Abstract
Loss of functional β-cell mass caused by lipotoxicity is a key pathogenic factor in the development of type 2 diabetes mellitus (T2DM). We have previously reported that sphingosine kinase (SK)1 is an endogenous protector of β-cells against lipotoxicity. The current study reports that SK2, another isoform of SK, is a crucial mediator of lipotoxicity in β-cells. Exposure of β-cells to palmitatic acid (PA), a saturated free fatty acid, resulted in a nearly 2-fold increase in SK2 expression, which paralleled the induction of cell death in a similar dose- and time-dependent fashion. Silencing SK2 expression by its specific small interfering RNAs significantly inhibited PA-induced cell death and caspase-3 activation, whereas overexpression of SK2 promoted lipotoxicity in β-cells. Mechanistically, upon exposure to PA, endogenous SK2 was shuttled from the nucleus to the cytoplasm, where it interacted with B-cell lymphoma–extra-large (Bcl-xL), leading to mitochondrial apoptotic pathway activation and cell death. By blocking SK2 translocation and its interaction with Bcl-xL, either the nuclear export signal mutant (L423A/L425A) or the BH3 domain mutant (L219A) of SK2 significantly attenuated β-cell lipotoxicity. Furthermore, SK2 deficiency in mice significantly prevented the loss of β-cell mass, preserved insulin production, and ameliorated the diabetic phenotype in an established T2DM model induced by feeding a high-fat diet accompanied by administration of streptozotocin. These findings provide the first evidence, in vitro and in vivo, of a critical role for SK2 in mediating β-cell lipotoxicity and the progression of diabetes.—Song, Z., Wang, W., Li, N., Yan, S., Rong, K., Lan, T., Xia, P. Sphingosine kinase 2 promotes lipotoxicity in pancreatic β-cells and the progression of diabetes.
Keywords: apoptosis, type 2 diabetes, β-cell biology, sphingolipids
Type 2 diabetes mellitus (T2DM) is a common metabolic disease worldwide and is characterized by insulin resistance and a progressive loss of functional pancreatic β-cell mass. Lipotoxicity in β-cells, arising from excess exposure to free fatty acids, such as palmitatic acid (PA), has been recognized as a key pathogenic factor causing the loss of functional β-cell mass, especially in obese individuals (1–3). Thus, understanding the mechanism of lipotoxicity in β-cells under metabolic stress is imperative for creating new strategies to prevent and treat diabetes.
Sphingosine kinase (SK) is a signaling enzyme that catalyzes sphingosine to generate sphingosine 1-phosphate (S1P). Acting through its receptors or perhaps through some intracellular targets, S1P has been broadly implicated in the regulation of multiple biologic processes, including cell growth and differentiation, tumorigenesis, inflammation, and metabolic homeostasis (4–7). The disordered SK/S1P signaling axis has been postulated to contribute to the pathogenesis and progression of various diseases, such as cancer and diabetes (4–7). Two isoforms of SK, SK1 and SK2, exist. Double knockout of SK1 and SK2 in mice results in embryonic lethality due to severe defects in neural and vascular development, highlighting the important function of this signaling enzyme (8). However, the single ablation of either gene does not lead to evident abnormality under normal physical conditions (9, 10), suggesting a redundant and complementary role of the 2 isoenzymes. Although SK1 and SK2 share similar catalytic activities, they possess different kinetic properties and distinct subcellular localization and differ in their biologic functions (6, 11). For instance, SK1 is often regarded as a potent antiapoptotic factor and mitogenic stimulator, whereas SK2 is reported to suppress cell growth or to promote cell death (4, 6, 12). Besides the proapoptotic effect of SK2, some recent studies have depicted an antiapoptotic, mitogenic, or even tumorigenic activity of SK2 under certain conditions (13, 14). Such a discrepancy regarding the role of SK2 is thought to be attributable to its differential expression levels, subcellular localizations, and cell type specificities (13). We have previously reported that SK1 plays a critical role in protecting β-cells against lipotoxicity through an S1P receptor–dependent mechanism (15). SK1 deficiency causes a significant loss of β-cell mass due to increased apoptosis in high-fat diet (HFD)-induced obese mice, leading to the onset of diabetes, revealing a pivotal role of SK1 in pancreatic β-cell survival (15). However, little is known about the role of SK2 in β-cells. In this study, we provide the first evidence that SK2, playing an opposing role of SK1, promotes β-cell lipotoxicity in vitro and in vivo. The proapoptotic effect of SK2 appears dependent on its translocation from the nucleus to the cytoplasm but independent of catalytic activity. SK2 deficiency prevents the loss of β-cell mass and thus ameliorates the diabetic phenotype in a diabetic animal model induced by feeding animals an HFD accompanied by administration of streptozotocin (STZ). Thus, the current findings illustrate a critical role for endogenous SK2 in β-cell lipotoxicity and suggest a potential intervention target for prevention of T2DM, especially for those with obesity.
MATERIALS AND METHODS
Cell culture and treatments
MIN6 and INS-1 cells were maintained in DMEM and RPMI 1640 medium, respectively, containing 10% (v/v) FCS at 37°C with 5% CO2. PA (MilliporeSigma, Shanghai, China) was dissolved in 0.1 M NaOH by heating at 90°C for 10 min, and then a stock solution was prepared containing 5 mM PA coupled with 5% (w/v) fatty acid–free bovine serum albumin (MilliporeSigma). ABC294640 (MedChemexpress, Princeton, NJ, USA) was dissolved in DMSO at 5 mM as a stock solution. For treatment of cells, the stock PA or ABC294640 solution was added to serum-free medium at the indicated concentrations for a desired time period.
Plasmids, small interfering RNAs, and transfections
Human SK2 cDNA (GenBank accession number NG-029867.1) was FLAG epitope-tagged at the 3′ end and subcloned into a pcDNA3 vector as previously described (14). Mutations on wild-type (WT) SK2, including nuclear export signal (NES) mutant (L423A/L425A) and a BH3 domain mutant (L219A), were generated using the HieffMut Site-Directed Mutagenesis Kit (Yeasen, Shanghai, China). All plasmid sequences were confirmed in the DNA Sequencing Facility (Biokea, Shanghai, China). Small interfering RNA (siRNA) against SK2 and its control siRNAs were purchased from GenePharma (Shanghai, China). FuGene HD or Lipofectamine 2000 reagents were used for transfection according to the manufacturer’s protocol.
Cell viability and cell death assays
Cell viability was assayed using a 3-(4, 5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide Cell Proliferation and Cytotoxicity Assay kit (Beyotime, Shanghai, China) according to the manufacturer’s protocol. For cell death assays, cells were stained with 1 μg/ml propidium iodide and Annexin V-FITC (Beyotime) followed by flow cytometry analysis. For mitochondrial transmembrane potential assays, flow cytometry analysis was carried out in cells stained with the JC-1 Assay kit (Beyotime) according to the manufacturer’s protocol.
Western blotting and immunoprecipitations
Western blotting and immunoprecipitations were conducted according to the standard protocol as previously described (16). Antibodies used in this study included those against caspase 3, tubulin, histone, B-cell lymphoma–extra-large (Bcl-xL), cytochrome c, COX IV, insulin, and FLAG (Cell Signaling Technology, Danvers, MA, USA); anti–β-actin (MilliporeSigma); and anti-SK2 (Annova, San Diego, CA, USA).
SK activity assays
SK1 and SK2 activities were measured in cell lysates using N-(7-nitro-2–1,3-benzoxadia-zol-4-yl)-d-erythro–sphingosine and ATP as substrate and isoform-selective assay buffers as previously described (14, 17).
Cell immunofluorescence assay
Immunofluorescence microscopic analysis was conducted as previously described (18). Briefly, after the indicated treatment, cells were fixed with 4% paraformaldehyde solution for 20 min and permeabilized with 0.1% Triton X-100 in PBS for 15 min. Cells were blocked with 5% serum in PBS for 20 min, incubated with the indicated primary antibodies at 4°C overnight, washed 3 times, and then incubated for 1 h with the secondary antibodies conjugated with Alexa Fluor in the dark. DAPI was then added, and cells were examined using a fluorescence microscope (Olympus, Tokyo, Japan).
Mitochondria and cytosolic fractionations
Mitochondrial and cytoplasmic fractions were isolated by differential centrifugations using a Cell Mitochondria Isolation Kit (Beyotime) according to the manufacturer’s instructions. Briefly, the treated cells were harvested, suspended in mitochondria isolation buffer, and homogenized by 30 passes through a 26.5-gauge needle. After centrifugation at 600 g for 10 min, the supernatants were transferred to a fresh tube and recentrifuged at 11,000 g for 30 min at 4°C. The supernatants were collected as cytosolic fractions, and the mitochondrial fractions were extracted from the pellets using lysis buffer from the kit.
Animals
Animal studies were approved by the Animal Use and Care Committee of Fudan University and conformed with the U.S. National Institutes of Health (NIH) U.S. Department of Health and Human Services Public Health Service Policy on Humane Care and Use of Laboratory Animals (NIH publication No. 15-8013; Bethesda, MD, USA). SK2−/− mice and the control WT littermates (gifts from Dr. Richard Proia, NIH) were derived on the same C57BL/6 background; these animals have been well characterized and have been used in a number of studies. All mice were housed in a temperature-controlled, pathogen-free environment on a 12-h light/dark cycle and had ad libitum access to food and water. After a 2-wk period of acclimation, WT and SK2−/− mice, aged 6–8 wk, were randomly assigned to 2 groups. The control group (n = 6) was fed regular chow ad libitum, and the High Fat Diet (HFD)/STZ group (n = 9) was fed an HFD containing 60% fat, 20% protein, and 20% carbohydrate (D12492; Research Diets, New Brunswick, NJ, USA) throughout the 16-wk experimental period. Four weeks after HFD feeding, mice were injected i.p. with 30 mg/kg per day STZ (MilliporeSigma) for a continuous 5 d period followed by additional 12-wk HFD feeding as previously described (19). Control mice received vehicle injections (sodium citrate buffer, 2%). Mice were weighed, and fasting blood glucose levels were measured (Accu-chek; Roche, Indianapolis, IN, USA) each week throughout the experimental period. Levels of plasma insulin were determined after 6 h starvation using Mouse Insulin ELISA kits (EMD Millipore, Bedford, MA, USA).
Oral glucose tolerance test and insulin tolerance test
Oral glucose tolerance test (OGTT) and insulin tolerance test were performed at 4 wk after completion of STZ or vehicle injections. After 6 h of food deprivation, mice were orally administered D-glucose at 2 mg/g body weight or i.p. injected insulin at 0.5 mU/g body weight. Glucose levels were measured from tail bleeds using a glucometer (LifeScan, Milpitas, CA, USA) at specified time points during the course of tests.
Islet morphology and TUNEL
Islet morphology analysis was performed as previously described (15). At least 3 sections (5 μm) of pancreas tissue from each mouse were mounted on slides for examinations. Apoptotic β-cells were determined by the TUNEL method in formalin-fixed pancreas tissues using an in situ Cell Death Detection kit (Roche) according to the manufacturer’s protocol. After TUNEL staining, sections were fluorescence stained with anti-insulin antibodies and DAPI counterstaining.
Statistical analysis
All data were analyzed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Comparisons were conducted using Mann-Whitney or ANOVA with Tukey’s post hoc tests for 2 or multiple groups, respectively. The level of statistical significance was set at P < 0.01.
RESULTS
SK2 promotes lipotoxicity in β-cells independent of its enzymatic activity
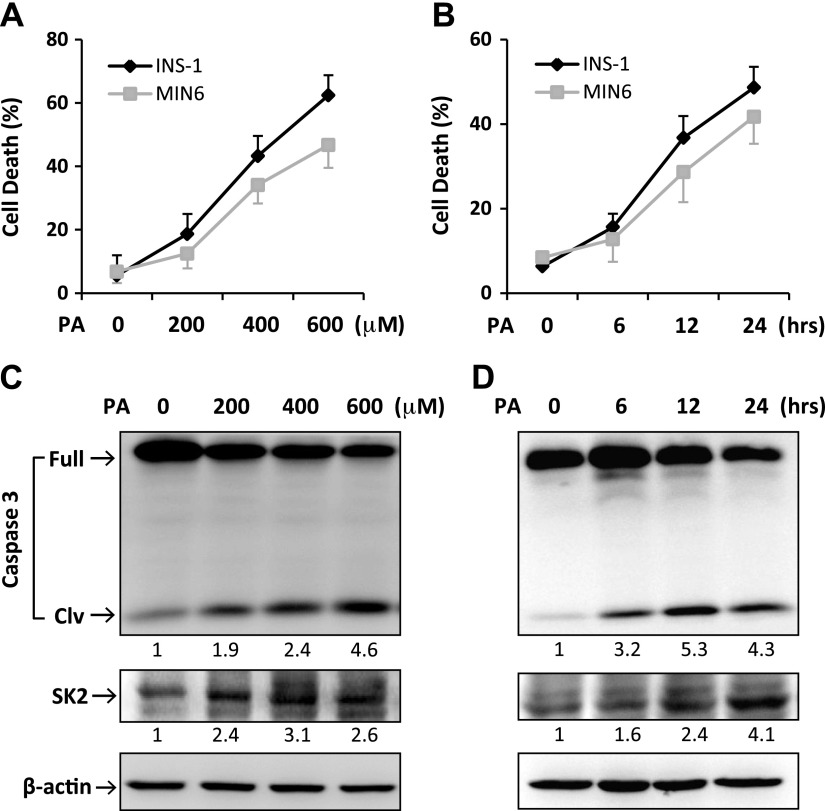
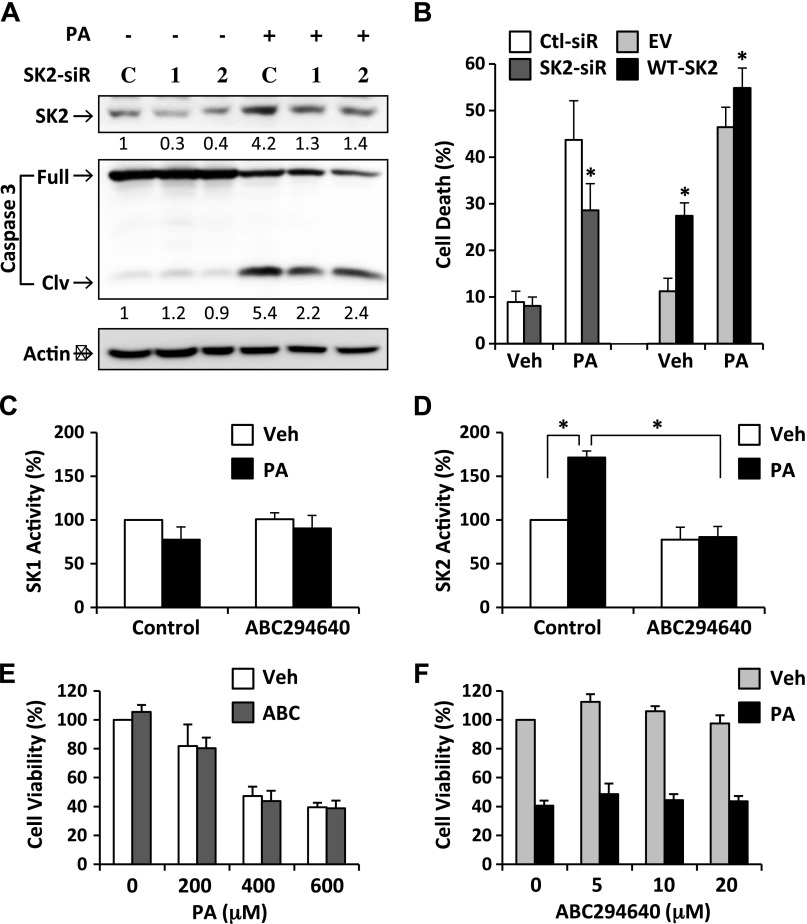
In keeping with our previous report and other studies (15, 16, 20), exposure to PA resulted in a dose- and time-dependent increase in apoptotic cell death and cleavage of caspase-3 in both INS-1 and MIN6 β-cell lines (Fig. 1A–D), indicating a potent proapoptotic effect of PA. In parallel with the PA-induced lipotoxicity, SK2 expression was significantly increased in β-cells by PA treatment in a similar dose- and time-dependent fashion (Fig. 1C, D), suggesting a potential link between SK2 expression and β-cell lipotoxicity. To further clarify such a link, we synthesized 2 siRNAs that specifically target SK2. The 2 SK2-siRNA significantly down-regulated SK2 expression by ∼80 and 65%, respectively (Fig. 2A). By knocking down SK2 expression, both siRNAs significantly inhibited PA-induced cleavage of caspase-3 and cell death in β-cells (Fig. 2A, B). By contrast, overexpression of SK2 in INS-1 β-cells significantly promoted cell death under both basal and lipotoxic conditions (Fig. 2B). Collectively, these data illustrate an important role of SK2 in mediating lipotoxicity in β-cells.
Figure 1.
PA induced apoptotic cell death and SK2 expression in β-cells. MIN6 and INS-1 cells were treated for 24 h with PA at increasing concentrations as indicated (A, C) or with PA at 400 μM for the indicated time points (B, D). A, B) The treated cells were costained with propidium iodide (PI) and annexin V–FITC (AV) followed by flow cytometric analysis. “Cell death” refers to the percentage of cells encompassing both AV single-positive and AV/PI double-positive cells. Error bars represent sd of the mean value from 3 independent experiments. C, D) Levels of full-length/cleaved (Clv) caspase-3 and SK2 expression were determined by Western blotting. Numbers below lanes indicate band intensity relative to that of the loading control β-actin. The blots represent 4 independent experiments.
Figure 2.
Role of SK2 in β-cell lipotoxicity. A) INS-1 β-cells were transfected with 2 synthesized siRNAs targeting SK2 (SK2-siR1,2) and the controls (C-siR) followed by treatment with or without PA (400 μM) for 24 h. Levels of SK2 and full-length/cleaved (Clv) caspase-3 were determined by Western blotting. Numbers below lanes indicate band intensity relative to that of the loading control β-actin. The blots represent 3 independent experiments. B) INS-1 β-cells were transfected with the control siRNA (Ctl-siR), SK2-siR1, WT-SK2, or a control empty vector (EV) followed by treatment with or without PA (400 μM) for 24 h. Cell death was analyzed by flow cytometry as described in Fig. 1. C, D) SK1 (C) and SK2 (D) activity was determined, respectively, in INS-1 β-cells treated for 24 h with 400 μM PA or with vehicle alone (Veh) after pretreatment with or without 10 μM ABC294640. E, F) Cell viability was determined in INS-1 β-cells treated for 24 h with PA at increasing concentrations as indicated after pretreatment with 10 μM ABC294640 (E) or treated with 400 μM PA after pretreatment with ABC294640 at 0, 5, 10, and 20 μM (F). Data are expressed as mean ± sd (n = 3). *P < 0.001.
To verify whether the proapoptotic effect of SK2 depends on its catalytic activity, we used a SK2-selective inhibitor, ABC294640 [3-(4-chlorophenyl)-adamantane-1 carboxylic acid (pyridin-4-yl-methyl) amide] that has been reported to specifically inhibit the enzymatic activity with a Ki of 9.8 µM (17). Consistent with the previous report (17), treatment of β-cells with ABC294640 resulted in significant inhibition of SK2 activity in both basal and PA-treated conditions (Fig. 2D), whereas there were no significant changes in SK1 activity (Fig. 2C). The suppression of β-cell viability induced by exposure to PA at various concentrations was not influenced by ABC294640 treatment at 10 µM (Fig. 2E). In addition, treatment with ABC294640 at a range of doses from 5 to 20 µM had no significant effect on the PA-induced β-cell death (Fig. 2F). The discrepancy between the effects of ABC294640 and SK2-siRNA on β-cell lipotoxicity suggests that the proapoptotic function of SK2 is unlikely mediated by its enzymatic activity.
Lipotoxic stress induces SK2 translocation from the nucleus to the cytoplasm in β-cells
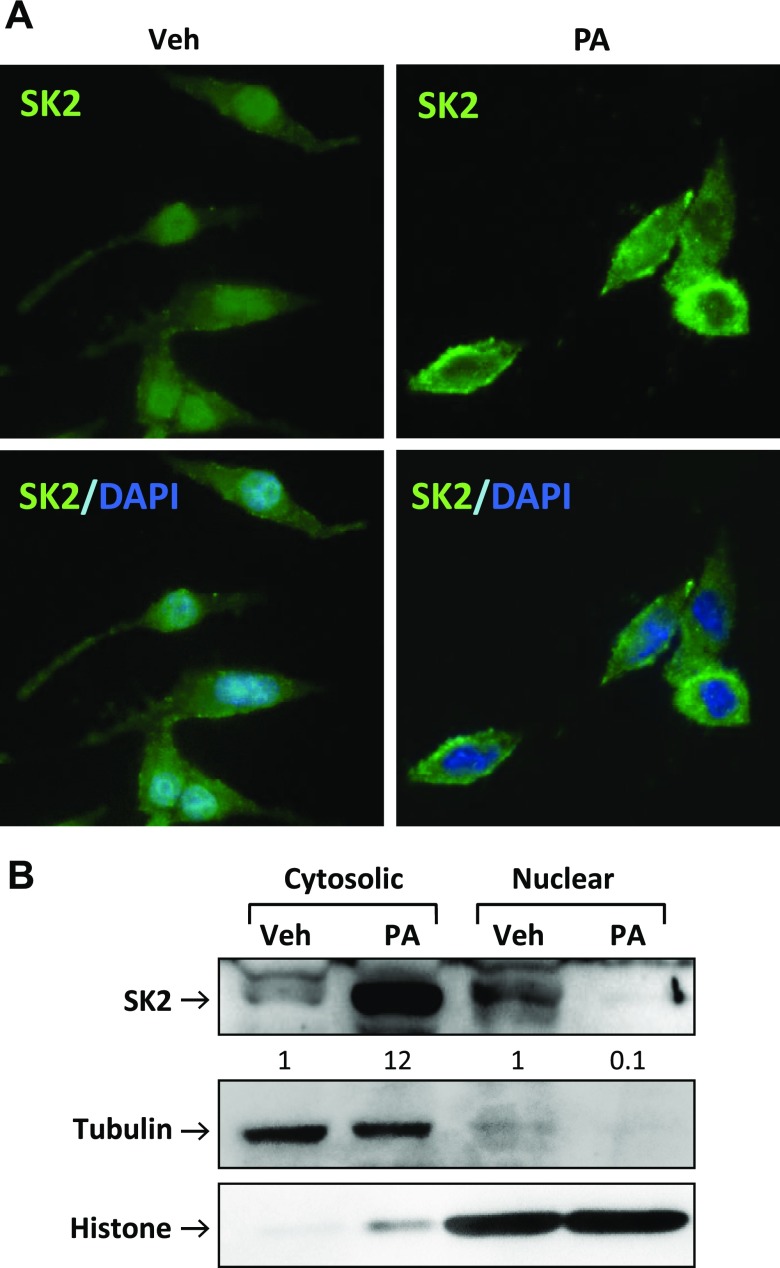
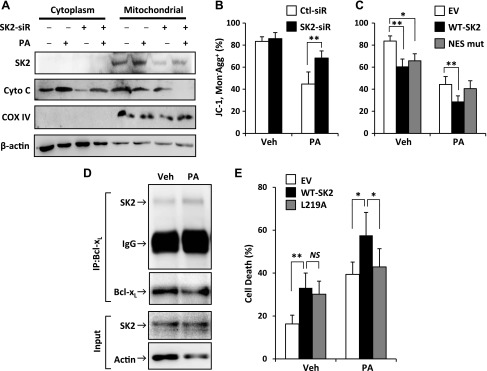
SK2 was originally identified as a nuclear protein that contains both nuclear localization signal and NES sequences and which shuttles between the nucleus and the cytoplasm of the cell (21, 22). We asked whether the proapoptotic function of SK2 is associated with its subcellular localization. The endogenous SK2 in β-cells was predominantly expressed in the nucleus under basal conditions, which is consistent with previous findings in other cell types (21, 22) (Fig. 3A). Treatment of β-cells with PA resulted in a dramatic redistribution of SK2 (i.e., increased cytoplasmic accumulation with a concomitant decrease in nuclear expression) (Fig. 3A). The subcellular localization of SK2 was further characterized by cell fractionation analysis, which confirmed nucleo/cytoplasmic shuttling of SK2 in β-cells in response to lipotoxic stress (Fig. 3B).
Figure 3.
PA induced SK2 translocation from the nucleus to the cytoplasm in β-cells. A) INS-1 β-cells were treated for 12 h with PA (400 μM) or with vehicle alone (Veh) and then fixed, permeabilized, and stained with antibodies against SK2 (green) and DAPI (blue), followed by epifluorescent microscopic analysis and imaging. Original magnification, ×40. B) The cytosolic and nuclear fractions were isolated from the treated INS-1 β-cells and immunoblotted with antibodies against SK2, tubulin, and histone. Numbers below lanes indicate band intensity relative to that of the loading control tubulin or histone. Representative images are shown from 3 independent experiments.
Blocking SK2 translocation protects β-cells against lipotoxicity
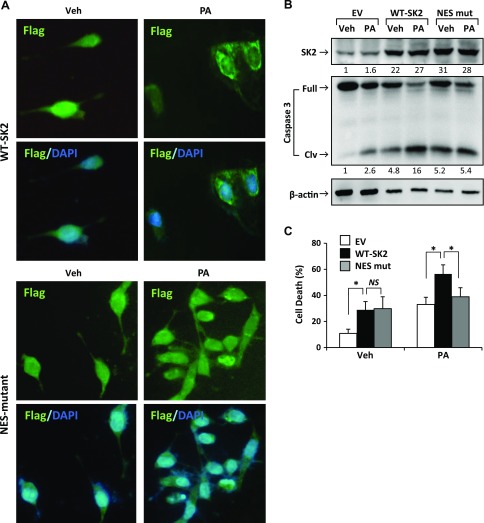
Having demonstrated PA-induced nucleo/cytoplasmic shuttling of SK2, we aimed to verify whether SK2 translocation is required for its proapoptotic effect in β-cells. To this end, we constructed an alanine substitution mutant in the NES-like sequence, L423A/L425A, according to the literature (22). In keeping with the above observation in endogenous SK2, both the overexpressed WT and NES-mutant SK2 displayed a predominantly nuclear localization in transfected β-cells under basal conditions (Fig. 4A). However, upon PA treatment, WT-SK2 shuttled from the nucleus into the cytoplasm, whereas NES-mutant SK2 failed to do so (Fig. 4A), indicating that the mutation effectively blocked PA-induced nuclear SK2 export. We then examined the effect of NES-mutant SK2 on lipotoxicity in β-cells. In line with the data shown in Fig. 2B, overexpression of WT-SK2 exacerbated the PA-induced caspase-3 activation and apoptosis in β-cells (Fig. 4B). However, NES-mutant SK2 had no significant effect on β-cell lipotoxicity, although it exhibited a proapoptotic activity similar to that of WT-SK2 under basal conditions (Fig. 4B, C). Together, these data suggest that SK2 promoted β-cell lipotoxicity through a mechanism relying on its nuclear exportation.
Figure 4.
Effect of NES-mutation on SK2 translocation and lipotoxicity in β-cells. INS-1 β-cells were transfected with Flag-tagged WT, NES-mutant SK2, or a control empty vector (EV) followed by treatment with PA (400 μM) or control vehicle (Veh) for 12 h. A) Treated cells were fixed and incubated with antibodies to the Flag epitope followed by the secondary antibodies conjugated with AlexaFluor (green) and DAPI (blue). Photomicrographs were taken of individual fields. Original magnification, ×40. B, C) Western blotting (B) and flow cytometry–based apoptosis (C) were conducted as detailed in Fig. 1. Data are expressed as mean ± sd (n = 3). NS, not significant. *P < 0.001.
SK2 activates the mitochondrial apoptotic pathway through its BH3 domain
SK2 has been identified as a putative BH3-only protein that interacts with a subgroup of the B-cell lymphoma (Bcl)-2 family proteins involved in the mitochondrial apoptotic pathway (23). As previously reported by us and others (16, 20), β-cell lipotoxicity is mediated chiefly through the mitochondrial apoptotic pathway. Exposure to PA markedly increased the release of cytochrome c from mitochondria into the cytosol (Fig. 5A). Consistently, PA treatment resulted in a significant reduction of mitochondrial transmembrane potential, as determined by the ratio of JC-1 monomers to its aggregates in β-cells (Fig. 5B). The action of PA on the mitochondrial pathway was significantly inhibited by the siRNA-mediated knockdown of SK2 (Fig. 5A, B). Furthermore, the PA-induced reduction of mitochondrial transmembrane potential was further reduced by overexpression of WT-SK2 but not by the NES-mutant (Fig. 5C). Taken together, these data indicate a functional connection between nucleo/cytoplasmic shuttling of SK2 and the mitochondrial apoptotic pathway activation in β-cells under lipotoxic stress.
Figure 5.
SK2 activated the mitochondrial apoptotic pathway through its BH3 domain. A) The cytoplasmic and mitochondrial fractions were isolated from INS-1 cells transfected with SK2-siR or control siRNA followed by treatment with or without PA (400 μM) for 24 h and immunoblotted for analyzing cytochrome c expression. B, C) Mitochondrial transmembrane potential was determined by flow cytometry with JC-1 staining in INS-1 cells transfected with SK2-siR or control siRNA (B) or Flag-tagged WT or NES-mutant SK2 (C), followed by treatment with or without PA (400 μM) for 24 h. D) Endogenous Bcl-xL was immunoprecipitated by anti–Bcl-xL antibodies and immunoblotted with anti-SK2 antibodies for analyzing their interaction in INS-1 cells that were treated with or without PA (400 μM) for 12 h. E) Cell death was analyzed by flow cytometry with Annexin V and propidium iodide double staining in INS-1 β-cells transfected with WT or BH3-mutant SK2 (L219A) and a control empty vector, followed by treatment with or without PA (400 μM) for 24 h. Data are expressed as mean ± sd (n = 3–4). NS, not significant. *P < 0.01, **P < 0.001.
To further investigate the role of SK2 in the mitochondrial apoptotic pathway, we sought to determine whether the PA-induced cytosolic translocation of SK2 could promote its interaction with the Bcl-2 family protein Bcl-xL. In agreement with a previous report (23) that shows the enforced overexpression of SK2 interaction with Bcl-xL, we detected only a faint band representing binding between endogenous SK2 and Bcl-xL by immunoprecipitation analysis in β-cells under basal conditions (Fig. 5D). However, the interaction of endogenous SK2 with Bcl-xL was enhanced after PA treatment (Fig. 5D), suggesting that SK2 might function as a BH3-only protein accounting for its promotion of β-cell lipotoxicity. To further this notion, we transfected β-cells with a substitute mutation of SK2 in the BH3 domain, L219A-SK2, which has been shown to completely block its binding to Bcl-xL without changing enzymatic activity (23). PA-induced lipotoxicity was significantly enhanced in β-cells overexpressing WT-SK2 but not L219A-SK2 (Fig. 5E). Collectively, these data suggest that the proapoptotic effect of SK2 in β-cells is attributable to its BH3 domain.
SK2 deficiency ameliorates the diabetic phenotype by preventing the loss of β-cell mass
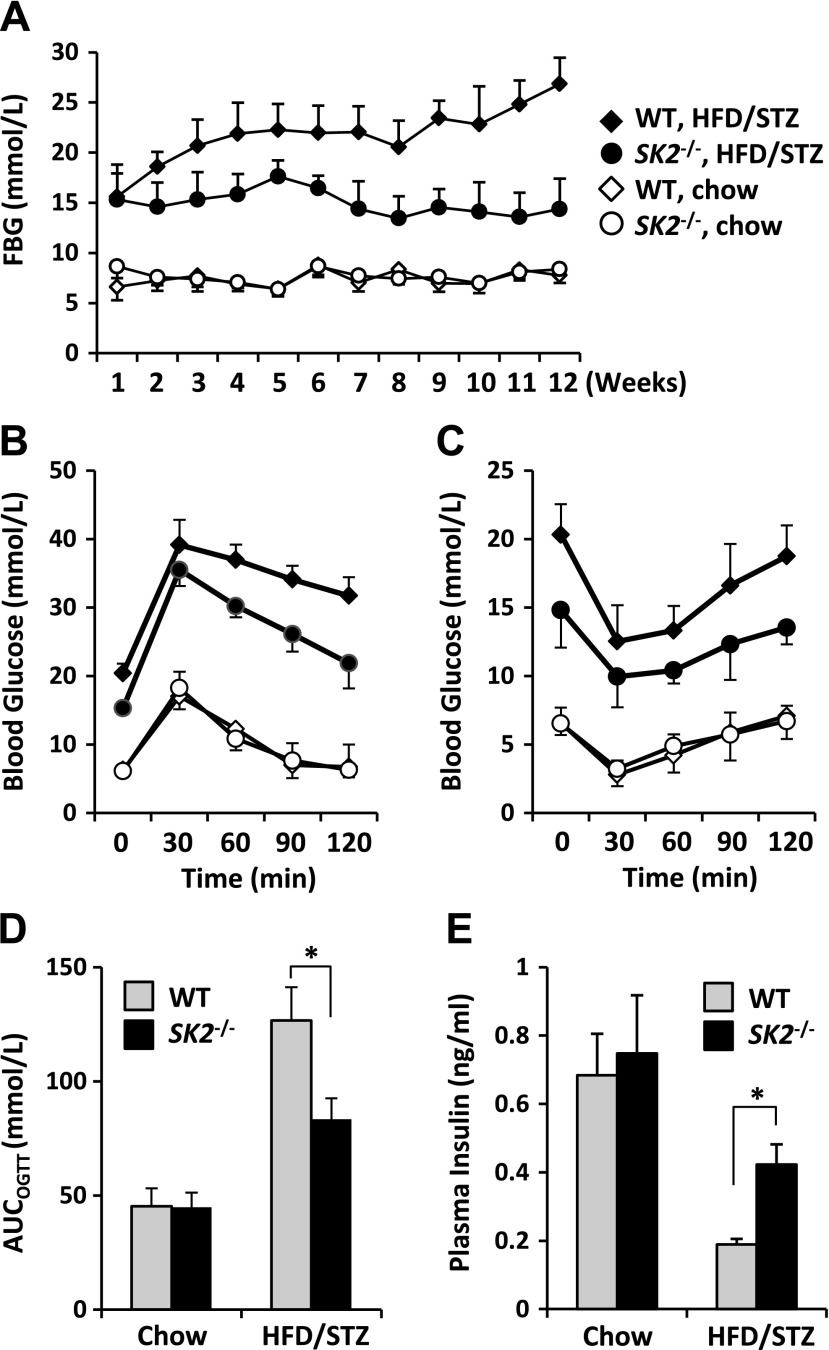
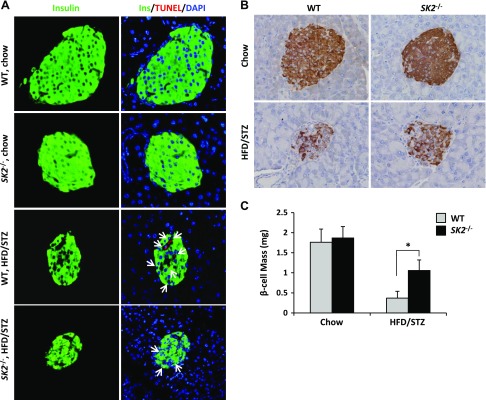
Loss of β-cell mass resulting from lipotoxicity is regarded as a key determinative factor for the development of T2DM. Having demonstrated the effect of SK2 in mediating β-cell lipotoxicity in vitro, we explored its role in diabetes. To this end, we applied an established T2DM animal model in SK2−/− mice and control WT littermates by feeding an HFD combined with administration of a small dose of STZ according to the literature (24). Levels of fasting blood glucose were sharply increased in both genotypes after STZ injection, indicating the onset of diabetes in these animals (Fig. 6A). The WT littermates exhibited a severe hyperglycemic level of >20 mM and maintained these high levels from the second week after the first injection of STZ until the end of the experiment (i.e., 12 wk), whereas the HFD/STZ-treated SK2−/− mice developed only mild hyperglycemia at levels of <16 mM. Correspondingly, whereas glucose tolerance tests showed diabetic profiles in HFD/STZ-treated WT and SK2−/− mice (Fig. 6B), the HFD/STZ-treated SK2−/− mice manifested a significant decrease in the area under curve, which integrates the glucose levels over the course of OGTT, compared with the control group (Fig. 6D). However, insulin tolerance tests revealed a similar degree of insulin resistance in HFD/STZ-treated WT and SK2−/− mice (Fig. 6C). Furthermore, the HFD/STZ-treated SK2−/− mice displayed a significantly higher level of circulating insulin compared with the control group, although insulin levels were markedly decreased in both genotypes after STZ administration (Fig. 6E). These data strongly suggest a protective effect of SK2−/− on β-cells in vivo. To further clarify the protective effect resulting from SK2 deficiency, we directly monitored in situ β-cell apoptosis by TUNEL staining, which identifies DNA fragmentation within apoptotic cells. As expected, positive TUNEL staining was detected in insulin-labeled cells in the HFD/STZ-treated WT mice, whereas few TUNEL-positive cells presented in the islets of SK2−/− mice exposed to HFD/STZ (Fig. 7A). In line with these observations, whereas HFD/STZ treatment resulted in a dramatic decrease in β-cell mass in both genotypes, SK2−/− mice exhibited a significantly larger β-cell mass than that of the control group after HFD/STZ treatment (Fig. 7B, C). Taken together, these data illustrate an important role of endogenous SK2 in promoting β-cell death and the development of diabetes in the type 2 diabetic animal model.
Figure 6.
SK2 deficiency ameliorated diabetic phenotypes. WT and SK2-deficient mice were treated with HFD/STZ or fed a chow diet accompanied by vehicle injections. A) Fasting blood glucose (FBG) levels were determined during the 12 wk experimental period. B, C) OGTT (B) and insulin tolerance test (C) were conducted in mice at 4 wk after STZ or vehicle injections as detailed in Materials and Methods. Labels in B and C are as same as in A. D) Area under curve integrates the glucose levels over the course of OGTT. E) Levels of plasma insulin were measured in mice starved for 6 h at the end of experiment (12 wk after HFD/STZ or control treatment). Data are expressed as mean ± sem (n = 6–9 mice/group). *P < 0.001.
Figure 7.
SK2 deficiency protected β-cells against apoptosis in HFD/STZ-treated mice. A) Representative images show the pancreatic sections from control or HFD/STZ-treated WT and SK2-deficient mice, fluorescence stained for insulin (green), TUNEL (red), and DAPI (blue). Arrowheads indicate apoptotic (TUNEL-positive) β-cells. B) Representative images show sections immunohistochemistry stained for insulin. C) β-Cell mass was determined based on insulin staining according to pancreas weight (milligrams) × islets per field area × β-cell numbers per islet area. Photomicrographs were taken of individual fields. Original magnification, ×40. At least 5 fields were taken from each section, and at least 3 pancreatic sections were counted from each animal. Data are expressed as mean ± sem. *P < 0.001.
DISCUSSION
In the present study, we demonstrated that SK2 plays a key role in mediating pancreatic β-cell lipotoxicity in an enzymatic activity–independent manner. SK2 deficiency significantly prevented the loss of β-cell mass associated with a reduction of apoptosis, increased insulin secretion, and ameliorated hyperglycemia in an established T2DM animal model. Thus, this study provides functional and mechanistic evidence showing the role of SK2 in β-cell lipotoxicity and the development and progression of diabetes.
Lipotoxicity that leads to cell death and loss of functional β-cell mass is a major pathogenic factor for the onset and progression of T2DM. Although insulin resistance is regarded as a key factor causing T2DM, diabetes develops only after the pancreatic β-cells fail to produce enough insulin to compensate for the increased metabolic demands (3, 25). Thus, the balance between β-cell replication and apoptosis under insulin resistance determines the onset of diabetes. Indeed, recent studies from autopsies have shown a significantly increased β-cell mass with evident β-cell neogenesis in obese humans (26). However, obese people who have impaired fasting glucose or T2DM manifest a 40–60% deficit of β-cell mass associated with elevated β-cell apoptosis (26). Under obese conditions, circulating free fatty acids are often elevated, especially the saturated ones such as PA, which exert potent lipotoxic effects toward pancreatic β-cells leading to apoptosis (1–3). As demonstrated in the present study, exposure to PA in INS-1 and MIN6 cells, the 2 most established β-cell lines, resulted in a dramatic increase in apoptosis in a dose- and time-dependent manner (Fig. 1A–D). Extensive studies of β-cell biology suggest that an aberrant ER stress response and activation of the mitochondrial apoptotic pathway are key mechanisms responsible for lipotoxicity in β-cells (25, 27). Our prior studies have demonstrated that SK1 plays a pivotal role in preventing β-cell lipotoxicity by ameliorating ER stress and inhibiting the mitochondrial apoptotic pathway via an S1P-dependent mechanism (15). S1P is an important signaling lipid that has been broadly implicated in the regulation of multiple biologic processes, including cell survival, proliferation, migration, and metabolic homeostasis (4, 7). Although SK1 and SK2 are responsible for the induction of S1P, they appear to function differently, especially regarding their role in the regulation of cell survival and growth (6, 11). Likewise, we reported herein that the endogenous SK2 plays an opposing role to SK1, profoundly promoting β-cell lipotoxicity in vitro and in vivo. We found that exposure of β-cells to PA resulted in a significant increase in SK2 expression, which was paralleled by the induction of cell death in a similar dose- and time-dependent fashion (Fig. 1A–D). Knocking down SK2 expression with its specific siRNAs significantly inhibited PA-induced cell death and caspase-3 activation, whereas overexpression of SK2 promoted lipotoxicity in β-cells (Fig. 2A, B). These findings clearly reveal a potent proapoptotic effect of SK2 on pancreatic β-cells under lipotoxic stress in vitro.
To further explore the role of SK2 in subjects with T2DM, we applied an established T2DM animal model by feeding mice an HFD and administering a small dose of STZ. Similar to the phenomena observed in humans with diabetes, HFD/STZ-induced diabetes in WT mice and caused insulin resistance, hyperglycemia, and loss of functional β-cell mass, which is consistent with previous reports (24). Compared with WT mice, SK2-deficient mice exhibited a significant amelioration in the diabetic phenotypes, as reflected by reduced hyperglycemia, improved OGTT, increased insulin production, decreased β-cell death, and alleviated loss of β-cell mass (Figs. 6 and 7). Moreover, insulin tolerance tests showed that HFD/STZ treatment induced comparable levels of insulin resistance in WT and SK2−/− mice (Fig. 6C). These data indicate that the ameliorated diabetic phenotype by SK2 deficiency is chiefly attributable to improved compensatory capacity of the β-cell mass. However, in the absence of a more precise analysis of insulin sensitivity in vivo in the current study, we cannot exclude the possibility that SK2 had an additional role in regulating insulin sensitivity. Furthermore, whether SK2 has a direct influence on insulin production and/or secretion in β-cells remains to be determined. Owing to a common limitation arising from global knockout of a gene, SK2 deficiency may have β-cell–independent effects that could account for amelioration of the diabetic phenotype. Therefore, additional studies using a β-cell–specific conditional knockout of SK2 are warranted.
Like SK1, SK2 is a key enzyme of the sphingolipid metabolic pathway. The biologic function of SK2 is principally ascribed to its enzymatic activity (i.e., the conversion of sphingosine to S1P). Indeed, despite conflicting data in the literature related to the proapoptotic or prosurvival role of SK2, multiple lines of evidence have demonstrated that the function of SK2 chiefly relies on its catalytic activity in a specific subcellular location (13). For instance, nuclear-localized SK2 has been shown to epigenetically regulate p21 and c-fos gene expression, leading to cell cycle arrest through S1P-mediated inhibition of histone deacetylase 1/2 activity (28). Other studies reported that mitochondria-localized SK2-induced production of S1P at this site was capable of promoting mitochondrial membrane permeabilization and cytochrome c release, resulting in apoptotic cell death (29, 30). More recently, SK2-derived intracellular S1P has been shown to promote epidermal growth factor–dependent chemotaxis and invasion of breast cancer cells (31) and to exacerbate insulin’s mitogenic action in cancer cells (14). The pharmacological inhibition of SK2, especially with the specific inhibitor ABC294640, has been shown to alleviate the progression of various diseases in animal models, such as osteoarthritis, rheumatoid arthritis, Crohn’s disease, diabetic retinopathy, and a multitude of malignancies [as reviewed by Neubauer and Pitson (13)]. However, we found that, although silencing SK2 expression by its specific siRNAs profoundly attenuates β-cell lipotoxicity, the SK2 inhibitor ABC294640 at a range of doses (5–20 µM) had no significant effect on lipotoxicity in β-cells (Fig. 2C), suggesting a catalytic activity–independent action of SK2.
Alternatively, the proapoptotic role of SK2 in β-cells appears to rely on its translocation from the nucleus to the cytoplasm. In agreement with previous reports on other cell types (21, 22), we found that endogenous SK2 was predominantly expressed in the nucleus of β-cells under basal conditions. Upon PA treatment, SK2 was dramatically redistributed from the nucleus to the cytoplasm (Fig. 3A, B). It has been documented that nucleo/cytoplasmic shuttling of SK2 was mediated by the NES in a protein kinase D–driven phosphorylation-dependent manner (22). Correspondingly, we applied the NES mutant SK2 (L423A/L425A) in this study, which effectively blocked PA-induced nuclear SK2 export by this mutant (Fig. 4A). The NES mutation was unable to promote β-cell lipotoxicity, although it exhibited a proapoptotic activity similar to that of WT-SK2 under basal conditions (Fig. 4B, C). These data strongly suggest that nucleo/cytoplasmic shuttling of SK2 is a key mechanism responsible for its role in promoting β-cell lipotoxicity. Thus, our findings provide additional evidence supporting the notion that the subcellular localization of SK2 is a critical determinant of its proapoptotic function, regardless of its catalytic activity.
In early studies, a putative BH3 domain was identified within SK2, which directly interacts with Bcl-xL, a prosurvival Bcl-2 family protein, exerting the proapoptotic function of SK2 (23). Although the interaction of SK2 with Bcl-xL was mostly observed in cells overexpressing SK2 in previous studies (23, 32), we observed that endogenous SK2 interacted with Bcl-xL in INS-1 β-cells. The interaction of SK2 with Bcl-xL was markedly enhanced in cells under lipotoxic stress (Fig. 5C), suggesting a mechanistic connection between the BH3 domain of SK2 and β-cell lipotoxicity. In agreement with previous studies (16, 20), PA-induced lipotoxicity in β-cells is chiefly mediated through the mitochondrial apoptotic pathway, as reflected in the reduced mitochondrial transmembrane potential and the increased release of cytochrome c into the cytosol (Fig. 5A, B). The finding that the siRNA-mediated knockdown of SK2 significantly inhibited PA-induced activation of the mitochondrial pathway (Fig. 5C) further suggests that the proapoptotic function of SK2 is likely associated with its BH3 domain. Indeed, PA-induced lipotoxicity was significantly enhanced in β-cells overexpressing WT-SK2 but not L219A-SK2, which has a mutated BH3 domain (Fig. 5D). It has been demonstrated that the mutant L219A-SK2 lost its ability to bind to Bcl-xL without alterations in enzymatic activity (23). Therefore, the data from L219A-SK2 further strengthen the notion that SK2 may function via its BH3 domain to promote β-cell lipotoxicity in an enzymatic-independent manner. However, recent studies have demonstrated that mitochondrial-localized SK2 activity (i.e., S1P generated at this site) was critical for Bcl-2–associated X protein (BAK)/Bcl-2 antagonist killer 1 (BAX) activation and apoptosis (30). Thus, it is of interest to test the functional connection between SK2/Bcl-xL interaction and BAK/BAX activation in future studies. It is also worth noting that, like the BH3 domain mutation, the NES-mutant SK2 also significantly attenuated the PA-induced reduction of mitochondrial transmembrane potential (Fig. 5B), indicating a mechanistic connection between nucleo/cytoplasmic shuttling of SK2 and its action on the mitochondrial apoptotic pathway.
In summary, the current study provides experimental evidence and mechanistic data showing a critical role of SK2 in promoting β-cell lipotoxicity. SK2 deficiency significantly protected β-cells against apoptosis, preserved insulin production, and thus alleviated the diabetic phenotype. These data, together with our previous findings related to the protective role of SK1 in β-cells (15, 27), suggest a new strategy that maintains a balance between SK1 and SK2 in β-cells for the prevention and treatment of diabetes.
ACKNOWLEDGMENTS
The authors thank Dr. Richard Proia (NIH, Bethesda, MD, USA) for providing SK2−/− and SK2+/+ mouse colonies and Dr. Carol Wadham (South Australia Pathology, Adelaide, Australia) for reviewing and editing the manuscript. This work was supported by National Natural Science Foundation of China Grants 81370937 and 81561128014 (to P.X.) and by a Fudan Distinguished Professorship (to P.X.). The authors declare no conflicts of interest.
Glossary
- Bcl
B-cell lymphoma
- Bcl-xL
B-cell lymphoma–extra-large
- HFD
high-fat diet
- NES
nuclear export signal
- OGTT
oral glucose tolerance test
- PA
palmitatic acid
- S1P
sphingosine 1-phosphate
- siRNA
small interfering RNA
- SK
sphingosine kinase
- STZ
streptozotocin
- T2DM
type 2 diabetes mellitus
- WT
wild type
AUTHOR CONTRIBUTIONS
P. Xia designed the research and wrote the manuscript; Z. Song, W. Wang, N. Li, S. Yan, and K. Rong performed the research; and Z. Song and T. Lan analyzed the data.
REFERENCES
- 1.Lee Y., Hirose H., Ohneda M., Johnson J. H., McGarry J. D., Unger R. H. (1994) Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. USA 91, 10878–10882 10.1073/pnas.91.23.10878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson R. P., Harmon J., Tran P. O., Poitout V. (2004) Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53(Suppl 1), S119–S124 10.2337/diabetes.53.2007.S119 [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo R. A. (2010) Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53, 1270–1287 10.1007/s00125-010-1684-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maceyka M., Harikumar K. B., Milstien S., Spiegel S. (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 10.1016/j.tcb.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia P., Wadham C. (2011) Sphingosine 1-phosphate, a key mediator of the cytokine network: juxtacrine signaling. Cytokine Growth Factor Rev. 22, 45–53 10.1016/j.cytogfr.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 6.Pitson S. M. (2011) Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 36, 97–107 10.1016/j.tibs.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 7.Hla T., Dannenberg A. J. (2012) Sphingolipid signaling in metabolic disorders. Cell Metab. 16, 420–434 10.1016/j.cmet.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizugishi K., Yamashita T., Olivera A., Miller G. F., Spiegel S., Proia R. L. (2005) Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25, 11113–11121 10.1128/MCB.25.24.11113-11121.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 10.1074/jbc.M406512200 [DOI] [PubMed] [Google Scholar]
- 10.Zemann B., Urtz N., Reuschel R., Mechtcheriakova D., Bornancin F., Badegruber R., Baumruker T., Billich A. (2007) Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol. Lett. 109, 56–63 10.1016/j.imlet.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 11.Maceyka M., Sankala H., Hait N. C., Le Stunff H., Liu H., Toman R., Collier C., Zhang M., Satin L. S., Merrill A. H., Jr., Milstien S., Spiegel S. (2005) SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 280, 37118–37129 10.1074/jbc.M502207200 [DOI] [PubMed] [Google Scholar]
- 12.Vadas M., Xia P., McCaughan G., Gamble J. (2008) The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim. Biophys. Acta 1781, 442–447 10.1016/j.bbalip.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 13.Neubauer H. A., Pitson S. M. (2013) Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 280, 5317–5336 10.1111/febs.12314 [DOI] [PubMed] [Google Scholar]
- 14.Dai L., Qi Y., Chen J., Kaczorowski D., Di W., Wang W., Xia P. (2014) Sphingosine kinase (SphK) 1 and SphK2 play equivalent roles in mediating insulin’s mitogenic action. Mol. Endocrinol. 28, 197–207 10.1210/me.2013-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi Y., Chen J., Lay A., Don A., Vadas M., Xia P. (2013) Loss of sphingosine kinase 1 predisposes to the onset of diabetes via promoting pancreatic β-cell death in diet-induced obese mice. FASEB J. 27, 4294–4304 10.1096/fj.13-230052 [DOI] [PubMed] [Google Scholar]
- 16.Qi Y., Xia P. (2012) Cellular inhibitor of apoptosis protein-1 (cIAP1) plays a critical role in β-cell survival under endoplasmic reticulum stress: promoting ubiquitination and degradation of C/EBP homologous protein (CHOP). J. Biol. Chem. 287, 32236–32245 10.1074/jbc.M112.362160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French K. J., Zhuang Y., Maines L. W., Gao P., Wang W., Beljanski V., Upson J. J., Green C. L., Keller S. N., Smith C. D. (2010) Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 333, 129–139 10.1124/jpet.109.163444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukocheva O., Wadham C., Gamble J., Xia P. (2015) Sphingosine-1-phosphate receptor 1 transmits estrogens’ effects in endothelial cells. Steroids 104, 237–245 10.1016/j.steroids.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Ji X., Li C., Ou Y., Li N., Yuan K., Yang G., Chen X., Yang Z., Liu B., Cheung W. W., Wang L., Huang R., Lan T. (2016) Andrographolide ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated renal oxidative stress and inflammation via Akt/NF-κB pathway. Mol. Cell. Endocrinol. 437, 268–279 10.1016/j.mce.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 20.Cunha D. A., Igoillo-Esteve M., Gurzov E. N., Germano C. M., Naamane N., Marhfour I., Fukaya M., Vanderwinden J. M., Gysemans C., Mathieu C., Marselli L., Marchetti P., Harding H. P., Ron D., Eizirik D. L., Cnop M. (2012) Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human β-cell apoptosis. Diabetes 61, 2763–2775 10.2337/db12-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igarashi N., Okada T., Hayashi S., Fujita T., Jahangeer S., Nakamura S. (2003) Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278, 46832–46839 10.1074/jbc.M306577200 [DOI] [PubMed] [Google Scholar]
- 22.Ding G., Sonoda H., Yu H., Kajimoto T., Goparaju S. K., Jahangeer S., Okada T., Nakamura S. (2007) Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J. Biol. Chem. 282, 27493–27502 10.1074/jbc.M701641200 [DOI] [PubMed] [Google Scholar]
- 23.Liu H., Toman R. E., Goparaju S. K., Maceyka M., Nava V. E., Sankala H., Payne S. G., Bektas M., Ishii I., Chun J., Milstien S., Spiegel S. (2003) Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 278, 40330–40336 10.1074/jbc.M304455200 [DOI] [PubMed] [Google Scholar]
- 24.Islam M. S., Wilson R. D. (2012) Experimentally induced rodent models of type 2 diabetes. Methods Mol. Biol. 933, 161–174 [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft F. M., Rorsman P. (2012) Diabetes mellitus and the β cell: the last ten years. Cell 148, 1160–1171 10.1016/j.cell.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 10.2337/diabetes.52.1.102 [DOI] [PubMed] [Google Scholar]
- 27.Song Z., Wang W., Xia P. (2016) Signaling pathways promoting beta cell survival under ER stress: role of sphingosine 1-phosphate and cellular inhibitor of apoptosis protein-1. In Endoplasmic Reticulum Stress: Regulation, Function and Role in Health and Disease (Wagner C., ed.), pp. 25–47, Nova Science Publisher, Inc., New York [Google Scholar]
- 28.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., Spiegel S. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 10.1126/science.1176709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strub G. M., Paillard M., Liang J., Gomez L., Allegood J. C., Hait N. C., Maceyka M., Price M. M., Chen Q., Simpson D. C., Kordula T., Milstien S., Lesnefsky E. J., Spiegel S. (2011) Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 25, 600–612 10.1096/fj.10-167502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chipuk J. E., McStay G. P., Bharti A., Kuwana T., Clarke C. J., Siskind L. J., Obeid L. M., Green D. R. (2012) Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148, 988–1000 10.1016/j.cell.2012.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adada M. M., Canals D., Jeong N., Kelkar A. D., Hernandez-Corbacho M., Pulkoski-Gross M. J., Donaldson J. C., Hannun Y. A., Obeid L. M. (2015) Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 29, 4654–4669 10.1096/fj.15-274340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song D. D., Zhang T. T., Chen J. L., Xia Y. F., Qin Z. H., Waeber C., Sheng R. (2017) Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain. Cell Death Dis. 8, e2912 10.1038/cddis.2017.289 [DOI] [PMC free article] [PubMed] [Google Scholar]