Abstract
The role of the ipsilaterally descending motor pathways in the recovery mechanisms after unilateral hemispheric damage is still poorly understood. Motor output reorganization was investigated in a 56-year-old male patient with acquired unilateral hemispheric atrophy due to Rasmussen encephalitis. In particular, the ipsilateral corticospinal pathways were explored using focal transcranial magnetic stimulation. In the first dorsal interosseous and wrist extensors muscles, the median amplitudes of the ipsilateral motor evoked potentials induced by transcranial magnetic stimulation in the patient were higher than those of 10 age-matched healthy control subjects. In the biceps brachii muscle, the median amplitudes of the ipsilateral motor evoked potentials were the second largest in the patient compared to the controls. This study demonstrated a reinforcement of ipsilateral motor projections from the unaffected motor cortex to the hemiparetic hand in a subject with acquired unihemispheric cortical damage.
Keywords: transcranial magnetic stimulation, motor cortex, ipsilateral motor evoked potentials, ipsilateral motor pathways, Rasmussen encephalitis, cortical atrophy, hemispheric damage
Chinese Library Classification No. R444; R741
Introduction
Ipsilaterally projecting, descending motor pathways have been anatomically and physiologically identified (Yakolev and Rakic, 1966; Wiesendanger, 1981). It has been reported that, in patients with unilateral cordotomies, the interruption of ipsilateral motor pathways by a second cordotomy immediately reversed all the motor functional recovery that had occurred after the first lesion (Nathan and Smith, 1973). These ipsilateral fibers could contribute to motor recovery independently from the crossed corticospinal tract. Indeed, the ipsilateral motor pathway is thought to be a normal motor control pathway and is accepted as one of the recovery mechanisms after unilateral hemispheric deficits.
Ipsilateral motor evoked potentials (MEPs) are thought to reflect the functional activity of uncrossed oligosynaptic projections, and can sometimes be recorded in proximal muscles of upper limbs using higher transcranial magnetic stimulation (TMS) intensities (Ziemann et al., 1999).
Ipsilateral MEPs have been reported in some congenital pathologies, including hemiplegic cerebral palsy (Farmer et al., 1991; Carr et al., 1993; Maegaki et al., 1997) and congenital mirror movements (Farmer et al., 1990; Konagaya et al., 1990; Britton et al., 1991; Capaday et al., 1991; Cohen et al., 1991; Danek et al., 1992; Cincotta et al., 1994; Kanouchi et al., 1997; Mayston et al., 1997), as well as in several acquired lesions, such as cerebral stroke (Benecke et al., 1991; Hömbeg et al., 1991; Turton et al., 1996; Netz et al., 1997), or following hemispherectomy (Benecke et al., 1991). On the other hand, MEPs can be recorded in ipsilateral muscles also in healthy subjects (Wassermann et al., 1991, 1994; Basu et al., 1994; Ziemann et al., 1999).
We report a rare case of Rasmussen encephalitis of late onset. The Rasmussen encephalitis has been first described by the neurosurgeon Theodore Rasmussen and his co-workers in the late 1950s (Rasmussen et al., 1958). It is a severe and progressive immune-mediated disease leading to unihemispheric brain atrophy, which is characterized by progressive hemiplegia, cognitive decline, and drug-resistant focal epilepsy (Varadkar et al., 2014). Approximately 10% of the reported cases begin in the adolescence or adulthood (Oguni et al., 1991; Dupont et al., 2017).
A TMS study was performed to investigate the involvement of ipsilateral descending motor pathways in this adult case diagnosed with Rasmussen encephalitis and affected by an acquired unihemispheric cortical atrophy.
Participants and Methods
Participants
The 56-years old man presented with epilepsia partialis continua, which was successfully treated with Levetiracetam (Teva) 750 mg twice daily. He developed a cognitive decline as well as visual and motor disturbances within a year of epilepsy onset. The neurological examination revealed right hemianopia, mild right hemiparesis, and a slight hypoesthesia on the right side.
Brain magnetic resonance imaging (MRI) revealed a left hemisphere atrophy, as well as T2/FLAIR hyperintensities in cortical and subcortical areas of the left frontal, temporal, and occipital lobes (Figure 1).
Figure 1.
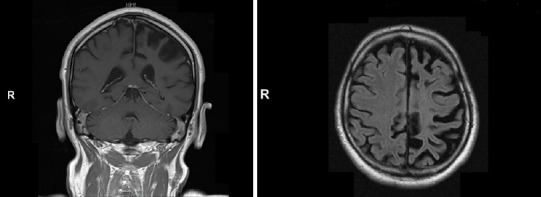
MRI images in the patient with acquired unilateral hemispheric atrophy due to Rasmussen encephalitis.
Coronal (A) and axial (B) brain MRI showed a left hemisphere atrophy, and a T2/Fluid-attenuated inversion recovery hyperintense signal in cortical and subcortical regions of the left frontal, temporal and occipital lobes. MRI: Magnetic resonance imaging.
Ten healthy age-matched right-handed control individuals were recruited from the community.
The tests used in this study were performed for clinical purposes using routine techniques, thus ethical approval was not sought. All participants received written information on methods and provided written informed consent for their inclusion in the present study.
Experimental procedures
The right-handed patient and 10 healthy control individuals were seated in a comfortable chair. Surface eletromyography from the different target muscles was recorded using Ag-AgCl cup electrodes with a belly-tendon montage. After electromyography (EMG) signal amplification and 10 Hz–2 kHz bandpass filtering, an IBM 486 AT-compatible laboratory computer (A/D rate, 5 kHz) was used for off-line analysis. Magnetic stimulation was performed using a figure-of-eight-shaped coil connected to a high-power Magstim® 2002 magnetic stimulator (The Magstim Company Ltd. Whitland Industrial Estate Spring Gardens Whitland Carmarthenshire SA34 0HR, UK).
Ipsilateral MEPs were examined in separate blocks of trials in the first dorsal interosseus (FDI), opponens pollicis, wrist flexors, wrist extensors, and biceps brachii muscles. In all muscles, the MEPs were recorded at about 30% of the maximal voluntary isometric contraction, determined by a strain gauge force transducer. Breaks between trials allowed avoiding fatigue.
Experiments started testing the ipsilateral FDI. Single pulses of TMS were delivered over the hand area of the contralateral motor cortex to determine the optimal coil position to induce MEPs in the target muscle. For recording the ipsilateral MEPs in the other muscles, the coil location was adjusted to produce the highest MEP amplitudes in the homologous muscle of the contralateral limb; the coil orientation was identical to the one eliciting the largest ipsilateral MEPs in the FDI. A stimulus intensity of 100% of maximum stimulator output was used. Five trials were collected for all conditions.
Resting motor threshold (RMT) was defined as the lowest stimulus intensity resulting in a MEP of ≥ 50 μV peak-to-peak amplitude in at least 5 out of 10 trials at rest (Rossini et al., 2015). Muscle relaxation was controlled by continuous auditory EMG biofeedback. The averaged peak-to-peak amplitude of the contralateral MEP was measured in the single trials. To quantitatively analyze the ipsilateral MEPs, single-trials of the EMG from 20 trials were rectified and the averages were calculated. An ipsilateral MEP was considered present when the poststimulus EMG exceeded the prestimulus EMG by at least one standard deviation for at least 5 ms. The EMG peak (ΔEMG, in μV) was expressed as:
ΔEMG = (ipsilateral MEP - prestimulus EMG) × duration of ipsilateral MEP
The duration was indicated by the length of the time during which the poststimulus EMG exceeded the prestimulus EMG. The ipsilateral MEP onset latency was defined as the beginning of this period.
The threshold for ipsilateral MEPs was determined using different stimulus intensities: 1.0, 1.5, 1.75, 2.0, and 2.25, 2.5 × the active motor threshold (AMT) for the contralateral MEP. AMT was defined as the nimimum stimulus intensity that elicites a MEP (about 200 μV in 50% of 10 trials) during isometric muscle contraction at approximately 10% maximum (Rossini et al., 2015).
Statistical analysis
Since we studied only one patient, statistical inference about the differences between the patient and the control populations was not meaningful. Instead, we provide a graphical comparison of the MEP sizes for the different subject/muscle combinations in Figure 2. This figure was produced using the statistical software R (version 3.5. obtained from https://www.R-project.org/, accessed on January 5th 2019; R Core Team, 2018) and the R package “tidyverse” (version 1.2.1. obtained from the Comprehensive R Archive Network (CRAN) https://cran.r-project.org/web/packages/tidyverse/index.html accessed on January 5, 2019, provided by the Vienna University of Economics and Business, Vienna, Austria; Wickham, 2017). Values in this section are given as the mean ± standard deviation.
Figure 2.
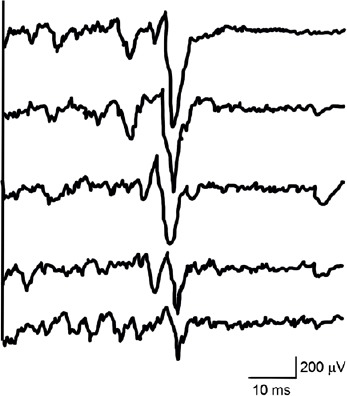
Five consecutive electromyography recordings of ipsilateral motor evoked potentials in the active left first dorsal interosseous of the patient.
Transcranial magnetic stimulation was applied to the left motor cortex at maximum stimulator output. It can be seen the marked trial-to-trial variability in motor evoked potential size and latency.
Results
Ipsilateral MEPs were recorded in the FDI, the wrist extensors and biceps brachii muscles in most or all of the control participants.
The amplitude of the ipsilateral MEP was on average 395.1 ± 313.9 μV in the FDI, 278.5 ± 22.7 μV in the wrist extensors and 245.7 ± 207.4 μV in the biceps brachii in the whole sample (Figure 3).
Figure 3.
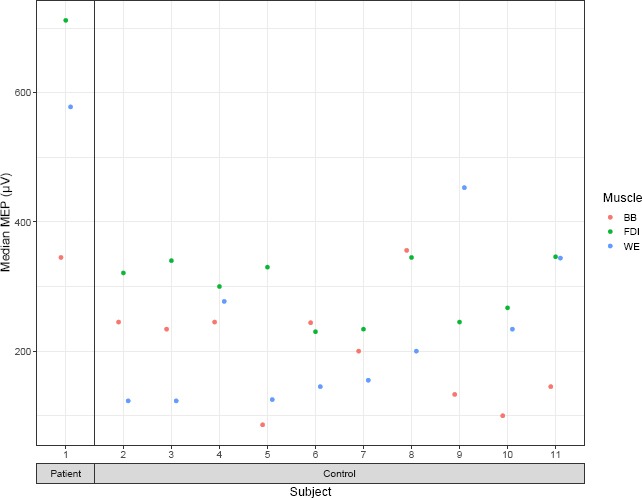
The MEPs for each participant and muscle.
Different colors correspond to the muscle groups. MEP: Motor evoked potential; BB: biceps brachii; FDI: first dorsal interosseous; WE: wrist extensors.
In the patient, MEP was on average 682.6 ± 163.1 μV in the FDI, 538.2 ± 309.1 μV in the wrist extensors and 402 ± 275.1 μV in the biceps brachii (Figure 2).
In the opponens pollicis and wrist flexors muscles, all control participants showed no response. By contrast, a small MEP was found in the patient in both muscles (213 ± 168 μV and 186 ± 103 μV, respectively).
Ipsilateral MEPs showed a higher threshold compared with contralateral MEPs, and there was a difference between control participants and patient. The mean threshold of ipsilateral MEPs was 2.3 × the AMT of the contralateral FDI in the control participants, the threshold was 1.75× the AMT in the patient (Table 1).
Table 1.
The ratio of the ipsilateral to the contralateral RMT in the FDI for each participant
| Patient | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Ipsilateral RMT/ Contralateral RMT | 1.75 | 2.25 | 2.50 | 2.50 | 2.00 | 2.25 | 2.50 | 2.25 | 2.00 | 2.25 | 2.25 |
RMT: Resting motor threshold; FDI: first dorsal interosseus.
Discussion
In the patient, ipsilateral MEPs were elicited in all the examined muscles of the affected side. In the FDI, wrist extensors and biceps brachii muscles the motor responses were increased compared with the control participants.
There is evidence that the ipsilateral motor projections play a crucial role in thecontrol of ipsilateral movements. These pathways largely contribute to the functional recovery after injury (Tazoe and Perez, 2014), but the extent to which the ipsilateral corticofugal projections are engaged in voluntary motor activity in healthy humans and following unihemispheric damage is still largely unknown.
Our results suggest that the motor outputs in the undamaged hemisphere are changed in a subject with hemispheric atrophy. This functional reorganization includes the unmasking of ipsilateral corticospinal projections.
In stroke patients, ipsilateral responses evoked from the unaffected hemisphere were found to be more relevant in the poorly recovered subjects (Turton et al., 1996; Netz et al., 1997). Therefore, in our patient the mechanisms underlying this reorganization might not be beneficial for functional recovery.
Several different physiological mechanisms have been proposed for the ipsilateral MEPs. In patients with congenital pathologies, such as hemiplegic cerebral palsy or Klippel-Feil syndrome, the onset latency was identical in the ipsilateral and contralateral MEP. Moreover, single motor-unit EMG recordings from homologous muscles pairs revealed short-lasting central peaks in the cross-correlogram, suggesting axonal branching of crossed corticospinal fibers (Farmer et al., 1990, 1991; Carr et al., 1993). The existence of fast-conducting uncrossed, monosynaptic, corticomotoneuronal pathway was considered to be a plausible explanation in patients with persistent mirror movements. Indeed, in these patients the ipsilateral MEP have the same latencies but higher amplitudes than the contralateral MEP (Mayston et al., 1997).
The expected delay of near zero between the ipsi- and contralateral MEPs is in contrast with the results in our patient. Therefore, in these adult subjects TMS might be able to activate corticofugal motor projections other than the fast-conducting crossed corticomotoneuronal fibers.
The characteristics of the ipsilateral MEP illustrated by the present study are rather more compatible with oligosynaptic ipsilateral projections, such as the corticoreticulospinal or corticopropriospinal tract (Ziemann et al., 1999).
In agreement with our findings, the latency of ipsilateral MEPs was more prolonged than the contralateral MEPs by 5-14 ms in patients with brain damage which is acquired in later stages of life (Benecke et al., 1991; Carr et al., 1993; Netz et al., 1997) and in normal subjects (Wassermann et al., 1991, 1994; Basu et al., 1994; Netz et al., 1997). It has been supposed that these delayed ipsilateral MEPs with delayed onset latencies are determined by unmasking of slower conducting ipsilateral corticospinal pathways from the undamaged hemisphere (Netz et al., 1997), in particular a corticoreticulospinal projection (Benecke et al., 1991; Wassermann et al., 1994).
The different directional preference for contralateral MEP compared with ipsilateral MEP and interhemispheric inhibition indicates that the ipsilateral effects are mediated by populations of cortical neurons that differ from those activating the motor neurons of the corticospinal tract (Chen and Young, 2003).
Similar to those previously reported, corticofugal motor neurons other than the fast conducting crossed monosynaptic corticomotoneuronal projections can be activated by TMS. Our results also indicate ipsilateral oligosynaptic pathways, in particular corticoreticulospinal or corticopropriospinal fibers, as the route for the ipsilateral MEP.
It should be considered that studies employing TMS to detect MEPs in ipsilateral muscles are hampered by the difficulty in providing unilateral focal stimulation without influencing the motor cortex of the contralateral hemisphere or subcortical structures (Alawieh et al., 2017).
Despite some limitations of this study in terms of size, statistical significance, inability to eliminate confounders, our results demonstrated that the undamaged hemisphere develops plastic changes in central motor organization after atrophy of the contralateral hemisphere. The reinforcement of the ipsilateral corticospinal pathway probably contributes to residual motor function in subjects with unilateral brain damage.
Additional file: Open peer review report 1 (105.4KB, pdf) .
Footnotes
Conflicts of interest: All authors report no conflicts of interest.
Financial support: None.
Institutional review board statement: The tests used in this study were performed for clinical purposes using routine techniques, thus ethical approval was not sought. All participants received written information on methods and provided written informed consent for their inclusion in the present study.
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals developed by the International Committee of Medical Journal Editors.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of the Christian Doppler Clinic in Salzburg (Austria).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data will not be available.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Leonardo Cocito, Universita degli Studi di Genova, Italy.
P-Reviewer: Cocito L; C-Editors: Zhao M, Li CH; T-Editor: Liu XL
References
- 1.Alawieh A, Tomlinson S, Adkins D, Kautz S, Feng W. Preclinical and clinical evidence on ipsilateral corticospinal projections: implication for motor recovery. Transl Stroke Res. 2017;8:529–540. doi: 10.1007/s12975-017-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu AP, Turton A, Lemon RN. Activation of ipsilateral upper limb muscles by transcranial magnetic stimulation. J Physiol. 1994;479:144–145. [Google Scholar]
- 3.Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp Brain Res. 1991;83:419–426. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- 4.Britton TC, Meyer BU, Benecke R. Central motor pathways in patients with mirror movements. J Neurol Neurosurg Psychiatry. 1991;54:505–510. doi: 10.1136/jnnp.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. J Physiol. 1991;440:243–255. doi: 10.1113/jphysiol.1991.sp018706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Cohen LG, Hallett M. Role of the ipsilateral motor cortex in voluntary movement. Role of the ipsilateral motor cortex in voluntary movement. Can J Neurol Sci. 1997;24:284–291. doi: 10.1017/s0317167100032947. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 9.Cincotta M, Ragazzoni A, de Scisciolo G, Pinto F, Maurri S, Barontini F. Abnormal projection of corticospinal tracts in a patient with congenital mirror movements. Neurophysiol Clin. 1994;24:427–434. doi: 10.1016/s0987-7053(05)80075-9. [DOI] [PubMed] [Google Scholar]
- 10.Cohen LG, Meer J, Tarkka I, Bierner S, Leiderman DB, Dubinsky RM, Sanes JN, Jabbari B, Branscum B, Hallett M. Congenital mirror movements. Abnormal organization of motor pathways in two patients. Brain. 1991;114:381–403. doi: 10.1093/brain/114.1.381. [DOI] [PubMed] [Google Scholar]
- 11.Danek A, Heye B, Schroedter R. Cortically evoked motor responses in patients with Xp22.3-linked Kallmann's syndrome and in female gene carriers. Ann Neurol. 1992;31:299–304. doi: 10.1002/ana.410310312. [DOI] [PubMed] [Google Scholar]
- 12.Dupont S, Gales A, Sammey S, Vidailhet M, Lambrecq V. Late-onset Rasmussen encephalitis: A literature appraisal. Autoimmun Rev. 2017;16:803–810. doi: 10.1016/j.autrev.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Farmer SF, Ingram DA, Stephens JA. Mirror movements studied in a patient with Klippel-Feil syndrome. J Physiol. 1990;428:467–484. doi: 10.1113/jphysiol.1990.sp018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41:1505–1510. doi: 10.1212/wnl.41.9.1505. [DOI] [PubMed] [Google Scholar]
- 15.Hömberg V, Stephan KM, Netz J. Transcranial stimulation of motor cortex in upper motor neurone syndrome: its relation to the motor deficit. Electroencephalogr Clin Neurophysiol. 1991;81:377–388. doi: 10.1016/0168-5597(91)90027-u. [DOI] [PubMed] [Google Scholar]
- 16.Kanouchi T, Yokota T, Isa F, Ishii K, Senda M. Role of the ipsilateral motor cortex in mirror movements. J Neurol Neurosurg Psychiatry. 1997;62:629–632. doi: 10.1136/jnnp.62.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konagaya Y, Mano Y, Konagaya M. Magnetic stimulation study in mirror movements. J Neurol. 1990;237:107–109. doi: 10.1007/BF00314672. [DOI] [PubMed] [Google Scholar]
- 18.Maegaki Y, Maeoka Y, Ishii S, Shiota M, Takeuchi A, Yoshino K, Takeshita K. Mechanisms of central motor reorganization in pediatric hemiplegic patients. Neuropediatrics. 1997;28:168–174. doi: 10.1055/s-2007-973695. [DOI] [PubMed] [Google Scholar]
- 19.Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann's syndrome. I. A neurophysiological study. Brain. 1997;120:1199–1216. doi: 10.1093/brain/120.7.1199. [DOI] [PubMed] [Google Scholar]
- 20.Nathan PW, Smith MC. Effects of two unilateral cordotomies on the motility of the lower limbs. Brain. 1973;9:471–494. doi: 10.1093/brain/96.3.471. [DOI] [PubMed] [Google Scholar]
- 21.Netz J, Lammers T, Hömberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- 22.Oguni H, Andermann F, Rasmussen TB. The natural history of the syndrome of chronic encephalitis and epilepsy: a study of the MNI series of forty-eight cases. In: Chronic encephalitis and epilepsy–Rasmussen's syndrome. In: Andermann F, editor. Boston: Butterworth-Heinemann; 1991. pp. 7–35. [Google Scholar]
- 23.R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Accessed on January 5, 2019]. R: A language and environment for statistical computing. URL https://www.R-project.org/ [Google Scholar]
- 24.Rasmussen T, Olszewski J, Lloyd-Smith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8:435–445. doi: 10.1212/wnl.8.6.435. [DOI] [PubMed] [Google Scholar]
- 25.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tazoe T, Perez MA. Selective activation of ipsilateral motor pathways in intact humans. J Neurosci. 2014;34:13924–13934. doi: 10.1523/JNEUROSCI.1648-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 28.Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, Vincent A, Mathern GW, Cross JH. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13:195–205. doi: 10.1016/S1474-4422(13)70260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassermann EM, Fuhr P, Cohen LG, Hallett M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology. 1991;41:1795–1799. doi: 10.1212/wnl.41.11.1795. [DOI] [PubMed] [Google Scholar]
- 30.Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- 31.Wassermann EM, Pascual-Leone A, Hallett M. Cortical motor representation of the ipsilateral hand and arm. Exp Brain Res. 1994;100:121–132. doi: 10.1007/BF00227284. [DOI] [PubMed] [Google Scholar]
- 32.Wickham H. tidyverse: Easily Install and Load the ‘Tidyverse’. R package version 1.2.1. [Accessed on January 5, 2019];2017 https://CRAN.R-project.org/package=tidyverse . [Google Scholar]
- 33.Wiesendanger M. The pyramidal tract. Its structure and function. In: Motor coordination. Handbook of behavioural neurobiology 5. In: Tow AL, Luchei ES, editors. New York: Plenum; 1981. pp. 401–491. [Google Scholar]
- 34.Yakolev PI, Rakic P. Patterns of decussation of bulbar pyramids and distribution of pyramidal tracts on two sides of the spinal cord. Trans Am Neurol Assoc. 1966;91:366–367. [Google Scholar]
- 35.Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518:895–906. doi: 10.1111/j.1469-7793.1999.0895p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


