Abstract
Critical functions of immune cells require them to rapidly change their shape and generate forces in response to cues from their surrounding environment. However, little is known about how soluble factors that may be present in the microenvironment modulate key aspects of cellular mechanobiology—such as immune cell deformability and force generation—to impact functions such as phagocytosis and migration. Here we show that signaling by soluble stress hormones through β-adrenoceptors (β-AR) reduces the deformability of macrophages; this is dependent on changes in the organization of the actin cytoskeleton and is associated with functional changes in phagocytosis and migration. Pharmacologic interventions reveal that the impact of β-AR signaling on macrophage deformability is dependent on actin-related proteins 2/3, indicating that stress hormone signaling through β-AR shifts actin organization to favor branched structures rather than linear unbranched actin filaments. These findings show that through remodeling of the actin cytoskeleton, β-AR-mediated stress hormone signaling modulates macrophage mechanotype to impact functions that play a critical role in immune response.—Kim, T.-H., Ly, C., Christodoulides, A., Nowell, C. J., Gunning, P. W., Sloan, E. K., Rowat, A. C. Stress hormone signaling through β-adrenergic receptors regulates macrophage mechanotype and function.
Keywords: deformability, phagocytosis, chemotaxis, actin
Immune cells sense and integrate information from diverse environmental cues (1–3). In response to physiologic and psychologic stressors, fibers of the peripheral sympathetic nervous system release catecholaminergic stress hormones including norepinephrine into major organ systems to induce a “fight-or-flight” response; epinephrine is released from the adrenal gland into circulation (4, 5). Norepinephrine and epinephrine bind to β-adrenergic receptors (β-ARs) to mediate changes in immune cell function. Such neuroendocrine-immune communication is evolutionarily conserved (6) and critical for modulating immune response [reviewed in (7)]. Macrophages are essential for immune function: inflammation triggers blood-circulating monocytes to be recruited and differentiate into macrophages (8). Resident macrophages are present in major organs and perform crucial functions in tissue homeostasis, such as eliminating infectious agents, mediating inflammation, and repairing tissue damage (9–11). β-AR signaling induces molecular changes in macrophages, including up-regulation of immune receptor CD14 and increased secretion of cytokines including TNF-α, IL-6, and IL-10 (12–14), as well as changes in integrin affinity (15). β-AR signaling has also been shown to affect phagocytosis, but studies reveal inconsistent results (13, 15). However, despite the importance of stress hormones in modulating immune cell function, little is understood about the physical and molecular mechanisms that underlie β-AR regulation of macrophage functions including migration and phagocytosis.
The mechanical phenotype—or mechanotype—of macrophages is intimately linked to their function (16, 17). The compliance of macrophages has been associated with their advection through narrow channels and migration through micron-scale pores (18). Macrophage phagocytosis has also been associated with cell mechanical properties (19). The link between mechanotype and functions can be explained as phagocytosis and migration require force generation and changes in cellular shape, which are regulated by shared molecular mediators. A primary determinant of the mechanical stability of macrophages is the actin cytoskeleton, which also provides critical force-generating machinery for the cell (18, 20). Protrusions and cellular shape changes generated by actin polymerization and branching—which are mediated by the actin-related protein 2/3 (Arp2/3) complex and formins—are required for functions such as migration and phagocytosis (21–24). However, it is poorly defined how stress hormone signaling modulates actin organization and dynamics to regulate macrophage mechanical properties and function. Such knowledge would provide mechanistic insight into how cells alter their physical properties in response to a soluble physiologic cue.
Here we investigate how β-AR signaling modulates macrophage mechanotype and key functions of phagocytosis and migration. Using U937 human myeloid cells and RAW 264.7 mouse myeloid cells, we show that signaling through β-AR reduces the deformability of differentiated macrophages but not of precursor monocytes. β-AR regulation of cell deformability is dependent on remodeling of the actin cytoskeleton and is associated with changes in phagocytosis and migration. These findings show that β-AR signaling, which is activated by soluble stress hormones, induces changes in macrophage mechanotype and critical functions of phagocytosis and migration. To our knowledge, this is the first report of how a physiologic cue regulates cellular physical phenotype and physiologic functions by shifting the balance between branched and unbranched actin filament populations.
MATERIALS AND METHODS
Cells and reagents
The human histomonocytic (U937) cell line [American Type Culture Collection (ATCC), Manassas, VA, USA] was maintained in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, West Sacramento, CA, USA) and 1% penicillin and streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). Macrophage-like cells were differentiated by treatment with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) in serum-free medium for 24 h (25). The murine macrophage (RAW 264.7) cell line (ATCC) was maintained in DMEM (Thermo Fisher Scientific) supplemented with 10% FBS and 1% penicillin and streptomycin (Thermo Fisher Scientific). All cells were maintained at 37°C, 5% CO2.
The β-AR agonist (isoproterenol) and antagonist (propranolol) were from MilliporeSigma (Burlington, MA, USA). The adenylyl cyclase activator forskolin (MilliporeSigma) was used at 10 µM for 24 h. To inhibit polymerization of F-actin, we treated cells with 2 μM cytochalasin D (Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h. To inhibit the activity of Arp2/3 and formins, cells were treated with 50 µM CK666 (MilliporeSigma) or 20 µM SMIFH2 (MilliporeSigma) for 24 h. To chelate intracellular Ca2+, cells were treated with 10 μM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis-acetoxymethyl ester (Thermo Fisher Scientific) for 24 h.
Quantitative RT-PCR
To measure levels of β-adrenoceptors in U937 cells before and after PMA-differentiation into macrophages, TRIzol reagent (Thermo Fisher Scientific) was used to extract total RNA from cells according to the manufacturer’s protocol. cDNA was obtained using SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific) starting from 1 µg of RNA. Quantitative PCR was performed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) and SYBR Green Real-Time PCR Master Mixes (Thermo Fisher Scientific) with the following primers: GAPDH (forward: 5′-tgcaccaccaactgcttagc-3′, reverse: 5′-ggcatggactgtggtcatgag-3′), TLR4 (forward: 5′-agacctgtccctgaaccctat-3′, reverse: 5′-cgatggacttctaaaccagcca-3′), CCR2 (forward: 5′-ccacatctcgttctcggtttatc-3′, reverse: 5′-cagggagcaccgtaatcataatc-3′), CD14 (forward: 5′-acgccagaaccttgtgagc-3′, reverse: 5′-gcatggatctccacctctactg-3′), CD163 (forward: 5′-tttgtcaacttgagtcccttcac-3′, reverse: 5′-tcccgctacacttgttttcac-3′), CSF1R (forward: 5′-gggaatcccagtgatagagcc-3′, reverse: 5′-ttggaaggtagcgttgttggt-3′), CD68 (forward: 5′-cttctctcattcccctatggaca-3′, reverse: 5′-gaaggacacattgtactccacc-3′), CD16/FCGR3A (forward: 5′-cctcctgtctagtcggtttgg-3′, reverse: 5′-tcgagcaccctgtaccattga-3′), CD71/TFRC (forward: 5′-accattgtcatatacccggttca-3′, reverse: 5′-caatagcccaagtagccaatcat-3′).
Flow cytometry
To confirm differentiation of U937 cells, we quantified the canonical macrophage surface marker, CD11b. Differentiated and undifferentiated U937 cells were harvested in ice-cold PBS containing 10% FBS solution. We then immunolabeled 1 × 106 cells with 0.75 µg of phycoerythrin-conjugated CD11b antibody (301306; BioLegend, San Diego, CA, USA) for 30 min at room temperature. After being washed with PBS, cells were analyzed with FACSAria II High-Speed Cell Sorter with FACSDiva software (BD Biosciences, San Jose, CA, USA) and FlowJo software (FlowJo, Ashland, Oregon, USA) at the University of California, Los Angeles (UCLA) Broad Stem Cell Research Center Flow Cytometry Core. To measure β2AR expression levels, we fixed cells with 4% paraformaldehyde and labeled them with 0.60 µg of FITC-conjugated β2AR antibody (sc-271322; Santa Cruz Biotechnology) before analyzing by flow cytometry.
Phagocytosis assay
To quantify phagocytosis, we measured the uptake of pH-sensitive dye (pHrodo) conjugated E. coli bioparticles (26) into macrophages using an automated live cell imaging system (IncuCyte Zoom; Essen BioScience, Ann Arbor, MI, USA) (27). Because the fluorescence of the pHrodo dye conjugated to E.coli bioparticles is sensitive to pH, the fluorescence increases once the particles are internalized through phagocytosis. PMA-differentiated U937 cells were treated with forskolin (10 µM) or isoproterenol (100 nM) with or without inhibitors of actin polymerization, branching, and nucleation (2 µM cytochalasin D, 50 µM CK666, and 20 µM SMIFH2). After 1 h of incubation, 10 µg/well of pHrodo conjugated E. coli bioparticles (Thermo Fisher Scientific) were added, and images were then acquired every 2 h for 48 h. To quantify the total green object area (square micrometers per image), which indicates the number of internalized particles, images were analyzed using IncuCyte Zoom software (Essen BioScience).
Chemotaxis assay
Chemotaxis of macrophages was measured using a transwell migration assay. We seeded 8 × 105 U937 cells on polycarbonate membranes with 5 µm-diameter pores (Transwell Inserts; Corning, Corning, NY, USA) and differentiated cells as previously described. After 24 h, forskolin (10 µM), propranolol (10 µM), or isoproterenol (100 nM) with or without other actin-perturbing drugs were added in both upper inserts and lower wells. To induce chemotaxis, the chemoattractant, 10% FBS, was added only to the lower wells. After incubation for 6 h at 37°C, we determined the number of cells that had migrated through to the lower well by staining the remaining cells in the lower well with Hoechst 33342 (10 µg/ml) for 30 min at room temperature. To quantify chemotaxis efficacy, we counted the number of cells that migrated into the lower well by acquiring at least 5 images per well using a charge-coupled device camera (AxioCam MRm; Carl Zeiss, Oberkochen, Germany) mounted on an inverted fluorescence microscope (Carl Zeiss) with ×10 objective (Zeiss EC Plan-NEOFLUAR, NA:0.3) equipped with X-Cite 120Q light source.
Quantitative image analysis
To quantify levels of F-actin, 1 × 106 undifferentiated U937 cells were plated on a glass (Millicell Ez Slides; MilliporeSigma) coated with 100 μg/ml of Matrigel (Corning) followed by differentiation with PMA. To identify single cells, we labeled both F-actin (phalloidin) and DNA (DRAQ5). Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, and then incubated for 1 h at room temperature with phalloidin (Alexa Fluor 546-conjugated Phalloidin, 1:200; Thermo Fisher Scientific) and DRAQ5 (1:1000; Thermo Fisher Scientific) diluted in 1% (w/v) bovine serum albumin in PBS with 0.3% Triton X-100. To acquire images, we used a laser-scanning confocal microscope (LSM 5; Carl Zeiss) equipped with argon (488 and 514 nm), HeNe (543 nm), and Red Diode (633 nm) lasers, and a ×63 objective (NA1.2 W Corr UV-VIS-IR, C-Apochromat; Carl Zeiss). For quantification of F-actin levels, we used a custom script to perform watershed segmentation and extract cell regions to measure actin intensity per cell (ImageJ; National Institutes of Health, Bethesda, MD, USA) (28).
Parallel microfiltration
To measure the deformability of cells, which is indicated by their ability to filter through 5 µm pores of a membrane with applied pressure, we used parallel microfiltration (PMF) as described in our previous studies (29, 30). Briefly, cells were treated with drugs for 24 h, and we prepared a suspension of single cells by trypsin treatment for 3 min followed by scraping to harvest additional cells that remain adhered. Cells were counted (TC20; Bio-Rad) to generate a suspension of 5 × 105 cells in culture media, which is placed in a well of the PMF device. To drive the cell suspension to pass through the 5 μm pore-polycarbonate membrane, we applied an air pressure of 2.0 kPa for 30 s. Retention was determined by measuring the mass of the cell suspension that was retained at the end of the filtration timecourse compared with the initial mass loaded (massfinal/massinitial). Higher retention indicates a population of cells that has a higher probability to occlude pores compared with a suspension of cells that more readily transit through pores. Cells with higher elastic modulus as measured by atomic force microscopy tend to have a higher probability of occluding micron-scale pores (31, 32).
Quantitative deformability cytometry
To measure the deformability of single cells, we used quantitative deformability cytometry as described in our previous studies (31, 33). Briefly, cell suspensions were flowed through polydimethylsiloxane (PDMS) microfluidic devices with channels that were 5 μm × 10 μm (width × height); the timescale of cell transit, or transit time, provides a measure of cell deformability (34). We flowed suspensions of 1.5 × 106 cells/ml through q-DC devices using a driving pressure of 41 kPa and captured 2500 frames/sec using a CMOS camera (Phantom) mounted on an inverted microscope (Carl Zeiss) equipped with a ×20/0.40 NA objective. We performed post acquisition analysis using Matlab (MathWorks, Natick, MA, USA) to determine the transit time for individual cells (https://github.com/knybe/RowatLab-DC-Analysis).
Statistical analyses
All experiments were performed at least 3 independent times. Statistical significance between control and treated groups was determined with an unpaired t test or 1-way ANOVA with Tukey’s multiple comparison post hoc analysis using Graphpad Prism 5 (GraphPad Software, La Jolla, CA, USA).
RESULTS
β-AR signaling reduces the deformability of macrophages
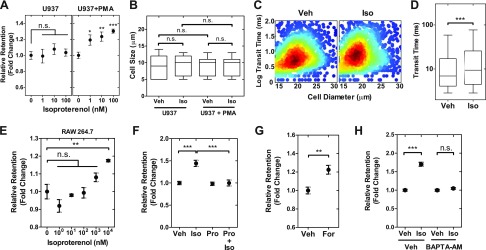
To determine whether β-AR signaling drives physical changes in myeloid cells, we examined the effect of the β-AR agonist isoproterenol on the deformability of monocytes and macrophages. First we investigated the human U937 monocyte cell line that can be differentiated into macrophage-like cells by treatment with PMA (35, 36). PMA-differentiated U937 cells have been widely used as a model for macrophages because they express macrophage marker proteins and exhibit similar behaviors to macrophages including phagocytosis and chemotaxis (37–39). Differentiation of U937 cells into macrophage-like cells following PMA treatment was confirmed by the up-regulated transcription of key macrophage markers including CD14, CSF1R, and CD163 determined by quantitative RT-PCR (qRT-PCR) and the increased surface expression of CD11b measured by flow cytometry (Supplemental Fig. S1A–D). Using qRT-PCR, we confirmed that both precursor monocytes (undifferentiated U937 cells) and macrophages (PMA-treated U937 cells) express the β-AR subtypes, ADRB1, ADRB2, and ADRB3 (Supplemental Fig. S1E). To determine effects of β-AR activation on both monocyte and macrophage physical properties, we treated cells with isoproterenol and measured their ability to deform through micron-scale pores using PMF (29, 30). In this assay, air pressure is applied to drive a suspension of cells through 5 µm diameter pores over a relatively short timescale of 30 s, and the volume of fluid retained above the membrane is determined; the retention volume depends on the number of pores that have been occluded by cells, which is largely determined by the physical properties of cells, including cell size and deformability (29). Before filtration, cells were treated with isoproterenol across a range of concentrations from 1 to 100 nM, which have been shown to induce intracellular signaling and invasion of cancer cells (40, 41). We found that isoproterenol had no effect on the retention of monocytes (Fig. 1A), suggesting that β-AR signaling does not change monocyte physical properties. By contrast, we observed a significant increase in retention of macrophages with increasing isoproterenol dose, suggesting β-AR signaling changes macrophage physical properties in a concentration-dependent manner (Fig. 1A). We found no significant changes in cell size distributions with or without isoproterenol (Fig. 1B), suggesting that increased retention of macrophages with β-AR signaling is due to decreased cell deformability. To determine whether the differential effect of β-AR signaling in monocytes and macrophages resulted from different levels of β-AR, we used flow cytometry to measure surface levels of β2AR, which is the dominant β-AR subtype in myeloid cells (14). We found no significant differences in β2AR levels in U937 cells before and after PMA treatment (Supplemental Fig. S1F–H). Nor did we find any difference in levels of ADRB2 transcripts measured by qRT-PCR (Supplemental Fig. S1E). Taken together, these data indicate that stress hormone signaling through β-AR selectively modulates the mechanotype of macrophages.
Figure 1.
β-AR signaling activation reduces the deformability of macrophages. A) Retention of monocyte (U937) and macrophage (U937+PMA) cells was measured using parallel microfiltration after isoproterenol treatment for 24 h. Retention is measured by the mass of cell suspension that remains in the top well after applying 2 kPa pressure for 30 s relative to the initial mass. Higher retention indicates that cells are more likely to occlude the 5 µm pores. B) Cell size distribution of monocytes and macrophages in vehicle control (Veh) and isoproterenol (Iso)-treated groups. C) Density scatterplot shows data from quantitative deformability cytometry. We measure cell size and transit time, or the timescale for macrophages to pass through the 5 × 10 μm gap of a microfluidic channel (n > 1600). D) Boxplot shows the lower and upper quartiles with median (line). Whiskers show the 10–90th percentiles (P < 0.0001). E) Isoproterenol effects on mouse macrophage (RAW 264.7) cells. F–H) Relative retention measured by PMF following 24 h treatment with Iso (100 nM) and the β-blocker propranolol (Pro) (10 μM) (F). Pro+Iso indicates cotreatment. Adenylyl cyclase activator forskolin (For; 10 μM) (G). Calcium chelator BAPTA-AM [1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis-acetoxymethyl ester] (10 μM) (H). One-way ANOVA test with Tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant. Results represent data from at least 3 independent experiments (N = 3).
To validate the β-AR–induced changes in deformability using an independent method, we used quantitative deformability cytometry to measure the timescale for individual macrophages to transit through 5 µm constrictions in a microfluidic device (31, 33); cells that are stiffer have longer transit times (33). Activation of β-AR signaling increased the transit time (TT) of macrophages (median TTVeh vs. TTIso: 6.9 vs. 9.4 ms, P < 0.0001) (Fig. 1C, D), consistent with findings by PMF that cells are becoming less deformable with β-AR activation. To investigate whether the β-AR–induced changes in cell deformability are generalizable to other myeloid cells, we measured the retention of the mouse macrophage cell line RAW 264.7 following treatment with the β-AR agonist isoproterenol. Consistent with findings for U937-derived macrophages, β-AR activation resulted in increased retention of RAW 264.7 cells, indicating a concentration-dependent reduction in cellular deformability (Fig. 1E); we note that a slight increase in deformability was observed for 1 nM of isoproterenol treatment, which is not seen in the human U937 cell line. Although the trend of increased retention with isoproterenol treatment was consistent across both human and mouse cells, the increase in retention for the mouse cells occurred at a higher isoproterenol concentration (∼1000 nM) compared with the human cells (∼1 nM), consistent with the lower expression of Adrb2 in RAW 264.7 cells (42).
To determine whether the effects of isoproterenol on macrophage deformability are modulated by signaling through β-AR—rather than nonspecific off-target effects—we treated cells with the β-AR antagonist propranolol to compete binding of the β-AR agonist at the receptor. We found that propranolol itself does not modulate cell deformability (P = 0.73; Fig. 1F), but propranolol blocked the increased retention with isoproterenol, confirming that signaling through β-AR mediates the effects on macrophage deformability (Fig. 1F). To identify the downstream signaling pathway that regulates cellular deformability, we manipulated other mediators downstream in the β-AR signaling pathway (41). β-AR is a Gαs-protein coupled receptor that activates adenylyl cyclase to elevate levels of intracellular cAMP and calcium (32, 41). To directly activate adenylyl cyclase, we treated U937 macrophages with forskolin and found increased retention, consistent with reduced deformability (Fig. 1G). To determine the role of intracellular Ca2+, we treated the cells with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis-acetoxymethyl ester to chelate intracellular calcium and found that isoproterenol no longer modulated cell deformability (Fig. 1H). Taken together, these results support that activation of β-AR signaling pathway modulates macrophage deformability.
F-actin mediates β-AR–induced changes in cell deformability
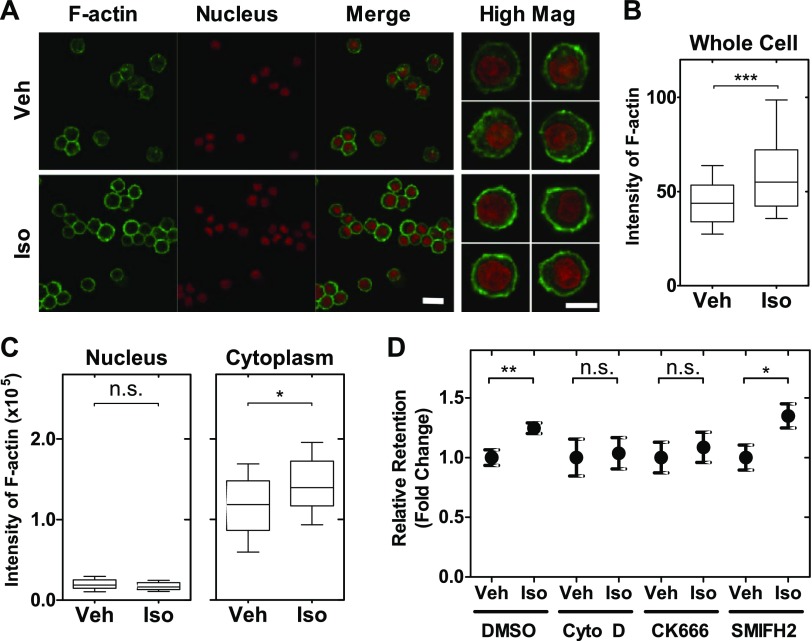
The actin cytoskeleton is a major determinant of cell deformability (43, 44). Specifically, levels of filamentous (F)-actin are critical for regulating cellular shape and mechanotype (45, 46). To investigate whether the effect of β-AR signaling on macrophage deformability was linked to changes in the actin cytoskeleton, we imaged F-actin using phalloidin staining and confocal microscopy. Quantitative image analysis revealed that treatment with isoproterenol for 24 h increased phalloidin intensity by 1.4-fold—indicating increased F-actin density (P < 0.001) (Fig. 2A, B). We observed that the increase in F-actin intensity occurred in the cytoplasm, but not in the nuclear region (P = 0.11; Fig. 2A–C). These analyses investigated actin distribution across a 2 µm-thick midplane section of individual cells, and future studies that image actin through the axial dimension of cells will be important to identify spatial differences in actin throughout the cell body. Nonetheless, consistent with these initial findings, we reported previously that β-AR signaling increases F-actin in tumor cells on similar timescales (32).
Figure 2.
Actin contributes to changes in cell mechanotype induced by β-AR. A) Representative images of macrophages treated with either vehicle or isoproterenol for 24 h followed by staining to visualize F-actin (phalloidin) and nucleus (DRAQ5). Scale bar, 20 μm. High Mag (Original magnification, ×63): representative images are confocal sections acquired at the midplane of individual cells with a slice thickness of 2 µm. Scale bar, 10 μm. B) Quantitative analysis of F-actin intensity in vehicle or isoproterenol-treated cells (n > 1000). Boxplot shows the lower and upper quartiles with median (line). Whiskers show the 10–90th percentiles. Unpaired t test. ***P < 0.001. C) Quantitative analysis of F-actin intensity in nuclear and cytoplasmic regions from high mag images (n = 30). Boxplot shows the lower and upper quartiles with median (line). Whiskers show the 10–90th percentiles. Unpaired t test. *P < 0.05. D) Macrophage retention measured by PMF. Cells are treated with inhibitors of F-actin polymerization [cytochalasin D (Cyto D) 2 μM for 1 h], Arp2/3 activity (CK666, 50 μM for 24 h), or formin activity (SMIFH2, 20 μM for 24 h). One-way ANOVA test with Tukey’s multiple comparison test. *P < 0.05, **P < 0.01. N.s., not significant. Results represent data from at least 3 independent experiments (N = 3).
To further explore the role of the actin cytoskeleton in β-AR regulation of macrophage deformability, we next examined the role of different actin filament populations. There are 2 major actin filament populations that are nucleated by formins and the Arp2/3 complex. Formins nucleate linear actin filaments and promote elongation, resulting in long, linear filaments (47, 48). The Arp2/3 complex also nucleates actin filaments, but generates branched F-actin filament networks that tend to be ∼10× shorter and have ∼20× faster turnover than formin-nucleated filaments (44, 49, 50). We found that treatment with the Arp2/3 inhibitor, CK666, or inhibitor of actin polymerization, cytochalasin D, blocked the ability of isoproterenol to increase macrophage retention, indicating that β-AR regulation of deformability is dependent on actin polymerization and branching. By contrast, treatment with the formin-inhibitor SMIFH2 did not reverse the effect of isoproterenol on retention (P = 0.02), indicating that formins are not a major determinant of β-AR regulation of macrophage deformability (Fig. 2D). These findings suggest that the effects of β-AR activation on macrophage deformability are predominantly mediated by Arp2/3-associated shorter, branched filament networks rather than unbranched filaments.
Activation of β-AR signaling increases macrophage phagocytosis and migration
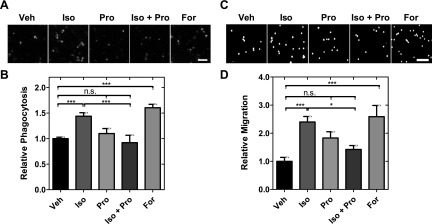
Macrophages respond to chemotactic cues within tissues and move to the site of infection to eliminate pathogens by phagocytosis. To investigate whether β-AR signaling modulates these key macrophage functions, we treated macrophages with isoproterenol and measured phagocytosis and migration. Isoproterenol treatment increased phagocytosis of fluorescent E.coli bioparticles by 44% compared with vehicle-treated cells (P < 0.001) (Fig. 3A, B and Supplemental Fig. S1I). Pretreatment with the β-AR antagonist propranolol blocked isoproterenol-enhanced E.coli internalization, showing the effect of isoproterenol on phagocytosis is dependent on the β-AR. In contrast, forskolin treatment enhanced phagocytosis, demonstrating a role for cAMP in regulating phagocytosis (Fig. 3A, B); these findings are consistent with modulation of phagocytosis by the β-AR signaling pathway.
Figure 3.
β-AR activation increases phagocytosis and motility in macrophages. A) Representative images from phagocytosis assay showing cells treated with vehicle control (Veh), isoproterenol (Iso), propranolol (Pro), or forskolin (For) for 25 h. Green signal shows internalized E.coli bioparticles after drug treatments. Scale bar, 50 μm. B) Relative phagocytosis shows number of internalized beads relative to vehicle control. Bars show means ± se. One-way ANOVA test with Tukey’s multiple comparison test. ***P < 0.001. C) Representative images from transwell migration assay. Scale bar, 100 μm. D) Number of migrated cells is determined by counting the number of nuclei in bottom chamber. One-way ANOVA test with Tukey’s multiple comparison test. *P < 0.05, ***P < 0.001. N.s., not significant. Results represent data from at least 3 independent experiments (N = 3).
To investigate the effect of β-AR activation on migration, we used a transwell migration assay to quantify migration of macrophages through 5 µm pores toward a serum gradient. We found that isoproterenol increased the number of cells that migrated through the porous membrane toward a serum gradient by 2.4-fold (P < 0.001), reflecting that β-AR activation enhances macrophage migration (Fig. 3C, D). Enhanced migration was also observed with forskolin treatment, but blocked by propranolol (Fig. 3C, D); these findings are consistent with regulation through the β-AR signaling pathway.
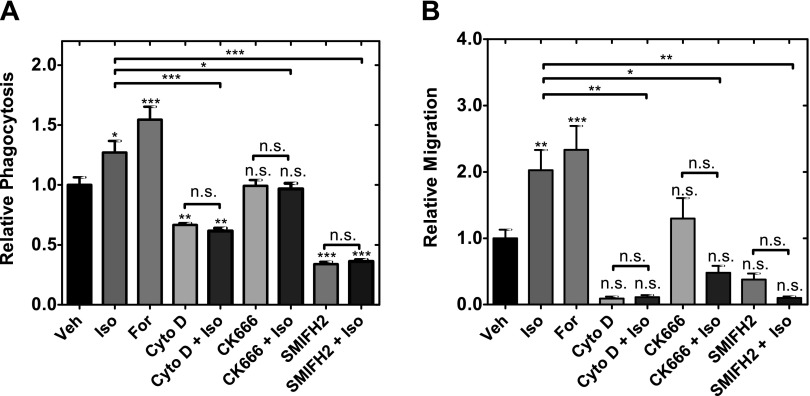
Because actin remodeling is critical for the β-AR–induced change in cell deformability, we next asked whether changes in actin filament organization also contribute to the β-AR-mediated increase in macrophage phagocytosis and migration. To determine the effects of changes in actin organization on phagocytosis and migration, we treated macrophages with inhibitors to block actin nucleation, polymerization, and branching. We found that inhibiting actin polymerization (Cyto D) or nucleation of unbranched filaments (SMIFH2) reduced basal levels of phagocytosis (Fig. 4A) and migration (Fig. 4B) and prevented isoproterenol from increasing either phagocytosis or migration. Interestingly, we observed the Arp2/3 inhibitor CK666 had no significant effects on basal levels of phagocytosis and migration, but blocked the β-AR–induced increase in phagocytosis and migration (Fig. 4).
Figure 4.
β-AR signaling regulates macrophage functions through changes in actin organization. A) Phagocytosis is measured in the presence of actin polymerization inhibitor (2 µM cytochalasin D), Arp2/3 inhibitor (50 µM CK666), or formin inhibitor (20 µM SMIFH2), whereas β-AR signaling is activated with 100 nM isoproterenol for 24 h. Statistical significance indicated by asterisks shows comparisons to vehicle control unless indicated. One-way ANOVA test with Tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001. B) Quantitative analysis of chemotaxis after 24 h of drug treatment. Statistical significance indicated by asterisks shows comparisons to vehicle control unless indicated. One-way ANOVA test with Tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001. N.s., not significant. Results represent data from at least 3 independent experiments (N = 3).
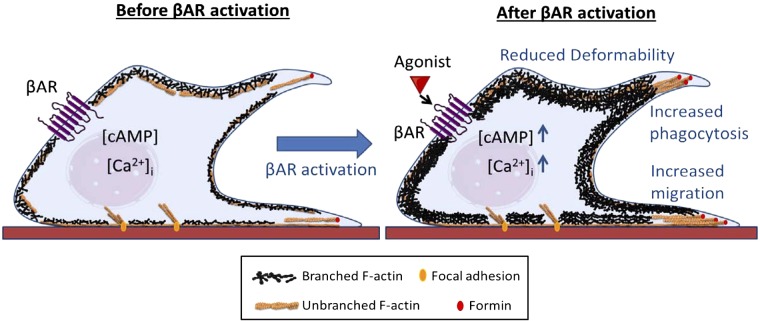
Taken together our findings show that β-AR modulation of actin organization and dynamics regulates phagocytosis and migration (Fig. 5), which are required for immune response. Our results also suggest that β-AR signaling regulates macrophage deformability by shifting the balance between longer, linear branched filaments of the actin cytoskeleton in favor of a highly dynamic network that exhibits more branching and shorter filaments (Fig. 5).
Figure 5.
Proposed mechanism for β-AR regulation of macrophage deformability and function. Activation of β-AR signaling with agonist results in reduced whole cell deformability and increased branched, Arp2/3-nucleated actin in the cytoplasm. β-AR activation also enhances macrophage functions such as phagocytosis and migration.
DISCUSSION
We show here that β-AR signaling triggers changes in cell mechanotype, phagocytosis, and migration that are mediated by changes in the organization of actin (Fig. 5). Although there is evidence that cell mechanical properties are linked with immune cell functions (16, 17, 51), mechanistic knowledge of how physiologic cues elicit such physical changes is still developing. Our results discriminate between the roles of formin-nucleated and Arp2/3-nucleated actin filaments in stress hormone regulation of macrophage deformability: our findings suggest that β-AR activation shifts the balance between actin filament populations to favor more highly branched, Arp2/3-mediated structures rather than linear unbranched filaments (Fig. 5). These results align well with recent observations of actin organization at adherens junctions, where bundles of nonmuscle myosin II-associated linear actin filaments run parallel to the plasma membrane and Arp2/3-associated branched networks emanate from these bundles and appear to push against the membrane (52). Our findings suggest that a similar organization may exist in macrophages where bundles of linear actin filaments provide basal stiffness; consistent with this, previous studies show that linear actin filaments are a major determinant of cell stiffness (44). Here, we show that the shift toward branched actin networks induced by β-AR activation is a major contributor to the observed decrease in cellular deformability. Given that Arp-2/3-nucleated filaments have ∼20× faster turnover than formin-nucleated filaments (44), it is intriguing to speculate that the shift to the more highly dynamic, Arp2/3-nucleated actin structures with stress hormone stimulation may enable cells to more quickly sample their environment and respond appropriately to external stimuli.
The finding that β-AR regulation of macrophage deformability depends on Arp2/3 suggests that both the organization and dynamics of actin may be important for regulation of cell mechanotype by soluble stress hormones. Branched actin structures proximal to the plasma membrane (52) counter the contractile forces generated by nonmuscle myosin II to increase cortical tension and cell stiffness (53, 54). Kinetic processes of actin nucleation and branching also contribute to cellular stiffness and force generation (44, 53, 55), which enable cells to effectively generate protrusive structures—such as lamellipodia and podosomes—to enhance key functions of phagocytosis and motility (22, 23, 56–59).
By demonstrating a key role for actin in β-AR regulation of phagocytosis and motility, the findings presented here provide mechanistic insight into previous studies that found increased phagocytosis of primary macrophages with β-AR activation (13) as well as observations that macrophage deformability is associated with phagocytosis (19, 59). Our results show that inhibition of Arp2/3 or formin activity blocks the β-AR–induced increase in phagocytosis, consistent with the role of actin polymerization and branching in generating protrusions required to engulf particles (60–62). The activity of Arp2/3 and formins is also critical for the motility of macrophages and dendritic cells (63–65), where force generation is required for the formation of protrusive structures that promote invasion (66, 67). Arp2/3 was also recently identified to contribute to the deformation of the nucleus during the migration of cells through narrow gaps (64). It is plausible that β-AR activation may enhance the ability of cells to move through micron-scale pores via increased nuclear translocation mediated by Arp2/3-generated forces. Cell motility is also sensitive to myosin II activity (68), and our previous findings in cancer cells showed that a β2AR–actin-myosin II axis mediates cell stiffness and motility (32). Future studies will investigate how β-AR signaling regulates the interplay between branched, Arp2/3-nucleated actin and myosin II, which is associated primarily with linear, formin-nucleated filaments (69, 70).
Although associations between cell mechanotype and function have been observed in other immune cell contexts, the molecular mechanisms have not been well understood. The ability of neutrophils to deform through narrow gaps is critical for their chemotactic functions. Indeed, neutrophils show increased compliance and faster advection through 12 µm narrow microfluidic channels; by contrast, macrophages exhibit reduced steady-state viscosity compared with neutrophils, which may be a physiologic adaptation for enhanced migration through tissues (18). The mechanotype of T cells influences the size of immune synapses formed with antigen presenting cells (APCs) and thus plays a crucial role in their signaling and activation: the more compliant cytoskeleton of active T cells compared with naïve T cells results in formation of larger immune synapses with APCs, because a more compliant T cell can more readily deform around or “hug” the APC (71). These findings suggest that cellular deformability serves as an important characteristic in predicting T cell interactions with APCs and their subsequent activation. Consistent with our findings that actin organization and dynamics link macrophage mechanotype and function, the actin-severing factor cofilin was found to be critical for modulating T cell deformability and immune synapses with APCs (71). Actin remodeling is also critical for intracellular trafficking and receptor internalization and sensitization (72–74); in future research it will be important to determine how these cellular functions are regulated by signaling through β-AR. Further studies of the biophysical and molecular mechanisms of immune cell functions will provide a more detailed understanding of the range of soluble and mechanical cues that regulate immune cell behaviors. For example, matrix stiffness regulates immune cell mechanotype (75, 76), cytokine production (77–79), and phagocytosis (19) and also determines migration mode (80, 81). Understanding how multiple soluble and mechanical cues in the microenvironment contribute to immune cell behaviors will be important to define immune response in physiologic and disease contexts.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Sherie L. Morrison [University of California, Los Angeles (UCLA)] for providing U937 cell line, and the UCLA Statistical Consulting Group for advice on data analysis. Flow cytometry ImageStream, and IncuCyte analyses were performed in the UCLA Flow Cytometry Core Facility, which is supported by U.S. National Institutes of Health (NIH) Awards CA-16042 (National Cancer Institute) and AI-28697 (National Institute of Allergy and Infectious Diseases) and by the Jonsson Comprehensive Cancer Center, the Center for AIDS Research, and the David Geffen School of Medicine at UCLA. This work was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA Clinical and Translational Science Institute Grant UL1TR000124, the University of California Cancer Research Coordinating Committe (CRR-18-52690), the National Science Foundation (CAREER DBI-1254185 to A.C.R.), and the Australian National Health and Medical Research Council (NHMRC) APP1147498. The authors declare no conflicts of interest.
Glossary
- APC
antigen presenting cell
- β-AR
β-adrenergic receptor
- Arp2/3
actin-related protein 2/3
- FBS
fetal bovine serum
- PMA
phorbol 12-myristate 13-acetate
- PMF
parallel microfiltration
- qRT-PCR
quantitative RT-PCR
- TT
transit time
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T.-H. Kim, E. K. Sloan, and A. C. Rowat designed the experiments; T.-H. Kim, C. Ly, and A. Christodoulides performed experiments; T.-H. Kim, C. Ly, C. J. Nowell, P. W. Gunning, E. K. Sloan, and A. C. Rowat analyzed and interpreted data; and T.-H. Kim, P. W. Gunning, E. K. Sloan, and A. C. Rowat wrote the manuscript.
REFERENCES
- 1.Okabe Y., Medzhitov R. (2014) Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157, 832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen K. D., Qiu Y., Cui X., Goh Y. P. S., Mwangi J., David T., Mukundan L., Brombacher F., Locksley R. M., Chawla A. (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S., Amit I. (2014) Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls A. J., Wen S. W., Hall P., Hickey M. J., Wong C. H. Y. (2018) Activation of the sympathetic nervous system modulates neutrophil function. J. Leukoc. Biol. 103, 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirth T., Westendorf A. M., Bloemker D., Wildmann J., Engler H., Mollerus S., Wadwa M., Schäfer M. K.-H., Schedlowski M., del Rey A. (2014) The sympathetic nervous system modulates CD4(+)Foxp3(+) regulatory T cells via noradrenaline-dependent apoptosis in a murine model of lymphoproliferative disease. Brain Behav. Immun. 38, 100–110 [DOI] [PubMed] [Google Scholar]
- 6.Cao X., Aballay A. (2016) Neural inhibition of dopaminergic signaling enhances immunity in a cell-non-autonomous manner. Curr. Biol. 26, 2398 [DOI] [PubMed] [Google Scholar]
- 7.Dantzer R. (2018) Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 98, 477–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epelman S., Lavine K. J., Randolph G. J. (2014) Origin and functions of tissue macrophages. Immunity 41, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.-A., Mardani F., Seifi B., Mohammadi A., Afshari J. T., Sahebkar A. (2018) Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 233, 6425–6440 [DOI] [PubMed] [Google Scholar]
- 10.Honold L., Nahrendorf M. (2018) Resident and monocyte-derived macrophages in cardiovascular disease. Circ. Res. 122, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Zhang X. (2017) Pivotal regulators of tissue homeostasis and cancer: macrophages. Exp. Hematol. Oncol. 6, 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiarella S. E., Soberanes S., Urich D., Morales-Nebreda L., Nigdelioglu R., Green D., Young J. B., Gonzalez A., Rosario C., Misharin A. V., Ghio A. J., Wunderink R. G., Donnelly H. K., Radigan K. A., Perlman H., Chandel N. S., Budinger G. R. S., Mutlu G. M. (2014) β2-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J. Clin. Invest. 124, 2935–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthu K., He L.-K., Szilagyi A., Strotmon P., Gamelli R. L., Shankar R. (2010) ß-adrenergic stimulation increases macrophage CD14 expression and E. coli phagocytosis through PKA signaling mechanisms. J. Leukoc. Biol. 88, 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izeboud C. A., Mocking J. A., Monshouwer M., van Miert A. S., Witkamp R. F. (1999) Participation of beta-adrenergic receptors on macrophages in modulation of LPS-induced cytokine release. J. Recept. Signal Transduct. Res. 19, 191–202 [DOI] [PubMed] [Google Scholar]
- 15.Serio M., Potenza M. A., Montagnani M., Mansi G., Mitolo-Chieppa D., Jirillo E. (1996) Beta-adrenoceptor responsiveness of splenic macrophages in normotensive and hypertensive rats. Immunopharmacol. Immunotoxicol. 18, 247–265 [DOI] [PubMed] [Google Scholar]
- 16.Lam J., Herant M., Dembo M., Heinrich V. (2009) Baseline mechanical characterization of J774 macrophages. Biophys. J. 96, 248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekpenyong A. E., Toepfner N., Fiddler C., Herbig M., Li W., Cojoc G., Summers C., Guck J., Chilvers E. R. (2017) Mechanical deformation induces depolarization of neutrophils. Sci. Adv. 3, e1602536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekpenyong A. E., Whyte G., Chalut K., Pagliara S., Lautenschläger F., Fiddler C., Paschke S., Keyser U. F., Chilvers E. R., Guck J. (2012) Viscoelastic properties of differentiating blood cells are fate- and function-dependent. PLoS One 7, e45237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel N. R., Bole M., Chen C., Hardin C. C., Kho A. T., Mih J., Deng L., Butler J., Tschumperlin D., Fredberg J. J., Krishnan R., Koziel H. (2012) Cell elasticity determines macrophage function. PLoS One 7, e41024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bufi N., Saitakis M., Dogniaux S., Buschinger O., Bohineust A., Richert A., Maurin M., Hivroz C., Asnacios A. (2015) Human primary immune cells exhibit distinct mechanical properties that are modified by inflammation. Biophys. J. 108, 2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rougerie P., Miskolci V., Cox D. (2013) Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunol. Rev. 256, 222–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kage F., Winterhoff M., Dimchev V., Mueller J., Thalheim T., Freise A., Brühmann S., Kollasser J., Block J., Dimchev G., Geyer M., Schnittler H.-J., Brakebusch C., Stradal T. E. B., Carlier M.-F., Sixt M., Käs J., Faix J., Rottner K. (2017) FMNL formins boost lamellipodial force generation. Nat. Commun. 8, 14832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labernadie A., Bouissou A., Delobelle P., Balor S., Voituriez R., Proag A., Fourquaux I., Thibault C., Vieu C., Poincloux R., Charrière G. M., Maridonneau-Parini I. (2014) Protrusion force microscopy reveals oscillatory force generation and mechanosensing activity of human macrophage podosomes. Nat. Commun. 5, 5343 [DOI] [PubMed] [Google Scholar]
- 24.Chitu V., Pixley F. J., Macaluso F., Larson D. R., Condeelis J., Yeung Y.-G., Stanley E. R. (2005) The PCH family member MAYP/PSTPIP2 directly regulates F-actin bundling and enhances filopodia formation and motility in macrophages. Mol. Biol. Cell 16, 2947–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hida A., Kawakami A., Nakashima T., Yamasaki S., Sakai H., Urayama S., Ida H., Nakamura H., Migita K., Kawabe Y., Eguchi K. (2000) Nuclear factor-kappaB and caspases co-operatively regulate the activation and apoptosis of human macrophages. Immunology 99, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan C. P., Park C. S., Lau B. H. (1993) A rapid and simple microfluorometric phagocytosis assay. J. Immunol. Methods 162, 1–7 [DOI] [PubMed] [Google Scholar]
- 27.Kapellos T. S., Taylor L., Lee H., Cowley S. A., James W. S., Iqbal A. J., Greaves D. R. (2016) A novel real time imaging platform to quantify macrophage phagocytosis. Biochem. Pharmacol. 116, 107–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi D., Kaur Gill N., Santiskulvong C., Sifuentes J., Dorigo O., Rao J., Taylor-Harding B., Ruprecht Wiedemeyer W., Rowat A. C. (2015) Screening cell mechanotype by parallel microfiltration. Sci. Rep. 5, 17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill N. K., Gill N. K., Qi D., Kim T.-H., Chan C., Nguyen A., Nyberg K. D., Rowat A. C. (2017) A protocol for screening cells based on deformability using parallel microfiltration. Protoc. Exch. doi: 10.1038/protex.2017.101 [Google Scholar]
- 31.Nyberg K. D., Hu K. H., Kleinman S. H., Khismatullin D. B., Butte M. J., Rowat A. C. (2017) Quantitative deformability cytometry: rapid, calibrated measurements of cell mechanical properties. Biophys. J. 113, 1574–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim T.-H., Gill N. K., Nyberg K. D., Nguyen A. V., Hohlbauch S. V., Geisse N. A., Nowell C. J., Sloan E. K., Rowat A. C. (2016) Cancer cells become less deformable and more invasive with activation of β-adrenergic signaling. J. Cell Sci. 129, 4563–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyberg K. D., Scott M. B., Bruce S. L., Gopinath A. B., Bikos D., Mason T. G., Kim J. W., Choi H. S., Rowat A. C. (2016) The physical origins of transit time measurements for rapid, single cell mechanotyping. Lab Chip 16, 3330–3339 [DOI] [PubMed] [Google Scholar]
- 34.Hoelzle D. J., Varghese B. A., Chan C. K., Rowat A. C. (2014) A microfluidic technique to probe cell deformability. J. Vis. Exp. 91, e51474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M. Y., Wu M. C. (1992) Induction of human monocyte cell line U937 differentiation and CSF-1 production by phorbol ester. Exp. Hematol. 20, 974–979 [PubMed] [Google Scholar]
- 36.Yang L., Dai F., Tang L., Le Y., Yao W. (2017) Macrophage differentiation induced by PMA is mediated by activation of RhoA/ROCK signaling. J. Toxicol. Sci. 42, 763–771 [DOI] [PubMed] [Google Scholar]
- 37.Groot-Kormelink P. J., Fawcett L., Wright P. D., Gosling M., Kent T. C. (2012) Quantitative GPCR and ion channel transcriptomics in primary alveolar macrophages and macrophage surrogates. BMC Immunol. 13, 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verhoeckx K. C. M., Bijlsma S., de Groene E. M., Witkamp R. F., van der Greef J., Rodenburg R. J. T. (2004) A combination of proteomics, principal component analysis and transcriptomics is a powerful tool for the identification of biomarkers for macrophage maturation in the U937 cell line. Proteomics 4, 1014–1028 [DOI] [PubMed] [Google Scholar]
- 39.Harris P., Ralph P. (1985) Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J. Leukoc. Biol. 37, 407–422 [DOI] [PubMed] [Google Scholar]
- 40.Creed S. J., Le C. P., Hassan M., Pon C. K., Albold S., Chan K. T., Berginski M. E., Huang Z., Bear J. E., Lane J. R., Halls M. L., Ferrari D., Nowell C. J., Sloan E. K. (2015) β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 17, 145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pon C. K., Lane J. R., Sloan E. K., Halls M. L. (2016) The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J. 30, 1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin J. F., Jin F. J., Li N., Guan H. T., Lan L., Ni H., Wang Y. (2015) Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 48, 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacQueen L. A., Thibault M., Buschmann M. D., Wertheimer M. R. (2012) Electromechanical deformation of mammalian cells in suspension depends on their cortical actin thicknesses. J. Biomech. 45, 2797–2803 [DOI] [PubMed] [Google Scholar]
- 44.Fritzsche M., Erlenkämper C., Moeendarbary E., Charras G., Kruse K. (2016) Actin kinetics shapes cortical network structure and mechanics. Sci. Adv. 2, e1501337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prentki M., Chaponnier C., Jeanrenaud B., Gabbiani G. (1979) Actin microfilaments, cell shape, and secretory processes in isolated rat hepatocytes. Effect of phalloidin and cytochalasin D. J. Cell Biol. 81, 592–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai M. A., Frank R. S., Waugh R. E. (1994) Passive mechanical behavior of human neutrophils: effect of cytochalasin B. Biophys. J. 66, 2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schönichen A., Geyer M. (2010) Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim. Biophys. Acta 1803, 152–163 [DOI] [PubMed] [Google Scholar]
- 48.Chesarone M. A., Goode B. L. (2009) Actin nucleation and elongation factors: mechanisms and interplay. Curr. Opin. Cell Biol. 21, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pizarro-Cerdá J., Chorev D. S., Geiger B., Cossart P. (2017) The diverse family of arp2/3 complexes. Trends Cell Biol. 27, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollard T. D., Beltzner C. C. (2002) Structure and function of the Arp2/3 complex. Curr. Opin. Struct. Biol. 12, 768–774 [DOI] [PubMed] [Google Scholar]
- 51.Di Cerbo A., Rubino V., Morelli F., Ruggiero G., Landi R., Guidetti G., Canello S., Terrazzano G., Alessandrini A. (2018) Mechanical phenotyping of K562 cells by the micropipette aspiration technique allows identifying mechanical changes induced by drugs. Sci. Rep. 8, 1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Efimova N., Svitkina T. M. (2018) Branched actin networks push against each other at adherens junctions to maintain cell-cell adhesion. J. Cell Biol. 217, 1827–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fletcher D. A., Mullins R. D. (2010) Cell mechanics and the cytoskeleton. Nature 463, 485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gabriele S., Benoliel A.-M., Bongrand P., Théodoly O. (2009) Microfluidic investigation reveals distinct roles for actin cytoskeleton and myosin II activity in capillary leukocyte trafficking. Biophys. J. 96, 4308–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cojoc D., Difato F., Ferrari E., Shahapure R. B., Laishram J., Righi M., Di Fabrizio E. M., Torre V. (2007) Properties of the force exerted by filopodia and lamellipodia and the involvement of cytoskeletal components. PLoS One 2, e1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dimchev G., Steffen A., Kage F., Dimchev V., Pernier J., Carlier M.-F., Rottner K. (2017) Efficiency of lamellipodia protrusion is determined by the extent of cytosolic actin assembly. Mol. Biol. Cell 28, 1311–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isogai T., van der Kammen R., Leyton-Puig D., Kedziora K. M., Jalink K., Innocenti M. (2015) Initiation of lamellipodia and ruffles involves cooperation between mDia1 and the Arp2/3 complex. J. Cell Sci. 128, 3796–3810 [DOI] [PubMed] [Google Scholar]
- 58.Revach O.-Y., Weiner A., Rechav K., Sabanay I., Livne A., Geiger B. (2015) Mechanical interplay between invadopodia and the nucleus in cultured cancer cells. Sci. Rep. 5, 9466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazur M. T., Williamson J. R. (1977) Macrophage deformability and phagocytosis. J. Cell Biol. 75, 185–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffmann A.-K., Naj X., Linder S. (2014) Daam1 is a regulator of filopodia formation and phagocytic uptake of Borrelia burgdorferi by primary human macrophages. FASEB J. 28, 3075–3089 [DOI] [PubMed] [Google Scholar]
- 61.May R. C., Caron E., Hall A., Machesky L. M. (2000) Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat. Cell Biol. 2, 246–248 [DOI] [PubMed] [Google Scholar]
- 62.May R. C., Machesky L. M. (2001) Phagocytosis and the actin cytoskeleton. J. Cell Sci. 114, 1061–1077 [DOI] [PubMed] [Google Scholar]
- 63.Bendell A. C., Williamson E. K., Chen C. S., Burkhardt J. K., Hammer D. A. (2017) The Arp2/3 complex binding protein HS1 is required for efficient dendritic cell random migration and force generation. Integr. Biol. 9, 695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thiam H.-R., Vargas P., Carpi N., Crespo C. L., Raab M., Terriac E., King M. C., Jacobelli J., Alberts A. S., Stradal T., Lennon-Dumenil A.-M., Piel M. (2016) Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nat. Commun. 7, 10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller M. R., Miller E. W., Blystone S. D. (2017) Non-canonical activity of the podosomal formin FMNL1γ supports immune cell migration. J. Cell Sci. 130, 1730–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jevnikar Z., Mirković B., Fonović U. P., Zidar N., Švajger U., Kos J. (2012) Three-dimensional invasion of macrophages is mediated by cysteine cathepsins in protrusive podosomes. Eur. J. Immunol. 42, 3429–3441 [DOI] [PubMed] [Google Scholar]
- 67.Li A., Dawson J. C., Forero-Vargas M., Spence H. J., Yu X., König I., Anderson K., Machesky L. M. (2010) The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr. Biol. 20, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vicente-Manzanares M., Ma X., Adelstein R. S., Horwitz A. R. (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van den Dries K., Meddens M. B. M., de Keijzer S., Shekhar S., Subramaniam V., Figdor C. G., Cambi A. (2013) Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nat. Commun. 4, 1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rottner K., Faix J., Bogdan S., Linder S., Kerkhoff E. (2017) Actin assembly mechanisms at a glance. J. Cell Sci. 130, 3427–3435 [DOI] [PubMed] [Google Scholar]
- 71.Thauland T. J., Hu K. H., Bruce M. A., Butte M. J. (2017) Cytoskeletal adaptivity regulates T cell receptor signaling. Sci. Signal. 10, eaah3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y., Shen H., Liu H., Feng H., Liu Y., Zhu X., Liu X. (2017) Arp2/3 complex controls T cell homeostasis by maintaining surface TCR levels via regulating TCR+ endosome trafficking. Sci. Rep. 7, 8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wheeler D., Sneddon W. B., Wang B., Friedman P. A., Romero G. (2007) NHERF-1 and the cytoskeleton regulate the traffic and membrane dynamics of G protein-coupled receptors. J. Biol. Chem. 282, 25076–25087 [DOI] [PubMed] [Google Scholar]
- 74.Itagaki K., Kannan K. B., Singh B. B., Hauser C. J. (2004) Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J. Immunol. 172, 601–607 [DOI] [PubMed] [Google Scholar]
- 75.Oakes P. W., Patel D. C., Morin N. A., Zitterbart D. P., Fabry B., Reichner J. S., Tang J. X. (2009) Neutrophil morphology and migration are affected by substrate elasticity. Blood 114, 1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mennens S. F. B., Bolomini-Vittori M., Weiden J., Joosten B., Cambi A., van den Dries K. (2017) Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci. Rep. 7, 17511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Previtera M. L., Sengupta A. (2015) Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One 10, e0145813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McWhorter F. Y., Davis C. T., Liu W. F. (2015) Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 72, 1303–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blakney A. K., Swartzlander M. D., Bryant S. J. (2012) The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A. 100, 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Goethem E., Poincloux R., Gauffre F., Maridonneau-Parini I., Le Cabec V. (2010) Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J. Immunol. 184, 1049–1061 [DOI] [PubMed] [Google Scholar]
- 81.Salmon H., Franciszkiewicz K., Damotte D., Dieu-Nosjean M.-C., Validire P., Trautmann A., Mami-Chouaib F., Donnadieu E. (2012) Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.