Abstract
Immunodeficient mice engrafted with human peripheral blood mononuclear cells (PBMCs) support preclinical studies of human pathogens, allograft rejection, and human T-cell function. However, a major limitation of PBMC engraftment is development of acute xenogeneic graft-versus-host disease (GVHD) due to human T-cell recognition of murine major histocompatibility complex (MHC). To address this, we created 2 NOD-scid IL-2 receptor subunit γ (IL2rg)null (NSG) strains that lack murine MHC class I and II [NSG–β-2-microglobulin (B2M)null (IA IE)null and NSG-(Kb Db)null (IAnull)]. We observed rapid human IgG clearance in NSG-B2Mnull (IA IE)null mice whereas clearance in NSG-(Kb Db)null (IAnull) mice and NSG mice was comparable. Injection of human PBMCs into both strains enabled long-term engraftment of human CD4+ and CD8+ T cells without acute GVHD. Engrafted human T-cell function was documented by rejection of human islet allografts. Administration of human IL-2 to NSG-(Kb Db)null (IAnull) mice via adeno-associated virus vector increased human CD45+ cell engraftment, including an increase in human regulatory T cells. However, high IL-2 levels also induced the development of GVHD. These data document that NSG mice deficient in murine MHC support studies of human immunity in the absence of acute GVHD and enable evaluation of human antibody therapeutics targeting human T cells.—Brehm, M. A., Kenney, L. L., Wiles, M. V., Low, B. E., Tisch, R. M., Burzenski, L., Mueller, C., Greiner, D. L., Shultz, L. D. Lack of acute xenogeneic graft-versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression.
Keywords: humanized, HU-PBL-SCID, GVHD, immunodeficient, humanized
Immunodeficient mice engrafted with human immune systems are valuable preclinical tools for studying human immunity in a small animal model. In the early 2000s, a number of laboratories developed immunodeficient mice with genetic mutations in the IL-2 receptor common γ chain (for reviews, see refs 1–6). These mice support engraftment with high levels of human immune and hematopoietic cells. Although a number of mouse strains bearing the IL-2 receptor subunit γ (IL2rg)null (NSG) mutation have been previously described (4), the NOD-scid IL2rgnull [NSG or NOD/Shi-scid/IL-2Rγnull (NOG)] strains are the most widely used as recipients of human cells and tissues (7, 8). These mice lack T, B, and NK cells, and have defects in innate immunity. In addition, the NSG and NOG strains have a humanlike polymorphism in the Sirpa gene, which controls macrophage recognition and the removal of foreign cells via the Sirp-α/CD47 axis. The Sirpa allele in NSG and NOG mice supports enhanced engraftment of human cells and tissues (9, 10).
A number of human tissues and cell populations have been engrafted into immunodeficient mice to model human biology and immunity (2, 6). One approach is the engraftment of human peripheral blood mononuclear cells, or PBMCs [termed the Hu–peripheral blood leukocyte (PBL)–SCID model], first described in 1988 (11). Human T cells are the predominant cell type that engrafts in this model, whereas engraftment of other cell populations—such as B, myeloid, or NK cells—is relatively low. The Hu-PBL-SCID model has been used to study human infectious agents, tissue transplantation, and human T-cell immune function (2, 12–14). One of the primary uses of this model is the study of acute graft-versus-host disease (GVHD) (15–25), a major problem in clinical hematopoietic stem cell transplantation (26). NSG mice engrafted with human PBMCs develop an acute xenogeneic GVHD-like disease upon recognition of the murine cells and tissues by mature human T cells (23).
Although useful for the study of human GVHD, studies of other human immune functions using the Hu-PBL-SCID model have been limited by the brief window of time available to conduct experiments before the PBMC recipients succumb to GVHD (23). We have reported that murine major histocompatibility complex (MHC) class I and II antigens are the major components that are recognized by mature human T cells during development of acute xenogeneic GVHD in NSG mice (23). These findings were based on earlier observations following injection of PBMCs into NSG mice lacking murine MHC class I or MHC class II antigens. Human CD4+ T cells predominated in MHC class I–deficient NSG mice, whereas human CD8+ T cells predominated in MHC class II–deficient NSG mice, demonstrating that robust expansion of the respective cell subsets depends on the type of murine MHC expressed (24). Moreover, NSG MHC class I–deficient mice engrafted with PBMCs exhibited delayed development of GVHD when compared with NSG or NSG MHC class II–deficient mice, suggesting that human CD8+ T cells are potent effectors in the GVHD disease (24).
To address the development of GVHD following human PBMC injection, a strain of NOG mice lacking both MHC class I and II has recently been described (25). It was reported that the PBMC-engrafted MHC-deficient NOG mice had longer lifespans than those of the parental NOG strain, and that the engrafted cells were able to generate cytotoxic T-cell activity against human tumor cells as well as NK cell–mediated cytotoxicity against NK-sensitive targets following treatment with anti–programmed death-1 (PD-1) antibody (Ab). To extend these observations, we now describe the generation of 2 models of NSG mice that lack both murine MHC class I and II. In the first model, IgG clearance is extremely rapid, whereas in the second model, IgG clearance is much slower and comparable to that observed in NSG mice. Injection of human PBMCs into both strains led to long-term engraftment of functional human T cells and lack of acute GVHD. Our data suggest NSG mice deficient in murine MHC class I and II can be used to study human immunity and the therapeutic effects of human Ab–based drugs on the human immune system in the absence of acute GVHD.
MATERIALS AND METHODS
Mice
All mice used in these studies were raised in the breeding colonies of L.D.S. at The Jackson Laboratory (Bar Harbor, ME, USA). NSG mice have been previously described (8). NSG mice were maintained through sib matings. NOD.Cg-Prkdcscid H2-K1tm1Bpe H2-Ab1em1Mvw H2-D1tm1Bpe Il2rgtm1Wjl/SzJ [NSG-(Kb Db)null (IAnull)] mice were developed using transcription activator–like effector nucleases (TALENs). Exon 2 of the H2-Ab1 gene was targeted in NOD.Cg-Prkdcscid H2-K1tm1Bpe H2-D1tm1Bpe Il2rgtm1Wjl/SzJ [NSG-(Kb Db)null] (27) embryos. The cytoplasmic microinjection was performed in homozygous NSG-(Kb Db)null fertilized oocytes, delivering 50 ng/μl of each TALEN mRNA (left TALEN targeting: TGGGGCGGCCAGACGCCG; right TALEN targeting: TCGCTCCAGGATCTCCGG), prepared in RNase-Free TE supplemented with RNasin (Promega, Madison, WI, USA) at a final concentration of 0.2 U/μl. To screen for potential candidates, PCR was performed using the following primers: (forward, 5′-TTCGTGTACCAGTTCATGGGCG-3′; reverse, 5′-GATGCCTAACCGACCACTTT-3′), which produces an 853 bp wild-type amplicon. The mutant allele that was fixed in this line yields a 292 bp product using these primers. Sanger sequencing was used to characterize this allele as an INDEL (net +7/−568), though it has 2 distinct deletions (61 bp, 507 bp) separated by a 39 bp “island” of intact sequence as well as a 7 bp insertion (5′-CCGTCAC-3′). The mutant PCR product is shown below; bracketed bases indicate the deletion sites and the insertion is underlined: 5′-TTCGTGTACCAGTTCATGGGCGAGTGCTACTTCACCAACGGGACGCAGCGCATACGATATGTGACCAGATACATCTACAACCGGGAGGAGTACGTGCGCTACGACAGCGACGTGGGCGAGCACCGCGCGGTGACCGAGCTGGGGCGGCCAGACGCCGAGTA[CA]CAACTACGAGGGGCCGGAGACCCACACCTCCCTGCGG[CCCGTCACA]ACTCATTTCCGTTTCCAGCACACTCCCTGATACCCCCAGAGCCTCTCACCCGTGATGCCAATTAAAGTGGTCGGTTAGGCATC-3′. The offspring carrying the null IAb allele (H2-Ab1em1Mvw) were identified by PCR and the null IAb allele was fixed to homozygosity. NSG-(Kb Db)null (IAnull) mice are maintained through homozygous sib mating. MHC class II molecules are heterodimers comprised of both α and β chains (28). Mice on the NOD background (H2g7) express both the IA α and β chains and express a functional IAg7 protein. H2g7 mice also express an IE β chain but have a deletion mutation within the IE α chain and therefore do not express a functional IE protein (29). Hence disruption of the IA-β chain eliminates all expression of MHC-class II in NSG mice.
NOD.Cg–β-2-microglobulin (B2m)tm1Unc Prkdcscid H2dlAb1-Ea Il2rgtm1Wjl/SzJ [NSG-B2Mnull (IA IE)null] were made by intercrossing NOD.Cg-B2mtm1Unc Prkdcscid Il2rgtm1Wjl/SzJ (NSG-B2Mnull) mice (23) with NOD.Cg-PrkdcscidH2dlAb1-Ea1Il2rg tm1Wjl/SzJ [NSG-(IA IE)null] (28) and intercrossing the F1 progeny followed by selecting the NSG mice doubly homozygous for the B2mtm1Unc and H2dlAb1-Ea alleles. The NSG-B2Mnull (IA IE)null mice were maintained through sib mating. MHC class I is a heterodimer comprised of a heavy chain and a B2M chain which are noncovalently linked, and both are required for cell surface expression of the class I complex. Mutations that disrupt expression of B2M abrogate the cell surface expression of MHC class I (30).
To create the NOD.Cg-Prkdcscid H2-K1tm1Bpe H2-Ab1em1Mvw H2-D1tm1Bpe Il2rgtm1Wjl Tg(Ins2-HBEGF)6832Ugfm/Sz transgene [NSG–rat insulin promoter (RIP)–diphtheria toxin receptor (DTR) (Kb Db)null (IAnull) strain], we backcrossed the Tg(Ins2-HBEGF)6832Ugfm, RIP-DTR transgene, onto the NSG strain (31, 32), and then crossed the NSG-DTR strain with the NSG-(Kb Db)null (IAnull) strain to create the NSG-RIP-DTR (Kb Db)null (IAnull) strain. These mice are maintained by sib mating of mice homozygous for the disrupted alleles and for the transgene.
All animals were housed in a specific pathogen–free facility in microisolator cages and given autoclaved food and acidified autoclaved water at the Jackson Laboratory or alternated weekly between acidified autoclaved water and sulfamethoxazole-trimethoprim–medicated water (Goldline Laboratories, Fort Lauderdale, FL, USA) at the University of Massachusetts Medical School. All animal procedures were done in accordance with the guidelines of the Animal Care and Use Committee of the Jackson Laboratory and the University of Massachusetts Medical School and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). Supplemental Figure S1 and Supplemental Table S1 provide a direct comparison of the relevant strains used for experiments (28, 29, 33–36).
Abs and flow cytometry
The phenotypes of murine cells in the NSG MHC knockout mice were determined as described (8). Anti-murine mAbs were purchased as FITC, phycoerythrin, allophycocyanin, or peridinin chlorophyll protein conjugates to accommodate 4-color flow cytometric analysis. Immune-competent NOD/ShiLtJ (NOD) and C57BL/6 (B6) mice (data not shown) were run with each experiment to ensure correct MHC staining. The B6 mice were included to control for carryover of the linked MHC II gene region adjacent to the classically knocked-out Ea genes, which was made in 129 embryonic stem cells and backcrossed to NSG to make NSG-B2Mnull (IA IE)null mice. Spleens were snipped into small pieces in 1 ml of 200 U/ml collagenase D in DMEM without serum on ice. Two additional milliliters of collagenase D solution were added and the splenocytes were vortexed. Cells were incubated in a 37°C water bath for 30 min with occasional vortexing and mixing. The cells were washed and suspended in Gey’s RBC lysing buffer (8.3 g/L NH4Cl, 1 g/liter KHCO3, pH 7.2; all reagents from MilliporeSigma, Burlington, MA, USA), mixed and incubated 1 min on ice. Cells were then washed with flow cytometry (FACS) buffer and stained for 30 min at 4°C, washed twice with FACS buffer, suspended in 250 μl of FACS buffer and stained with propidium iodide, and 100,000 events analyzed on a BD Biosciences LSR II Flow Cytometer (San Jose, CA, USA). Anti-mouse Abs used were anti-H2Kb (clone AF6-885), H2Kd (SF1-1.1), CD11b (M1/70), CD11c (N418), I-Ab,d IEk,d (M5/114), Ly6G (1A8), Ly6c (HK1.4), and I-Ag7 (10-2.16).
Human immune cell populations were monitored in PBMC-engrafted mice using mAbs specific to the following human antigens: CD45 (clone HI30), CD3 (clone UCHT1), CD4 (clone RPA-T4), CD8 (clone RPA-T8), CD20 (clone 2H7) CD45RA (clone HI100), CCR7 (clone G043H7), PD-1 (clone EH12.2H7), and granzyme B (clone GB11), purchased from either eBioscience (San Diego, CA, USA), BD Biosciences, or BioLegend (San Diego, CA, USA). Murine cells were identified and excluded from analysis by staining with a mAb specific to murine CD45 (clone 30-F11; BD Biosciences).
Single-cell splenic suspensions were prepared from engrafted mice, and whole blood was collected in heparin. Single cell suspensions of 1 × 106 cells or 100 μl of whole blood were washed with FACS buffer (PBS supplemented with 2% fetal bovine serum and 0.02% sodium azide) and then preincubated with rat anti-mouse FcR11b mAb (clone 2.4G2; BD Biosciences) to block binding to murine FcRs. Specific mAbs were then added to the samples and incubated for 30 min at 4°C. Stained samples were washed and fixed with 2% paraformaldehyde for cell suspensions or treated with BD FACS lysing solution for whole blood. At least 50,000 events were acquired on LSRII or FACSCalibur instruments (BD Biosciences). For human cell phenotyping, murine cells were identified and excluded from analysis by staining with a mAb specific to murine CD45 (clone 30-F11; BD Biosciences). Data analysis was performed with FlowJo (Treestar, Ashland, OR, USA) software.
Collection of human PBMCs
Human PBMCs were obtained from healthy volunteers under signed informed consent in accordance with the Declaration of Helsinki and approval from the Institutional Review Board of the University of Massachusetts Medical School. PBMCs were collected in heparin, purified by Ficoll-Hypaque density centrifugation, and suspended in Roswell Park Memorial Institute medium for injection into mice at the cell doses indicated. In some experiments, pheresis leukopaks were obtained as anonymous discarded units from the blood bank at the University of Massachusetts Medical Center.
GVHD protocol
Mice were injected intraperitoneally with various doses of PBMCs. Mice were weighed 2–3 times/wk and the appearance of GVHD-like symptoms, including weight loss (>20%), hunched posture, ruffled fur, reduced mobility, and tachypnea, was used to determine when mice would be euthanized. This is indicated as time of survival.
Human islet transplantation
The procurement and use of human islets were performed under protocols approved by the institutional review board of the University of Massachusetts Medical School. Human islets designated for research were obtained from Prodo Laboratories (Aliso Viejo, CA, USA). Then, 4000 human islet equivalents (IEQs) were transplanted into the spleens of NSG-RIP-DTR (Kb Db)null (IAnull) mice. NSG-RIP-DTR (Kb Db)null (IAnull) mice were treated with 40 ng diphtheria toxin 2–4 d prior to islet transplantation. Hyperglycemia (>400 mg/dl) was confirmed using an Accu-Chek Active glucometer (Roche, Basel, Switzerland). Blood glucose levels were checked at twice-weekly intervals following transplantation to monitor islet graft function. C-peptide levels were detected in plasma using an ELISA kit specific to human C-peptide (Alpco, Salem, NH, USA). Total insulin content within transplanted spleens was determined as previously described (37) using an ELISA kit specific to human insulin (Alpco).
Double-stranded adeno-associated virus vectors
The double-stranded (ds) adeno-associated virus (AAV) vectors were engineered and packaged as previously described (38). Briefly, full-length cDNA encoding human IL-2 or EGFP was subcloned into a dsAAV plasmid (39) containing the murine preproinsulin II promoter. dsAAV vector packaging with serotype 8 capsid protein was carried out as previously described (40, 41) or produced by the Viral Vector Core at the University of Massachusetts Medical School Horae Gene Therapy Center (Worcester, MA, USA). Recipient mice were intraperitoneally injected with 2.5 × 1011 particles of the purified AAV8-huIL-2 (AAV-IL-2).
Statistical analyses
To compare individual pairwise groupings, we used 1-way ANOVA or 2-way ANOVA with Bonferroni posttests and Kruskal–Wallis test with Dunn’s posttest for parametric and nonparametric data, respectively. Significant differences were assumed for values of P < 0.05. Statistical analyses were performed using GraphPad Prism software v.4.0c (La Jolla, CA, USA).
RESULTS
Phenotypic characterization of NSG mice and 2 strains of NSG MHC class I/II double-knockout mice
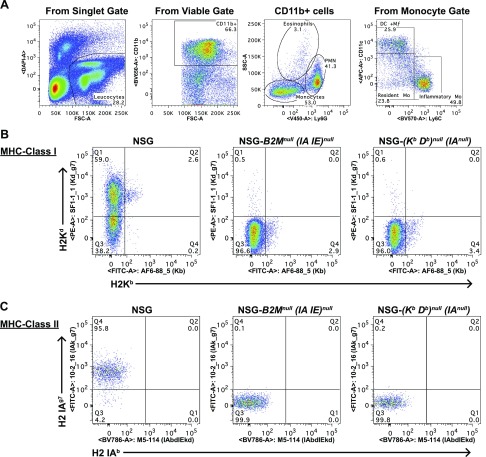
We created 2 NSG mouse strains that are doubly deficient in MHC class I and II, the NSG-(Kb Db)null (IAnull) and NSG-B2Mnull (IA IE)null knockout strains. The absence of MHC class I and II in both strains was confirmed by flow cytometry (Fig. 1). To compensate for the lack of immune cells expressing readily detectable levels of mouse MHC II, we enzymatically disaggregated spleens and gated on CD11c+ cells to analyze the dendritic cell population. Figure 1A demonstrates the gating strategy of excluding doublets and dead cells and proceeds to gate on monocyte-derived dendritic cells (CD11b+ Ly6cdim CD11c+). The NSG mouse demonstrates the expected staining pattern of H2Kd positive, H2Kb negative for MHC class I (Fig. 1B), and I-Ag7 positive, I-Ab negative for MHC class II (Fig. 1C). Both the NSG-(Kb Db)null (IAnull) and the NSG-B2Mnull (IA IE)null knockout mice strains lack MHC class I and II molecules normally expressed by NOD and C57BL/6 mice.
Figure 1.
Representative flow cytometry of MHC class I and II expression in NSG, NSG-(Kb Db)null (IAnull), and NSG-B2Mnull (IA IE)null mice. Splenic monocyte–derived dendritic cells from NSG, NSG-(Kb Db)null (IAnull), and NSG-B2Mnull (IA IE)null knockout mice were analyzed by flow cytometry. A) Monocyte-derived dendritic cells were identified in viable cells as CD11b+, Ly6Gdim, CD11c+, and Ly6C−. B, C) Monocyte derived dendritic cells recovered from each strain were evaluated for expression of murine H2Kd and H2Kb (B), and murine H2 IAg7 and H2 IAb (C). Representative staining is shown for all stains (n = 2).
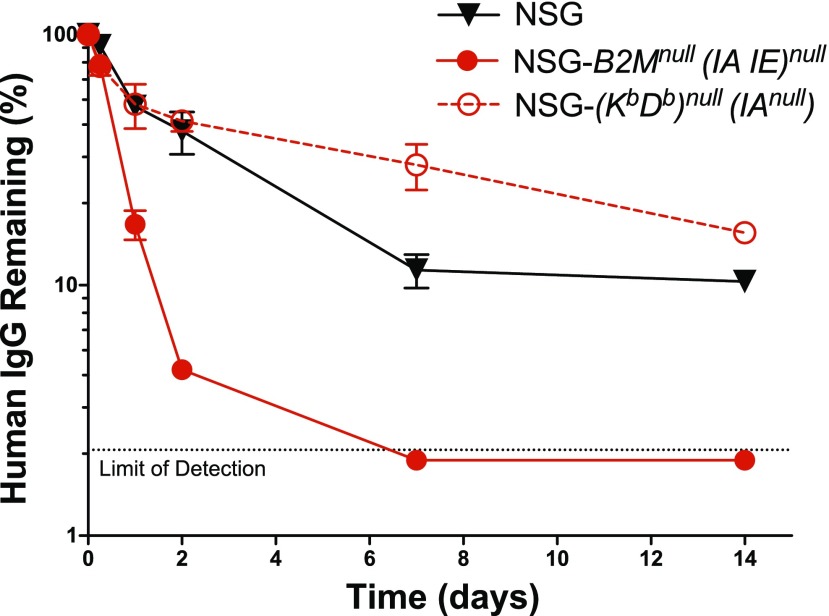
Due to the requirement of B2M for appropriate expression of murine neonatal FcR (42), the receptor responsible for prolonging the half-life of IgG in the circulation (43), we compared the clearance of human IgG in both stocks of mice. Mice received an injection (200 μg, i.v.) of human IgG and were bled at intervals for ELISA analysis of circulating human IgG. The first bleed at 2 min postinjection was considered 100% serum IgG. We observed rapid clearance of human IgG in NSG-B2Mnull (IA IE)null mice whereas IgG clearance in NSG-(Kb Db)null (IAnull) mice was much slower and similar to that observed in NSG mice (Fig. 2).
Figure 2.
Human IgG half-life in the serum of NSG, NSG-(Kb Db)null (IAnull), and NSG-B2Mnull (IA IE)null mice. Mice received an injection (200 μg, i.v.) of human IgG and were bled at the indicated time points to recover serum. Serum was used for ELISA analysis of circulating human IgG. The first bleed at 2 min postinjection was considered to be 100% serum IgG. Each point represents the mean ± se of IgG in 5 males, 2–3 mo of age.
Survival of PBMC-engrafted NSG mice and NSG-MHC class I knockout, NSG-MHC class II knockout, and NSG-MHC I/II knockout mice
NSG-(Kb Db)null (IAnull)
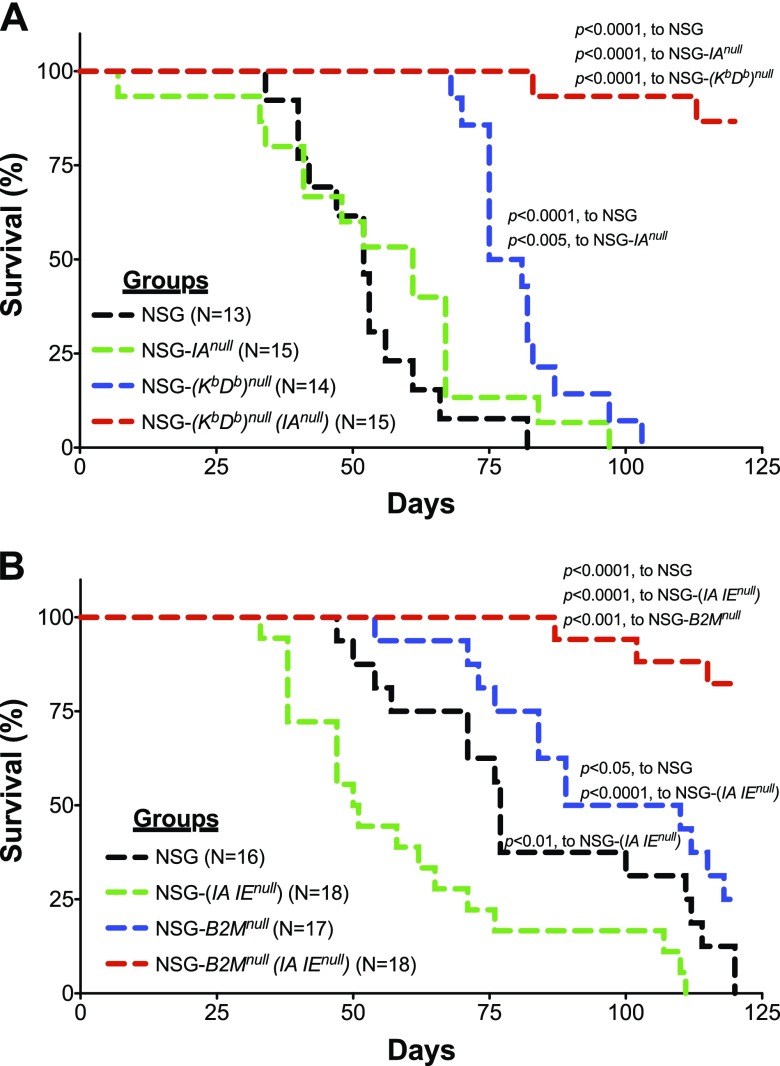
We first determined whether the absence of mouse MHC class I and II altered the incidence and kinetics of xenogeneic GVHD following human PBMC engraftment into NSG MHC I/II knockout mice. NSG strains deficient in MHC class I, MHC class II, or the 2 NSG double-knockout strains were engrafted with 10 × 106 PBMCs, and their survival was compared with that of the NSG mice. As previously reported (23), both NSG and NSG-(IAnull) mice showed relatively short survival times, similar to those observed in NSG mice (23). By contrast, as expected, NSG-(Kb Db)null mice had a longer period of survival than did the NSG mice (23). When both MHC class I and II were knocked out in NSG-(Kb Db)null (IAnull) mice, however, survival was >100 d, with 13/15 MHC I/II knockout mice exhibiting no symptoms of GVHD for up to 125 d (Fig. 3A).
Figure 3.
Survival of NSG mice lacking the expression of mouse MHC class I and II following injection of PBMCs. Recipient mice were intravenously injected with 10 × 106 PBMCs and were monitored for overall health and survival. A) NSG, NSG-(IAnull), NSG-(Kb Db)null, and NSG-(Kb Db)null (IAnull) mice received PBMCs. The data are representative of 3 independent experiments. B) NSG, NSG-(IA IE)null, NSG-B2Mnull, and NSG- B2Mnull (IA IE)null mice received PBMCs. The data are representative of 3 independent experiments. Survival distribution between groups was tested using the logrank test.
NSG-B2Mnull (IA IE)null
Similar extended survival results were observed in PBMC-engrafted NSG-B2Mnull (IA IE)null mice. For this MHC I/II knockout strain, we used the NSG-B2Mnull strain as the control rather than the NSG-(Kb Db)null strain. Again, as expected (23), NSG and NSG-(IAnull) knockout mice engrafted with human PBMCs demonstrated relatively short survival times. Survival of NSG-B2Mnull mice was significantly higher. As observed in NSG-(Kb Db)null (IAnull) mice, long-term survival of PBMC-engrafted NSG-B2Mnull (IA IE)null mice was achieved, with 15/18 surviving to the termination of the experiment (125 d) with no symptoms of GVHD (Fig. 3B).
Human cell chimerism in PBMC-engrafted NSG mice and NSG-MHC class I knockout, NSG-MHC class II knockout, and NSG-MHC I/II knockout mice
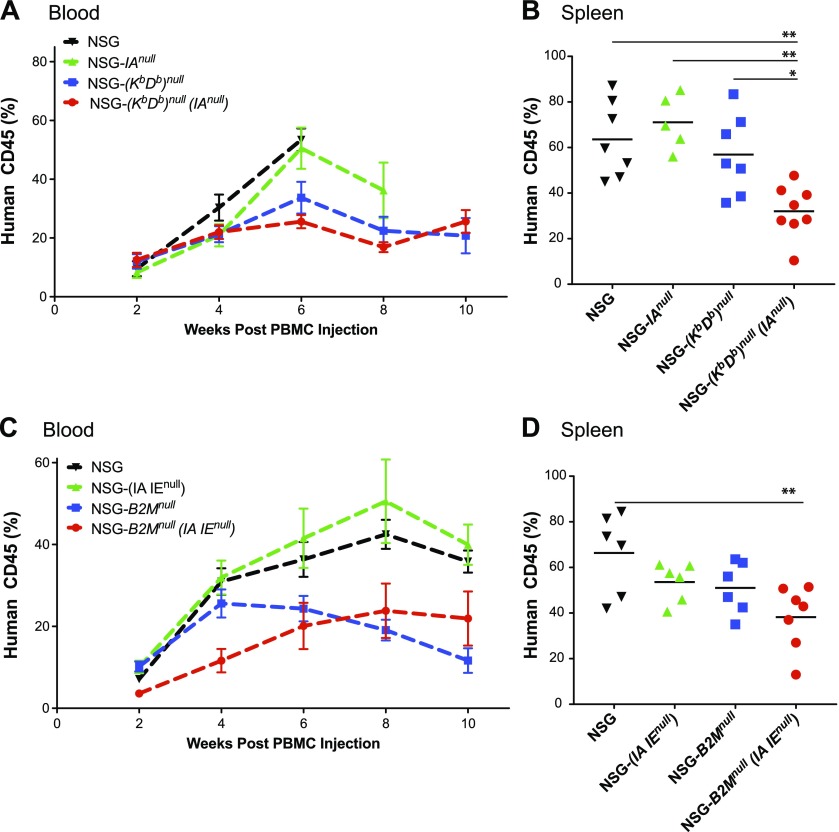
The long-term survival of PBMC-engrafted NSG MHC I/II knockout mice could be the result of either a lack of human cell engraftment or a lack of GVHD due to the absence of MHC class I and II. To distinguish between these 2 possibilities, we injected 10 × 106 PBMC IP into both NSG MHC I/II knockout strains and compared the levels of CD45+ cells in the circulation over time with NSG, NSG class I knockout, and MHC class II knockout mice.
NSG-(Kb Db)null (IAnull) mice
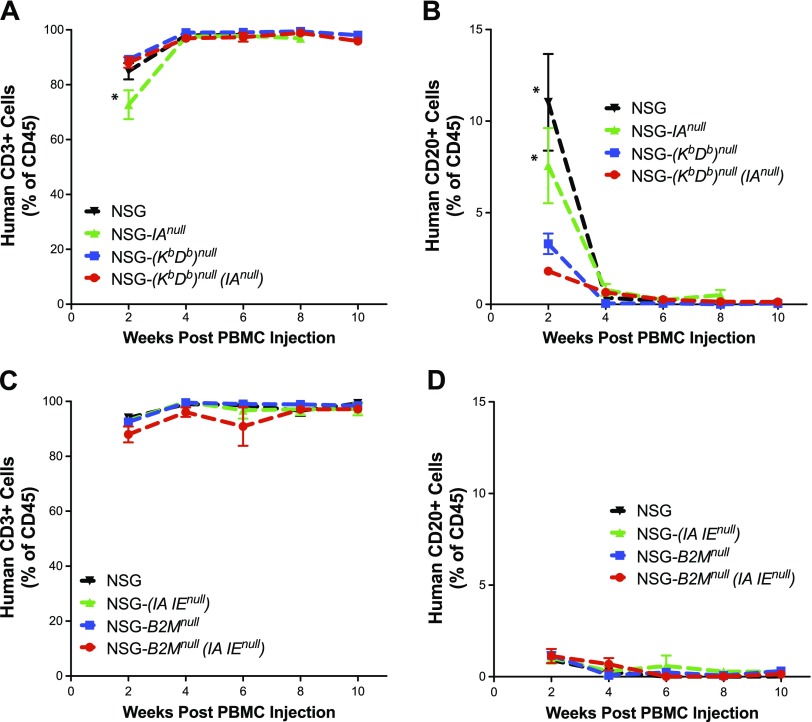
As expected (23), we observed that human CD45 cell engraftment increased rapidly in NSG mice and NSG-(IAnull) mice (Fig. 4A). The percentages of circulating human CD45+ cells over time were lower in NSG-(Kb Db)null and NSG-(Kb Db)null (IAnull) mice than in NSG and NSG-(IAnull) mice. In the spleen, the percentages of human CD45+ cells in NSG-(IAnull) and NSG-(Kb Db)null mice were comparable to that observed in NSG mice, but the percentages of human CD45+ cells in the spleens of NSG-(Kb Db)null (IAnull) mice were significantly lower than in the other 3 strains (Fig. 4B).
Figure 4.
Human CD45+ cell chimerism levels in PBMC-engrafted NSG mice lacking the expression of both mouse MHC class I and II. Recipient mice were intravenously injected with 10 × 106 PBMCs and were assessed for levels of human cell chimerism by determining the proportion of human CD45+ cells in the peripheral blood (A, C) and spleens (B, D). A) Human cell chimerism levels were monitored in the blood of NSG, NSG-(IAnull), NSG-(Kb Db)null, and NSG-(Kb Db)null (IAnull) mice injected with PBMCs over a 10-wk period. The data are representative of 3 independent experiments. A 2-way ANOVA was used to determine significant differences between groups at each time point. Week 6: NSG vs. NSG-(Kb Db)null, P < 0.01; NSG vs. NSG-(Kb Db)null (IAnull), P < 0.001; NSG-(IAnull) vs. NSG-(Kb Db)null, P < 0.01; and NSG -(IAnull) vs. NSG-(Kb Db)null (IAnull), P < 0.001. B) Human cell chimerism levels were monitored in the spleens of NSG, NSG-(IAnull), NSG-(Kb Db)null, and NSG-(Kb Db)null (IAnull) mice injected with PBMCs when mice were euthanized after developing GVHD or at 10 wk post–PBMC injection. A 1-way ANOVA was used to determine significant differences between groups. *P < 0.05, **P < 0.01. C) Human cell chimerism levels were monitored in the blood of NSG, NSG-(IA IE)null, NSG-B2Mnull, and NSG-B2Mnull (IA IE)null mice injected with PBMCs over a 10-wk period. The data are representative of 3 independent experiments. A 2-way ANOVA was used to determine significant differences between groups at each time point. Week 4: NSG vs. NSG-B2Mnull (IA IE)null; P < 0.01; NSG-(IA IE)null vs. NSG-B2Mnull (IA IE)null, P < 0.01; and NSG-B2Mnull vs. NSG-B2Mnull (IA IE)null, P < 0.05. Week 6: NSG vs. NSG-B2Mnull (IA IE)null, P < 0.05; NSG-(IA IE)null vs. NSG-B2Mnull, P < 0.05; and NSG-(IA IE)null vs. NSG-B2Mnull (IA IE)null, P < 0.01. Week 8: NSG vs. NSG-B2Mnull, P < 0.001; NSG vs. NSG-B2Mnull (IA IE)null, P < 0.01; NSG-(IA IE)null vs. NSG-B2Mnull, P < 0.001; and NSG-(IA IE)null vs. NSG-B2Mnull (IA IE)null, P < 0.01. Week 10: NSG vs. NSG-B2Mnull, P < 0.01; NSG vs. NSG-B2Mnull (IA IE)null, P < 0.01; NSG-(IA IE)null vs. NSG-B2Mnull, P < 0.01; and NSG-(IA IE)null vs. NSG-B2Mnull (IA IE)null, P < 0.01. D) Human cell chimerism levels were monitored in the spleens of NSG, NSG-(IA IE)null, NSG-B2Mnull, and NSG-B2Mnull (IA IE)null mice injected with PBMCs when mice were euthanized after developing GVHD or at 10 wk post–PBMC injection. A 1-way ANOVA was used to determine significant differences between groups. **P < 0.01.
NSG-B2Mnull (IA IE)null mice
In these experiments, we used the NSG-B2Mnull strain as the NSG MHC class I knockout control. As observed in the NSG, NSG-(IAnull) mice, NSG-(Kb Db)null and NSG-(Kb Db)null (IAnull) mice, the percentages of circulating human CD45+ cells were higher in the NSG and NSG-(IAnull) mice than in NSG-B2Mnull and NSG-B2Mnull (IA IE)null mice (Fig. 4C). The percentages of human CD45+ cells in the spleen of NSG-B2Mnull (IA IE)null mice were significantly decreased (Fig. 4D).
Engraftment of human T and B cells in PBMC-engrafted NSG, NSG-MHC class I knockout, NSG-MHC class II knockout, and NSG-MHC I/II knockout mice
NSG-(Kb Db)null (IAnull)
As expected (23), we observed that circulating human CD45+ cells were predominately CD3+ T cells in NSG, NSG-(IAnull), and NSG-(Kb Db)null mice (Fig. 5A). Similarly, the majority of CD45+ cells in NSG-(Kb Db)null (IAnull) mice were also CD3+ T cells. In the NSG and NSG-(IAnull) mice, there were readily detectable numbers of CD20+ B cells 2 wk post-engraftment, but these were essentially undetectable 4 wk post-engraftment (Fig. 5B).
Figure 5.
Engraftment of human T and B cells in PBMC-engrafted NSG mice lacking the expression of both mouse MHC class I and II. Recipient mice were injected intravenously with 10 × 106 PBMCs, and mice were monitored for levels of human CD3+ T (A, C) and CD20+ B (B, D) cells in peripheral blood. A, B) NSG (N = 7), NSG-(IAnull) (n = 5), NSG-(Kb Db)null (n = 7), and NSG-(Kb Db)null (IAnull) (n = 8) mice received PBMCs. C, D) NSG (6), NSG-(IA IE)null (n = 6), NSG-B2Mnull (n = 5), and NSG-B2Mnull (IA IE)null (n = 7) mice received PBMCs. The data are representative of 3 independent experiments. A 2-way ANOVA was used to determine significant differences between groups at each time point. *P < 0.05.
NSG-B2Mnull (IA IE)null
For this comparison we again used NSG-B2Mnull mice as MHC class I knockout control. PBMC engraftment in NSG-B2Mnull (IA IE)null mice consisted of predominately CD3+ T cells, similar to NSG, NSG-(IAnull), and NSG-B2Mnull mice (Fig. 5C). Although human CD20+ B cells were readily apparent in the NSG and NSG-IAnull mice at 2 wk in the first experiments (Fig. 5B), levels were significantly lower in all 4 strains examined in Fig. 5D, likely reflecting variability in donor PBMCs. The variability between PBMC donors is mostly likely attributed to differences in T- and B-cell levels, and activation status of the cells (unpublished results).
Phenotypic analysis of human T cells engrafted in NSG, NSG-(IAnull), NSG-(Kb Db)null, and NSG-(Kb Db)null (IAnull) mice injected with PBMCs
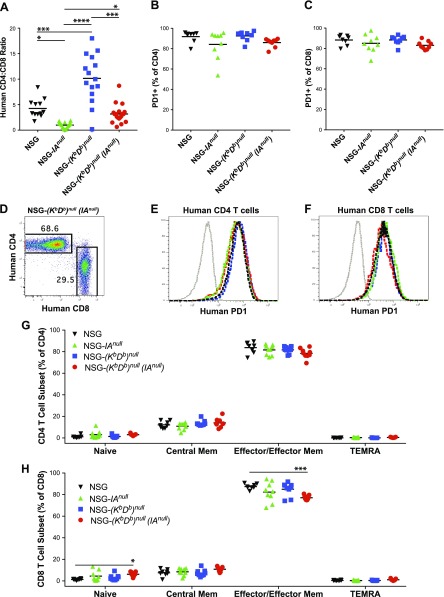
The CD4:CD8 ratio in NSG mice at 4 wk post PBMC-engraftment, as expected (44), was ∼4:1 (Fig. 6A). By contrast, very few CD4+ T cells were detected in NSG-(IAnull) mice, whereas relatively high levels of CD4+ T cells engrafted in NSG-(Kb Db)null mice, resulting in very low and very high CD4:CD8 ratios, respectively. The CD4:CD8 ratio of CD3+ T cells in NSG-(Kb Db)null (IAnull) mice was similar to that observed in NSG mice (Fig. 6A), suggesting that neither human T-cell subset had a selective advantage for engraftment in mice lacking both MHC class I and MHC class II. The majority of CD4+ and CD8+ T cells in all 4 strains expressed the activation marker PD-1 (Fig. 6B, C). A representative histogram of CD4+CD3+ and CD8+CD3+ T cells (Fig. 6D) and of PD-1 staining of CD4+ and CD8+ cells is shown (Fig. 6E, F). To determine the differentiation state of the CD4+ and CD8+ T cells, we stained each subset for CD45RA and CCR7. CD45RA+CCR7+ cells represent naive T cells, CD45RA−CCR7+ cells represent central memory T cells, CD45RA−CCR7− cells represent T-effector/effector memory T cells, and CD45RA+CCR7− cells represent terminally differentiated effector memory CD45RA+ T (TEMRA) cells (45, 46). In both the CD4+ (Fig. 6G) and CD8+ T cell populations (Fig. 6H), very few naive T cells were observed in blood at 4 wk post-PBMC injection. A few central memory CD4+ and CD8+ T cells were detected, whereas almost no TEMRA CD4+ or CD8+ T cells were present. The majority of CD4+ and CD8+ T cells were effector/effector memory CD45RA−CCR7− T cells (Fig. 6G, H).
Figure 6.
Phenotypic analysis of human T cells engrafting in PBMC-injected NSG, NSG-(IAnull), NSG-(Kb Db)null, and NSG-(Kb Db)null (IAnull) mice. Recipient mice were intravenously injected with 10 × 106 PBMCs, and at 4 wk postinjection mice were monitored for levels of human CD3+/CD4+ and CD3+/CD8+ T cells (A, D) and T cell phenotype (B, C, E–H) in peripheral blood. A) Levels of CD4+ and CD8+ T cells were determined by flow cytometry and expressed as a ratio of CD4+ to CD8+ T cells. B, C) PD-1 expression by CD4+ and CD8+ T cells was determined by flow cytometry. D–F) Representative CD4, CD8, and PD-1 staining is shown. G, H) CD4+ and CD8+ T cells were evaluated for expression of CD45RA and CCR7 by flow cytometry. Percentages of T-cell subsets are shown with naive CD45RA+/CCR7+ cells, central memory CD45RA−/CCR7+ cells, effector/effector memory CD45RA−/CCR7− cells, and TEMRA CD45RA+/CCR7− cells. The data are representative of 2 independent experiments. A 1-way ANOVA was used to determine significant differences between groups. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Phenotypic analysis of human T cells engrafting in NSG, NSG-(IA IE)null, NSG-B2Mnull, and NSG-B2Mnull (IA IE)null mice injected with PBMCs
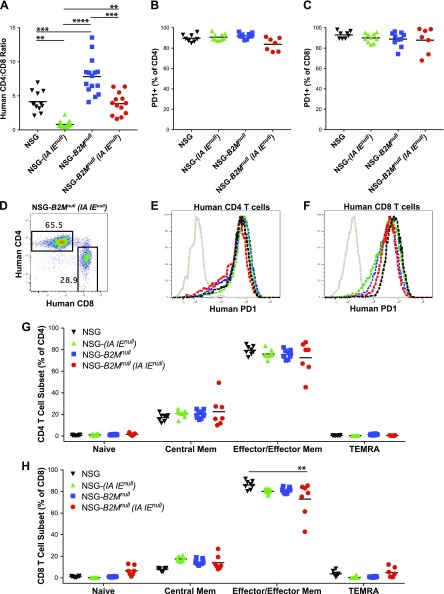
The CD4:CD8 T-cell ratios in NSG mice were again ∼4:1 (Fig. 7A). MHC class II (IA IE)null knockout and class I B2Mnull knockout mice similarly had CD4:CD8 low and high T-cell ratios, respectively, as observed in the NSG-(IAnull) and NSG-(Kb Db)null mice (Fig. 6A). NSG-B2Mnull (IA IE)null mice (Fig. 7A) showed an ∼4:1 CD4:CD8 ratio that is similar to that observed in NSG and in NSG-(Kb Db)null (IAnull) mice (Fig. 6A). The majority of CD4 (Fig. 7B) and CD8 (Fig. 7C) cells in all 4 MHC knockout strains expressed the activation marker PD-1. Representative histograms of CD4 and CD8 staining (Fig. 7D) and of CD4 (Fig. 7E) and CD8 (Fig. 7F) staining with anti-PD-1 are shown. In all 4 strains, although there were few CD4 (Fig. 7G) or CD8 (Fig. 7H) naive or TEMRA cells observed, some central memory cells were present. The majority of T cells exhibited the CD45RA−CCR7+ effector/effector memory phenotype (Fig. 7G, H).
Figure 7.
Phenotypic analysis of human T cells engrafting in PBMC-injected NSG, NSG-(IA IE)null, NSG-B2Mnull, and NSG-B2Mnull (IA IE)null mice. Recipient mice were intravenously injected with 10 × 106 PBMCs, and at 4 wk postinjection mice were monitored for levels of human CD3+/CD4+ and CD3+/CD8+ T cells (A, D) and T-cell phenotype (B, C, E–H) in peripheral blood. A) Levels of CD4+ and CD8+ T cell were determined by flow cytometry and expressed as a ratio of CD4+ to CD8+ T cells. B, C) PD-1 expression by CD4+ and CD8+ T cells was determined by flow cytometry. D–F) Representative CD4, CD8, and PD-1 staining is shown. G, H) CD4+ and CD8+ T cells were evaluated for expression of CD45RA and CCR7 by flow cytometry. Percentages of T-cell subsets are shown with naive CD45RA+/CCR7+ cells, central memory CD45RA−/CCR7+ cells, effector/effector memory CD45RA−/CCR7− cells, and TEMRA CD45RA+/CCR7− cells. The data are representative of 2 independent experiments. A 1-way ANOVA was used to determine significant differences between groups. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Engrafted human T cells in NSG-(Kb Db)null (IAnull) mice are functional
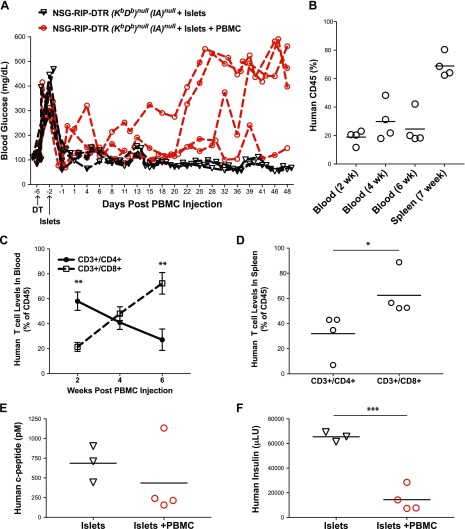
We have previously reported that injection of human PBMCs into NSG mice engrafted with human allogeneic islets leads to islet allograft rejection (47). To determine if the human immune cells engrafted in NSG MHC I/II knockout mice were functional, we created a new mouse strain, NSG-RIP-DTR (Kb Db)null (IAnull). Injection of diphtheria toxin (DT) into mice expressing the DTR under the control of the RIP led to murine β cell death and hyperglycemia (48). Injection of NSG-RIP-DTR (Kb Db)null (IAnull) mice with DT led to the rapid development of diabetes (Fig. 8A). Intrasplenic transplantation of 4000 human IEQs restored normoglycemia in the mice within 1–2 d. These mice were then divided into 2 groups. To confirm the function of the human islets in the absence of an allogeneic immune system, one islet-transplanted group was intraperitoneally injected with 50 × 106 allogeneic PBMCs whereas the other group received no PBMCs. Control mice that received only human islets remained normoglycemic throughout the experimental period (n = 3). By contrast, 3 of the 4 mice that received allogeneic human PBMCs reverted to hyperglycemia after 3–4 wk (Fig. 8A).
Figure 8.
Rejection of human islet allografts in PBMC-engrafted NSG-RIP-DTR (Kb Db)null (IAnull) mice. Recipient NSG-RIP-DTR (Kb Db)null (IAnull) mice were generated as described in Materials and Methods. A) NSG-RIP-DTR (Kb Db)null (IAnull) mice were treated with 40 ng of DT 6 d before PBMC injection, and then implanted with human islets (4000 IEQs) by intrasplenic injection. On d 0, one group of mice was intraperitoneally injected with 50 × 106 human PBMCs, and one group was left untreated. Blood glucose levels were monitored; mice with blood glucose levels over 300 mg/dl for 2 consecutive tests were considered diabetic. B) Mice were monitored for levels of human cell chimerism by determining the proportion of CD45+ cells in the peripheral blood over 6 wk and in spleen at 7 wk. C, D) Levels of CD3+/CD4+ and CD3+/CD8+ T cells were evaluated in peripheral blood and spleen. E) Levels of circulating human C-peptide in plasma was determined by ELISA at wk 6. F) Total insulin content from spleens of islet-engrafted mice was determined at wk 7 by ELISA. The data are representative of 2 independent experiments. Student’s t test was used to determine significant differences between groups. *P < 0.05, **P < 0.01, ***P < 0.005.
The engraftment levels of human CD45+ cells in PBMC-injected, islet-transplanted mice trended toward higher percentages in the blood over time, and up to ∼70% human CD45+ cells were detected in the spleen at 7 wk post–PBMC injection. This level of human CD45+ cell engraftment in NSG-RIP-DTR (Kb Db)null (IAnull) mice was higher than in PBMC-engrafted NSG-(Kb Db)null (IAnull) mice (Fig. 8B) and was consistent with the number of human PBMCs (50 × 106) that were injected into the NSG-RIP-DTR (Kb Db)null (IAnull) mice, a 5-fold increase over the 10 × 106 cells injected into NSG-(Kb Db)null (IAnull) mice. The CD4:CD8 T-cell ratio changed significantly in the blood over the course of the experiment as the percentage of CD4+ T cells decreased (Fig. 8C). At the termination of the experiment, the ratios of CD4:CD8 T cells in the spleen also showed a significant increase of CD8+ T cells (Fig. 8D). The levels of human C-peptide in the blood at 6 wk was decreased in 3 of the 4 islet-engrafted mice that received human PBMCs; the 1 mouse that did not revert to hyperglycemia had levels of C-peptide similar to that observed in islet recipients that were not injected with allogeneic PBMCs (Fig. 8E). In all 4 mice that were given allogeneic PBMCs, however, the level of human insulin observed in the islet grafts was significantly lower than that found in control islet transplant recipients (Fig. 8F).
Modulation of engrafted human T cells by treatment with dsAAV-IL-2 in NSG and NSG-(KbDb)null (IAnull) mice transplanted with PBMCs
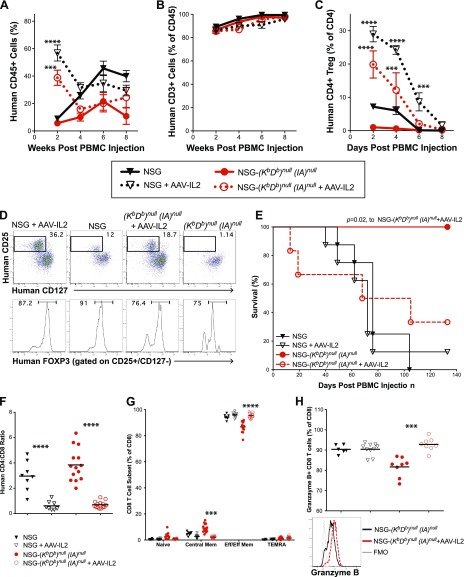
Having shown that the engrafted human T cells in the NSG-(Kb Db)null (IAnull) mice are functional (Fig. 8A) but do not mediate acute GVHD (Fig. 3), we next determined whether administration of human recombinant IL-2 could modulate the T cell populations. We have previously shown that administration of a dsAAV8 vector encoding human IL-2 (dsAAV8-huIL-2) increased human regulatory T (Treg) cells in NSG mice humanized by engraftment of human fetal liver and thymus [i.e., the BLT model (49)]. In the current study, injection of dsAAV8-huIL-2 led to a transient expansion of human CD45+ cells in the blood of NSG and NSG-(Kb Db)null (IAnull) mice engrafted with 10 × 106 PBMCs for 2 wk (Fig. 9A). dsAAV8-huIL-2 did not alter the proportion of human CD45+ cells that were CD3+ over 8 wk (Fig. 9B). However, there was a significant increase in the proportion of CD4+ T cells that expressed a Treg phenotype (CD4+CD25+CD127−FOXP3+) at 2, 4, and 6 wk in NSG mice, and at 2 and 4 wk in NSG-(Kb Db)null (IAnull) mice post–PBMC injection (Fig. 9C). Representative staining of CD4+ T cells with CD25 and CD127 Abs is shown in the top row and the expression of FOXP3 in CD4+CD25+CD127− T cells in NSG and NSG-(Kb Db)null (IAnull) mice with or without administration of dsAAV8-huIL-2 is shown in the bottom row (Fig. 9D). The relative percentage of Treg cells declined steadily from 2 to 8 wk in mice treated with AAV-IL-2 and were present at levels similar to untreated mice by 8 wk post PBMC engraftment. NSG and NSG-(Kb Db)null (IAnull) mice injected with dsAAV8-huIL-2 have detectable human IL-2 in blood as early as 2 wk postinjection (219 ± 48 and 262 ± 40 pg/ml, respectively).
Figure 9.
Expression of human IL-2 in PBMC-engrafted NSG mice and NSG-(Kb Db)null (IAnull) mice enhances survival of human CD4+ Treg. Recipient NSG and NSG-(Kb Db)null (IAnull) mice were intraperitoneally injected with 2.5 × 1011 particles of dsAAV8-huIL-2 or PBS. Two weeks later mice were intraperitoneally injected with 1 × 106 PBMCs. A–C) Levels of human CD45+ cells (A), CD3+ T cells (B) and CD4+/CD25+/CD127−/FOXP3+ Treg cells (C) were determined by flow cytometry. A 2-way ANOVA was used to determine significant differences between groups. ***P < 0.005, ****P < 0.001. D) Representative staining of CD4+ T cells for CD25, CD127 and FOXP3 is shown for all groups. E) Survival of recipient mice was monitored, and survival distribution between groups was determined using the logrank test. F, G) For the graphs shown, closed black triangles represent NSG mice, open black triangles represent NSG mice injected with AAV-IL-2, closed red circles represent NSG-(Kb Db)null (IAnull) mice and open red circles represent NSG-(Kb Db)null (IAnull) mice injected with dsAAV8-huIL-2. F) Levels of CD4+ and CD8+ T cells were determined by flow cytometry and expressed as a ratio of CD4+ to CD8+ T cells. G) CD8+ T cells were evaluated for expression of CD45RA and CCR7 by flow cytometry. Percentages of T-cell subsets are shown for naive CD45RA+/CCR7+ cells, central memory CD45RA−/CCR7+ cells, effector/effector memory CD45RA−/CCR7− cells, and TEMRA CD45RA+/CCR7− cells. H) Granzyme B expression by CD8+ T cells was determined by flow cytometry and representative staining is shown. Student’s t test was used to determine significant differences between mice treated with AAV-IL-2 and controls. The data are representative of 3 independent experiments. ***P < 0.005, ****P < 0.001.
However, the administration of dsAAV8-huIL-2 shortened the survival of NSG-(Kb Db)null (IAnull) mice to that observed in NSG mice and NSG mice treated with dsAAV8-huIL-2 (Fig. 9E). The injection of dsAAV8-huIL-2 also altered the CD4:CD8 T-cell ratio to that of predominately CD8+ T cells in both NSG and NSG-(Kb Db)null (IAnull) mice when compared with controls (Fig. 9F). In addition, treatment with dsAAV8-huIL-2 increased the level of effector/effector memory CD8+ T cells and decreased the level of central memory CD8+ T cells as compared to untreated NSG-(Kb Db)null (IAnull) mice (Fig. 9G). No changes were observed in CD4+ T-cell subsets following treatment with dsAAV8-huIL-2 (data not shown). Correlated to the increase in percentages of CD8+ effector/effector memory T cells in dsAAV8-huIL-2 treated NSG-(Kb Db)null (IAnull) mice was an increase in the percentage of granzyme B–expressing CD8+ T cells (Fig. 9H).
DISCUSSION
Humanized mice have been widely used to model human immune cell function in vivo (1–6). In the Hu-PBL-SCID model, a major limitation of studying human T-cell function is the rapid development of fatal xenogeneic GVHD that not only shortens the experimental time window but also confounds the analysis of human T cell function due to the underlying ongoing acute GVHD that eventually kills the mice (15–25). In the present study, we have overcome this limitation by eliminating expression of murine MHC class I and II in NSG mice. Using two different NSG MHC class I/II knockout mouse models, we engrafted human PBMCs in mice lacking murine MHC, but these mice failed to develop acute GVHD-like disease for ≤125 d after PBMC engraftment. The engrafted human T cells remained functional, as demonstrated by their ability to reject human islet allografts. Moreover, the human T cells could be modulated in vivo, as seen after dsAAV8-huIL-2 injection. Administration of dsAAV8-huIL-2, however, resulted in the restoration of a wasting GVHD. Importantly, in the NSG-(Kb Db)null (IAnull) strain, human IgG clearance was comparable to that observed in NSG mice whereas IgG clearance in the NSG-B2Mnull (IA IE)null strain was extremely rapid.
We have previously shown that the primary xenogeneic targets of engrafted human PBM T cells in NSG mice are the murine MHC class I and II molecules (23). In that study, we knocked out expression of the B2M molecule, which is required for MHC class I expression (50), therby extending the survival time of PBMC-injected NSG mice. NOD and NSG mice do not express the IE molecule (34), and by knocking out the IAb gene, we eliminated the expression of MHC class II in NSG mice (23). The survival of NSG-MHC class II IAb knockout mice was only minimally extended over NSG mice, and in the present study, we failed to see a significant increase in survival of NSG (IA IE)null mice over that of NSG mice. In vitro analyses in our previous report documented that knocking out either MHC class I or II reduced the in vitro mixed-lymphocyte xenoreactivity of the human CD8+ and CD4+ T cells, respectively, suggesting that the majority of xenoreactivity was mainly directed against the murine MHC molecules. This was confirmed by the near absence of T-cell proliferation in response to antigen-resenting cells from NSG mice lacking both MHC class I and II expression (23).
NOG mice lacking both murine MHC class I (β2m) and II (IAb) have recently been described (25). The strategy to create the NOG-MHC I/II knockout was to cross the β2mnull allele from NOD-scid β2mnull mice (51) and the Iabnull allele from a MHC class II–deficient B6 mouse (52) onto the NOG background. NOG-MHC I/II knockout mice survived for ≤70 d following the injection of 1 × 107 human PBMCs. The engrafted T cells inhibited human tumor cell line growth following the injection of anti-PD-1, due to induction of cytotoxic T cell and NK cell killing (25). In our studies, we generated 2 strains of NSG-MHC class I/II–deficient mice: one based on NSG-β2mnull mice crossed with NSG (IA IE)null mice (23), and one by knocking out the Ia gene in NSG-(KbDb)null mice (27). β2m is required for FcRn expression (42), and controls the half-life of IgG in the circulation (43). Accordingly, we observed rapid clearance of human IgG in NSG-B2Mnull (IA IE)null knockout mice, that lack a functional FcRn, whereas IgG clearance in NSG-(Kb Db)null (IAnull) mice was prolonged and similar to that observed in NSG mice, as these strains express a functional FcRn. It would be expected that the NOG MHC I/II knockout mice that are based on a B2M deficiency for knocking out expression of MHC class I would also show rapid IgG clearance (25). This is important because many of the new biologic therapeutics entering the market are Ab-based drugs, and preclinical studies using these drugs will be best performed in NSG-(Kb Db)null (IAnull) mice rather than NSG-B2Mnull (IA IE)null mice.
Engraftment and function of human PBMCs in both NSG strains of MHC I/II knockout mice and the respective control single MHC knockout mice were assessed by injection of 10 × 106 PBMCs and by monitoring mice for engraftment, weight loss and survival. The survival kinetics of NSG, NSG MHC class I and II knockout mice were similar to what has been previously reported (23). By contrast, we observed that a majority of both strains of NSG MHC I/II knockout mice survived to the end of the observation period, ∼125 d. NOG MHC I/II knockout mice were observed for 70 d after PBMC engraftment for survival (25). Prolonged survival of NSG-B2Mnull and NSG-(Kb Db)null mice correlated with lower levels of engrafted human CD45+ cells relative to NSG mice and in NSG class II knockout mice, peaking at ∼20%.
The predominant CD45+ cell subset in engrafted NSG, NSG class I or II knockout, and NSG MHC I/II knockout mice was CD3+ T cells, with few other cell populations engrafting beyond 2 wk. Interestingly, in NSG mice deficient in MHC class II, the CD4:CD8 T-cell ratio was low, indicating that murine MHC class II was a major driver for human CD4+ T cell expansion. By contrast, in NSG mice deficient in MHC class I, the CD4:CD8 T-cell ratio was high, indicating the murine MHC class I was a major driver for human CD8 T cell expansion. The CD4:CD8 T-cell ratio in NSG MHC I/II knockout mice was ∼4:1, similar to that found in NSG mice, where there was no selective pressure for expansion of either T-cell subset. In all strains of NSG mice tested, effector/effector memory CD45RA−CCR7−CD3+ T cells were the predominant CD4+ and CD8+ T-cell population. This observation suggests that even though a wasting-like syndrome was not observed, engrafted CD4+ and CD8+ T cells nevertheless became activated.
We have previously reported that human PBMC-engrafted NSG mice reject human islet allografts (47). To test the ability of human PBMCs to reject islet allografts in hyperglycemic NSG MHC I/II knockout mice, we developed the NSG-RIP-DTR (Kb Db)null (IAnull) strain. This new model permits the complete, specific, and permanent ablation of murine pancreatic β cells, avoiding the broadly toxic effects of diabetogenic drugs such as streptozotocin (31, 32). Human PBMCs readily engrafted in NSG-RIP-DTR (Kb Db)null (IAnull) mice that had been rendered hyperglycemic and restored to normoglycemia by engraftment of human islets. The human islet allografts were rejected as evidenced by recurrent hyperglycemia, marked by reduced circulating C-peptide and decreased insulin content of the islet grafts. Interestingly, the islet allograft recipients had an increased frequency of CD8+ T cells in both the blood and the spleen over time. This suggests that the presence of islet allografts preferentially stimulated and expanded the cytotoxic CD8+ T-cell population. These data indicate that human PBMC function can be evaluated in NSG MHC I/II knockout mice in the absence of an ongoing GVHD response.
Many of the drugs being advanced to the clinic are immune modulators, and one of these entering clinical trials is the administration of recombinant IL-2. High dose IL-2 has been used for cancer therapy (53, 54) whereas low dose IL-2 has been applied toward the treatment of autoimmune diseases (55, 56). To determine if IL-2 can modulate human T cells in NSG-(Kb Db)null (IAnull) mice, we administered an injection of dsAAV8-huIL-2 (41). High doses of the dsAAV8-huIL-2 vector led to elevated human IL-2 levels in the circulation and increased levels of Treg cells; however, the elevated levels of IL-2 also led to the development of GVHD in NSG-(Kb Db)null (IAnull) mice. This suggests that the engrafted human T cells in NSG-(Kb Db)null (IAnull) mice are responsive to immunomodulatory drugs, and that the inability to mediate GVHD in these NSG MHC I/II knockout mice can be overcome by strong T cell activation and expansion. Although the major targets of the xenoresponse, murine MHC class I and II molecules, have been eliminated there are multiple other xenoantigens that may stimulate mature human T cells, including murine minor MHC antigens and murine antigens cross-presented to the human CD8+ T cells. High levels of IL-2 appear to lead to activation of the engrafted human T cells that is sufficient to initiate and mediate a lethal GVHD, even in the presence of increased proportions of human Treg cells.
In summary, we have described 2 new models of NSG mice that are deficient in MHC class I and II and have demonstrated that PBMC engraftment does not lead to lethal GVHD. We have also shown that the engrafted T cells are functional, reject islet allografts, and are responsive to immunomodulators such as IL-2. Finally, we have overcome the major limitation of IgG clearance rate in NSG-B2Mnull (IA IE)null mice through the development of the NSG-(Kb Db)null (IAnull) strain, which has IgG clearance rates similar to that of NSG mice. These new models of NSG MHC I/II knockout mice will facilitate the study of human immunity in the absence of GVHD and permit evaluation of clinical uses of Ab-based therapeutics.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported, in part, by U.S. National Institutes of Health (NIH) Office of the Director Grant 1R24 OD018259 and the NIH National Institute of Diabetes and Digestive and Kidney Diseases–supported Human Islet Research Network (https://hirnetwork.org) Grants UC4 DK104218 (to M.A.B., D.L.G., and L.D.S.), CA034196 (to L.D.S.), 1R01 AI132963 (to M.A.B. and L.D.S.), HL131471 (to C.M.), 1DP3DK111898 (to M.A.B.), DK098252 (to C.M.), and 1R01 DK1035486 (R.M.T. and M.A.B.). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. M.A.B. and D.L.C. are consultants for The Jackson Laboratory. The authors declare no conflicts of interest.
Glossary
- AAV
adeno-associated virus
- Ab
antibody
- B6
C57BL/6
- ds
double-stranded
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- FcRn
neonatal FcR
- GVHD
graft-versus-host disease
- IEQ
islet equivalent
- IL2rg
IL-2 receptor subunit γ
- MHC
major histocompatibility complex
- NOD
NOD/ShiLtJ
- NOG
NOD/Shi-scid/IL-2Rγnull
- NSG
NOD-scid IL2rgnull
- PBL
peripheral blood lymphocyte
- PBMC
peripheral blood mononuclear cell
- PD-1
programmed death-1
- RIP
rat insulin promoter
- TALEN
transcription activator–like effector nuclease
- TEMRA
effector memory CD45RA+ T
- Treg
regulatory T
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. A. Brehm designed and performed research, analyzed data, and wrote the manuscript; L. L. Kenney performed research and analyzed data; M. V. Wiles, B.E. Low and C. Mueller contributed new reagents; R. M. Tisch contributed new reagents and reviewed the manuscript; L. Burzenski performed research and analyzed data; D. L. Greiner analyzed data and wrote the manuscript; and L. D. Shultz designed and performed research, analyzed data, and wrote the manuscript.
REFERENCES
- 1.Theocharides A. P. A., Rongvaux A., Fritsch K., Flavell R. A., Manz M. G. (2016) Humanized hemato-lymphoid system mice. Haematologica 101, 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brehm M. A., Bortell R., Verma M., Shultz L. D., Greiner D. L. (2016) Humanized mice in translational immunology. In Translational Immunology: Mechanisms and Pharmacological Approaches (Tan S.-L., ed.), pp. 285–326, Elsevier, Amsterdam: [Google Scholar]
- 3.Ito R., Takahashi T., Katano I., Ito M. (2012) Current advances in humanized mouse models. Cell. Mol. Immunol. 9, 208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shultz L. D., Brehm M. A., Garcia-Martinez J. V., Greiner D. L. (2012) Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12, 786–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shultz L. D., Ishikawa F., Greiner D. L. (2007) Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 [DOI] [PubMed] [Google Scholar]
- 6.Walsh N. C., Kenney L. L., Jangalwe S., Aryee K.-E., Greiner D. L., Brehm M. A., Shultz L. D. (2017) Humanized mouse models of clinical disease. Annu. Rev. Pathol. 12, 187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K., Heike T., Nakahata T. (2002) NOD/SCID/(γc)null mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182 [DOI] [PubMed] [Google Scholar]
- 8.Shultz L. D., Lyons B. L., Burzenski L. M., Gott B., Chen X., Chaleff S., Kotb M., Gillies S. D., King M., Mangada J., Greiner D. L., Handgretinger R. (2005) Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 [DOI] [PubMed] [Google Scholar]
- 9.Strowig T., Rongvaux A., Rathinam C., Takizawa H., Borsotti C., Philbrick W., Eynon E. E., Manz M. G., Flavell R. A. (2011) Transgenic expression of human signal regulatory protein alpha in Rag2−/−γc−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. USA 108, 13218–13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takenaka K., Prasolava T. K., Wang J. C. Y., Mortin-Toth S. M., Khalouei S., Gan O. I., Dick J. E., Danska J. S. (2007) Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 8, 1313–1323 [DOI] [PubMed] [Google Scholar]
- 11.Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. (1988) Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335, 256–259 [DOI] [PubMed] [Google Scholar]
- 12.Akkina R., Allam A., Balazs A. B., Blankson J. N., Burnett J. C., Casares S., Garcia J. V., Hasenkrug K. J., Kashanchi F., Kitchen S. G., Klein F., Kumar P., Luster A. D., Poluektova L. Y., Rao M., Sanders-Beer B. E., Shultz L. D., Zack J. A. (2016) Improvements and limitations of humanized mouse models for HIV research: NIH/NIAID “Meet the Experts” 2015 workshop summary. AIDS Res. Hum. Retroviruses 32, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brehm M. A., Wiles M. V., Greiner D. L., Shultz L. D. (2014) Generation of improved humanized mouse models for human infectious diseases. J. Immunol. Methods 410, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenney L. L., Shultz L. D., Greiner D. L., Brehm M. A. (2016) Humanized mouse models for transplant immunology. Am. J. Transplant. 16, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham S., Choi J.-G., Ye C., Manjunath N., Shankar P. (2015) IL-10 exacerbates xenogeneic GVHD by inducing massive human T cell expansion. Clin. Immunol. 156, 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham S., Guo H., Choi J.-G., Ye C., Thomas M. B., Ortega N., Dwivedi A., Manjunath N., Yi G., Shankar P. (2017) Combination of IL-10 and IL-2 induces oligoclonal human CD4 T cell expansion during xenogeneic and allogeneic GVHD in humanized mice. Heliyon 3, e00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali N., Flutter B., Sanchez Rodriguez R., Sharif-Paghaleh E., Barber L. D., Lombardi G., Nestle F. O. (2012) Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS One 7, e44219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruck F., Belle L., Lechanteur C., de Leval L., Hannon M., Dubois S., Castermans E., Humblet-Baron S., Rahmouni S., Beguin Y., Briquet A., Baron F. (2013) Impact of bone marrow-derived mesenchymal stromal cells on experimental xenogeneic graft-versus-host disease. Cytotherapy 15, 267–279 [DOI] [PubMed] [Google Scholar]
- 19.Gregoire-Gauthier J., Fontaine F., Benchimol L., Nicoletti S., Selleri S., Dieng M. M., Haddad E. (2015) Role of natural killer cells in intravenous immunoglobulin–induced graft-versus-host disease inhibition in NOD/LtSz-scidIL2rg−/− (NSG) mice. Biol. Blood Marrow Transplant. 21, 821–828 [DOI] [PubMed] [Google Scholar]
- 20.Hatano R., Ohnuma K., Yamamoto J., Dang N. H., Yamada T., Morimoto C. (2013) Prevention of acute graft-versus-host disease by humanized anti-CD26 monoclonal antibody. Br. J. Haematol. 162, 263–277 [DOI] [PubMed] [Google Scholar]
- 21.Hilger N., Glaser J., Müller C., Halbich C., Müller A., Schwertassek U., Lehmann J., Ruschpler P., Lange F., Boldt A., Stahl L., Sack U., Oelkrug C., Emmrich F., Fricke S. (2016) Attenuation of graft-versus-host-disease in NOD scid IL-2Rγ−/− (NSG) mice by ex vivo modulation of human CD4+ T cells. Cytometry A 89, 803–815 [DOI] [PubMed] [Google Scholar]
- 22.Ito R., Katano I., Kawai K., Yagoto M., Takahashi T., Ka Y., Ogura T., Takahashi R., Ito M. (2017) A novel xenogeneic graft-versus-host disease model for investigating the pathological role of human CD4+ or CD8+ T cells using immunodeficient NOG mice. Am. J. Transplant. 17, 1216–1228 [DOI] [PubMed] [Google Scholar]
- 23.King M. A., Covassin L., Brehm M. A., Racki W., Pearson T., Leif J., Laning J., Fodor W., Foreman O., Burzenski L., Chase T. H., Gott B., Rossini A. A., Bortell R., Shultz L. D., Greiner D. L. (2009) Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host–like disease and the role of host major histocompatibility complex. Clin. Exp. Immunol. 157, 104–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pino S., Brehm M. A., Covassin-Barberis L., King M., Gott B., Chase T. H., Wagner J., Burzenski L., Foreman O., Greiner D. L., Shultz L. D. (2010) Development of novel major histocompatibility complex class I and class II–deficient NOD-SCID IL2R gamma chain knockout mice for modeling human xenogeneic graft-versus-host disease. In Mouse Models for Drug Discovery, Vol. 602, (Proetzel G., and Wiles M. V., eds.), pp. 105–117, Springer, Amsterdam, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashizawa T., Iizuka A., Nonomura C., Kondou R., Maeda C., Miyata H., Sugino T., Mitsuya K., Hayashi N., Nakasu Y., Maruyama K., Yamaguchi K., Katano I., Ito M., Akiyama Y. (2017) Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin. Cancer Res. 23, 149–158 [DOI] [PubMed] [Google Scholar]
- 26.Ferrara J. L., Levine J. E. (2006) Graft-versus-host disease in the 21st century: new perspectives on an old problem. Semin. Hematol. 43, 1–2 [DOI] [PubMed] [Google Scholar]
- 27.Covassin L., Jangalwe S., Jouvet N., Laning J., Burzenski L., Shultz L. D., Brehm M. A. (2013) Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin. Exp. Immunol. 174, 372–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen L., Labrecque N., Engberg J., Dierich A., Svejgaard A., Benoist C., Mathis D., Fugger L. (1999) Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA 96, 10338–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podolin P. L., Pressey A., DeLarato N. H., Fischer P. A., Peterson L. B., Wicker L. S. (1993) I-E+ nonobese diabetic mice develop insulitis and diabetes. J. Exp. Med. 178, 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bevan M. J. (2010) The earliest knockouts. J. Immunol. 184, 4585–4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai C., Kayton N. S., Shostak A., Poffenberger G., Cyphert H. A., Aramandla R., Thompson C., Papagiannis I. G., Emfinger C., Shiota M., Stafford J. M., Greiner D. L., Herrera P. L., Shultz L. D., Stein R., Powers A. C. (2016) Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J. Clin. Invest. 126, 1857–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C., Loehn M., Jurczyk A., Przewozniak N., Leehy L., Herrera P. L., Shultz L. D., Greiner D. L., Harlan D. M., Bortell R. (2015) Lixisenatide accelerates restoration of normoglycemia and improves human beta-cell function and survival in diabetic immunodeficient NOD–scid IL-2rgnull RIP-DTR mice engrafted with human islets. Diabetes Metab. Syndr. Obes. 8, 387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharya A., Dorf M. E., Springer T. A. (1981) A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J. Immunol. 127, 2488–2495 [PubMed] [Google Scholar]
- 34.Hattori M., Buse J. B., Jackson R. A., Glimcher L., Dorf M. E., Minami M., Makino S., Moriwaki K., Kuzuya H., Imura H., Strauss W. M., Seidman J. G., Eisentbarth G. S. (1986) The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science 231, 733–735 [DOI] [PubMed] [Google Scholar]
- 35.Lefranc M.-P., Duprat E., Kaas Q., Tranne M., Thiriot A., Lefranc G. (2005) IMGT unique numbering for MHC groove G-DOMAIN and MHC superfamily (MhcSF) G-LIKE-DOMAIN. Dev. Comp. Immunol. 29, 917–938 [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki T., Matsuda Y., Toyonaga T., Miyazaki J., Yazaki Y., Yamamura K. (1992) Prevention of autoimmune insulitis in nonobese diabetic mice by expression of major histocompatibility complex class I Ld molecules. Proc. Natl. Acad. Sci. USA 89, 9519–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harlan D. M., Barnett M. A., Abe R., Pechhold K., Patterson N. B., Gray G. S., June C. H. (1995) Very-low-dose streptozotocin induces diabetes in insulin promoter-mB7-1 transgenic mice. Diabetes 44, 816–823 [DOI] [PubMed] [Google Scholar]
- 38.He Y., Weinberg M. S., Hirsch M., Johnson M. C., Tisch R., Samulski R. J., Li C. (2013) Kinetics of adeno-associated virus serotype 2 (AAV2) and AAV8 capsid antigen presentation in vivo are identical. Hum. Gene Ther. 24, 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarty D. M., Monahan P. E., Samulski R. J. (2001) Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8, 1248–1254 [DOI] [PubMed] [Google Scholar]
- 40.Grieger J. C., Choi V. W., Samulski R. J. (2006) Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1, 1412–1428 [DOI] [PubMed] [Google Scholar]
- 41.Johnson M. C., Garland A. L., Nicolson S. C., Li C., Samulski R. J., Wang B., Tisch R. (2013) β-cell–specific IL-2 therapy increases islet Foxp3+Treg and suppresses type 1 diabetes in NOD mice. Diabetes 62, 3775–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghavan M., Bjorkman P. J. (1996) Fc receptors and their interactions with immunoglobulins. Annu. Rev. Cell Dev. Biol. 12, 181–220 [DOI] [PubMed] [Google Scholar]
- 43.Roopenian D. C., Akilesh S. (2007) FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 [DOI] [PubMed] [Google Scholar]
- 44.Wagar E. J., Cromwell M. A., Shultz L. D., Woda B. A., Sullivan J. L., Hesselton R. M., Greiner D. L. (2000) Regulation of human cell engraftment and development of EBV-related lymphoproliferative disorders in Hu-PBL-scid mice. J. Immunol. 165, 518–527 [DOI] [PubMed] [Google Scholar]
- 45.Kumar B. V., Connors T. J., Farber D. L. (2018) Human T cell development, localization, and function throughout life. Immunity 48, 202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van den Broek T., Borghans J. A. M., van Wijk F. (2018) The full spectrum of human naive T cells. Nat. Rev. Immunol. 18, 363–373 [DOI] [PubMed] [Google Scholar]
- 47.King M., Pearson T., Shultz L. D., Leif J., Bottino R., Trucco M., Atkinson M. A., Wasserfall C., Herold K. C., Woodland R. T., Schmidt M. R., Woda B. A., Thompson M. J., Rossini A. A., Greiner D. L. (2008) A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin. Immunol. 126, 303–314 [DOI] [PubMed] [Google Scholar]
- 48.Thorel F., Népote V., Avril I., Kohno K., Desgraz R., Chera S., Herrera P. L. (2010) Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durost P. A., Aryee K. E., Manzoor F., Tisch R. M., Mueller C., Jurczyk A., Shultz L. D., Brehm M. A. (2018) Gene therapy with an adeno-associated viral vector expressing human interleukin-2 alters immune system homeostasis in humanized mice. Hum. Gene Ther. 29, 352–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raulet D. H. (1993) MHC class I-deficient mice. Adv. Immunol. 55, 381–421 [DOI] [PubMed] [Google Scholar]
- 51.Christianson S. W., Greiner D. L., Hesselton R. A., Leif J. H., Wagar E. J., Schweitzer I. B., Rajan T. V., Gott B., Roopenian D. C., Shultz L. D. (1997) Enhanced human CD4+ T cell engraftment in β2-microglobulin-deficient NOD-scid mice. J. Immunol. 158, 3578–3586 [PubMed] [Google Scholar]
- 52.Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. (1991) Mice lacking MHC class II molecules. Cell 66, 1051–1066 [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg S. A. (2014) IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192, 5451–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sim G. C., Radvanyi L. (2014) The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 25, 377–390 [DOI] [PubMed] [Google Scholar]
- 55.Koreth J., Matsuoka K.-I., Kim H. T., McDonough S. M., Bindra B., Alyea E. P., III, Armand P., Cutler C., Ho V. T., Treister N. S., Bienfang D. C., Prasad S., Tzachanis D., Joyce R. M., Avigan D. E., Antin J. H., Ritz J., Soiffer R. J. (2011) Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saadoun D., Rosenzwajg M., Joly F., Six A., Carrat F., Thibault V., Sene D., Cacoub P., Klatzmann D. (2011) Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.