Abstract
Nicotinamide adenine dinucleotide phosphate oxidase (NOX) is a multisubunit enzyme complex that utilizes nicotinamide adenine dinucleotide phosphate to produce superoxide anions and other reactive oxygen species. Under normal circumstances, reactive oxygen species mediate a number of important cellular functions, including the facilitation of adaptive immunity. In pathogenic circumstances, however, excess reactive oxygen species generated by NOX promotes apoptotic cell death. In ischemic stroke, in particular, it has been shown that both NOX activation and derangements in glucose metabolism result in increased apoptosis. Moreover, recent studies have established that glucose, as a NOX substrate, plays a vital role in the pathogenesis of reperfusion injury. Thus, NOX inhibition has the potential to mitigate the deleterious impact of hyperglycemia on stroke. In this paper, we provide an overview of this research, coupled with a discussion of its implications for the development of NOX inhibition as a strategy for the treatment of ischemic stroke. Both inhibition using apocynin, as well as the prospect of developing more specific inhibitors based on what is now understood of the biology of NOX assembly and activation, will be highlighted in the course of our discussion.
Keywords: nicotinamide adenine dinucleotide phosphate oxidase, stroke, nicotinamide adenine dinucleotide phosphate oxidase inhibitors, reactive oxygen species, ischemia/reperfusion, neuroprotection, hyperglycolysis, NADPH, NOX
Introduction
Ischemic stroke remains the second leading cause of death worldwide, despite significant efforts to better understand its biology and to intervene in its pathogenesis (Chandra et al., 2017; Li et al., 2017). Even so, mortality alone hardly accounts for the total burden imposed by this disease. Leaving survivors with profound neurological deficits, stroke is the leading cause of major disability worldwide. Moreover, the acute therapies currently available are limited to recannualization procedures (mechanical or pharmacological) that are too time-sensitive to be a viable option for most patients (Ji, 2015; Kim et al., 2017). It is therefore crucial that research aimed at elucidating the mechanisms of ischemia-mediated damage continues to be pursued vigorously: successful interventions in these pathways possess nearly unparalleled potential to alleviate the suffering of patients.
One mechanism that has been the focus of recent attention is the cerebral hyperglycolysis induced by ischemia (Schurr, 2002) and thought to potentiate stroke-induced brain damage (Kochanski et al., 2013). Following ischemia/reperfusion, the brain significantly upregulates its glycolytic rate, its expression of glucose transporters 1 and 3 (Shen et al., 2016), and lactate levels, resulting in lactic acidosis (Schurr, 2002). The increased glucose is consumed via anaerobic respiration and further shunted through the hexose monophosphate pathway, increasing nicotinamide adenine dinucleotide phosphate (NADPH) levels and activating NADPH oxidase (NOX), as seen by increases in NOX activity, NOX subunit expression (p47-phox, p67-phox, and gp91-phox), and cell death found after ischemia/reperfusion (Tang et al., 2012; Yao et al., 2017).
NOX itself is a family of essential enzyme complexes expressed in many different tissues throughout the body. NOX is best-known for its involvement in the antimicrobial respiratory burst by which free radical production occurs in the cells involved in innate immunity (Carbone et al., 2015). Upon activation via assembly of its multiple subunits, NOX uses NADPH to catalyze the reduction of molecular oxygen to the superoxide anion (O2 •–). This production of reactive oxygen species (ROS) has been increasingly recognized as an important component of various cellular events, including bio-signaling and apoptotic regulation (Sumimoto et al., 2005; D’Autréaux and Toledano, 2007). In addition to its normal physiologic functions, NOX is intimately involved in the pathways leading to brain damage caused by ischemia/reperfusion injury in stroke (Tang et al., 2012; Zhao et al., 2016). Because of this participation in ischemia/reperfusion pathophysiology and its pervasive expression, NOX has emerged as an attractive therapeutic target. In particular, inhibition of NOX may prove to be a promising treatment for ischemic stroke.
NOX Subcellular Location, Structure and Subunit Activation
The NOX complex contains a membrane-bound component, as well as a cytosolic component. At rest, the catalytic center of NOX is comprised of the two tightly complexed membrane-integrated flavocytochromes, gp91-phox and p22-phox. In the cytosol, the cytosolic components contain p47-phox, p67-phox, and p40-phox and the small GTPase Rac1/Rac2; p40-phox and p67-phox are often complexed prior to activation (Yu et al., 1998; Sumimoto et al., 2005; Carbone et al., 2015). During NOX activation, phosphorylation unmasks a binding region on p47-phox, allowing it to definitively bind p67-phox to form a trimeric cytosolic complex (Tsunawaki and Yoshikawa, 2000; Lapouge et al., 2002). Subsequently, p47-phox mediates translocation of the cytosolic complex to the membrane, where it binds principally to p22-phox, resulting in assembly of the active NOX complex and activation of gp91-phox, the catalytic subunit (Ago et al., 2003). As the catalytic core, gp91-phox levels are measured as a surrogate for the extent of NOX complex formation. The gp91-phox NOX protein family is comprised of membrane-spanning structures with NADPH- (or NADH-) binding domains, using NADPH as electron donors for molecular oxygen to produce the superoxide anion (O2 •–, a precursor for other reactive oxygen species) (Yu et al., 1998; Cairns et al., 2012). Thus, NOX requires glucose metabolism to provide the NADPH necessary for NOX complex formation and function (Suh et al., 2008; Tang et al., 2012).
All of the major NOX subunits (p22-phox, p47-phox, p67-phox and gp91-phox) have been found in the brain (Bedard and Krause, 2007; Montezano and Touyz, 2012; Tang et al., 2012), in which, upon phosphorylation following ischemia, the active complex is assembled as described above (Bokoch and Knaus, 2003). Thus, upregulation of these subunits has been found to correlate with increased NOX activity (Takeya et al., 2003). The multiplicity of steps in this complex activation process provides the opportunity for specific modulation prior to and during activation of NOX (Groemping and Rittinger, 2005; Sumimoto et al., 2005).
Another aspect of the NOX family is its plentiful isoforms, comprised of NOX 1–5, dual oxidase (DUOX) 1 and 2, with slight variations in its subunits. In NOX2, the gp91-phox isoform is present (Tang et al., 2012). Of these isoforms, NOX2 and NOX4 are the most involved in ischemia/reperfusion injury (Zhang et al., 2015; Lou et al., 2018). NOX2, mostly present in microglia and circulating immune cells, dominates in inflammatory driven conditions such as reperfusion and is upregulated in ischemic stroke with concurrent increases in microglial activation (Tang et al., 2012). NOX 4, usually present at very low physiologic levels in the brain, is upregulated in ischemia/reperfusion pathologies to further contribute to acute oxidative damage in the reperfusion period (Yao et al., 2017; Lou et al., 2018). To illustrate, a NOX4 knockout model has demonstrated attenuated oxidative stress, blood-brain barrier disruption, neuronal death, and mortality (Kleinschnitz et al., 2010). Isoform specificity yields another target to which therapies can be tailored to enhance treatment specificity.
NOX-Mediated Pathogenesis
Glucose metabolism
Ischemia is well-known to induce cerebral hyperglycolysis. Moreover, hyperglycemia during stroke worsens outcomes, independent of diabetes or pre-ischemic blood glucose concentrations (Ribo et al., 2005; Li et al., 2017). Several studies have findings that identify glucose as the requisite electron donor for reperfusion-induced neuronal superoxide production and establish a previously unrecognized mechanism by which hyperglycemia can exacerbate ischemic brain injury (Takeya et al., 2003; Yip et al., 2016): the glucose excess generated by ischemia is shunted into the hexose monophosphate pathway (Figure 1), where it reduces NADP+ to NADPH (Suh et al., 2008). This newly formed NADPH is then itself utilized as an electron donor, in this case by NOX to generate the ROS (Kruyt et al., 2010) that damage the brain during post-ischemic hyperglycolysis (Kochanski et al., 2013). For instance, post-ischemic superoxide production and neuronal death were reversed in neuronal cultures when glucose was absent, NOX was inactivated or the hexose monophosphate shunt producing NADPH from glucose was inhibited within the culture (Takeya et al., 2003). Analogously, neuronal superoxide production and death were reduced by the glucose antimetabolite 2-deoxyglucose and exacerbated by increased blood glucose concentrations in a murine stroke model. Inactivating NADPH oxidase, either by use of apocynin or by deletion of the p47-phox subunit, was found to block neuronal superoxide production and to negate the detrimental effects of hyperglycemia (Suh et al., 2008). Additionally, Ding et al. found increased NOX activation, NOX subunit levels, and resulting apoptotic cell death in rat models with post-ischemic hyperglycolysis that was exacerbated by early rehabilitation (Shen et al., 2016; Tang et al., 2018). Thus, hyperglycemia provides the necessary substrates via the hexose monophosphate shunt to facilitate NOX-induced ROS production.
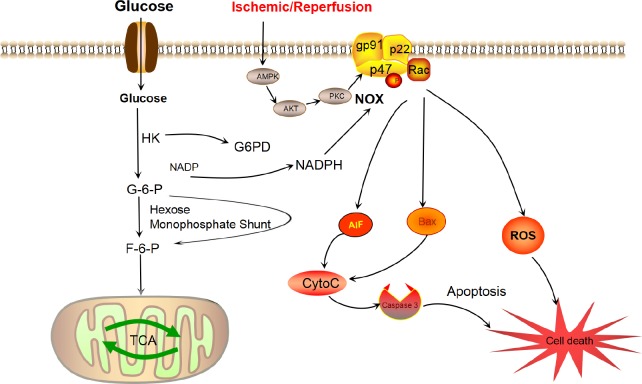
Figure 1.
The nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase (NOX) complex and its subunits are shown with its activation sequence and selected downstream effects.
In the NOX complex, two membrane subunits (gp91-phox, or its homologs, and p22-phox) comprise the catalytic core of NOX, while several cytosolic subunits (p47-phox, p67-phox, p40-phox) and the G-protein Rac are all essential components for assembly and activation. Stroke induces adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)/phosphorylated protein kinase B (Akt)/protein kinase C (PKC) activation, which can activate NOX via phosphorylation. When activated, NOX produces superoxide ion by transferring an electron from NADPH to molecular oxygen. NADPH is derived from glucose, which enters the cell and is converted to glucose-6-phosphate (G-6-P) by glycolytic kinases. The G-6-P is then shunted through the hexose monophosphate shunt to produce NADPH by donating an electron to NADP+. The NADPH thus produced, along with molecular oxygen, acts as a substrate for NOX to produce reactive oxygen species (ROS). In disease states, ROS overproduction results in cell death, both directly and indirectly. In such cases, NOX can induce the release of apoptosis-inducing factor (AIF), cytochrome C and Bcl-2-associated X protein (BAX), resulting in the activation of caspase 3-mediated apoptotic cell death. TCA: Tricarboxylic acid cycle; F-6-P: fructose-6-phosphate; G6PD: glucose-6-phosphate 1-dehydrogenase; HK: hexokinase.
Hyperglycemia also potentiates NOX activity through a more direct mechanism – through pathological cellular signaling. Excess glucose has been found to activate protein kinase C and thus downstream, activate NOX via phosphorylation and facilitate ROS production to further worsen outcomes in ischemic stroke. Hyperglycemia-amplified protein kinase C expression increased NOX-induced ROS production in an in vitro simulation of the blood-brain barrier. The resulting increases in p47-phox phosphorylation and NOX activation caused blood-brain barrier breakdown and apoptosis. Such effects were mitigated by inhibiting protein kinase C expression, with subsequent decreases in p47-phox phosphorylation and NOX activation (Kleinschnitz et al., 2010; Shao and Bayraktutan, 2014). Thus excess glucose contributes twofold to NOX activation.
NOX activation and reactive oxygen species-mediated cell death
Within the brain, NOX has been identified as an important generator of ROS both in physiologic and pathologic conditions, particularly stroke (Kahles and Brandes, 2012). Stroke-induced elevations in NOX expression, activity, and ROS-mediated damage have been widely observed following ischemia (Tang et al., 2012; Kahles and Brandes, 2013; Kochanski et al., 2013). Ischemia itself makes the brain particularly vulnerable to ROS damage. Upon cessation of blood flow, mitochondria in affected brain regions incur injury and cannot detoxify the influx of oxygen that results from reperfusion (Tang et al., 2014b). During reperfusion, the concurrent influx of excess glucose is increasingly shunted into the hexose monophosphate pathway, whereby glucose-6-phosphate is used to generate increased NADPH (Figure 1) (Brennan-Minnella et al., 2015; Thibodeau A et al., 2016). Thus, because plentiful substrates are present due to post-ischemic hyperglycemia, increased NOX activation produces ROS in pathogenic quantity. These ROS then, through lipid peroxidation, directly damage cells by compromising membrane integrity and precipitating damage to organelles (Rastogi et al., 2016).
To prevent ROS overproduction, NOX activity is regulated at the level of its activation via phosphorylation. Phosphorylation occurs due to stimuli such as phagocytosed particles or cellular stresses, including cerebral ischemia (Jiang et al., 2011; Kim et al., 2017). Though the pathways for activation have not been fully elucidated, multiple independent pathways are involved in physiologic and pathologic NOX phosphorylation and action. In ischemia, many studies have implicated the protein kinase C isoforms, particularly via the 5′-monophosphate-activated protein kinase/phosphorylated protein kinase B/protein kinase C pathway, as well as several other potential upstream factors such as transforming growth factor-β and myosin light chain kinase (Zhang et al., 2015; Cai et al., 2017; Lou et al., 2018). Multiple experiments have illustrated NOX activation in the brain, such as that of Zheng et al. (2014), in which oxygen-glucose deprivation of brain slices yielded upregulation of NOX and concurrent increased production of ROS. In another study, inhibition of NOX by casein kinase 2 led to neuronal survival. Conversely, activation of an NADPH oxidase subunit resulted in increased oxidative stress that caused the release of apoptogenic factors from mitochondria, damaged DNA, and was associated with neuronal death after ischemia and reperfusion (Kim et al., 2012).
Once generated by NOX, ROS mediate cellular damage in a variety of ways: superoxide may cause oxidative damage on its own; it can generate more radical species with its unpaired electron; it may produce peroxynitrite with nitric oxide, which can scavenge and prevent the function of nitric oxide, can induce further damage by nitration in most biomolecules, and is considered an important cause of post-stroke apoptosis (Broughton et al., 2009); alternatively, it may be enzymatically dismutated to hydrogen peroxide and oxygen, the former of which is a membrane-permeable oxidizer of cellular macromolecules and can generate DNA-damaging hydroxyl radicals in the presence of transition metals. The resulting ROS can cause direct damage to DNA and pervasive lipid peroxidation pertaining cellular membranes. Moreover, ROS sources may also damage cellular signaling cascades, causing apoptosis (Kim et al., 2016; Ma et al., 2017). Dysregulated NOX production of ROS may disrupt the signaling cascades in which NOX can be intimately involved. Cells damaged in these ways may die either of apoptosis or necrosis, depending on several factors including cell type, stage in development, and type of stimulus (i.e., severity of ischemic injury) (Dirnagl et al., 1999). One mechanism of apoptosis thought to be activated by NOX-generated radicals involves apoptosis-inducing factor. Upon activation by oxidative stress, poly (ADP-ribose) polymerase-1, a DNA-repairing enzyme that acts to mitigate radical damage, generates poly (ADP-ribose), which is sensed as a death signal by neurons. Poly (ADP-ribose) binds apoptosis-inducing factor, triggering its liberation from the mitochondrial outer membrane; in general, pro-apoptotic molecules are released following oxidative augmentation of mitochondrial membrane permeability, and these converge to activate caspase-3, causing apoptosis (Kim et al., 2012) through destruction of nuclear DNA repair enzymes (Khoshnam et al., 2017).
Numerous factors influencing stroke outcomes have proven to modulate NOX activity, the most recent of which is glucose itself. These factors’ newly identified function as NOX regulators suggest novel mechanisms for their effects on ischemic brain injury (Brennan-Minnella et al., 2015). It has been found, for instance, that stroke-induced brain injury in rats leads to activation of NADPH oxidase through a signaling pathway implicating adenosine 5′-monophosphate-activated protein kinase/phosphorylated protein kinase B/protein kinase C (Figure 1) (Tang et al., 2014a; Cai et al., 2017; Geng et al., 2017). In another study, infarct volume was attenuated in mice treated with a NOX inhibitor; this inhibition was associated with significant improvement in neurological function, as well as attenuation of neuronal apoptosis and expression of Bax (Bcl-2-associated X protein)/Bcl-2(B-cell lymphoma 2), cytochrome C and cleaved caspase 3 (Song et al., 2013). Finally, in a study that investigated its role in ischemia-reperfusion injury to the peri-infarct region, NOX was implicated in ROS-mediated damage to DNA, suggesting its involvement in peri-infarct neurodegeneration and inhibition of neurogenesis in this region; NOX knockdown was found to facilitate survival and development of neuronal progenitor cells and to improve functional recovery after stroke (Choi et al., 2015).
Therapeutic NOX Inhibition on Cell Death
Reduced NOX activity can protect cells from the oxidative stress and consequent cell death that otherwise occurs after ischemia. Excess ROS creates oxidative stress known to exacerbate ischemic damage, particularly through reperfusion. Antioxidant-focused therapies targeting ROS production and activity have demonstrated improved experimental outcomes. Neuronal superoxide production, of which NOX is the major source, contributes to cell death during both glutamate excitotoxicity and stroke; regulation of NOX activity can thus alter stroke outcomes (Brennan-Minnella et al., 2015). To illustrate this, the increased blood-brain barrier permeability, infarct size, and hemorrhage with tissue-type plasminogen activator-induced reperfusion in a hyperglycemic rat model was prevented with apocynin-mediated NOX inhibition (Won et al., 2011). Apocynin is a nonspecific NOX inhibitor that has been shown in rat models of stroke to prevent NOX activation and to reduce cytosolic ROS production (Kleinschnitz et al., 2010). In vitro and in vivo models with both the nonspecific NOX inhibitor diphenyleneiodonium and NOX-siRNA have also been shown to attenuate cytosolic ROS levels (Carbone et al., 2015). Genetic deletion of NOX was found to significantly reduce disruption of the blood-brain barrier and infarct size after ischemia/reperfusion in mice (Kahles et al., 2007; Zhang et al., 2016). Similarly, after ischemia and reperfusion, NOX knockout mice demonstrated reduced infarct size and reduced post-infarct inflammation, implicating NOX in both inflammation and infarct progression in stroke (Chen et al., 2011). It has been demonstrated in a thromboembolic stroke model that other known neuroprotective modalities may exert their effect by NOX inhibition: after reperfusion by recombinant tissue plasminogen activator, combining normobaric oxygen with either hypothermia or ethanol conferred neuroprotection through modulation of NOX activation.
The recognition of oxidative stress as a pathological mechanism of post-stroke neurodegeneration, as well as the inhibition of NOX investigated as a consequence of this recognition, has been considered a conceptual breakthrough in stroke therapy (Radermacher et al., 2013). The advantages of a targeted central nervous system NOX inhibitor that would inhibit the production of superoxide by non-phagocytic cells are evident (Cairns et al., 2012; Kim et al., 2017), as both NOX inhibition and genetic deletion of certain NOX isoforms have sizable and demonstrable improvements in experimental stroke outcomes (Kahles and Brandes, 2012). Unfortunately, no such inhibitors have yet demonstrated clinical viability. The currently studied inhibitors possess insufficient safety and specificity for development in humans, with limited ability to target specific tissues or specific NOX isoforms and with many off-target effects. For instance, diphenyleneiodonium, in addition to impairing NOX-associated flavoprotein-mediated superoxide generation, also inhibits other metabolically important flavoenzymes (Kim et al., 2017). Another example is afforded by preclinical studies of the NOX inhibitor apocynin, which appears to inhibit superoxide generation by preventing p47-phox translocation to the membrane-bound NOX components: dose-dependent efficacy data for stroke is variable and suggests a narrow therapeutic window, outside of which there is risk of brain hemorrhage exacerbation (Kim et al., 2017). Other limitations of apocynin are that it acts on NOX1 and 2 more than NOX4 isoform and requires myeloperoxidase to become active. As a result, apocynin can only act on NOX in leukocytes, and in other locations such as vascular cells or smooth muscle cells, it acts as an antioxidant rather than an inhibitor (Heumüller et al., 2008; Kim et al., 2017). Therefore, its functionality and specificity must be determined more clearly before it can be translated clinically. VAS2870, a small molecule inhibitor of NOX isoforms that reduced lesion size and neurological deficits in a stroke model, provides one final example: in addition to inhibiting NOX, this molecule has been found to exert off-target thiol alkylation that may replicate ROS in redox sequences (Sun et al., 2012; Kim et al., 2017). However, with current studies elucidating both mechanisms of NOX action and the specific NOX isoforms involved, particularly NOX 2 and 4 in stroke, a wide range of potential specific targets for therapy are becoming apparent. Recent research using known inhibitors has substantially increased our knowledge of the role of NOX in central nervous system diseases, and it is this knowledge that will provide a framework for the development of specific, potent, and safe NOX inhibitors for clinical use in the future.
Conclusion
By generating ROS levels in excess of what the body is capable of handling through endogenous antioxidant defense mechanisms, NOX is a principal contributor to oxidative stress. Such disproportionate increases in ROS lead to increases in lipid peroxidation, membrane degeneration, matrix metalloprotease production, DNA damage, the propagation of dysfunctional and damaging signaling cascades, and, ultimately, cell death (Tang et al., 2012; Qin et al., 2017). Moreover, as the primary generator of ROS in the central nervous system, NOX is inextricably involved in the perpetuation of cellular damage in the wake of ischemic stroke. Accordingly, interventions aimed at downregulating the excessive, pathogenic function of this enzyme complex possess considerable promise in the effort to mitigate post-stroke damage, and ultimately to improve quality of life for afflicted patients.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest.
Financial support: This work was partially supported by Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation Research and Development Service (to YD), as well as the National Natural Science Foundation of China (81501141), Beijing New Star of Science and Technology Program of China (xx2016061), Beijing Tongzhou District Financial Fund, and Scientific Research Common Program of Beijing Municipal Commission of Education, China (KM201610025028) (to XG).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was partially supported by Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation Research and Development Service (to YD), as well as the National Natural Science Foundation of China (81501141), Beijing New Star of Science and Technology Program of China (xx2016061), Beijing Tongzhou District Financial Fund, and Scientific Research Common Program of Beijing Municipal Commission of Education, China (KM201610025028) (to XG).
C-Editors: Zhao M, Yu J; L-Editor: Song LP; T-Editor: Liu XL
References
- 1.Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci U S A. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 4.Brennan-Minnella AM, Won SJ, Swanson RA. NADPH oxidase-2: linking glucose, acidosis, and excitotoxicity in stroke. Antioxid Redox Signal. 2015;22:161–174. doi: 10.1089/ars.2013.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 6.Cai L, Stevenson J, Geng X, Peng C, Ji X, Xin R, Rastogi R, Sy C, Rafols JA, Ding Y. Combining normobaric oxygen with ethanol or hypothermia prevents brain damage from thromboembolic stroke via PKC-Akt-NOX modulation. Mol Neurobiol. 2017;54:1263–1277. doi: 10.1007/s12035-016-9695-7. [DOI] [PubMed] [Google Scholar]
- 7.Cairns B, Kim JY, Tang XN, Yenari MA. NOX inhibitors as a therapeutic strategy for stroke and neurodegenerative disease. Curr Drug Targets. 2012;13:199–206. doi: 10.2174/138945012799201676. [DOI] [PubMed] [Google Scholar]
- 8.Carbone F, Teixeira PC, Braunersreuther V, Mach F, Vuilleumier N, Montecucco F. Pathophysiology and treatments of oxidative injury in ischemic stroke: focus on the phagocytic NADPH oxidase 2. Antioxid Redox Signal. 2015;23:460–489. doi: 10.1089/ars.2013.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra A, Stone CR, Du X, Li WA, Huber M, Bremer R, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease III: Stroke. Brain Circ. 2017;3:66–77. doi: 10.4103/bc.bc_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Kim GS, Okami N, Narasimhan P, Chan PH. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 2011;42:341–348. doi: 10.1016/j.nbd.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi DH, Kim JH, Lee KH, Kim HY, Kim YS, Choi WS, Lee J. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PLoS One. 2015;10:e0116814. doi: 10.1371/journal.pone.0116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 13.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 14.Geng X, Li F, Yip J, Peng C, Elmadhoun O, Shen J, Ji X, Ding Y. Neuroprotection by chlorpromazine and promethazine in severe transient and permanent ischemic stroke. Mol Neurobiol. 2017;54:8140–8150. doi: 10.1007/s12035-016-0280-x. [DOI] [PubMed] [Google Scholar]
- 15.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 17.Ji X. Forward thinking in stroke treatment: advances in cerebrovascular reperfusion and neurorehabilitation. Brain Circ. 2015;1:1–2. [Google Scholar]
- 18.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 19.Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69:2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahles T, Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid Redox Signal. 2013;18:1400–1417. doi: 10.1089/ars.2012.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 22.Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38:1167–1186. doi: 10.1007/s10072-017-2938-1. [DOI] [PubMed] [Google Scholar]
- 23.Kim GS, Jung JE, Narasimhan P, Sakata H, Yoshioka H, Song YS, Okami N, Chan PH. Release of mitochondrial apoptogenic factors and cell death are mediated by CK2 and NADPH oxidase. J Cereb Blood Flow Metab. 2012;32:720–730. doi: 10.1038/jcbfm.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Park J, Lee JE, Yenari MA. NOX inhibitors - a promising avenue for ischemic stroke. Exp Neurobiol. 2017;26:195–205. doi: 10.5607/en.2017.26.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KS, Kwak JW, Lim SJ, Park YK, Yang HS, Kim HJ. Oxidative stress-induced telomere length shortening of circulating leukocyte in patients with obstructive sleep apnea. Aging Dis. 2016;7:604–613. doi: 10.14336/AD.2016.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8:e1000479. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochanski R, Peng C, Higashida T, Geng X, Huttemann M, Guthikonda M, Ding Y. Neuroprotection conferred by post-ischemia ethanol therapy in experimental stroke: an inhibitory effect on hyperglycolysis and NADPH oxidase activation. J Neurochem. 2013;126:113–121. doi: 10.1111/jnc.12169. [DOI] [PubMed] [Google Scholar]
- 28.Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 29.Lapouge K, Smith SJ, Groemping Y, Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox. J Biol Chem. 2002;277:10121–10128. doi: 10.1074/jbc.M112065200. [DOI] [PubMed] [Google Scholar]
- 30.Li WA, Geng X, Ding Y. Stroke is a global epidemic: new developments in clinical and translational cerebrovascular diseases research. Neurol Res. 2017;39:475–476. doi: 10.1080/01616412.2017.1330307. [DOI] [PubMed] [Google Scholar]
- 31.Lou Z, Wang AP, Duan XM, Hu GH, Song GL, Zuo ML, Yang ZB. Upregulation of NOX2 and NOX4 mediated by TGF-β signaling pathway exacerbates cerebral ischemia/reperfusion oxidative stress injury. Cell Physiol Biochem. 2018;46:2103–2113. doi: 10.1159/000489450. [DOI] [PubMed] [Google Scholar]
- 32.Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, Brann DW. NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener. 2017;12:7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montezano AC, Touyz RM. Reactive oxygen species and endothelial function--role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol. 2012;110:87–94. doi: 10.1111/j.1742-7843.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- 34.Qin YY, Li M, Feng X, Wang J, Cao L, Shen XK, Chen J, Sun M, Sheng R, Han F, Qin ZH. Combined NADPH and the NOX inhibitor apocynin provides greater anti-inflammatory and neuroprotective effects in a mouse model of stroke. Free Radic Biol Med. 2017;104:333–345. doi: 10.1016/j.freeradbiomed.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Radermacher KA, Wingler K, Langhauser F, Altenhofer S, Kleikers P, Hermans JJ, Hrabe de Angelis M, Kleinschnitz C, Schmidt HH. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal. 2013;18:1418–1427. doi: 10.1089/ars.2012.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastogi R, Geng X, Li F, Ding Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci. 2016;10:301. doi: 10.3389/fncel.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribo M, Molina C, Montaner J, Rubiera M, Delgado-Mederos R, Arenillas JF, Quintana M, Alvarez-Sabín J. Acute hyperglycemia state is associated with lower tPA-induced recanalization rates in stroke patients. Stroke. 2005;36:1705–1709. doi: 10.1161/01.STR.0000173161.05453.90.9f. [DOI] [PubMed] [Google Scholar]
- 38.Schurr A. Lactate, glucose and energy metabolism in the ischemic brain (review) Int J Mol Med. 2002;10:131–136. [PubMed] [Google Scholar]
- 39.Shao B, Bayraktutan U. Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C-ssI and prooxidant enzyme NADPH oxidase. Redox Biol. 2014;2:694–701. doi: 10.1016/j.redox.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen J, Huber M, Zhao EY, Peng C, Li F, Li X, Geng X, Ding Y. Early rehabilitation aggravates brain damage after stroke via enhanced activation of nicotinamide adenine dinucleotide phosphate oxidase (NOX) Brain Res. 2016;1648:266–276. doi: 10.1016/j.brainres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Song W, Huo T, Guo F, Wang H, Wei H, Yang Q, Dong H, Wang Q, Xiong L. Globular adiponectin elicits neuroprotection by inhibiting NADPH oxidase-mediated oxidative damage in ischemic stroke. Neuroscience. 2013;248:136–144. doi: 10.1016/j.neuroscience.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 42.Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 44.Sun QA, Hess DT, Wang B, Miyagi M, Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1, 2, 3-triazolo[4,5-d]pyrimidine (VAS2870) Free Radic Biol Med. 2012;52:1897–1902. doi: 10.1016/j.freeradbiomed.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 46.Tang H, Pan CS, Mao XW, Liu YY, Yan L, Zhou CM, Fan JY, Zhang SY, Han JY. Role of NADPH oxidase in total salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: implication of AMPK/Akt/PKC. Microcirculation. 2014a;21:615–627. doi: 10.1111/micc.12140. [DOI] [PubMed] [Google Scholar]
- 47.Tang P, Dang H, Huang J, Xu T, Yuan P, Hu J, Sheng JF. NADPH oxidase NOX4 is a glycolytic regulator through mROS-HIF1α axis in thyroid carcinomas. Sci Rep. 2018;8:15897. doi: 10.1038/s41598-018-34154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang X, Zhong W, Tu Q, Ding B. NADPH oxidase mediates the expression of MMP-9 in cerebral tissue after ischemia-reperfusion damage. Neurol Res. 2014b;36:118–125. doi: 10.1179/1743132813Y.0000000266. [DOI] [PubMed] [Google Scholar]
- 49.Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res. 2012;34:338–345. doi: 10.1179/1743132812Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thibodeau A, Geng X, Previch LE, Ding Y. Pyruvate dehydrogenase complex in cerebral ischemia-reperfusion injury. Brain Circulation. 2016;2:61–66. doi: 10.4103/2394-8108.186256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsunawaki S, Yoshikawa K. Relationships of p40(phox) with p67(phox) in the activation and expression of the human respiratory burst NADPH oxidase. J Biochem. 2000;128:777–783. doi: 10.1093/oxfordjournals.jbchem.a022815. [DOI] [PubMed] [Google Scholar]
- 52.Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011;70:583–590. doi: 10.1002/ana.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao H, Ago T, Kitazono T, Nabika T. NADPH oxidase-related pathophysiology in experimental models of stroke. Int J Mol Sci. 2017;18:E2123. doi: 10.3390/ijms18102123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yip J, Geng X, Shen J, Ding Y. Cerebral gluconeogenesis and diseases. Front Pharmacol. 2016;7:521. doi: 10.3389/fphar.2016.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu L, Quinn MT, Cross AR, Dinauer MC. is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A. 1998) Gp91(phox;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang HF, Li TB, Liu B, Lou Z, Zhang JJ, Peng JJ, Zhang XJ, Ma QL, Peng J, Luo XJ. Inhibition of myosin light chain kinase reduces NADPH oxidase-mediated oxidative injury in rat brain following cerebral ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:953–963. doi: 10.1007/s00210-015-1125-2. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Wu J, Duan X, Tian X, Shen H, Sun Q, Chen G. NADPH oxidase: a potential target for treatment of stroke. Oxid Med Cell Longev. 2016;2016:5026984. doi: 10.1155/2016/5026984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao H, Han Z, Ji X, Luo Y. Epigenetic regulation of oxidative stress in ischemic stroke. Aging Dis. 2016;7:295–306. doi: 10.14336/AD.2015.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng J, Li G, Chen S, Bihl J, Buck J, Zhu Y, Xia H, Lazartigues E, Chen Y, Olson JE. Activation of the ACE2/Ang-(1-7)/Mas pathway reduces oxygen-glucose deprivation-induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience. 2014;273:39–51. doi: 10.1016/j.neuroscience.2014.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]