Abstract
Common neurodegenerative diseases of the central nervous system are characterized by progressive damage to the function of neurons, even leading to the permanent loss of function. Gene therapy via gene replacement or gene correction provides the potential for transformative therapies to delay or possibly stop further progression of the neurodegenerative disease in affected patients. Adeno-associated virus has been the vector of choice in recent clinical trials of therapies for neurodegenerative diseases due to its safety and efficiency in mediating gene transfer to the central nervous system. This review aims to discuss and summarize the progress and clinical applications of adeno-associated virus in neurodegenerative disease in central nervous system. Results from some clinical trials and successful cases of central neurodegenerative diseases deserve further study and exploration.
Keywords: nerve regeneration, central nervous system, gene therapy, neurodegenerative disease, viral vector, adeno-associated virus, Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, spinal muscular atrophy, neural regeneration
Introduction
The central nervous system is a complex and delicate system where many of the pervasive disease processes arise. These diseases include a broad range of pathological states and can affect global or local metabolism and function (Simonato et al., 2013). Neurodegenerative diseases, which commonly occur in the central nervous system, are defined by progressive nervous system dysfunction associated with atrophy of the nervous structures. The altered maintenance of proteostasis is considered as a general feature among neurodegenerative diseases (Gerakis and Hetz, 2018). Prototypical neurodegenerative diseases in the central nervous system include Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis and Huntington’s disease (Lin and Beal, 2006). In general, the most available therapeutic approaches have not been proven as an effective treatment for neurodegenerative diseases in the central nervous system. This is due to the lack of viable long-term delivery of therapeutic drugs to the central nervous system. Furthermore, the blood-brain barrier restricts the access of many systemic treatments and those that do pass often have effects on non-target areas compromising the widespread delivery of therapeutic drugs to the central nervous system (Lykken et al., 2018). Thus, there is a necessity for more effective strategies and therapies to be explored.
Currently, gene therapy has been developed as a novel strategy for treating both genetic inherited and acquired neurodegenerative diseases in the central nervous system. It can deliver a transgene to supply gene products that will permanently restore the missing functions or introduce a therapeutic gene to cells of the target tissue (Piguet et al., 2017). Both viral and non-viral vectors are commonly used vehicles for gene therapy (Bangde et al., 2017). In comparison with viral vectors, the instability and rapid clearance of non-viral vectors still need further resolution (Yin et al., 2014).
The viral vector-mediated gene therapy, which includes the vectors of lentiviruses, adenovirus, herpes simplex virus, vaccinia virus and adeno-associated virus (AAV), can deliver therapeutic genes directly to the central nervous system (Kotterman et al., 2015). However, the efficiency and safety of gene therapy are very dependent on the vectors. Many studies and developments have shifted toward the advancement of innovative viral vectors that could combine low genotoxicity and immunogenicity delivery with greater efficiency. Among these vectors, adeno-associated virus was regarded as a promising technological advance (Murlidharan et al., 2014).
The structure of AAV involves one single-stranded DNA with an inverted terminal repeat as the viral genome and one protein capsid (Cassinotti et al., 1988; Lentz et al., 2012; Pillay et al., 2016). Generally, it can package DNA to a size limit of less than 4.7 kb (Colella et al., 2018). AAV vectors have the ability to transduce distinctive cells or tissues by packaging non-genomic DNA that is not related to any central nervous system or neurodegenerative disease (Agbandje-McKenna and Kleinschmidt, 2011). AAV also combines low immunogenicity and less pathogenicity with long-lasting transgene expression in clinical applications. Some serotypes of AAV can cross the blood-brain barrier with ease. With an AAV2 capsid, the vector has been shown to target cerebral vascular endothelial cells specifically (Chen et al., 2009; Gray et al., 2010; McCown, 2011). After systemic administration with the AAV9 serotype, high expression was observed in the great mass of cerebral regions involving the substantia nigra par reticulata, hippocampus, cerebellum, motor cortex and cervical spinal cord (McLean et al., 2014).
The distinct structures of AAV-mediated gene therapy have allowed novel treatments of neurodegenerative diseases in the central nervous system, particularly with recombinant AAV. To analyze the application and related function of AAV, this review investigates the past, present and future statuses of AAV for the treatment of neurodegenerative diseases in the central nervous system. We performed literature searches by using PubMed, Scopus, Embase and Web of Science databases with no language restrictions. The time period ranged from July 1, 2005 to May 31, 2018. Search terms include “central nervous system” or “gene therapy” or “neurodegenerative disease” or “viral vector” or “adeno-associated virus” or “Alzheimer’s disease” or “Parkinson’s disease” or “Huntington’s disease” or “amyotrophic lateral sclerosis” or “spinal muscular atrophy” or “Canavan disease” or “Metachromatic leukodystrophy”. Eligibility criteria were as follows: All articles and reviews regarding AAV-mediated gene therapy for neurodegenerative diseases or any specific neurodegenerative disease of the central nervous system were included. In addition, we searched the reference lists of the retrieved papers for relevant articles. The full texts of the relevant articles were read to extract information on the topic of interest (summarized in Table 1). The procedures used generalized AAV-mediated gene therapy for the different neurodegenerative diseases of the central nervous system as follows.
Table 1.
AAV-mediated gene therapy in clinical trials for neurodegenerative diseases in the central nervous system
| Disease | Therapeutic gene | Serotype | Administration | Number of patients | Identifier | Clinical trial phase & Status | Remarks |
|---|---|---|---|---|---|---|---|
| Parkinson’s disease | GDNF | AAV2 | Putaminal | 25 | NCT01621581 | Phase I, active not recruiting | Convection-enhanced delivery method was performed to infuse virus vectors into the brain |
| NTN/CERE-120 | AAV2 | Substantia nigra and putamen | 60* | NCT00985517 | Phase I/II, active not recruiting | No difference was observed between AAV2-NTN-treated and sham-operated patients | |
| GAD | AAV2 | Subthalamic nucleus | 45 | NCT00643890 | Phase II, terminate | AAV2-GAD gene therapy improved UPDRS motor score at 6 months | |
| AADC | AAV2 | Intraputaminal | 10 | NCT00229736 | Phase I, complete | AAV2-AADC treatment was safe and that AADC gene expression was maintained at least 4 years after administration of therapy | |
| AADC | AAV2 | Intraputaminal | 6* | NCT02418598 | Phase I/II, active recruiting | Evaluating the safety, efficacy of intraputaminal administration of AAV2-AADC by stereotaxic surgery in advanced PD patients | |
| Alzheimer’s disease | APOE2 | AAVrh.10 | Intracisternal | 15* | NCT03634007 | Phase I, active not recruiting | The study will explore a maximum dose and attempt to convert APOE4 homozygotes to APOE2-APOE4. |
| NGF/CERE-110 | AAV2 | Basal forebrain | 49 | NCT00876863 | Phase II, active not recruiting | AAV-NGF had no benefit on cognition at 24 months after treatment | |
| Canavan disease | ASPA | AAV2 | Intraparenchymal | 21 | McPhee et al., 2006; Leone et al., 2012 | Phase I, complete | The gene therapy was tolerable with no severe adverse effects and delayed progression of brain atrophy |
| Spinal muscular atrophy | AVXS-101 | AAV9 | Intravenous | 15 | NCT02122952 | Phase I, complete | The study prolonged survival, improved motor function, and increased the CHOP INTEND scores |
| AVXS-101 | AAV9 | Intravenous | 44* | NCT03505099 | Phase III, active recruiting | This study will recruit patients with multiple copies of SMN2 | |
| Metachromic leukodystrophy | Arylsulfatase A | AAVrh.10 | Intracerebral | 5* | NCT01801709 | Phase I/II, active not recruiting | Safety and efficacy evaluated based on clinical, neuropsychological, radiological, electrophysiological and biological parameters |
*Estimated number of enrolled participants. AAV: Adeno-associated virus; GDNF: glial cell line-derived neurotrophic factor; NTN: neurturin; CERE: Ceregene; GAD: glutamic acid decarboxylase; UPDRS: unified PD rating scale; AADC: aromatic L-amino acid decarboxylase; PD: Parkinson’s disease; APOE: apolipoprotein E; NGF: nerve growth factor; ASPA: aspartoacylase; AVXS-101: AveXis-101; CHOP INTEND: Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; SMN2: survival motor neuron 2.
Alzheimer’s Disease
AD is one of the age-related neurodegenerative disorders and is accompanied by the progressive loss of cognitive function and impaired learning ability. It is associated with progressive deficiency in synapses and neurons of the cerebral cortex (Fol et al., 2016; Javidnia et al., 2017). The causes for most AD are still unknown, and several competing hypotheses have been proposed to elucidate the pathogenesis of the disease. In recent years, AAV has been used to test different hypotheses with the therapy having the advantages of long-term genome expression and fewer immune responses.
The accumulated evidence has indicated that neuroinflammation is one of the main reasons for AD (Heppner et al., 2015; Onyango, 2018; Wang et al., 2018a). It was reported that interleukin-2, which is considered to act as an inflammation controller, could be packaged in an AAV vector and applied to alleviate AD in mice model (Saadoun et al., 2011). AAV-interleukin-2 was able to remodel the hippocampus by rescuing spine density and improving synaptic plasticity that led to progressive recovery of memory ability with only rare side effects (Alves et al., 2017). In the neuroinflammatory process, microglia, which highly express the pro-inflammatory molecule glia maturation factor, have been proven to play another important role (Cornejo and von Bernhardi, 2016; Crotti and Ransohoff, 2016; Colonna and Butovsky, 2017). It was found that glia maturation factor was expressed in various regions of AD brains. However, reduction of glia maturation factor could protect neuronal degeneration in PD mouse models. Raikwar et al. (2018) applied AAV-mediated gene therapy to downregulate glia maturation factor gene expression in the reactive microglial cell line. The progression of neuroinflammation was inhibited after genome editing, indicating that the downregulation of AAV-mediated glia maturation factor might be a potential target for AD treatment (Raikwar et al., 2018).
In the amyloid hypothesis of AD, the overexpression or accumulation of amyloids induces inflammation, leading to neuronal death (Hardy and Selkoe, 2002; Tong et al., 2018; Zhang et al., 2018). Therefore, the amyloid precursor protein whose proteolysis generates amyloid beta is considered as a potential target for AD treatment. Kiyota et al. (2015) found that CD74 could bind to amyloid precursor protein and inhibit amyloid beta processing. Subsequently, they showed that AAV-mediated CD74 expression decreased the production of amyloid beta and improved the brain function in mice (Kiyota et al., 2015). Some therapeutic enzymes delivered by AAV, such as asparagine endopeptidase and neprilysin 2, also achieved positive effects in an AD animal model. These enzymes could rescue the loss of synaptic functions and delay cognitive deficits by degrading amyloid beta (Sasmita, 2018). These results have inspired a new approach to treating AD.
The degeneration of the nucleus basalis of Meynert, which can cause cortical cholinergic deficits, is considered another feasible pathogenesis in AD. To delay or even stop degeneration of the nucleus basalis of Meynert in patients, a Phase II clinical trial (Identifier: NCT00876863) that delivered nerve growth factor intracerebrally by AAV2 was performed. The rationale for this trial was based on preclinical animal research that showed nerve growth factor deprivation led to the degeneration of nucleus basalis of Meynert cells. Conversely, exposure to nerve growth factor rescued or increased cholinergic function in the deprived nucleus basalis of Meynert neurons (Rafii et al., 2014; Hunsberger et al., 2016; Cummings et al., 2017). However, in the clinical trial, no benefit of AAV2-nerve growth factor group was observed compared with sham surgery at 24 months post procedure. Due to this unsatisfactory result, more accurate and specific gene targeting is needed (Rafii et al., 2018).
For late-onset AD, apolipoprotein E (APOE) alleles have an effect on AD (Yi et al., 2014). One of APOE alleles, APOE4, which leads to the massive accumulation of amyloid beta in AD brain, can accelerate the likelihood of AD occurrence and reduce the age of onset. However, APOE2 serves a protective role in AD. In a mouse model, it was reported that AAV-mediated APOE2 delivery could alleviate symptoms of AD (Zhao et al., 2016). In addition, a Phase I clinical trial (Identifier: NCT03634007), which uses AAVrh.10 as the viral vector to deliver APOE2 to AD patients, is in process in 2018 (Table 1).
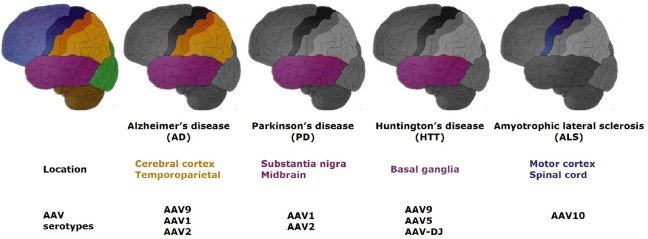
Taken together, AAV-mediated gene therapy has been applied in various pathogenesis of AD. Even though the Phase II clinical trial (AAV2-nerve growth factor) did not achieve the expected goal, AAV vectors can still provide a safe means to deliver therapy for AD (Figure 1).
Figure 1.
General locations of the main neurodegenerative diseases in the central nervous system and related AAV serotypes, which have been published.
The first picture on the left is the normal structure of the brain with each major region in a different color. The locations of the different disorders are shown in color. AAV: Adeno-associated virus.
Parkinson’s Disease
PD is another age and neuroinflammation related neurodegenerative disease caused by the progressive loss of dopaminergic neurons in the substantia nigra (Shi and Chen., 2017; Martinez and Peplow, 2018). Traditional anti-Parkinson agents, such as levodopa and dopamine agonists, are widely used in clinical treatment. However, as the disease progresses, these drugs become less effective and often lead to additional side effects. Lately, AAV-mediated gene therapy has been extensively explored for treating PD. It aims to rescue damaged neurons by delivering neurotrophic factors or rebuild functions by delivering key enzymes involved in dopamine synthesis and metabolism (O’Connor and Boulis, 2015). There have been some interesting reports on clinical trials of gene therapy application in PD since 2013 (Table 1).
Neurturin is a member of the glial cell line-derived neurotrophic factor family of ligands. Some animal experiments showed that neurturin was able to rescue dopaminergic neurons and improve motor function (Gash et al., 1996; Emborg et al., 2009; Herzog et al., 2013). After preliminary clinical tests, a clinical trial was conducted using AAV2-mediated neurturin (Cere-120) gene delivery (Identifier: NCT00985517). In this Phase II trial, 51 advanced PD patients who received AAV2-neurturin-treated or sham-operated agent were randomly divided into two groups. The agent was administered directly into the putamen and substantia nigra. However, 15 months post-administration, clinical outcomes were evaluated and showed that there was no improvement in AAV2-NRTN-treated patients. This result might be caused by the downregulation of neurturin and glial cell line-derived neurotrophic factor receptors in advanced PD patients (Warren Olanow et al., 2015).
Aromatic L-amino acid decarboxylase (AADC), the inhibitor of premature conversion of levodopa to dopamine, is another treatment for PD. One Phase I clinical trial (Identifier: NCT00229736) using AAV2-AADC to delay the decarboxylation of levodopa in nigrostriatal dopamine neurons indicated that AAV2-mediated AADC gene therapy was safe. Although AADC expression was stable over the four years in patients, the unified PD rating scale was improved only in the first 12 months (Christine et al., 2009; Mittermeyer et al., 2012). Recently, a magnetic resonance imaging-guided delivery system with convection enhanced delivery used in a non-human primate experiment showed a better effect of the treatment (Zeiss et al., 2017). Based on this, the intraputaminal infusion of AAV2-AADC by stereotaxic surgery was used in another Phase II clinical trial (Identifier: NCT02418598). This clinical trial is now recruiting, and the results should be interesting.
Glutamic acid decarboxylase (GAD) is considered another target in PD treatment. It is the rate-limiting enzyme for regulating gamma-aminobutyric acid production. Recently, a controlled, blinded Phase II trial that delivered AAV2-GAD by directly injecting to the subthalamic nucleus showed promising efficacy (Identifier: NCT00643890). AAV2-GAD gene therapy improved unified the PD rating scale motor score at six months. However, the improvement of patients in the treatment group did not exceed the existing treating methods, so the study was terminated (LeWitt et al., 2011).
Despite some unsatisfactory clinical trial results, the research and exploration of PD treatment has not stopped. Oh et al. (2015) demonstrated that combined transcription factors, Nurr1 and Foxa2, delivered by an AAV vector could protect midbrain dopamine neurons and improve motor behaviors in mice. Another repulsive guidance molecule a, which regulates axon guidance and neuronal apoptosis, could also be a target. Repulsive guidance molecule a is upregulated markedly in the substantia nigra of PD patients. To verify the effect of repulsive guidance molecule a on dopaminergic neurons, repulsive guidance molecule a was delivered to the midbrain of mice using an AAV vector. It was reported that repulsive guidance molecule a influenced the PD process when analyzed at molecular, anatomical and behavioral levels (Oh et al., 2015; Korecka et al., 2017).
Huntington’s Disease
Huntington’s disease is an inherited gene disorder, which leads to progressive degeneration/death of striatal neurons (Ramaswamy and Kordower, 2012; Boussicault et al., 2016). Researchers are dedicating time and effort to clarify the pathogenesis of Huntington’s disease. Korean scientists showed that an AAV vector, serotype DJ, could generate a novel juvenile-mouse model of Huntington’s disease (Jang et al., 2018). After treatment with AAV-DJ, the behaviors of mice in the model replicated the dysfunction of motor neurons and neurodegeneration observed in Huntington’s disease. The changes in animal behavior were accompanied by extensive neural cell apoptosis, the upregulation of inflammatory cytokines and activation of glia. This model illustrated that the use of AAV vector could help understand the mechanisms in the Huntington’s disease striatum and explore therapeutic strategies for Huntington’s disease (Jang et al., 2018).
One AAV vector-mediated gene therapy in a Huntington’s disease animal model showed robust therapeutic effects (Dufour et al., 2014; Vagner et al., 2016). It is reported that Huntington’s disease transgenic sheep, with human mutant HTT, were conducted for Huntington’s disease study. When AAV9-mediated miRNA, which targeted exon 48, was delivered to Huntington’s disease sheep striatum, human mutant (m) HTT mRNA and 50–80% proteins encoded by mutant Huntington gene were eliminated six months post injection. Moreover, the Iba1-positive microglia could be detected at the same level as the control group (Pfister et al., 2018). In another experiment, a SIRT3 gene delivered by AAV vector protected Huntington’s disease mice from neurodegeneration by blocking mitochondrial oxidative stress and maintaining neuronal bioenergy (Cheng et al., 2016). In addition, AAV5 noticeably reduced the mutant Huntington mRNA and protein when delivering the Huntington-lowering gene in minipigs. These results illustrate the safety and effectiveness of AAV vectors in treating Huntington’s disease. Figure 1 summarizes research to date on the brain areas associated with each degenerative disease and the AAV vectors used to apply therapies. The laboratory and preliminary clinical research on Huntington’s disease has reached the stage where treatment could start to be applied clinically (Evers et al., 2018).
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis, also known as Lou Gehrig’s disease, is a neuromuscular disease. Amyotrophic lateral sclerosis is characterized by degeneration of the motor cortex, brainstem, and spinal cord, along with atrophy in motor neurons and muscles. The incidence of amyotrophic lateral sclerosis is only 4.3 per 100,000 in the United States (Pfister et al., 2018). However, amyotrophic lateral sclerosis is a disease which leads to paralysis and premature death (Bertram and Tanzi, 2005; Yamashita et al., 2013). Recently, AAV9-mediated human insulin-like growth factor 1 was applied to the amyotrophic lateral sclerosis mouse model. This research showed that AAV9-mediated insulin-like growth factor 1 therapy decreased the amount of apoptotic motor neurons and prolonged the lifespan of amyotrophic lateral sclerosis mice. The pathway has been associated with the blocking of phosphorylated p38 mitogen-activated protein kinase and c-Jun-N terminal kinase in the spinal cord (Wang et al., 2018b).
The suppression of toxic mutant superoxide dismutase 1 (SOD1) has been considered one of the most promising treatments for amyotrophic lateral sclerosis for the last few decades. Biferi et al. (2017) reported that AAV10, packaged in a modified U7 small nuclear RNA, reduced the mutant SOD1 level through regulating exon skipping of the mutant SOD1 pre-mRNA in an amyotrophic lateral sclerosis mouse model. The treatment prevented the loss of body weight, relieved neuromuscular function damage and led to an increase in survival of SOD1-G93A mice. Li et al. (2017) pointed out that the injection speed of AAV10 affected the transduction of the spinal cord and dorsal root ganglia. If the injection speed was decreased, the transduction efficiency was increased (Biferi et al., 2017; Li et al., 2017). These encouraging results of the application of AAV in amyotrophic lateral sclerosis should inspire further research in patients (Figure 1).
Spinal Muscular Atrophy
Spinal muscular atrophy is an inherited neuromuscular disorder caused by motor neuron degeneration in the anterior horns of the spinal cord and brain stem (Parente and Corti, 2018). Spinal muscular atrophy leads to progressive weakness and atrophy of muscle. Respiratory muscle failure is usually the fatal factor for spinal muscular atrophy patients. Spinal muscular atrophy arises from the loss of survival motor neuron 1 (SMN1) gene, which encodes the essential protein, SMN protein, for alpha motor neurons (Kariyawasam et al., 2018). In spinal muscular atrophy patients the SMN1 gene is replaced by a mutated identical gene, SMN2, which encodes an unstable and partially functional protein due to the splicing of exon 7 (Scoto et al., 2018). The clinical severity of the disease is associated with the onset age and the number of SMN2 copies. Based on this, spinal muscular atrophy is classified as four phenotypes (Lefebvre et al., 1997; Parsons et al., 1998; Messina, 2018; Tizzano and Zafeiriou, 2018). Since the pathological mechanism of spinal muscular atrophy has been explained, many emerging strategies focus on the regulation of the deficiency gene. Recently, the application of AAV9 to deliver a functional SMN1 gene has been considered as a promising candidate for treating spinal muscular atrophy. Firstly, the studies in mice have verified that AAV9-AVXS-101 achieves the expression of SMN1 gene in the spinal cord after an intravenous administration. Moreover, the pathological symptoms of the spinal muscular atrophy mice have also been corrected (Foust et al., 2010; Passini et al., 2010; Valori et al., 2010). After verification in the laboratory, some clinical trials have been initiated (Table 1). The first Phase I trial (Identifier: NCT02122952) that tested the safety and efficiency of intravenous delivery of AAV9-AVXS-101 to 15 spinal muscular atrophy type I infants has been completed. The outcomes showed that the 15 patients who received a single intravenous administration of AAV9-AVXS-101 were all alive (an age of at least 20 months) along with improved motor function and increased CHOP INTEND scores up to August 2017 (Mendell et al., 2017). These results encourage great confidence in curing spinal muscular atrophy. In the meantime, other AAV-mediated spinal muscular atrophy clinical trials are being initiated as part of a wide application of AAV gene therapy. The latest Phase III trial (Identifier: NCT03505099) characterized by recruiting patients with multiple phenotypes was just posted in 2018.
Canavan Disease
Canavan disease is a neurodegenerative disease defined by diffused spongiform white matter degeneration in the brain, dysmyelination and intramyelinic oedema along with consequent deterioration of psychomotor development (Matalon and Michals-Matalon, 1999; Kumar et al., 2006; Hoshino and Kubota, 2014). The pathology of Canavan disease is caused by inactive aspartoacylase, which leads to the toxic accumulation of N-acetylaspartate. Currently, traditional treatments for Canavan disease include the application of lithium, glyceryl triacetate, triheptanoin and oral N-acetylaspartate administration (Janson et al., 2005; Madhavarao et al., 2009; Arun et al., 2010; Baslow and Guilfoyle, 2013; Francis et al., 2014). However, it is evident that an efficient gene therapy for Canavan disease would be an ideal treatment if begun at an early age. A Phase I clinical trial which delivered AAV2-aspartoacylase intraparenchymally to Canavan disease patients has been completed (Table 1). A long-term follow up of gene delivery in 13 Canavan disease patients was published by Leone et al. (2012). Their results showed the treatment was well tolerated and no serious side effects occurred. The therapeutic benefits were decreased accumulation of brain N-acetylaspartate; delayed progressive brain atrophy; and less frequent seizures. Neurological examination showed significant motor function improvements in the younger patients treated for Canavan disease, suggesting the probable advantage of early-onset therapeutic intervention (McPhee et al., 2006; Leone et al., 2012; Ahmed and Gao, 2013).
Metachromatic Leukodystrophy
Metachromatic leukodystrophy is an inherited lysosomal disorder caused by deficiency of the enzyme arylsulfatase A. This results in excess accumulation of sulfatides in Schwann cells, oligodendrocytes, and brain neurons (van Rappard et al., 2015). Currently, there are several promising treatments available for metachromatic leukodystrophy along with bone marrow transplants, enzyme-replacement therapy and AAV-mediated gene therapy directly to the central nervous system (Rosenberg et al., 2016). A Phase I/II clinical trial of intracerebrally delivering AAVRh10-arylsulfatase A to metachromatic leukodystrophy patients is in process (Table 1). This clinical trial follows previous successful AAV-mediated gene therapy experiments in mice (Piguet et al., 2012; Miyake et al., 2014). The safety and efficacy of AAVRh10-arylsulfatase A on early-onset metachromatic leukodystrophy are being assessed by clinical, neuropsychological, radiological, electrophysiological and biological parameters. It could become a promising method to treat metachromatic leukodystrophy patients.
Conclusion
In the past decades, AAV has been developed as a promising nonpathogenic vector of gene therapy to treat neurodegenerative diseases in the central nervous system. It can deliver the therapeutic gene to the central nervous system directly and provide long-term and functional correction of missing or mutated genes. The development of different serotypes and genetic targets for different cells could assist in the curing of neurodegenerative diseases stemming from various causes. A powerful example of AAV and its established achievements are illustrated in the successful curing of spinal muscular atrophy in young infants.
However, there have been some poor results in other trials using AAV. Some of the failures may be attributed to the lack of complete and thorough knowledge concerning the diseases rather than the errors arising from the use of AAV itself. The clinical application of AAV faces other challenges. The neutralizing antibodies against AAV are considered as a major obstacle. The pre-existing antibodies which can neutralize AAV have severely limited the broad use of AAV vectors in clinical gene therapy (Ortolano et al., 2012; Rapti et al., 2012). Another limitation for the application of AAV is target specificity. The receptors for different serotypes of AAV are still being explored. To date, AAVR and galactose could bind more specifically to AAV2 and AAV9 respectively, but these receptors are located in many organs in the human body (Madigan and Asokan, 2016; Pillay et al., 2016). This means that it is difficult to deliver AAV2 or AAV9 to unique cells or tissue after a systemic administration.
Other issues, such as optimal administration methods and manufacturing problems, need to be addressed. These efforts should accelerate and improve the clinical application of AAV vectors in each neurodegenerative disease of the central nervous system. AAV is a promising vector in the new application of gene therapy and is deserving of more opportunities for further research, exploration, and implementation.
Additional file: Open peer review reports 1 (106.6KB, pdf) and 2 (106.6KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that they have no competing interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Panteleimon Giannakopoulos, University Hospitals of Geneva, Switzerland; Marvin Soriano-Ursua, Escuela Superrior de Medicina IPN, Mexico.
P-Reviewers: Giannakopoulos P, Soriano-Ursua M; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Dawes EA, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Agbandje-McKenna M, Kleinschmidt J. AAV capsid structure and cell interactions. Methods Mol Biol. 2011;807:47–92. doi: 10.1007/978-1-61779-370-7_3. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SS, Gao G. Gene therapy for Canavan’s disease takes a step forward. Mol Ther. 2013;21:505–506. doi: 10.1038/mt.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves S, Churlaud G, Audrain M, Michaelsen-Preusse K, Fol R, Souchet B, Braudeau J, Korte M, Klatzmann D, Cartier N. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain. 2017;140:826–842. doi: 10.1093/brain/aww330. [DOI] [PubMed] [Google Scholar]
- 4.Arun P, Madhavarao CN, Moffett JR, Hamilton K, Grunberg NE, Ariyannur PS, Gahl WA, Anikster Y, Mog S, Hallows WC, Denu JM, Namboodiri AM. Metabolic acetate therapy improves phenotype in the tremor rat model of Canavan disease. J Inherit Metab Dis. 2010;33:195–210. doi: 10.1007/s10545-010-9100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangde P, Atale S, Dey A, Pandit A, Dandekar P, Jain R. Potential gene therapy towards treating neurodegenerative diseases employing polymeric nanosystems. Curr Gene Ther. 2017;17:170–183. doi: 10.2174/1566523217666170510153845. [DOI] [PubMed] [Google Scholar]
- 6.Baslow MH, Guilfoyle DN. Canavan disease, a rare early-onset human spongiform leukodystrophy: insights into its genesis and possible clinical interventions. Biochimie. 2013;95:946–956. doi: 10.1016/j.biochi.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biferi MG, Cohen-Tannoudji M, Cappelletto A, Giroux B, Roda M, Astord S, Marais T, Bos C, Voit T, Ferry A, Barkats M. A new AAV10-U7-mediated gene therapy prolongs survival and restores function in an ALS mouse model. Mol Ther. 2017;25:2038–2052. doi: 10.1016/j.ymthe.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boussicault L, Alves S, Lamazière A, Planques A, Heck N, Moumné L, Despres G, Bolte S, Hu A, Pagès C, Galvan L, Piguet F, Aubourg P, Cartier N, Caboche J, Betuing S. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington's disease. Brain. 2016;139:953–970. doi: 10.1093/brain/awv384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassinotti P, Weitz M, Tratschin JD. Organization of the adeno-associated virus (AAV) capsid gene: mapping of a minor spliced mRNA coding for virus capsid protein 1. Virology. 1988;167:176–184. [PubMed] [Google Scholar]
- 11.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23:128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev. 2018;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornejo F, von Bernhardi R. Age-dependent changes in the activation and regulation of microglia. Adv Exp Med Biol. 2016;949:205–226. doi: 10.1007/978-3-319-40764-7_10. [DOI] [PubMed] [Google Scholar]
- 17.Crotti A, Ransohoff RM. Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity. 2016;44:505–515. doi: 10.1016/j.immuni.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2017. Alzheimers Dement (N Y) 2017;3:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufour BD, Smith CA, Clark RL, Walker TR, McBride JL. Intrajugular vein delivery of AAV9-RNAi prevents neuropathological changes and weight loss in Huntington’s disease mice. Mol Ther. 2014;22:797–810. doi: 10.1038/mt.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emborg ME, Moirano J, Raschke J, Bondarenko V, Zufferey R, Peng S, Ebert AD, Joers V, Roitberg B, Holden JE, Koprich J, Lipton J, Kordower JH, Aebischer P. Response of aged Parkinsonian monkeys to in vivo gene transfer of GDNF. Neurobiol Dis. 2009;36:303–311. doi: 10.1016/j.nbd.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evers MM, Miniarikova J, Juhas S, Valles A, Bohuslavova B, Juhasova J, Skalnikova HK, Vodicka P, Valekova I, Brouwers C, Blits B, Lubelski J, Kovarova H, Ellederova Z, van Deventer SJ, Petry H, Motlik J, Konstantinova P. AAV5-miHTT gene therapy demonstrates broad distribution and strong human mutant Huntingtin lowering in a Huntington’s disease Minipig Model. Mol Ther. 2018;26:2163–2177. doi: 10.1016/j.ymthe.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fol R, Braudeau J, Ludewig S, Abel T, Weyer SW, Roederer JP, Brod F, Audrain M, Bemelmans AP, Buchholz CJ, Korte M, Cartier N, Muller UC. Viral gene transfer of APPsα rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol. 2016;131:247–266. doi: 10.1007/s00401-015-1498-9. [DOI] [PubMed] [Google Scholar]
- 23.Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AH, Kaspar BK. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Francis JS, Markov V, Leone P. Dietary triheptanoin rescues oligodendrocyte loss, dysmyelination and motor function in the nur7 mouse model of Canavan disease. J Inherit Metab Dis. 2014;37:369–381. doi: 10.1007/s10545-013-9663-6. [DOI] [PubMed] [Google Scholar]
- 25.Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in Parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 26.Gerakis Y, Hetz C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 2018;285:995–1011. doi: 10.1111/febs.14332. [DOI] [PubMed] [Google Scholar]
- 27.Gray SJ, Woodard KT, Samulski RJ. Viral vectors and delivery strategies for CNS gene therapy. Ther Deliv. 2010;1:517–534. doi: 10.4155/tde.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 29.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 30.Herzog CD, Brown L, Kruegel BR, Wilson A, Tansey MG, Gage FH, Johnson EM, Jr, Bartus RT. Enhanced neurotrophic distribution, cell signaling and neuroprotection following substantia nigral versus striatal delivery of AAV2-NRTN (CERE-120) Neurobiol Dis. 2013;58:38–48. doi: 10.1016/j.nbd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Hoshino H, Kubota M. Canavan disease: clinical features and recent advances in research. Pediatr Int. 2014;56:477–483. doi: 10.1111/ped.12422. [DOI] [PubMed] [Google Scholar]
- 32.Hunsberger JG, Rao M, Kurtzberg J, Bulte JWM, Atala A, LaFerla FM, Greely HT, Sawa A, Gandy S, Schneider LS, Doraiswamy PM. Accelerating stem cell trials for Alzheimer’s disease. Lancet Neurol. 2016;15:219–230. doi: 10.1016/S1474-4422(15)00332-4. [DOI] [PubMed] [Google Scholar]
- 33.Jang M, Lee SE, Cho IH. Adeno-associated viral vector serotype DJ-mediated overexpression of N171-82Q-mutant Huntingtin in the striatum of juvenile mice is a new model for Huntington’s disease. Front Cell Neurosci. 2018;12:157. doi: 10.3389/fncel.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janson CG, Assadi M, Francis J, Bilaniuk L, Shera D, Leone P. Lithium citrate for Canavan disease. Pediatr Neurol. 2005;33:235–243. doi: 10.1016/j.pediatrneurol.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Javidnia M, Kurd-Misto BT, Moussa CE. An update on clinical trials targeting human tauopathies. Clin Trials Degener Dis. 2017;2:66–76. [Google Scholar]
- 36.Kariyawasam D, Carey KA, Jones KJ, Farrar MA. New and developing therapies in spinal muscular atrophy. Paediatr Respir Rev. 2018 doi: 10.1016/j.prrv.2018.03.003. doi: 10.1016/j.prrv.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Kiyota T, Zhang G, Morrison CM, Bosch ME, Weir RA, Lu Y, Dong W, Gendelman HE. AAV2/1 CD74 gene transfer reduces beta-amyloidosis and improves learning and memory in a mouse model of Alzheimer’s disease. Mol Ther. 2015;23:1712–1721. doi: 10.1038/mt.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korecka JA, Moloney EB, Eggers R, Hobo B, Scheffer S, Ras-Verloop N, Pasterkamp RJ, Swaab DF, Smit AB, van Kesteren RE, Bossers K, Verhaagen J. Repulsive guidance molecule a (RGMa) induces neuropathological and behavioral changes that closely resemble Parkinson’s disease. J Neurosci. 2017;37:9361–9379. doi: 10.1523/JNEUROSCI.0084-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotterman MA, Chalberg TW, Schaffer DV. Viral vectors for gene therapy: translational and clinical outlook. Annu Rev Biomed Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Mattan NS, de Vellis J. Canavan disease: a white matter disorder. Ment Retard Dev Disabil Res Rev. 2006;12:157–165. doi: 10.1002/mrdd.20108. [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, Dreyfuss G, Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- 42.Lentz TB, Gray SJ, Samulski RJ. Viral vectors for gene delivery to the central nervous system. Neurobiol Dis. 2012;48:179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT, Wang DJ, Assadi M, Goldfarb O, Goldman HW, Freese A, Young D, During MJ, Samulski RJ, Janson CG. Long-term follow-up after gene therapy for Canavan disease. Sci Transl Med. 2012;4:165ra163. doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, Tatter SB, Schwalb JM, Poston KL, Henderson JM, Kurlan RM, Richard IH, Van Meter L, Sapan CV, During MJ, Kaplitt MG, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 45.Li D, Liu C, Yang C, Wang D, Wu D, Qi Y, Su Q, Gao G, Xu Z, Guo Y. Slow intrathecal injection of rAAVrh10 enhances its transduction of spinal cord and therapeutic efficacy in a mutant SOD1 model of ALS. Neuroscience. 2017;365:192–205. doi: 10.1016/j.neuroscience.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 47.Lykken EA, Shyng C, Edwards RJ, Rozenberg A, Gray SJ. Recent progress and considerations for AAV gene therapies targeting the central nervous system. J Neurodev Disord. 2018;10:16. doi: 10.1186/s11689-018-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madhavarao CN, Arun P, Anikster Y, Mog SR, Staretz-Chacham O, Moffett JR, Grunberg NE, Gahl WA, Namboodiri AMA. Glyceryl triacetate for Canavan disease: a low-dose trial in infants and evaluation of a higher dose for toxicity in the tremor rat model. J Inherit Metab Dis. 2009;32:640. doi: 10.1007/s10545-009-1155-3. [DOI] [PubMed] [Google Scholar]
- 49.Madigan VJ, Asokan A. Engineering AAV receptor footprints for gene therapy. Curr Opin Virol. 2016;18:89–96. doi: 10.1016/j.coviro.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez B, Peplow PV. Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease. Neural Regen Res. 2018;13:1493–1506. doi: 10.4103/1673-5374.237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matalon R, Michals-Matalon K. Biochemistry and molecular biology of Canavan disease. Neurochem Res. 1999;24:507–513. doi: 10.1023/a:1022531829100. [DOI] [PubMed] [Google Scholar]
- 52.McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2011;11:181–188. doi: 10.2174/156652311795684759. [DOI] [PubMed] [Google Scholar]
- 53.McLean JR, Smith GA, Rocha EM, Hayes MA, Beagan JA, Hallett PJ, Isacson O. Widespread neuron-specific transgene expression in brain and spinal cord following synapsin promoter-driven AAV9 neonatal intracerebroventricular injection. Neurosci Lett. 2014;576:73–78. doi: 10.1016/j.neulet.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 54.McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, Shera D, Lioutermann L, Feely M, Freese A, Leone P. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- 55.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, Lowes L, Alfano L, Berry K, Church K, Kissel JT, Nagendran S, L’Italien J, Sproule DM, Wells C, Cardenas JA, Heitzer MD, Kaspar A, Corcoran S, Braun L, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 56.Messina S. New directions for SMA therapy. J Clin Med. 2018;7:E251. doi: 10.3390/jcm7090251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyake N, Miyake K, Asakawa N, Yamamoto M, Shimada T. Long-term correction of biochemical and neurological abnormalities in MLD mice model by neonatal systemic injection of an AAV serotype 9 vector. Gene Ther. 2014;21:427–433. doi: 10.1038/gt.2014.17. [DOI] [PubMed] [Google Scholar]
- 59.Murlidharan G, Samulski RJ, Asokan A. Biology of adeno-associated viral vectors in the central nervous system. Front Mol Neurosci. 2014;7:76. doi: 10.3389/fnmol.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connor DM, Boulis NM. Gene therapy for neurodegenerative diseases. Trends Mol Med. 2015;21:504–512. doi: 10.1016/j.molmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Oh SM, Chang MY, Song JJ, Rhee YH, Joe EH, Lee HS, Yi SH, Lee SH. Combined Nurr1 and Foxa2 roles in the therapy of Parkinson’s disease. EMBO Mol Med. 2015;7:510–525. doi: 10.15252/emmm.201404610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Onyango IG. Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease. Neural Regen Res. 2018;13:19–25. doi: 10.4103/1673-5374.224362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ortolano S, Spuch C, Navarro C. Present and future of adeno associated virus based gene therapy approaches. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:47–66. doi: 10.2174/187221412799015245. [DOI] [PubMed] [Google Scholar]
- 64.Parente V, Corti S. Advances in spinal muscular atrophy therapeutics. Ther Adv Neurol Disord. 2018;11:1756285618754501. doi: 10.1177/1756285618754501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parsons DW, McAndrew PE, Iannaccone ST, Mendell JR, Burghes AH, Prior TW. Intragenic telSMN mutations: frequency, distribution, evidence of a founder effect, and modification of the spinal muscular atrophy phenotype by cenSMN copy number. Am J Hum Genet. 1998;63:1712–1723. doi: 10.1086/302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Passini MA, Bu J, Roskelley EM, Richards AM, Sardi SP, O’Riordan CR, Klinger KW, Shihabuddin LS, Cheng SH. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J Clin Invest. 2010;120:1253–1264. doi: 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfister EL, DiNardo N, Mondo E, Borel F, Conroy F, Fraser C, Gernoux G, Han X, Hu D, Johnson E, Kennington L, Liu P, Reid SJ, Sapp E, Vodicka P, Kuchel T, Morton AJ, Howland D, Moser R, Sena-Esteves M, et al. Artificial miRNAs reduce human mutant Huntingtin throughout the striatum in a transgenic sheep model of Huntington’s disease. Hum Gene Ther. 2018;29:663–673. doi: 10.1089/hum.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piguet F, Alves S, Cartier N. Clinical gene therapy for neurodegenerative diseases: past, present, and future. Hum Gene Ther. 2017;28:988–1003. doi: 10.1089/hum.2017.160. [DOI] [PubMed] [Google Scholar]
- 69.Piguet F, Sondhi D, Piraud M, Fouquet F, Hackett NR, Ahouansou O, Vanier MT, Bieche I, Aubourg P, Crystal RG, Cartier N, Sevin C. Correction of brain oligodendrocytes by AAVrh. 10 intracerebral gene therapy in metachromatic leukodystrophy mice. Hum Gene Ther. 2012;23:903–914. doi: 10.1089/hum.2012.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS, Carette JE. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rafii MS, Tuszynski MH, Thomas RG, Barba D, Brewer JB, Rissman RA, Siffert J, Aisen PS. Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with Alzheimer disease: A Randomized Clinical Trial. JAMA Neurol. 2018;75:834–841. doi: 10.1001/jamaneurol.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rafii MS, Baumann TL, Bakay RA, Ostrove JM, Siffert J, Fleisher AS, Herzog CD, Barba D, Pay M, Salmon DP, Chu Y, Kordower JH, Bishop K, Keator D, Potkin S, Bartus RT. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimers Dement. 2014;10:571–581. doi: 10.1016/j.jalz.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Raikwar SP, Thangavel R, Dubova I, Selvakumar GP, Ahmed ME, Kempuraj D, Zaheer SA, Iyer SS, Zaheer A. Targeted gene editing of glia maturation factor in microglia: a novel Alzheimer’s disease Therapeutic Target. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1068-y. doi: 10.1007/s12035-018-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramaswamy S, Kordower JH. Gene therapy for Huntington’s disease. Neurobiol Dis. 2012;48:243–254. doi: 10.1016/j.nbd.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 75.Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, Zolotukhin S, Hajjar RJ, Weber T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther. 2012;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg JB, Kaminsky SM, Aubourg P, Crystal RG, Sondhi D. Gene therapy for metachromatic leukodystrophy. J Neurosci Res. 2016;94:1169–1179. doi: 10.1002/jnr.23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 78.Sasmita AO. Current viral-mediated gene transfer research for treatment of Alzheimer’s disease. Biotechnol Genet Eng Rev. 2018 doi: 10.1080/02648725.2018.1523521. doi: 10.1080/02648725.2018.1523521. [DOI] [PubMed] [Google Scholar]
- 79.Scoto M, Finkel R, Mercuri E, Muntoni F. Genetic therapies for inherited neuromuscular disorders. Lancet Child Adolesc Health. 2018;2:600–609. doi: 10.1016/S2352-4642(18)30140-8. [DOI] [PubMed] [Google Scholar]
- 80.Shi CK, Chen ZQ. Effect of microglia on iron metabolismin midbrain dopaminergic neurons and theunderlying mechanism: study protocol for an in vitro cellular experiment. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:1262–1267. [Google Scholar]
- 81.Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gray SJ, Lowenstein PR, Vandenberghe LH, Wilson TJ, Wolfe JH, Glorioso JC. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9:277–291. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tizzano EF, Zafeiriou D. Prenatal aspects in spinal muscular atrophy: From early detection to early presymptomatic intervention. Eur J Paediatr Neurol. 2018 doi: 10.1016/j.ejpn.2018.08.009. doi: 10.1016/j.ejpn.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 83.Tong BC, Wu AJ, Li M, Cheung KH. Calcium signaling in Alzheimer’s disease & therapies. Biochim Biophys Acta Mol Cell Res. 2018 doi: 10.1016/j.bbamcr.2018.07.018. doi: 10.1016/j.bbamcr.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 84.Vagner T, Dvorzhak A, Wojtowicz AM, Harms C, Grantyn R. Systemic application of AAV vectors targeting GFAP-expressing astrocytes in Z-Q175-KI Huntington’s disease mice. Mol Cell Neurosci. 2016;77:76–86. doi: 10.1016/j.mcn.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, Shaw PJ, Azzouz M. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci Transl Med. 2010;2:35ra42. doi: 10.1126/scitranslmed.3000830. [DOI] [PubMed] [Google Scholar]
- 86.van Rappard DF, Boelens JJ, Wolf NI. Metachromatic leukodystrophy: Disease spectrum and approaches for treatment. Best Pract Res Clin Endocrinol Metab. 2015;29:261–273. doi: 10.1016/j.beem.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Wang MM, Miao D, Cao XP, Tan L, Tan L. Innate immune activation in Alzheimer’s disease. Ann Transl Med. 2018a;6:177. doi: 10.21037/atm.2018.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang W, Wen D, Duan W, Yin J, Cui C, Wang Y, Li Z, Liu Y, Li C. Systemic administration of scAAV9-IGF1 extends survival in SOD1(G93A) ALS mice via inhibiting p38 MAPK and the JNK-mediated apoptosis pathway. Brain Res Bull. 2018b;139:203–210. doi: 10.1016/j.brainresbull.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 89.Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzog C, et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann Neurol. 2015;78:248–257. doi: 10.1002/ana.24436. [DOI] [PubMed] [Google Scholar]
- 90.Yamashita T, Chai HL, Teramoto S, Tsuji S, Shimazaki K, Muramatsu S, Kwak S. Rescue of amyotrophic lateral sclerosis phenotype in a mouse model by intravenous AAV9-ADAR2 delivery to motor neurons. EMBO Mol Med. 2013;5:1710–1719. doi: 10.1002/emmm.201302935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yi L, Wu T, Luo W, Zhou W, Wu J. A non-invasive, rapid method to genotype late-onset Alzheimer’s disease-related apolipoprotein E gene polymorphisms. Neural Regen Res. 2014;9:69–75. doi: 10.4103/1673-5374.125332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 93.Zeiss CJ, Allore HG, Beck AP. Established patterns of animal study design undermine translation of disease-modifying therapies for Parkinson’s disease. PLoS One. 2017;12:e0171790. doi: 10.1371/journal.pone.0171790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L, Yang J, Liu ZH. STEP61 negatively regulates amyloid beta-mediated ERK signaling pathway in Alzheimer’s disease cell model. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:4507–4512. [Google Scholar]
- 95.Zhao L, Gottesdiener AJ, Parmar M, Li M, Kaminsky SM, Chiuchiolo MJ, Sondhi D, Sullivan PM, Holtzman DM, Crystal RG, Paul SM. Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer’s disease mouse models. Neurobiol Aging. 2016;44:159–172. doi: 10.1016/j.neurobiolaging.2016.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.