Abstract
Accumulating evidence suggests that the abnormal aggregation of amyloid-β (Αβ) peptide in Alzheimer’s disease (AD) begins intraneuronally, within vesicles of the endosomal-lysosomal pathway where Aβ is both generated and degraded. Metalloproteases, including endothelin-converting enzyme (ECE)-1 and -2, reside within these vesicles and normally limit the accumulation of intraneuronally produced Aβ. In this study, we determined whether disruption of Aβ catabolism could trigger Aβ aggregation within neurons and increase the amount of Aβ associated with exosomes, small extracellular vesicles derived from endosomal multivesicular bodies. Using cultured cell lines, primary neurons, and organotypic brain slices from an AD mouse model, we found that pharmacological inhibition of the ECE family of metalloproteases increased intracellular and extracellular Aβ levels and promoted the intracellular formation of Aβ oligomers, a process that did not require internalization of secreted Aβ. In vivo, the accumulation of intraneuronal Aβ aggregates was accompanied by increased levels of both extracellular and exosome-associated Aβ, including oligomeric species. Neuronal exosomes were found to contain both ECE-1 and -2 activities, suggesting that multivesicular bodies are intracellular sites of Aβ degradation by these enzymes. ECE dysfunction could lead to the accumulation of intraneuronal Aβ aggregates and their subsequent release into the extracellular space via exosomes.—Pacheco-Quinto, J., Clausen, D., Pérez-González, R., Peng, H., Meszaros, A., Eckman, C. B., Levy, E., Eckman, E. A. Intracellular metalloprotease activity controls intraneuronal Aβ aggregation and limits secretion of Aβ via exosomes.
Keywords: Alzheimer’s disease, oligomer, ECE, multivesicular body
One of the central hallmarks of Alzheimer’s disease (AD) pathology is an extensive accumulation of extracellular amyloid deposits in brain parenchyma and vasculature (1). The amyloid deposits are primarily composed of amyloid-β (Aβ) peptide, which is produced by proteolysis of the amyloid precursor protein (APP) by β- and γ-secretases (2). Although most Aβ initially remains in a soluble state, it can acquire a β-sheet conformation that allows polymerization into insoluble fibrils, which are the building blocks of the amyloid deposits (1, 3). The formation of insoluble amyloid is sequential and is preceded by the formation of prefibrillar soluble species, known as Aβ oligomers (oligomeric Aβ or oAβ), which are widely considered major culprits in the synaptic dysfunction and neuronal loss typical of AD (4). The alterations in Aβ homeostasis associated with Aβ oligomerization in vivo, however, are far from understood.
Several factors have been found to regulate the formation of amyloid and the rate of aggregation, such as peptide sequence and concentration, interaction with membranes and other proteins, and pH (5, 6). In physiologic solutions in vitro, Aβ spontaneously aggregates at high nano- to low micromolar levels (7, 8), concentrations well above the estimated pico- to low nanomolar levels found in the interstitial fluid of human brains (9). For that reason, it has been proposed that Aβ aggregation starts intraneuronally, in cellular compartments that provide favorable conditions and accumulate enough Aβ to promote aggregation (10). This hypothesis is consistent with the increased intraneuronal Aβ immunoreactivity preceding extracellular amyloid formation seen in several AD mouse models (11) and in patients with Down syndrome (12, 13), who invariably develop early-onset AD (14).
Intraneuronal Aβ has been observed in vesicles within the endocytic route, including multivesicular bodies (MVBs) (15), recycling endosomes (16) and lysosomes, where several Aβ degrading enzymes, such as endothelin-converting enzyme (ECE)-1 and ECE-2 (17) and cathepsins (D, B, S and L), reside. Thus, one prevailing view is that intraneuronal Aβ represents a pool of endocytosed Aβ bound for degradation (18, 19). However, endocytic vesicles also contain the immediate Aβ precursor, APP C-terminal fragment (CTF)-β, as well as γ-secretase (20–22), and retain the ability to produce substantial amounts of Aβ. Under normal conditions, this pool of endogenous Aβ is constantly hydrolyzed by the ECE family of proteases, and in cellular models, ECE inhibition is necessary for observation of Aβ production within these compartments (17). Based on the unique convergence of Aβ production and degradation in endosomal-lysosomal (E-L) vesicles, we tested the hypothesis that disturbances in E-L proteostasis leads to the initiation of the amyloidogenic process independently from Aβ internalization. Furthermore, because small extracellular vesicles known as exosomes are derived from MVBs and their secretion may represent a mechanism for the release of intraneuronal Aβ aggregates (23), we sought to determine whether intravesicular accumulation of Aβ is associated with increased exosomal Aβ.
MATERIALS AND METHODS
SH-SY5Y-APP cell culture and extraction
The human neuroblastoma SH-SY5Y cell line (ATCC CRL-2266, RRID:CVCL_0019; American Type Culture Collection, Manassas, VA, USA) was stably transfected with pcDNA3 vector (Thermo Fisher Scientific, Waltham MA, USA), containing human wild-type APP695 cDNA (NM_201414.2) or empty vector as control. This cell line endogenously expresses mRNAs coding for all known isoforms of ECE-1, 2 major isoforms of ECE-2, and both neprilysin (NEP) and NEP-2 (see Results), although NEP enzymatic activity is reportedly negligible (24). Cells were maintained in DMEM supplemented with 10% fetal bovine serum, glutamine, and penicillin/streptomycin and containing 400 μg/ml G418 for selection. Twelve-well plate polyester membrane Transwell inserts (Corning, Corning, NY, USA) with a 0.4-µm pore size were used for coculture experiments. For Aβ extraction, cells grown in 12-well plates were scraped with cold PBS and lysed with 100 µl of either 0.1% Triton X-100 in PBS or RIPA buffer [25 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS (pH 7.6)] containing Halt Protease Inhibitor Cocktail with EDTA (Thermo Fisher Scientific).
Determination of ECE1, ECE2, MME, and MMEL1 expression by reverse-transcription PCR
Total RNA was prepared from SH-SY5Y-APP cells and human temporal cortex [from the Banner Sun Health Research Institute Brain and Body Donation Program (25)] using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA). After treatment with DNase I, mRNA was converted to cDNA with SuperScript IV reverse transcriptase and random primers (Thermo Fisher Scientific). Expression of 4 different ECE1 transcripts, each coding for a different protein isoform, was assessed by using a common reverse primer (5′-CCACAGGCGTAGCTGAAGAA-3′) and isoform-specific forward primers: ECE1 variant 1 (NM_001397.2), 5′-CATCCCATCCCGGCCAC-3′; ECE1 variant 2 (NM_001113349.1), 5′-ATTTGGCCTTGCAGATGTCG-3′; ECE 1 variant 3 (NM_001113347.1), 5′-CCGAGCTGCTTGACTCTCTG-3′; and ECE1 variant 4 (NM_001113348.1), 5′-GAGCTGGGAATCGGGAGC-3′. Expression of 3 different ECE2 transcripts (variant 2, NM_001037324.2; variant 4, NM_001100120.1; and variant 5, NM_001100121.1) was determined by using forward primer 5′-CCGACTCCACCATGAACGTC-3′ and reverse primer 5′-GTGCTTCAGTATGGCCTGGT-3′. Expression of membrane metalloendopeptidase (MME; NM_007289.3 and all other known transcripts coding for NEP) was determined using forward primer 5′-AGCTGCTCGACTGATCCAAA-3′ and reverse primer 5′-GTTGCTACTGGCCACCCATA-3′. Expression of membrane metalloendopeptidase–like-1 (MMEL1; NM_033467.3 and all other known transcripts coding for NEP-2) was determined with forward primer 5′-GGAACCGTGTGACGACTTCT-3′ and reverse primer 5′-AGTCCTACGGTCTCGTTCCA-3′. PCR amplification (35 cycles) was performed with MyTaq HS Red mix (Bioline, Taunton, MA, USA) and 0.4 µM each primer (Integrated DNA Technologies, Coralville, IA, USA). PCR products were detected by gel electrophoresis and ethidium bromide staining.
ELISA
Aβ species were measured by sandwich ELISA with specific mouse monoclonal antibodies, as described by Scheuner et al. (26). For human Aβ40 measurement, antibody MM27-33.1.1 (raised against human Aβ1-16) was used for capture, and horseradish-peroxidase (HRP)–conjugated Aβ40-specific antibody MM32-13.1.1 (raised against Aβ35-40) was used for detection. For mouse Aβ40, antibody MM44-32.4.1 (raised against mouse Aβ1-16) was used for capture and MM32-13.1.1-HRP for detection. For human and mouse Aβ42 measurement, anti-Aβ42 antibody MM26-2.1.3 (Aβ35-42) was used for capture and HRP-conjugated 4G8 antibody (Aβ17-24) was used for detection. Aβ antibodies besides 4G8 (Covance, Princeton, NJ, USA) were from Mayo Clinic (Rochester, MN, USA). Levels of oligomeric Aβ were quantified with a commercially available sandwich ELISA (Biosensis, Thebarton, SA, Australia) that both captures and detects Aβ oligomers with the N-terminal Aβ monoclonal antibody MOAB-2.
Estimation of intravesicular Aβ concentration
SH-SY5Y-APP cells grown in a 4-chamber slide with a surface area of 1.8 cm2/well were treated with 100 µM of the metalloprotease inhibitor phosphoramidon (PA) for 48 h. Levels of intracellular Aβ40 were measured by sandwich ELISA in 0.1% Triton X-100 cell extracts. To quantify the number and size of Aβ-containing vesicles, intracellular Aβ was detected by immunofluorescence in sister wells grown under the same conditions and confluence. Cells were fixed with methanol at −20°C for 10 min, and nonspecific binding was blocked with 5% bovine serum albumin/0.1% Tween-20/PBS. After incubation with rabbit anti-β-tubulin class III (04-1049, RRID:AB1977542; MilliporeSigma, Burlington, MA, USA) and mouse anti-human Aβ antibody MM27-33.1.1, bound antibodies were detected by incubation with 488-Alexa Fluor–conjugated donkey anti-mouse and 546-Alexa Fluor donkey anti-rabbit antibodies (Thermo Fisher Scientific). The fluorescent dye Hoechst 33258 (AnaSpec, Fremont, CA, USA) was used to label nuclei. Using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA), we thresholded fluorescence micrographs (mean, 50 per well) and converted them to binary images to quantify the number and the size of the Aβ-containing vesicles. The total moles of Aβ measured per well by ELISA was divided by the number of vesicles per well, and the mean vesicle volume, to estimate the concentration of Aβ per vesicle. PA treatment does not alter the levels of APP or APP metabolites other than Aβ, and the specificity of antibody MM27-33.1.1 for detection of intravesicular Aβ in PA-treated SH-SY5Y-APP cells has been reported (17).
Mice
TgCRND8 mice overexpressing human APP KM670/671NL (Swedish mutation) and V717F (Indiana mutation) under control of the hamster prion gene promoter, were a kind gift from Dr. Paul Fraser (University of Toronto, ON, Canada) (27). Experimental mice [transgenic and nontransgenic (wild-type) littermates, both male and female] were generated by breeding male TgCRND8 mice and female B6C3F1/J hybrid mice (100010; The Jackson Laboratory, Bar Harbor, ME, USA). Pups were genotyped by PCR amplification of the human APP transgene from DNA extracted from ear punches, with the following primers: 5′-CTGACCACTCGACCAGGTTCTGGGT-3′ and 5′-GTGGATAACCCCTCCCCCAGCCTAGACCA-3′. Experiments were performed on 2-mo-old mice, before the onset of amyloid deposition; Aβ levels in TgCRND8 mice of this age do not differ between sexes (27). Mice were housed in ventilated microisolator cages in a temperature- and humidity-controlled room with a 12-h light/dark cycle. Mice had unrestricted access to food and water and were provided with nesting material and Shepherd shacks (Shepherd Specialty Papers, Watertown, TN, USA) for enrichment. Mice were euthanized by CO2 asphyxiation and brains for intravesicular and extracellular Aβ analysis were processed immediately without freezing. Brains used for exosomes isolation were frozen immediately on dry ice and stored at −80°C before processing. The use of mice was approved by the Institutional Animal Care and Use Committee at Biomedical Research Institute of New Jersey and followed American Veterinary Medical Association guidelines.
Intracerebroventricular injection
Mice were anesthetized with isoflurane (1.5–5%) and placed in a digital stereotaxic frame (Stoelting Co., Wood Dale, IL, USA). Analgesia was administered (0.1 ml 0.125% bupivacaine injected subcutaneously at the incision site and meloxicam 2 mg/kg injected subcutaneously in the scapular region). Body temperature was maintained with a heating pad. The skull was exposed through a 1-cm scalp incision, stereotaxic coordinates (0.9 mm posterior, 1.5 mm lateral to the bregma) were located and marked, and small burr holes were drilled in the skull with a dental drill. PA (118 nmol) or vehicle (0.9% saline) was injected in a total volume of 2 µl, with 1 µl injected into each lateral ventricle at a depth of 2.1 mm and a rate of 0.5 µl/min with a Hamilton glass microsyringe driven by a Micro4 microsyringe pump (World Precision Instruments, Sarasota, FL, USA).
Primary neuronal cultures
Litters of TgCRND8 mice were genotyped on postnatal day 0, and cultures were prepared from brain tissue pooled from several transgenic pups. For controls, cultures were prepared from nontransgenic (wild-type) littermates. Using previously published methods (28), brain tissue was disaggregated by mild trypsinization and cells were grown on poly-d-lysine–coated 24-well plates in neurobasal medium supplemented with B27. For Aβ quantification by ELISA, cells from single wells were extracted in 100 μl of RIPA buffer containing EDTA and protease inhibitor cocktail.
Organotypic brain slice cultures
Brains from TgCRND8 mouse pups (postnatal day 6–8) or nontransgenic littermates were cut into 400-μm–thick coronal sections with a McIlwain tissue chopper (Ted Pella, Redding, CA, USA) and quickly submerged in cold Earle’s balanced salt solution (EBSS; Thermo Fisher Scientific) containing 25 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]. Sections with an intact cortex and hippocampus were placed on 13-mm hydrophilic polytetrafluoroethylene membranes (0.45 μm pore size), resting on top of 30-mm diameter Millicell culture plate inserts (0.4 μm pore size) (MilliporeSigma) placed in a 6-well culture plate. Tissue was grown in minimum essential medium/Earle’s balanced salt solution with Glutamax (Thermo Fisher Scientific), 360 mM d-glucose with 25% horse serum plus penicillin, streptomycin, and nystatin, as described by De Simoni and Yu (29).
Separation of intravesicular/extracellular Aβ from brains
Mice were decapitated immediately after euthanasia and the cortex and hippocampus were quickly dissected. Using a motorized glass-Teflon Dounce homogenizer, the tissue was homogenized at a concentration of 50–100 mg wet weight per milliliter in cold 0.32 M sucrose/10 mM HEPES buffer containing protease inhibitor cocktail with EDTA. All centrifugation steps were performed at 4°C. Homogenates were initially centrifuged for 10 min at 1000 g to pellet nuclei, debris, and unbroken cells. The resulting supernatant was centrifuged at 16,000 g for 10 min to pellet intracellular vesicles, which were subsequently washed 3 times with homogenization buffer, lysed with RIPA buffer, and used directly for quantification of intravesicular Aβ (for transgenic mice) or further purified by solid-phase extraction (for wild-type mice, see below). The supernatant from the 16,000 g spin, enriched for interstitial and cytoplasmic proteins, was further spun at 120,000 g for 1 h to remove remaining intracellular or extracellular vesicles. The final supernatant was diluted 1.5 times with ELISA binding buffer {0.02 M sodium phosphate (pH 7.0), containing 0.002 M EDTA, 0.4 M NaCl, 0.2% bovine serum albumin, 0.05% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 0.4% Block Ace, 0.05% NaN3} and used to quantify extracellular-enriched Aβ.
Solid-phase extraction
Rodent Aβ was extracted from the intracellular vesicle fraction and from exosomes isolated from wild-type mouse brains, using a modified solid-phase extraction protocol (30). A 60-mg Oasis HLB 96-well plate (Waters, Milford, MA, USA) fitted in a vacuum manifold was conditioned with 1 ml methanol and equilibrated with 1 ml diH2O. Pelleted vesicles were resuspended in 1 ml 6 M guanidine HCl and run through the columns. Samples were washed sequentially with 1 ml 10 and 30% methanol, and the Aβ was eluted with 1 ml 2% NH4OH in 90% methanol. The eluted samples were vacuum centrifuged at 60°C until dry and resuspended in RIPA buffer for analysis by ELISA.
Lipid raft isolation
Cells grown to confluence in a T75 flask were scraped in cold PBS, pelleted by centrifugation at 16,000 g for 9 min, and incubated for 30 min at 4°C in buffer A [1% Triton X-100, 150 mM NaCl, 25 mM Tris-HCl, and 2.5 mM EDTA (pH 7.4)]. The resulting cell lysates were adjusted to 40% OptiPrep (MilliporeSigma) and 2 ml was loaded at the bottom of an ultracentrifuge tube. A discontinuous gradient consisting of 0, 20, 25, 30, and 35% OptiPrep (2 ml each) in buffer A was layered on top of the lysates. After centrifugation at 160,000 g for 5 h at 4°C, samples were collected in 500 µl aliquots from top to bottom.
Extracellular vesicle isolation
Extracellular vesicles (EVs) enriched with exosomes were isolated and purified from frozen mouse hemibrains, as described by Perez-Gonzalez et al. (31). In brief, frozen hemibrains were treated with 20 U/ml papain (Worthington, Lakewood, NJ, USA) in Hibernate A solution (HA, 3.5 ml/sample; BrainBits, Springfield, IL, USA) for 15 min at 37°C. The brain tissue was gently dissociated in 6.5 ml of cold HA supplemented with protease inhibitors, centrifuged at 300 g for 10 min at 4°C to discard the cells, and filtered. The filtrate was sequentially centrifuged at 4°C, at 2,000 g for 10 min and 10,000 g for 30 min, to discard membranes and debris, and at 100,000 g for 70 min to pellet EVs. The washed EV pellet was resuspended in 2 ml of 0.95 M sucrose solution and inserted into a discontinuous sucrose gradient (six 2-ml steps starting from 2.0 M sucrose up to 0.25 M). The sucrose gradient was centrifuged at 200,000 g for 16 h and fractions were collected from the top of the gradient, diluted in cold PBS, and centrifuged at 100,000 g for 70 min. Sucrose gradient fraction pellets were resuspended in 30 µl cold PBS and lysed in 30 µl 2× RIPA buffer. Western blot analysis of exosome markers Alix and TSG101 was used to evaluate the presence of exosomes in fractions b, c, and d and to quantify the number of exosomes released. Fraction c, which was the most enriched with exosomes, was selected for Aβ analysis. Exosomal protein content was determined by bicinchoninic acid assay (Thermo Fisher Scientific) and used to normalize Aβ measurements.
EVs were also isolated from conditioned medium of SH-SY5Y-APP cells grown in serum-free Opti-Mem (Thermo Fisher Scientific) for 48 h in 150 × 20-mm cell culture dishes. Medium was sequentially centrifuged at 2000 g for 20 min and 10,000 g for 60 min at 4°C to exclude cellular debris. EVs were pelleted by centrifugation at 100,000 g for 60 min at 4°C and washed twice by resuspending in PBS and pelleting again.
Western blot analysis
SH-SY5Y cells and mouse brain intracellular vesicles were extracted with RIPA buffer, electrophoresed on Novex 10–20% Tris-tricine gels (Thermo Fisher Scientific) and transferred to nitrocellulose membranes. Extracellular vesicle proteins were extracted in RIPA buffer, electrophoresed on 4–20% Tris-glycine gels (Criterion Precast Gel; Bio-Rad, Hercules, CA, USA), and transferred to PVDF membranes (Immobilon; MilliporeSigma). Membranes were blocked with 5% bovine serum albumin/PBS for 1 h followed by incubation with primary antibody. After the membranes were washed with 0.05% Tween-20/PBS, they were incubated with HRP–conjugated secondary antibody, washed and developed with ECL reagents (MilliporeSigma). Chemiluminescent signals were digitally recorded with an ImageQuant LAS 4000 (GE Healthcare Life Sciences, Marlborough, MA, USA). The rabbit anti-C-terminal APP antibody (A8717, RRID:AB_258409; MilliporeSigma) was used at a 1:3000 dilution. Antibodies against Alix (1:1000, ABC40, RRID:AB_10806218; MilliporeSigma) and TSG101 (1:1000, 4A10, RRID:AB_373239; GeneTex, Irvine, CA, USA) were used to identify exosomal markers, and an antibody against flotillin (1:1000, EP446Y, RRID:AB_838269; MilliporeSigma) was used to identify lipid raft-containing fractions.
Big endothelin-1 conversion assay
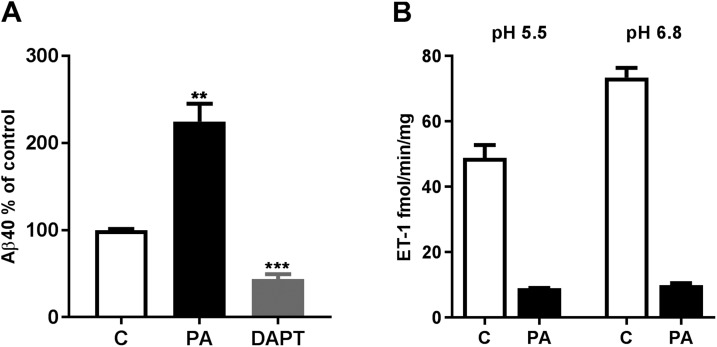
ECE activity was measured with a big endothelin (ET)-1 conversion assay (32), which depends on the specific cleavage of the inactive precursor, big ET-1, between positions Trp 21 and Val 22 to generate the active ET-1 peptide. In this assay, ECE-1 has optimal activity at pH 6.8 and ECE-2 at pH 5.5. Each enzyme is virtually inactive against big ET-1 at the pH optimum of the other [unlike big ET-1, Aβ and other neuropeptides are cleaved by both ECE-1 and -2 at acidic pH (33, 34)]. EVs from SH-SY5Y cells were solubilized in 20 mM Tris-HCl (pH 7.4), containing 250 mM sucrose and 2.5% C12E10 (polyoxyethylene-10-lauryl ether), and 2 µg protein was incubated with 100 nM recombinant human big ET-1 (Enzo Life Sciences, Farmingdale, NY, USA) in 50 µl of either 0.1 M sodium phosphate (pH 6.8) or 0.1 M sodium acetate buffer (pH 5.5) containing 0.5 M NaCl and protease inhibitor cocktail, without EDTA, supplemented with 1 µM thiorphan. The final C12E10 concentration was 0.2%. Reactions were performed in the presence or absence of PA (100 μM) for 1 h at 37°C and were stopped by the addition of EDTA (5 mM final concentration). Levels of ET-1 were measured by sandwich ELISA (R&D Systems, Minneapolis, MN, USA).
Chemicals and general reagents
General laboratory chemicals and protease inhibitors PA, E-64, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT), and thiorphan were purchased from MilliporeSigma.
Data analysis and graphing
The statistical and graphing software Prism 5.01 (GraphPad Software, La Jolla, CA, USA) was used for data analysis and graphical representation. For column graphs, column bars represent means ± sem. The unpaired Student’s t test was used for statistical analysis and P < 0.05 was considered significant. Data from a mouse with Aβ oligomer values >3 sd greater than the mean were removed from the analysis, as the mouse may have had early amyloid deposition. Every experiment was performed at least 2–5 times.
RESULTS
Inhibition of metalloprotease activity triggers the formation of intracellular Aβ oligomers
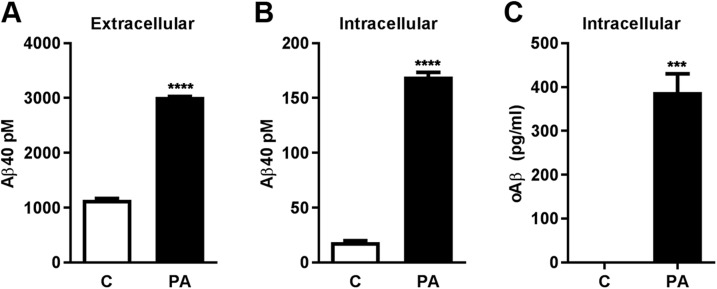
ECE-1 and -2 are members of the PA-sensitive M13 family of membrane-bound zinc metalloproteases that also includes NEP and NEP-2 (35). In SH-SY5Y cells overexpressing wild-type APP (SH-SY5Y-APP), inhibition of this protease family with PA or the more selective ECE inhibitor CGS 35066, but not the NEP/NEP-2 inhibitor thiorphan, produces a significant accumulation of intracellular Aβ within E-L vesicles and autophagosomes that can be detected by ELISA, Western blot, and immunofluorescence (17). To gauge whether the concentration of intracellular Aβ reached after PA treatment is sufficient to promote aggregation, we calculated the approximate intravesicular concentration of the peptide. By measuring the mean size (0.38 µm3) and number (1 × 106) of Aβ-positive vesicles per culture well by immunofluorescence microscopy and the amount of intracellular Aβ40 by ELISA in identically cultured sister wells (7.4 fmol), we determined the concentration of intravesicular Aβ40 in PA-treated cells to be ∼20 µM, within the range of Aβ concentrations known to favor aggregation in vitro, especially at acidic pH (36). Next, to determine whether the PA-mediated increase in intracellular Aβ triggers the formation of intracellular oAβ, we used an oAβ-specific ELISA that both captures and detects oAβ with the same N-terminal Aβ antibody (37, 38), making dimers the minimum detectable oligomer size. Within 48 h of PA treatment, elevation of intracellular and extracellular Aβ was accompanied by the formation of intracellular oAβ, which was undetectable in untreated cells (Fig. 1). oAβ was not detectable in the culture medium of SH-SY5Y-APP cells under any condition.
Figure 1.
Pharmacologic inhibition of metalloprotease activity leads to the intracellular accumulation of Aβ aggregates. A) Treatment of SH-SY5Y-APP cells with PA (100 μM, 48 h) produced a significant increase in extracellular Aβ, compared with nontreated cells (control, C). B, C) Intracellular levels of Aβ40 (B) and oAβ (C) were also significantly elevated in PA-treated cells (n = 3 per group). ***P < 0.001, ****P < 0.0001.
Intracellular Aβ accumulation does not require Aβ internalization
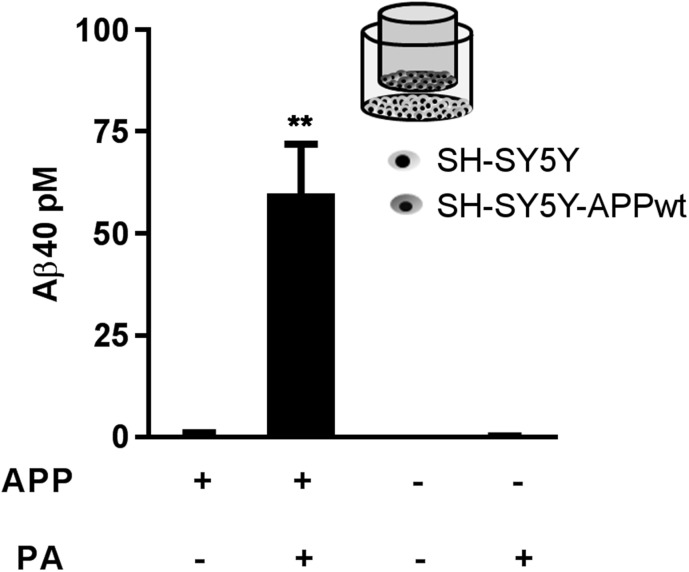
After establishing that disturbances in Aβ degradation within the E-L pathway can initiate intraneuronal Aβ aggregation, we sought to identify the source of the accumulated intracellular Aβ. Although it is commonly accepted that intracellular Aβ originates from reinternalization of secreted Aβ (18, 19), evidence that E-L vesicles can also produce Aβ endogenously (17) prompted us to test whether Aβ internalization was needed to cause intracellular Aβ accumulation in this model. Using Transwell permeable inserts, we cocultured empty vector–transfected SH-SY5Y cells with SH-SY5Y-APP cells, so that they shared the same medium but were not in direct contact. We hypothesized that if intracellular Aβ accumulation were related to impaired degradation of internalized Aβ, PA treatment would induce intracellular Aβ accumulation in both cell types, regardless of APP overexpression. This method also allowed us to study the effect of sustained levels of cell-produced and unmodified Aβ at nanomolar levels. After 48 h PA treatment, significant intracellular accumulation of Aβ was detectable only in APP-overexpressing cells (Fig. 2).
Figure 2.
The intracellular pool of Aβ accumulating after PA treatment does not have an extracellular origin. In a coculture of empty vector–transfected (APP−) and APP-transfected (APP+) SH-SY5Y cells, PA treatment (100 μM, 48 h) resulted in significantly increased intracellular Aβ levels only in cells overexpressing APP (n = 3 per group). **P < 0.01.
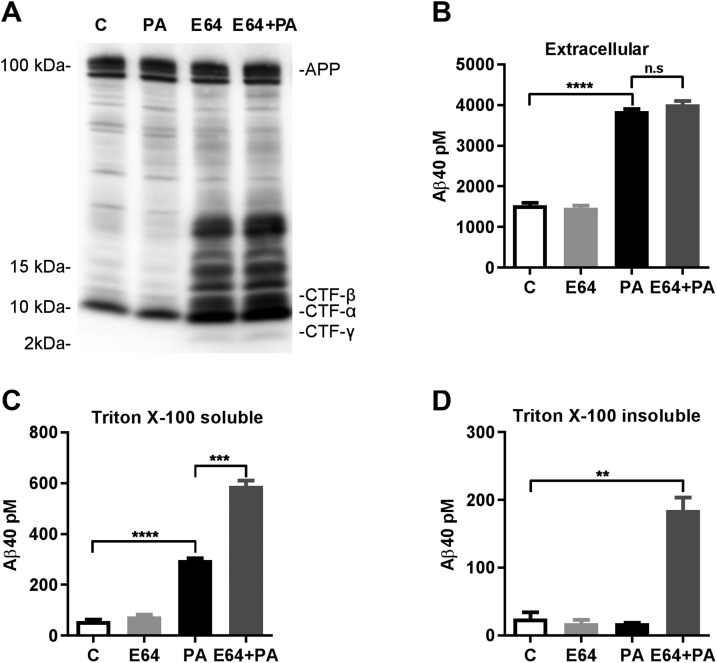
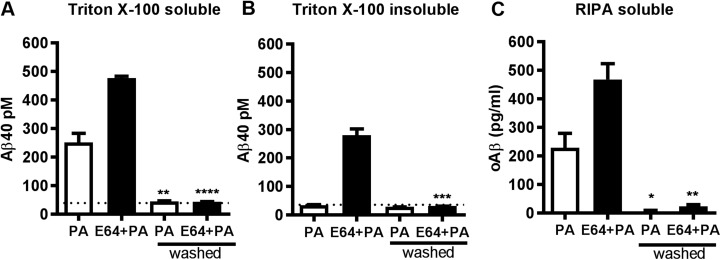
To further investigate whether intracellular Aβ originates endogenously, we treated SH-SY5Y-APP cells with the cysteine protease inhibitor E-64, which inhibits CTF degradation by cysteine cathepsins in the E-L pathway (39, 40), thus increasing the amount of substrate for intracellular Aβ production (Fig. 3A). Treatment with E-64 alone did not produce significant increases in either intracellular or secreted Aβ. However, combined treatment with E-64 and PA resulted in increases in intracellular levels of Aβ more than double those observed with PA treatment alone, with no additional effect on extracellular Aβ (Fig. 3B, C). The combined treatment also resulted in the accumulation of a Triton X-100–resistant pool of intracellular Aβ that required RIPA buffer for solubilization (Fig. 3D), suggesting that Aβ was bound to lipid rafts or acquired a state of aggregation that was resistant to Triton extraction, or both.
Figure 3.
Combined metalloprotease and cysteine protease inhibition increases pools of intracellular Aβ with different solubilities. A) Western blot analysis of SH-SY5Y-APP cell extracts with a C-terminal APP antibody showed that the cysteine protease inhibitor E-64 (100 μM) increased the accumulation of APP CTFs, without altering full-length APP levels. PA treatment did not alter levels of APP or APP CTFs. B) E-64, alone or in combination with PA (100 μM), had no effect on extracellular Aβ. C) E-64 alone had no effect on intracellular Aβ, but combined E-64 and PA treatment further increased Triton X-100 extractable intracellular Aβ, compared to PA alone. D) Subsequent extraction of the Triton-insoluble fraction with RIPA buffer revealed a specific accumulation of Triton-insoluble Aβ in E-64+PA treated cells that was not detected in cells treated with either inhibitor alone (n = 3 for all groups). n.s., not significant. **P < 0.01, ***P < 0.001, ****P < 0.0001.
The formation of soluble Aβ oligomers within intracellular vesicles can precede Aβ accumulation in lipid rafts
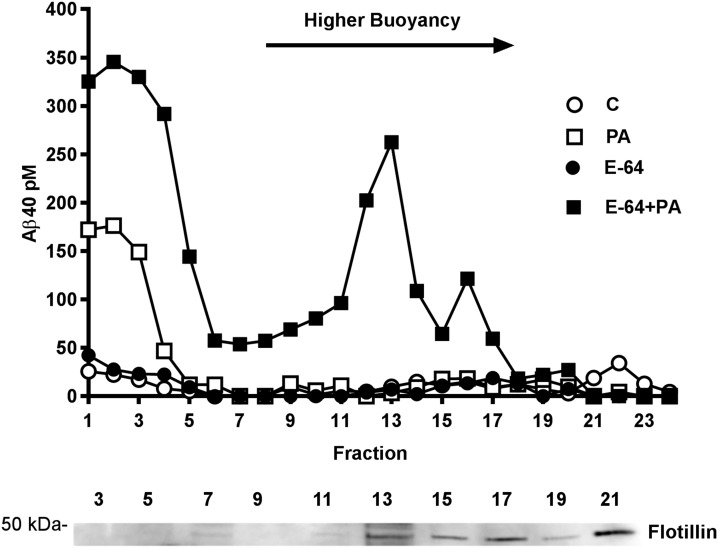
Because intraneuronal Aβ aggregation has been proposed to start with binding to lipid rafts (41, 42), high buoyancy cholesterol-rich membrane microdomains that are insoluble in cold Triton X-100, we examined whether Aβ interaction with lipid rafts preceded the formation of oAβ in our cell model. First, we determined whether the Triton-insoluble fraction of Aβ in cells treated with E-64 and PA represented Aβ bound to lipid rafts. Treated cells were incubated with cold 1% Triton X-100, and lipid rafts were separated by floatation on a discontinuous gradient. Consistent with the results of Triton extracts assayed directly by ELISA (Fig. 3C), Triton-soluble Aβ remaining at the bottom of the gradient in fractions 1–5 was found elevated in all groups treated with PA and was considerably higher in cells cotreated with E-64 (Fig. 4). In fractions of higher buoyancy corresponding to flotillin-containing lipid rafts, Aβ accumulation was detected exclusively in cells treated with E-64+PA.
Figure 4.
Intracellular Aβ interaction with lipid rafts. SH-SY5Y-APP cells lysed in cold 1% Triton X-100 were fractionated by centrifugation in a discontinuous gradient, and collected fractions were analyzed for Aβ40 by ELISA. In cells treated with E64+PA, a selective increase in Aβ level was seen in higher buoyancy fractions 12–13, in which the lipid raft marker flotillin was detected by Western blot. Both PA and E-64+PA treatments produced an elevation in Aβ levels in the bottom fractions (1–5), corresponding to Triton X-100 soluble proteins.
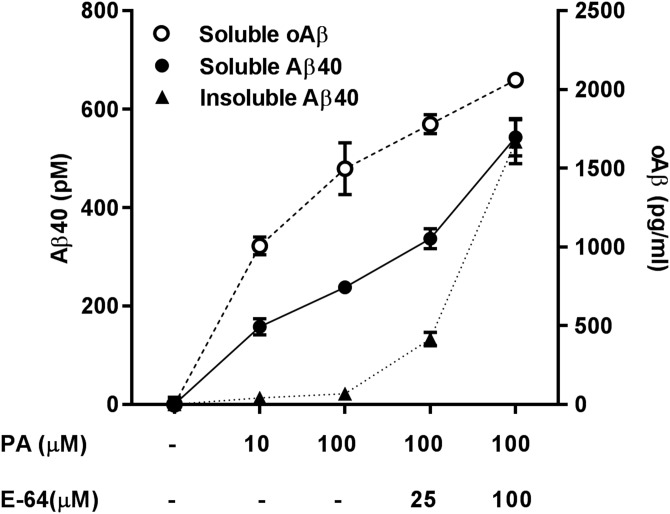
In a subsequent series of experiments, we studied the dynamics of Aβ aggregation and lipid raft interaction by treating cells with increasing doses of PA or E-64+PA and performing sequential extractions with Triton X-100 (soluble fraction) followed by RIPA buffer to solubilize lipid raft–bound (Triton-insoluble) Aβ. We observed that Triton-soluble oAβ was significantly elevated, even at the lowest dose of PA, coinciding with an elevation in soluble Aβ40 (Fig. 5). On the other hand, increases in insoluble Aβ40 were detected only after a considerable accumulation of these soluble species, and oAβ was not detectable in the insoluble fraction at any dose or combination of PA and E-64.
Figure 5.
Dynamics of intracellular Aβ oligomerization. In SH-SY5Y-APP cells, there was an incremental accumulation of Triton X-100 soluble intracellular Aβ40 with increasing doses of PA and E-64. Triton-insoluble (RIPA extractable) Aβ40 accumulation started at higher doses of E-64+PA but increased exponentially, reaching levels comparable to soluble Aβ40 at the highest doses tested. Whereas soluble oAβ was readily detectable at low levels of PA, oAβ was not detected in the Triton-insoluble fraction in any condition (n = 3 for all groups).
Soluble and insoluble pools of intracellular Aβ are initially reversible and can be cleared by restoring metalloprotease activity
We next examined the stability of the aggregated Aβ species and determined whether Aβ was irreversibly bound to lipid rafts, by washing out inhibitors and thereby restoring endogenous metalloprotease activity. Cells that had accumulated intracellular Aβ during the 48-h treatment period with PA or PA+E-64 were washed and incubated for an additional 48-h period without inhibitors. To avoid possible effects of exchanging cells into fresh medium, we incubated “washed” cells with conditioned medium from sister cultures. As illustrated in Fig. 6, after the 48-h washout period, restoration of protease activity resulted in nearly complete elimination of soluble and insoluble Aβ40 and oAβ.
Figure 6.
Intracellular Aβ can be cleared by restoring metalloprotease activity. Cells were incubated with inhibitors for 48 h, followed by continued incubation or exchange to conditioned medium without inhibitors (washed). A, B) After washing out the inhibitors, intracellular levels of Triton X-100–soluble (A) and Triton X-100–insoluble (RIPA extractable) (B) Aβ40 returned to control levels (indicated by the dotted line). C) Intracellular levels of RIPA-extractable oAβ were completely undetectable in control cells and nearly undetectable in drug-treated cells after the washout period. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Intracellular Aβ aggregation in primary neuronal cultures
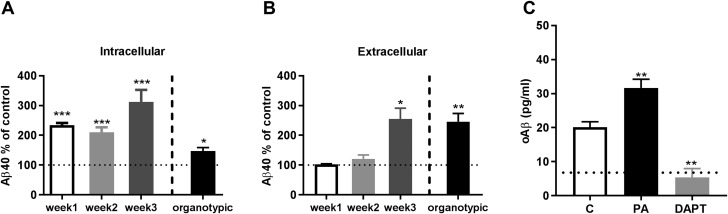
To test the relevance of the ECE family of proteases in maintaining Aβ homeostasis in differentiated neurons, we extended our study to corticohippocampal neuronal cultures and organotypic brain slices from TgCRND8 human APP transgenic mice (27). By measuring the response to PA treatment at different time points during the maturation of primary neuronal cultures, we found that intraneuronal Aβ was sensitive to PA at all stages of differentiation (Fig. 7A), whereas PA-induced elevations in extracellular Aβ were significant only in mature (3-wk-old) cultures (Fig. 7B). The magnitude of the Aβ elevation in 3-wk-old primary neuronal cultures was equivalent to that observed in organotypic slices from TgCRND8 mice. PA treatment failed to modulate extracellular Aβ in 1-wk-old primary neuronal cultures, but the >2-fold increase in intracellular Aβ at this time point was accompanied by a significant elevation in intraneuronal oAβ (Fig. 7C). Unlike in SH-SY5Y-APP cells, intracellular oAβ was already detectable even in untreated primary neurons from TgCRND8 mice and inhibition of Aβ production with the γ-secretase inhibitor DAPT reduced the signal of oAβ to background levels. Consistent with results in SH-SY5Y cells, treatment of mature neuronal cultures or organotypic slices with the more selective NEP/NEP-2 inhibitor, thiorphan, did not alter either intracellular or extracellular Aβ levels (Supplemental Fig. S1).
Figure 7.
Intracellular and extracellular Aβ are differentially modulated by PA treatment during the maturation of primary neuronal cultures. Primary neurons from TgCRND8 mice were treated with PA (100 μM, 48 h) after 1, 2, or 3 wk in culture. A) From the first week on, levels of intracellular human Aβ40 in PA-treated cells were significantly increased compared with control cells (level indicated by dotted line at 100%). B) Extracellular human Aβ40 was significantly increased by PA treatment only in mature (3-wk-old) cultures. The magnitude of the PA-induced increase in extracellular Aβ40 at that point was similar to the increase in PA-treated organotypic slice cultures (n = 3 for all groups). C) Unlike SH-SY5Y-APP cells, 1-wk-old primary neurons accumulated intracellular oAβ, even under control (C) conditions. The specificity of the oAβ signal from untreated cells was confirmed with the γ-secretase inhibitor DAPT (100 µM). The dotted line represents signal detected in cells from wild-type mice, which was equal to that obtained in cells treated with DAPT and considered background. Treatment of the primary neurons with PA produced a significant increase in RIPA-soluble intracellular oAβ (n = 4 for all groups). *P < 0.05, **P < 0.01, ***P < 0.001.
Acute PA treatment elevates extracellular and intracellular Aβ accumulation, in vivo, in brains of APP transgenic and wild-type mice
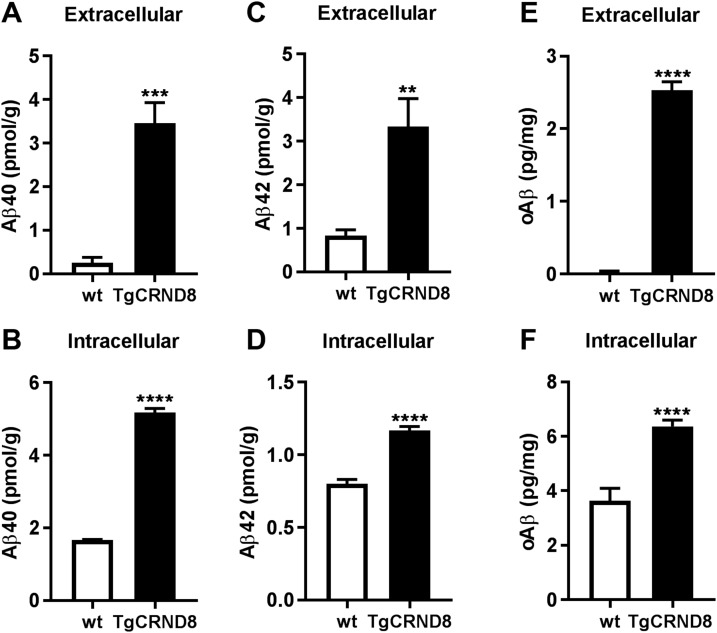
To further substantiate our findings in vivo, we separated soluble extracellular (interstitial) Aβ from the intravesicular pool, by homogenizing brains in iso-osmotic sucrose buffer lacking detergent, followed by sequential centrifugation. Using this approach, we determined that there was a significant amount of RIPA-soluble Aβ, including oAβ, present in intracellular vesicles isolated from brains of young (2-mo-old) TgCRND8 mice, before extracellular amyloid deposition began (Fig. 8).
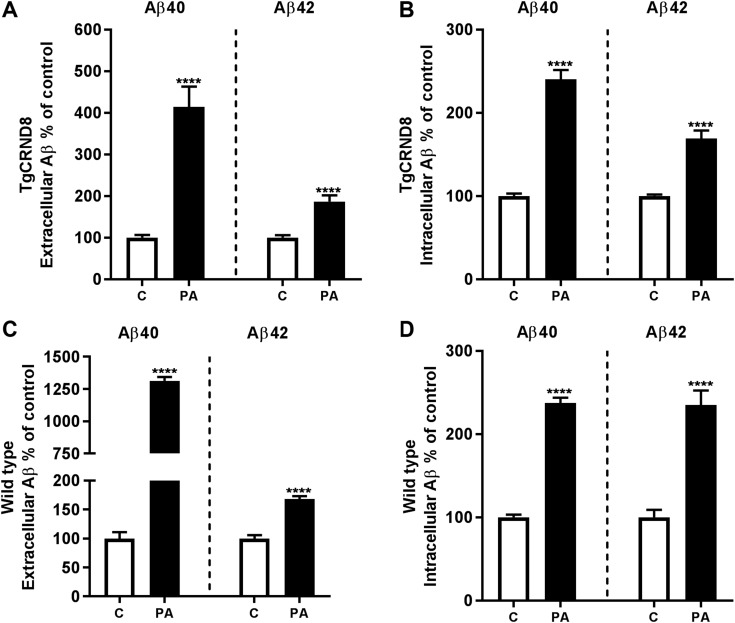
Figure 8.
Aβ species including oAβ are present in both extracellular and intracellular fractions from young TgCRND8 mouse brains. Levels of human Aβ40 (A, B), human/rodent Aβ42 (C, D), and oAβ (E, F) were measured in the Tris-soluble, extracellular enriched fraction and the RIPA-extracted intracellular vesicle fraction from 2-mo-old (preamyloid deposition) TgCRND8 mouse brains (n = 10). Identically prepared extracts from wild-type (wt) littermates (n = 5) were included as controls to validate Aβ measurements in transgenic mice. For every assay, the signal in TgCRND8 transgenic mice was significantly higher than in wild-type mice. **P < 0.01, ***P < 0.001, ****P < 0.0001.
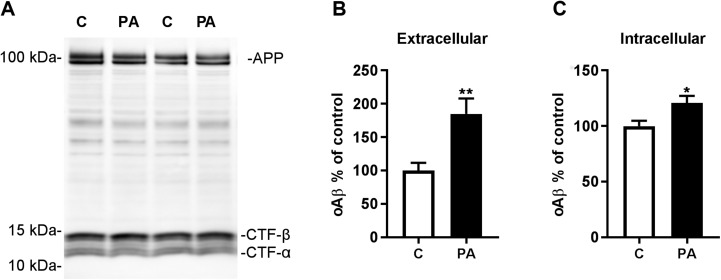
To corroborate the role of the ECE family of proteases in modulating this pool of intravesicular Aβ in vivo, we treated mice with PA by intracerebroventricular (icv) administration. Sixteen hours after a single icv injection of PA into brains of young TgCRND8 mice, we detected substantial elevations in Aβ40 and Aβ42 in both extracellular-enriched and intravesicular fractions (Fig. 9A, B). After a modified solid-phase extraction of endogenous rodent Aβ to increase sensitivity (30), we detected similar >2-fold surges in endogenous intravesicular Aβ40 and Aβ42 levels in wild-type mice injected with PA that were accompanied by large increases in the levels of soluble extracellular Aβ (Fig. 9C, D). Consistent with the elevations in Aβ being related to decreased degradation and not to increased production, PA treatment did not alter the levels of full-length APP or APP-CTFs (Fig. 10A).
Figure 9.
Intracerebroventricular administration of PA to TgCRND8 and wild-type mice increases extracellular and intracellular Aβ. Aβ species were measured 16 h after icv administration of PA to TgCRND8 mice (A, B) and wild-type mice (C, D). A, B) Levels of Tris-soluble extracellular (A) and RIPA-soluble intracellular (B) Aβ40 and Aβ42 were increased in transgenic mouse brains after PA injection. C, D) Levels of endogenous rodent Aβ40 and Aβ42 were also increased in extracellular (C) and intracellular (D) fractions of wild-type mouse brains after PA injection. Intracellular Aβ from wild-type mice was purified and concentrated by solid-phase extraction before measurement by ELISA (for TgCRND8 mice, n = 8 for Aβ40 and n = 10 for Aβ42; for wild-type mice, n = 6 for all groups). ****P < 0.0001.
Figure 10.
PA treatment elevates extracellular and intracellular oAβ in vivo. A) Western blot analysis of brains from PA-treated TgCRND8 mice showed no change in full-length APP or CTF levels. B, C) Levels of oAβ were elevated in extracellular (B) and intravesicular (C) fractions from TgCRND8 mice after PA treatment (n = 9 control and n = 10 PA). *P < 0.05, **P < 0.01.
As in SH-SY5Y-APP cells and organotypic slice cultures, oAβ levels were significantly increased in intracellular vesicles isolated from brains of PA-treated TgCRND8 mice. However, unlike in the in vitro models, increased oAβ was also detected in the extracellular-enriched fraction (Fig. 10B, C), raising the possibility that intracellular Aβ aggregates may be released into the extracellular space in vivo.
PA treatment increases exosome-associated Aβ and oAβ in TgCRND8 mice
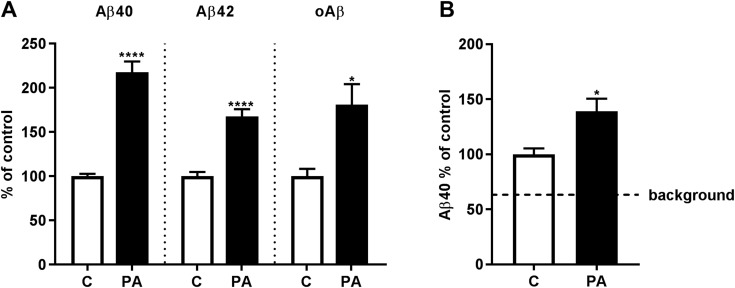
One way that intracellularly accumulated Aβ could reach the extracellular space is by the fusion of Aβ-containing MVBs with the plasma membrane, resulting in secretion of the endosomal lumenal contents and exosomal vesicles. In this way, both soluble and exosome-associated Aβ could be released into the interstitial fluid. To begin to test this notion, we evaluated whether the substantial increase in intravesicular Aβ detected after PA treatment was accompanied by increased levels of exosome-associated Aβ. First, using exosomes isolated from wild-type, APP knockout, and TgCRND8 mice, we established by ELISA, unequivocally, the presence of Aβ in exosomes from young (preamyloid deposition) TgCRND8 mice (Supplemental Fig. S2). Next, we measured Aβ levels in exosomes isolated from brains of TgCRND8 mice injected with PA and found significant increases in RIPA-soluble Aβ40 and -42 and oAβ (Fig. 11A). Finally, to provide further support for the physiologic relevance of Aβ secretion via exosomes, we repeated the experiment in wild-type mice and confirmed the presence of exosome-associated endogenous rodent Aβ that was increased in mice treated with PA (Fig. 11B).
Figure 11.
Exosome-associated Aβ is increased after PA treatment. Exosomes were isolated from brains 16 h after administration of PA or vehicle (control, C). A) RIPA-soluble exosome associated Aβ40, Aβ42, and oAβ were increased in exosomes isolated from PA-treated TgCRND8 mice. B) After solid-phase extraction and concentration of exosome-associated rodent Aβ40, significant increases were also detected in wild-type mice. The dashed line represents background signal from exosomes isolated from APP knockout mice (n = 6 per group for TgCRND8 mice, n = 5 per group for wild-type mice, n = 2 APP knockout mice). *P < 0.05, ****P < 0.0001.
Exosomes contain endogenous ECE activity
Among the M13 family members capable of degrading Aβ, only ECE-1 and -2 are known to be active in acidic intracellular vesicles. Certain isoforms of ECE-1 have been observed in MVBs in nonneuronal cells, including endothelial cells (43), and mRNAs coding for these isoforms are expressed in SH-SY5Y cells and in human brain (Supplemental Fig. S3). To determine whether neuronal exosomes are indeed derived from ECE-containing intracellular vesicles, we next isolated exosome-enriched EVs from the culture medium of SH-SY5Y-APP cells and measured ECE-1 and -2 activity, as well as Aβ content. Similar to results with exosomes from brain, Aβ was present in exosomes secreted from SH-SY5Y-APP cells grown under control conditions, and levels of the peptide increased after PA treatment and decreased with γ-secretase inhibition by DAPT (Fig. 12A). Consistent with MVBs being an intracellular site of Aβ degradation by ECEs, exosomes were found to contain both ECE-1 and -2 specific activity (Fig. 12B).
Figure 12.
Neuronal exosomes contain ECE-1 and -2 activity. A) Aβ was measured by sandwich ELISA in exosomes isolated from SH-SY5Y-APP cells grown in serum-free medium (control, C). Levels of the peptide increased with PA treatment and decreased with DAPT (100 µM, 48 h). B) SH-SY5Y-APP exosomes contained specific PA-sensitive activity for ECE-1 (at pH 6.8) and ECE-2 (at pH 5.5), measured with a big ET-1 conversion assay. **P < 0.01, ***P < 0.001.
DISCUSSION
A major challenge faced by the AD research community is deciphering how an otherwise soluble peptide ends up accumulating in large amounts in the brain in the form of insoluble amyloid deposits. E-L vesicles are considered an important catabolic route for extracellular Aβ, and alterations in E-L homeostasis have long been suspected of triggering abnormal accumulation and aggregation of internalized Aβ. Although the reported E-L dysfunction in AD (44) fits well with this hypothesis, in experimental models, inhibition of cathepsin activity or other disturbances in E-L function have produced conflicting results with respect to Aβ accumulation. Elevated levels of APP-CTFs are consistently observed in many of these models, but intraneuronal Aβ levels remain unchanged (45–47) or modestly elevated (48, 49). With the evidence provided in this report, we propose that endogenous production of Aβ in E-L vesicles is a major source of intraneuronal Aβ and that the ECE family of membrane-bound proteases plays a crucial role in buffering intraneuronal Aβ accumulation. Hence, E-L insults that increase β-CTF accumulation (the immediate precursor to Aβ) may only translate to Aβ accumulation and aggregation if ECE activity is also disrupted or overwhelmed. Moreover, because this intracellular pool of Aβ appears independent from extracellular Aβ, the amyloidogenic process can start intraneuronally, without any indication of perturbed Aβ secretion.
Using wild-type human APP-overexpressing cells, not bearing any mutations that alter the aggregation properties of Aβ or the Aβ42/Aβ40 ratio, we demonstrate that acute inhibition of PA-sensitive metalloproteases was sufficient to trigger intracellular Aβ oligomer formation. Combining metalloprotease inhibition with cysteine cathepsin inhibition, using E-64, provided additional insights into the oligomerization process in this cellular model. With the combined treatment, we found that with increasing accumulation of intravesicular Aβ, the peptide tended to form aggregates that remained in a soluble state. Association of Aβ with lipid rafts was detectable only after considerable accumulation of soluble monomeric and aggregated Aβ within the vesicle lumen. All these intravesicular Aβ species appeared to exist in a dynamic equilibrium during the time frame of our experiment (48 h) and restoring protease activity by washing out inhibitors cleared vesicles of all accumulated Aβ species, including insoluble pools. Although we cannot completely rule out direct hydrolysis of oAβ after inhibitors were removed, ECE-1 and -2 have small catalytic chamber openings (50) that limit the size of potential substrates, with Aβ42 being the largest known physiologic substrate. It is more likely that degradation of Aβ monomers shifted the equilibrium toward oAβ disaggregation. The observation that inhibition of Aβ production with DAPT in neuronal cultures also lowered intraneuronally accumulated oAβ further substantiates the reversibility of intravesicular Aβ aggregates, at least at the time points studied.
Although accumulation of intraneuronal Aβ is regarded as a defining point early in the progression of AD, so far evidence of its existence in vivo has been limited mainly to immunohistochemical findings. Staining for intraneuronal Aβ is challenging, however, because of antigen masking and potential cross-reactivity of Aβ antibodies with other APP metabolites. To curtail these limitations, we developed a method of studying the dynamics of intraneuronal Aβ accumulation and aggregation in vivo using brains from young TgCRND8 mice before the appearance of amyloid plaques. We separated secreted Aβ from the intravesicular pool by homogenizing brains without detergent, while maintaining the integrity of intracellular vesicles. Although limited by the fact that only soluble/diffusible forms of Aβ and oAβ were extracted into the aqueous extracellular-enriched fraction, this approach allowed us to firmly establish the presence of intravesicular Aβ and oAβ in a mouse model of AD before amyloid deposition, eliminating any possibility of contamination with Aβ dissociating from plaques. The physiologic relevance of the ECE family of proteases in regulating this intravesicular Aβ pool was confirmed in experiments in wild-type mice. Although overall levels of Aβ were 5–10 times lower, the magnitude of PA-induced increases in endogenous mouse Aβ mirrored those in TgCRND8 human APP transgenic mice. Because PA inhibits multiple members of the ECE (M13) family of proteases, we cannot rule out that enzymes besides the ECEs may contribute to the PA-induced effects on Aβ accumulation in vivo. It is important to note, however, that although endogenous Aβ levels are elevated in brains of mice deficient in ECE-1, ECE-2, NEP, or NEP-2 (51–53), it has been reported that PA treatment still significantly increases Aβ levels in brains of NEP/NEP-2 double-knockout mice (54).
By studying the effect of PA in vivo, we also obtained evidence that the activity of ECE family members modulates the level of exosome-associated Aβ. Exosomes originate from vesicles generated by the invagination of the outer membranes of late endosomes during the formation of MVBs. These vesicles then accumulate within the lumen of MVBs and are released into the extracellular space as exosomes after fusion with the plasma membrane. Consistent with their late endosomal origin, the membranes of MVBs as well as exosomes are rich in APP and APP-CTFs as well as α-, β, and γ-secretases (55), and several studies have indicated that exosomes also contain Aβ, which is thought to be bound to lipid rafts on the outer surface of the exosomal membrane (56, 57). In our study, exosomes, and hence late endosomal/MVB membranes, were also found to contain ECE-1 and -2 activity; the significant increase in exosome-associated Aβ after PA treatment implicates MVBs as intracellular sites where ECEs regulate Aβ concentration. The detection of ECE-regulated Aβ in exosomes isolated from the brains of wild-type mice further suggests that the release of intracellular Aβ content via exosome secretion is a physiologic process and not an artifact of mutant APP overexpression in transgenic mice. Although it is possible that exosomal membranes themselves produce Aβ or that soluble Aβ present in the extracellular space associates with exosomal membranes after exosome secretion, our results strongly suggest that Aβ produced within the lumen of MVBs associates intracellularly with exosomal membranes, before secretion. In support of this, the increase in exosome-associated Aβ in PA-treated mice was similar in magnitude to the increase in intracellular vesicle preparations and did not parallel increases in extracellular Aβ. Furthermore, the finding of oAβ associated with exosomes from APP transgenic mice before the onset of amyloid deposition supports the notion that exosomes are vehicles for the initiation and dissemination of extracellular amyloid pathology.
Little is currently known about whether certain subtypes of neurons are more or less susceptible to intracellular Aβ accumulation, but differential expression of ECEs may be a contributing factor. The expression patterns and subcellular localization of ECE-1 and ECE-2 differ significantly. ECE-2 localizes exclusively intracellularly, within membranes of E-L vesicles including autophagosomes, and in cultured cells, ECE-2 activity influences only intracellular Aβ (17). We recently discovered that ECE-2 expression is largely restricted to somatostatin-containing interneurons in the cortex and hippocampus (58). If these neurons normally require substantial ECE-2 activity to maintain intracellular Aβ homeostasis, it is conceivable that they are also especially vulnerable to insults that disrupt this activity.
In contrast to ECE-2, ECE-1 displays more widespread expression in the brain and regulates both intracellular and extracellular Aβ levels. Besides MVBs, different ECE-1 isoforms localize to the plasma membrane or to a wider variety of E-L vesicles, including recycling endosomes (59–61). The presence of ECE-1 in recycling endosomes provides it with the ability to regulate another pool of Aβ that is bound for secretion (33). Although the experiments described in this study were not designed to identify the specific M13 family member responsible for PA-induced changes in different pools of Aβ, inhibition of ECE-1 is likely to be at least partially responsible for the dramatic elevations in extracellular Aβ in brain after icv PA administration. The large increases in extracellular Aβ observed in both transgenic and wild-type mice treated with PA suggest that only a fraction of Aβ produced in ECE-containing compartments ever reaches the extracellular space. With the evidence presented, we can only speculate about the biologic reason for this “energetically expensive” mechanism, but ECE activity may represent a determinant step in modulating the putative function of Aβ as a neuroactive peptide (62).
In summary, our results demonstrate the existence of an intraneuronally produced pool of Aβ that is regulated by proteolytic degradation and can aggregate before secretion. As such, potential therapeutic agents that influence only interstitial Aβ may have suboptimal effects. Identifying the physiologic functions of intraneuronal and secreted Aβ and characterizing the consequences of their pathologic accumulation could eventually help in the development of new therapeutic approaches for AD.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Luis Marcelino and Joaquim Vieira (Biomedical Research Institute of New Jersey) for assistance with the mice and the Banner Sun Health Research Institute Brain and Body Donation Program (Sun City, AZ, USA), for the provision of human brain tissue. Research reported in this publication was supported in part by a grant from the Vanech Family Foundation (to J.P.-Q.), by U.S. National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke Grant NS073512 (to E.A.E.) and NIH National Institute on Aging Grants AG017617 and AG056732 (to E.L.). The Brain and Body Donation Program was supported by the NIH National Institute of Neurological Disorders and Stroke Grant U2418 NS072026 (National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders); the NIH National Institute on Aging Grant P30 AG19610 (Arizona Alzheimer’s Disease Core Center); the Arizona Department of Health Services Contract 211002 (Arizona Alzheimer’s Research Center); the Arizona Biomedical Research Commission Contracts 4001, 0011, 05-901 and 1001 (Arizona Parkinson's Disease Consortium), and the Michael J. Fox Foundation for Parkinson’s Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- CTF
C-terminal fragment
- DAPT
N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester
- ECE
endothelin-converting enzyme
- E-L
endosomal-lysosomal
- ET
endothelin
- EV
extracellular vesicle
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- icv
intracerebroventricular
- HRP
horseradish peroxidase
- MME
membrane metalloendopeptidases
- MVB
multivesicular body
- NEP
neprilysin
- oAβ
oligomeric amyloid-β
- PA
phosphoramidon
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Pacheco-Quinto and E. A. Eckman designed research; J. Pacheco-Quinto, D. Clausen, R. Pérez-González, H. Peng, A. Meszaros, and E. A. Eckman performed experiments and analyzed data; and J. Pacheco-Quinto, R. Pérez-González, C. B. Eckman, E. Levy, and E. A. Eckman interpreted results and wrote or revised the manuscript.
REFERENCES
- 1.Glenner G. G., Wong C. W. (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 10.1016/S0006-291X(84)80190-4 [DOI] [PubMed] [Google Scholar]
- 2.Haass C., Kaether C., Thinakaran G., Sisodia S. (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2, a006270 10.1101/cshperspect.a006270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roychaudhuri R., Yang M., Hoshi M. M., Teplow D. B. (2009) Amyloid beta-protein assembly and Alzheimer disease. J. Biol. Chem. 284, 4749–4753 10.1074/jbc.R800036200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe D. J. (2008) Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 192, 106–113 10.1016/j.bbr.2008.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masters C. L., Selkoe D. J. (2012) Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006262 10.1101/cshperspect.a006262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharadwaj P. R., Dubey A. K., Masters C. L., Martins R. N., Macreadie I. G. (2009) Abeta aggregation and possible implications in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 13, 412–421 10.1111/j.1582-4934.2009.00609.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellstrand E., Boland B., Walsh D. M., Linse S. (2010) Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chem. Neurosci. 1, 13–18 10.1021/cn900015v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novo M., Freire S., Al-Soufi W. (2018) Critical aggregation concentration for the formation of early Amyloid-β (1-42) oligomers. Sci. Rep. 8, 1783 10.1038/s41598-018-19961-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody D. L., Magnoni S., Schwetye K. E., Spinner M. L., Esparza T. J., Stocchetti N., Zipfel G. J., Holtzman D. M. (2008) Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 321, 1221–1224 10.1126/science.1161591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouras G. K., Almeida C. G., Takahashi R. H. (2005) Intraneuronal Aβ accumulation and origin of plaques in Alzheimer’s disease. Neurobiol. Aging 26, 1235–1244 10.1016/j.neurobiolaging.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 11.Billings L. M., Oddo S., Green K. N., McGaugh J. L., LaFerla F. M. (2005) Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45, 675–688 10.1016/j.neuron.2005.01.040 [DOI] [PubMed] [Google Scholar]
- 12.Gyure K. A., Durham R., Stewart W. F., Smialek J. E., Troncoso J. C. (2001) Intraneuronal Aβ-amyloid precedes development of amyloid plaques in Down syndrome. Arch. Pathol. Lab. Med. 125, 489–492 [DOI] [PubMed] [Google Scholar]
- 13.Mori C., Spooner E. T., Wisniewsk K. E., Wisniewski T. M., Yamaguch H., Saido T. C., Tolan D. R., Selkoe D. J., Lemere C. A. (2002) Intraneuronal Aβ42 accumulation in Down syndrome brain. Amyloid 9, 88–102 10.3109/13506120208995241 [DOI] [PubMed] [Google Scholar]
- 14.Colacurcio D. J., Pensalfini A., Jiang Y., Nixon R. A. (2018) Dysfunction of autophagy and endosomal-lysosomal pathways: roles in pathogenesis of Down syndrome and Alzheimer’s Disease. Free Radic. Biol. Med. 114, 40–51https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28988799&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi R. H., Milner T. A., Li F., Nam E. E., Edgar M. A., Yamaguchi H., Beal M. F., Xu H., Greengard P., Gouras G. K. (2002) Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 161, 1869–1879 10.1016/S0002-9440(10)64463-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cataldo A. M., Petanceska S., Terio N. B., Peterhoff C. M., Durham R., Mercken M., Mehta P. D., Buxbaum J., Haroutunian V., Nixon R. A. (2004) Aβ localization in abnormal endosomes: association with earliest Aβ elevations in AD and Down syndrome. Neurobiol. Aging 25, 1263–1272 10.1016/j.neurobiolaging.2004.02.027 [DOI] [Google Scholar]
- 17.Pacheco-Quinto J., Eckman E. A. (2013) Endothelin-converting enzymes degrade intracellular β-amyloid produced within the endosomal/lysosomal pathway and autophagosomes. J. Biol. Chem. 288, 5606–5615 10.1074/jbc.M112.422964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X., Crick S. L., Bu G., Frieden C., Pappu R. V., Lee J. M. (2009) Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-β peptide. Proc. Natl. Acad. Sci. USA 106, 20324–20329 10.1073/pnas.0911281106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich R. P., Tepper K., Rönicke R., Soom M., Westermann M., Reymann K., Kaether C., Fändrich M. (2010) Mechanism of amyloid plaque formation suggests an intracellular basis of Aβ pathogenicity. Proc. Natl. Acad. Sci. USA 107, 1942–1947 10.1073/pnas.0904532106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasternak S. H., Bagshaw R. D., Guiral M., Zhang S., Ackerley C. A., Pak B. J., Callahan J. W., Mahuran D. J. (2003) Presenilin-1, nicastrin, amyloid precursor protein, and gamma-secretase activity are co-localized in the lysosomal membrane. J. Biol. Chem. 278, 26687–26694 10.1074/jbc.M304009200 [DOI] [PubMed] [Google Scholar]
- 21.Kaether C., Schmitt S., Willem M., Haass C. (2006) Amyloid precursor protein and Notch intracellular domains are generated after transport of their precursors to the cell surface. Traffic 7, 408–415 10.1111/j.1600-0854.2006.00396.x [DOI] [PubMed] [Google Scholar]
- 22.Vetrivel K. S., Cheng H., Lin W., Sakurai T., Li T., Nukina N., Wong P. C., Xu H., Thinakaran G. (2004) Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem. 279, 44945–44954 10.1074/jbc.M407986200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy E. (2017) Exosomes in the diseased brain: first insights from in vivo studies. Front. Neurosci. 11, 142 10.3389/fnins.2017.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belyaev N. D., Nalivaeva N. N., Makova N. Z., Turner A. J. (2009) Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep. 10, 94–100 10.1038/embor.2008.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beach T. G., Adler C. H., Sue L. I., Serrano G., Shill H. A., Walker D. G., Lue L., Roher A. E., Dugger B. N., Maarouf C., Birdsill A. C., Intorcia A., Saxon-Labelle M., Pullen J., Scroggins A., Filon J., Scott S., Hoffman B., Garcia A., Caviness J. N., Hentz J. G., Driver-Dunckley E., Jacobson S. A., Davis K. J., Belden C. M., Long K. E., Malek-Ahmadi M., Powell J. J., Gale L. D., Nicholson L. R., Caselli R. J., Woodruff B. K., Rapscak S. Z., Ahern G. L., Shi J., Burke A. D., Reiman E. M., Sabbagh M. N. (2015) Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 35, 354–389 10.1111/neup.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. (1996) Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 2, 864–870 10.1038/nm0896-864 [DOI] [PubMed] [Google Scholar]
- 27.Chishti M. A., Yang D. S., Janus C., Phinney A. L., Horne P., Pearson J., Strome R., Zuker N., Loukides J., French J., Turner S., Lozza G., Grilli M., Kunicki S., Morissette C., Paquette J., Gervais F., Bergeron C., Fraser P. E., Carlson G. A., George-Hyslop P. S., Westaway D. (2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 276, 21562–21570 10.1074/jbc.M100710200 [DOI] [PubMed] [Google Scholar]
- 28.Beaudoin G. M., III, Lee S. H., Singh D., Yuan Y., Ng Y. G., Reichardt L. F., Arikkath J. (2012) Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 7, 1741–1754 10.1038/nprot.2012.099 [DOI] [PubMed] [Google Scholar]
- 29.De Simoni A., Yu L. M. (2006) Preparation of organotypic hippocampal slice cultures: interface method. Nat. Protoc. 1, 1439–1445 10.1038/nprot.2006.228 [DOI] [PubMed] [Google Scholar]
- 30.Lanz T. A., Schachter J. B. (2006) Demonstration of a common artifact in immunosorbent assays of brain extracts: development of a solid-phase extraction protocol to enable measurement of amyloid-β from wild-type rodent brain. J. Neurosci. Methods 157, 71–81 10.1016/j.jneumeth.2006.03.023 [DOI] [PubMed] [Google Scholar]
- 31.Pérez-González R., Gauthier S. A., Kumar A., Saito M., Saito M., Levy E. (2017) A method for isolation of extracellular vesicles and characterization of exosomes from brain extracellular space. Methods Mol. Biol. 1545, 139–151 10.1007/978-1-4939-6728-5_10 [DOI] [PubMed] [Google Scholar]
- 32.Emoto N., Yanagisawa M. (1995) Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J. Biol. Chem. 270, 15262–15268 10.1074/jbc.270.25.15262 [DOI] [PubMed] [Google Scholar]
- 33.Eckman E. A., Reed D. K., Eckman C. B. (2001) Degradation of the Alzheimer’s amyloid β peptide by endothelin-converting enzyme. J. Biol. Chem. 276, 24540–24548 10.1074/jbc.M007579200 [DOI] [PubMed] [Google Scholar]
- 34.Fahnoe D. C., Knapp J., Johnson G. D., Ahn K. (2000) Inhibitor potencies and substrate preference for endothelin-converting enzyme-1 are dramatically affected by pH. J. Cardiovasc. Pharmacol. 36(Suppl 1), S22–S25 10.1097/00005344-200036001-00009 [DOI] [PubMed] [Google Scholar]
- 35.Rawlings N. D., Barrett A. J., Thomas P. D., Huang X., Bateman A., Finn R. D. (2018) The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 46, D624–D632 10.1093/nar/gkx1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. (1992) Assembly and aggregation properties of synthetic Alzheimer’s A4/β amyloid peptide analogs. J. Biol. Chem. 267, 546–554 [PubMed] [Google Scholar]
- 37.Youmans K. L., Tai L. M., Nwabuisi-Heath E., Jungbauer L., Kanekiyo T., Gan M., Kim J., Eimer W. A., Estus S., Rebeck G. W., Weeber E. J., Bu G., Yu C., Ladu M. J. (2012) APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease. J. Biol. Chem. 287, 41774–41786 10.1074/jbc.M112.407957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youmans K. L., Tai L. M., Kanekiyo T., Stine W. B., Jr., Michon S. C., Nwabuisi-Heath E., Manelli A. M., Fu Y., Riordan S., Eimer W. A., Binder L., Bu G., Yu C., Hartley D. M., LaDu M. J. (2012) Intraneuronal Aβ detection in 5xFAD mice by a new Aβ-specific antibody. Mol. Neurodegener. 7, 8 10.1186/1750-1326-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinohara M., Sato N., Kurinami H., Takeuchi D., Takeda S., Shimamura M., Yamashita T., Uchiyama Y., Rakugi H., Morishita R. (2010) Reduction of brain β-amyloid (Aβ) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Aβ clearance. J. Biol. Chem. 285, 22091–22102 10.1074/jbc.M110.102277 [DOI] [Google Scholar]
- 40.Boland B., Kumar A., Lee S., Platt F. M., Wegiel J., Yu W. H., Nixon R. A. (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J. Neurosci. 28, 6926–6937 10.1523/JNEUROSCI.0800-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ariga T., McDonald M. P., Yu R. K. (2008) Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease: a review. J. Lipid Res. 49, 1157–1175 10.1194/jlr.R800007-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai A. Y., McLaurin J. (2010) Mechanisms of amyloid-beta peptide uptake by neurons: the role of lipid rafts and lipid raft-associated proteins. Int. J. Alzheimers Dis. 2011, 548380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdenaire O., Barret A., Schweizer A., Rohrbacher E., Mongiat F., Pinet F., Corvol P., Tougard C. (1999) Two di-leucine-based motifs account for the different subcellular localizations of the human endothelin-converting enzyme (ECE-1) isoforms. J. Cell Sci. 112, 3115–3125 [DOI] [PubMed] [Google Scholar]
- 44.Nixon R. A. (2017) Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J. 31, 2729–2743 10.1096/fj.201700359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boland B., Smith D. A., Mooney D., Jung S. S., Walsh D. M., Platt F. M. (2010) Macroautophagy is not directly involved in the metabolism of amyloid precursor protein. J. Biol. Chem. 285, 37415–37426 10.1074/jbc.M110.186411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tien N. T., Karaca I., Tamboli I. Y., Walter J. (2016) Trehalose alters subcellular trafficking and the metabolism of the Alzheimer-associated amyloid precursor protein. J. Biol. Chem. 291, 10528–10540 10.1074/jbc.M116.719286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cermak S., Kosicek M., Mladenovic-Djordjevic A., Smiljanic K., Kanazir S., Hecimovic S. (2016) Loss of cathepsin B and L leads to lysosomal dysfunction, NPC-like cholesterol sequestration and accumulation of the key Alzheimer’s proteins. PLoS One 11, e0167428 10.1371/journal.pone.0167428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keilani S., Lun Y., Stevens A. C., Williams H. N., Sjoberg E. R., Khanna R., Valenzano K. J., Checler F., Buxbaum J. D., Yanagisawa K., Lockhart D. J., Wustman B. A., Gandy S. (2012) Lysosomal dysfunction in a mouse model of Sandhoff disease leads to accumulation of ganglioside-bound amyloid-β peptide. J. Neurosci. 32, 5223–5236 10.1523/JNEUROSCI.4860-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vingtdeux V., Chandakkar P., Zhao H., d’Abramo C., Davies P., Marambaud P. (2011) Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-β peptide degradation. FASEB J. 25, 219–231 10.1096/fj.10-167361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bur D., Dale G. E., Oefner C. (2001) A three-dimensional model of endothelin-converting enzyme (ECE) based on the X-ray structure of neutral endopeptidase 24.11 (NEP). Protein Eng. 14, 337–341 10.1093/protein/14.5.337 [DOI] [PubMed] [Google Scholar]
- 51.Eckman E. A., Adams S. K., Troendle F. J., Stodola B. A., Kahn M. A., Fauq A. H., Xiao H. D., Bernstein K. E., Eckman C. B. (2006) Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J. Biol. Chem. 281, 30471–30478 10.1074/jbc.M605827200 [DOI] [PubMed] [Google Scholar]
- 52.Hafez D., Huang J. Y., Huynh A. M., Valtierra S., Rockenstein E., Bruno A. M., Lu B., DesGroseillers L., Masliah E., Marr R. A. (2011) Neprilysin-2 is an important β-amyloid degrading enzyme. Am. J. Pathol. 178, 306–312 10.1016/j.ajpath.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N. P., Gerard C., Hama E., Lee H. J., Saido T. C. (2001) Metabolic regulation of brain Aβ by neprilysin. Science 292, 1550–1552 10.1126/science.1059946 [DOI] [PubMed] [Google Scholar]
- 54.Hanson L. R., Hafez D., Svitak A. L., Burns R. B., Li X., Frey W. H., II, Marr R. A. (2011) Intranasal phosphoramidon increases beta-amyloid levels in wild-type and NEP/NEP2-deficient mice. J. Mol. Neurosci. 43, 424–427 10.1007/s12031-010-9460-8 [DOI] [PubMed] [Google Scholar]
- 55.Vingtdeux V., Hamdane M., Loyens A., Gelé P., Drobeck H., Bégard S., Galas M. C., Delacourte A., Beauvillain J. C., Buée L., Sergeant N. (2007) Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem. 282, 18197–18205 10.1074/jbc.M609475200 [DOI] [PubMed] [Google Scholar]
- 56.Rajendran L., Honsho M., Zahn T. R., Keller P., Geiger K. D., Verkade P., Simons K. (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 103, 11172–11177 10.1073/pnas.0603838103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Gonzalez R., Gauthier S. A., Kumar A., Levy E. (2012) The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem. 287, 43108–43115 10.1074/jbc.M112.404467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pacheco-Quinto J., Eckman C. B., Eckman E. A. (2016) Major amyloid-β-degrading enzymes, endothelin-converting enzyme-2 and neprilysin, are expressed by distinct populations of GABAergic interneurons in hippocampus and neocortex. Neurobiol. Aging 48, 83–92 10.1016/j.neurobiolaging.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller L., Barret A., Etienne E., Meidan R., Valdenaire O., Corvol P., Tougard C. (2003) Heterodimerization of endothelin-converting enzyme-1 isoforms regulates the subcellular distribution of this metalloprotease. J. Biol. Chem. 278, 545–555 10.1074/jbc.M208949200 [DOI] [PubMed] [Google Scholar]
- 60.Valdenaire O., Lepailleur-Enouf D., Egidy G., Thouard A., Barret A., Vranckx R., Tougard C., Michel J. B. (1999) A fourth isoform of endothelin-converting enzyme (ECE-1) is generated from an additional promoter molecular cloning and characterization. Eur. J. Biochem. 264, 341–349 10.1046/j.1432-1327.1999.00602.x [DOI] [PubMed] [Google Scholar]
- 61.Schweizer A., Valdenaire O., Nelböck P., Deuschle U., Dumas Milne Edwards J. B., Stumpf J. G., Löffler B. M. (1997) Human endothelin-converting enzyme (ECE-1): three isoforms with distinct subcellular localizations. Biochem. J. 328, 871–877 10.1042/bj3280871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puzzo D., Gulisano W., Arancio O., Palmeri A. (2015) The keystone of Alzheimer pathogenesis might be sought in Aβ physiology. Neuroscience 307, 26–36 10.1016/j.neuroscience.2015.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.