Abstract
Allergic conjunctivitis (AC) is one of the most common ocular surface diseases in the world. In AC, T helper type 2 (Th2) immune responses play central roles in orchestrating inflammatory responses. However, the roles of lipid mediators in the onset and progression of AC remain to be fully explored. Although previous reports have shown the beneficial effects of supplementation of ω-3 fatty acids in asthma or atopic dermatitis, the underlying molecular mechanisms are poorly understood. In this study, a diet rich in ω-3 fatty acids alleviated AC symptoms in both early and late phases without affecting Th2 immune responses, but rather by altering the lipid mediator profiles. The ω-3 fatty acids completely suppressed scratching behavior toward the eyes, an allergic reaction provoked by itch. Although total serum IgE levels and the expression levels of Th2 cytokines and chemokines in the conjunctiva were not altered by ω-3 fatty acids, eosinophil infiltration into the conjunctiva was dramatically suppressed. The levels of ω-6–derived proinflammatory lipid mediators, including those with chemoattractant properties for eosinophils, were markedly reduced in the conjunctivae of ω-3 diet–fed mice. Dietary ω-3 fatty acids can alleviate a variety of symptoms of AC by altering the lipid mediator profile.—Hirakata, T., Lee, H.-C., Ohba, M., Saeki, K., Okuno, T., Murakami, A., Matsuda, A., Yokomizo, T. Dietary ω-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating Th2 immune responses.
Keywords: eicosanoid, inflammation, hay fever, lipidomics, fish oil
Allergic conjunctivitis (AC) is one of the most common ocular diseases in the world, affecting up to 30–40% of the general population (1). Those experiencing it have several symptoms, such as itch, conjunctival redness, lid and conjunctival edema, and discharge (2, 3). AC is characterized by activation of CD4+ T helper type 2 (Th2) cells, and production of Th2-associated cytokines [e.g., interleukin (IL)-4, -5, and -13], and of IgE, which activates mast cells (4). These Th2 immune responses are also well-known as the cause of asthma, food allergy, and atopic dermatitis. In addition to histamine, activated mast cells produce and release various inflammatory lipid mediators, such as prostaglandins (PGs) and leukotrienes (LTs), which elicit the symptoms in the early phase of the allergic response (2). These inflammatory lipid mediators, such as PGD2 and LTB4, also play a role in promoting eosinophil migration into the conjunctiva (5). These eosinophils release inflammatory mediators and cause the late-phase allergic symptoms (6, 7). Despite the importance of these inflammatory lipid mediators in such allergic diseases, there are no reports on the lipid mediator profile in conjunctival tissue with AC in vivo. Currently, antihistamine, steroid (glucocorticoid), or immunosuppressive eye drops are the main treatment for AC; eye drops targeting lipid mediators have not been developed, in part because of the lack of direct evidence on the roles of lipid mediators in AC in vivo.
The ω-3 and -6 polyunsaturated fatty acids (PUFAs) are essential fatty acids. Arachidonic acid (AA), one of the most abundant ω-6 PUFAs found in mammals, is the precursor of prostanoids and leukotrienes, most of which promote inflammation. Mammalian cells cannot convert ω-6 to -3 fatty acids because they lack the enzyme responsible for these reactions. Because ω-3 and -6 fatty acids are metabolized by essentially the same enzymatic pathways in a competitive manner, intake of ω-3 fatty acids results in reduction of AA and AA-derived mediators (8, 9). An imbalance of the ω-6/ω-3 fatty acids ratio in the diet is implicated in various diseases (10, 11); however, the present Western diet contains very low amounts of ω-3 fatty acids, with a ω-6:ω-3 ratio of 15–20:1 (12). Previous epidemiologic studies reported a correlation between the Westernization of diet and an increase in allergic diseases (13–16). It is noteworthy that some reports showed the efficacy of dietary ω-3 fatty acids for asthmatic and atopic patients (17–20). However, lipidomic analyses were not performed in these studies, and therefore the underlying molecular mechanisms of the beneficial effects of ω-3 fatty acids remain unclear.
We investigated the efficacy of dietary ω-3 fatty acids on AC in an experimental AC (EAC) mouse model using ragweed (RW) pollen. Mice were fed chow containing either linseed oil, which is rich in an ω-3 fatty acid (α-linolenic acid), or soy oil, which is rich in an ω-6 fatty acid linoleic acid, for 2 mo. AC symptoms were markedly alleviated in mice fed ω-3 fatty acids. Eosinophil infiltration into the conjunctiva was also suppressed by dietary ω-3 fatty acids. However, the magnitude of typical Th2 immune responses elicited by RW pollen was comparable between both groups. Finally, lipidomics analysis of PUFA metabolites revealed that dietary ω-3 fatty acids resulted in the dramatic reduction of various proinflammatory lipid mediators derived from AA and an increase in eicosapentaenoic acid (EPA)–derived metabolites in the conjunctival tissue.
MATERIALS AND METHODS
Ethics statement
All animal experiments were approved by the Ethics Committee for Animal Experiments in Juntendo University (Approval 300124). All procedures in the study were performed in accordance with the approved guidelines and regulations.
Mice
Female 4-wk-old wild-type BALB/c mice were purchased from Sankyo (Shizuoka, Japan) and maintained for 2 mo on AIN-93M, which contains 4% soy oil, or modified AIN-93M, which contains 4% linseed oil instead of soy oil (Oriental Yeast, Tokyo, Japan) (21, 22). All animals were raised in specific-pathogen–free conditions and provided with food and water ad libitum.
AC mouse model
Four-week-old wild-type female BALB/c mice were fed either the ω-3 or -6 diet for 2 mo. The first sensitization was performed 30 d after commencing the diet. The mouse EAC model was induced as described by Asada et al. (23). In brief, pollen from the short ragweed (RW; Polysciences, Warrington, PA, USA) was emulsified with Imject Alum Adjuvant (Thermo Fisher Scientific, Waltham, MA, USA). At d 0, 50 µl emulsified RW pollen (50 µg RW pollen with 50 µl alum) or 50 µl PBS as the control was injected into the left hind foot and root of the tail subcutaneously, and serum was collected from the tail vein. Two weeks later (d 14), a second sensitization was performed by injecting the reagents into the right hind foot. From d 26 to 29, the eyes of the mice were challenged daily by eye drops containing RW pollen in PBS (2 mg in 10 µl/eye) or PBS 10 µl alone as the control. At 24 h after the final eye drop challenge, the eyeballs (with lids and conjunctival tissue) were collected.
Clinical score
The severity of allergic symptoms in the EAC model was quantified with clinical score (mean of scores in right and left eyes). The clinical score consisted of 4 factors (chemosis, redness, lid edema, and discharge) based on the criteria of Magone et al. (24). The assay was performed in the same time course as previously described (23, 25). In brief, the clinical score was calculated on d 29, 20 min after the final eye drop challenge, and on d 30, 24 h after the final challenge.
Assay of scratching behavior
Mice were placed in an acrylic cage and videotaped for 40 min immediately after the final challenge. The number of scratching actions toward the eyes with the forepaw and hindpaw was counted during video playback. Grooming, a gesture of wiping the head and face from the back of the ear, was not counted as scratching behavior.
Histologic analysis
Eyeballs (with conjunctival tissues and eyelids) were dissected and fixed in 4% paraformaldehyde in PBS, embedded in paraffin, sectioned (3-µm thickness), and stained with Giemsa (Merck, GmbH, Darmstadt, Germany). Infiltrating eosinophils in the lamina propria mucosa of the tarsal and bulbar conjunctiva were counted from the section of the central portion of the eye, which included the pupil and optic nerve head as previously described (23, 26).
Flow cytometry
Peripheral blood was collected with a heparinized syringe. The samples were immediately mixed with 2% dextran in a ratio of 1:1 and left to stand for 20 min. The leukocyte-rich plasma was mixed with 3 volumes of hemolysis buffer (17 mM Tris HCl, 140 mM NH4Cl, pH 7.6) to lyse red blood cells. Cells were preincubated with 5 mg/ml anti-mouse FcγR II/III antibody (clone 2.4G2) followed by staining with anti-mouse CD45.2-PerCP (clone 104; eBioscience, San Diego CA, USA), anti-mouse CD11b-allophycocyanin (clone M1/70; eBioscience), and anti-mouse Siglec-F-PE (clone E50-2440; BD Biosystems, San Jose, CA, USA) antibodies on ice for 30 min. Cells were analyzed on a FACS Calibur (BD Biosystems) using CellQuest software (BD Immunocytometry Systems, San Jose, CA, USA) and FlowJo software (Ashland, OR, USA).
Real-time PCR
Conjunctival tissues were isolated from mouse eyes and immediately submerged in RNAlater solution (Thermo Fisher Scientific). Total RNA was extracted from the tissue with an RNA Isolation Kit (NucleoSpin II; Macherey-Nagel GmbH, Duren, Germany). cDNAs were prepared with the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) with random and oligo-dT mixed primers. The target genes were detected by real-time PCR using FastStart DNA Green Master (Roche Diagnostics, Mannheim, Germany) in a LightCycler (Roche Diagnostics). Relative mRNA expression levels were calculated by normalizing to the expression of β-actin. The sequences of primers used are listed in Supplemental Table S1.
Measurement of serum IgE levels
Serum IgE levels at d 0 (before the sensitization) and d 30 (24 h after the final challenge) were quantified with a mouse IgE ELISA kit (ELISA Max; BioLegend, San Diego, CA, USA), according to the manufacturer’s protocol.
Quantification of lipid mediators in mouse conjunctiva
Lipid mediators in each sample were quantified by HPLC-tandem mass spectrometry (HPLC/MS/MS), as described by Ohba et al. (27). Eyes were collected, and conjunctival tissue was separated carefully. Lipids were extracted from the conjunctiva of the right eye with methanol containing deuterium-labeled internal standards. Each sample was diluted with water to yield a final methanol concentration of 20%, and then loaded on Oasis HLB cartridges (Waters, Milford, MA, USA). The column was subsequently washed with petroleum ether, followed by 15% methanol, and then water, each containing 0.1% formic acid. The samples were eluted with 200 µl methanol containing 0.1% formic acid. The lipids were injected on a Prominence HPLC system (Shimadzu, Kyoto, Japan) and a TSQ Quantum Ultra Triple-Stage Quadrupole Mass Spectrometer (Thermo Fisher Scientific) equipped with heated electrospray ionization interface. Mobile phases A and B comprised water and acetonitrile:formic acid (100:0.1 v/v), respectively. The gradient conditions were as follows: 0–7 min, 37% B; 7–19 min, 37–90% B (linear gradient); 19–21 min, 100% B; 21–22.5 min, 37% B; and flow rate, 0.12 ml/min. The separation column was a Capcell Pak C18 MGS3 (1 × 100 mm; Shiseido, Tokyo, Japan) and the trapping column was an Opti-Guard Mini C18 (1 × 15 mm; Optimize Technologies, Oregon City, OR, USA). The separation column temperature was set at 46°C. Lipids were detected in negative ESI mode under the following conditions: spray voltage, 2500 V; capillary temperature, 225°C; vaporizer temperature, 250°C; sheath gas (N2) pressure, 40 psi; ion sweep gas pressure, 0 psi; auxiliary gas pressure, 10 psi; collision gas (Ar) pressure, 1.5 mTorr; and the EZ method (scheduled selected random monitoring method) with a cycle time of 1.0 s. Mass spectrometer parameters including tube lens, collision energy, precursor ion, and product ion were optimized for each compound. Data analysis and quantitative calculations were performed with Xcalibur 2.1 software (Thermo Fisher Scientific).
Statistical analysis
All data are expressed as means ± se. Statistical significance was calculated with Prism 7 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Dietary ω-3 fatty acids improved allergic symptoms
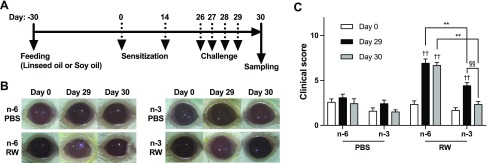
BALB/c wild-type female mice were fed chow containing 4% linseed oil (rich in ω-3 fatty acids) or 4% soy oil (rich in ω-6 fatty acids) as a control, for 2 mo, beginning 30 d before the first sensitization. They were sensitized twice on d 0 and 14 by subcutaneously injecting RW pollen, and challenged 4 times with eye drops containing RW daily from d 26 to 29 (Fig. 1A). During the course of the study, body weight was not significantly different between both groups (Supplemental Fig. S1). In the EAC mouse model, early-phase allergic symptoms occur about 20 min after the challenge and late-phase allergic symptoms at about 24 h after the challenge (24). Thus, to evaluate the severity of AC symptoms in both phases, a clinical score based on chemosis, conjunctival redness, lid enema, and discharge was calculated on d 29, 20 min after the fourth eye drop, and on d 30, 24 h after the fourth eye drop (Fig. 1B and Supplemental Fig. S2). As predicted, RW treatment caused severe AC symptoms in the control ω-6 diet group on d 29, and the symptoms remained severe until d 30 (Fig. 1C). Notably, mice fed ω-3 fatty acids showed milder AC symptoms on d 29, and the symptoms were almost resolved within the next 24 h.
Figure 1.
An ω-3 diet attenuates experimental AC. A) The protocol used in the mouse model of RW pollen–induced EAC is shown. Mice were fed with chow containing 4% linseed (ω-3 diet; n-3) or soy (ω-6 diet; n-6) oil for 30 d before sensitization with RW pollen. PBS was used as a control. B) Representative photos of mouse eyes. C) Clinical score was calculated at d 0, 29, and 30 (n = 6–7 at each time point). The total score consisted of the sum of scores for 4 factors—chemosis, conjunctival redness, lid edema, and discharge—each scored from 0 to 3 points, depending on the severity. Data are representative of 4 independent experiments, in which comparable results were obtained. Data were analyzed by Mann-Whitney U test. Data are expressed as means ± se. **P < 0.01, ω-6 vs. ω-3; ††P < 0.01, PBS vs. RW; §§P < 0.01, d 29 vs. d 30.
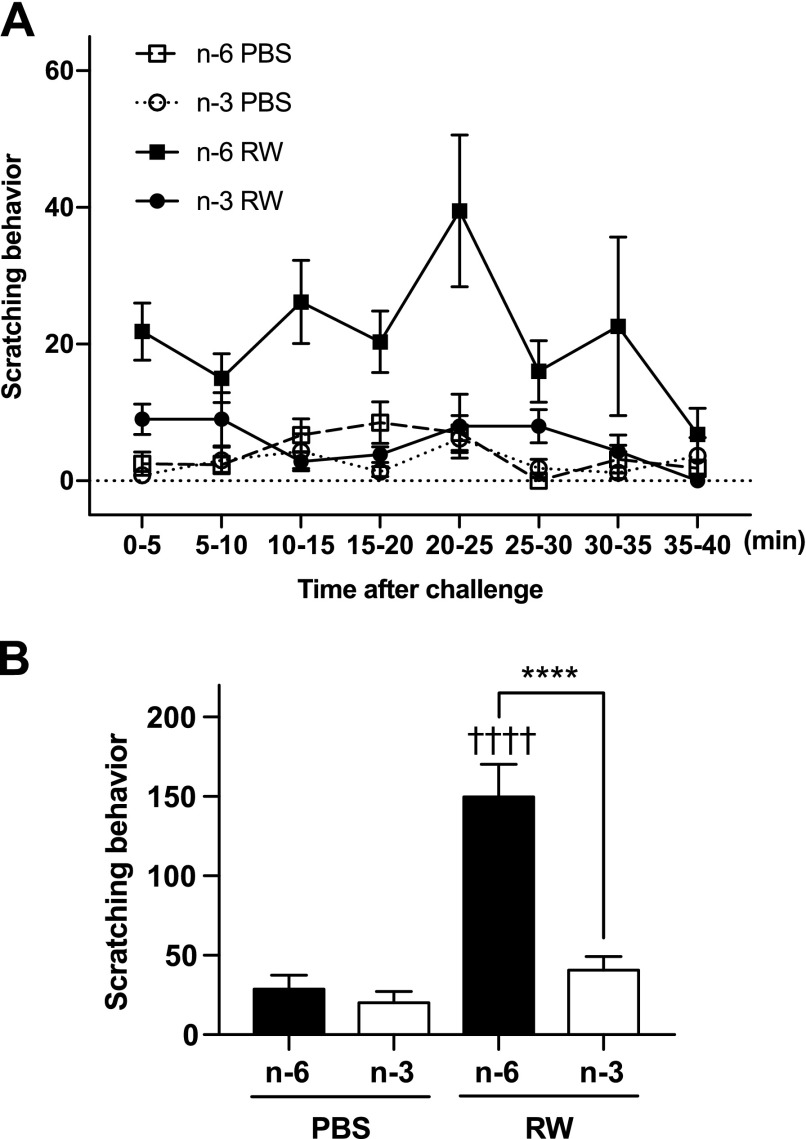
Itch is one of the chief complaints and reduces quality of life (QOL) in AC patients. Scratching behavior is used as an index of itch response in rodents (28). We monitored mice for 40 min immediately after administering the final eye drops and evaluated the scratching behavior by counting the number of episodes of paws brought to the eyes. A previous study using an EAC mouse model showed that the most severe allergic symptoms occur at 20 min after challenge (24). Similar to the previous studies (24, 29), in the present EAC model, mice treated with RW repeated the scratching behavior toward the eyes, and the frequency of scratch was highest between 20 and 25 min (Fig. 2A). Remarkably, in mice fed ω-3 fatty acids, scratching behavior was almost completely suppressed (Fig. 2). These results suggest that dietary ω-3 fatty acids suppress a variety of allergic symptoms that occur in both the early and late phases of AC.
Figure 2.
An ω-3 diet attenuates AC-induced itch. A) Scratching behavior was monitored immediately after the final challenge and counted for 40 min. B) The total number of scratching actions during 40 min (n = 6). Data were analyzed by 1-way ANOVA and Tukey’s multiple-comparison test. Data are expressed as means ± se. ****P < 0.0001, ω-6 vs. ω-3; ††††P < 0.0001, PBS vs. RW.
Eosinophil infiltration was attenuated in the conjunctiva of ω-3–fed mice
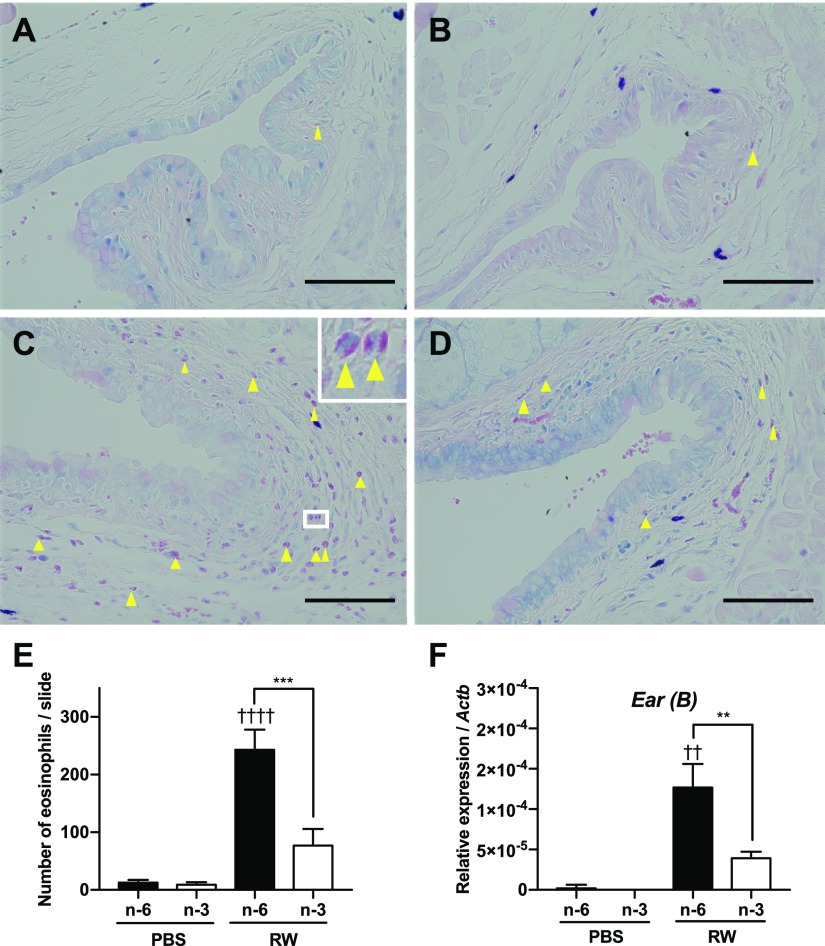
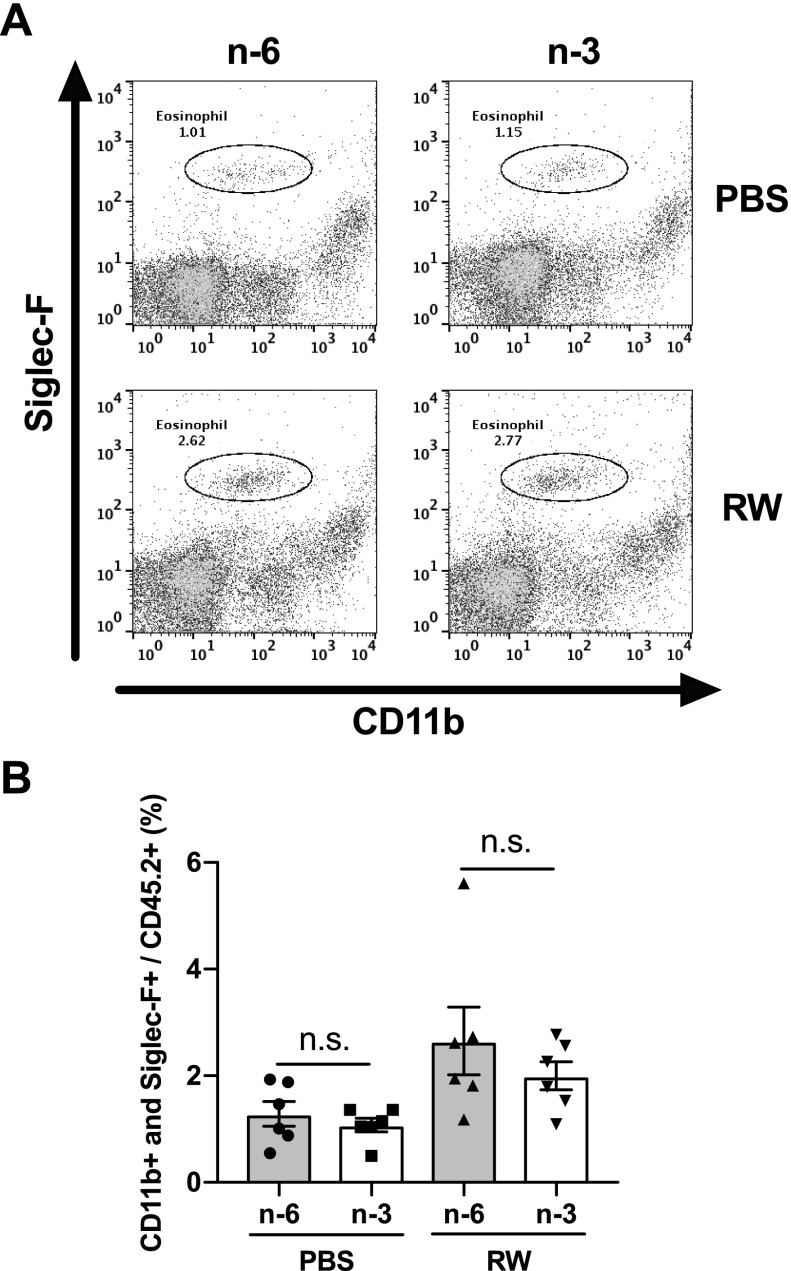
Generally, eosinophils are absent in the normal conjunctiva (2, 30). However, infiltrated eosinophils are observed in the conjunctiva of patients with AC (31). These infiltrated eosinophils promote allergic symptoms by releasing major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPX), and proinflammatory lipid mediators (32). In the EAC model, a large number of infiltrated eosinophils were observed in the conjunctiva of the ω-6 diet group treated with RW pollen (Fig. 3A, C, E). On the other hand, the number of infiltrated eosinophils was markedly lower in the ω-3–fed mice (Fig. 3B, D, E). Quantitative RT-PCR analyses of conjunctival tissue showed that the expression levels of eosinophil-associated RNase subfamily B (Fig. 3F) and eosinophil-associated RNase subfamily A [Ear (A); Supplemental Fig. S3] were also lower in the ω-3 diet group than in the ω-6 diet group. In addition, the expression levels of other genes known to be active in eosinophils, MBP (Mbp), ECP2 (Ecp2), and EPX (Epx) were also lower in the ω-3 diet group than in the ω-6 diet group (Supplemental Fig. S3). Next, we investigated the effect of dietary ω-3 fatty acids on eosinophils in the peripheral blood. The percentage of eosinophils (CD45.2+, CD11b+, Siglec-F+) in the leukocytes increased in both diet groups by RW; however, there were no differences between the ω-6 and ω-3 groups (Fig. 4). These results confirm that dietary ω-3 fatty acids suppress eosinophil infiltration of the conjunctiva.
Figure 3.
Reduced eosinophil migration in the conjunctiva of ω-3–fed mice. A–D) Sections containing conjunctiva were stained with Giemsa stain, and eosinophils were counted. Arrows: eosinophils. Scale bars, 50 µm. The conjunctiva of a mouse fed an ω-6 diet (n-6) treated with PBS (A) or with RW pollen (C). The conjunctiva of a mouse fed an ω-3 diet (n-3) treated with PBS (B) or created with RW pollen (D). E) The number of eosinophils infiltrating conjunctiva. F) mRNA level of eosinophil-associated RNase subfamily B mRNA measured by real-time PCR (n = 3). E, F) Data are expressed as means ± se. Data were analyzed by 1-way ANOVA and Tukey’s multiple-comparison test. **P < 0.01, ***P < 0.001, ω-6 vs. ω-3; ††P < 0.01, ††††P < 0.0001, PBS vs. RW.
Figure 4.
Dietary ω-3 fatty acids do not affect eosinophils in the peripheral blood. A, B) The percentage of eosinophils in the peripheral blood was analyzed by flow cytometry (n = 6). Data are expressed as means ± se and the individual values are plotted on the bar graph. Data were analyzed by 1-way ANOVA and Tukey’s multiple comparison test.
Th2 cytokines and chemokines were not altered by the ω-3 diet
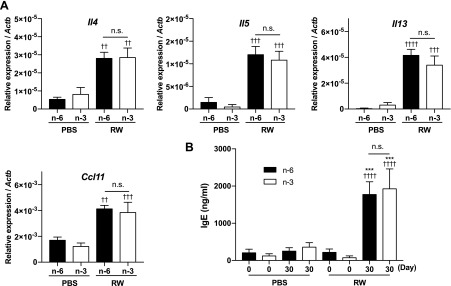
Next, we examined whether dietary ω-3 fatty acids affect Th2 immune responses elicited by RW pollen. Unexpectedly, the gene expression levels of Th2 cytokines (Il4, Il5, and Il13) and a chemokine (Ccl11) in the conjunctiva of RW-treated mice were not different between ω-6 and -3 diet groups (Fig. 5A). Moreover, the serum IgE level was not different between these 2 groups (Fig. 5B). These results suggest that the effects of ω-3 fatty acids are not attributable to the alteration of Th2 immune responses in the EAC mouse model.
Figure 5.
Th2 immune responses are not modulated by dietary ω-3 fatty acids. A) mRNA expression levels of Th2 cytokines and chemokines in the conjunctiva were measured by real-time PCR (n = 6–7). B) Serum IgE level was measured by ELISA (n = 6–7). A, B) Data are expressed as means ± se, analyzed by 1-way ANOVA and Tukey’s multiple-comparison test. ***P < 0.001, ω-6 vs. ω-3; ††P < 0.01, †††P < 0.001, ††††P < 0.0001, PBS vs. RW.
The ω-3 diet alters the conjunctival lipid mediator profile
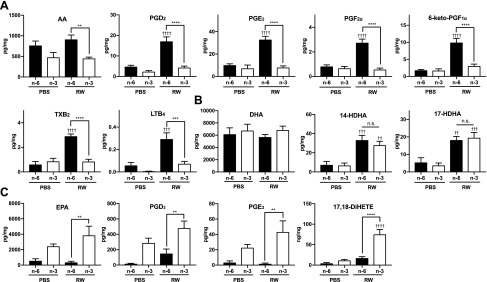
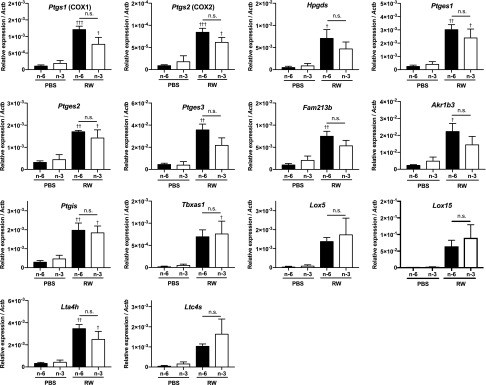
To further investigate the underlying mechanisms, various PUFAs and their metabolites in the conjunctiva were measured by HPLC/MS/MS (Fig. 6). Metabolite maps of lipid mediators and their biosynthetic enzymes are shown in Supplemental Fig. S4. Compared with the group fed ω-6, the level of free AA was decreased by 50% in mice fed ω-3 fatty acids in both the RW pollen–treated and PBS-treated groups (Fig. 6A), which is as expected because linseed oil contains less linoleic acid, the precursor of AA, than soy oil. The amounts of AA-derived lipid mediators, such as PGD2, PGE2, PGF2α, PGI2 (represented by its stable metabolite 6-keto-PGF1α), thromboxane A2 (TXA2; represented by its stable metabolite TXB2), and LTB4, were markedly elevated by RW pollen in mice fed ω-6 fatty acids (Fig. 6A). By contrast, in ω-3–fed mice treated with RW pollen, the elevation of these mediators was almost completely suppressed (Fig. 6A), even though the mRNA expression levels of cyclooxygenases [COXs; PG synthases (Ptgs1/2), lipoxygenases (Lox5/15), terminal PG synthases (Hpgds, Ptges1/2/3, Fam213b, Akr1b3, Ptgis, and Tbxas1), LTA4 hydrolase (Lta4h), and LTC4 synthase (Ltc4s)] were not significantly different between the 2 groups (Fig. 7). This may be explained by the excessive amount (3–4-fold higher than that of AA) of free EPA present in the conjunctiva of ω-3–fed mice (Fig. 6A, C). EPA acts as a competitive substrate of AA for AA oxygenases, such as COX and 5-lipoxygenase (5-LOX) (8, 33). Indeed, PGD3 and PGE3, the COX metabolites of EPA (34), were markedly produced in the conjunctiva of the ω-3–fed RW group, whereas only a slight amount of these metabolites was found in the conjunctiva of the ω-6–fed groups (Fig. 6C). Notably, the concentration of PGD3 in the conjunctiva of mice fed ω-3 fatty acids was much higher (28-fold) than PGD2 in the ω-6–fed RW group (Fig. 6A, C). Other AA metabolites were also reduced in mice fed ω-3 fatty acids (Supplemental Fig. S5). These results indicate that ω-3 fatty acids are competitively metabolized in the synthetic pathways of AA-derived metabolites, thereby leading to the reduction of proinflammatory lipid mediators.
Figure 6.
Lipidomics analysis of the conjunctiva. Various lipid mediators derived from AA (A), DHA (B), and EPA (C) in conjunctivae were measured by LC/MS/MS (n = 4–5). Data are expressed as means ± se, analyzed by 1-way ANOVA and Tukey’s multiple-comparison test. **P < 0.01, ***P < 0.001, ****P < 0.0001, ω-6 vs. ω-3; ††P < 0.01, †††P < 0.001, ††††P < 0.0001, PBS vs. RW.
Figure 7.
mRNA expression levels of fatty acid metabolizing enzymes. mRNA expression levels of eicosanoid biosynthetic enzymes, cyclooxygenase1/2 (Cox1/2), hematopoietic PGD synthase (Hpgds), PGE synthase 1/2/3 (Ptges1/2/3), PGF synthase (Fam213b and Akr1b3), PGI synthase (Ptgis), TXA synthase 1 (Txas1), LTA4 hydrolase (Lta4h), and LTC4 synthase (Ltc4s), were evaluated by real-time PCR (n = 3). Data represent means ± se, analyzed by 1-way ANOVA and Tukey’s multiple-comparison test. †P < 0.05, ††P < 0.01, †††P < 0.001, control vs. RW.
A derivative of EPA, 17,18-epoxyeicosatetraenoic acid (17,18-EpETE) has recently been identified as an anti-allergic lipid mediator derived from EPA (21). Although we did not measure 17,18-EpETE per se, our lipidomics analysis revealed that its downstream product 17,18-dihydroxyeicosatetraenoic acid (17,18-DiHETE; Fig. 6C), a vicinal diol derived from 17,18-EpETE, which also shows mild anti-allergic properties (21), was dramatically elevated by RW in the conjunctiva of mice fed ω-3 fatty acids (Fig. 6C). Thus, in addition to promoting a reduction in proinflammatory lipid mediators, intake of ω-3 fatty acids lead to the production of EPA-derived metabolites that may exert anti-allergic effects on AC.
DISCUSSION
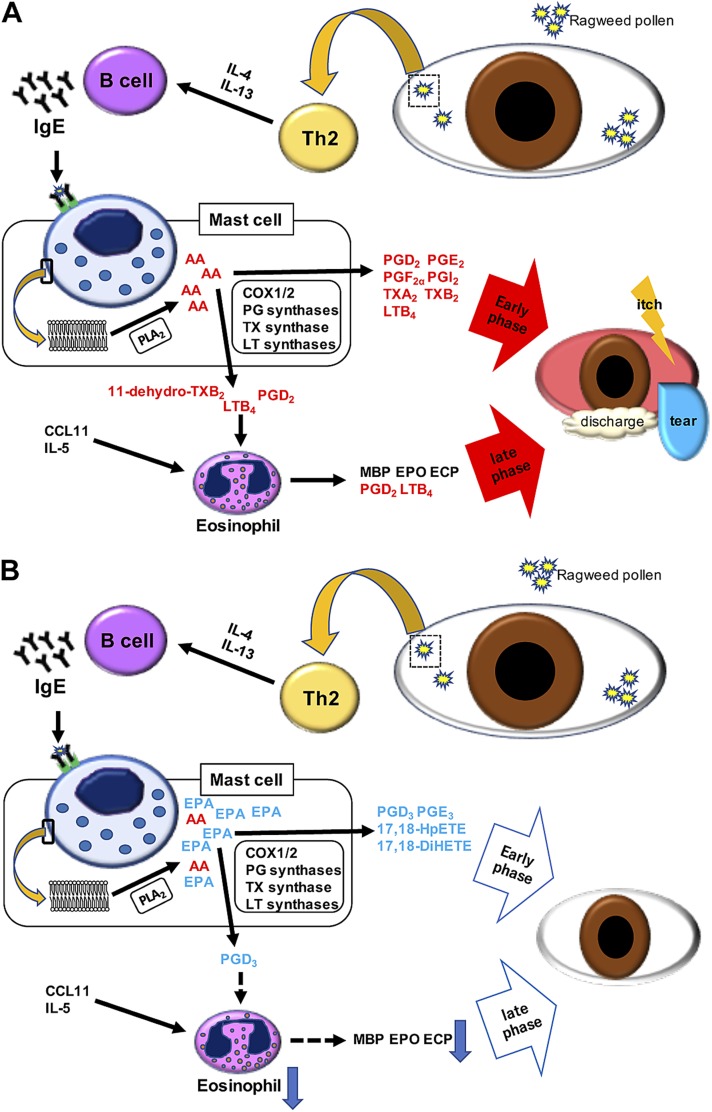
This study is the first report that shows the beneficial effects of dietary ω-3 fatty acids on AC in mice. In the presence of allergen in the conjunctiva, a cascade of Th2 immune responses are evoked and a variety of AA-derived inflammatory mediators are released from mast cells and eosinophils, thereby causing various AC symptoms such as conjunctival redness, discharge, and itch (Fig. 8A). We found that a diet of ω-3 fatty acids alleviates AC symptoms without altering Th2 immune responses (Th2 cytokines, chemokines, and serum IgE) (Fig. 8B). In mice fed a control ω-6 diet, fatty acid metabolizing enzymes convert free AA released from cell membranes to various proinflammatory lipid mediators, such as prostanoids (PGD2, PGE2, PGF2α, and TXA2) and LTB4 (Fig. 8A). The ω-3 diet almost completely suppressed the production of these AA-derived inflammatory lipid mediators, probably through providing the competitive substrates (i.e., EPA and its downstream metabolites) for the synthetic enzymes of AA metabolites in the conjunctiva (Fig. 8B). Therefore, we conclude that dietary ω-3 fatty acids alleviate AC, mainly through altering the lipid mediator profile, including reduction of proinflammatory ω-6–derived mediators and production of anti-inflammatory and proresolving ω-3–derived mediators. Although previous reports showed the efficacy of supplementation of ω-3 fatty acids on other types of allergic diseases in humans (19, 35–39), the underlying molecular mechanisms of these beneficial effects remain largely unexplained because of the lack of lipidomics measurements in human samples. Considering the limited availability of human conjunctival tissues, the murine AC model combined with MS-based lipidomics would be one of the most suitable and practical approaches to describe the impact of dietary ω-3 fatty acids on lipid mediator profiles of the mammalian conjunctival tissues. Our results provide encouraging evidence for the use of dietary ω-3 fatty acids in patients with AC. Future studies using human conjunctival samples, though challenging, will support the metabolic effects of ω-3 fatty acids on the human conjunctiva.
Figure 8.
Mechanisms of AC suppression by ω-3 diet. A) The control diet: Th2 cells produce IL-4 and -13, which activate B cells. These activated B cells produce IgE, which activates mast cells. In the activated mast cells, free AA is produced from the phospholipid membrane by PLA2, and proinflammatory lipid mediators are synthesized by enzymes such as oxygenases and terminal prostanoid synthases. These proinflammatory lipid mediators elicit allergic symptoms in the early phase such as conjunctival redness, discharge, and itch. These lipid mediators such as PGD2 and LTB4 also play a role in attracting eosinophils. Infiltrated eosinophils produce inflammatory mediators and cause the late-phase allergic symptoms. B) The ω-3 diet: dietary ω-3 fatty acids do not alter the Th2 immune response, but increase the EPA content in the membrane phospholipids. Free EPA liberated by PLA2 competes with AA, and reduces the production of AA-derived mediators, resulting in the reduction of proinflammatory lipid mediators. Furthermore, lipid mediators derived from EPA function as anti-allergic or proresolving mediators. This altered lipid profile leads to the alleviation of AC in both the early and late phases.
In the EAC mouse model, dietary ω-3 fatty acids attenuated AC symptoms in both the early and late phases. In Th2-mediated allergy, the early phase reactions occur within minutes after allergen exposure (40). In the process of a Th2 immune response, first, allergen is taken up and processed by antigen-presenting cells, such as dendritic cells, and these activated cells induce differentiation of naïve T-cells into Th2 cells. Th2 cells produce IL-4 and -13, which stimulate B-cells. B-cells then produce IgE, which activates mast cells with its antigen (4). Finally, the activated mast cells release various mediators causing the early-phase allergic symptoms (4, 41). In the present study, the levels of these Th2 cytokines and IgE were not altered by dietary ω-3 fatty acids (Fig. 5). Similar to our results, Kunisawa et al. (21) reported antiallergic effects of dietary ω-3 fatty acids in a food allergy mouse model without affecting the serum IgE level. These findings indicate that the preventive and therapeutic effects of dietary ω-3 fatty acids on Th2-mediated allergy are not brought about by the modulation of Th2 immune responses, and that mast cell activation occurs independently of the ω-3:ω-6 ratio in the diet, at least in the current study.
In activated mast cells, cytosolic phospholipase A2 (cPLA2) releases AA from phospholipids, and subsequently prostanoids and leukotrienes, collectively called eicosanoids, are produced by COX, PG synthase, and lipoxygenase pathways (41). These lipid mediators cause vasodilation (conjunctival redness), vascular permeability (chemosis and lid edema), and the secretion of mucus (tear and discharge) (40). They also stimulate nociceptors, which results in itching (40). Our results showed that the production of inflammatory lipid mediators derived from AA such as PGs, TXA2, and LTB4 were almost completely suppressed in the conjunctiva by feeding mice with ω-3 fatty acids. Nonsteroidal anti-inflammatory drugs (NSAIDs) block COX and thereby reduce prostanoids. A previous report showed that NSAIDs had therapeutic effects on AC in a meta-analysis of randomized studies (42). Ballas et al. (43) reported that the eye drops containing 0.5% of the NSAID keratolac improved allergic symptoms such as conjunctival inflammation, itching, swollen eyes, discharge, and tears in randomized, double-blind, clinical human studies. These findings suggest that reducing inflammatory lipid mediators is a good therapeutic strategy for the treatment of AC. However, NSAIDs may cause side effects such as corneal epithelial injury, ulceration, and perforation (44). Moreover, NSAIDs do not block lipoxygenases, and in some cases, NSAIDs are known to increase the production of LTs by shunting AA from the COX to the LT pathway. Thus, ω-3 fatty acids may be more suitable for prevention and therapy for AC as they have fewer side effects and can reduce AA metabolites produced through both COX and lipoxygenase pathways.
Another novel finding of the current study is the marked elevation in the conjunctivae of mice given the ω-3 diet (especially when treated with RW pollen) of PGD3 and PGE3, the alternative eicosanoids synthesized from EPA through the COX pathway. Notably, the PGD2 receptor DP1 prefers PGD3 over PGD2 (34), and PGD3 inhibits the migration of neutrophils by antagonizing DP1 (45, 46). Thus, in addition to the reduction of proinflammatory lipid mediators, EPA-derived metabolites may also contribute to the alleviation of allergic symptoms by antagonizing their receptors and thereby inhibiting the downstream signaling pathways of the cells mediating allergic reactions. Furthermore, our lipidomics analysis suggest that intake of the ω-3 diet increases the level of EPA-derived anti-allergic metabolites 17,18-EpETE (detected as 17,18-DiHETE) in the conjunctiva. Therefore, dietary ω-3 fatty acids may exert antiallergic effects through multiple mechanisms in AC.
Eosinophils play important roles in the late-phase reaction, which occurs 12–24 h after allergen exposure (25). Both IL-5 and chemokine (C-C motif) ligand (CCL)-11 (eotaxin) are well known as attractants that promote eosinophil migration. IL-5, which is released from Th2 cell and mast cells, promotes the growth of eosinophils in bone marrow, and supports eosinophil survival (32, 47, 48). CCL11 mainly plays a role in attracting eosinophils (49, 50). In the present study, IL-5 and CCL11 were induced by RW pollen in both the ω-6 and -3 diet groups. Consistently, the percentage of eosinophils in the peripheral blood was increased in both diet groups by RW pollen (Fig. 4B). In addition, increase in these chemoattractants likely contributed to the mild increase in eosinophil infiltration of the conjunctiva in the RW-treated ω-3 diet group (Fig. 3E). There are, however, other mechanisms that can explain the large difference in the number of infiltrated eosinophils in the conjunctiva between the ω-6 and ω-3 diet groups (Fig. 3A–E), such as the marked contrast in eicosanoid production, which was revealed by our lipidomics analysis. Lipid mediators such as PGD2, LTB4, and 11-dehydro-TXB2 (a metabolite of TXA2) are also known as attractants of eosinophils (5, 6, 51, 52). Indeed, eosinophils express receptors for these lipid mediators (5, 52), and some studies have shown that blocking these receptors is efficacious for damping allergy (51, 53). For example, in an allergic asthmatic mouse model, knockout (KO) mice lacking BLT1, the high-affinity LTB4 receptor, showed a decreased eosinophil infiltration of the airways (54). In the same model, DP1 KO mice also showed reduced eosinophil infiltration, and thereby failed to develop airway hyperreactivity in allergic asthmatic mouse model (55). Notably, PGD2 and LTB4 also act as chemoattractants for eosinophils into the conjunctiva (6, 56, 57). Thus, instead of alteration in the levels of protein mediators, such as IL-5 and CCL11, the reduction of lipid mediators, especially PGD2 and LTB4, is likely to be the main mechanism through which eosinophil infiltration was reduced in the ω-3–fed mice. Infiltrated eosinophils cause the late-phase allergic symptoms by releasing inflammatory lipid mediators, such as PGD2, LTB4, LTC4, and TXA2, and granule-stored cationic proteins such as MBP, ECP, and EPX (5–7, 58). We confirmed that the expression levels of granule-stored cationic proteins were reduced in the conjunctivae of mice fed ω-3 fatty acids (Supplemental Fig. S3). These findings suggest that dietary ω-3 fatty acids reduce the production of lipid mediators that recruit eosinophils, resulting in decreased eosinophil infiltration and inflammatory mediators produced by these cells.
In this study, itching was also attenuated in the ω-3 diet groups. Previous studies showed that PGD2, PGE2, PGF2α, and PGI2 were reported to elicit scratching of the ocular surface (59–61). Andoh et al. (29) showed that LTB4 elicited itch in the mouse conjunctiva through BLT1, and the effective doses of LTB4 were 1:10,000 or less than that needed for histamine, whereas LTC4, LTD4, and LTE4 did not induce scratching (59). TXA2 elicits hindpaw scratching through its receptor, thromboxane receptor, in the skin of mice (62). Thus, in AC, dietary ω-3 fatty acids suppressed itch, probably by depleting itch-eliciting AA-derived lipid mediators, in the conjunctiva.
Metabolites of ω-3 PUFAs, such as the resolvin E (RvE) series, resolvin D (RvD) series, maresine-1, and protectin D1, are collectively called SPMs. Previous reports revealed therapeutic effects of SPMs for Th2-mediated allergy. RvD1 dampens IgE production by B-cells in asthma patients (63) and modulates allergic airway responses by decreasing IL-5, eosinophils, and proinflammatory mediators in the ovalbumin-induced asthma mouse model (64). In the same model, RvE1 also decreased IL-13, IgE, and eosinophil infiltration (65). Although several studies have suggested the beneficial effects of SPMs on ocular diseases (66–69), there is no direct evidence on whether SPMs have any efficacy for AC. In the present study, none of the SPMs mentioned above was detected in the conjunctiva. We speculate that their absence was mainly related to the small sample size (1 conjunctival tissue weighs only about 10 mg), because the sources of SPMs [free EPA, docosahexaenoic acid (DHA) or AA] and the biosynthetic enzymes required for the production of SPMs were present in conjunctiva. Indeed, DHA was abundant in conjunctiva of mice tested in this study, regardless of the difference in diet, and 17-hydroxydocosahexaenoic acid (a precursor of the RvD series derived from DHA) and 14-hydroxy-docosahexaenoic acid (a precursor of maresine-1 derived from DHA) were both elevated in mice treated with RW (Fig. 6B). RvD series SPMs are synthesized from DHA by 15-LOX and 5-LOX, and marsine-1 is synthesized from DHA by 12/15-LOX (70–72). The expression levels of these enzymes were also elevated in the RW groups (Fig. 7). To understand the roles of these SPMs in AC, modulation of their levels by a DHA-rich diet or by direct targeting may be required.
In summary, this study revealed that dietary ω-3 fatty acids alleviate AC in both the early and late phases, by suppressing the production of various proinflammatory lipid mediators derived from AA, which are involved in the pathogenesis of AC. In general, Th2 immune responses are regarded as the most important drug targets in allergy; however, the preventive and therapeutic effects of dietary ω-3 fatty acids described in this study were not through modulating Th2 immune responses. Instead, at least in the present AC model, lipid mediators derived from AA seem to play a major role in eliciting allergic symptoms. Although the drugs currently on the market for treating AC can inhibit the production of either PGs or LTs, none blocks both pathways. Thus, altering the lipid mediator profile with dietary ω-3 fatty acids may be a safe and practical approach for the prevention and therapeutics of AC, especially given that ω-3 fatty acid consumption is currently declining worldwide.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank members of the Laboratory of Morphology and Image Analysis Research Support Center at the Juntendo University Graduate School of Medicine for technical assistance with microscopy. This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT)/Japan Society for the Promotion of Sport Grants-in-Aid for Scientific Research (KAKENHI) (18K16246 to H.-C.L.; 15K08316 and 18K06923 to K.S.; 15KK0320 and 16K08596 to T.O.; 16K11303 to A.Matsuda; and 15H05904, 15H04708, and 18H02627 to T.Y.), and grants from the Naito Foundation, the Ono Medical Research Foundation, the Uehara Memorial Foundation, the Mitsubishi Foundation, the Takeda Science Foundation, and, in part, by Grant-in-Aid S1311011 from the Foundation of Strategic Research Projects in Private Universities from MEXT, and institutional grants for Environmental and Gender-Specific Medicine and the Atopy Research Center at the Juntendo University Graduate School of Medicine. The authors declare no conflicts of interest.
Glossary
- 17,18-EpETE
17,18-epoxyeicosatetraenoic acid
- AA
arachidonic acid
- AC
allergic conjunctivitis
- CCL
chemokine (C-C motif) ligand
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- DHA
docosahexaenoic acid
- EAC
experimental allergic conjunctivitis
- ECP
eosinophil cationic protein
- EPA
eicosapentaenoic acid
- EPX
eosinophil peroxidase
- MS/MS
tandem mass spectrometry
- LOX
lipoxygenase
- LT
leukotriene
- MBP
major basic protein
- NSAID
nonsteroidal anti-inflammatory drug
- PG
prostaglandin
- PUFA
polyunsaturated fatty acid
- Rv
resolvin
- RW
ragweed
- SPM
specialized proresolving mediator
- Th2
T helper type 2
- TX
thromboxane
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. Hirakata, H.-C. Lee, A. Murakami, A. Matsuda, and T. Yokomizo designed all experiments; T. Hirakata and M. Ohba performed the experiments; T. Hirakata, H.-C. Lee, K. Saeki, T. Okuno, A. Matsuda, and T. Yokomizo acquired and analyzed the data; and T. Hirakata, H.-C. Lee, K. Saeki, A. Matsuda, and T. Yokomizo wrote the manuscript.
REFERENCES
- 1.Singh K., Axelrod S., Bielory L. (2010) The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J. Allergy. Clin. Immunol. 126, 778–783.e6 [DOI] [PubMed] [Google Scholar]
- 2.Ono S. J., Abelson M. B. (2005) Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 115, 118–122 [DOI] [PubMed] [Google Scholar]
- 3.Kari O., Saari K. M. (2010) Updates in the treatment of ocular allergies. J. Asthma Allergy 3, 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane-Myers A. (2001) The pathogenesis of allergic conjunctivitis. Curr. Allergy Asthma Rep. 1, 550–557 [DOI] [PubMed] [Google Scholar]
- 5.Luna-Gomes T., Bozza P. T., Bandeira-Melo C. (2013) Eosinophil recruitment and activation: the role of lipid mediators. Front. Pharmacol. 4, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luna-Gomes T., Magalhães K. G., Mesquita-Santos F. P., Bakker-Abreu I., Samico R. F., Molinaro R., Calheiros A. S., Diaz B. L., Bozza P. T., Weller P. F., Bandeira-Melo C. (2011) Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J. Immunol. 187, 6518–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampson A. P. (2000) The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy 30(Suppl 1), 22–27 [DOI] [PubMed] [Google Scholar]
- 8.Beermann C., Neumann S., Fußbroich D., Zielen S., Schubert R. (2016) Combinations of distinct long-chain polyunsaturated fatty acid species for improved dietary treatment against allergic bronchial asthma. Nutrition 32, 1165–1170 [DOI] [PubMed] [Google Scholar]
- 9.Whelan J. (1996) Antagonistic effects of dietary arachidonic acid and n-3 polyunsaturated fatty acids. J. Nutr. 126(Suppl 4), 1086S–1091S [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos A. P. (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 60, 502–507 [DOI] [PubMed] [Google Scholar]
- 11.Rupp H., Wagner D., Rupp T., Schulte L. M., Maisch B. (2004) Risk stratification by the “EPA+DHA level” and the “EPA/AA ratio” focus on anti-inflammatory and antiarrhythmogenic effects of long-chain omega-3 fatty acids. Herz 29, 673–685; erratum: 805 [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos A. P. (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56, 365–379 [DOI] [PubMed] [Google Scholar]
- 13.Devereux G. (2006) The increase in the prevalence of asthma and allergy: food for thought. Nat. Rev. Immunol. 6, 869–874 [DOI] [PubMed] [Google Scholar]
- 14.Devenny A., Wassall H., Ninan T., Omran M., Khan S. D., Russell G. (2004) Respiratory symptoms and atopy in children in Aberdeen: questionnaire studies of a defined school population repeated over 35 years. BMJ 329, 489–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devereux G., Seaton A. (2005) Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 115, 1109–1117; quiz 1118 [DOI] [PubMed] [Google Scholar]
- 16.Fogarty A., Britton J. (2000) The role of diet in the aetiology of asthma. Clin. Exp. Allergy 30, 615–627 [DOI] [PubMed] [Google Scholar]
- 17.Farjadian S., Moghtaderi M., Kalani M., Gholami T., Hosseini Teshnizi S. (2016) Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine 85, 61–66 [DOI] [PubMed] [Google Scholar]
- 18.Bisgaard H., Stokholm J., Chawes B. L., Vissing N. H., Bjarnadóttir E., Schoos A. M., Wolsk H. M., Pedersen T. M., Vinding R. K., Thorsteinsdóttir S., Følsgaard N. V., Fink N. R., Thorsen J., Pedersen A. G., Waage J., Rasmussen M. A., Stark K. D., Olsen S. F., Bønnelykke K. (2016) Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N. Engl. J. Med. 375, 2530–2539 [DOI] [PubMed] [Google Scholar]
- 19.Hansen S., Strøm M., Maslova E., Dahl R., Hoffmann H. J., Rytter D., Bech B. H., Henriksen T. B., Granström C., Halldorsson T. I., Chavarro J. E., Linneberg A., Olsen S. F. (2017) Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J. Allergy. Clin. Immunol. 139, 104–111.e4 [DOI] [PubMed] [Google Scholar]
- 20.Dunstan J. A., Mori T. A., Barden A., Beilin L. J., Taylor A. L., Holt P. G., Prescott S. L. (2003) Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J. Allergy Clin. Immunol. 112, 1178–1184 [DOI] [PubMed] [Google Scholar]
- 21.Kunisawa J., Arita M., Hayasaka T., Harada T., Iwamoto R., Nagasawa R., Shikata S., Nagatake T., Suzuki H., Hashimoto E., Kurashima Y., Suzuki Y., Arai H., Setou M., Kiyono H. (2015) Dietary ω3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci. Rep. 5, 9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunisawa J., Hashimoto E., Ishikawa I., Kiyono H. (2012) A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS One 7, e32094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asada Y., Nakae S., Ishida W., Hori K., Sugita J., Sudo K., Fukuda K., Fukushima A., Suto H., Murakami A., Saito H., Ebihara N., Matsuda A. (2015) Roles of epithelial cell-derived type 2-initiating cytokines in experimental allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 56, 5194–5202 [DOI] [PubMed] [Google Scholar]
- 24.Magone M. T., Chan C. C., Rizzo L. V., Kozhich A. T., Whitcup S. M. (1998) A novel murine model of allergic conjunctivitis. Clin. Immunol. Immunopathol. 87, 75–84 [DOI] [PubMed] [Google Scholar]
- 25.Matsuba-Kitamura S., Yoshimoto T., Yasuda K., Futatsugi-Yumikura S., Taki Y., Muto T., Ikeda T., Mimura O., Nakanishi K. (2010) Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int. Immunol. 22, 479–489 [DOI] [PubMed] [Google Scholar]
- 26.Ishida W., Fukuda K., Kajisako M., Sumi T., Matsuda H., Yagita H., Fukushima A. (2012) B and T lymphocyte attenuator regulates the development of antigen-induced experimental conjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 250, 289–295 [DOI] [PubMed] [Google Scholar]
- 27.Ohba M., Saeki K., Koga T., Okuno T., Kobayashi Y., Yokomizo T. (2018) Profiling of bioactive lipids in different dendritic cell subsets using an improved multiplex quantitative LC-MS/MS method. Biochem. Biophys. Res. Commun. 504, 562–568 [DOI] [PubMed] [Google Scholar]
- 28.Kuraishi Y., Nagasawa T., Hayashi K., Satoh M. (1995) Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur. J. Pharmacol. 275, 229–233 [DOI] [PubMed] [Google Scholar]
- 29.Andoh T., Sakai K., Urashima M., Kitazawa K., Honma A., Kuraishi Y. (2012) Involvement of leukotriene B4 in itching in a mouse model of ocular allergy. Exp. Eye Res. 98, 97–103 [DOI] [PubMed] [Google Scholar]
- 30.Nakazawa Y., Oka M., Takehana M. (2017) Model for studying anti- allergic drugs for allergic conjunctivitis in animals. Open Med. (Wars.) 12, 231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abelson M. B., Madiwale N., Weston J. H. (1983) Conjunctival eosinophils in allergic ocular disease. Arch. Ophthalmol. 101, 555–556 [DOI] [PubMed] [Google Scholar]
- 32.Stone K. D., Prussin C., Metcalfe D. D. (2010) IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 125(2 Suppl 2), S73–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giudetti A. M., Cagnazzo R. (2012) Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 99, 57–67 [DOI] [PubMed] [Google Scholar]
- 34.Wada M., DeLong C. J., Hong Y. H., Rieke C. J., Song I., Sidhu R. S., Yuan C., Warnock M., Schmaier A. H., Yokoyama C., Smyth E. M., Wilson S. J., FitzGerald G. A., Garavito R. M., Sui X., Regan J. W., Smith W. L. (2007) Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J. Biol. Chem. 282, 22254–22266 [DOI] [PubMed] [Google Scholar]
- 35.Foiles A. M., Kerling E. H., Wick J. A., Scalabrin D. M., Colombo J., Carlson S. E. (2016) Formula with long-chain polyunsaturated fatty acids reduces incidence of allergy in early childhood. Pediatr. Allergy Immunol. 27, 156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Best K. P., Sullivan T., Palmer D., Gold M., Kennedy D. J., Martin J., Makrides M. (2016) Prenatal fish oil supplementation and allergy: 6-year follow-up of a randomized controlled trial. Pediatrics 137, e20154443 [DOI] [PubMed] [Google Scholar]
- 37.Ciaccio C. E., Girdhar M. (2014) Effect of maternal ω3 fatty acid supplementation on infant allergy. Ann. Allergy Asthma Immunol. 112, 191–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Vaz N., Meldrum S. J., Dunstan J. A., Martino D., McCarthy S., Metcalfe J., Tulic M. K., Mori T. A., Prescott S. L. (2012) Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics 130, 674–682 [DOI] [PubMed] [Google Scholar]
- 39.DINO Steering Committee (2011) High-dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics 128, e71–e77 [DOI] [PubMed] [Google Scholar]
- 40.Galli S. J., Tsai M., Piliponsky A. M. (2008) The development of allergic inflammation. Nature 454, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leonardi A. (2002) The central role of conjunctival mast cells in the pathogenesis of ocular allergy. Curr. Allergy Asthma Rep. 2, 325–331 [DOI] [PubMed] [Google Scholar]
- 42.Swamy B. N., Chilov M., McClellan K., Petsoglou C. (2007) Topical non-steroidal anti-inflammatory drugs in allergic conjunctivitis: meta-analysis of randomized trial data. Ophthalmic Epidemiol. 14, 311–319 [DOI] [PubMed] [Google Scholar]
- 43.Ballas Z., Blumenthal M., Tinkelman D. G., Kriz R., Rupp G. (1993) Clinical evaluation of ketorolac tromethamine 0.5% ophthalmic solution for the treatment of seasonal allergic conjunctivitis. Surv. Ophthalmol. 38, 141–148 [DOI] [PubMed] [Google Scholar]
- 44.Guidera A. C., Luchs J. I., Udell I. J. (2001) Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology 108, 936–944 [DOI] [PubMed] [Google Scholar]
- 45.Tull S. P., Yates C. M., Maskrey B. H., O’Donnell V. B., Madden J., Grimble R. F., Calder P. C., Nash G. B., Rainger G. E. (2009) Omega-3 fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 7, e1000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calder P. C. (2013) Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 75, 645–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clutterbuck E. J., Hirst E. M., Sanderson C. J. (1989) Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood 73, 1504–1512 [PubMed] [Google Scholar]
- 48.Yamaguchi Y., Hayashi Y., Sugama Y., Miura Y., Kasahara T., Kitamura S., Torisu M., Mita S., Tominaga A., Takatsu K. (1988) Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J. Exp. Med. 167, 1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teixeira M. M., Wells T. N., Lukacs N. W., Proudfoot A. E., Kunkel S. L., Williams T. J., Hellewell P. G. (1997) Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J. Clin. Invest. 100, 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitaura M., Nakajima T., Imai T., Harada S., Combadiere C., Tiffany H. L., Murphy P. M., Yoshie O. (1996) Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J. Biol. Chem. 271, 7725–7730 [DOI] [PubMed] [Google Scholar]
- 51.Böhm E., Sturm G. J., Weiglhofer I., Sandig H., Shichijo M., McNamee A., Pease J. E., Kollroser M., Peskar B. A., Heinemann A. (2004) 11-Dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J. Biol. Chem. 279, 7663–7670 [DOI] [PubMed] [Google Scholar]
- 52.Grant G. E., Rokach J., Powell W. S. (2009) 5-Oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Mediat. 89, 98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peinhaupt M., Sturm E. M., Heinemann A. (2017) Prostaglandins and their receptors in eosinophil function and as therapeutic targets. Front. Med. (Lausanne) 4, 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terawaki K., Yokomizo T., Nagase T., Toda A., Taniguchi M., Hashizume K., Yagi T., Shimizu T. (2005) Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J. Immunol. 175, 4217–4225 [DOI] [PubMed] [Google Scholar]
- 55.Matsuoka T., Hirata M., Tanaka H., Takahashi Y., Murata T., Kabashima K., Sugimoto Y., Kobayashi T., Ushikubi F., Aze Y., Eguchi N., Urade Y., Yoshida N., Kimura K., Mizoguchi A., Honda Y., Nagai H., Narumiya S. (2000) Prostaglandin D2 as a mediator of allergic asthma. Science 287, 2013–2017 [DOI] [PubMed] [Google Scholar]
- 56.Woodward D. F., Hawley S. B., Williams L. S., Ralston T. R., Protzman C. E., Spada C. S., Nieves A. L. (1990) Studies on the ocular pharmacology of prostaglandin D2. Invest. Ophthalmol. Vis. Sci. 31, 138–146 [PubMed] [Google Scholar]
- 57.Spada C. S., Woodward D. F., Hawley S. B., Nieves A. L. (1986) Leukotrienes cause eosinophil emigration into conjunctival tissue. Prostaglandins 31, 795–809 [DOI] [PubMed] [Google Scholar]
- 58.Costa J. J., Weller P. F., Galli S. J. (1997) The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA 278, 1815–1822 [PubMed] [Google Scholar]
- 59.Woodward D. F., Nieves A. L., Friedlaender M. H. (1996) Characterization of receptor subtypes involved in prostanoid-induced conjunctival pruritus and their role in mediating allergic conjunctival itching. J. Pharmacol. Exp. Ther. 279, 137–142 [PubMed] [Google Scholar]
- 60.Nakajima M., Goh Y., Azuma I., Hayaishi O. (1991) Effects of prostaglandin D2 and its analogue, BW245C, on intraocular pressure in humans. Graefes Arch. Clin. Exp. Ophthalmol. 229, 411–413 [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto T., Igarashi A., Hoshina F., Yamada M., Toyoda Y., Notsu Y., Kohno S. (2003) Effects of nonsteroidal anti-inflammatory drugs on experimental allergic conjunctivitis in Guinea pigs. J. Ocul. Pharmacol. Ther. 19, 569–577 [DOI] [PubMed] [Google Scholar]
- 62.Andoh T., Nishikawa Y., Yamaguchi-Miyamoto T., Nojima H., Narumiya S., Kuraishi Y. (2007) Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J. Invest. Dermatol. 127, 2042–2047 [DOI] [PubMed] [Google Scholar]
- 63.Kim N., Ramon S., Thatcher T. H., Woeller C. F., Sime P. J., Phipps R. P. (2016) Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur. J. Immunol. 46, 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogerio A. P., Haworth O., Croze R., Oh S. F., Uddin M., Carlo T., Pfeffer M. A., Priluck R., Serhan C. N., Levy B. D. (2012) Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. 189, 1983–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aoki H., Hisada T., Ishizuka T., Utsugi M., Kawata T., Shimizu Y., Okajima F., Dobashi K., Mori M. (2008) Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 367, 509–515 [DOI] [PubMed] [Google Scholar]
- 66.Livne-Bar I., Wei J., Liu H. H., Alqawlaq S., Won G. J., Tuccitto A., Gronert K., Flanagan J. G., Sivak J. M. (2017) Astrocyte-derived lipoxins A4 and B4 promote neuroprotection from acute and chronic injury. J. Clin. Invest. 127, 4403–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodges R. R., Li D., Shatos M. A., Serhan C. N., Dartt D. A. (2016) Lipoxin A4 counter-regulates histamine-stimulated glycoconjugate secretion in conjunctival goblet cells. Sci. Rep. 6, 36124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lippestad M., Hodges R. R., Utheim T. P., Serhan C. N., Dartt D. A. (2017) Resolvin D1 increases mucin secretion in cultured rat conjunctival goblet cells via multiple signaling pathways. Invest. Ophthalmol. Vis. Sci. 58, 4530–4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biteman B., Hassan I. R., Walker E., Leedom A. J., Dunn M., Seta F., Laniado-Schwartzman M., Gronert K. (2007) Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 21, 2257–2266 [DOI] [PubMed] [Google Scholar]
- 70.Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serhan C. N., Yang R., Martinod K., Kasuga K., Pillai P. S., Porter T. F., Oh S. F., Spite M. (2009) Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 206, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.