 control group; ▴▴P < 0.01, vs. SAH + siwnt1 group. SAH: Subarachnoid hemorrhage; NF: nuclear factor; PPAR: peroxisome proliferator-activated receptor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Figure 8.
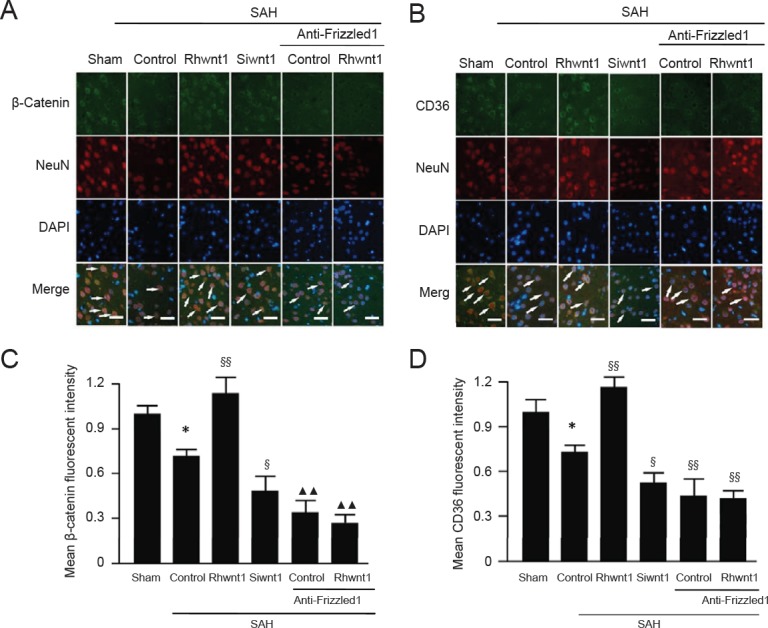
Changes in β-catenin and CD36 following rhwnt1, siwnt1, and anti-Frizzled1 intervention after experimental SAH.
(A, B) Double immunofluorescence analysis by fluorescence microscopy (original magnification, 100×) with antibodies for β-catenin and CD36 (green) and neuronal marker (NeuN; red). Nuclei were fluorescently labeled with DAPI (blue). Arrows indicate β-catenin and CD36 immunoreactive neurons. Scale bars: 32 μm. (C, D) Mean fluorescent intensity in the sham group was used as the standard. Data are shown as the mean ± SEM (n = 6; one-way analysis of variance followed by Scheffé F post hoc test). *P < 0.05, vs. sham group; §P < 0.05, §§P < 0.01, vs. SAH + control group; ▴▴P < 0.01, vs. SAH + siwnt1 group. SAH: Subarachnoid hemorrhage; DAPI: 4′,6-diamidino-2-phenylindole.
Discussion
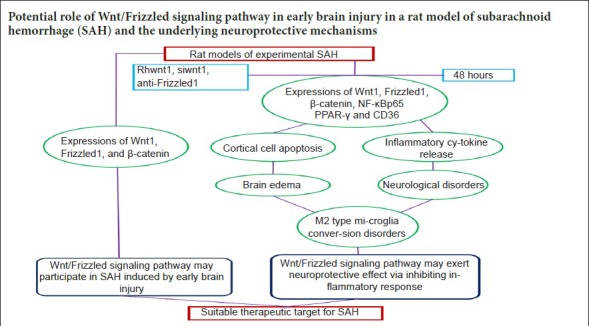
Experiment 1 showed decreased levels of Wnt1, Frizzled1, and β-catenin in brain tissue and neurons after SAH, with the most noticeable inhibition time point being 48 hours after SAH. In experiment 2, we found the following results: first, rhwnt1 treatment up-regulated the SAH-induced decrease in Wnt1, β-catenin, PPAR-γ, and CD36, and further down-regulated the SAH-induced decrease in NF-κBp65 in brain tissue and neurons. Meanwhile, siwnt1RNA had a down-regulatory effect. Anti-Frizzled1 inhibited levels of Frizzled1, β-catenin, PPAR-γ, and CD36, and enhanced NF-κBp65 in brain tissue and neurons, but had no effect on Wnt1 levels. Anti-Frizzled1 effects were partially reversed by rhwnt1 treatment. Further, rhwnt1 treatment alleviated brain edema, cortical apoptosis, and impaired neurological behavior after experimental SAH, while siwnt1RNA and anti-Frizzled1 had an aggravated effect that was partially reversed by rhwnt1 treatment. Ultimately, rhwnt1 treatment promoted microglia conversion into M2-type, and inhibited inflammatory cytokine release. Again, siwnt1RNA and anti-Frizzled1 exerted an opposite effect. Based on the above results, we hypothesized that decreased Wnt1 protein secretion and expression occurs after SAH, which is accompanied by suppression of the Wnt/Frizzled signaling pathway. Extracellular Wnt1 did not interact with Frizzled1, which was mediated by β-catenin accumulation inside the cell. Decreased β-catenin content in the nucleus decreased PPAR-γ expression, which combined with the transcription factor, TCF/LEF. Alternatively, reduction of PPAR-γ expression down-regulated expression of the membrane receptor, CD36, and promoted conversion of microglia to a M2 classification. Since low concentrations of intranuclear PPAR-γ were unable to effectively antagonize NF-κB, relevant inflammatory signaling pathways were activated. Consequently, release of a large number of inflammatory factors was inevitable, which directly caused nerve cell damage and exacerbated early brain injury (hypothesis figure of inflammation-induced neural damage mediated by Wnt/Frizzled1 signaling pathway suppression after SAH; Figure 9).
Figure 9.
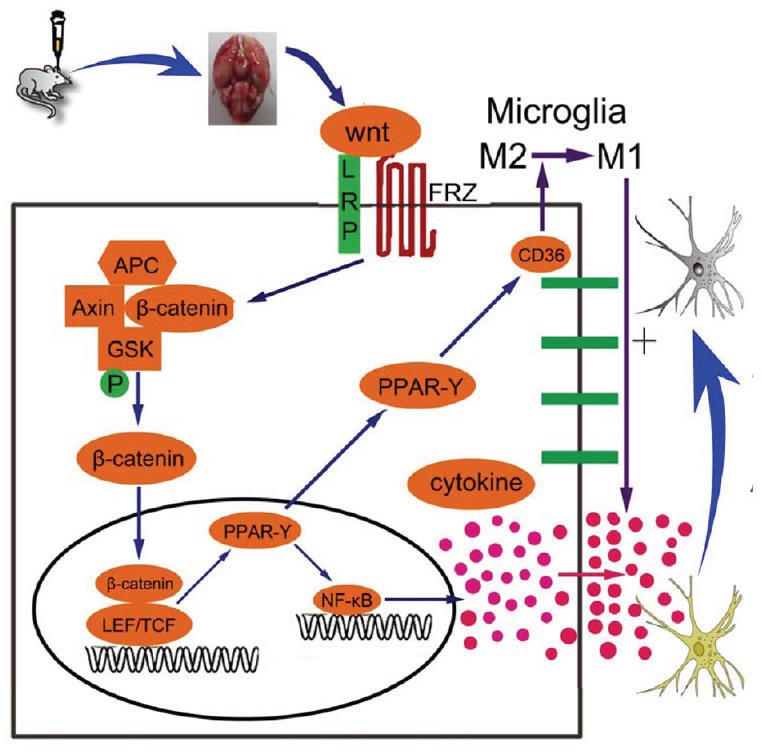
Schematic showing the role of the Wnt/Frizzled signaling pathway and potential mechanisms of the neuroprotective effect on early brain injury.
Briefly, the Wnt/Frizzled signaling pathway is suppressed after SAH, which is accompanied by markedly decreasing Wnt1 protein secretion and expression. Weak interaction between extracellular Wnt1 and Frizzled1 cannot be mediated by sufficient β-catenin content inside the cell, and reduced β-catenin in the nucleus decreases PPAR-γ expression. Reduced PPAR-γ down-regulates CD36 expression, which promotes M2 microglia conversion. Alternatively, as low concentrations of intranuclear PPAR-γ cannot effectively antagonize nuclear factor-κB (NF-κB) factor, relevant inflammatory signaling pathways are activated, and release of inflammatory factors is inevitable, directly causing nerve cell damage and intensified early brain injury. SAH: Subarachnoid hemorrhage; PPAR: peroxisome proliferator-activated receptor; APC: adenomatous Polyposis Coli; FRZ: Frizzled; GSK: glycogen synthase kinase; LEF/TCF: lymphoid enhancer factor/T cell factor.
It is generally known that the inflammatory reaction is an important host defense reaction to brain injury after SAH. Accumulative evidence has shown that excessive inflammatory responses following SAH aggravate brain injury, including blood-brain barrier disruption, brain edema aggravation, and neuronal cell death (Guo et al., 2016; Chaudhry et al., 2017; Frontera et al., 2017; Yuan et al., 2018). Activation of inflammation following SAH results from microglial activation, perihematomic inflammation reaction, infiltration of blood-derived inflammatory cells, and release of inflammatory cytokines (including IL-1β, IL-6, and TNF-α) (Yin et al., 2017; Schneider et al., 2018). At present, the Wnt/Frizzled signaling pathway is strongly associated with common inflammatory diseases. For example, it is highly expressed in a mouse model of chronic asthma as well as human patients, and regulates development of airway remodeling in chronic asthma. Blocking this pathway dampens airway remodeling by down-regulating TGF-β and tenascin C/PDGFR, resulting in subepithelial fibrosis and smooth muscle hyperplasia (Kwak et al., 2015). Recently, investigators have suggested that the Wnt/Frizzled signaling pathway might play important roles in different central nervous system diseases (Sorcini et al., 2017). These findings demonstrate that TWS119, an inhibitor of glycogen synthase kinase 3β (a key node in the Wnt/Frizzled signaling pathway), reduced recombinant tissue plasminogen activator-induced hemorrhagic transformation and attenuated blood-brain barrier disruption, possibly through activating the Wnt/Frizzled signaling pathway by increasing β-catenin, claudin-3, and ZO-1 protein expression and suppressing glycogen synthase kinase 3β expression (Wang et al., 2016). Another study showed that galangin might function as a multi-target drug for treating ischemic stroke by improving the microenvironment of neurovascular units. This included ameliorating neurological defects and neurovascular unit damage after middle cerebral artery occlusion via activating Wnt/β-catenin and hypoxia-inducible factor-1α/vascular endothelial growth factor signaling pathways (Wu et al., 2016). In addition, the Wnt/β-catenin signaling pathway is relevant to cell apoptosis and expression of proliferating cell nuclear antigen in rat brain, regulating the balance between cell apoptosis and cell proliferation following intracerebral hemorrhage induction (Zhou et al., 2014; Ma et al., 2018).
PPAR-γ is a ligand-dependent transcription factor that can regulate expression of the scavenger receptor, CD36, which is of significance for phagocytic activity and microglial phenotypes (Tyagi et al., 2011; Li et al., 2015; Zhao et al., 2015; Flores et al., 2016). NF-κB is a transcription factor that modulates production of many pro-inflammatory enzymes, chemokines, and adhesion molecules, resulting in massive amplification of the secondary inflammatory response after cerebral stroke (Gan et al., 2016; Peng et al., 2018; Yue et al., 2018). In both in vitro and in vivo experiments, agonists of PPAR-γ as therapeutic targets, decrease expression of pro-inflammatory determinants (including IL-1β, TNF-α, matrix metalloproteinase-9, and inducible nitric oxide synthase) via interaction with the transcription factor, NF-κB, and inhibiting DNA binding of NF-κB (Wu et al., 2016). In addition, TAK-242, a TLR4 inhibitor, remarkably relieved inflammatory injury after intracerebral hemorrhage, and improved neurological deficits by up-regulating CD36 expression in microglia, increasing phagocytic capacity of microglia, and improving hematoma absorption in intracerebral hemorrhage mice (Fang et al., 2014). At present, the internal relationship between the Wnt/Frizzled signaling pathway, PPAR-γ, CD36, NF-κB, and inflammatory cytokine release is still not elaborated in experimental SAH. Nevertheless, in this study, reduced PPAR-γ expression directly led to down-regulation of CD36 and phagocytic capacity of microglia, and was associated with suppression of the Wnt/Frizzled signaling pathway after SAH. Alternatively, activation of the Wnt/Frizzled signaling pathway generated an opposite effect.
This current study has some limitations. First, we have not determined the precise mechanism of the Wnt/Frizzled signaling pathway in regulating PPAR-γ expression. In addition, it is not possible to accurately evaluate microglia type conversion using immunofluorescence, and flow cytometry is more suitable. Nevertheless, in conclusion, our study provides comprehensive and adequate evidence supporting the potential effects of Wnt/Frizzled on an experimental SAH rat model and its underlying neuroprotective mechanisms. First, rhwnt1 ameliorated protein expression of key nodes (specifically, Wnt1, Frizzled1, and β-catenin), as well as brain edema, cortical cell apoptosis, and neurological defects induced by SAH. Siwnt1RNA and anti-Frizzled treatment had an opposite effect. Moreover, rhwnt1 promoted microglia conversion into M2-type and inhibited inflammatory cytokine release via regulation of PPAR-γ, CD36, and NF-κBp65 expression. All data in these experiments confirm that the neuroprotective effect of the Wnt/Frizzled signaling pathway might result from inhibition of inflammatory responses after SAH. Therefore, the Wnt/Frizzled signaling pathway may function as a multi-target in the treatment of hemorrhagic stroke.
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interests regarding the publication of this paper.
Financial support: This study was supported by the Natural Science Foundation of Anhui Province of China, No. 1508085QH184 (to YW). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study was approved by Animal Ethics Committee of Anhui Medical University and First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China (approval No. LLSC-20180202) in May 2017.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the Natural Science Foundation of Anhui Province of China, No. 1508085QH184 (to YW).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: James R, Pack M, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Amado NG, Predes D, Moreno MM, Carvalho IO, Mendes FA, Abreu JG. Flavonoids and Wnt/β-catenin signaling: potential role in colorectal cancer therapies. Int J Mol Sci. 2014;15:12094–12106. doi: 10.3390/ijms150712094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae WJ, Ehrlich AK, Chan PY, Teixeira AM, Henegariu O, Hao L, Shin JH, Park JH, Tang WH, Kim ST, Maher SE, Goldsmith-Pestana K, Shan P, Hwa J, Lee PJ, Krause DS, Rothlin CV, McMahon-Pratt D, Bothwell AL. The wnt antagonist dickkopf-1 promotes pathological type 2 cell-mediated inflammation. Immunity. 2016;44:246–258. doi: 10.1016/j.immuni.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhry SR, Güresir E, Vatter H, Kinfe TM, Dietrich D, Lamprecht A, Muhammad S. Aneurysmal subarachnoid hemorrhage lead to systemic upregulation of IL-23/IL-17 inflammatory axis. Cytokine. 2017;97:96–103. doi: 10.1016/j.cyto.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Fu Q, Li D, Wu Y, Sun S, Zhang X. Wnt3a suppresses Pseudomonas aeruginosa-induced inflammation and promotes bacterial killing in macrophages. Mol Med Rep. 2016;13:2439–2446. doi: 10.3892/mmr.2016.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conzen C, Becker K, Albanna W, Weiss M, Bach A, Lushina N, Steimers A, Pinkernell S, Clusmann H, Lindauer U. The acute phase of experimental subarachnoid hemorrhage: intracranial pressure dynamics and their effect oncerebral blood flowand autoregulation. Transl Stroke Res. 2018 doi: 10.1007/s12975-018-0674-3. doi: 10.1007/s12975-018-0674-3. [DOI] [PubMed] [Google Scholar]
- 6.Dang B, Li H, Xu X, Shen H, Wang Y, Gao A, He W, Wang Z, Chen G. Cyclophilin a/cluster of differentiation 147 interactions participate in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2015;43:e369–e381. doi: 10.1097/CCM.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 7.Fan LF, He PY, Peng YC, Du QH, Ma YJ, Jin JX, Xu HZ, Li JR, Wang ZJ, Cao SL, Li T, Yan F, Gu C, Wang L. Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic Biol Med. 2017;112:336–349. doi: 10.1016/j.freeradbiomed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Fang H, Chen J, Lin S, Wang P, Wang Y, Xiong X, Yang Q. CD36-mediated hematoma absorption following intracerebral hemorrhage: negative regulation by TLR4 signaling. J Immunol. 2014;192:5984–5992. doi: 10.4049/jimmunol.1400054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng D, Wang B, Ma Y, Shi W, Tao K, Zeng W, Cai Q, Zhang Z, Qin H. The Ras/Raf/Erk pathway mediates the subarachnoid hemorrhage-induced apoptosis of hippocampal neurons through phosphorylation of p53. Mol Neurobiol. 2016;53:5737–5748. doi: 10.1007/s12035-015-9490-x. [DOI] [PubMed] [Google Scholar]
- 10.Flores JJ, Klebe D, Rolland WB, Lekic T, Krafft PR, Zhang JH. PPARγ-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol Dis. 2016;87:124–33. doi: 10.1016/j.nbd.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frontera JA, Provencio JJ, Sehba FA, McIntyre TM, Nowacki AS, Gordon E, Weimer JM, Aledort L. The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care. 2017;26:48–57. doi: 10.1007/s12028-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan P, Gao Z, Zhao X, Qi G. Surfactin inducing mitochondria-dependent ROS to activate MAPKs, NF-κB and inflammasomes in macrophages for adjuvant activity. Sci Rep. 2016;6:39303. doi: 10.1038/srep39303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Mishra A, Howland E, Zhao C, Shukla D, Weng T, Liu L. Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood. 2015;126:2220–2229. doi: 10.1182/blood-2015-02-622233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Z, Hu Q, Xu L, Guo ZN, Ou Y, He Y, Yin C, Sun X, Tang J, Zhang JH. Lipoxin a4 reduces inflammation through formyl peptide receptor 2/p38mapk signaling pathway in subarachnoid hemorrhage rats. Stroke. 2016;47:490–497. doi: 10.1161/STROKEAHA.115.011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han XR, Wen X, Wang YJ, Wang S, Shen M, Zhang ZF, Fan SH, Shan Q, Wang L, Li MQ, Hu B, Sun CH, Wu DM, Lu J, Zheng YL. MicroRNA-140-5p elevates cerebral protection of dexmedetomidine against hypoxic-ischaemic brain damage via the Wnt/β-catenin signalling pathway. J Cell Mol Med. 2018;22:3167–3182. doi: 10.1111/jcmm.13597. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Han YW, Liu XJ, Zhao Y, Li XM. Role of oleanolic acid in maintaining BBB integrity by targeting p38MAPK/VEGF/Src signaling pathway in rat model of subarachnoid hemorrhage. Eur J Pharmacol. 2018;839:12–20. doi: 10.1016/j.ejphar.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi-Monfared A, Firouzi M, Bahrami Z, Zahednasab H, Harirchian MH. Minocycline decreases CD36 and increases CD44 in LPS-induced microglia. J Neuroimmunol. 2018;317:95–99. doi: 10.1016/j.jneuroim.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Huang XP, Peng JH, Pang JW, Tian XC, Li XS, Wu Y, Li Y, Jiang Y, Sun XC. peli1 contributions in microglial activation, neuroinflammatory responses and neurological deficits following experimental subarachnoid hemorrhage. Front Mol Neurosci. 2017;10:398. doi: 10.3389/fnmol.2017.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SM, Mun BR, Lee SJ, Joh Y, Lee HY, Ji KY, Choi HR, Lee EH, Kim EM, Jang JH, Song HW, Mook-Jung I, Choi WS, Kang HS. TREM2 promotes Aβ phagocytosis by upregulating C/EBPα-dependent CD36 expression in microglia. Sci Rep. 2017;7:11118. doi: 10.1038/s41598-017-11634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak HJ, Park DW, Seo JY, Moon JY, Kim TH, Sohn JW, Shin DH, Yoon HJ, Park SS, Kim SH. The Wnt/β-catenin signaling pathway regulates the development of airway remodeling in patients with asthma. Exp Mol Med. 2015;47:e198. doi: 10.1038/emm.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Faustino J, Woo MS, Derugin N, Vexler ZS. Lack of the scavenger receptor CD36 alters microglial phenotypes after neonatal stroke. J Neurochem. 2015;135:445–452. doi: 10.1111/jnc.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Liu W, Yin J, Chen Y, Guo S, Fan H, Li X, Zhang X, He X, Duan C. TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAHrats through the SOCS3/STAT3 pathway. J Neuroinflammation. 2018;15:231. doi: 10.1186/s12974-018-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma S, Deng X, Yang Y, Zhang Q, Zhou T, Liu Z. The lncRNA LINC00675 regulates cell proliferation, migration, and invasion by affecting Wnt/β-catenin signaling in cervical cancer. Biomed Pharmacother. 2018;108:1686–1693. doi: 10.1016/j.biopha.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Miller BA, Turan N, Chau M, Pradilla G. Inflammation, vasospasm, and brain injury after subarachnoid hemorrhage. Biomed Res Int 2014. 2014:384342. doi: 10.1155/2014/384342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishikawa H, Suzuki H. Implications of periostin in the development of subarachnoid hemorrhage-induced brain injuries. Neural Regen Res. 2017;12:1982–1984. doi: 10.4103/1673-5374.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada T, Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen Res. 2017;12:193–196. doi: 10.4103/1673-5374.200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Y, Jin J, Fan L, Xu H, He P, Li J, Chen T, Ruan Wu, Chen G. Rolipram attenuates early brain injury following experimental subarachnoid hemorrhage in rats: possibly via regulating the sirt1/nf-κb pathway. Neurochem Res. 2018;43:785–795. doi: 10.1007/s11064-018-2480-4. [DOI] [PubMed] [Google Scholar]
- 28.Savarraj JP, McGuire MF, Parsha K, Hergenroeder G, Bajgur S, Ahn S, Zhu L, Espino E, Chang T, Blackburn S, Kim DH, Dash P, Choi HA. Disruption of thrombo-inflammatory response and activation of a distinct cytokine cluster after subarachnoid hemorrhage. Cytokine. 2018;111:334–341. doi: 10.1016/j.cyto.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Schneider UC, Xu R, Vajkoczy P. Inflammatory events following subarachnoid hemorrhage (SAH) Curr Neuropharmacol. 2018;16:1385–1395. doi: 10.2174/1570159X16666180412110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi X, Fu Y, Zhang S, Ding H, Chen J. Baicalin attenuates subarachnoid hemorrhagic brain injury by modulating blood-brain barrier disruption, inflammation, and oxidative damage in mice. Oxid Med Cell Longev. 2017;2017:1401790. doi: 10.1155/2017/1401790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM. The Wnt/β-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediators Inflamm. 2014;2014:310183. doi: 10.1155/2014/310183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva GO, Zhang Z, Cucco C, Oh M, Camargo CHR, Nör JE. NöraLRP6 signaling is necessary for vasculogenic differentiation of human dental pulp stem cells. J Endod. 2017;43:S25–S30. doi: 10.1016/j.joen.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorcini D, Bruscoli S, Frammartino T, Cimino M, Mazzon E, Galuppo M, Bramanti P, Al-Banchaabouchi M. Wnt/β-catenin signaling induces integrin α4β1 in t cells and promotes a progressive neuroinflammatory disease in mice. J Immunol. 2017;199:3031–3041. doi: 10.4049/jimmunol.1700247. [DOI] [PubMed] [Google Scholar]
- 34.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueba Y, Aratake T, Onodera KI, Higashi Y, Hamada T, Shimizu T, Shimizu S, Yawata T, Nakamura R, Akizawa T, Ueba T, Saito M. Attenuation of zinc-enhanced inflammatory M1 phenotype of microglia by peridinin protects against short-term spatial-memory impairment following cerebral ischemia in mice. Biochem Biophys Res Commun. 2018;507:476–483. doi: 10.1016/j.bbrc.2018.11.067. [DOI] [PubMed] [Google Scholar]
- 36.Wang CX, Xie GB, Zhou CH, Zhang XS, Li T, Xu JG, Li N, Ding K, Hang CH, Shi JX, Zhou ML. Baincalein alleviates early brain injury after experimental subarachnoid hemorrhage in rats: possible involvement of TLR4/NF-κB-mediated inflammatory pathway. Brain Res. 2014;1594:245–255. doi: 10.1016/j.brainres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Li M, Wang Y, Li Q, Deng G, Wan J, Yang Q, Chen Q, Wang J. Gsk-3β inhibitor tws119 attenuates rtpa-induced hemorrhagic transformation and activates wnt/β-catenin signaling pathway after acute ischemic stroke in rats. Mol Neurobiol. 2016;53:7028–7036. doi: 10.1007/s12035-015-9607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Gao A, Xu X, Dang B, You W, Li H, Yu Z, Chen G. The neuroprotection of lysosomotropic agents in experimental subarachnoid hemorrhage probably involving the apoptosis pathway triggering by cathepsins via chelating intralysosomal iron. Mol Neurobiol. 2015;52:64–77. doi: 10.1007/s12035-014-8846-y. [DOI] [PubMed] [Google Scholar]
- 39.Wu C, Chen J, Chen C, Wang W, Wen L, Gao K, Chen X, Xiong S, Zhao H, Li S. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci Rep. 2015;5:16151. doi: 10.1038/srep16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu JS, Tsai HD, Cheung WM, Hsu CY, Lin TN. PPAR-γ ameliorates neuronal apoptosis and ischemic brain injury via suppressing NF-κB-driven p22phox transcription. Mol Neurobiol. 2016;53:3626–3645. doi: 10.1007/s12035-015-9294-z. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi M, Zhou C, Nanda A, Zhang JH. a canine double-hemorrhagemodel. Stroke. 2004;35:1750–1755. doi: 10.1161/01.STR.0000129898.68350.9f. [DOI] [PubMed] [Google Scholar]
- 42.Yan F, Cao S, Li J, Dixon B, Yu X, Chen J, Gu C, Lin W, Chen G. Pharmacological Inhibition of PERK attenuates early brain injury after subarachnoid hemorrhage in rats through the activation of akt. Mol Neurobiol. 2017;54:1808–1817. doi: 10.1007/s12035-016-9790-9. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Zhao Y, Zhang L, Fan H, Qi C, Zhang K, Liu X, Fei L, Chen S, Wang M, Kuang F, Wang Y, Wu S. RIPK3/MLKL-mediated neuronal necroptosis modulates the M1/M2 polarization of microglia/macrophages in the ischemic cortex. Cereb Cortex. 2018;28:2622–2635. doi: 10.1093/cercor/bhy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye ZN, Zhuang Z, Wu LY, Liu JP, Chen Q, Zhang XS, Zhou ML, Zhang ZH, Li W, Wang XL, Hang CH. Expression and cell distribution of leukotriene B4 receptor 1 in the rat brain cortex after experimental subarachnoid hemorrhage. Brain Res. 2016;1652:127–134. doi: 10.1016/j.brainres.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Yin C, Huang GF, Sun XC, Guo Z, Zhang JH. Dlk silencing attenuated neuron apoptosis through jip3/ma2k7/jnk pathway in early brain injury after sah in rats. Neurobiol Dis. 2017;103:133–143. doi: 10.1016/j.nbd.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan ZJ, He XY, Yuan P, Zheng XM, Li XG. Morroniside improves the neurological function in intracerebral hemorrhage rats by inhibiting inflammatory response. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:1217–1222. [Google Scholar]
- 47.Yue Y, Stone S, Lin W. Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis. Neural Regen Res. 2018;13:1507–1515. doi: 10.4103/1673-5374.237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuksel S, Tosun YB, Cahill J, Solaroglu I. Early brain injury following aneurysmal subarachnoid hemorrhage: emphasis on cellular apoptosis. Turk Neurosurg. 2012;22:529–533. doi: 10.5137/1019-5149.JTN.5731-12.1. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci. 2018;12:306. doi: 10.3389/fncel.2018.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, Gonzales N, Aronowski J. Pleiotropic role of PPARγ in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-κB. CNS Neurosci Ther. 2015;21:357–366. doi: 10.1111/cns.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng VZ, Wong GKC. Neuroinflammation responses after subarachnoid hemorrhage: a review. J Clin Neurosci. 2017;42:7–11. doi: 10.1016/j.jocn.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Zhou L, Deng L, Chang NB, Dou L, Yang CX. Cell apoptosis and proliferation in rat brains after intracerebral hemorrhage: role of Wnt/β-catenin signaling pathway. Turk J Med Sci. 2014;44:920–927. doi: 10.3906/sag-1308-100. [DOI] [PubMed] [Google Scholar]
- 53.Zu G, Guo J, Che N, Zhou T, Zhang X. Protective effects of ginsenoside Rg1 on intestinal ischemia/reperfusion injury-induced oxidative stress and apoptosis via activation of the Wnt/β-catenin pathway. Sci Rep. 2016;6:38480. doi: 10.1038/srep38480. [DOI] [PMC free article] [PubMed] [Google Scholar]