Abstract
Background:
Hand-to-genital contact is hypothesized to be a transmission mode of HPV of genus Alphapapillomavirus. We compared the relative importance of hand-to-genital and genital-to-genital HPV transmission between sexual partners.
Methods:
We recruited and followed-up female university students 18-24 years old and their male sexual partners in Montréal, Canada (2005-2011). Partners provided hand and genital samples, which we tested for DNA of 36 HPV types using PCR. We assessed predictors of incident type-specific HPV detections using Cox proportional hazards models.
Findings:
264 women and 291 men had valid hand samples. The hazard ratio (HR) of incident female genital HPV detection was 5·0 (95% confidence interval [CI] 1·5-16·4) when her partner was same type hand-positive vs -negative, but adjustment for his genital HPV status reduced the HR to 0·5 (95%CI 0·1-1·8). Similarly, the HR of incident male genital HPV detection was 17·9 (95%CI 7·9-38·5) when his partner was same type hand-positive vs negative, but adjustment for her genital HPV status reduced the HR to 2·3 (95%CI 0·9-6·2). Conversely, the HR of type-specific incident genital HPV detections associated with partner genital HPV positivity was 19·3 (95%CI 11·8-31·8) for females and 28·4 (95%CI 15·4-52·1) for males after adjustment for their hand HPV status.
Interpretation:
Clinicians can reassure their patients that HPV transmission is unlikely to occur through hand-to-genital contact. The majority of genital HPV infections are likely caused by genital-to-genital sexual transmission.
Funding:
Canadian Institutes for Health Research, National Institutes of Health, Fonds de la Recherche en Santé du Québec, Merck & Co. Ltd.
Introduction
Human papillomavirus (HPV) types of the genus Alphapapillomavirus (alpha) are sexually transmitted infections, many of which cause anogenital and oropharyngeal cancers in men and women.1 Alpha HPV types mainly infect mucosal tissues.2 Because HPV prevalence is highly correlated with the number of recent and lifetime genital sex partners, a large body of evidence suggests alpha HPV types are mainly transmitted through genital-to-genital contact.3 Over the years, some have also speculated as to the possibility of hand-to-genital transmission of alpha HPV types.4–8 This hypothesis is supported by the frequent detection of alpha HPV DNA on hands and under fingernails,8–10 as well as the high concordance between hand and genital HPV types in the same person6,9 and between partners.8 However, there has always been skepticism as to the importance of hand-to-genital transmission of alpha HPV types.11 The high correlation between hand and genital HPV detections makes it difficult in most studies to separate cause from effect and the directionality of transmission. It remains unclear to what extent HPV transmission events occur from hand-to-genital or genital-to-hand contact.
The general public is becoming more aware of HPV due to the availability of vaccines and cervical cancer screening with HPV tests, and many may have questions and anxieties regarding the transmission and risk of HPV infections.12,13 Clinicians and public health workers must be able to inform the public on the modes and risks of transmission of HPV. It is therefore important to elucidate the importance of hand-to-genital transmission, and provide public health messages based on strong scientific evidence. This could assuage fears of inadvertently transmitting HPV to partners or of becoming infected from hand-to-genital contacts.
Our objective was to determine whether hand-to-genital transmission of HPV is supported by examining cross-sectional and prospective dynamics of hand and genital HPV detection in couples, or whether the detection of HPV DNA in the hand is merely carriage that is incidental to genital infections.
Methods
Study design and data collection
We used data from the HPV Infection and Transmission Among Couples through Heterosexual Activity (HITCH) study, which examined the transmission of HPV in young, heterosexual, newly-formed couples. Study procedures have been previously described.14–17 Briefly, HITCH was a prospective cohort study which enrolled young female university and college students aged 18-24 years old and their male partners ≥18 years old in Montréal, Canada, during 2005-2011. Participants were recruited through promotional materials distributed on campus and student venues. Eligible couples needed to have initiated sexual activity within the past 6 months. The ethical review committees of McGill University, Concordia University, and the Centre Hospitalier de l’Université de Montreal approved the study. All participants provided written informed consent.
Women were examined at clinic visits at baseline and every 4- to 6-months for up to 24 months. Men had a baseline visit and a single follow-up visit approximately 4 months later. Participants answered self-administered behavioral questionnaires and provided biologic samples for HPV testing at each study visit. Initially, only genital samples were collected, but beginning in 2008 we started collecting hand samples during the first two visits (at enrolment and 4 months) when both men and women were scheduled to attend. If a couple terminated their relationship during the study, the participants were encouraged to enrol any new eligible partner, though this was not required for continued participation.
These participants provided additional hand samples corresponding with the first two visits of their newly recruited partner, which were included in analyses. Most hand samples came from couples recruited after 2008; there were however some exceptions where hand samples were taken from individuals recruited previously who recruited new partners into the study after 2008, or whose second visit occurred following the start of hand sample collection.
Participants were instructed to wash their hands with soap and water prior to hand sampling. An ultra-fine emery paper was used to exfoliate the palmar surface of the index and middle fingers before sampling with a Dacron swab. Wearing latex gloves, the study nurse used a cytobrush to swab the fingertips and under the nails of the dominant hand. Women self-collected vaginal specimens using a Dacron swab, after being instructed by the study nurse. For men, the nurse collected epithelial cells from the penis and scrotum in separate sample containers using gentle exfoliation with ultra-fine emery paper followed by swabbing with a Dacron swab. The Dacron swabs were placed into vials with Preservcyt™ (Hologic, Marlborough, MA, USA), agitated to release cells, and then discarded; the emery papers were also placed in the respective vials. Samples were processed and DNA extracted as previously described.17
HPV DNA testing
Genital and hand specimens were tested by a polymerase chain reaction using the Linear Array HPV genotyping assay (LA-HPV) (Roche Molecular Systems, Alameda, CA, USA).18 This technique detects DNA from 36 mucosal HPV genotypes of the Alphapapillomavirus genus (6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82 [including its subtype IS39], 83, 84, and 89). β-globin DNA was co-amplified to assess DNA integrity of samples and to control for the presence of cells. A sample was considered valid if β-globin DNA was detected. An individual was considered hand type-specific HPV positive if either the fingernail or finger samples were positive for a given HPV type. A man was considered genital type-specific HPV positive if either the penile or scrotal samples were positive for a given HPV type.
Statistical analysis
We restricted analyses to clinic visits where participants had valid hand samples. The unit of analysis was type-specific HPV positivity in the hand or genital samples at each clinic visit. Each participant therefore contributed multiple observations to each analysis with 36 different HPV types and with multiple clinic visits.
Prevalent HPV analyses.
We calculated the observed/expected (O/E) ratio of the probability of detecting the same HPV type in participants’ hand and genital samples during a given visit, summed over all 36 HPV types:
Where oi, is the observed number of visits where both hand and genital samples are positive for HPV type i, ai is the observed number of hand samples positive for HPV type i, bi is the observed number of genital samples positive for HPV type i, and n is the number of visits with valid hand and genital samples. O/E ratios above 1 indicates a type-specific co-detection pattern occurs more often than would be expected if the probability of HPV infection were completely independent between hand and genitals, whereas ratios below 1 indicate a type-specific co-detection pattern occurs less often than would be expected if HPV were distributed completely independently across hands and genitals. The 95% confidence intervals (95%CI) were generated using block bootstrapping with 1000 resamples of participants19. We used multilevel logistic regression models to assess whether type-specific hand and genital HPV positivity was associated with same-type positivity at other sites during the same visit. Multilevel models included a random intercept for each participant. This accounts for potentially correlated data due to repeated measurements on the same person (multiple HPV types and multiple clinic visits). For partner-level analyses, we further restricted the analyses to study visits in which couples both had valid samples taken on the same day. In a separate analysis, we analyzed whether the questionnaire-reported frequency of performing hand-genital and vaginal sex were associated with the hand-genital concordance between partners. We averaged both partners’ reports for this analysis.
Incident HPV analyses.
We plotted the cumulative risks of incident HPV acquisition using Kaplan-Meier curves. We assessed whether incident hand and genital HPV detection were associated with HPV positivity at other self- and partner-sites at the preceding visit using Cox proportional hazards models with a random effect for each participant (log-normal frailty model) and Efron’s approximate likelihood.20,21 We restricted prospective analyses to individuals with at least two visits with valid hand samples, who were type-specific HPV negative at the baseline visit at the analyzed site, and who had valid HPV data at the baseline visit for the other self and partner sample sites. Because HPV infections are asymptomatic, we imputed incident HPV acquisitions as occurring mid-way through the interval when the individual became HPV positive. Participants who had no incident type-specific HPV detection were censored at their last study visit with a valid hand sample. In sensitivity analyses, we fitted a fixed effects Cox model using interval-censoring methods to assess whether midpoint imputation affected results.
Due to the infectious nature of HPV, positivity at different sites is expected to be highly correlated. To control for confounding, we included type-specific HPV positivity at all other exposure sites as predictors in multivariable logistic and Cox regression models. The objective was to determine which sites were the strongest predictors of type-specific HPV detection at the outcome site, unconfounded by same-type HPV positivity at other sites. Statistical analyses were done using SAS 9.4 and R.
Role of the funding source
The study sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Hand samples available for analysis
There were 264 women and 291 men (37 recruited after baseline) who provided at least one valid hand HPV DNA sample during 479 and 489 visits, respectively (Figure 1). The concomitant genital DNA samples were valid in 473 and 483 [99%] of male and female visits. The participants included in the present analyses had a mean age of 20·9 years (standard deviation 2·4) for women and 22·9 years (standard deviation 4·0) for men, and reported a median of 4 vaginal sex acts/week. More characteristics of this subset of HITCH participants are presented in Supplementary Table 1.
Figure 1. Flow chart of HITCH study participants and exclusions.
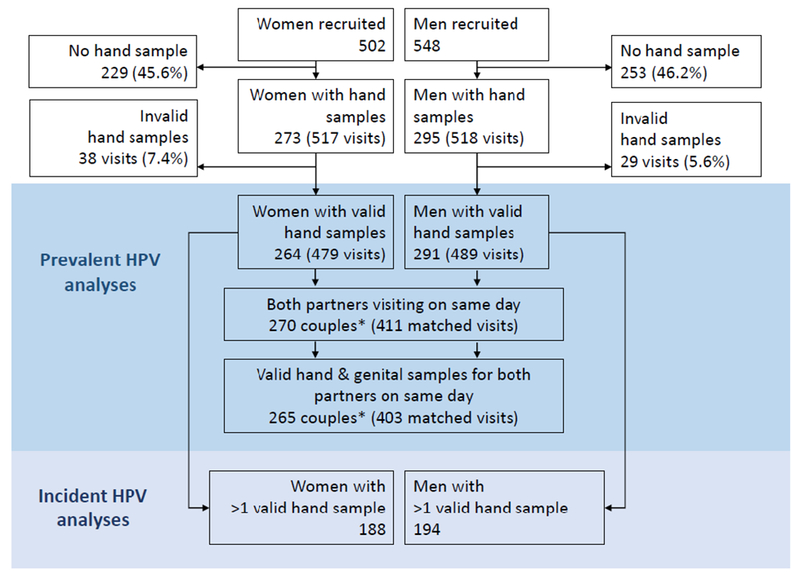
Hand sampling began half-way through study recruitment in 2008. Invalid hand and genital samples had no detected β-globin DNA. *There are more couples (270) than women (264) in cross-sectional partnered analyses because some women recruited multiple male partners in the study and were part of more than one couple.
There were 188 women and 194 men who had more than one study visit with a valid hand sample and were included in prospective analyses. The median number of visits was 2 (range 1-5) for women and 2 (range 1-2) for men. Women were followed for a median (interquartile range [IQR]) of 141 (IQR 113-194) days, and men for a median of 135 (IQR 112-176) days.
HPV positivity in the hands and genitals
Across women’s visits, there were 300 HPV types detected in 479 hand samples and 748 HPV types detected in 483 vaginal samples. Across men’s visits, there were 352 HPV types detected in 489 hand samples and 903 HPV types detected in 483 genital samples. The prevalence of at least one HPV type was 35·5% [170/479] in female hand samples, 36·4% [178/489] in male hand samples, 59·8% [283/473] in female genital samples, and 63·4% [306/483] in male genital samples (Table 1). Across individual HPV types, the type-specific HPV prevalence was nearly always lower in hand than in genital samples (Supplementary Table 2).
Table 1.
HPV DNA type-specific number of detections and prevalence in valid hand samples and concomitant genital samples.
| HPV group | Sex | Total HPV type detections (n)a |
HPV prevalenceb |
|||||
|---|---|---|---|---|---|---|---|---|
| Hand | Genital | Either Hand or Genital | Hand (women N=479 men N=489) | Genital (women N=473 men N=483) | ||||
| n | (%) | n | (%) | |||||
| Any | Women | 300 | 748 | 805 | 170 | 35·5% | 283 | 59·8% |
| Men | 352 | 903 | 959 | 178 | 36·4% | 306 | 63·4% | |
| HRC | Women | 126 | 326 | 344 | 95 | 19·8% | 203 | 42·9% |
| Men | 143 | 356 | 384 | 105 | 21·5% | 218 | 45·1% | |
| LRC | Women | 126 | 291 | 317 | 99 | 20·7% | 186 | 39·3% |
| Men | 135 | 386 | 399 | 101 | 20·7% | 226 | 46·8% | |
HPV=Human papillomavirus; HR=high risk types; LR=low risk types; N=denominator.
Number of type-specific detections at all visits. Each individual may contribute multiple HPV type detections if samples are positive for multiple types.
Proportion of samples that are positive for any of the group’s HPV types across visits. Denominators (N) are the number of valid hand and genital samples over all visits.
HR: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68. LR: HPV 6, 11, 40, 42, 44, 54, 61, 62, 71, 72, 81, 83, 84, 89.
HPV co-detection patterns in the hands and genitals
The probability of detecting the same HPV type in both an individual’s hand and genital samples was 16-18 times higher than expected if HPV types were independently distributed across hand and genital samples (Figure 2A-B). The probability of detecting the same HPV type in both partners’ hand and/or genital samples was 3.8-32.8 times higher than expected if HPV types were independently distributed across partnerships (Figure 2C). This over-representation of partners concurrently positive for the same-type and under-representation of co-negative samples when a partner is HPV positive at any site would be expected if there is cross-site HPV transmission, either within individuals or between sex partners. The especially lower than expected observed cases of same-type hand positive/genital negative (0/E=0.16-0.19) than observed cases of same-type hand-negative/genital-positive (O/E=0.69) may possibly reflect asymmetry in transmission or clearance between sites.
Figure 2. Observed to expected ratios of type-specific detection patterns by site pooled over all HPV types in women A), in men B), and in partnerships C).
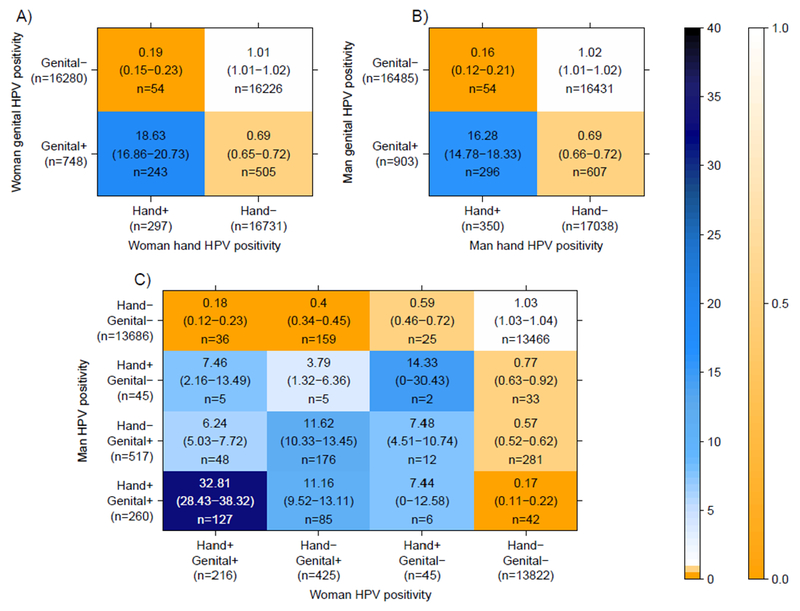
Analyses are restricted to visits with concurrently valid hand and genital samples (473 for women, 483 for men, and 403 for couples). Marginal totals (the denominators) are derived from the number of visits with concurrent valid hand & genital samples * 36 HPV types. Marginal totals are lower in C) than in A-B) because partnership analyses are restricted to the visits where both partners had valid hand and genital samples taken on the same day. The vertical graded color bar indicates the magnitude of the observed-to-expected ratios. Numbers in parentheses represent the 95% percentiles of 1000 block bootstrap resamples.
Table 2 shows the probability of type-specific HPV positivity at a given site, stratified by sex and same-type HPV positivity at other sites. For instance, the probability of being hand HPV positive for a type was respectively 45·2% [141/312] for women and 53·8% [141/262] for men if their partner’s hand was positive for that type at the same visit. The probability of being genital HPV positive for a type was respectively 81·8% [243/297] for women and 84·6% [296/350] for men if their own hand sample was positive for that type at the same visit.
Table 2.
Probability and odds ratios of hand and genital HPV DNA positivity stratified by HPV positivity at other sites at the same visit, pooled over all HPV types.
| Outcome | Exposure site | HPV type-specific positivity probability (exposure site positives)a n/Nd (%) | HPV type-specific positivity probability (exposure site negatives)b n/Nd (% | Univariate OR (95% CI) | Adjusted for all sitesc OR (95% CI) |
|---|---|---|---|---|---|
| Women | |||||
| Hand HPV positivity | Genital (own) | 243/748 (32·5%) | 54/16280 (0·3%) | 170·1 (121·0-239·0) | 49·5 (30·6-80·2) |
| Genital (partner) | 194/792 (24·5%) | 68/13860 (0·5%) | 74·3 (53·9-102·5) | 3·0 (1·8-4·9) | |
| Hand (partner) | 141/312 (45·2%) | 121/14484 (0·8%) | 127·0 (88·8-181·7) | 6·7 (4·2-10·4) | |
| Genital HPV positivity | Hand (own) | 243/297 (81·8%) | 505/16731 (3·0%) | 189·4 (131·7-272·5) | 50·7 (30·5-84·3) |
| Genital (partner) | 436/777 (56·1%) | 205/13731 (1·5%) | 96·4 (76·4-121·5) | 44·5 (34·1-58·0) | |
| Hand (partner) | 222/306 (72·6%) | 423/14346 (3·0%) | 105·6 (76·5-145·8) | 4·1 (2·7-6·3) | |
| Men | |||||
| Hand HPV positivity | Genital (own) | 296/903 (32·8%) | 54/16485 (0·3%) | 189·3 (133·3-268·7) | 54·4 (34·5-85·9) |
| Genital (partner) | 222/645 (34·4%) | 84/14007 (0·6%) | 104·7 (76·1-144·2) | 5·1 (3·3-7·8) | |
| Hand (partner) | 141/262 (53·8%) | 171/14534 (1·2%) | 133·5 (92·0-193·7) | 7·3 (4·7-11·4) | |
| Genital HPV positivity | Hand (own) | 296/350 (84·6%) | 607/17038 (3·6%) | 205·0 (142·0-296·1) | 51·8 (32·1-83·6) |
| Genital (partner) | 436/641 (68·0%) | 341/13867 (2·5%) | 102·4 (80·5-130·3) | 46·7 (35·4-61·5) | |
| Hand (partner) | 194/262 (74·1%) | 598/14390 (4·2%) | 82·0 (57·9-116·1) | 2·5 (1·5-4·1) | |
CI=Confidence interval; HPV=human papillomavirus; n=numerator; N=denominator; OR=Odds ratio.
Probability that the outcome site is HPV DNA type-specific positive if the exposure site is same-type positive.
Probability that the outcome site is HPV DNA type-specific positive if the exposure site is same-type negative.
Hand positivity: mutually adjusted for genital (own), genital (partner), and hand (partner) exposure site positivity; Genital positivity: mutually adjusted for hand (own), genital (partner), and hand (partner) exposure site positivity.
The denominators are 36 HPV types * the number of visits where both the exposure and the outcome site samples were valid and taken on the same day. Numbers may be higher than in Figure 2C, which is restricted to visits with complete data where both partners had valid hand and genital samples taken on the same day, a more stringent criteria (4 concurrent valid samples instead of 2).
Variables associated with hand HPV positivity
Prevalent hand HPV.
In univariate cross-sectional analyses (Table 2), men and women were substantially more likely to be hand type-specific HPV positive if their own genitals or their partner’s hands and genitals were also positive for same HPV type. However, once we adjusted for positivity at all sites, HPV positivity in the hand remained most strongly associated with same-type positivity in the individual’s own genitals. Women and men were respectively 49·5 (95%CI: 30·6-80·2) and 54·4 (95%CI: 34·5-85·9) times more likely to be hand HPV positive if they were genital HPV positive for the same HPV type than if they were genital HPV negative, after adjusting for HPV positivity of their partner’s samples. Hand HPV positivity was substantially less correlated with the partner’s hand or genital status once we accounted for this intra-individual hand-genital correlation. The male partners who reported performing more hand-genital sex on their female partner had a higher probability of being hand positive for their partner’s genital types, but the overall association was not significant (Supplementary Table 3). Individuals who had more frequent vaginal sex were more likely to have the same HPV type in the hand as in their partner’s genitals (OR 2·1 [95%CI: 1·1-3·9] for women and 2·7 [95%CI: 1·3-5·4] for men who have vaginal sex >4 times a week vs ≤2 times a week), but the relationship was no longer significant after we controlled for HPV positivity in their own genitals (Supplementary Table 3)·
Incident hand HPV.
In incident HPV analyses (Table 3), once we adjusted for HPV positivity at all other sites at the previous visit, a woman was most likely to have an incident hand HPV detection if she was first genital positive for that HPV type (hazard ratio [HR] 17·9, 95%CI: 8·8-36·5) at the previous visit. She was also significantly more likely to have an incident hand HPV detection if her partner was genital positive for that HPV type (HR 5·9, 95%CI: 2·8-12·4), but not if he was hand positive for the same type (HR 1·4, 95%CI: 0·7-2·8) at the previous visit. Adjusting for positivity at all other sites, a man was more likely to have an incident hand HPV detection if he was first genital positive for that HPV type (HR 7·2, 95%CI: 3·4-15·5) or if his partner was type-specific genital positive for that HPV type (HR 9·5, 95%CI: 4·3-21·1) at the previous visit, but not if she was hand positive (HR 1·0, 95%CI: 0·4-2·7). Sensitivity analyses with interval-censored proportional hazard models provided very similar estimates (Supplementary Table 4). Kaplan-Meier curves of cumulative incident hand HPV detections are presented in Figure 3A-B.
Table 3.
Incidence rate and hazard ratios of type-specific incident hand and genital HPV DNA positivity stratified by HPV positivity at other sites at the previous visit, pooled over all HPV types.
| Exposure site positive |
Exposure site negative |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Exposure site | Number at riska | Events (n)a | Incidence rate (/100 years) | Number at riska | Events (n)a | Incidence rate (/100 years) | Univariate HR (95% CI) | Adjusted for all sitesb HR (95% CI) |
| Women | |||||||||
| Incident hand | Genital (own) | 223 | 45 | 50·6 | 7218 | 33 | 1·0 | 51·0 (31·4–82·7) | 17·9 (8–8-36–5) |
| positivity | Genital (partner) | 284 | 39 | 32·5 | 6633 | 30 | 1·0 | 33·8 (20·2–56·5) | 5·9 (2–8-12–4) |
| Hand (partner) | 80 | 16 | 48·3 | 6909 | 54 | 1·7 | 26·7 (14·3-49·8) | 1·4 (0·7-2·8) | |
| Incident genital | Hand (own) | 34 | 5 | 29·9 | 7140 | 101 | 3·1 | 9·8 (3·8-25·4) | 2·7 (0·8-8·5) |
| positivity | Genital (partner) | 164 | 28 | 38·2 | 6435 | 62 | 2·1 | 19·2 (12·0-30·8) | 19·3 (11·8-31·8) |
| Hand (partner) | 41 | 3 | 15·5 | 6630 | 87 | 2·9 | 5·0 (1·5-16·4) | 0·5 (0·1-1·8) | |
| Men | |||||||||
| Incident hand | Genital (own) | 221 | 34 | 45·8 | 6496 | 42 | 1·6 | 27·4 (16·7-45·0) | 7·2 (3·4-15·5) |
| positivity | Genital (partner) | 170 | 28 | 50·2 | 6090 | 37 | 1·5 | 31·7 (18·5-54·2) | 9·5 (4·3-21·1) |
| Hand (partner) | 57 | 8 | 39·2 | 6238 | 57 | 2·3 | 14·3 (6·4-32·3) | 1·0 (0·4-2·7) | |
| Incident genital | Hand (own) | 25 | 3 | 29·8 | 6496 | 89 | 3·4 | 8·7 (2·6-29·5) | 0·5 (0·1-3·1) |
| positivity | Genital (partner) | 90 | 22 | 75·5 | 5921 | 58 | 2·4 | 33·3 (19·4-57·1) | 28·4 (15·4-52·1) |
| Hand (partner) | 37 | 8 | 59·5 | 6005 | 72 | 3·0 | 17·4 (7·9-38·5) | 2·3 (0·9-6·2) | |
CI=Confidence interval; HPV=human papillomavirus; HR=Hazard ratio; n=number of events.
The number at risk is 36 HPV types * the number of instances where both the exposure and the outcome baseline site samples were valid and taken on the same day, and which have valid follow-up data for the outcome site. Total numbers at risk and number of events vary between rows because some individuals contribute to some analyses but not others if one of their samples is invalid.
Incident hand positivity: mutually adjusted for genital (own), genital (partner), and hand (partner) exposure site positivity; Incident genital positivity: mutually adjusted for hand (own), genital (partner), and hand (partner) exposure site positivity.
Figure 3. Cumulative incidence of hand and genital type-specific HPV detections.
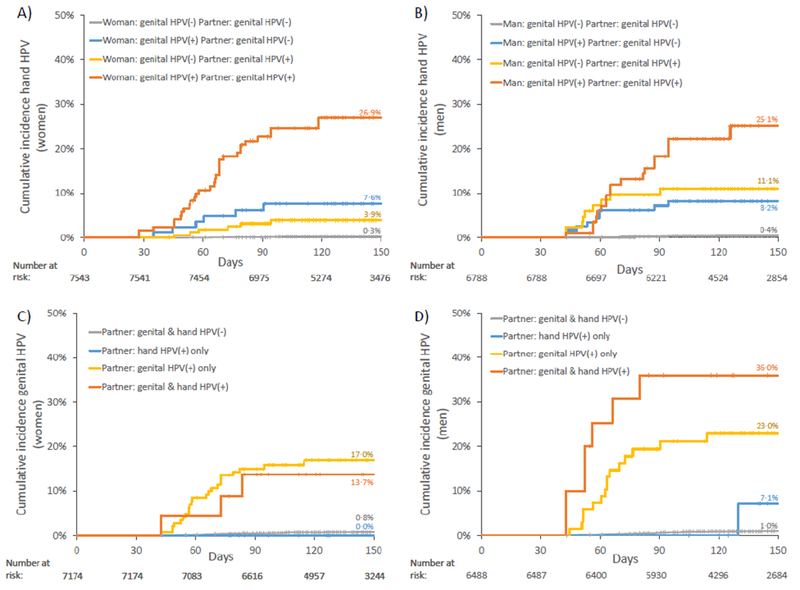
A) Incident hand HPV detections in women; B) incident hand HPV detections in men; C) incident genital HPV detections in women; C) incident genital HPV detections in men. Results are stratified by partner’s hand and genital same-type HPV positivity at the previous visit, and by own same-type genital positivity at the previous visit (for A-B). Results are pooled over all HPV types.
Variables associated with genital HPV positivity
Prevalent genital HPV.
In univariate cross-sectional analyses (Table 2), men and women were substantially more likely to be genital type-specific HPV positive if their own hand or their partner’s hand or genital samples were positive for the same HPV type. However, once we adjusted for positivity at all sites, HPV positivity in the genitals was substantially less associated with same type HPV positivity in the partner’s hands. For example, while women were 105·6 times more likely to be genital HPV positive if their partner was same type hand positive than if he was negative, this association declined to 4·1 (95%CI: 2·7-6·3) upon conditioning on same-type HPV positivity at other sites.
Incident genital HPV.
In prospective analyses (Table 3), once we adjusted for HPV positivity at all other sites, both women and men were most likely to have an incident genital HPV detection if their partner was genital-positive for that HPV type at the previous visit (HR 19·3 [95%CI: 11·8-31·8] and 28·4 [95%CI: 15·4-52·1], respectively). The incidence of genital HPV detections was not significantly associated with their own or their partner’s hand HPV positivity once we accounted for their partner’s same-type genital HPV positivity at the previous visit. Sensitivity analyses with interval-censored proportional hazard models provided very similar estimates (Supplementary Table 4). Kaplan-Meier curves of cumulative incident genital HPV detections are presented in Figure 3C-D. There were no incident female genital HPV detections in couples where the male partner’s hand was the only HPV positive site at the previous visit (Figure 3C). There was only one incident male genital HPV89 detection in couples where the female partner’s hand was the only HPV89-positive site at the previous visit (Figure 3D); this man reported having ended the relationship and having new sexual partners during the interval, so he may have acquired HPV89 from another partner.
HPV Persistence
If an HPV type was detected in a hand sample, the probability of detecting the same type in the next hand sample visit was 26·4% (95%CI: 18·8-36·3) [40/151] for women and 36·0% (95%CI: 27·7-46·8) [58/161] for men. If an HPV type was detected in a genital sample, the probability of detecting the same type in the next genital sample visit was 69·0% (95%CI: 60·6-78·6) [234/339] for women and 75·2% (95%CI: 66·6-84·9) [267/355] for men.
Discussion
The importance of hand-to-genital transmission has to date been uncertain. While some have proposed that hand-to-genital transmission is plausible based on a high observed concordance between hands and genitals within individuals and between partners,6,8 others have deemed it unlikely, due to the transience of HPV detected on the hands9 and doubts as to whether sufficient live virus is present on the hands or shed via exfoliation for successful transmission.11 In this study, we found that the detection of alpha type HPV on the hands is common in men and women, but that it is most likely to concurrently occur with a same-type HPV genital infection. Alpha HPV DNA detection in the hands alone without a same-type genital infection within the same individual or their sexual partner occurs substantially less often than expected due to chance alone. Both HPV positivity in the individual’s own genitals and their partner’s genitals were important predictors of incident hand HPV detection. This suggests that the majority of alpha HPV DNA detection in the hands in couples is due to self-inoculation from the person’s own genitals or from the partner’s genitals rather than hand-to-hand transmission. Conversely, hand HPV positivity was not a significant predictor of incident same type genital HPV detections after we accounted for partner genital HPV positivity. This suggests that the majority of incident genital infections are caused by genital-to-genital transmission, and that hand-to-genital transmission is unlikely to substantially contribute to the sexual transmission of alpha HPV types.
We found similar cross-site concordance rates as previous studies, which found that if HPV was detected in the hand, there was >60% probability that the person’s or their partner’s genitals were positive for the same type.8,9 Others have also estimated high rates of genital-to-hand transmission rates.7,8,10 A strength of this study relative to previous studies was that we had sufficient data to adjust our analyses for HPV positivity at different sites. This allowed us to study the directionality of transmission while taking into account confounding due to other routes of transmission, which to date had been a major challenge for studying hand HPV transmission. Our results suggest that the high observed genital-hand HPV concordance is due to genital-to-hand transmission rather than hand-to-genital transmission. A limitation of our data is that we still had very few observations from individuals exposed to a partner who is only hand type-specific positive in incident genital detection analyses (17 for women and 17 for men), and that there were very few couples in HITCH who were not having vaginal sex. This limited our ability to completely rule out hand-to-genital HPV transmission. However, if there is hand-to-genital transmission, our study suggests it is unlikely to be an important mode of HPV transmission. The low prevalence of hand HPV positivity independent of genital positivity also further supports that the hand is unlikely to be an important reservoir of transmission. While we did not find that the frequency of hand-genital sex was significantly associated with hand-genital HPV partner concordance, we had low statistical power for this analysis because very few couples reported never performing hand-genital sex.
It is impossible to determine whether the detection of HPV DNA represents an active infection at a site or merely the deposition of virions and/or free viral DNA. Participants had been asked to wash their hands with soap previously to sampling in order to reduce the likelihood of detecting contaminations in hand samples. We had concluded in a previous analysis that up to 14·1% of genital HPV detections in HITCH might be partner deposition from recent sexual activity,22 but it is unknown what proportion of HPV DNA detected in the hand represents deposition. The low persistence of hand HPV between visits in this and a previous study9 suggests many hand detections are likely to be depositions. Alpha HPV types are thought to mostly infect the genitals or the oropharynx.2 However, mouse models suggest that alpha HPV infections may become established in cutaneous tissues after skin trauma.23 Reports of cutaneous squamous cell carcinomas (SCC) (Bowen’s disease) on the hands linked with alpha HPV types suggest that alpha HPV can infect the hands in some cases.5,24,25 The same HPV type is often found in both cervical and finger samples of patients with a history of both SCC of the fingers and SCC of the cervix.25 Because the diagnosis of cervical cancer generally predates that of SCC of the finger, this finding is also consistent with transmission mostly occurring from genital-to-hand. Regardless of whether the HPV is present due to deposition or due to an active hand infection, we did not find that HPV detected on the hands substantially increased genital infection risk.
Compared to our study population, the general population is older, and has a higher proportion of individuals who report no recent sexual partners or vaginal sex.26 It is therefore likely that most hand HPV detections in the general population are due to self-inoculation rather than partner deposition, as the exposure to an infected partner is lower in the general population. The associations we measured between HPV positivity in the genitals and hands are likely generalizable to most heterosexual populations. Our results may not be generalizable to non-heterosexual partnerships, as the relative importance of different modes of HPV transmission may be different.
The transmission modes of HPV are important for shaping the public health narrative surrounding HPV. HPV testing is becoming widely implemented in many countries for cervical cancer screening. There are likely to be increasing numbers of women who will learn for the first time that they are HPV positive and who will have questions and concerns regarding their HPV diagnosis, including how they acquired it.12,13 The information that HPV transmission is largely transmitted by sexual genital contact may lead some to feel shame over having a sexually transmitted infection; however, the reassurance that HPV is highly common and that most people will become infected in their lifetime may reduce this stigma.13,27 Given that transmission is most likely to occur from genital-to-genital contact, this may also be an opportunity to emphasize the preventive benefits of condoms to reduce HPV transmission to partners.28 Clinicians might also reassure women that hand-to-genital transmission of HPV to self or to others is not as efficient a mechanism of transmission as genital intercourse, based on our results.
Our results do not necessarily indicate that hand-to-genital HPV transmission does not occur, because it is easier to reject than to prove a null hypothesis of no transmission. However, our study does bolster the assertion that if there is hand-to-genital transmission, it is unlikely to be an important mode of transmission of genital HPV infections in sexual partnerships. Our study suggests that genital alpha HPV detections are more likely caused by genital-to-genital transmission, and that most alpha HPV DNA detections in the hand are likely caused by either genital-to-hand deposition and/or transmission (either from one’s own genitals or from a partner’s genitals). The high cumulative incidence of detection of HPV in the hand suggests genital-to-hand HPV deposition is common. However, detection of HPV in the hand should not be cause for concern as it is unlikely to substantially increase the risk of genital HPV transmission to oneself or to one’s partners.
Supplementary Material
Research in context.
Evidence before this study
While hand-to-genital transmission has long been hypothesized as a mode of HPV transmission, there has been very little published data on the prevalence and incidence of HPV on hands to test this hypothesis. We performed a literature review by searching Pubmed for (“Papillomavirus Infections/transmission”[MeSH Terms] OR “Papillomavirus Infections”[MeSH Terms] OR “Alphapapillomavirus”[MeSH Terms] OR “HPV”[Title/Abstract]) AND (“Hand/virology”[MeSH Terms] OR “Fingers/virology”[MeSH Terms] OR “Disease Transmission, Infectious”[MeSH Terms]) and for (“hand” and “HPV” and “transmission”). We identified 5 studies that examined Alphapapillomavirus type concordance between hand and genital sites or incidence of transmission between sites in the past 20 years. The conclusions from these studies have been conflicting, with some concluding that hand-to-genital transmission is possible, others concluding it is unlikely, and others concluding it is unclear. The main limitations of previous studies have been a small sample size and/or the lack of data on sexual partners to assess sexual transmission.
Added value of this study
This is the largest study to date of sex partners with genital and hand HPV data. We controlled for confounding due to the correlation in HPV positivity at multiple sites to assess the directionality of HPV transmission between sites and between partners. Our results provide the strongest evidence to date that genital HPV acquisition is unlikely to be due to hand-to-genital transmission, occurs mostly as a result of genital-to-genital contact, and that most HPV DNA on the hands is likely from self-inoculation from the genitals.
Implications of the available evidence
Due to the carcinogenicity of many HPV types, cervical cancer screening is increasingly done using HPV testing. Many women will become aware that they are HPV positive and have questions regarding how they contracted HPV, and the risk of transmission to their partners. Our results suggest that clinicians can reassure their patients that transmission is unlikely to occur through hand contact.
Acknowledgements
This work was supported by CIHR [operating grant 68893 and team grant 83320 to ELF, and Fellowship Awards to TM & MW]; the US National Institutes of Health [grant AI073889 to ELF]; the Réseau FRSQ Fonds de la Recherche en Santé du Québec AIDS and Infectious Disease Network (SIDA-MI) [support for optimization of molecular techniques to FC]; and supplementary and unconditional funding by Merck-Frosst Canada Ltd and Merck & Co Ltd. The funders played no role in the writing of the manuscript, the collection/analysis of the data, or the decision to submit it for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
TM & MW report postdoctoral grants from the Canadian Institutes of Health Research (CIHR) during the conduct of the study. KL reports grants from the Sigrid Juselius Foundation and the Finnish Medical Foundation. ELF reports grants from CIHR, grants from Merck, and grants from the National Institutes of Health during the conduct of the study; personal fees from Roche, personal fees from BD, personal fees from Merck, and personal fees from GlaxoSmithKline outside of the submitted work. FC reports grants from Reseau FRQS-SIDA during the conduct of the study and grants from Becton Dickinson outside of the submitted work. ANB, PPT, and MEZ have no conflicts of interest to disclose.
References
- 1.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016; 4(9): e609–16. [DOI] [PubMed] [Google Scholar]
- 2.Egawa N, Egawa K, Griffin H, Doorbar J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015; 7(7): 3863–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine 2006; 24, Supplement 3: S52–S61. [DOI] [PubMed] [Google Scholar]
- 4.Fairley CK, Gay NJ, Forbes A, Abramson M, Garland SM. Hand-genital transmission of genital warts? An analysis of prevalence data. Epidemiol Infect 1995; 115(1): 169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsuishi T, Sata T, Matsukura T, Iwasaki T, Kawashima M. The presence of mucosal human papillomavirus in Bowen’s disease of the hands. Cancer 1997; 79(10): 1911–7. [DOI] [PubMed] [Google Scholar]
- 6.Sonnex C, Strauss S, Gray JJ. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex Transm Infect 1999; 75(5): 317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of Human Papillomavirus in Heterosexual Couples. Emerging infectious diseases 2008; 14(6): 888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widdice L, Ma Y, Jonte J, et al. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis 2013; 207(8): 1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winer RL, Hughes JP, Feng Q, et al. Detection of genital HPV types in fingertip samples from newly sexually active female university students. Cancer Epidemiol Biomarkers Prev 2010; 19(7): 1682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis 2007; 196(8): 1128–36. [DOI] [PubMed] [Google Scholar]
- 11.Mindel A, Tideman R. HPV transmission--still feeling the way. Lancet 1999; 354(9196): 2097–8. [DOI] [PubMed] [Google Scholar]
- 12.Patel H, Moss EL, Sherman SM. HPV PRIMARY CERVICAL SCREENING IN ENGLAND: WOMEN’S AWARENESS AND ATTITUDES. Psychooncology 2018. [DOI] [PubMed] [Google Scholar]
- 13.McRae J, Martin C, O’Leary J, Sharp L. “If you can’t treat HPV, why test for it?” Women’s attitudes to the changing face of cervical cancer prevention: a focus group study. BMC Womens Health 2014; 14: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology 2010; 21(1): 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis 2010; 37(1): 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burchell AN, Coutlee F, Tellier P-P, Hanley J, Franco EL. Genital Transmission of Human Papillomavirus in Recently Formed Heterosexual Couples. Journal of Infectious Diseases 2011; 204(11): 1723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burchell AN, Rodrigues A, Moravan V, et al. Determinants of prevalent human papillomavirus in recently formed heterosexual partnerships: a dyadic-level analysis. J Infect Dis 2014; 210(6): 846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coutlee F, Rouleau D, Petignat P, et al. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear array HPV genotyping test. J Clin Microbiol 2006; 44(6): 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelo C, Ripley B. boot: Bootstrap R (S-Plus) Functions R package version 1.3-20. 2017. [Google Scholar]
- 20.Ripatti S, Palmgren J. Estimation of multivariate frailty models using penalized partial likelihood. Biometrics 2000; 56(4): 1016–22. [DOI] [PubMed] [Google Scholar]
- 21.Efron B The Efficiency of Cox’s Likelihood Function for Censored Data. Journal of the American Statistical Association 1977; 72(359): 557–65. [Google Scholar]
- 22.Malagon T, Burchell AN, El-Zein M, et al. Estimating HPV DNA Deposition Between Sexual Partners Using HPV Concordance, Y Chromosome DNA Detection, and Self-reported Sexual Behaviors. J Infect Dis 2017; 216(10): 1210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handisurya A, Day PM, Thompson CD, et al. Murine skin and vaginal mucosa are similarly susceptible to infection by pseudovirions of different papillomavirus classifications and species. Virology 2012; 433(2): 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clavel CE, Huu VP, Durlach AP, Birembaut PL, Bernard PM, Derancourt CG. Mucosal oncogenic human papillomaviruses and extragenital Bowen disease. Cancer 1999; 86(2): 282–7. [PubMed] [Google Scholar]
- 25.Forslund O, Nordin P, Hansson BG. Mucosal human papillomavirus types in squamous cell carcinomas of the uterine cervix and subsequently on fingers. Br J Dermatol 2000; 142(6): 1148–53. [DOI] [PubMed] [Google Scholar]
- 26.Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). The Lancet 2013; 382(9907): 1781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connor M, Costello L, Murphy J, et al. ‘I don’t care whether it’s HPV or ABC, I just want to know if I have cancer.’ Factors influencing women’s emotional responses to undergoing human papillomavirus testing in routine management in cervical screening: a qualitative study. Bjog 2014; 121(11): 1421–9. [DOI] [PubMed] [Google Scholar]
- 28.Winer RL, Hughes JP, Feng Q, et al. Condom Use and the Risk of Genital Human Papillomavirus Infection in Young Women. New England Journal of Medicine 2006; 354(25): 2645–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


