Abstract
Peroxisomes are essential organelles for the specialized oxidation of a wide variety of fatty acids, but they are also able to degrade fatty acids that are typically handled by mitochondria. Using a combination of pharmacological inhibition and clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein 9 genome editing technology to simultaneously manipulate peroxisomal and mitochondrial fatty acid β-oxidation (FAO) in HEK-293 cells, we identified essential players in the metabolic crosstalk between these organelles. Depletion of carnitine palmitoyltransferase (CPT)2 activity through pharmacological inhibition or knockout (KO) uncovered a significant residual peroxisomal oxidation of lauric and palmitic acid, leading to the production of peroxisomal acylcarnitine intermediates. Generation and analysis of additional single- and double-KO cell lines revealed that the D-bifunctional protein (HSD17B4) and the peroxisomal ABC transporter ABCD3 are essential in peroxisomal oxidation of lauric and palmitic acid. Our results indicate that peroxisomes not only accept acyl-CoAs but can also oxidize acylcarnitines in a similar biochemical pathway. By using an Hsd17b4 KO mouse model, we demonstrated that peroxisomes contribute to the plasma acylcarnitine profile after acute inhibition of CPT2, proving in vivo relevance of this pathway. We summarize that peroxisomal FAO is important when mitochondrial FAO is defective or overloaded.—Violante, S., Achetib, N., van Roermund, C. W. T., Hagen, J., Dodatko, T., Vaz, F. M., Waterham, H. R., Chen, H., Baes, M., Yu, C., Argmann, C. A., Houten, S. M. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4.
Keywords: fatty acid oxidation, mitochondria, organellar crosstalk, acylcarnitine, CPT2 deficiency
Mitochondrial long-chain fatty acid β-oxidation (FAO) plays a key role in energy homeostasis because it is a main energy source during fasting (1). Patients with inborn errors of mitochondrial FAO may present with hypoglycemia and with skeletal and cardiac muscle disease, illustrating the physiologic importance of this pathway in humans (1). Peroxisomes were originally also shown to oxidize long-chain fatty acids, such as palmitate akin to mitochondria (2, 3). The identification of peroxisomal disorders, however, led to the characterization of specific substrates for the peroxisomal FAO machinery, such as very long-chain fatty acids, long-chain dicarboxylic acids (DCAs), 2-methyl-branched fatty acids, and bile acid precursors (4). Although potentially important in patients with a mitochondrial FAO disorder, the metabolic interplay between peroxisomes and mitochondria (5) and a more general role for peroxisomes in the oxidation of long-chain fatty acids have remained largely unexplored.
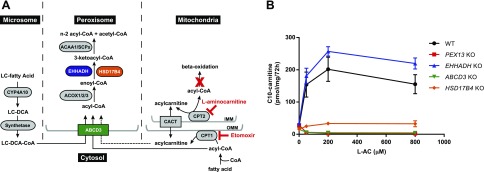
It is known that peroxisomal FAO contributes to the oxidation of long-chain fatty acids in liver and heart (6–8), but the molecular and biochemical mechanisms and physiologic relevance of this pathway are unknown. In addition, it is well established that, under specific conditions such as fasting or in the case of a mitochondrial FAO defect, long-chain fatty acids destined for mitochondria can be ω-oxidized to DCAs by microsomal cytochrome P450 enzymes of the CYP4A family. These long-chain DCAs are then transported to the peroxisome, where they are shortened to medium-chain DCAs such as adipic acid. Indeed, the peroxisomal enzyme enoyl-CoA hydratase/3-hydroxy acyl-CoA dehydrogenase (EHHADH; L-bifunctional protein) is involved in the production of medium-chain DCAs during fasting and dietary intake of medium-chain fatty acids (9, 10). Furthermore, we have recently demonstrated in vitro that, in the presence of a defect in mitochondrial FAO, fatty acids can also be redirected to the peroxisomes without prior ω-oxidation (11) (Fig. 1A).
Figure 1.
Peroxisomal fatty acid β-oxidation of lauric acid in HEK-293 cells. A) Schematic representation of the microsomal, peroxisomal, and mitochondrial fatty acid metabolism. 1) Microsomal ω-oxidation converts long-chain fatty acids to long-chain DCAs, which are subsequently directed to the peroxisome. 2) Long-chain DCA- and acyl-CoAs (and possibly acylcarnitines, as demonstrated in this study) enter the peroxisome for further oxidation. Peroxisomal FAO consists of 4 steps catalyzed by different enzymes and leading to the formation of a chain-shortened acyl-CoA and acetyl-CoA that leave the peroxisome unesterified or as an acylcarnitine. 3) Fatty acids enter mitochondria through the carnitine shuttle, which is composed by CPT1, CPT2, and the mitochondrial transporter carnitine acylcarnitine translocase (CACT, SLC25A20). Fatty acids are activated to the respective acyl-CoA ester in the cytosol and converted to acylcarnitines by the action of CPT1, a transmembrane protein of the outer mitochondrial membrane (OMM). These acylcarnitines are then transported across the inner mitochondrial membrane (IMM) by CACT and once in the matrix are reconverted back to acyl-CoAs that will undergo mitochondrial FAO. This cycle can reverse its action to export intramitochondrially accumulating acyl-CoAs as acylcarnitine esters. l-AC inhibits CPT2, and etomoxir inhibits CPT1. B) Production of C10-carnitine in the extracellular medium of KO cell lines after loading with C12:0 in the presence of increasing concentrations of the CPT2 inhibitor l-AC. Data are the mean ± sem of duplicates of 2 independent experiments. All experiments were carried out with 2 clonal cell lines. In 2-way ANOVA with Tukey’s multiple comparisons test, both genotype and l-AC concentration were shown to have a significant effect. The multiple comparisons test yielded significant results at all used l-AC concentrations (except for 0 µM). All KO clones except for the EHHADH KO differed significantly from WT HEK-293 cells.
Beadle and Tatum (12) demonstrated that genes control specific chemical reactions, now known as the 1 gene–1 enzyme hypothesis. Their work was based on the ability to establish mutant Neurospora strains by X-ray and the characterization of the resulting vitamin B auxotrophy. For many years, biochemical genetics research took advantage of similar tools, often using the yeast Saccharomyces cerevisiae. Forward genetic screens in this model organism have revealed the identity of many genes involved in conserved biochemical pathways, such as the PEX genes in peroxisome biogenesis (13). S. cerevisiae was the first eukaryote for which the genome was sequenced, which, combined with a relatively simple strategy to disrupt genes, made this yeast ideal for reverse genetics studies. Although RNAi approaches, and later zinc finger nucleases and transcription activator–like effector-based nucleases, enabled similar studies in human cells, the development of the clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein 9 (Cas9) genome editing technology has significantly advanced the application of biochemical genetics to human cell lines. The main reason for this is that the CRISPR-Cas9 technology allows for the efficient and fast generation of a complete gene knockout (KO) in different cell lines (14). Additional advantages include the possibility to target multiple genes and to generate double or even triple KO cell lines.
Here we used a combination of pharmacological inhibition and CRISPR-Cas9 genome editing technology to identify essential players in the metabolic crosstalk between mitochondria and peroxisomes. We demonstrated in vivo relevance of this pathway using different mouse models with acute FAO inhibition. Taken together, our findings suggest that there is a tight functional link between mitochondria and peroxisomes whereby peroxisomal FAO can serve as a compensatory mechanism in case of mitochondrial FAO defects or mitochondrial overload.
MATERIALS AND METHODS
Materials
Minimal essential medium was obtained from MilliporeSigma (Burlington, MA, USA). DMEM, penicillin, streptomycin, and Lipofectamine 2000 were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Fetal bovine serum was from BioWhittaker Lonza (Basel, Switzerland). The CRISPR-Cas9 plasmid [pSpCas9(BB)-2A–green fluorescent protein (GFP); PX458] was obtained from Addgene (Watertown, MA, USA). HEK-293 cells were obtained from American Type Culture Collection (Manassas, VA, USA). The acylcarnitine internal standard mix containing 2H9-carnitine, 2H3-acetylcarnitine (C2), 2H3-propionylcarnitine (C3), 2H3-butyrylcarnitine (C4), 2H9-isovalerylcarnitine (C5), 2H3-octanoylcarnitine (C8), 2H9-myristoylcarnitine (C14), and 2H3-palmitoylcarnitine (C16) was from Cambridge Isotope Laboratories (Tewksbury, MA, USA). Bovine serum albumin (fatty acid free), l-carnitine, lauric acid, palmitic acid, lauroylcarnitine, and palmitoylcarnitine were obtained from MilliporeSigma. l-Aminocarnitine [l-AC; (R)-aminocarnitine, minimum 97%] was obtained from Toronto Research Chemical Inc. (Toronto, ON, Canada). (R)-(+)-Etomoxir sodium salt was from Tocris Bioscience (Bristol, United Kingdom). All other chemicals were of analytical grade.
Cell culture conditions
HEK-293 cells were cultured in DMEM with 4.5 g/L glucose, 584 mg/L l-glutamine, and 25 mM Hepes supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified atmosphere of 5% CO2 at 37°C.
Generation of CRISPR-Cas9 KO cell lines
The generation of gene KO cell lines using the CRISPR-Cas9 genome editing technique was performed essentially as previously described (14), with minor modifications. For each gene, 3 different guides were chosen and cloned into the pSpCas9(BB)-2A–GFP vector. After plasmid purification, HEK-293 cells were transfected using Lipofectamine 2000. Forty-eight hours after transfection, the transfected cells with GFP signal were sorted as single cells into 96-well plates by fluorescence-activated cell sorting. The clonal cells were cultured for ∼2 wk and collected for genomic DNA extraction.
We selected 2 independent clonal KO cell lines for PEX13, EHHADH, HSD17B4, carnitine palmitoyltransferase (CPT)1A, CPT2/PEX13, CPT2/ABCD3, and CPT2/CPT1A. Only 1 clonal KO cell line was obtained for ABCD3 and CPT2. To simplify the mutation analysis, we selected clones with homozygous or compound heterozygous nonsense mutations. HEK-293 cells are near triploid, with 62–70 chromosomes per cell (15, 16). This implies that cell lines with an apparent homozygous mutation harbored identical triallelic mutations. In apparent compound heterozygous cell lines, one of the mutations must be biallelic. Mutation analysis was performed by direct Sanger sequencing of the genomic DNA. Primers were chosen to flank the region surrounding the respective guides for each gene (PCR products between 250 and 500 bp). A list of the established cell lines and their mutations is provided in Supplemental Table S1. All mutations were predicted to be disease causing (i.e., probably deleterious by MutationTaster) (17). In addition, functional assays were performed to validate all the generated KO cell lines except the EHHADH KO cell lines. This was due to undetectable protein levels by immunoblotting (GeneTex GTX81126). The PEX13 KO cell lines were validated using fluorescence microscopy (Supplemental Fig. S1B). The HSD17B4 and ABCD3 KO cell lines were validated using immunoblot (Supplemental Fig. S1C, D). The ABCD3 KO cell lines were validated also by rescuing the phenotype by reintroducing ABCD3. The CPT2 and CPT2/CPT1A KO cell lines were validated by their aberrant acylcarnitine production.
Visualization of peroxisomes using fluorescence microscopy
Fluorescence microscopy was performed essentially as previously described. Briefly, HEK-293 cell lines were transfected with a plasmid encoding an enhanced GFP-SKL (18, 19). The SKL is the peroxisomal targeting sequence (i.e., PTS1) that will direct the GFP to the peroxisome, enabling its visualization by fluorescence microscopy. PEX13 KO cells had no detectable peroxisomes (Supplemental Fig. S1B).
Immunoblot analysis
The HSD17B4 and ABCD3 KO cell lines were validated by immunoblotting. We used a mouse monoclonal antibody against HSD17B4 at 1:1000 (ab128565; Abcam, Cambridge, MA, USA) and a rabbit polyclonal against PMP70 at 1:500 (ABCD3, PA1-650; Thermo Fisher Scientific). The selected HSD17B4 KO cell lines had undetectable protein levels (Supplemental Fig. S1C). The ABCD3 single-KO and CPT2/ABCD3 double-KO cell lines had undetectable ABCD3 protein levels (Supplemental Fig. S1D).
Analysis of fatty acid and acylcarnitine metabolism in HEK-293 cells
The acylcarnitine profiling in HEK-293 cells was performed essentially as previously described (11, 20) with minor modifications. HEK-293 cells were seeded in 24-well plates (∼100 µg protein per well) and incubated overnight at 37°C. The following day, the media was removed, and 500 µl of the incubation mixture was added to each well. Incubation mixtures contained minimal essential medium supplemented with 0.4% bovine serum albumin (fatty acid free), 0.4 mM l-carnitine and 120 µM lauric acid (C12:0), 120 µM palmitic acid (C16:0), 25 µM lauroylcarnitine (C12-carnitine), or 25 µM palmitoylcarnitine (C16-carnitine). For the inhibition studies, etomoxir and l-AC were added to the incubation mixture at final concentrations of 10 and 0–800 µM, respectively. After incubation for 72 h in a humidified CO2 incubator (5% CO2, 95% air) at 37°C, the medium was collected, and the cells were washed and resuspended in 100 µl of RIPA buffer to measure protein content using the bicinchoninic acid assay and human serum albumin as standard.
To 20 µl of medium, 100 µl of internal standard mix in methanol was added containing 3.8 µM 2H9-carnitine, 0.95 µM 2H3-C2–carnitine, 0.19 µM 2H3-C3–carnitine, 2H3-C4–carnitine, 2H9-C5–carnitine, 2H3-C8–carnitine, 2H9-C14–carnitine, and 0.38 µM 2H3-C16-carnitine. After derivatization of the produced acylcarnitines with 3 N 1-butanol/HCl (Regis Technologies, Morton Grove, IL, USA), these intermediates were quantified by electrospray ionization tandem mass spectrometry (6460 Triple Quad MS; Agilent Technologies, Santa Clara, CA, USA) using an established procedure for clinical testing.
Oxidation of [1-14C] C16:0 and [1-14C] C16-carnitine was measured as previously described (21).
ABCD3 overexpression
CPT2 KO and CPT2/ABCD3 KO cell lines were transfected with a plasmid containing the human ABCD3 transporter or the empty vector (pcDNA3) (22). Transfection was performed with Lipofectamine 2000 following the supplier’s instructions. After incubation for 24 h, the cells were loaded with C12:0 or C12-carnitine. The medium was analyzed after 72 h incubation as previously described.
Animals
All animal experiments were approved either by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai and comply with the Guide for the Care and use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) or by the Research Ethical Committee of the KU Leuven (181/2015). Ehhadh KO mice (B6; 129P2-Ehhadhtm1Jkr) (23) were generously provided by Dr. Janardan K. Reddy (Feinberg School of Medicine, Northwestern University, Chicago, IL, USA). These mice were crossed with C57BL/6N to create a first-generation progeny (B6129PF1). Ehhadh+/− F1 mice were intercrossed to generate an experimental cohort of B6129PF2 mice. From this F2 cohort, male wild-type (WT) and Ehhadh KO mice were selected. Mice were kept on regular chow (PicoLab Rodent Diet 20; LabDiet, St. Louis, MO, USA) and analyzed between 6 and 8 mo of age. Hsd17b4 KO (also called Mfp2−/−) mice were obtained by intercrossing heterozygous mice on a Swiss/Webster background as previously described (24). All animals received an intraperitoneal injection of vehicle (0.9% NaCl), l-AC (16 mg/kg), or etomoxir (50 mg/kg) at the end of the afternoon followed by overnight food withdrawal. Mice were euthanized by exsanguination via the vena cava inferior after pentobarbital anesthesia (100 mg/kg, i.p.). Blood was collected for the preparation of EDTA plasma, and organs were snap frozen in liquid nitrogen and stored at −80°C for future analyses. Plasma was used for acylcarnitine analysis as previously described. Blood was also collected from the submandibular vein after 8 h of having food withheld or in the random fed state with similar results. Ultraperformance liquid chromatography tandem mass spectrometry analysis of acylcarnitines was performed on pooled plasma from all animals within the groups (25, 26). Hsd17b4 KO mice and controls received an injection of l-AC (16 mg/kg, i.p.). Blood for plasma isolation was collected from the tail vein after 8 h of having food withheld. Two groups of mice were analyzed: a group 4 wk of age (n = 4 WT, n = 4 KO) and a group 16–17 wk of age (n = 4 WT, n = 4 KO).
RESULTS
Peroxisomal β-oxidation of lauric acid in HEK-293 cells
HEK-293 cells are easily genetically modified by CRISPR-Cas9 genome editing (14). Here we evaluated whether HEK-293 can be used to measure the transfer of mitochondrial substrates to the peroxisome, as suggested in our previous work in primary skin fibroblasts (11). To this end, we generated HEK-293 KO cells for PEX13, a crucial gene in peroxisomal biogenesis (27). PEX13 KO cells had no detectable peroxisomes as demonstrated by the cytosolic localization of GFP-SKL, whereas control cells showed the typical peroxisomal punctuated fluorescence pattern (Supplemental Fig. S1B).
Long-chain fatty acids [e.g., palmitic acid (C16:0)] and the medium-chain fatty acid lauric acid (C12:0) require the action of the carnitine shuttle before they can be oxidized in the mitochondrial matrix (28) (Fig. 1A). We have shown that, upon inhibition of CPT2 with l-AC, fatty acids can be rerouted to the peroxisome for alternative FAO, resulting in the production of medium- and short-chain acylcarnitines that accumulate in the extracellular media (11). l-AC reveals the production of peroxisomal acylcarnitines not only due to its general inhibition of mitochondrial FAO (29) but also because it prevents peroxisomal acylcarnitines from entering the mitochondria for full oxidation (11).
To measure peroxisomal acylcarnitine production, control and PEX13 KO HEK-293 cells were incubated with C12:0 and increasing concentrations of l-AC. Media of control HEK-293 cells incubated with l-AC and C12:0 showed accumulation of C12-, C10-, C8-, and C6-carnitine. Media of the PEX13 KO cells failed to accumulate C10-, C8-, and C6-carnitine, proving their peroxisomal origin (Fig. 1B and Supplemental Fig. S1E). Thus, HEK-293 cells are capable of oxidizing C12:0 in the peroxisome. For the remainder of the paper, we focus on C10-carnitine as the main marker for peroxisomal fatty acid oxidation.
HSD17B4 and ABCD3 are essential for oxidation of lauric acid
Peroxisomal FAO follows a mechanism that involves dehydrogenation, hydration, a second dehydrogenation, and thiolytic cleavage, similar to mitochondrial FAO (4). The second and third step are catalyzed by the D- and L-bifunctional protein encoded by HSD17B4 and EHHADH, respectively (Fig. 1A). Both genes are expressed in HEK-293 cells, although EHHADH is expressed at a relatively low level (Supplemental Fig. S1A). We used CRISPR-Cas9 genome editing to generate KO cell lines for both genes. Next, we loaded the cells with C12:0 and inhibited the carnitine shuttle with l-AC to monitor the peroxisomal acylcarnitine production. Although EHHADH KO cells produced levels of C10-carnitine similar to controls, this metabolite was severely reduced in HSD17B4 KO cells (Fig. 1B). This indicates that HSD17B4 is important for the peroxisomal oxidation of medium-chain fatty acids, whereas EHHADH is either not involved or is not essential in HEK-293 cells.
Next, we focused on the peroxisomal import of fatty acids. This process is mediated by 3 ATP-binding cassette transporters (ABCD1, -2, and -3). Based on the reported expression levels of these ABC transporters in HEK-293 cells (Supplemental Fig. S1A) and the established substrate specificities (30), we speculated that ABCD3 would transport C12:0 to the peroxisome. Therefore, we targeted this gene using CRISPR-Cas9 genome editing generating ABCD3 KO cell lines. Upon loading with C12:0 and l-AC, ABCD3 KO cells failed to accumulate C10-carnitine (Fig. 1B), suggesting that transport mediated by ABCD3 is crucial in the peroxisomal degradation of medium-chain fatty acids.
CPT2 KO cells further implicate ABCD3 in peroxisomal lauric acid metabolism
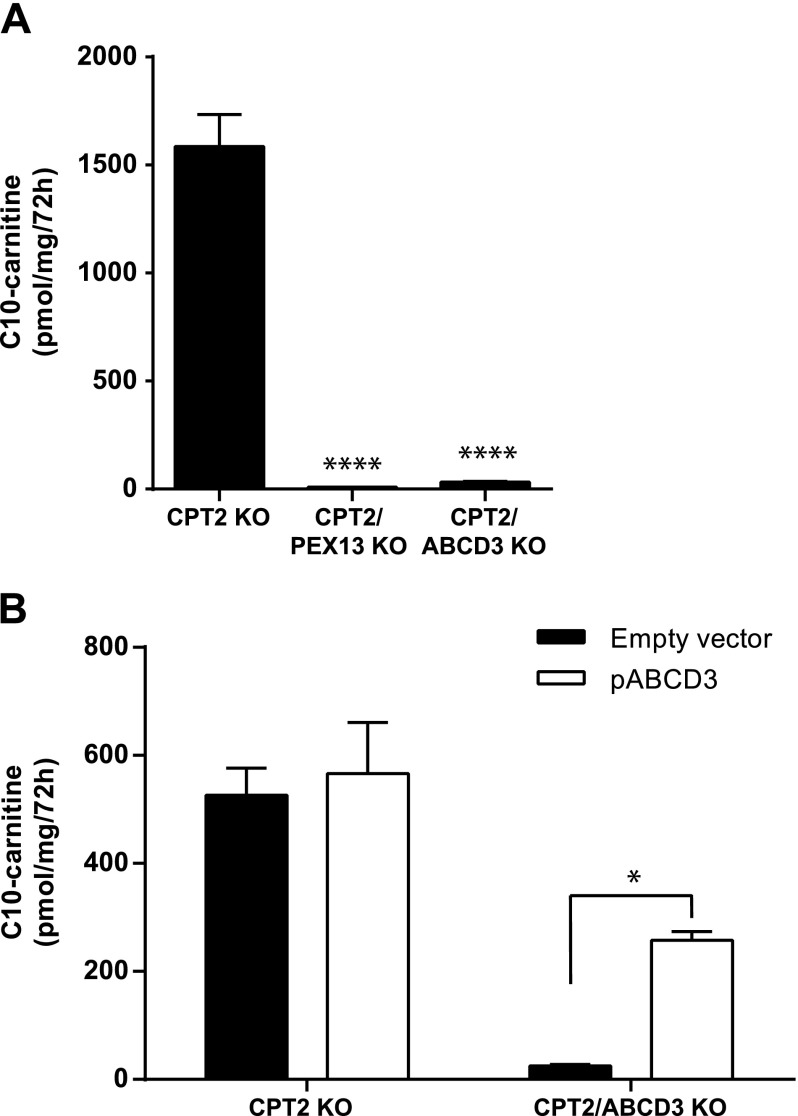
To avoid potential problems of the pharmacological inhibition of mitochondrial FAO using l-AC, we next generated CPT2 KO cell lines using CRISPR-Cas9 genome editing in control HEK-293 and the existing PEX13 and ABCD3 KO cell lines. Incubation of CPT2 KO cells with C12:0 led to a more pronounced accumulation of C10-carnitine when compared with the condition in which CPT2 was inhibited by l-AC (Figs. 1B and 2A). This difference may be due to a lack of specificity of l-AC for CPT2 at higher concentrations or the competitive nature of the inhibition (29). We note again that there is also peroxisomal production of C8- and C6-carnitine (Supplemental Fig. S1F). The production of C10-carnitine is absent in CPT2/PEX13 and CPT2/ABCD3 double-KO cells, confirming that ABCD3 is involved in the peroxisomal generation of this intermediate (Fig. 2A).
Figure 2.
ABCD3 is essential for peroxisomal FAO of lauric acid. A) Production of C10-carnitine in the extracellular medium of CPT2, CPT2/PEX13, and CPT2/ABCD3 KO cell lines after incubation with C12:0. Statistical significance was tested using 1-way ANOVA with Dunnett’s multiple comparisons test. ****P < 0.0001. B) Production of C10-carnitine in the extracellular medium of CPT2 and CPT2/ABCD3 KO cell lines transfected for 24 h with an empty plasmid or a plasmid containing the human transporter ABCD3 (pABCD3) after incubation with C12:0. Statistical significance was tested using an ordinary 2-way ANOVA with Bonferroni’s multiple comparisons test. Data are the mean ± sem of duplicates of 2 independent experiments. All experiments were carried out with 2 clonal cell lines. *P < 0.05.
To further establish the role of ABCD3 in this pathway, we overexpressed ABCD3 in the CPT2 KO and CPT2/ABCD3 KO cell lines and loaded these cells with C12:0. Overexpression of ABCD3 restored the production of C10-carnitine in CPT2/ABCD3 KO cells (Fig. 2B). These data unequivocally establish that ABCD3 is required for transport of medium-chain fatty acids across the peroxisomal membrane.
Peroxisomal β-oxidation of palmitic acid in HEK-293 cells
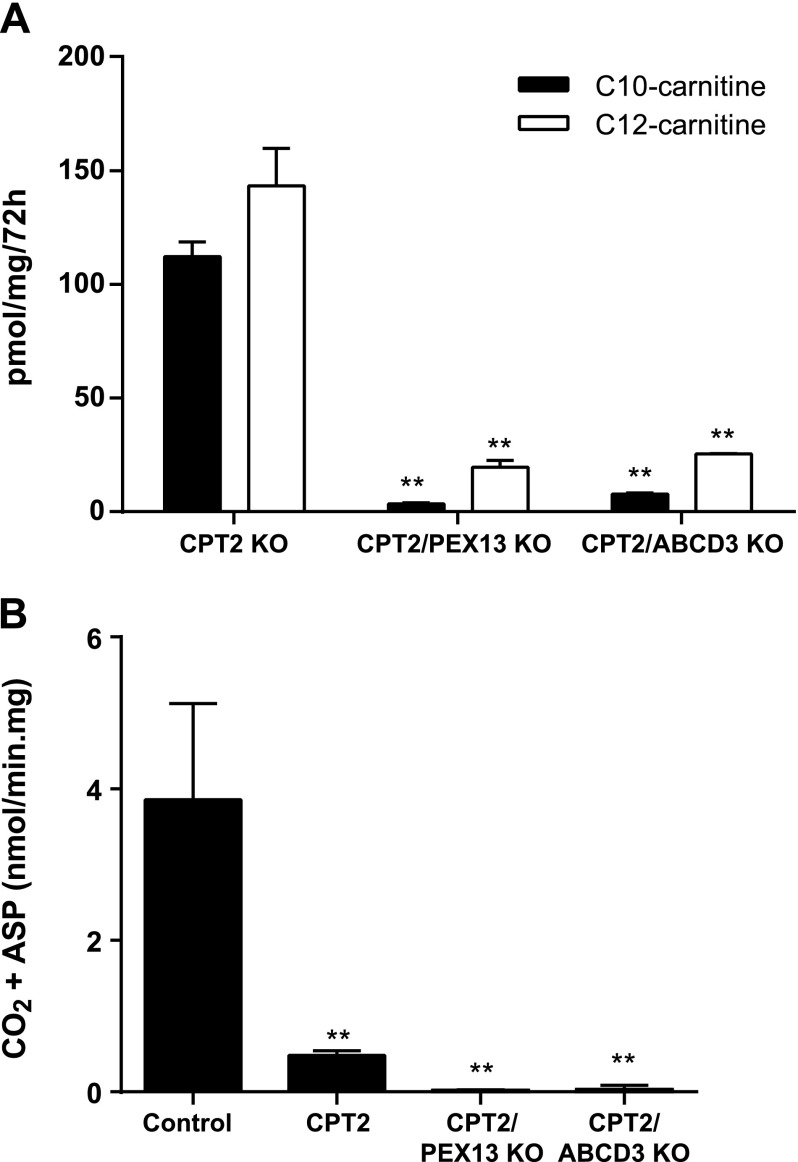
We next investigated peroxisomal metabolism of C16:0, which is a common fatty acid in most diets. Upon loading with C16:0, we were unable to detect C10- and C12-carnitine after inhibition of CPT2 with l-AC, but these metabolites were detectable in CPT2 KO cells. The levels of C10- and C12-carnitine were lower in media of CPT2/PEX13 and CPT2/ABCD3 KO cells when compared with CPT2 KO cells, evidencing their peroxisomal origin (Fig. 3A and Supplemental Fig. S1G). These data indicate that C16:0 can also be metabolized in peroxisomes when mitochondrial FAO is defective.
Figure 3.
Peroxisomal FAO of palmitic acid in HEK-293 cells. A) Production of C10- and C12-carnitine in the extracellular medium of CPT2, CPT2/PEX13, and CPT2/ABCD3 KO cell lines after incubation with palmitate (C16:0). Statistical significance was tested using 2-way ANOVA with Bonferroni’s multiple comparisons test. Data are the mean ± sem of duplicates of 2 independent experiments. All experiments were carried out with 2 clonal cell lines. **P < 0.01. B) Production of CO2 and acid-soluble products (ASP) from [1-14C] C16:0 in CPT2, CPT2/PEX13, and CPT2/ABCD3 KO cell lines. Statistical significance was tested using 1-way ANOVA with Dunnett’s multiple comparisons test. Data are means ± sd. We used 3 control, 1 CPT2, 1 CPT2/PEX13, and 2 CPT2/ABCD3 independently generated KO cell lines. Each cell line was analyzed in triplicate. **P < 0.01.
To provide additional evidence for the peroxisomal oxidation of long-chain fatty acids, we measured production of CO2 and acid-soluble products from [1-14C] C16:0. C16:0 oxidation in CPT2 KO cells was 13% of control HEK-293 cells but was clearly detectable (Fig. 3B). C16:0 oxidation decreased to 1% in CPT2/PEX13 and CPT2/ABCD3 KO (Fig. 3B). This further proves that peroxisomes can contribute significantly to long-chain FAO.
Peroxisomes can accept acylcarnitines as substrate
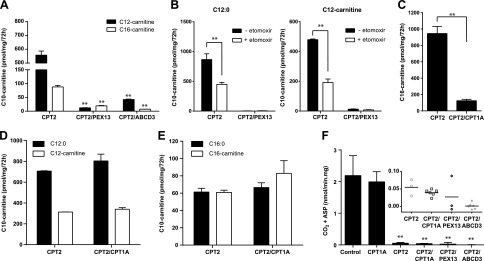
After identifying some of the molecular players in this pathway, we aimed to further characterize the mechanism by which these fatty acids are transported into the peroxisome. The peroxisomal ABC transporters are thought to transport acyl-CoA intermediates. The acyl-CoA is hydrolyzed by intrinsic thioesterase activity of the transporter during transit, leaving a free fatty acid in the peroxisome (31). Our previous work using a combination of CPT1 and CPT2 inhibitors suggested that C12:0 has better access to the peroxisome as C12-carnitine than as the acyl-CoA derivative (11). Consistently, we now show that C12- and C16-carnitine can undergo peroxisomal FAO when CPT2 is blocked in a process that depends on ABCD3 (Fig. 4A). To further investigate whether peroxisomes can accept acylcarnitines as substrate, we inhibited CPT1 using the irreversible inhibitor etomoxir, preventing the reconversion of the acylcarnitine into the respective CoA ester (Fig. 1A). The CPT2 KO cell lines incubated with etomoxir and C12-carnitine still showed production of peroxisomal C10-carnitine (Fig. 4B), which suggests that C12-carnitine has direct access to the peroxisome.
Figure 4.
Peroxisomes can accept acylcarnitines as substrate. A) Production of C10-carnitine after incubation of CPT2, CPT2/PEX13, and CPT2/ABCD3 KO cells with lauroylcarnitine (C12-carnitine) and palmitoylcarnitine (C16-carnitine). B) Production of C10-carnitine after incubation of CPT2 and CPT2/PEX13 KO cells with C12:0 and C12-carnitine, with and without etomoxir inhibition. C) Production of C16-carnitine after incubation of CPT2 and CPT2/CPT1A KO cell lines with C16:0. D) Production of C10-carnitine after incubation of CPT2 and CPT2/CPT1A KO cell lines with C12:0 or C12-carnitine. E) Production of C10-carnitine after incubation of CPT2 and CPT2/CPT1A KO cell lines with C16:0 or C16-carnitine. Statistical significance was tested using 2-way ANOVA with Bonferroni’s multiple comparisons test. Data are means ± sem of duplicates of 2 independent experiments. All experiments were carried out with 2 clonal cell lines. **P < 0.01. F) Production of CO2– and acid-soluble products (ASP) from [1-14C] C16-carnitine in CPT1A, CPT2, CPT2/CPT1A, CPT2/PEX13, and CPT2/ABCD3 KO cell lines. Statistical significance was tested using 1-way ANOVA with Dunnett’s multiple comparisons test. Data are means ± sd. We used 3 control, 2 CPT1A, 1 CPT2, 2 CPT2/CPT1A, 1 CPT2/PEX13, and 2 CPT2/ABCD3 independently generated KO cell lines. Each cell line was analyzed in triplicate. The inset shows the individual data points for the indicated cell lines. **P < 0.01.
To overcome potential unintended effects of pharmacologic inhibition using etomoxir, we removed the activity of both acyltransferases by generating CPT2/CPT1A double-KO cell lines. The successful targeting of CPT1A is confirmed by a pronounced defect in C16-carnitine production after incubation of the double-KO cells with C16:0 (Fig. 4C). We incubated CPT2 KO and CPT2/CPT1A KO cells with C12:0, C12-carnitine, C16:0, or C16-carnitine (Fig. 4D for C12 and Fig. 4E for C16). The production of the peroxisomal C10-carnitine from all these substrates was not affected by the additional loss of CPT1A (Fig. 4D, E). This strongly suggests that C12 and C16 not only have direct access to the peroxisome as acyl-CoAs but also as acylcarnitines. The accumulation of C10-carnitine after incubation with C12-carnitine is 50% in comparison with C12:0 incubation, indicating that the transport of C12-carnitine esters may be less efficient than that of C12-CoA, or that the peroxisomal conversion of acylcarnitines into the respective acyl-CoAs (likely via carnitine octanoyltransferase) may be a rate-limiting step (Fig. 4D).
Overall, our data suggest that peroxisomes accept the CoA and carnitine ester of C12:0 and C16:0 as substrate in a mechanism possibly involving ABCD3. We next measured production of CO2 and acid-soluble products from [1-14C] C16-carnitine. Oxidation of C16-carnitine was not impaired in CPT1A KO HEK-293 cells, which illustrates that extracellular C16-carnitine is effectively used for mitochondrial oxidation and that its (enzymatic) hydrolysis is negligible. Oxidation of C16-carnitine in CPT2 and CPT2/CPT1A KO was detectable, but in only 2% of control HEK-293 cells. Oxidation of C16-carnitine in CPT2/PEX13 and CPT2/ABCD3 was virtually undetectable (Fig. 4F). Overall, our results indicate that peroxisomes have the ability to accept acylcarnitines as substrates.
Peroxisomal acylcarnitines are detectable in plasma of mice after pharmacological inhibition of FAO
Multiple case reports of CPT2-deficient patients describe that, in addition to the prominent increase in plasma C16- and C18:1-carnitine, there are elevations of C12- and C14-acylcarnitines (32–37). Our work suggests that these metabolites have a peroxisomal origin.
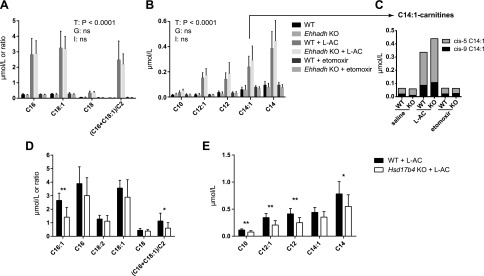
To study peroxisomal FAO of long-chain fatty acids in a mitochondrial FAO defect in vivo, we administered l-AC and etomoxir to WT mice followed by overnight food withdrawal. As expected, CPT2 inhibition resulted in pronounced accumulation of long-chain acylcarnitine species in the plasma of these animals, specifically C16-, C18:1-, and C18-carnitine. Treated animals also present a significantly higher ratio (C16+C18:1)/C2, which is commonly used for the clinical diagnosis of CPT2 deficiency (Fig. 5A). We also observed an increased concentration of several other acylcarnitines in the treated animals, including C10:1-, C10-, C12:1-, C12-, C14:1-, and C14-carnitine (Fig. 5B). Additional quantification of the C14:1-carnitines using ultraperformance liquid chromatography tandem mass spectrometry analysis demonstrated that the majority was cis-5–tetradecenoylcarnitine (Fig. 5C). In contrast to cis-9–tetradecenoate (myristoleic acid), cis-5–tetradecenoate is not a dietary fatty acid but is derived from oleate oxidation (38). Mitochondrial FAO inhibition with etomoxir did not lead to significant accumulation of any acylcarnitine species (Fig. 5A, B). Analysis of liver acylcarnitines yielded results similar to plasma (data not shown). We conclude that, given the inhibition of mitochondrial FAO at the level of CPT2, the in vivo accumulation of these nondietary C10-, C12-, and C14-acylcarnitines in plasma is likely mediated by peroxisomal oxidation.
Figure 5.
Peroxisomal acylcarnitines in plasma of WT, Ehhadh KO, and Hsd17b4 KO mice treated with l-AC. A) Concentrations of the C16-, C18:1-, and C18-carnitines and the ratio (C16+C18:1)/C2. B) Concentrations of the C10-, C12-, and C14-carnitines. C) Proportion of cis-5-tetradecenoylcarnitine and myristoleoylcarnitine (cis-9) of the C14:1-carnitine. Plasma acylcarnitine concentrations were measured in WT and Ehhadh KO mice held without food overnight and treated with saline (n = 7 WT, n = 5 KO), l-AC (n = 6 WT and 6 KO), or etomoxir (n = 6 WT, n = 6 KO). Data are means ± sd. A 2-way ANOVA was performed, and the results are displayed in a table for each graph. G, genotype effect; I, interaction term; ns, not significant; T, treatment effect. D) Concentrations of the C16:1-, C16-, C18:2-, C18:1-, and C18-carnitines and the ratio (C16+C18:1)/C2. E) Concentrations of the C10-, C12-, and C14-carnitines. Plasma acylcarnitine concentrations were measured in WT and Hsd17b4 KO mice held without food for 8 h and treated with l-AC (n = 8 WT, n = 8 KO). Data are means ± sd. The significance was determined using a Mann-Whitney U test. *P < 0.05, **P < 0.01.
Similar to our in vitro experiments, we next investigated whether D-bifunctional (HSD17B4) or L-bifunctional (EHHAHD) protein is involved in the production of peroxisomal acylcarnitines in vivo upon CPT2 inhibition. For this, we administered l-AC to Hsd17b4 KO, Ehhadh KO, and WT mice. The concentrations of C10-, C12:1-, C12-, C14-, and C16:1-carnitine were significantly lower in Hsd17b4 KO mice compared with the controls. The concentrations of C16-, C18:2-, C18:1-, and C18-carnitine were not different between Hsd17b4 KO and WT mice (Fig. 5D, E). The results in the Ehhadh KO mice were not different compared with their WT counterparts (Fig. 5A, B). Taken together, these animal studies indicate that CPT2 inhibition leads to the production of peroxisomal acylcarnitines. HSD17B4 appears to be the major peroxisomal bifunctional enzyme involved in the generation of these intermediates.
DISCUSSION
The potential role of peroxisomal FAO as an alternative pathway when mitochondrial FAO is impaired or overloaded has remained largely unexplored. We have previously shown that when mitochondrial FAO is defective in fibroblasts, peroxisomes can take up and oxidize C12:0, a fatty acid that is normally metabolized within the mitochondria (11). To define the molecular players of this process, we used CRISPR-Cas9 genome editing to perform a classic reverse genetics study in which we disrupted genes of interest in mitochondrial or peroxisomal FAO and observed the biochemical phenotype by measuring the production of the peroxisomal acylcarnitines, such as C10-carnitine. We aimed to identify the enzymes and transporters involved in the peroxisomal FAO of medium- and long-chain fatty acids when mitochondrial FAO is defective. We started by investigating the role of l- and d-bifunctional proteins (encoded by EHHADH and HDS17B4, respectively) that catalyze the second and third step in peroxisomal FAO. EHHADH was an attractive candidate gene because it is highly inducible and has an established role in the oxidation of long- and medium-chain DCAs (9, 10). However, upon inhibition of mitochondrial FAO and loading with C12:0, EHHADH KO cell lines produced C10-carnitine levels similar to control cell lines. This suggests that this enzyme is not involved, or at least it is not essential, in the peroxisomal degradation of medium-chain fatty acids in HEK-293 cells. In contrast, HSD17B4 KO cells incubated with C12:0 showed a marked decrease in the production of C10-carnitine, which indicates that this enzyme is involved in the peroxisomal FAO of C12:0. In contrast to PEX13 KO cell lines, the HSD17B4 KO produced small amounts of C10-carnitine (Fig. 1B). Given the predicted severity of the HSD17B4 mutations and the resulting absence of the protein, it is unlikely that this is due to residual HSD17B4 activity. Therefore, we speculate that, despite its low expression in HEK-293 cells, EHHADH can partially compensate for the loss of HSD17B4. We obtained similar results in animal studies in which we administered l-AC to WT, Ehhadh KO, and Hsd17b4 KO mice. The concentration of C10-, C12:1-, and C12-carnitine increased upon l-AC administration. We observed no differences in the plasma concentration of these acylcarnitines between WT and Ehhadh KO animals. These metabolites were significantly lower in Hsd17b4 KO mice but were not absent. This proves their peroxisomal origin but also indicates that HSD17B4 is not the only source, with EHHADH being the most likely alternative source. The identity of the enzymes that catalyze the first and last step in peroxisomal FAO will be addressed in future studies.
We also studied the mechanism by which mitochondrial FAO substrates cross the peroxisomal membrane. The peroxisomal transporter ABCD3 is well expressed in HEK-293 cells and is known to transport a variety of fatty acids, including C16:0 (30). ABCD3 KO cell lines failed to produce C10-carnitine from both C12:0 and C16:0. This proves that ABCD3 is crucial in the transport of mitochondrial substrates into peroxisomes in HEK-293 cells. ABCD1 and ABCD2 have been demonstrated to transport C16:0 as well (30). ABCD1 is minimally expressed in HEK-293 cells, whereas expression of ABCD2 expression is absent, which likely explains their negligible contribution to the peroxisomal oxidation of C16:0 and C12:0 in the HEK-293 cell system. This expression profile of the peroxisomal ABC transporters in HEK-293 cells mirrors the expression profile in major fatty acid–catabolizing organs, such as liver and kidney (39). We therefore argue that ABCD3 likely plays a major role in the transport of mitochondrial substrates into peroxisomes in physiologically relevant tissues. However, we cannot exclude the possibility that ABCD1 and ABCD2 can contribute to this process in tissues with significant expression of these transporters.
We have shown here that specific metabolites accumulating in mitochondrial FAO defects can be directed to the peroxisome for alternative FAO. The peroxisomal ABC transporters are known to accept acyl-CoAs as substrate. There are currently 2 models for acyl-CoA transport, 1 where acyl-CoAs enter the peroxisome directly (40) and another where the acyl-CoA is hydrolyzed during transport and re-esterified in the peroxisomal matrix (31). The ABC transporters contain intrinsic thioesterase activity, which allows cleavage of the CoA moiety (41). This second model of transport is currently favored. Our data suggest that acylcarnitines might be an alternative substrate for ABCD3. To investigate this, we used the CPT2 KO cell model and disrupted CPT1A activity either pharmacologically or by using CRISPR-Cas9. The impairment of CPT1A prevents the conversion of acyl-CoAs into acylcarnitines but also prevents the reverse activity in which the acylcarnitine is converted into an acyl-CoA. We show that in the CPT2/CPT1A double-KO cell lines, C12- and C16-carnitine are oxidized in the peroxisome, which is evidenced by the production of peroxisomal acylcarnitines and a low but detectable oxidation of [1-14C] C16-carnitine. This indicates that, in addition to acyl-CoAs, acylcarnitines are a possible substrate for peroxisomes. This finding may not be completely unexpected given the strong evidence that peroxisomes are able to produce acylcarnitines (42–48). One potential pitfall of our experiment may be the presence of carboxylesterases. These enzymes can catalyze the hydrolysis of many endogenous compounds, including acylcarnitines (49, 50). Thus, in a CPT2/CPT1A double-KO cell line, a carboxylesterase may hydrolyze the acylcarnitine, and the resulting fatty acid could then be reactivated to an acyl-CoA. Carboxylesterases, however, are mainly localized in the endoplasmic reticulum, and such a mechanism seems therefore unlikely. Indeed, our results show that extracellular C16-carnitine is an effective substrate for mitochondrial FAO, and thus hydrolysis of the carnitine ester appears negligible. Definite proof that acylcarnitines can be directly transported into peroxisomes by ABCD3 or other transporters will require reconstitution of these proteins in liposomes and subsequent transport studies. Unfortunately, such studies have proven challenging and have not been reported.
It has been speculated that acylcarnitines cross the peroxisomal membrane through unspecific membrane channels (such as PXMP2), which are permeable to compounds up to 400 Da (51). We show that medium- and long-chain acylcarnitines depend on the ABCD3 transporter to reach the peroxisomal matrix for FAO, arguing against a model in which these metabolites simply diffuse into the peroxisome.
The incubation of CPT2 KO cells with C16:0 led to the production of peroxisomal C10- and C12-carnitine intermediates, illustrating that peroxisomes can handle these abundant dietary fatty acids. Analysis of plasma acylcarnitine profiles in patients diagnosed with CPT2 deficiency has also revealed an accumulation of medium-chain acylcarnitines (32–37). Using l-AC in mice, we reproduced a similar acylcarnitine profile with a pronounced increase in C10, C12, and C14 saturated and unsaturated species. Fatty acids with these chain lengths are not part of a regular mouse chow or human diet. Therefore, similar to the cell-based experiments, these acylcarnitines must be derived from peroxisomal degradation of the long-chain fatty acids. Indeed, the concentrations of C10-, C12:1-, and C12-carnitine were lower in Hsd17b4 KO mice after administration of l-AC. These results establish that peroxisomal FAO can be an alternative in vivo pathway when mitochondrial FAO is defective. Of note, inhibition of mitochondrial FAO using etomoxir in mice did not lead to a detectable production of peroxisomal acylcarnitine species. We speculate that, in etomoxir-treated animals, the accumulating long-chain acyl-CoA esters will be partially oxidized in the peroxisome. However, because the mitochondrial CPT2 is functional, the resulting peroxisomal medium-chain acylcarnitines can be transferred to the mitochondria and fully oxidized, explaining their absence in the plasma acylcarnitine profile.
In summary, we have demonstrated, in vitro and in vivo, that peroxisomes accept and oxidize medium- and long-chain fatty acids in a pathway that involves HSD17B4 and ABCD3. Our work suggests that peroxisomal FAO is a relevant pathway for alternative metabolism in mitochondrial long-chain FAO deficiencies such as CPT2, very long-chain acyl-CoA dehydrogenase, and long-chain hydroxyacyl-CoA dehydrogenase deficiency. Future studies should consider this pathway in the pathophysiology and treatment of long-chain FAO deficiencies.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Janet Koster (Academic Medical Center) for providing the ABCD3 plasmid, Ethellyn Panta and Tara Singh (Clinical Biochemical Genetics, Icahn School of Medicine at Mount Sinai) for assistance with the acylcarnitine measurements, Charles L. Hoppel (Case Western Reserve University, Cleveland, OH, USA) for quantification of the C14:1-carnitines, and Benny Das (University of Leuven) for technical assistance. Research reported in this publication was supported by U.S. Natiional Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Award R01DK113172. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- Cas9
clustered regularly interspaced short palindromic repeats associated protein 9
- CPT
carnitine palmitoyltransferase
- CRISPR
clustered regularly-interspaced short palindromic repeats
- DCA
dicarboxylic acid
- EHHADH
enoyl-CoA hydratase/3-hydroxy acyl-CoA dehydrogenase
- FAO
fatty acid β-oxidation
- GFP
green fluorescent protein
- KO
knockout
- l-AC
l-aminocarnitine
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Violante, C. A. Argmann, and S. M. Houten designed the research; S. Violante, N. Achetib, C. W. T. van Roermund, J. Hagen, and T. Dodatko performed the research; F. M. Vaz, H. R. Waterham, H. Chen, M. Baes, and Chunli Yu contributed new reagents or analytic tools; S. Violante and S. M. Houten analyzed the data; and S. Violante and S. M. Houten wrote the manuscript.
REFERENCES
- 1.Houten S. M., Violante S., Ventura F. V., Wanders R. J. (2016) The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu. Rev. Physiol. 78, 23–44 [DOI] [PubMed] [Google Scholar]
- 2.Lazarow P. B. (1978) Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J. Biol. Chem. 253, 1522–1528 [PubMed] [Google Scholar]
- 3.Lazarow P. B. (1977) Three hypolipidemic drugs increase hepatic palmitoyl-coenzyme A oxidation in the rat. Science 197, 580–581 [DOI] [PubMed] [Google Scholar]
- 4.Wanders R. J., Waterham H. R. (2006) Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75, 295–332 [DOI] [PubMed] [Google Scholar]
- 5.Schrader M., Costello J., Godinho L. F., Islinger M. (2015) Peroxisome-mitochondria interplay and disease. J. Inherit. Metab. Dis. 38, 681–702 [DOI] [PubMed] [Google Scholar]
- 6.Reszko A. E., Kasumov T., David F., Jobbins K. A., Thomas K. R., Hoppel C. L., Brunengraber H., Des Rosiers C. (2004) Peroxisomal fatty acid oxidation is a substantial source of the acetyl moiety of malonyl-CoA in rat heart. J. Biol. Chem. 279, 19574–19579 [DOI] [PubMed] [Google Scholar]
- 7.Bian F., Kasumov T., Thomas K. R., Jobbins K. A., David F., Minkler P. E., Hoppel C. L., Brunengraber H. (2005) Peroxisomal and mitochondrial oxidation of fatty acids in the heart, assessed from the 13C labeling of malonyl-CoA and the acetyl moiety of citrate. J. Biol. Chem. 280, 9265–9271 [DOI] [PubMed] [Google Scholar]
- 8.Kasumov T., Adams J. E., Bian F., David F., Thomas K. R., Jobbins K. A., Minkler P. E., Hoppel C. L., Brunengraber H. (2005) Probing peroxisomal beta-oxidation and the labelling of acetyl-CoA proxies with [1-(13C)]octanoate and [3-(13C)]octanoate in the perfused rat liver. Biochem. J. 389, 397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houten S. M., Denis S., Argmann C. A., Jia Y., Ferdinandusse S., Reddy J. K., Wanders R. J. (2012) Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J. Lipid Res. 53, 1296–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding J., Loizides-Mangold U., Rando G., Zoete V., Michielin O., Reddy J. K., Wahli W., Riezman H., Thorens B. (2013) The peroxisomal enzyme L-PBE is required to prevent the dietary toxicity of medium-chain fatty acids. Cell Rep. 5, 248–258 [DOI] [PubMed] [Google Scholar]
- 11.Violante S., Ijlst L., Te Brinke H., Koster J., Tavares de Almeida I., Wanders R. J., Ventura F. V., Houten S. M. (2013) Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. Biochim. Biophys. Acta 1831, 1467–1474 [DOI] [PubMed] [Google Scholar]
- 12.Beadle G. W., Tatum E. L. (1941) Genetic control of biochemical reactions in Neurospora. Proc. Natl. Acad. Sci. USA 27, 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramani S. (1993) Protein import into peroxisomes and biogenesis of the organelle. Annu. Rev. Cell Biol. 9, 445–478 [DOI] [PubMed] [Google Scholar]
- 14.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bylund L., Kytölä S., Lui W. O., Larsson C., Weber G. (2004) Analysis of the cytogenetic stability of the human embryonal kidney cell line 293 by cytogenetic and STR profiling approaches. Cytogenet. Genome Res. 106, 28–32 [DOI] [PubMed] [Google Scholar]
- 16.Lin Y. C., Boone M., Meuris L., Lemmens I., Van Roy N., Soete A., Reumers J., Moisse M., Plaisance S., Drmanac R., Chen J., Speleman F., Lambrechts D., Van de Peer Y., Tavernier J., Callewaert N. (2014) Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 5, 4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz J. M., Cooper D. N., Schuelke M., Seelow D. (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods 11, 361–362 [DOI] [PubMed] [Google Scholar]
- 18.Waterham H. R., Koster J., van Roermund C. W., Mooyer P. A., Wanders R. J., Leonard J. V. (2007) A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 356, 1736–1741 [DOI] [PubMed] [Google Scholar]
- 19.Ebberink M. S., Mooijer P. A., Gootjes J., Koster J., Wanders R. J., Waterham H. R. (2011) Genetic classification and mutational spectrum of more than 600 patients with a Zellweger syndrome spectrum disorder. Hum. Mutat. 32, 59–69 [DOI] [PubMed] [Google Scholar]
- 20.Ventura F. V., Costa C. G., Struys E. A., Ruiter J., Allers P., Ijlst L., Tavares de Almeida I., Duran M., Jakobs C., Wanders R. J. (1999) Quantitative acylcarnitine profiling in fibroblasts using [U-13C] palmitic acid: an improved tool for the diagnosis of fatty acid oxidation defects. Clin. Chim. Acta 281, 1–17 [DOI] [PubMed] [Google Scholar]
- 21.Wanders R. J., Denis S., Ruiter J. P., Schutgens R. B., van Roermund C. W., Jacobs B. S. (1995) Measurement of peroxisomal fatty acid beta-oxidation in cultured human skin fibroblasts. J. Inherit. Metab. Dis. 18 (Suppl 1), 113–124 [DOI] [PubMed] [Google Scholar]
- 22.Ferdinandusse S., Jimenez-Sanchez G., Koster J., Denis S., Van Roermund C. W., Silva-Zolezzi I., Moser A. B., Visser W. F., Gulluoglu M., Durmaz O., Demirkol M., Waterham H. R., Gökcay G., Wanders R. J., Valle D. (2015) A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum. Mol. Genet. 24, 361–370 [DOI] [PubMed] [Google Scholar]
- 23.Qi C., Zhu Y., Pan J., Usuda N., Maeda N., Yeldandi A. V., Rao M. S., Hashimoto T., Reddy J. K. (1999) Absence of spontaneous peroxisome proliferation in enoyl-CoA Hydratase/L-3-hydroxyacyl-CoA dehydrogenase-deficient mouse liver: further support for the role of fatty acyl CoA oxidase in PPARalpha ligand metabolism. J. Biol. Chem. 274, 15775–15780 [DOI] [PubMed] [Google Scholar]
- 24.Baes M., Huyghe S., Carmeliet P., Declercq P. E., Collen D., Mannaerts G. P., Van Veldhoven P. P. (2000) Inactivation of the peroxisomal multifunctional protein-2 in mice impedes the degradation of not only 2-methyl-branched fatty acids and bile acid intermediates but also of very long chain fatty acids. J. Biol. Chem. 275, 16329–16336 [DOI] [PubMed] [Google Scholar]
- 25.Minkler P. E., Stoll M. S., Ingalls S. T., Kerner J., Hoppel C. L. (2015) Validated method for the quantification of free and total carnitine, butyrobetaine, and acylcarnitines in biological samples. Anal. Chem. 87, 8994–9001 [DOI] [PubMed] [Google Scholar]
- 26.Minkler P. E., Stoll M. S., Ingalls S. T., Kerner J., Hoppel C. L. (2015) Quantitative acylcarnitine determination by UHPLC-MS/MS: going beyond tandem MS acylcarnitine “profiles.” Mol. Genet. Metab. 116, 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterham H. R., Ebberink M. S. (2012) Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim. Biophys. Acta 1822, 1430–1441 [DOI] [PubMed] [Google Scholar]
- 28.Violante S., Ijlst L., Te Brinke H., Tavares de Almeida I., Wanders R. J., Ventura F. V., Houten S. M. (2013) Carnitine palmitoyltransferase 2 and carnitine/acylcarnitine translocase are involved in the mitochondrial synthesis and export of acylcarnitines. FASEB J. 27, 2039–2044 [DOI] [PubMed] [Google Scholar]
- 29.Chegary M., Te Brinke H., Doolaard M., Ijlst L., Wijburg F. A., Wanders R. J., Houten S. M. (2008) Characterization of L-aminocarnitine, an inhibitor of fatty acid oxidation. Mol. Genet. Metab. 93, 403–410 [DOI] [PubMed] [Google Scholar]
- 30.Van Roermund C. W., Ijlst L., Wagemans T., Wanders R. J., Waterham H. R. (2014) A role for the human peroxisomal half-transporter ABCD3 in the oxidation of dicarboxylic acids. Biochim. Biophys. Acta 1841, 563–568 [DOI] [PubMed] [Google Scholar]
- 31.Baker A., Carrier D. J., Schaedler T., Waterham H. R., van Roermund C. W., Theodoulou F. L. (2015) Peroxisomal ABC transporters: functions and mechanism. Biochem. Soc. Trans. 43, 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurvitz H., Klar A., Korn-Lubetzki I., Wanders R. J., Elpeleg O. N. (2000) Muscular carnitine palmitoyltransferase II deficiency in infancy. Pediatr. Neurol. 22, 148–150 [DOI] [PubMed] [Google Scholar]
- 33.Fontaine M., Briand G., Largilliere C., Degand P., Divry P., Vianey-Saban C., Mousson B., Vamecq J. (1998) Metabolic studies in a patient with severe carnitine palmitoyltransferase type II deficiency. Clin. Chim. Acta 273, 161–170 [DOI] [PubMed] [Google Scholar]
- 34.Anichini A., Fanin M., Vianey-Saban C., Cassandrini D., Fiorillo C., Bruno C., Angelini C. (2011) Genotype-phenotype correlations in a large series of patients with muscle type CPT II deficiency. Neurol. Res. 33, 24–32 [DOI] [PubMed] [Google Scholar]
- 35.Yamada K., Bo R., Kobayashi H., Hasegawa Y., Ago M., Fukuda S., Yamaguchi S., Taketani T. (2017) A newborn case with carnitine palmitoyltransferase II deficiency initially judged as unaffected by acylcarnitine analysis soon after birth. Mol. Genet. Metab. Rep. 11, 59–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wieser T. (2004) Carnitine palmitoyltransferase II deficiency. In GeneReviews (Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., eds.), University of Washington, Seattle, WA, USA: [PubMed] [Google Scholar]
- 37.Albers S., Marsden D., Quackenbush E., Stark A. R., Levy H. L., Irons M. (2001) Detection of neonatal carnitine palmitoyltransferase II deficiency by expanded newborn screening with tandem mass spectrometry. Pediatrics 107, E103 [DOI] [PubMed] [Google Scholar]
- 38.Chegary M., Brinke H., Ruiter J. P., Wijburg F. A., Stoll M. S., Minkler P. E., van Weeghel M., Schulz H., Hoppel C. L., Wanders R. J., Houten S. M. (2009) Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim. Biophys. Acta 1791, 806–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.GTEx Consortium (2015) Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiesinger C., Kunze M., Regelsberger G., Forss-Petter S., Berger J. (2013) Impaired very long-chain acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J. Biol. Chem. 288, 19269–19279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Marcos Lousa C., van Roermund C. W., Postis V. L., Dietrich D., Kerr I. D., Wanders R. J., Baldwin S. A., Baker A., Theodoulou F. L. (2013) Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. USA 110, 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhoeven N. M., Roe D. S., Kok R. M., Wanders R. J., Jakobs C., Roe C. R. (1998) Phytanic acid and pristanic acid are oxidized by sequential peroxisomal and mitochondrial reactions in cultured fibroblasts. J. Lipid Res. 39, 66–74 [PubMed] [Google Scholar]
- 43.Choi S. J., Oh D. H., Song C. S., Roy A. K., Chatterjee B. (1995) Molecular cloning and sequence analysis of the rat liver carnitine octanoyltransferase cDNA, its natural gene and the gene promoter. Biochim. Biophys. Acta 1264, 215–222 [DOI] [PubMed] [Google Scholar]
- 44.Miyazawa S., Ozasa H., Osumi T., Hashimoto T. (1983) Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J. Biochem. 94, 529–542 [DOI] [PubMed] [Google Scholar]
- 45.Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. (1984) Properties of purified carnitine acyltransferases of mouse liver peroxisomes. J. Biol. Chem. 259, 13089–13095 [PubMed] [Google Scholar]
- 46.Farrell S. O., Bieber L. L. (1983) Carnitine octanoyltransferase of mouse liver peroxisomes: properties and effect of hypolipidemic drugs. Arch. Biochem. Biophys. 222, 123–132 [DOI] [PubMed] [Google Scholar]
- 47.Jakobs B. S., Wanders R. J. (1995) Fatty acid beta-oxidation in peroxisomes and mitochondria: the first, unequivocal evidence for the involvement of carnitine in shuttling propionyl-CoA from peroxisomes to mitochondria. Biochem. Biophys. Res. Commun. 213, 1035–1041 [DOI] [PubMed] [Google Scholar]
- 48.Verhoeven N. M., Jakobs C., ten Brink H. J., Wanders R. J., Roe C. R. (1998) Studies on the oxidation of phytanic acid and pristanic acid in human fibroblasts by acylcarnitine analysis. J. Inherit. Metab. Dis. 21, 753–760 [DOI] [PubMed] [Google Scholar]
- 49.Furihata T., Hosokawa M., Nakata F., Satoh T., Chiba K. (2003) Purification, molecular cloning, and functional expression of inducible liver acylcarnitine hydrolase in C57BL/6 mouse, belonging to the carboxylesterase multigene family. Arch. Biochem. Biophys. 416, 101–109 [DOI] [PubMed] [Google Scholar]
- 50.Satoh T., Hosokawa M. (2006) Structure, function and regulation of carboxylesterases. Chem. Biol. Interact. 162, 195–211 [DOI] [PubMed] [Google Scholar]
- 51.Antonenkov V. D., Hiltunen J. K. (2012) Transfer of metabolites across the peroxisomal membrane. Biochim. Biophys. Acta 1822, 1374–1386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.