Abstract
As mechanisms controlling redox homeostasis become impaired with aging, exaggerated oxidant stress may cause disproportional oxidation of cell membranes and circulating phospholipids (PLs), leading to the formation of truncated oxidized PL products (Tr-OxPLs), which exhibit deleterious effects. This study investigated the role of elevated Tr-OxPLs as a factor exacerbating inflammation and lung barrier dysfunction in an animal model of aging. Mass spectrometry analysis of Tr-OxPL species in young (2–4 mo) and aging (18–24 mo) mice revealed elevated basal levels of several products [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphocholine (POVPC), 1-palmitoyl-2-glutaroyl-sn-glycero-phosphocholine, lysophosphocholine, 1-palmitoyl-2-(9-oxo-nonanoyl)-sn-glycero-3-phosphocholine, 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine, O-1-O-palmitoyl-2-O-(5,8-dioxo-8-hydroxy-6-octenoyl)-l-glycero-3-phosphocholine, and others] in the aged lungs. An intratracheal (i.t.) injection of bacterial LPS caused increased generation of Tr-OxPLs in the lungs but not in the liver, with higher levels detected in the aged group. In addition, OxPLs clearance from the lung tissue after LPS challenge was delayed in the aged group. The impact of Tr-OxPLs on endothelial cell (EC) barrier compromise under inflammatory conditions was further evaluated in the 2-hit cell culture model of acute lung injury (ALI). EC barrier dysfunction caused by cell treatment with a cytokine mixture (CM) was augmented by cotreatment with low-dose Tr-OxPLs, which did not significantly affect endothelial function when added alone. Deleterious effects of Tr-OxPLs on inflamed ECs stimulated with CM were associated with further weakening of cell junctions and more robust EC hyperpermeability. Aged mice injected intratracheally with TNF-α exhibited a more pronounced elevation of cell counts and protein content in bronchoalveolar lavage (BAL) samples. Interestingly, intravenous administration of low POVPC doses—which did not affect BAL parameters alone in young mice exposed to i.t. TNF-α challenge—augmented lung injury to the levels observed in aged mice stimulated with TNF-α alone. Inhibition of Tr-OxPL generation by ectopic expression of PL-specific platelet-activating factor acetylhydrolase 2 (PAFAH2) markedly reduced EC dysfunction induced by CM, whereas PAFAH2 pharmacologic inhibition augmented deleterious effects of cytokines on EC barrier function. Moreover, exacerbating effects of PAFAH2 inhibition on TNF-α–induced lung injury were observed in vivo. These results demonstrate an age-dependent increase in Tr-OxPL production under basal conditions and augmented Tr-OxPL generation upon inflammatory stimulation, suggesting a major role for elevated Tr-OxPLs in more severe ALI and delayed resolution in aging lungs.—Ke, Y., Karki, P., Kim, J., Son, S., Berdyshev, E., Bochkov, V. N., Birukova, A. A., Birukov, K. G. Elevated truncated oxidized phospholipids as a factor exacerbating ALI in the aging lungs.
Keywords: lung injury, vascular permeability, inflammation
Vulnerability to various stresses, such as inflammation and oxidative damage, increases with age. The elderly population becomes more susceptible to a wide variety of infections, and to respiratory infections in particular. Individuals >70 yr with influenza infections exhibit a 35-fold increase in mortality (1). Elderly patients with sepsis have mortality rates of 30–40%, compared with 4–5% for younger patients (2). A common theory of aging is based on the oxidant stress theory: increasing levels of reactive oxygen species (ROS) in cells with increasing age result in various forms of oxidative modifications of proteins, lipids, and DNA that eventually lead to loss of function or development of pathologic reactions. Indeed, the decline in antioxidant capacity with age strongly correlates with increased risk of mortality from various causes (3). As a result, oxidative damage due to increased production of ROS and reactive nitrogen species is highly associated with acute lung injury (ALI) and may be even more severe in aging lungs (4).
Phospholipids (PLs) containing unsaturated fatty acids, such as arachidonic acid in sn-2 position, serve as a major building block of cell membranes, and they are also present in circulating lipoproteins. PLs participate in many cellular and organ functions, but may undergo oxidation by lipoxygenases or ROS and reactive nitrogen species in various pathologic conditions such as ventilator-induced lung injury, trauma, or septic inflammation (5–8). Under these conditions, lung vascular barrier function is largely compromised and truncated oxidized phospholipid products (Tr-OxPLs) such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphocholine (POVPC), 1-palmitoyl-2-glutaroyl-sn-glycero-phosphocholine (PGPC), and lysophosphocholine (lyso-PC) increase permeability in EC monolayer cultures (9, 10), thus potentially contributing to endothelial barrier disruption and lung injury in vivo. Given the reduction of antioxidant capacity associated with aging and reflected by decreased expression and activation of antioxidant enzymes, reduced glutathione buffering capacity, and other alterations (11, 12), the production of Tr-OxPLs may have an increased role in more severe ALIs observed in an aging population, and this question warrants further investigation.
This study tested a hypothesis that aging-related decompensation of redox balance leads to increased production of Tr-OxPL products under basal conditions and further augments OxPL generation under inflammatory conditions, thus leading to further exacerbation of lung injury and lung vascular endothelial barrier compromise. Using combination of biochemical and functional assays performed in the in vitro and animal models of lung endothelial inflammation and barrier dysfunction, we analyzed the levels of Tr-OxPL products in the lungs of young (2–4 mo old) and aging (18–24 mo old) mice under basal conditions and following inflammatory insult. We further investigated effects of Tr-OxPL elevation on the magnitude of barrier dysfunction in cultured human lung EC and in lungs of LPS-challenged mice. Finally, we tested whether control of Tr-OxPL levels by expression of PL-specific platelet-activating factor acetylhydrolase 2 (PAFAH2) is a feasible approach to suppress vascular permeability caused by inflammatory mediators.
MATERIALS AND METHODS
Cell culture and reagents
Human pulmonary artery endothelial cells (HPAECs) were obtained from Lonza (Basel, Switzerland) and used at passages 5–8. All experiments were performed in EGM growth medium (Lonza) containing 2% fetal bovine serum unless otherwise specified. Texas Red–conjugated phalloidin and Alexa Fluor 488–labeled secondary antibodies were purchased form Molecular Probes (Eugene, OR, USA). We obtained 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine, POVPC, PGPC, 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (PazPC), O-1-O-palmitoyl-2-O-(5,8-dioxo-8-hydroxy-6-octenoyl)-l-glycero-3-phosphocholine (KOdiAPC), 1-palmitoyl-2-(9-oxo-nonanoyl)-sn-glycero-3-phosphocholine (PONPC), and lyso-PC from Avanti Polar Lipids (Alabaster, AL, USA). The PLs were dissolved in chloroform and stored at −70°C. PAFAH2 inhibitor SB480848 was purchased from Cayman Chemical (Ann Arbor, MI, USA). Unless otherwise specified, all other biochemical reagents were obtained from MilliporeSigma (Burlington, MA, USA). DNA plasmid encoding Myc-FLAG–tagged PAFAH2 was obtained from OriGene (Rockville, MD, USA). Transient transfections of human lung ECs with PAFAH2 plasmid were carried out using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) as recommended by the manufacturer. After 24 h of transfection, cells were treated overnight with agonists of interest and used for permeability measurements, biochemical assays, or immunofluorescence analysis.
Animal studies
All animal care and treatment procedures were approved by the Institutional Animal Care and Use Committees of the University of Maryland and the University of Chicago. Young (2–4 mo) C57BL/6J male and female mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and aging (18–24 mo) C57BL/6J male and female mice were obtained from the Aged Rodent Colonies Biologic Resources Branch of the U.S. National Institutes of Health (NIH) National Institute on Aging (Bethesda, MD, USA). Animals were handled according to the Guide for the Care and Use of Laboratory Animals (NIH). There were no observed sex differences in the outcomes of our experiments. Either bacterial LPS (0.75 mg/kg body weight; Escherichia coli O55:B5), TNF-α [10 µg/kg, intratracheal (i.t.)] or a small volume of sterile water (20–30 µl, i.t.) was injected using a 20-gauge catheter (Exelint International, Redondo Beach, CA, USA). In select experiments, to mimic elevated Tr-OxPL levels in the aging group, POVPC (10 μg/kg) or sterile saline solution was administered to the young group concurrently and 5 h after TNF-α instillation by intravenous injection in the external jugular vein. Animals were killed on d 1–6 by exsanguination under anesthesia. Bronchoalveolar lavage (BAL) was performed using 1 ml of sterile HBSS buffer, and ELISA measurements of cell count, protein concentration, and cytokine levels in BAL samples were conducted as previously described (13). To analyze LPS-induced lung vascular leaks, Evans blue dye (30 ml/kg) was injected into the external jugular vein 2 h before termination of the experiment, as described in Meliton et al. (14).
In vivo optical imaging
After an i.t. injection of inflammatory agents, animals were injected via the tail vein with 100 µl of 2 nmol Angiosense 680 EX Imaging agent (NEV10054EX; PerkinElmer, Waltham, MA, USA). Fluorescent optical imaging was performed at indicated times after the inflammatory challenge using Xenogen IVIS 200 Spectrum (PerkinElmer). Mice were exposed to isoflurane anesthesia with O2 through a gas anesthesia manifold and placed on the imaging stage. Acquisition and image analysis were performed with Living Image software (v.4.3.1), as previously described (15).
Mass spectrometry analysis of oxidized phospholipids in tissue samples
Standards and reagents
Methanol, water, and chloroform [liquid chromatography (LC)/mass spectrometry (MS) or HPLC grade] were purchased from Thermo Fisher Scientific. We obtained 21:0/22:6-phosphatidylcholine (21:0/22:6-PC) from Avanti Polar Lipids. The standards were dissolved in methanol and stored at −80°C.
Lipid extraction
Tubes containing frozen lung samples were weighed; the weight of the tissue was calculated later by subtracting the weight of the empty tube. The samples (typical wet weight 1–1.5 g) were ground in a mortar containing liquid nitrogen and poured into a 50 ml plastic tube containing 6 ml of methanol/acetic acid (3%) and butylated hydroxytoluene (BHT, 0.01%). After the liquid nitrogen evaporated, the tubes were purged with argon gas and incubated for 20 min on ice with periodic vortexing. After centrifugation (5 min; 3000 g; 4°C), 2.5 ml of supernatant was transferred to acid-washed glass tubes and 100 µl of internal standards (2 nmol 1,2-dinonanoyl-sn-glycero-3-phosphocholine + 15 pmol 1,2-ditridecanoyl-sn-glycero-3-phosphocholine dissolved in methanol) were added. The samples were washed a further 3 times with 5 ml of hexane/BHT (0.01%) with vigorous vortexing. After adding 5 ml of chloroform/BHT (0.01%) and 1.9 ml of formic acid (0.7 M), the samples were vortexed, the lower phase was transferred to 8-ml glass tubes and dried under the stream of argon gas. The lipids were dissolved in 1 ml chloroform, transferred to autosampler glass vials, dried, and stored at −70°C for further analysis.
HPLC–tandem MS analysis of OxPLs in lung tissue
The analytical HPLC–tandem MS (MS/MS) procedure was described in detail in our previous publication (16). Briefly, PLs were separated on a core shell–type C18 column (Kinetex 2.6 µm, 50 × 3 mm ID; Phenomenex, Torrance, CA, USA) eluted with a linear gradient of water and methanol both containing 5 mM ammonium formate and 0.1% formic acid. Detection of PLs by MS/MS was performed using a 4000 QTrap triple quadrupole linear ion trap hybrid mass spectrometer system equipped with a Turbo V electrospray ion source (Thermo Fisher Scientific) in a positive mode using phosphocholine (m/z 184) as a diagnostic production. Each sample was analyzed in 2 HPLC-MS/MS runs. For the analysis of OxPLs, the lipid extracts were dissolved in 100 µl of MS solvent and 10 µl were injected onto the column. The data were collected during the first 8 min, after which the eluent was switched to waste. To analyze the unoxidized PLs, the samples dissolved in 100 µl were further diluted 1000-fold and 10 µl were injected. The data were collected for 15 min.
EC permeability assays
Measurements of transendothelial electrical resistance
Cellular barrier properties were analyzed by measurements of transendothelial electrical resistance (TER) across confluent human pulmonary endothelial monolayers using an electrical cell-substrate impedance sensing system (Applied BioPhysics, Troy, NY, USA) as previously described (17).
EC permeability assay for macromolecules
EC permeability to macromolecules was monitored using an express permeability testing assay (18) available from MilliporeSigma (Vascular Permeability Imaging Assay, 17-10398). In permeability visualization experiments, after EC stimulation with appropriate agonist, FITC-avidin tracer was added directly to the culture medium for 3 min before the experiment ended. Unbound FITC-avidin was washed out with PBS (pH 7.4, 37°C), cells were fixed with 3.7% formaldehyde in PBS (10 min, room temperature), and visualization of FITC-avidin immobilized on the bottoms of coverslips precoated with biotinylated collagen was performed using the Nikon Eclipse TE 300 imaging system (Nikon, Tokyo, Japan) equipped with a digital camera (DKC 5000; Sony, Tokyo, Japan); ×10 objective lenses were used. Images were processed in Adobe Photoshop 7.0 (San Jose, CA, USA).
Cytokine measurement
The concentrations of sICAM-1 (soluble intercellular adhesion molecule 1) and KC (mouse IL-8 homolog) in mouse BAL fluid were measured using mouse-specific ELISA kits (R&D Systems, Minneapolis, MN, USA). Absorbance was read at 450 nm within 30 min by a Victor ×5 multilabel plate reader (PerkinElmer). Standard curves were generated with expected minimum detectable concentration of 8 pg/ml.
Immunofluorescence and image analysis
Following agonist stimulation, ECs were fixed in 3.7% formaldehyde in PBS for 10 min at 4°C and were then washed with PBS, permeabilized with 0.1% Triton X-100 in PBS for 30 min at room temperature and blocked with 2% bovine serum albumin in PBS for 30 min. Incubation with VE-cadherin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was performed in blocking solution (2% bovine serum albumin in PBS) for 1 h at room temperature followed by staining with Alexa 488–conjugated secondary antibody. Actin filaments were stained with Texas Red–conjugated phalloidin diluted in the blocking solution. After immunostaining, the slides were analyzed using a Nikon Eclipse TE300 inverted microscope connected to a Spot RT monochrome digital camera and image processor (Diagnostic Instruments, Sterling Heights, MI, USA). The images were acquired using SPOT acquisition software (v. 3.5; Diagnostic Instruments) and processed in Adobe Photoshop 7.0.
Statistical analysis
Results are expressed as the mean ± sd of 3–5 independent experiments. Stimulated samples were compared with controls using an unpaired Student’s t test. For comparisons involving multiple groups, we used 1-way ANOVA followed by the post hoc Fisher’s test. Values of P < 0.05 were considered statistically significant.
RESULTS
Aging mice (18–24 mo) develop more severe ALI in response to LPS, than young mice (2–4 mo)
We evaluated the parameters of barrier dysfunction and lung inflammation caused by an i.t. injection of LPS in young (2–4 mo) and aging (18–24 mo) mice. BAL was performed 18 h after LPS challenge, as described in Materials and Methods. LPS challenge increased inflammatory cell count (Fig. 1A) and protein content (Fig. 1B) in BAL samples, and this effect was more prominent in the aging group (Fig. 1A, B). The differences in the magnitude of LPS-induced lung barrier dysfunction in young and aging groups were further assessed by measuring Evans blue accumulation in the lung tissue, which indicates a vascular leak. LPS induced noticeable Evans blue leakage from the vascular space into the lung parenchyma, which was further augmented in the aging group (Fig. 1C). Next, LPS-induced lung cytokine production was tested in young and aging mice. The data show that LPS-induced elevation of the mouse IL-8 homolog KC and sICAM-1 in BAL samples was augmented in the aging group (Fig. 1D).
Figure 1.
Aged mice are more susceptible to LPS-induced ALI. Both sets of mice (young, 2–4 vs. old, 18–24 mo) were challenged with LPS (0.75 mg/kg, i.t.) for 24 h. A, B) BAL was collected from mice to analyze total cell count (A) and total protein content (B); n = 10, *P < 0.05. C) Vehicle (saline) or LPS-challenged mice were injected with Evans blue dye (30 mg/kg, i.v.) 2 h before the end of the experiment; lung vascular permeability was then assessed by visualizing Evans blue–stained lungs. The quantitative analysis of Evans blue–labeled albumin extravasation was performed by spectrophotometric analysis of Evans blue extracted from the lung tissues; n = 4. *P < 0.05. D) The levels of proinflammatory cytokines KC (a mouse IL-8 homolog) and sICAM were determined by ELISA. Data are expressed as means ± sd; n = 4. *P < 0.05. E) Accumulation of fluorescent Angiosense 680 EX imaging agent in mice lungs reflecting a vascular leak was evaluated at the indicated time points after instillation of LPS (0.75 mg/kg, i.t., 1–6 d) and presented in arbitrary colors, as described in Materials and Methods. The bar graph displays a quantitative analysis of imaging data; n = 4. *P < 0.05.
The time course of the lung vascular leak in young and in aging mice treated with LPS was further monitored using the noninvasive fluorescence optical imaging approach. Lung accumulation of intravenously injected tracer Angiosense 680 EX was evaluated in the same animals prospectively 1, 2, 3, and 6 d after LPS treatment. Accumulation of this fluorescent tracer reflecting lung inflammation and vascular barrier compromise was observed 24 h after LPS injection, gradually declining by d 6 (Fig. 1E). Importantly, lung dysfunction was noticeably increased in the aging mice group, and recovery of lung function was also delayed in aging mice when compared with their young counterparts. Taken together, these changes in the lungs indicate development of more severe pulmonary interstitial edema, fluid accumulation in alveolar space, and inflammatory cell infiltration caused by bacterial compounds in the aging mice.
MS analysis of oxidized PLs species in control and LPS-challenged young and aging lungs
Non-enzymatic oxidation of PLs containing mono- and polyunsaturated fatty acids can be initiated by free radicals and nonradical ROS or reactive nitrogen species and leads to formation of full-length oxygenated PLs or Tr-OxPLs. Increased levels of oxidized PLs present in the injured lung may exhibit profound effects on pulmonary EC functions including modulation of the inflammatory response and of EC barrier regulation (14, 19–21). In particular, Tr-OxPLs exhibit strong barrier-disrupting effects on pulmonary vascular ECs (9, 10, 22).
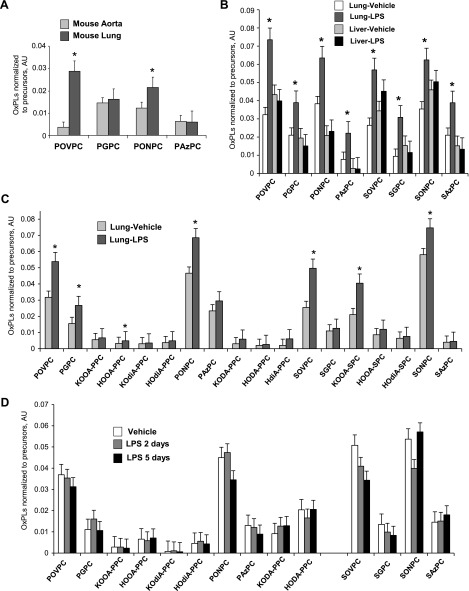
We performed comparative analysis of Tr-OxPL levels in mouse lung and nonlung (aorta) tissue using LC-MS/MS techniques. The results showed higher basal levels of several Tr-OxPL species—POVPC, PGPC, and PONPC—in the lungs than in the aorta. POVPC was among the most prominent OxPL products detected in the lung (Fig. 2A). Of note, i.t. injection of LPS caused increase in Tr-OxPL species in the lung, without increasing Tr-OxPLs in the liver (Fig. 2B). These results reflect organ-specific elevation of Tr-OxPLs upon inflammatory stimulation.
Figure 2.
MS analysis of Tr-OxPLs species in young and aged mice. A) MS determination of the indicated Tr-OxPLs in the lung and aorta tissues of mice was performed; n = 3, *P < 0.05. B, C) Mice were challenged with LPS (0.75 mg/kg, i.t., 6 h) followed by analysis of Tr-OxPLs by MS; n = 3. *P < 0.05. D) Change in the levels of Tr-OxPLs in mice lungs after 2 and 5 d of LPS exposure (0.75 mg/kg, i.t.) was determined; n = 3. *P < 0.05.
Further analysis of Tr-OxPL levels was performed in lungs stimulated with LPS. Lung tissue levels of several Tr-OxPL products, such as POVPC, PGPC, PONPC, SOVPC, KOOA-SPC, and SONPC, were increased by a 6 h i.t. injection of LPS (Fig. 2C). LPS-induced elevation of Tr-OxPL species was transient, and LC-MS/MS analysis of lung samples at 2 and 5 d after LPS challenge did not reveal elevated Tr-OxPL products (Fig. 2D).
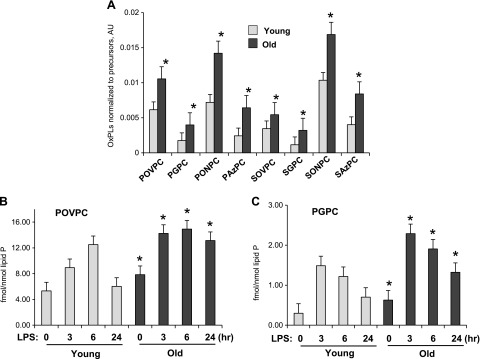
Weakening of the antioxidant defense and activation of redox-dependent pathologic processes in aging populations is well recognized (12, 23, 24). We next evaluated Tr-OxPL profiles in the lungs of control and LPS-stimulated young and aging mice. The results show statistically significant increase of basal levels of a number of Tr-OxPL species in the aging mice (Fig. 3A).
Figure 3.
Basal Tr-OxPLs levels are higher in aging lungs. A) Basal levels of Tr-OxPLs were determined by MS in lungs of young and old mice; n = 4, *P < 0.05. B, C) A set of old and young mice were challenged with LPS (0.75 mg/kg, i.t.) for the indicated time periods, and the levels of POVPC (B) and PGPC (C) were determined by MS; n = 4. *P < 0.05.
The aforementioned results (Figs. 1 and 2) show more pronounced and sustained LPS-induced lung injury in aging mice than in young mice. We next evaluated the time course of Tr-OxPL levels in LPS-challenged young and aging lungs. Analysis of the time course of an LPS-induced Tr-OxPL increase in young and aging mice showed transient elevation of POVPC and PGPC levels in LPS-treated young mice, which declined by 24 h poststimulation. In contrast, higher levels of Tr-OxPLs were detected in the lungs of LPS-treated aging mice, and this increase was sustained, remaining elevated for ≤24 h posttreatment (Fig. 3B, C).
Ox-TrPLs increase endothelial permeability
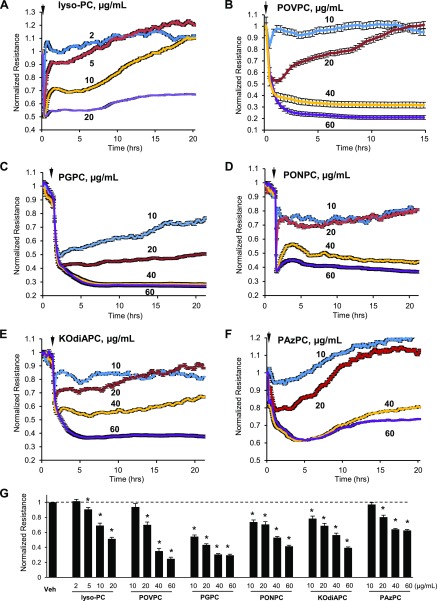
The results of HPLC-MS/MS analysis showed increased generation of several Tr-OxPL species in the lungs upon inflammatory stimulation. Using single purified Tr-OxPL products from the list of molecules detected by MS analysis and up-regulated after LPS challenge, we evaluated their effects on HPAEC permeability, which was monitored by measurements of TER in vitro. Treatment of cells with Tr-OxPL was performed at a concentration range of 2–60 µg/ml. The same dose range (10–60 µg/ml) was used for all compounds except lyso-PC (2–20 µg/ml), which exhibited a more potent barrier-disrupting effect. At the selected concentration range, lyso-PC, PONPC POVPC, PGPC, KOdiAPC, and PazPC caused rapid TER decline in a dose-dependent manner (Fig. 4A–F). Bar graphs presented in Fig. 4G show dose-dependent permeability changes induced by Tr-OxPL after 4 h of treatment.
Figure 4.
Tr-OxPLs cause an increase in permeability in pulmonary lung ECs. A–F) HPAEC monolayers grown on gold electrodes were exposed (marked by arrows) to indicated concentrations of lyso-PC (A), POVPC (B), PGPC (C), PONPC (D), KOdiAPC (E), or PazPC (F). TER was subsequently monitored over time. G) The bar graph represents quantitative analysis of TER data after 4 h of cell stimulation; n = 4. *P < 0.05.
Ox-TrPLs augment endothelial permeability caused by inflammatory agonists
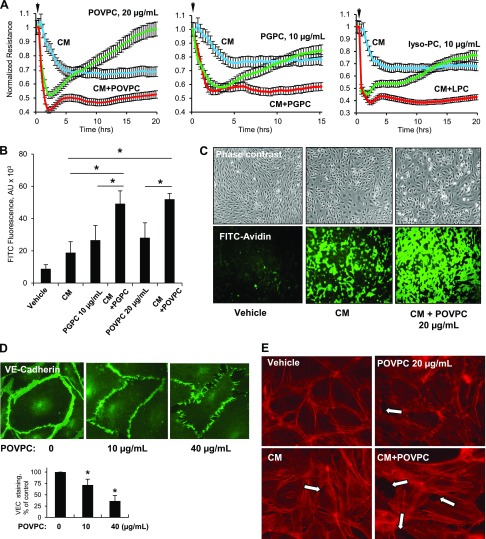
The results of direct Tr-OxPL effects on EC permeability strongly suggest their role is to modulate the EC barrier under pathologic conditions. Furthermore, the results of LC-MS/MS analysis showed augmented elevation of Tr-OxPLs in the aging lungs under inflammatory conditions. Certain products of PL oxidation inhibit TLR4-mediated inflammatory cascade triggered by TLR4 ligands (i.e., LPS) through competitive inhibition of the CD14–LPS interaction, which leads to the blocking of TLR4 signaling activation (25, 26). To avoid potential direct interference by Tr-OxPLs with agonist-induced activation of the TLR4 inflammatory cascade, in the next experiments we stimulated HPAECs with a combination of cytokines [cytokine mixture (CM), comprising 5 ng/ml each of IL-1β, TNF-α, and IFN-γ] known to induce TLR4-independent EC inflammation and lung injury (27). To model aging conditions characterized by elevated Tr-OxPL, CM-challenged pulmonary ECs were cotreated with Tr-OxPL at submaximal concentrations. Therefore, in the next experiments we stimulated HPAECs with CM combined with Tr-OxPL cotreatment at submaximal concentrations to model aging conditions. Separate stimulation with CM, POVPC, PGPC, or lyso-PC (20, 10, and 10 µg/ml, respectively) caused moderate TER decline, reflecting increased EC permeability. Remarkably, the combined treatment with same doses of CM and any of 3 Tr-OxPL products caused a synergistic effect indicated by a further drop in TER (Fig. 5A). Of note, at very low concentrations (<2 µg/ml), Tr-OxPL did not affect EC permeability, even when added in combination with inflammatory agonists (data not shown).
Figure 5.
Tr-OxPLs augment EC barrier disruption in 2-hit model of in vitro lung injury. HPAECs were challenged (marked by arrows) with a low dose of a mixture of cytokines (CM, containing IL-1β, TNF-α, and IFN-γ, 5 ng/ml each) and POVPC, PGPC, or lyso-PC (20, 10, and 10 µg/ml, respectively) either alone or in combination. A) Cell resistance was monitored for the indicated time periods; normalized TER values are presented. B, C) HPAECs grown on 96-well plates (B) or glass coverslips (C) coated with biotinylated gelatin were exposed to CM and Tr-OxPLs for 4 h. At the end of the stimulation, FITC-avidin (25 µg/ml) was added for 3 min and excess unbound FITC-avidin was washed away with PBS. FITC fluorescence was measured using a Victor ×5 plate reader (n = 6) (B) or visualized by fluorescence microscopy (C). D) Cell junction remodeling induced by the indicated concentrations of POVPC for 4 h was analyzed by immunofluorescence staining with VE-cadherin antibody. The bar graph represents quantitative analysis of imaging data; n = 3. E) Cells were challenged with CM and POVPC for 4 h either separately or in combination, and F-actin was visualized by immunofluorescence staining with Texas Red phalloidin. Paracellular gaps are marked by arrows. *P < 0.05.
Analysis of EC monolayer permeability for macromolecules using FITC-labeled avidin as a tracer (as described in Materials and Methods) confirmed the results of TER measurements and demonstrated the augmented EC permeability response to cotreatment with CM and low doses of either PGPC or POVPC (Fig. 5B). Visualization of EC monolayer permeability caused by CM or CM combined with low-dose POVPC is presented in Fig. 5C.
Immunofluorescence analysis of cell junctions and cytoskeletal remodeling in POVPC-treated pulmonary ECs showed some weakening of VE-cadherin peripheral staining in ECs treated with a low dose of POVPC (10 µg/ml), but visible disruption of VE-cadherin positive adherens junctions and paracellular gap formation in ECs treated with 40 µg/ml POVPC (Fig. 5D). We next used low-dose POVPC, CM, or a combination of the two to evaluate their effects on F-actin remodeling. Although low-dose POVPC or CM alone did not significantly affect F-actin arrangement, their combination markedly augmented the formation of stress fibers and paracellular gaps, leading to disruption of the endothelial monolayer (Fig. 5E).
Cotreatment of young mice with low-dose POVPC and TNF-α recapitulates the severity of TNF-α–induced ALI observed in aging mice
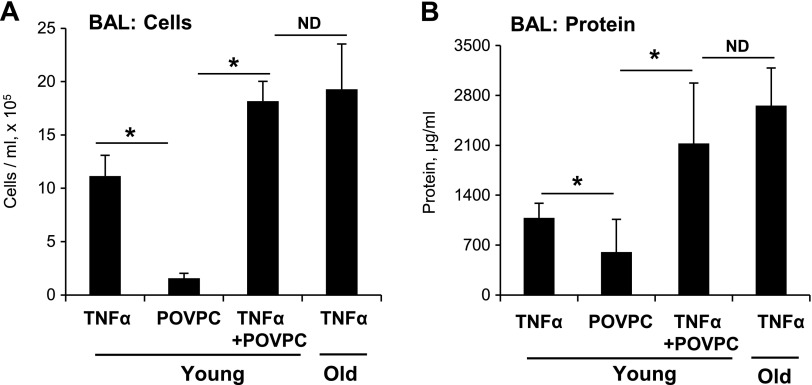
The results of this study show increased levels of Tr-OxPLs in aging mouse lungs affected by inflammation. In cell culture, the addition of Tr-OxPLs augmented the barrier-disrupting effects of CM on pulmonary ECs. To further test the role of Tr-OxPL elevation in the more severe course of ALI in the aging group, we modeled the elevated aging-related Tr-OxPL background by intravenously administration of POVPC to TNF-α–challenged young mice. The parameters of TNF-α–induced lung barrier dysfunction were compared between the following groups: young + TNF-α; young + TNF-α + POVPC; and aging + TNF-α. Cotreatment with TNF-α and POVPC increased the cell count (Fig. 6A) and protein content (Fig. 6B) in BAL samples of young mice, which was similar to the effect of TNF-α alone observed in aging mice (Fig. 6).
Figure 6.
A combination of Tr-OxPL and inflammatory agonist challenges in young mice mimics severe ALI induced in aged mice. Young mice were exposed to TNF-α (10 µg/kg, i.t.) or POVPC (10 mg/kg, i.v.) separately or in combination for 24 h. Mice were killed and BAL was analyzed for total cell count (A) and protein content (B). For comparison, old mice were also challenged with same dose of TNF-α. Results are expressed as means ± sd; n = 4. *P < 0.05.
Rescue strategies: modulation of EC barrier dysfunction and lung injury by PAFAH2
ECs stimulated with proinflammatory agonists generate sufficient oxidizing radicals to overwhelm normal cellular defenses and form Tr-OxPLs that trigger deleterious cell responses (28). Although they act as barrier-disrupting and proinflammatory agents, Tr-OxPLs (but not their PL precursors) are also substrates for a group of highly specific phospholipases, the group VII class of PAF acetylhydrolases. These enzymes specifically hydrolyze the fatty acyl fragment that remains esterified in the sn-2 position of the PL glycerol backbone after fragmentation of the oxidized fatty acyl residue (29). Among several PAF hydrolases, PAFAH2 is a highly specific oxidized PL phospholipase, which may inactivate proinflammatory Tr-OxPLs (30).
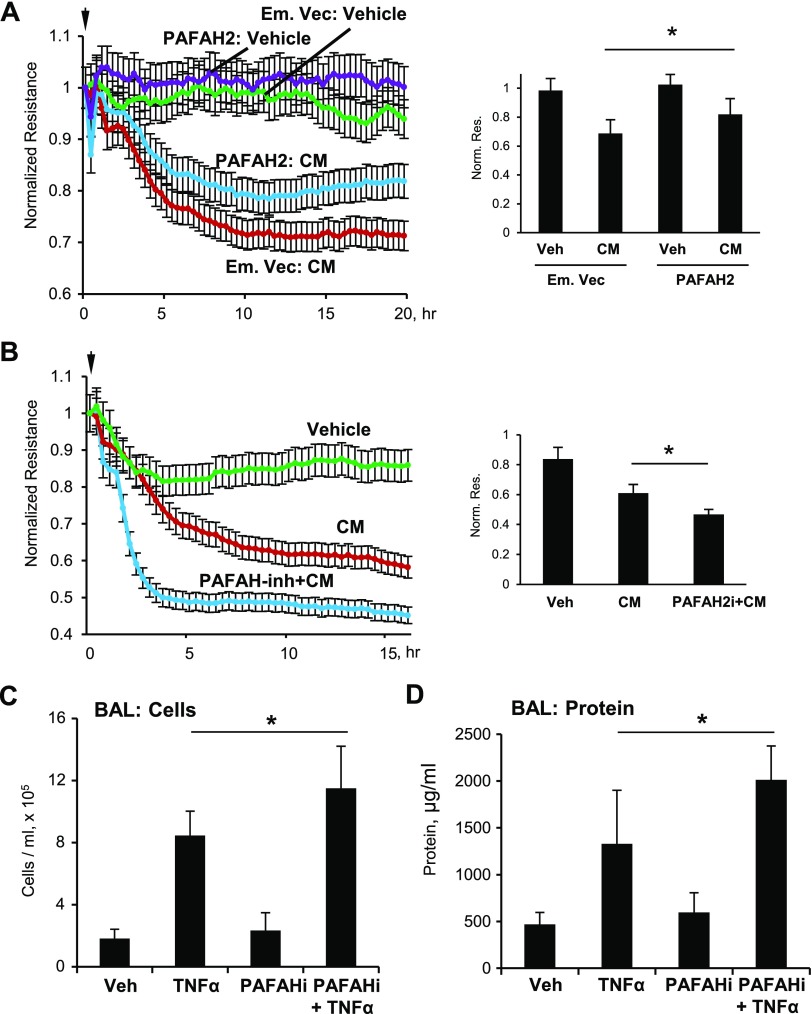
To evaluate PAFAH2 as a potential rescue strategy reducing aging-associated Tr-OxPL elevation and exacerbation of inflammatory EC barrier dysfunction, we performed ectopic expression of PAFAH2 in HPAECs, followed by the CM challenge and permeability measurements. PAFAH2 expression significantly attenuated the drop in TER caused by EC treatment with CM (Fig. 7A). In turn, EC pretreatment with a pharmacologic PAFAH2 inhibitor, SB-480848, exacerbated CM-induced EC barrier dysfunction monitored by TER measurements (Fig. 7B). This augmenting effect of PAFAH2 inhibition on inflammatory response was further tested in the animal model of TNF-α–induced lung injury. Intravenous pretreatment of mice with SB-480848 30 min prior to TNF-α challenge augmented increases in BAL cell count and protein content caused by TNF-α alone (Fig. 7C, D).
Figure 7.
PAFAH2 attenuates CM- or TNF-α–induced lung injury in vitro and in vivo, respectively. A) HPAECs were transfected with an empty vector or full-length human PAFAH2 overexpressing plasmid (Addgene) for 24 h followed by treatment with CM (IL-1β, TNF-α and IFN-γ, 5 ng/ml each). TER was monitored for 20 h. The bar graph represents quantitative analysis of TER data after 15 h of cell stimulation; n = 4. *P < 0.05. B) Cells were pretreated with PAFAH2 inhibitor SB-480848 (1 µM) for 60 min followed by CM. Then, EC permeability was assessed by measuring TER for 16 h. The bar graph represents quantitative analysis of TER data after 15 h of cell stimulation; n = 4. *P < 0.05. C, D) C57/BL6 mice were exposed to TNF-α (10 μg/kg, i.t.) and PAFAH2 inhibitor SB-480848 (50 mg/kg, i.v.) concurrently, and an extra dose of SB-48048 (50 mg/kg, i.v.) was injected 5 h later. After 24 h, BAL was collected to analyze total cells (C) and protein content (D). Data are expressed as means ± sd; n = 3. *P < 0.05.
DISCUSSION
Impaired antioxidant defense (deregulated redox balance control) is a well-recognized factor associated with aging, which significantly impacts both physiologic and pathologic processes (4, 24). Aging populations suffer from more severe lung injury caused by bacterial pathogens, delayed recovery, and increased mortality (12, 31). Although the impact of redox disbalance in more severe inflammatory response observed in aging population is evident, specific mediators generated as result of activated oxidation and contributing to more severe lung injury, inflammation and barrier dysfunction have not been precisely characterized.
This study demonstrates for the first time a mechanism of more severe lung vascular endothelial barrier dysfunction caused by proinflammatory agents in aging lungs due to elevation of Tr-OxPLs as a result of weakened antioxidant defense. This is also the first report characterizing basal levels of oxidized PLs in young and aging lungs and the global changes in the spectrum of Tr-OxPLs in young and aging lungs exposed to proinflammatory agents.
Compared with the lungs of young animals, aging lungs exhibited increased basal levels of Tr-OxPL products and dramatically up-regulated Tr-OxPL production upon stimulation with LPS or TNF-α. Interestingly, a modest increase in basal levels of Tr-OxPLs in the aging lungs did not lead to lung barrier dysfunction, suggesting intrinsic compensation for Tr-OxPLs’ adverse effects in physiologic conditions.
Among the Tr-OxPL products induced by LPS challenge, elevation of PONPC, POVPC, PGPC, KOdiAPC, and PazPC levels in the lungs was most prominent. Interestingly, this effect was restricted to the lungs; no changes in the Tr-OxPL spectrum were found in the liver tissue. These results suggest that local elevation of Tr-OxPL in the inflamed organ may exacerbate ongoing inflammation and vascular dysfunction without affecting other organs.
The effects of Tr-OxPLs on lung barrier function identified in the LPS-challenged lungs were further studied in cell models. The results showed that all of the tested Tr-OxPL compounds caused a pronounced increase in lung EC permeability. These Tr-OxPLs displayed a time course similar to that of TER decline, which was consistent with the effects of PGPC and lyso-PC described in our previous studies (9, 32).
For in vivo studies, Tr-OxPL levels measured in mouse lungs were normalized to the total lipid phosphorus. Conversion to Tr-OxPL per gram of tissue indicates a 2–3 fold increase in POVPC (0.355 ± 0.061 and 0.617 ± 0.106 µg/ml), PGPC (0.098±0.033 and 0.159 ± 0.033 µg/ml), PONPC (0.645 ± 0.214 and 1.586 ± 0.628 µg/ml), and PazPC (0.0839 ± 0.048 and 0.222 ± 0.072 µg/ml) content in control and inflamed lungs, respectively. It is important to note that Tr-OxPL levels measured in lung tissue samples may not reflect actual local concentrations in pulmonary vasculature; in fact, they may be even higher. These factors may represent a model limitation; however, experimental selection of subthreshold Tr-OxPL concentrations for cell culture experiments with activation by proinflammatory agonists may be an adequate approach to studying functional interactions between altered Tr-OxPL balance and severity of pathogen-induced EC and lung inflammation.
More pronounced elevation of Tr-OxPLs observed in LPS-challenged aging lungs was associated with more severe ALI, as shown by increased protein content, cell counts, and cytokine levels in BAL samples, and by increased vascular leakage indicated by Evans blue lung extravasation assay. Optical imaging of LPS-challenged lungs also showed more pronounced and sustained lung barrier dysfunction in the aging group, whereas LPS-induced injury in young mice was less severe and recovered more quickly (Fig. 1E).
Analysis of the time course of LPS-induced Tr-OxPL elevation was performed using POVPC and PGPC as Tr-OxPL representatives and showed pronounced increase in their levels in young mice after 3 and 6 h post–LPS challenge with decline to basal level within 24 h. By contrast, Tr-OxPL elevation in the lungs of LPS-challenged aging mice was sustained for ≤24 h with higher levels of POVPC and PGPC at each time point. Does Tr-OxPL elevation in the injured lung represent a pathologic mechanism, or do Tr-OxPLs merely serve as passive bystanders? To address this question, we cotreated pulmonary EC in vitro with submaximal concentrations of Tr-OxPL compounds—POVPC, PGPC, and lyso-PC—as well as inflammatory cytokines (CM). Each of the Tr-OxPL compounds augmented EC barrier decline caused by the CM. Similar results were observed in animal model of LPS-induced lung injury in young mice coinjected with POVPC at a dose that does not affect the lung barrier per se. Again, cotreatment with POVPC augmented TNF-α–induced lung barrier dysfunction and inflammatory response to the level observed in aging mice challenged with TNF-α alone. These modeling experiments strongly suggest Tr-OxPLs as a mechanistic cause of exacerbated lung injury response to bacterial pathogens in aging individuals.
Of note, intravenous administration of Tr-OxPLs (POVPC) alone at higher concentrations did not significantly affect inflammatory cell counts in the BAL samples but rather increased lung permeability, indicated by rapid elevation of BAL protein content (data not shown). This permeability effect of POVPC may be due to direct effects on vascular EC junctions. Our previous studies on pulmonary ECs demonstrated rapid Tr-OxPL–induced tyrosine phosphorylation of transmembrane adherens junction protein VE-cadherin, which caused disassembly of the VE-cadherin–p120–catenin–b-catenin protein complex, VE-cadherin internalization, and disassembly of EC cell–cell junctions. This process was attenuated by inhibition of ROS and src tyrosine kinase signaling in cultured pulmonary endothelium (9, 33, 34).
In addition to nonenzymatic oxidative cleavage of PLs, we also cannot exclude involvement of phospholipases. For example, LPS-activated group V phospholipase A2 may increase pulmonary EC permeability through direct hydrolytic action on membrane PLs yielding lyso-PLs such as lyso-PC and lysophosphatidic acid (35). These events, combined with increased generation of Tr-OxPLs as a result of aging-associated deregulation of redox balance, may further boost inflammation and lung barrier dysfunction.
Uncontrolled oxidant stress is indeed a major contributing factor to acute respiratory distress syndrome (ARDS) and sepsis (36, 37), and different antioxidant therapies show some benefits in preclinical ARDS models (38). However, redox signaling plays an essential role in many physiologic mechanisms, and antioxidant treatments, although effective in specific ROS-centric disease conditions, likely exert multiple off-target effects on other systems. In contrast, specific elimination of deleterious Tr-OxPLs may be a more targeted mechanism-based approach to alleviate ARDS severity in aging population. Thus, induction of PAFAH2, a highly specific oxidized PL phospholipase shown to inactivate proinflammatory Tr-OxPLs (30), was tested in this study as a more targeted approach to neutralize deleterious effects of Tr-OxPLs. Remarkably, expression of PAFAH2 improved barrier properties of HPAEC monolayers treated with CM, whereas pharmacologic inhibition of PAFAH2 exacerbated EC barrier dysfunction in vitro and augmented ALI parameters in TNF-α-treated mice. These results suggest that therapeutic strategies to reduce Tr-OxPL production or circulating levels may prove to be efficient approach to attenuate severity of ALI in aging population and accelerate recovery. These findings support the importance of prophylactic general antioxidant treatment of the aging population at risk and additional pharmacologic approaches to stimulate Tr-OxPL clearance in the settings of lung injury and inflammation.
There are various manifestations of oxidative stress in old age, including lipid peroxidation, DNA oxidation, protein oxidation, and a shift in the redox states of thiol/disulfide redox couples such as glutathione, cysteine, and albumin (11). Analysis of basal membrane PL oxidation in old rats (24 mo) showed a 2–3-fold increase compared with their adult (5 mo) counterparts (39). Age-related increase in PL oxidation was linked to reduced expression of key enzymatic and nonenzymatic antioxidants such as glutathione and several superoxide dismutases. The decline in antioxidant capacity with age strongly correlates with increased risk of mortality from various causes (3). Accordingly, oxidative damage due to increased production of ROS provoked nonenzymatic ROS-triggered generation of proinflammatory Tr-OxPLs and augmented lung injury caused by infectious and noninfectious insults (4, 19). Our previous studies have demonstrated that such proinflammatory Tr-OxPLs promote vascular endothelial permeability (10). We described redox-dependent signaling mechanisms driving Tr-OxPL disruptive effects (9). Taken together, then, previous reports and the present data strongly suggest that increased generation of Tr-OxPLs in aging lungs results from down-regulation of antioxidant enzymatic and nonenzymatic defense mechanisms, leading to increased ROS production and activation of nonenzymatic, ROS-dependent mechanisms of PL oxidation, which is in turn associated with more severe ALI.
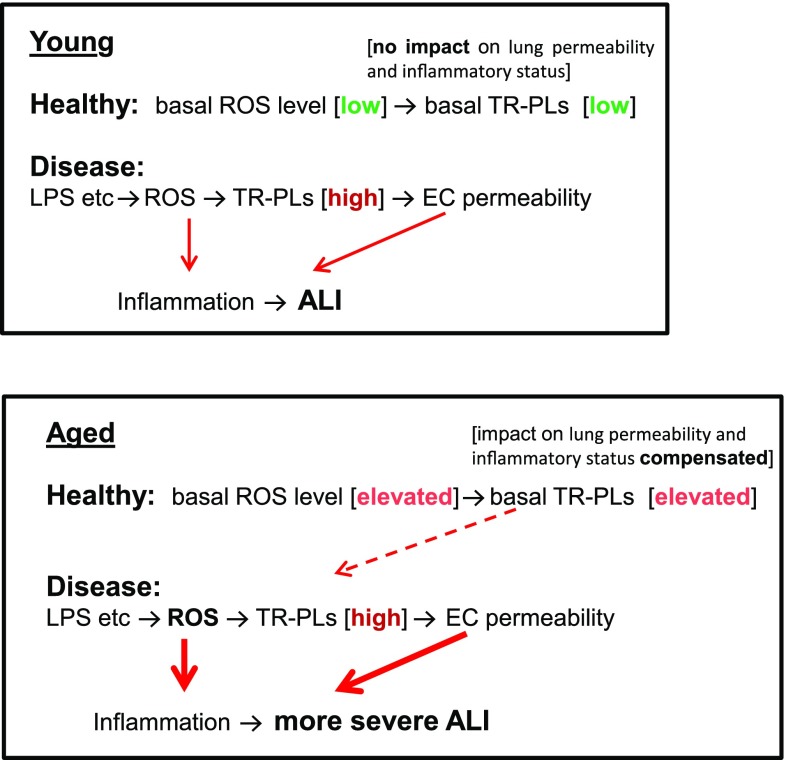
The results of this study are summarized in Fig. 8. In aged individuals, antioxidant defense is reduced and basal Tr-OxPLs are elevated. This state is compensated for, however, and basal levels of Tr-OxPLs do not cause deleterious effects. Tr-OxPL production in aging lungs caused by inflammation is further exacerbated due to age-associated weakening of antioxidant defense mechanisms. As a result, increased Tr-OxPL generation in aging mice augments EC barrier dysfunction and lung inflammation via multiple mechanisms: disassembly of cell–cell junctions; RhoA- and redox-dependent coactivation of the NF-κB cascade, known to augment inflammation and lung barrier dysfunction (15, 40, 41); expression of adhesion molecules and cytokines; and increased infiltration of inflammatory cells into the lungs. Altogether, these factors increase severity of ALI and delay recovery, a complication of disease frequently observed in aging populations. The results of this study further advance our understanding of the pathologic mechanisms behind more severe lung inflammation in the aging population and provide insights into future strategies to confront age-related exacerbation of organ injury and inflammation by inhibiting production or accelerating breakdown of bioactive truncated PLs.
Figure 8.
Schematic model showing the role of Tr-OxPLs in inducing ALI in young and old mice. Basal levels of Tr-OxPL oxidation in the lungs of aged mice increase, although they do not considerably affect basal lung barrier properties and inflammatory status. However, aging-related redox disbalance leads to augmented Tr-OxPL generation upon inflammatory insults by agents such as LPS. As a result, elevated Tr-OxPLs levels exacerbate LPS-induced lung inflammation and barrier dysfunction, leading to more severe ALI in aged groups.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants HL076259 (to K.G.B.), HL087823, HL107920, and HL130431; NIH National Institute on Aging Grant AG048231 (to K.G.B.); and NIH National Institute of General Medical Sciences Grants GM122940 (to K.G.B.) and GM114171 (to A.A.B.). The authors declare no conflicts of interest.
Glossary
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- BHT
butylated hydroxytoluene
- CM
cytokine mixture
- EC
endothelial cell
- HPAEC
human pulmonary artery endothelial cell
- i.t.
intratracheal
- KOdiAPC
O-1-O-palmitoyl-2-O-(5,8-dioxo-8-hydroxy-6-octenoyl)-l-glycero-3-phosphocholine
- LC
liquid chromatography
- lyso-PC
lysophosphocholine
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- PAFAH2
phospholipid-specific platelet-activating factor acetylhydrolase 2
- PazPC
1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine
- PGPC
1-palmitoyl-2-glutaroyl-sn-glycero-phosphocholine
- PL
phospholipid
- PONPC
1-palmitoyl-2-(9-oxo-nonanoyl)-sn-glycero-3-phosphocholine
- POVPC
1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphocholine
- ROS
reactive oxygen species
- TER
transendothelial electrical resistance
- Tr-OxPL
truncated oxidized phospholipid product
AUTHOR CONTRIBUTIONS
Y. Ke collected, analyzed, and interpreted data, and drafted the article; P. Karki collected data and drafted the article; J. Kim collected data; S. Son collected data; E. Berdyshev collected data and critically revised the article; V. N. Bochkov collected data and critically revised the article; A. A. Birukova analyzed and interpreted data and critically revised the article; K. G. Birukov conceived and designed the study, analyzed and interpreted data, and critically revised the article. All authors reviewed the results and approved the final version of the manuscript.
REFERENCES
- 1.Webster R. G. (2000) Immunity to influenza in the elderly. Vaccine 18, 1686–1689 10.1016/S0264-410X(99)00507-1 [DOI] [PubMed] [Google Scholar]
- 2.Stengle J., Dries D. (1994) Sepsis in the elderly. Crit. Care Nurs. Clin. North Am. 6, 421–427 10.1016/S0899-5885(18)30501-X [DOI] [PubMed] [Google Scholar]
- 3.Akbaraly T. N., Favier A., Berr C. (2009) Total plasma carotenoids and mortality in the elderly: results of the Epidemiology of Vascular Ageing (EVA) study. Br. J. Nutr. 101, 86–92 10.1017/S0007114508998445 [DOI] [PubMed] [Google Scholar]
- 4.Starr M. E., Ueda J., Yamamoto S., Evers B. M., Saito H. (2011) The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free Radic. Biol. Med. 50, 371–380 10.1016/j.freeradbiomed.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennathur S., Bergt C., Shao B., Byun J., Kassim S. Y., Singh P., Green P. S., McDonald T. O., Brunzell J., Chait A., Oram J. F., O’brien K., Geary R. L., Heinecke J. W. (2004) Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 279, 42977–42983 10.1074/jbc.M406762200 [DOI] [PubMed] [Google Scholar]
- 6.Kalyanaraman B. (2004) Nitrated lipids: a class of cell-signaling molecules. Proc. Natl. Acad. Sci. USA 101, 11527–11528 10.1073/pnas.0404309101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow J. D., Roberts L. J. (2002) The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am. J. Respir. Crit. Care Med. 166(Suppl 1), S25–S30 10.1164/rccm.2206011 [DOI] [PubMed] [Google Scholar]
- 8.Sprong R. C., Winkelhuyzen-Janssen A. M., Aarsman C. J., van Oirschot J. F., van der Bruggen T., van Asbeck B. S. (1998) Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am. J. Respir. Crit. Care Med. 157, 1283–1293 10.1164/ajrccm.157.4.9508063 [DOI] [PubMed] [Google Scholar]
- 9.Birukova A. A., Starosta V., Tian X., Higginbotham K., Koroniak L., Berliner J. A., Birukov K. G. (2013) Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl. Res. 161, 495–504 10.1016/j.trsl.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birukov K. G., Bochkov V. N., Birukova A. A., Kawkitinarong K., Rios A., Leitner A., Verin A. D., Bokoch G. M., Leitinger N., Garcia J. G. (2004) Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ. Res. 95, 892–901 10.1161/01.RES.0000147310.18962.06 [DOI] [PubMed] [Google Scholar]
- 11.Dröge W. (2002) Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- 12.Hecker L. (2018) Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am. J. Physiol. Lung Cell. Mol. Physiol. 314, L642–L653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu P., Birukova A. A., Xing J., Sammani S., Murley J. S., Garcia J. G., Grdina D. J., Birukov K. G. (2009) Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur. Respir. J. 33, 612–624 10.1183/09031936.00014808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meliton A. Y., Meng F., Tian Y., Sarich N., Mutlu G. M., Birukova A. A., Birukov K. G. (2015) Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L550–L562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukova A. A., Meng F., Tian Y., Meliton A., Sarich N., Quilliam L. A., Birukov K. G. (2015) Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochim. Biophys. Acta 1852, 778–791 10.1016/j.bbadis.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruber F., Bicker W., Oskolkova O. V., Tschachler E., Bochkov V. N. (2012) A simplified procedure for semi-targeted lipidomic analysis of oxidized phosphatidylcholines induced by UVA irradiation. J. Lipid Res. 53, 1232–1242 10.1194/jlr.D025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birukova A. A., Birukov K. G., Smurova K., Adyshev D., Kaibuchi K., Alieva I., Garcia J. G., Verin A. D. (2004) Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 18, 1879–1890 10.1096/fj.04-2328com [DOI] [PubMed] [Google Scholar]
- 18.Dubrovskyi O., Birukova A. A., Birukov K. G. (2013) Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab. Invest. 93, 254–263 10.1038/labinvest.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y. H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J. S., Slutsky A. S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C. J., Penninger J. M. (2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249 10.1016/j.cell.2008.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Z., Li J., Yang L., Mu Y., Xie W., Pitt B., Li S. (2004) Inhibition of LPS- and CpG DNA-induced TNF-α response by oxidized phospholipids. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L808–L816 10.1152/ajplung.00220.2003 [DOI] [PubMed] [Google Scholar]
- 21.Nonas S., Birukova A. A., Fu P., Xing J., Chatchavalvanich S., Bochkov V. N., Leitinger N., Garcia J. G., Birukov K. G. (2008) Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit. Care 12, R27 10.1186/cc6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochkov V. N., Leitinger N., Birukov K. G. (2006) Role of oxidized phospholipids in acute lung injury. Curr. Respir. Med. Rev. 2, 27–37 10.2174/157339806775486182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkel T., Holbrook N. J. (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Xia S., Kalionis B., Wan W., Sun T. (2014) The role of oxidative stress and inflammation in cardiovascular aging. BioMed Res. Int. 2014, 615312 10.1155/2014/615312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oskolkova O. V., Afonyushkin T., Preinerstorfer B., Bicker W., von Schlieffen E., Hainzl E., Demyanets S., Schabbauer G., Lindner W., Tselepis A. D., Wojta J., Binder B. R., Bochkov V. N. (2010) Oxidized phospholipids are more potent antagonists of lipopolysaccharide than inducers of inflammation. J. Immunol. 185, 7706–7712 10.4049/jimmunol.0903594 [DOI] [PubMed] [Google Scholar]
- 26.Von Schlieffen E., Oskolkova O. V., Schabbauer G., Gruber F., Blüml S., Genest M., Kadl A., Marsik C., Knapp S., Chow J., Leitinger N., Binder B. R., Bochkov V. N. (2009) Multi-hit inhibition of circulating and cell-associated components of the toll-like receptor 4 pathway by oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 29, 356–362 10.1161/ATVBAHA.108.173799 [DOI] [PubMed] [Google Scholar]
- 27.Bhatia M., Moochhala S. (2004) Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 202, 145–156 10.1002/path.1491 [DOI] [PubMed] [Google Scholar]
- 28.Foulks J. M., Weyrich A. S., Zimmerman G. A., McIntyre T. M. (2008) A yeast PAF acetylhydrolase ortholog suppresses oxidative death. Free Radic. Biol. Med. 45, 434–442 10.1016/j.freeradbiomed.2008.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stremler K. E., Stafforini D. M., Prescott S. M., McIntyre T. M. (1991) Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J. Biol. Chem. 266, 11095–11103 [PubMed] [Google Scholar]
- 30.McIntyre T. M. (2012) Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: formation, targets, and inactivation. Biochim. Biophys. Acta 1818, 2456–2464 10.1016/j.bbamem.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thannickal V. J., Murthy M., Balch W. E., Chandel N. S., Meiners S., Eickelberg O., Selman M., Pardo A., White E. S., Levy B. D., Busse P. J., Tuder R. M., Antony V. B., Sznajder J. I., Budinger G. R. (2015) Blue journal conference. Aging and susceptibility to lung disease. Am. J. Respir. Crit. Care Med. 191, 261–269 10.1164/rccm.201410-1876PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heffern C. T., Pocivavsek L., Birukova A. A., Moldobaeva N., Bochkov V. N., Lee K. Y., Birukov K. G. (2013) Thermodynamic and kinetic investigations of the release of oxidized phospholipids from lipid membranes and its effect on vascular integrity. Chem. Phys. Lipids 175-176, 9–19 10.1016/j.chemphyslip.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birukova A. A., Lee S., Starosta V., Wu T., Ho T., Kim J., Berliner J. A., Birukov K. G. (2012) A role for VEGFR2 activation in endothelial responses caused by barrier disruptive OxPAPC concentrations. PLoS One 7, e30957 10.1371/journal.pone.0030957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starosta V., Wu T., Zimman A., Pham D., Tian X., Oskolkova O., Bochkov V., Berliner J. A., Birukova A. A., Birukov K. G. (2012) Differential regulation of endothelial cell permeability by high and low doses of oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine. Am. J. Respir. Cell Mol. Biol. 46, 331–341 10.1165/rcmb.2011-0153OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz N. M., Desai A., Meliton L. N., Meliton A. Y., Zhou T., Leff A. R., Dudek S. M. (2012) Group V phospholipase A2 increases pulmonary endothelial permeability through direct hydrolysis of the cell membrane. Pulm. Circ. 2, 182–192 10.4103/2045-8932.97604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chabot F., Mitchell J. A., Gutteridge J. M., Evans T. W. (1998) Reactive oxygen species in acute lung injury. Eur. Respir. J. 11, 745–757 [PubMed] [Google Scholar]
- 37.Prauchner C. A. (2017) Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns 43, 471–485 10.1016/j.burns.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K. I., Tamura F., Sugizaki T., Kawahara M., Kuba K., Imai Y., Mizushima T. (2017) Evaluation of lecithinized superoxide dismutase for the prevention of acute respiratory distress syndrome in animal models. Am. J. Respir. Cell Mol. Biol. 56, 179–190 [DOI] [PubMed] [Google Scholar]
- 39.Cao L., Leers-Sucheta S., Azhar S. (2004) Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J. Steroid Biochem. Mol. Biol. 88, 61–67 10.1016/j.jsbmb.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 40.József L., Khreiss T., El Kebir D., Filep J. G. (2006) Activation of TLR-9 induces IL-8 secretion through peroxynitrite signaling in human neutrophils. J. Immunol. 176, 1195–1202 10.4049/jimmunol.176.2.1195 [DOI] [PubMed] [Google Scholar]
- 41.Guo F., Tang J., Zhou Z., Dou Y., Van Lonkhuyzen D., Gao C., Huan J. (2012) GEF-H1-RhoA signaling pathway mediates LPS-induced NF-κB transactivation and IL-8 synthesis in endothelial cells. Mol. Immunol. 50, 98–107 10.1016/j.molimm.2011.12.009 [DOI] [PubMed] [Google Scholar]