Abstract
The RNA-binding protein LIN28 is known to regulate cell fate, tissue growth, and pluripotency; however, a unified understanding of its role at the cellular level has not been achieved. Here, we address its developmental activity in mammalian postnatal neurogenesis. Constitutive expression of LIN28 in progenitor cells of the mouse subventricular zone (SVZ) caused several distinct effects: 1) the number of differentiated neurons in the olfactory bulb was dramatically reduced, whereas the relative abundance of 2 neuronal subtypes was significantly altered, 2) the population of proliferating neural progenitors in the SVZ was reduced, whereas the proportion of neuroblasts was increased, and 3) the number of astrocytes was reduced, occasionally causing them to appear early. Thus, LIN28 acts at a poststem cell/predifferentiation step, and its continuous expression caused a precocious phenotype unlike in other experimental systems. Furthermore, for the first time in a vertebrate system, we separate the majority of the biologic role of LIN28 from its known activity of blocking the microRNA let-7 by using a circular RNA sponge. We find that although LIN28 has a multifaceted role in the number and types of cells produced during postnatal neurogenesis, it appears that its action through let-7 is responsible for only a fraction of these effects.—Romer-Seibert, J. S., Hartman, N. W., Moss, E. G. The RNA-binding protein LIN28 controls progenitor and neuronal cell fate during postnatal neurogenesis.
Keywords: let-7, circRNA, developmental timing
Developmental timing is a key aspect of proper tissue and organ formation in which distinct cell types are generated through a series of steps from common progenitors. Numerous regulators of developmental timing have been identified in Caenorhabiditis elegans, some of which are conserved in mammals, of which LIN28 is a well-known example. In C. elegans, constitutive expression of LIN28 causes a reiterative phenotype and prevents final maturation, whereas absence of LIN28 causes cells to skip developmental stages (1, 2). LIN28 can induce pluripotent stem cells in combination with sex-determining region Y box 2 (Sox2), octamer-binding transcription factor 4 (Oct4), and Nanog, and has therefore been thought to be a stem cell factor (3, 4). It has been shown to promote proliferation in human and mouse embryonic stem cells, as well as neuroprogenitor cells (5–8).
LIN28 is an RNA-binding protein containing a unique combination of 2 RNA-binding domains: a cold-shock domain and a pair of CCHC motifs (9). The best understood molecular activity of LIN28 is to inhibit the expression of another conserved developmental timing regulator, the micro (mi)RNA let-7 (10–12). When LIN28 is highly expressed, mature let-7 is absent, and as LIN28 is down-regulated, mature let-7 accumulates (10, 13, 14). Substantially less understood is the molecular activity of LIN28 that is independent of let-7 (13, 15, 16). Numerous potential targets have been identified, but a mechanism is not yet clear (5, 6, 8, 13, 17).
In most tissues, LIN28 is expressed in stem cells or other progenitor cells and is temporally repressed to display an on-early/off-late expression profile, including in Drosophila and Xenopus embryos and in a variety of vertebrate tissues (9, 16, 18). In developing mammalian tissues, LIN28 is often found expressed in pluripotent cells and some committed, but still not fully differentiated, cells (7, 13, 19–21). Although it is known to regulate both cell fate and tissue growth and at times to promote an undifferentiated state, thus far, a unified understanding of the biologic role of LIN28 at the cellular level has not been achieved.
The developing nervous system is an important setting for understanding developmental timing. The ordered generation of different cell types and the critical switch from stem cells to committed progenitors require precise regulation to ensure the proper number and types of differentiated neural cells (22). In the embryo, neural progenitors divide and differentiate according to a regular and deterministic program that dictates the number and types of cells produced (23). A cell-intrinsic developmental timing mechanism has been suggested to play an important role in the determination of the clone size of progenitors and the neuronal cell fates (24–27). After birth, neurogenesis occurs in 2 special niches of the mouse brain: the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles. In the SVZ, neural stem cells (NSCs) divide asymmetrically to maintain their own population and to produce transit-amplifying cells (TACs) (28, 29). The NSCs and TACs are identified by their expression of Sox2 and are collectively termed neural progenitor cells (NPCs). Following several rounds of division, the TACs will further differentiate to immature, migratory neurons, known as neuroblasts (NBs) (28). These NBs migrate along the rostral migratory stream (RMS) to the olfactory bulb (OB) (30, 31). They undergo radial migration throughout the OB and terminally differentiate into interneurons.
To explore the function of LIN28 in mammalian neural development, we used electroporation to target the NSCs that line the lateral ventricle in the SVZ of neonatal mice (32–37). Plasmids injected into the lateral ventricle are taken up by NSCs and continue to be expressed in their progeny (Fig. 1A, left). NSC daughter cells leave the SVZ and travel through the RMS into the OB, where they differentiate into mature neurons (Fig. 1A, right). As green fluorescence protein (GFP) is used to mark all cells that take up DNA—as well as their descendants in the SVZ, RMS, and OB—all comparisons between experimental and control regarding marker staining are normalized to the number of plasmid-containing cells. Thus, alterations in clone expansion and cell fate can be inferred by the comparison of experimental and control. This system provides an opportunity to study self-renewal, proliferation, differentiation, and cell-fate choices in a genetically accessible model.
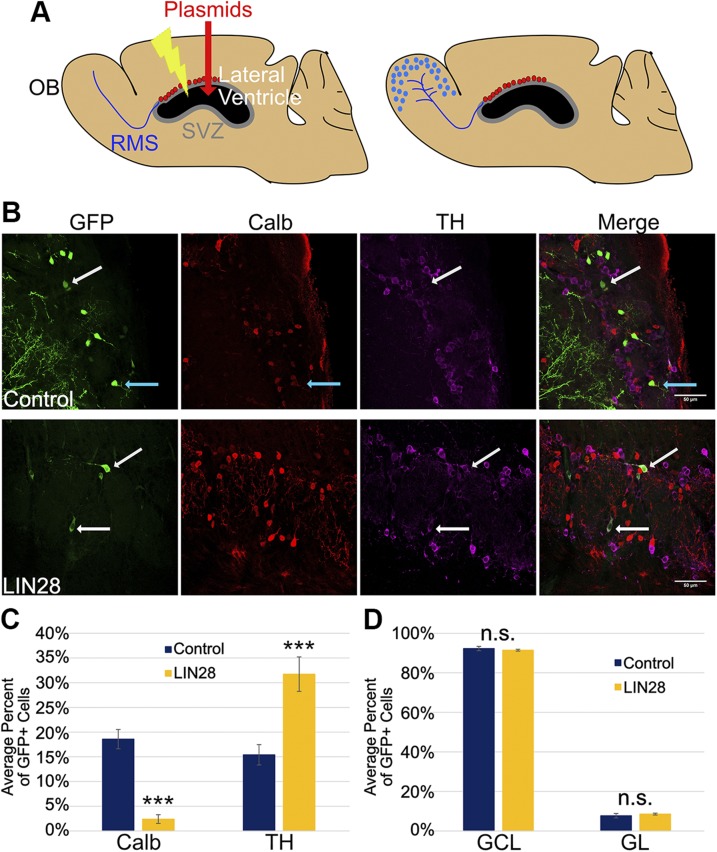
Figure 1.
LIN28 alters cell fate in the GL of the OB at 21 DPE. A) Schematic of a sagittal mouse brain section depicting the electroporation protocol. A) At 0 DPE (left), plasmids are injected into the lateral ventricle, and then electrical impulses are administered so the DNA is incorporated in the NSCs of the SVZ (red dots). The NSCs of the SVZ produce progeny that migrate through the RMS to the OB at 14 DPE or later (right). B) Representative ×40 micrographs showing GFP (green), Calb (red), and TH (purple) immunostaining in the GL. Upper: control. Lower: LIN28. White arrows depict GFP/TH+ neurons; teal arrows depict a GFP/Calb+ neuron. Original scale bars, 50 μM. C) Bar graph depicting the average percent of GFP+ cells positive for Calb or TH at 21 DPE; n = 9 slices (control), n = 10 slices (LIN28::GFP). D) The average percent of GFP+ cells present in either the GCL or GL layers of the OB at 21 DPE; n = 7 slices. C, D) Data presented as means ± sem. ***P ≤ 0.005 vs. control, Student’s t test. N.s., not significant.
As LIN28 is normally down-regulated, as pluripotent cells progress toward differentiation, we investigated the effects of constitutive expression on the number and types of cells produced by clones of NSCs during postnatal neurogenesis. In addition, we assessed the degree to which these effects are a result of the inhibition of LIN28 of let-7, using a novel, circular RNA (circRNA) to inhibit let-7 activity.
MATERIALS AND METHODS
Animals
Wild-type CD1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). All of the animals used in this study were maintained on a 12 h light/dark cycle with ad libitum access to food and water. All of the experiments involving live animals were performed in accordance with the guidelines and regulations of the Institutional Animal Care and Use Committee at Stockton University.
Postnatal electroporation
Electroporation was performed as previously described (32–37). Postnatal d (PN)0–1 CD1 pups were injected with 1 μl plasmid DNA (1–2 μg/μl) with 0.1% Fast Green as a tracer dye directly into the lateral ventricle using a pulled borosilicate glass pipette. Five square pulses of 50 ms duration with 950 ms intervals at 100 V were applied using a pulse ECM830 generator and platinum tweezer-type electrodes (model 520; BTX Harvard Apparatus, Holliston, MA, USA). Pups were then allowed to recover. Experiments were terminated at 1, 3, 7, and 21 d postelectroporation (DPE). All experiments used littermate controls with a minimum of 3 mice per condition (≥3 for control and ≥3 for the experimental condition). The experimental n is given in each figure legend as the total number of slices from the indicated number of mice (32–37). An example of the variability seen from mouse to mouse and slice to slice between control and LIN28 is shown in Supplemental Fig. S1C, D, respectively.
Tissue preparation
Mice were deeply anesthetized with isoflurane. The brains were quickly removed and incubated in 4% paraformaldehyde in 1× PBS at 4°C overnight. They were then transferred to 30% sucrose in 1× PBS and incubated at 4°C overnight with rocking. Brains were embedded in optimal cutting temperature compound and stored at −80°C. Preserved brains were sliced into 50 or 100 µM coronal sections or 40 μM sagittal sections at −20°C on a cryostat (Microm HM 550; Thermo Fisher Scientific, Waltham, MA, USA). Slices were stored in antifreeze at −20°C.
Primary neural progenitor cell culture
Primary neural progenitor cultures were prepared from SVZ regions of P2 CD1 mouse pups. The brain was removed and placed into cold HBSS, supplemented with 5× penicillin/streptomycin. The brain was cut coronally, and the SVZ was microdissected and the tissue minced. The tissue was incubated for 15 min in Accutase, and the enzymatic digestion was stopped by an equal amount of inhibition solution (Stemcell Technologies, Seattle, WA, USA). The resulting cell suspension was washed by adding and resuspending in 10 ml DMEM/F12 (MilliporeSigma, Burlington, MA, USA). The cells were resuspended in neural proliferation medium (1:1 mixture of DMEM/F12 medium and neurobasal medium supplemented with 1× B27, 1× N2, 1× penicillin/streptomycin, 1× Glutamax, 25 μg/ml bovine serum albumin, 20 ng/ml fibroblast growth factor 2, and 20 ng/ml epidermal growth factor) and strained with a 70 μM pore cell filter. Primary NSCs of the SVZ were plated at 5 × 104, cultured in neural proliferation medium, and grown on laminin-treated chamber slides. Growth factors were removed, and 1.6 × 104 NSCs were plated and differentiated for 5 d.
Immunostaining and antibodies
Undifferentiated and differentiated NSCs were fixed in 4% paraformaldehyde for 15 min. Fixed cells were washed and blocked in Block solution for 1 h at room temperature. Antibodies were diluted in 5% normal goat serum and 2% bovine serum albumin in 1× PBS block and incubated for 1 h at room temperature: rat glial fibrillary acidic protein (GFAP; 1:500; Thermo Fisher Scientific, Waltham, MA, USA), rabbit Ki67 (1:250; Neomarkers, Fremont, CA, USA), rabbit doublecortin (DCX; 1:400; Cell Signaling Technology, Danvers, MA, USA), rat LIN28 (1:500), and rabbit Nestin (1:1000; Abcam, Cambridge, MA, USA). Goat anti-rat Alexa Fluor 568 and anti-chicken 633 (1:1000; Thermo Fisher Scientific), and goat anti-rabbit DyLight 550 and 633 (1:1000; Thermo Fisher Scientific) were used as secondary antibodies. Cells were then stained with DAPI (1:1000; Thermo Fisher Scientific), washed, and preserved with Prolong Gold Antifade Reagent (Thermo Fisher Scientific). Cells were coverslipped and examined by confocal microscopy. For in vivo experiments, each staining was replicated in slices from at least 3 mice in a region of interest (SVZ, OB, or RMS). Slices were washed 3 times in 1× PBS. Slices were incubated in 100% methanol for 20 min at 4°C, washed, and then blocked for 1 h at room temperature in Block solution. Slices were then incubated for 48 h at 4°C in primary antibodies diluted in the block. Primary antibodies included the following: rat GFAP (1:500; Thermo Fisher Scientific), rabbit Ki67 (1:250; Neomarkers), rabbit Sox2 (1:100; Abcam), rabbit DCX (1:400; Cell Signaling Technology), rabbit calbindin (Calb; 1:500; Abcam), chicken tyrosine hydroxalase (TH; 1:1000; Abcam), and cleaved Caspase 3 (1:400; Cell Signaling Technology). Slices were washed as above and then incubated for 1.5 h at room temperature in secondary antibodies diluted in the block. Secondary antibodies included the following: goat anti-rat Alexa Fluor 568 and anti-chicken Alexa Fluor 633 (1:1000; Thermo Fisher Scientific) and goat anti-rabbit DyLight 550 and 633 (1:1000; Thermo Fisher Scientific). Slices were washed and incubated in DAPI (1:1000; Thermo Fisher Scientific) for 15 min at room temperature. Slices were washed once again, placed on slides, covered in Prolong Gold Antifade Reagent (Thermo Fisher Scientific), and then coverslipped.
Image acquisition and analysis
Images were acquired on a confocal microscope (Leica True Confocal Scanner SPE). Each staining was replicated in 3–4 slices for each mouse. Images were reconstructed using FIJI ImageJ software for cell counting. Cells were counted and marked for each image in each channel. DAPI was used to verify cells. Raw counts were recorded in Excel. The total number of GFP+ cells was counted, and the percent of the GFP+cells positive for a lineage-specific antigen was calculated. For Calb/TH of the OB glomerular layer (GL), images were taken of the entire layer. For total neurons of the OB, images were taken of the entire OB, and montage images were reconstructed using FIJI ImageJ (https://fiji.sc/; U.S. National Institutes of Health, Bethesda, MD, USA).
Dendrite analysis
GFP/tdTomato+ neurons were captured using confocal microscopy. Only neurons that could be followed from the top of apical dendrites to the tips of the basal dendrites were used. Twenty neurons total for each condition from 3 brains each were analyzed. Neurons were traced using the NeuronJ (https://imagescience.org/meijering/software/neuronj/) plugin of FIJI ImageJ (https://fiji.sc/), and total dendritic length was calculated using the measure-tracings function. These tracings were used to assess dendritic complexity, determined by Sholl analysis in FIJI ImageJ (https://imagej.net/Sholl_Analysis).
RNA isolation and purification
Mouse brains were put into ice-cold 1× HBSS. OBs were removed using a razor and put into 1 ml Qiazol lysis reagent (Qiagen, Germantown, MD, USA). The tissue was manually homogenized using a pipette. Homogenate was incubated in Qiazol for 5 min at room temperature. Chloroform was added to each sample, mixed vigorously, and incubated for 3 min at room temperature. Phases were separated by centrifugation at 12,000 g for 15 min at 4°C. The top phase was transferred to a new tube, mixed with 100% isopropanol, and incubated for 10 min at room temperature. Samples were centrifuged at 12,000 g for 10 min at 4°C. Pellets were saved, washed in 70% ethanol, and centrifuged at 7500 g for 5 min at 4°C. Pellets were briefly air dried and resuspended in nuclease-free H2O.
RNA DNase treatment, cDNA generation, and Taqman real-time PCR
DNA was removed from 200 ng RNA using Turbo DNase (Ambion, Austin, TX, USA), following the manufacturers protocol. Ten nanograms of DNase-treated RNA was input into a cDNA reaction using the components of the Taqman Reverse Transcription Reagents Kit (Thermo Fisher Scientific). Specific primers (×5) for hsa-let-7g and snoRNA202 (endogenous control) were generated and provided in the Taqman MicroRNA Assays Kit by Thermo Fisher Scientific (PN4427975). cDNA was generated using the following thermocycles: 30 min at 16°C, 30 min at 42°C, 5 min at 85°C, and infinite hold at 4°C. Real-time PCR tubes were set up containing 20× assay specific for hsa-let-7g and snoRNA202 (Taqman Reverse Transcription Reagents Kit), 2× Taqman Universal PCR Master Mix No AmpErase Uracil N-Glycosylase (Thermo Fisher Scientific), nuclease-free H2O, and 5 μl cDNA sample. Each sample was done in triplicate. Levels were assessed using relative quantification on a 7500 Real-Time PCR System (Thermo Fisher Scientific).
Plasmids and plasmid construction
LIN28-expressing and GFP control plasmids were reported previously (13, 38). Renilla (pARG-RL) and luciferase (pARG-FF) cell culture reporter plasmids were provided by Houbaviy and colleagues (39). Oligonucleotides for a let-7A target site (see Supplemental Fig. S4B) was cloned into the 3′UTR of the luciferase plasmid. The circRNA-expressing plasmids contained the cytomegalovirus early enhancer element and chicken β-actin (CAG) promoter (lacking the intron), the inverted repeat sequences, and splice sites from the pcDNA3-ciRS-7 plasmid of Hansen et al.(40) and the circle-forming sequence cloned into a BamHI site located between the splice acceptor and donor. The let-7 sponge sequence was generated by GeneArt (Thermo Fisher Scientific) to have 36 18-bp sites with complementarity to let-7a, -d, -f, and -g (Supplemental Table S1). The sponge sequence was cloned into the BamHI site of the circRNA plasmid in both orientations to generate the let-7 sponge and control circRNAs. The in vivo tomato reporter plasmid was generated by amplification of 5 let-7 target sites from the psiCHECK2-let-7, 8× plasmid (#20931; Addgene, Cambridge, MA, USA), and insertion of them into the 3′UTR of the pCAG-tdTomato (41). pCAG-GFP was obtained from Addgene (#11150). circRNA expression in C. elegans was achieved using the constitutive epidermal col-10 promoter, followed by 667 bp of the Caenorhabditis remanei ama-1 intron 2 that included the splice acceptor and an NgoMI site in exon 3. This was followed by the 654 bp, 36 site let-7 sponge sequence in either the forward or reverse orientation, which was followed by 178 bp Crama-1 that included the last 19 bp of exon 3, the splice donor sequence, and part of intron 3. This was followed by the first 489 bp of the intron 2 sequence reversed to make a hairpin, followed by the unc-54 3′UTR. The sequence of the C. elegans circRNA-expressing contstruct is given in Supplemental Table S3.
P19 cell culture
Mouse P19 cells were acquired from American Type Culture Collection (Manassas, VA, USA). P19 cells were cultured in α minimum essential medium (MilliporeSigma); supplemented with 2.5% fetal bovine serum, 7.5% bovine serum, and 50 U/ml penicillin/streptomycin; and grown in 37°C and 5% CO2. Cell lines were transfected using the Xfect Transfection Reagent. Cells were grown for 48 h and then split 1:10 in 10 cm cell-culture dishes. Cells were grown for 48 additional h, and then integrated cell lines were selected by 2 μg/ml puromycin (MilliporeSigma). Individual colonies were selected using trypsin-soaked, 3 mm cloning discs. Colonies were grown in individual wells. Cell lines were confirmed using the Dual Luciferase Reporter Systems (Promega, Fitchburg, WI, USA). Sponge cell lines were harvested, and DNA was purified using the Genomic DNA Extraction protocol of the DNAeasy Blood and Tissue Kit (Qiagen). Taq polymerase (Denville Scientific, Holliston, MA, USA) PCR was used to confirm plasmid DNA. LIN28 was confirmed using fluorescence microscopy. Clonal P19 cell line differentiation was induced using 5 × 10−7 M retinoic acid (MilliporeSigma) and grown on bacterial Petri dishes for 5 d. After this time, cell aggregates in suspension were harvested and dissociated. Cells (2 × 106) were plated on tissue-culture dishes and grown in α minimum essential medium, supplemented with N2 supplement (Thermo Fisher Scientific). Cells were fed every-other day over the time course. Cells (1 × 106) were harvested at d 0 and 14 for luciferase assays and Western blot.
Luciferase assay
Cell pellets were thawed on ice and resuspended in 300 μl 1× Passive lysis buffer. Pellets were incubated for 15 min at room temperature. Aliquots of lysis were placed into technical replicates. Firefly and Renilla luciferase assays were completed with the Dual Luciferase Reporter Systems kit (Promega) on a Glomax 20/20 dual injector luminometer (Promega).
Western blot
OBs were microdissected from the brain, homogenized, and lysed in RIPA buffer (with Halt, 5 mM EDTA, and DNaseI) on ice. Cell pellets were thawed and lysed in Nonidet P-40 buffer (1% Nonidet P-40 buffer, 50 mM Tris HCl, pH 8.0, 150 mM NaCl) on ice for 45 min. Lysed cells were centrifuged for 20 min at 14,000 rpm in 4°C. The supernate was saved and concentration assessed at 595 nm using a spectrophotometer and the Protein Concentration Assay Reagent (Bio-Rad, Hercules, CA, USA) to generate standards. Twenty micrograms of total protein was loaded into a 10% SDS-PAGE gel and separated for 1 h at 200 V. A polyvinylidene fluoride membrane was activated, and proteins were transferred at 30 V for 1 h at 4°C. Membranes were blocked in 10% milk in 1× PBS for 1 h at room temperature. Primary antibodies were incubated overnight at 4°C and diluted in 5% milk in 1× PBS with Tween 20: mouse GFAP (1:1000; MilliporeSigma), mouse TuJ1 (1:1000; Covance, Princeton, NJ, USA), mouse Oct3/4 (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA), rat LIN28 (1:500), and mouse β-actin (1:1000; MilliporeSigma). Bound primary antibodies detected using horseradish peroxidase-conjugated anti-mouse (1:10,000) and anti-rat (1:12,500) were incubated for 1 h at room temperature and diluted in 5% milk in 1× PBS Tween 20.
C. elegans
Nematodes were grown under standard conditions at 20°C. Nomarski differential interference contrast microscopy was used to score adult lateral alae. The developmental stage was assessed by the extent of gonad and germ-line development. All images were taken with a Zeiss Axioplan2 microscope (Carl Zeiss AG, Oberkochen, Germany). Transgenes containing circRNA constructs also contained coinjection markers col-19::GFP and ttx-3::GFP.
Statistical analysis
Student’s t test for 2 samples was used and completed in spreadsheets provided by an online resource to calculate significance (42). Two-way ANOVA was used to assess statistical significance for the Sholl analysis using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA) and paired t test for the luciferase assay.
RESULTS
Constitutive LIN28 alters cell fate in the OB of the mouse
To study LIN28 in this system, we constitutively expressed the open-reading frame of LIN28 fused to GFP under the CAG promoter (13). CAG-GFP was used as a control. To establish the consistency of our procedure, we assessed whether the LIN28-expressing plasmid electroporated at the same rate as controls. When coelectroporated with a plasmid expressing tdTomato (a red fluorescent protein), LIN28::GFP was found to mark 97 and 96% of the all red and green fluorescent cells at 1 and 3 DPE. GFP alone marked 98 and 97% of all of the red and green fluorescent cells at 1 and 3 DPE in the SVZ, respectively. Thus, comparisons between experimental and control regarding marker staining were normalized to the number of GFP+ cells (not to total DAPI+ cells). We also determined that <1% of the GFP+ cells expressed cleaved Caspase 3, a marker for apoptosis, in both control and LIN28 conditions, and their difference was not statistically significant (Supplemental Fig. S1A). Furthermore, the electroporation procedure involved sweeping of the electrodes from dorsal to lateral to ensure uniformity in treating the dorsal, dorsolateral, and lateral regions of the SVZ (35). To confirm this, we viewed GFP at 1 DPE and found that both the control and LIN28 plasmids were electroporated throughout both the dorsal and lateral regions of the SVZ (Supplemental Fig. S1B).
To begin to understand the role of LIN28 in postnatal neural development, we first asked whether constitutive LIN28 expression alters neuronal cell fate in the OB at 21 DPE. Two populations of periglomerular neurons were identified using TH and Calb immunostaining. An even distribution of TH+ and Calb+ neurons was seen in the control (Fig. 1B, upper), whereas a bias toward TH+ neurons was observed in the constitutive LIN28 condition (Fig. 1B, lower). In control conditions, Calb+ neurons comprised 17.8% of the GFP+ population in the GL (Fig. 1C). This population was significantly reduced to 2.5% in the presence of constitutive LIN28 expression. The TH+ population was dramatically increased: the TH+ neurons more than doubled from 15.3 to 31.7% (Fig. 1C). To test whether these changes were the result of mislocalization of migrating cells, we examined the distribution of GFP+ neurons in each of the layers. The vast majority of neurons that migrate into the OB from the SVZ localizes to either the GL or granule cell layer (GCL). In both control and LIN28, 92 and 91%, respectively, of GFP+ neurons localized to the GCL and 8 and 9% to the GL, demonstrating that improper tangential migration was not a major factor in the cell-fate measurements (Fig. 1D). Therefore, we conclude that these findings show that expression of LIN28 influences cell fate in the postnatal OB and can bias developing neurons toward the TH+ periglomerular fate.
Constitutive LIN28 reduces the total number of neurons in the OB
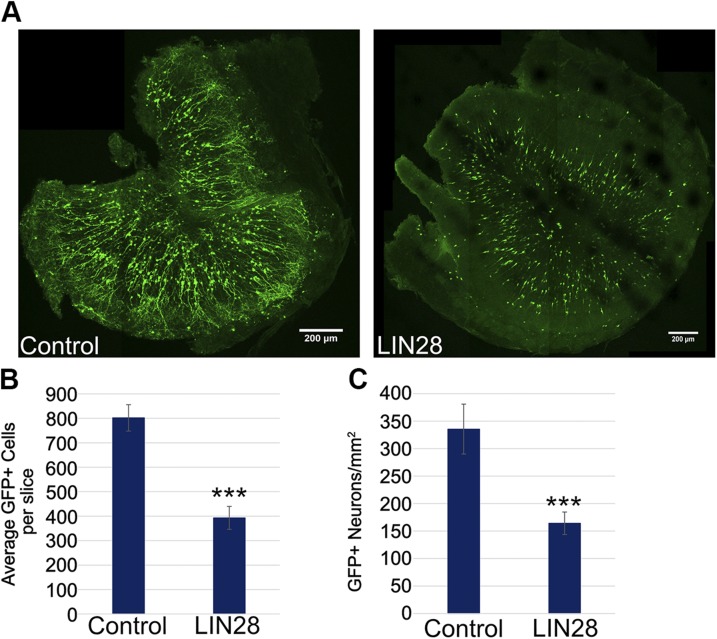
During our studies, we observed that whereas constitutive expression of LIN28 increased TH+ neurons relative to Calb+ neurons, it conspicuously reduced overall the amount of newly generated neurons in the OB (Fig. 2A). The mean number of neurons at 21 DPE in each histologic slice was reduced in ∼50% in the LIN28 condition compared with the control (Fig. 2B). In addition, LIN28 expression reduced the number of neurons per area also by 50% compared with control (Fig. 2C). Constitutive LIN28 appeared to influence the progress of neurogenesis at some stage earlier than differentiation in the OB.
Figure 2.
LIN28 reduces the total number of neurons in the OB at 21 DPE. A) Representative ×10 micrographs displaying total GFP+ cells in the OB of control (left) and LIN28 (right) at 21 DPE. Original scale bars, 200 μM. B) Bar graph showing the average number of total GFP+ neurons in each OB slice at 21 DPE; n = 7 slices (control), n = 10 slices (LIN28::GFP). C) The average number of GFP+ neurons per area (square millimeters) at 21 DPE; n = 7 slices. B, C) Data are presented as means ± sem. ***P ≤ 0.005 vs. control, Student’s t test.
As seen in Fig. 2A, we observed what appeared to be distinct morphology differences between granule GFP+ neurons in control and LIN28 (Supplemental Fig. S2A). Compared with control, LIN28::GFP-expressing granule neurons appeared to lack long branching apical dendrites based on the extent of the fluorescence. With the use of 2 fluorescence markers, where only one is attached to LIN28, we determined that LIN28 is excluded from the apical dendrites, which gave the appearance of altered morphology (Supplemental Fig. S2B). Furthermore, we determined directly that constitutive LIN28 expression does not alter dendritic length and branching complexity in granule neurons (Supplemental Fig. S2C, D).
Constitutive LIN28 reduces the neural progenitor population but does not affect the NSCs
To determine the underlying cause of reduced neuron production, we investigated LIN28 expression during lineage amplification in the SVZ. First, we assessed the expression of endogenous LIN28 in cells derived from the SVZ of untreated animals. Primary NSCs were cultured and stained for endogenous LIN28 and markers for neuronal precursors: GFAP, a marker for NSCs, and Nestin, a marker for NPCs. Of the cultured cells, 100% were positive for GFAP, and 99% were positive for Nestin (Fig. 3A). We observed that 100% of these cells were also positive for LIN28, which is consistent with previous reports showing LIN28 expression in NSCs (Figs. 3A) (6, 7). To induce differentiation of cultured, explanted NSCs into NBs, we removed growth factors for 5 d and found that the resulting cells costained with LIN28 and DCX, a marker for NBs (Fig. 3B). In contrast, we found that LIN28 was not detected in neurons of the OB (Fig. 3C). These findings indicate that endogenous LIN28 is expressed in stem and progenitor cells of the SVZ, as well as cells that differentiated as NBs, but downregulated by the time they differentiate as neurons in the OB.
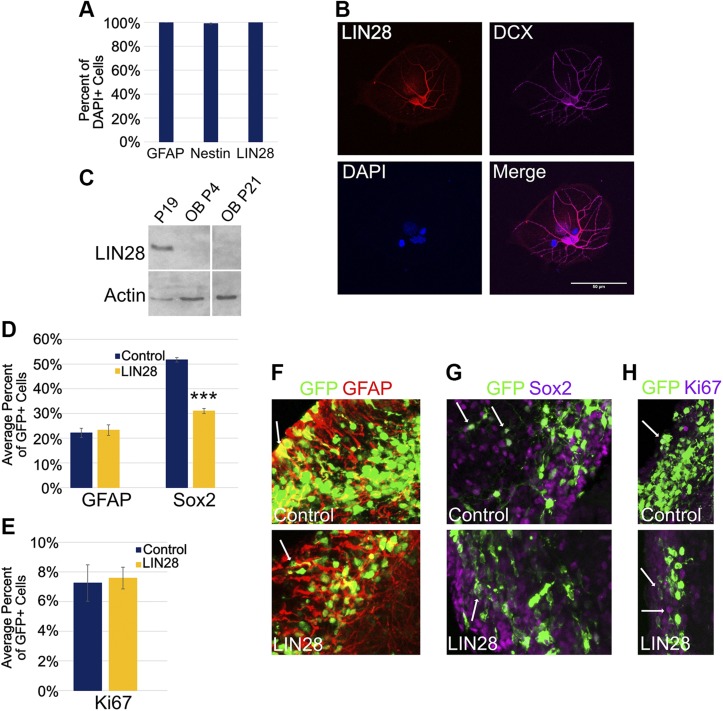
Figure 3.
LIN28 is expressed in NPCs and at 3 DPE, affects the cell types of the SVZ differently. A) Percent of explanted primary DAPI+ NSCs positive for GFAP, Nestin, and LIN28. B) Micrographs (×40) depicting immunostaining of explanted, differentiated NSCs derived from the SVZ. LIN28 (red), DCX (purple), and DAPI (blue). Original scale bar, 50 μM. C) Western blot of LIN28 protein expression in undifferentiated P19 cells (positive control) and the OB in mice at postnatal d 4 and 21; n = 4 mice at each time point. Actin was used as a loading control. D) Bar graph showing the average percent of GFP+ cells positive for GFAP, a marker for NSCs and Sox2, a marker for NPCs at 3 DPE; n = 8 slices (control-GFAP), n = 7 slices (LIN28::GFP-GFAP), and n = 12 slices (Sox2). E) Bar graph depicting the percent of GFP+ cells positive for Ki67, a marker for active proliferation at 3 DPE; n = 12 slices (control), n = 9 slices (LIN28::GFP). F–H) Representative ×30 micrographs depicting immunostaining of NSCs (GFAP, red) (F), NPCs (Sox2, purple) (G), and actively proliferating cells (Ki67, purple) (H). Arrows depict examples of positive cells in each stain. A, D, E) Data presented as means ± sem. ***P ≤ 0.005 vs. control, Student’s t test.
We next compared the proportion of 2 cell populations in the SVZ, NSCs, and TACs in electroporated animals under experimental and control conditions. NSCs exhibit radial glial properties, divide slowly, self renew, and generate all of the progeny cell populations of the SVZ. TACs, on the other hand, are fast-dividing cells that undergo several rounds of division. At 3 DPE, 22% of the GFP+ cells stained positive for GFAP, which we found to be similar under both control and LIN28-expressing conditions (23%; Fig. 3D, F). By contrast, immunostaining for Sox2, a marker for both NSCs and TACs, revealed a difference between control and LIN28-expressing conditions: 52% of the GFP+ cells were positive for Sox2 in control; however, the Sox2+ population was reduced to 31% of GFP+ cells by constitutive expression of LIN28 (Fig. 3D, G).
The reduction of the highly proliferative Sox2+ population suggested that constitutive LIN28 expression might have had its first effect in these cells. There are 2 possible ways to affect proliferation in this situation: 1) the proportion of proliferating cells is reduced, or 2) the proportion of proliferating cells remains the same, but the proliferative stage is shortened; i.e., the number of cell-division cycles is reduced by premature differentiation. With the use of Ki67 as a marker of actively dividing cells, we observed that 7.3 and 7.6%, respectively, of the GFP+ cells were actively proliferating in both the control and LIN28 conditions, suggesting no substantial difference in the proportion of proliferating cells (Fig. 3E, H). Taken together, these data suggest that constitutive LIN28 expression, although having no effect in NSCs, reduced the proliferation of TACs.
LIN28 promotes an increase in the NB population
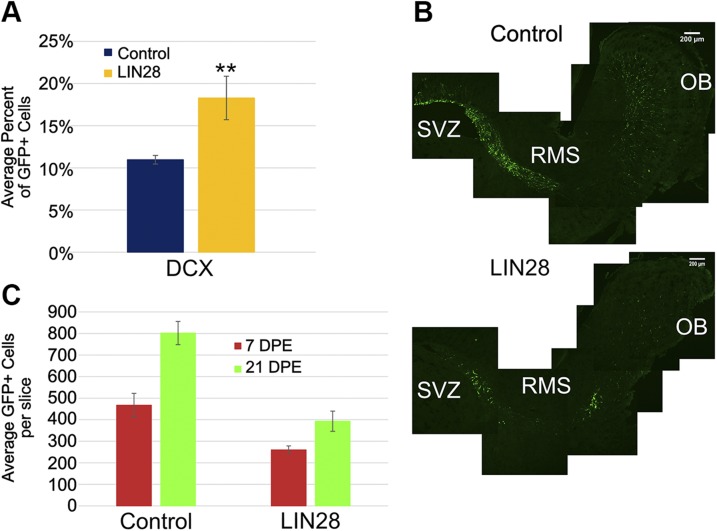
Our observation that endogenous LIN28 is expressed in NBs derived from explanted NSCs (Fig. 3C) suggested that LIN28 could have some function in NBs in vivo. We examined this population in the SVZ at 3 DPE using DCX as a marker. Immunostaining revealed that constitutive LIN28 promoted a 60% increase compared with control: 11% of the GFP+ cells were positive for DCX in control, which increased to 18% with constitutive LIN28 expression (Fig. 4A). As NBs are migratory immature neurons, we looked at sagittal sections of the SVZ, RMS, and OB at 7 DPE to see whether these cells strayed from the RMS. In both control and constitutive LIN28 conditions, the GFP+ cells remained in the RMS and followed the path from SVZ to OB properly (Fig. 4B). To see whether these NBs left the SVZ earlier than normal, we counted the total neurons in the OB at 7 DPE and compared them with the total neurons at 21 DPE. If LIN28 NBs were exiting the SVZ and migrating to the OB at the same time as the control, then we would expect a similar percentage of neurons comparing 7–21 DPE. Control conditions at 7 DPE averaged 467 GFP+ neurons vs. 260 in the LIN28 condition (Fig. 4C). The control at 7 DPE was 58% of the 21 DPE totals, whereas LIN28 was 66% of the 21 DPE total. This suggested more NBs had migrated to the OB in the presence of constitutive LIN28 compared with control. It is possible that we may have underestimated the proportion of NBs in the SVZ that LIN28 promoted, as some may have exited the region earlier than in the control and therefore, escaped our counts. Therefore, it appears that LIN28 was not only endogenously expressed in NBs, but it also promoted an increase in this population. The increase in this NB population caused increased exit from the SVZ, entry into the RMS and ultimately, migration to the OB.
Figure 4.
LIN28 increases the NB population in the SVZ at 3 DPE. A) Bar graph showing the average percent of GFP+ cells positive for DCX, a marker for NBs, at 3 DPE; n = 7 slices (control), n = 6 slices (LIN28::GFP). B) Representative ×10 sagittal micrographs showing the GFP+ cells (green) in the SVZ, RMS, and OB for control (upper) and LIN28 (lower) at 7 DPE. Original scale bars, 200 μM. C) Bar graph displaying the average total GFP+ cells at 7 and 21 DPE. Data (21 DPE) were presented earlier in Fig. 2; n = 11 slices (control), n = 7 slices (LIN28::GFP). A, C) Data are presented as means ± sem. **P ≤ 0.001 vs. control, Student’s t test.
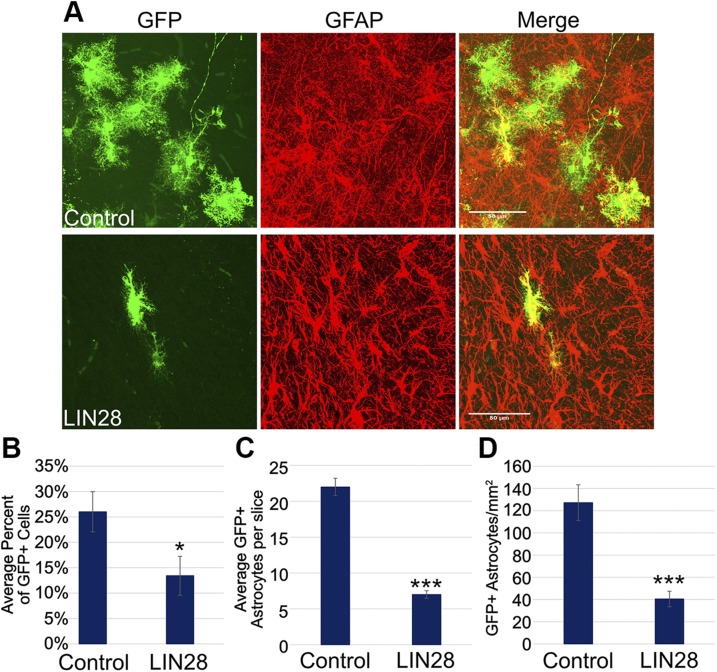
LIN28 causes early astrogliogenesis but ultimately, a reduction in the total number of astrocytes
At 3 DPE, we occasionally observed production of GFP+ astrocytes following LIN28 electroporation well before they should normally appear. Although the appearance of these astrocytes was infrequent and difficult to quantify as a result of low numbers, we saw this with independent electroporations of LIN28 plasmids, and early astrocytes were never present in control conditions at this time point. We further explored the glial population at other time points to determine whether LIN28 altered astrogliogenesis. To do this, we looked at GFP+ astrocytes in the striatum at 21 DPE, the time when the population of astrocytes peaks. We found the astrocyte populations were distinctly different between the control and constitutive LIN28 (Fig. 5A). We quantified astrocytes in the striatum bordering the SVZ as a percent of the GFP+ cells. LIN28 reduced the number of astrocytes by 50%: 26% in control and 13% in constitutive LIN28 conditions (Fig. 5B). The control averaged 22 GFP+ astrocytes in the striatum per slice, whereas constitutive LIN28 averaged 7 GFP+ astrocytes per slice (Fig. 5C). In addition, we quantified the number of astrocytes per area (square millimeters) in each condition. Constitutive LIN28 significantly reduced the astrocytes per area by 68% (Fig. 5D). These data suggest that constitutive expression of LIN28 occasionally caused early astrocyte production but by the same token, caused a reduction in the quantity of astrocytes overall.
Figure 5.
LIN28 reduces the total number of astrocytes at 21 DPE in the striatum. A) Representative ×40 micrographs showing GFP (green)/GFAP+ (red) astrocytes in the striatum of control (upper) and LIN28 (lower) at 21 DPE. Original scale bars, 50 μM. B) Bar graph displaying the average percent of GFP+ cells that are positive astrocytes in the striatum bordering the SVZ at 21 DPE; n = 12 slices (control), n = 15 slices (LIN28::GFP). C) The average number of GFP+ astrocytes in the striatum per slice at 21 DPE; n = 7 slices. D) Bar chart depicting the average number of GFP+ astrocytes per area (square millimeters) at 21 DPE; n = 7 slices. B–D) Data are presented as means ± sem. *P ≤ 0.05, ***P ≤ 0.005 vs. control, Student’s t test.
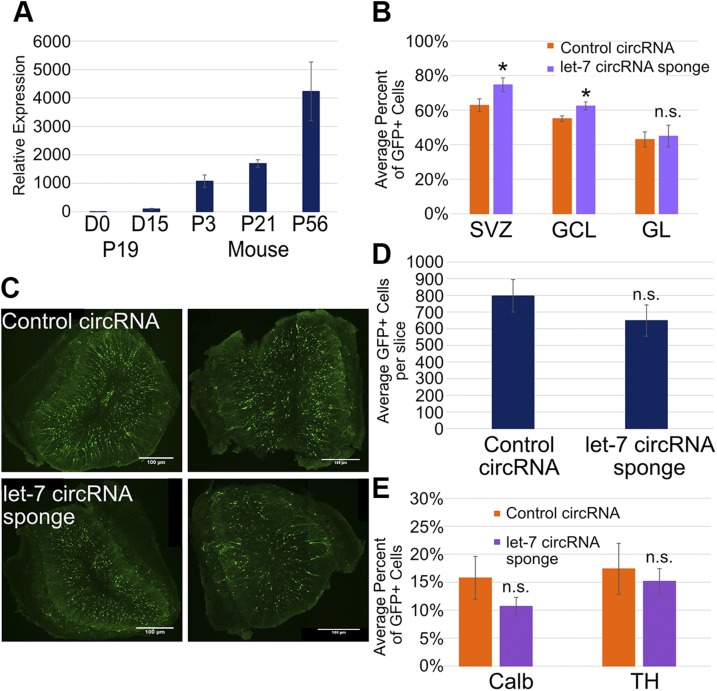
let-7 expression increases in the OB as the mouse ages
LIN28 is best known to block the maturation of the miRNA let-7 (12). Previous work in our lab (13, 14) demonstrated that LIN28 possesses 2 separable activities: one to inhibit let-7 accumulation and the other that is independent of this process. To begin to address whether let-7 has a role in postnatal neurogenesis, we first measured the level of mature let-7 in the OB at different PN ages—PN3, newborn mice; PN21, weaned mice; and PN56, sexually mature adult mice—compared with undifferentiated and differentiated (d 0 and 15, respectively) PN19 embryonal carcinoma cells (Fig. 6A). LIN28 is highly expressed at d 0 in PN19 cells, blocking mature let-7, and as it is downregulated upon differentiation into neurons and astrocytes, let-7 levels increase ∼100-fold (Fig. 6A) (13). In the OB at PN3, let-7 expression was 10 times higher than D15 PN19 cells (Fig. 6A). The level of let-7 increased ∼1.6-fold by PN21 and ∼4-fold by PN56 mice. Thus, let-7 was highly expressed in the OB, consistent with LIN28 being off in neurons (Fig. 3C). Furthermore, even though the OB was established before birth, and let-7 was already highly expressed in PN3 mice, neurons expressed increasing levels of let-7 during the transition to adulthood.
Figure 6.
Relative levels of mature let-7 increase over time. let-7 has a minor effect on cell fate and total neurons in the OB at 21 DPE. A) Bar graph showing that relative levels of let-7g in the OB determined use of real-time PCR, normalized to the endogenous control snoRNA202. P, postnatal. P19 cells were used as a positive (d 15) and negative (d 0) control; n = 4 mice at each time point. B) Bar graph depicting the average percent of GFP+ cells expressing tdTomato at 21 DPE in the SVZ and the GCL and GL of the OB; n = 6 slices (control), n = 4 slices (LIN28::GFP). C) Representative ×10 micrographs displaying total GFP+ cells (green) in the OB of control circRNA (upper left and right) and let-7 circRNA sponge (lower left and right) at 21 DPE. Original scale bars, 100 μM. D) Bar graph showing the average total GFP+ neurons in each OB slice at 21 DPE; n = 7 slices (control), n = 7 slices (LIN28::GFP). E) The average percent of GFP+ cells positive for Calb or TH at 21 DPE; n = 5 slices (control), n = 7 slices (LIN28::GFP). A, B, D, E) Data are presented as means ± sem. *P ≤ 0.05 vs. control, Student’s t test.
A circRNA sponge was used to inhibit let-7 activity
We next investigated whether some or all of the effects of LIN28 on postnatal neurogenesis might be a result of an inhibition of let-7. To do this, we sought a means to inhibit let-7 in cells developing from the SVZ that is independent of LIN28. circRNA sponges have been shown to sequester miRNAs effectively away from their mRNA targets, thereby reducing or eliminating their regulatory effects (40). Therefore, we endeavored to block let-7 activity directly using such a sponge. To target multiple members of the let-7 family, we designed an miRNA sponge containing 36 repeats of diverse let-7 target sites, 18 nt apart, directed at 4 members of the family: let-7a, -d, -f, and -g (Supplemental Table S1) (13). Based on the designs of Hansen et al. (40), constructs were generated to express the let-7 sponge stably as RNA circles: the order of the splice acceptor and donor was reversed, resulting in circle formation and a long, inverted repeat to bring the splice sites together (Supplemental Fig. S3A). As a control, we generated a version that contained the same 36 repeats but in the opposite orientation, resulting in a repetitive sequence that cannot bind let-7 miRNAs.
To test the efficacy of the circRNA sponge, we measured its ability to phenocopy a let-7 mutant in C. elegans (43). We found that nematodes expressing the let-7 sponge resembled a let-7-deficient worm in their developmental phenotypes and in the prevention of expression of a differentiation-specific reporter (Supplemental Fig. S3B, C and Supplemental Table S2). These results indicate that the let-7 circRNA sponge was a potent inhibitor of let-7 in C. elegans.
We next tested the efficacy of the let-7 circRNA sponge in mouse PN19 cells undergoing neurogenic differentiation in vitro. We used a luciferase reporter assay where the open-reading frame of firefly luciferase was constitutively expressed under the CAG promoter, and the 3′UTR contained a let-7a target sequence (Supplemental Fig. S4A, B). Clonal cell lines were generated expressing a luciferase reporter, a Renilla luciferase reporter for normalization, and a test plasmid expressing the let-7 circRNA sponge, a control circRNA, LIN28::GFP, or empty vector sequence. All cell lines were confirmed to express luciferase, Renilla, and the circRNAs or LIN28::GFP as intended. In undifferentiated cells (d 0), there is no let-7 present, but as the cells differentiate into neurons and astrocytes, let-7 levels increase until the end of the time course (d 14). As expected, luciferase was highly expressed at d 0 in all conditions (Supplemental Fig. S4C). Upon differentiation, let-7 activity would increase unless blocked, and luciferase expressed from a let-7-regulated reporter would be correspondingly downregulated. With both empty vector and control circRNA, luciferase was down-regulated at d 14; however, luciferase expression remained high in the presence of LIN28 and the let-7 circRNA sponge (Supplemental Fig. S4C). Thus, the let-7 circRNA sponge was as effective as LIN28 in blocking let-7 during PN19 neurogenic differentiation in vitro.
Previously, we showed that constitutive LIN28 inhibited gliogenesis of PN19 cells in vitro, and so we asked whether direct inhibition of let-7 had the same effect (13). GFAP was detected by immunoblot at d 14 in the control circRNA cell line but absent in the LIN28 line (Supplemental Fig. S4D). When the let-7 sponge was expressed, d 14 cells were positive for GFAP, indicating that gliogenesis had occurred. This result suggested that LIN28 blocked gliogenesis in differentiating PN19 cells independent of let-7.
Direct inhibition of let-7 activity alters neuron fate and abundance in the OB
To block let-7 activity directly in cells developing from the SVZ we electroporated a sponge-expressing construct into mouse NSCs. To demonstrate that the sponge was functional in vivo, we electroporated the let-7 circRNA sponge or the control circRNA with CAG-GFP and a repressible CAG-tdTomato reporter containing 5 let-7 target sites in the 3′UTR. If let-7 is expressed and active, then we would expect red tdTomato fluorescence to be down-regulated. If the let-7 circRNA sponge is able to inhibit let-7 activity effectively in vivo, then red fluorescence would continue. In the SVZ at 21 DPE, 63% of GFP+ cells showed red fluorescence in the control condition, whereas 75% of GFP+ cells showed red fluorescence in the let-7 circRNA sponge condition (Fig. 6B). Likewise, more GFP+ granule neurons showed red fluorescence in the presence of the let-7 circRNA (62%) than in the presence of the control circRNA (55%). Red tdTomato expression was comparable in the control circRNA (43%) and let-7 circRNA sponge (45%) conditions in the periglomerular neurons. The results of the reporter assay indicated that the let-7 circRNA sponge effectively blocked let-7 activity in vivo.
The incorporation of the let-7 circRNA sponge produced a small, statistically insignificant but quantifiable decrease in GFP+ neurons in the OB at 21 DPE compared with the control circRNA. The control circRNA averaged 797 neurons per slice, consistent with CAG-GFP control, whereas the let-7 circRNA sponge reduced total GFP+ neurons to 649 per slice, a 19% reduction (Fig. 6C, D). In addition, the let-7 sponge reduced both populations of periglomerular neurons: Calb+ from 15.8 to 10.7% and TH+ from 17.4 to 15.2% (Fig. 6E). Although these populations were reduced compared with their respective control populations, they were both still within the wild-type-reported ranges in the literature (44). As direct inhibition of let-7 activity in cells developing from the SVZ had only a modest effect on neuron fate and abundance in the OB, a significant fraction of the effects of constitutive LIN28 may be independent of its inhibition of let-7.
DISCUSSION
Constitutive expression of LIN28 in cells derived from the SVZ caused several distinct effects on postnatal neurogenesis in the mouse: it dramatically reduced the number of differentiated neurons, it altered the relative abundance of 2 neuronal subtypes, it reduced the population of proliferating neural progenitors in the SVZ, whereas increasing the proportion of NBs, it promoted exit of NBs from the SVZ, and it reduced the number of astrocytes and occasionally causing them to appear early. Direct inhibition of let-7, one of the targets of LIN28, caused a small change in differentiated neurons, consistent with its involvement in a subset of the phenotypes of LIN28.
LIN28 is often described as a pluripotency factor that promotes proliferation, so we expected that LIN28 might affect the growth of the NSC population in the SVZ, yet it did not. Our finding that endogenous LIN28 is expressed in cultured primary NSCs may explain constitutive lack of effect of LIN28: changes in these cells would not occur if LIN28 were already expressed in them. However, we might expect the highly proliferative TACs to be altered by constitutive LIN28, likely expanding and extending the proliferative stage. In fact, the opposite occurred, and the TAC population was significantly reduced. We observed that the proportion of actively proliferating cells was unchanged by constitutive LIN28. Furthermore, neither electroporation efficiency nor an increase in apoptosis was a factor in the TAC reduction.
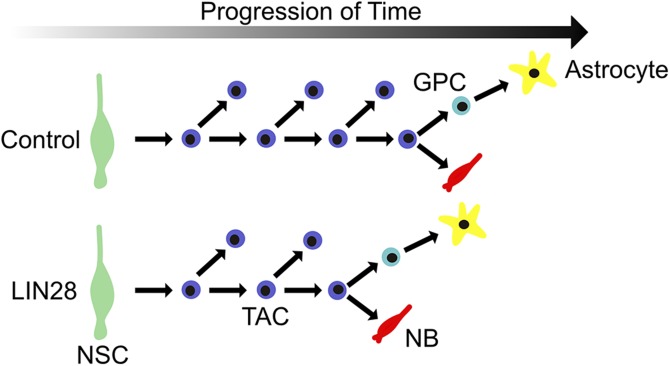
The reduction in proliferating TACs may be connected to the fact that constitutive LIN28 promotes an increase in the NB population. Over the course of normal postnatal neurogenesis in the SVZ, NSCs divide slowly, and then self renew, and ultimately form TACs, which divide rapidly. The TACs undergo several rounds of division and then further differentiate to NBs (29, 45). We propose that when LIN28 is constitutively expressed, the TACs exit the proliferation stage early, thus promoting the NB population (Fig. 7). This would also explain the premature appearance of astrocytes, where the cell-fate switch to astrogliogenesis is also moved up in time. Thus, LIN28 does not govern specific cell behaviors, such as division, maturation, or differentiation itself, but controls the timing of cell type- and lineage-specific decisions. Importantly, the shortening of the proliferation stage of TAC ultimately reduces the overall number of its descendants. Therefore, although LIN28 promotes a proportional increase in the NB population, the total number of all cell types (NPCs, NBs, astrocytes, and neurons) is reduced.
Figure 7.
LIN28 causes TACs to differentiate prematurely to NBs and causes early astrogliogenesis. Control (upper); LIN28 (lower). NSC, green; TAC, blue; NB, red. GPC, glial progenitor cell (light blue).
The question arises of whether the effects of constitutive LIN28 on the cell-fate changes in the OB relate to the premature switch from proliferation to differentiation in the SVZ. Loss of paired box 6, which is normally highly expressed in TACs of the SVZ, resulted in the reduction of the TH+ population, demonstrating that periglomerular cell-fate choices were determined quite early during neurogenesis (46). Therefore, with the promotion of a switch from the proliferating TAC stage to the NB stage, constitutive LIN28 may have also affected a contemporaneous switch concerning cell-fate decisions in the peri-GL. The periglomerular subtypes are generated in a specific order: TH+ first and Calb+ second during embryonic development (47). If this relationship is also true in postnatal neurogenesis, then the preferential generation of LIN28 of TH+ neurons, an earlier fate, occurred at the expense of Calb+ neurons, a later fate.
Our experiments with cultured, primary NSCs revealed that endogenous LIN28 was expressed in both neural progenitors and NBs. We have not been able to achieve sensitive staining of histologic sections using our anti-LIN28 antibodies, so we do not know the levels of LIN28 in each of these populations in vivo. Nevertheless, we must consider how exogenous expression of LIN28 can alter the behavior of TACs and NBs in which LIN28 is already endogenously expressed. From investigations into muscle development, it was found that different levels of constitutive LIN28 caused different outcomes (20). This is not unique to LIN28; it has been shown for another pluripotency factor Oct3/4 that different levels of the factor cause different outcomes: an increase in expression caused differentiation, whereas loss of expression resulted in loss of pluripotency (48). We speculate that the absolute level—not merely presence or absence—of LIN28 in a developing cell plays a role in its cell type-specific proliferation or differentiation choices. Although LIN28 may be endogenously expressed in TACs, higher levels may promote a switch from proliferation to differentiation into the NB stage.
Our findings run counter to prevailing narratives about LIN28. First, constitutive expression of LIN28 in C. elegans and some mammalian systems is generally thought to promote proliferation and tissue growth over differentiation. However, in our in vivo experiments, constitutive LIN28 appears to reduce proliferation at one developmental stage (TACs) and cause precocious development of one cell type (astrocytes). Detailed studies in C. elegans show that LIN28 acts primarily in a developmental timing mechanism where cells of different lineages use that timing information in different ways: in some cases, to proliferate if appropriate and in other cases, to stay quiescent (1, 49). We should not be surprised to find as we learn more about the activity of LIN28 in different developmental contexts that its up- or downregulation causes cell behaviors particular to a given tissue and a developmental time point. LIN28 is not simply a promoter of proliferation and an undifferentiated state (16). Furthermore, the expression of LIN28 in NBs was surprising, only because that cell type is already committed to a neuronal lineage. LIN28 is also expressed in regenerating muscle and erythroblasts, also committed lineages (20, 21).
Although the most well-known molecular activity of LIN28 is to inhibit the maturation of the let-7 miRNA, in several different systems, LIN28 has been demonstrated to operate by other mechanisms (10–14, 16, 18). As the secondary mechanism is not well defined, we are unable to determine what fraction of the biologic roles of LIN28 is attributable to either mechanism. In C. elegans, the majority of the activity of LIN28 is let-7 independent (14). Therefore, we attempted to shed some light on whether constitutive effects of LIN28 in postnatal development were a result of its inhibition of let-7. To do this, we developed a circRNA sponge specific for let-7 based on the work of Hansen et al. (40). Although other sponge methods have been used, the circular form is based on naturally occurring miRNA sponges, enhancing RNA stability within the cell and potentially targeting multiple family members at one time. Our let-7 circRNA contains 36 repeats of target sites, 18 nt apart, that were optimized to 4 different let-7 variants observed in neurogenesis. This circRNA sponge is effective in 3 systems: C. elegans, in vitro neurogenesis, and postnatal neurogenesis. Despite its measured effectiveness at inhibiting let-7 activity, our observations suggest that let-7 makes a minor contribution to the effects that we observed and that other activities of LIN28 may account for the majority of the phenotypes.
Petri et al. (50) recently demonstrated a decline in radial migration of NBs in the OB following knockdown of let-7 using a different kind of sponge. Although it is difficult to compare the results directly, we note that they introduced their lentiviral vectors at 10 wk into the RMS, whereas we introduced our sponge at PN0–PN1 into the SVZ and assessed it at 3 wk. We showed there is a dramatic rise in let-7 levels in the OB over time. It is difficult to know, given the state of our knowledge, when relevant let-7 targets are acting and being repressed to control proper postnatal neurogenesis.
Various candidates have been proposed for the let-7-independent targets of LIN28. LIN28 may promote translation through direct binding of a target’s mRNA (5, 17, 51–55). This, in combination with its association with eukaryotic translation initiation factors 3β and 4E in messenger RNPs and polyribosomes, could suggest a role in promoting translation (20, 56). Furthermore, loss of LIN28 results in a shift of target mRNAs from the polysomal to the nonpolysomal fraction, further supporting a positive role in translation (6). The mammalian target of rapamycin (mTOR) pathway plays a key role in cell-fate choices in the SVZ, and differential expression of LIN28 has been shown to affect the levels of downstream mTOR targets during embryonic neural development (8, 36). Approximately 80% of NBs in the SVZ expressed phosphorylated eukaryotic translation initiation factor 4E-binding protein, indicative of active mTOR and cap-dependent translation (36). This could suggest that LIN28 is positively regulating members of the mTOR pathway and cap-dependent translation to promote NB differentiation. Other candidate targets have been proposed that may or may not make sense in neural development, but all of these remain to be examined.
Importantly, neurogenesis has been shown to differ between primate and rodent species in the developmental timing of neurogenic clones, an evolutionary phenomenon termed heterochrony (27, 57). Although multiple factors are likely to govern the specific switches from symmetric to asymmetric divisions and then to differentiation and the lengths of the proliferative and neurogenic phases, we speculate that previously identified developmental timing regulators, such as LIN28, have roles in this process. We demonstrate here that the expression of LIN28 does indeed influence the number and types of cells produced from a clone of postnatal NSCs, and it does so in a way that suggests important dynamics in its regulation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank John Rokita and the animal laboratory staff at Stockton University for animal care, Dr. Hristo Houbaviy (Rowan University) for expert advice and plasmids, and Kevin Kemper and Madeleine Minutillo (Rowan University) for comments on the manuscript. This work was funded, in part, by the U.S. National Institutes of Health, National Institute of Neurological Disorders and Stroke, Grant R15NS092026-01A1 (to N.W.H). The authors declare no conflicts of interest.
Glossary
- CAG
cytomegalovirus early enhancer element and chicken β-actin
- Calb
calbindin
- circRNA
circular RNA
- DCX
doublecortin
- DPE
days postelectroporation
- GCL
granule cell layer
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescence protein
- GL
glomerular layer
- miRNA
microRNA
- mTOR
mammalian target of rapamycin
- NB
neuroblast
- NPC
neural progenitor cell
- NSC
neural stem cell
- OB
olfactory bulb
- Oct3/4
octamer-binding transcription factor 3/4
- PN
postnatal day
- RMS
rostral migratory stream
- Sox2
sex-determining region Y box 2
- SVZ
subventricular zone
- TAC
transit-amplifying cell
- TH
tyrosine hydroxalase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. S. Romer-Seibert executed all cell culture and experiments, data analysis and statistical analysis for P19 cell culture, mouse and NSC culture, performed all mouse postnatal electroporations (except for 2), designed and compiled all of the figures, made the supplemental tables, and wrote the original manuscript; N. W. Hartman performed 2 postnatal electroporations and provided oversight for the mouse project and edits and original text for the manuscript; and E. G. Moss completed all of the C. elegans work, data analysis, and statistical analysis, designed the sponge sequence, provided oversight for the P19 and mouse projects, made the supplemental tables, and provided edits and original text for the manuscript.
REFERENCES
- 1.Ambros V., Horvitz H. R. (1984) Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409–416 [DOI] [PubMed] [Google Scholar]
- 2.Moss E. G., Lee R. C., Ambros V. (1997) The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 88, 637–646 [DOI] [PubMed] [Google Scholar]
- 3.Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., Nie J., Jonsdottir G. A., Ruotti V., Stewart R., Slukvin I. I., Thomson J. A. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 [DOI] [PubMed] [Google Scholar]
- 4.Hanna J., Saha K., Pando B., van Zon J., Lengner C. J., Creyghton M. P., van Oudenaarden A., Jaenisch R. (2009) Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu B., Zhang K., Huang Y. (2009) Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA 15, 357–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng S., Chen L. L., Lei X. X., Yang L., Lin H., Carmichael G. G., Huang Y. (2011) Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells 29, 496–504 [DOI] [PubMed] [Google Scholar]
- 7.Cimadamore F., Amador-Arjona A., Chen C., Huang C. T., Terskikh A. V. (2013) SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc. Natl. Acad. Sci. USA 110, E3017–E3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M., Yang S. L., Herrlinger S., Liang C., Dzieciatkowska M., Hansen K. C., Desai R., Nagy A., Niswander L., Moss E. G., Chen J. F. (2015) Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development 142, 1616–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss E. G., Tang L. (2003) Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev. Biol. 258, 432–442 [DOI] [PubMed] [Google Scholar]
- 10.Newman M. A., Thomson J. M., Hammond S. M. (2008) Lin-28 interaction with the let-7 precursor loop mediates regulated microRNA processing. RNA 14, 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rybak A., Fuchs H., Smirnova L., Brandt C., Pohl E. E., Nitsch R., Wulczyn F. G. (2008) A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 10, 987–993 [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan S. R., Daley G. Q., Gregory R. I. (2008) Selective blockade of microRNA processing by Lin28. Science 320, 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balzer E., Heine C., Jiang Q., Lee V. M., Moss E. G. (2010) LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development 137, 891–900 [DOI] [PubMed] [Google Scholar]
- 14.Vadla B., Kemper K., Alaimo J., Heine C., Moss E. G. (2012) lin-28 controls the succession of cell fate choices via two distinct activities. PLoS Genet. 8, e1002588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu H., Shah S., Shyh-Chang N., Shinoda G., Einhorn W. S., Viswanathan S. R., Takeuchi A., Grasemann C., Rinn J. L., Lopez M. F., Hirschhorn J. N., Palmert M. R., Daley G. Q. (2010) Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat. Genet. 42, 626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faas L., Warrander F. C., Maguire R., Ramsbottom S. A., Quinn D., Genever P., Isaacs H. V. (2013) Lin28 proteins are required for germ layer specification in Xenopus. Development 140, 976–986 [DOI] [PubMed] [Google Scholar]
- 17.Qiu C., Ma Y., Wang J., Peng S., Huang Y. (2010) Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 38, 1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsialikas J., Romer-Seibert J. (2015) LIN28: roles and regulation in development and beyond. Development 142, 2397–2404 [DOI] [PubMed] [Google Scholar]
- 19.Yang D. H., Moss E. G. (2003) Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr. Patterns 3, 719–726 [DOI] [PubMed] [Google Scholar]
- 20.Polesskaya A., Cuvellier S., Naguibneva I., Duquet A., Moss E. G., Harel-Bellan A. (2007) Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 21, 1125–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vasconcellos J. F., Fasano R. M., Lee Y. T., Kaushal M., Byrnes C., Meier E. R., Anderson M., Rabel A., Braylan R., Stroncek D. F., Miller J. L. (2014) LIN28A expression reduces sickling of cultured human erythrocytes. PLoS One 9, e106924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConnell S. K. (1985) Migration and differentiation of cerebral cortical neurons after transplantation into the brains of ferrets. Science 229, 1268–1271 [DOI] [PubMed] [Google Scholar]
- 23.Gao P., Postiglione M. P., Krieger T. G., Hernandez L., Wang C., Han Z., Streicher C., Papusheva E., Insolera R., Chugh K., Kodish O., Huang K., Simons B. D., Luo L., Hippenmeyer S., Shi S. H. (2014) Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 159, 775–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian X., Goderie S. K., Shen Q., Stern J. H., Temple S. (1998) Intrinsic programs of patterned cell lineages in isolated vertebrate CNS ventricular zone cells. Development 125, 3143–3152 [DOI] [PubMed] [Google Scholar]
- 25.Qian X., Shen Q., Goderie S. K., He W., Capela A., Davis A. A., Temple S. (2000) Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28, 69–80 [DOI] [PubMed] [Google Scholar]
- 26.Espinosa J. S., Luo L. (2008) Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J. Neurosci. 28, 2301–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otani T., Marchetto M. C., Gage F. H., Simons B. D., Livesey F. J. (2016) 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doetsch F., Caillé I., Lim D. A., García-Verdugo J. M., Alvarez-Buylla A. (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 [DOI] [PubMed] [Google Scholar]
- 29.Costa M. R., Ortega F., Brill M. S., Beckervordersandforth R., Petrone C., Schroeder T., Götz M., Berninger B. (2011) Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development 138, 1057–1068 [DOI] [PubMed] [Google Scholar]
- 30.Lois C., Alvarez-Buylla A. (1994) Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145–1148 [DOI] [PubMed] [Google Scholar]
- 31.Lois C., García-Verdugo J. M., Alvarez-Buylla A. (1996) Chain migration of neuronal precursors. Science 271, 978–981 [DOI] [PubMed] [Google Scholar]
- 32.Lacar B., Young S. Z., Platel J. C., Bordey A. (2010) Imaging and recording subventricular zone progenitor cells in live tissue of postnatal mice. Front. Neurosci. 4 pii: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platel J. C., Dave K. A., Gordon V., Lacar B., Rubio M. E., Bordey A. (2010) NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron 65, 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feliciano D. M., Quon J. L., Su T., Taylor M. M., Bordey A. (2012) Postnatal neurogenesis generates heterotopias, olfactory micronodules and cortical infiltration following single-cell Tsc1 deletion. Hum. Mol. Genet. 21, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feliciano D. M., Lafourcade C. A., Bordey A. (2013) Neonatal subventricular zone electroporation. J. Vis. Exp. e50197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartman N. W., Lin T. V., Zhang L., Paquelet G. E., Feliciano D. M., Bordey A. (2013) mTORC1 targets the translational repressor 4E-BP2, but not S6 kinase 1/2, to regulate neural stem cell self-renewal in vivo. Cell Reports 5, 433–444 [DOI] [PubMed] [Google Scholar]
- 37.Mahoney C., Feliciano D. M., Bordey A., Hartman N. W. (2016) Switching on mTORC1 induces neurogenesis but not proliferation in neural stem cells of young mice. Neurosci. Lett. 614, 112–118 [DOI] [PubMed] [Google Scholar]
- 38.Matsuda T., Cepko C. L. (2004) Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 101, 16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S., Aksoy M., Shi J., Houbaviy H. B. (2014) Evolution of the miR-290-295/miR-371-373 cluster family seed repertoire. PLoS One 9, e108519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen T. B., Jensen T. I., Clausen B. H., Bramsen J. B., Finsen B., Damgaard C. K., Kjems J. (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 [DOI] [PubMed] [Google Scholar]
- 41.Pathania M., Torres-Reveron J., Yan L., Kimura T., Lin T. V., Gordon V., Teng Z. Q., Zhao X., Fulga T. A., Van Vactor D., Bordey A. (2012) miR-132 enhances dendritic morphogenesis, spine density, synaptic integration, and survival of newborn olfactory bulb neurons. PLoS One 7, e38174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald J. H. (2014) Handbook of Biological Statistics, 3rd ed., Sparky House Publishing, Baltimore, MD, USA [Google Scholar]
- 43.Reinhart B. J., Slack F. J., Basson M., Pasquinelli A. E., Bettinger J. C., Rougvie A. E., Horvitz H. R., Ruvkun G. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906 [DOI] [PubMed] [Google Scholar]
- 44.Nagayama S., Homma R., Imamura F. (2014) Neuronal organization of olfactory bulb circuits. Front. Neural Circuits 8, 98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponti G., Obernier K., Guinto C., Jose L., Bonfanti L., Alvarez-Buylla A. (2013) Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc. Natl. Acad. Sci. USA 110, E1045–E1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohwi M., Osumi N., Rubenstein J. L., Alvarez-Buylla A. (2005) Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J. Neurosci. 25, 6997–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batista-Brito R., Close J., Machold R., Fishell G. (2008) The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci. 28, 3966–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niwa H., Miyazaki J., Smith A. G. (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 24, 372–376 [DOI] [PubMed] [Google Scholar]
- 49.Rougvie A. E., Moss E. G. (2013) Developmental transitions in C. elegans larval stages. Curr. Top. Dev. Biol. 105, 153–180 [DOI] [PubMed] [Google Scholar]
- 50.Petri R., Pircs K., Jönsson M. E., Åkerblom M., Brattås P. L., Klussendorf T., Jakobsson J. (2017) let-7 regulates radial migration of new-born neurons through positive regulation of autophagy. EMBO J. 36, 1379–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu B., Huang Y. (2009) Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element. Nucleic Acids Res. 37, 4256–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng C., Neumeister V., Ma W., Xu J., Lu L., Bordeaux J., Maihle N. J., Rimm D. L., Huang Y. (2012) Lin28 regulates HER2 and promotes malignancy through multiple mechanisms. Cell Cycle 11, 2486–2494 [DOI] [PubMed] [Google Scholar]
- 53.Lei X. X., Xu J., Ma W., Qiao C., Newman M. A., Hammond S. M., Huang Y. (2012) Determinants of mRNA recognition and translation regulation by Lin28. Nucleic Acids Res. 40, 3574–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li N., Zhong X., Lin X., Guo J., Zou L., Tanyi J. L., Shao Z., Liang S., Wang L. P., Hwang W. T., Katsaros D., Montone K., Zhao X., Zhang L. (2012) Lin-28 homologue A (LIN28A) promotes cell cycle progression via regulation of cyclin-dependent kinase 2 (CDK2), cyclin D1 (CCND1), and cell division cycle 25 homolog A (CDC25A) expression in cancer. J. Biol. Chem. 287, 17386–17397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hafner M., Max K. E., Bandaru P., Morozov P., Gerstberger S., Brown M., Molina H., Tuschl T. (2013) Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition. RNA 19, 613–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balzer E., Moss E. G. (2007) Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 4, 16–25 [DOI] [PubMed] [Google Scholar]
- 57.Betizeau M., Dehay C. (2016) From stem cells to comparative corticogenesis: a bridge too far? Stem Cell Investig. 3, 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.