Abstract
Colonization of the gut by certain probiotic Lactobacillus reuteri strains has been associated with reduced risk of inflammatory diseases and colorectal cancer. Previous studies pointed to a functional link between immunomodulation, histamine production, and folate metabolism, the central 1-carbon pathway for the transfer of methyl groups. Using mass spectrometry and NMR spectroscopy, we analyzed folate metabolites of L. reuteri strain 6475 and discovered that the bacterium produces a 2-carbon-transporting folate in the form of 5,10-ethenyl-tetrahydrofolyl polyglutamate. Isotopic labeling permitted us to trace the source of the 2-carbon unit back to acetate of the culture medium. We show that the 2C folate cycle of L. reuteri is capable of transferring 2 carbon atoms to homocysteine to generate the unconventional amino acid ethionine, a known immunomodulator. When we treated monocytic THP-1 cells with ethionine, their transcription of TNF-α was inhibited and cell proliferation reduced. Mass spectrometry of THP-1 histones revealed incorporation of ethionine instead of methionine into proteins, a reduction of histone-methylation, and ethylation of histone lysine residues. Our findings suggest that the microbiome can expose the host to ethionine through a novel 2-carbon transporting variant of the folate cycle and modify human chromatin via ethylation.—Röth, D., Chiang, A. J., Hu, W., Gugiu, G. B., Morra, C. N., Versalovic, J., Kalkum, M. The two-carbon folate cycle of commensal Lactobacillus reuteri 6475 gives rise to immunomodulatory ethionine, a source for histone ethylation.
Keywords: ethenyltetrahydrofolate, probiotic bacteria, microbiome, posttranslational modification, lysine ethylation
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (1), and they possess the ability to restore the composition of and introduce beneficial functions to intestinal microbiota. The beneficial functions of probiotics include suppression of pathogens, modulation of the immune system, fortification of the intestinal barrier, and exertion of different metabolic effects via secretion of specific bacterial enzymes or metabolites (2). In particular, probiotic Lactobacillus reuteri exerts anti-inflammatory, antimicrobial, and immunomodulatory effects on the host. L. reuteri is indigenous to the gastrointestinal tract of various mammalian species including humans, mice, rat, and pigs (3, 4). Patients with inflammatory bowel diseases have a reduced number of intestinal lactobacilli (5–8), and oral supplementation of probiotic Lactobacillus species can effectively improve the intestinal inflammation and gut barrier function in patients with Crohn’s disease and ulcerative colitis (9–12). However, the underlying mechanisms of probiotics’ effects on the host are still largely unknown.
The metabolites produced by the intestinal microbiota can directly affect the host through various mechanisms. Commensal bacterial fermentation of nondigestible carbohydrates leads to increased concentration of the luminal short-chain fatty acids (SCFAs) including acetate, butyrate, and propionate (13, 14). These SCFAs contribute to different functions in the health of the host, but in general, a greater increase in SCFA production in the colon results in protective effects including the reduction of the risks for developing gastrointestinal disorders, cancers, and cardiovascular diseases (15). In addition, intestinal microbiota play an important role in synthesizing vitamins and in catabolizing proteins to amino acid and other metabolites that include biogenic amines, immunomodulatory compounds, and other signaling molecules (16). The immunoregulatory compound histamine, generated through decarboxylation of l-histidine, is produced by several microbes that express histidine decarboxylase (17, 18). In contrast to mast cell-derived histamine and allergic inflammation, bacterial-derived histamines yielded anti-inflammatory effects in mammalian hosts, depending on the relative distribution of histamine receptors. Specifically, histamine production by L. reuteri ATCC 6475 suppresses the production of proinflammatory cytokines in intestinal epithelial cells and macrophages in vitro (17, 19, 20). Administration of live L. reuteri 6475, cell-free culture supernatants, or histamine suppresses the intestinal inflammation in mouse colitis models (19–21).
Moreover, L. reuteri produces high levels of folate (vitamin B9) (22, 23). Folate is essential for several metabolic preprocesses involved in the transfer and formation of 1-carbon (C1) units. This C1 metabolism is critical for the purine synthesis, thymidylate synthesis, methylation reaction, and the metabolism of several amino acids (24). Furthermore, folate synthesis is associated with the immunomodulatory function and histamine production of L. reuteri 6475. Recently, we identified the production of 5,10-methenyl-tetrahydrofolyl (5,10-methenyl-THF) polyglutamate by L. reuteri 6475 using matrix-assisted laser desorption/ionization (MALDI)- time-of-flight (TOF) (25). Inactivation of the bifunctional dihydrofolate synthase/folypolyglutamate synthase type 2 (folC2) gene inhibited the biosynthesis of 5,10-methenyl-THF polyglutamate as well as L. reuteri–mediated production of histamine, suppression of proinflammatory cytokines in macrophages, and the anti-inflammatory effect in a 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced mouse colitis (25). Nevertheless, how folate synthesis contributes to the immunosuppressive phenotype of L. reuteri 6475 requires further investigation.
In this study, we demonstrate that L. reuteri 6475 produces a novel form of folate that is analogous to 5,10-methenyl-THF, but incorporates an additional methyl group at the folate’s reactive center. This new form, 5,10-methylmethenyl-THF polyglutamate, or short 5,10-ethenyltetrahydrofolyl (5,10-EtTHF) polyglutamate, is created by incorporation of 2 carbon atoms from acetate. EtTHF is biologically active and can transfer 2 carbons onto homocysteine, resulting in the formation of the amino acid ethionine. Ethionine is an analog to methionine; it contains an ethyl group instead of a methyl group at the sulfur. We show that ethionine has an immunosuppressive effect on LPS-stimulated THP-1 monocytes, which import and use this unconventional amino acid. Hereby, ethionine can suppress histone methylation. Furthermore, it leads to histone ethylation and direct incorporation of ethionine into proteins instead of methionine.
MATERIALS AND METHODS
Reagents were purchased from MilliporeSigma (Burlington, MA, USA) unless otherwise described. Stable isotope labeled 15N3-13C6 L-histidine was from Cambridge Isotope Laboratories (Tewksbury, MA, USA).
L. reuteri culture
L. reuteri was cultured in Lactobacillus defined medium (LDM) 3 or 4 in an anaerobic hood (Bactron IV; Sheldon Manufacturing, Cornelius, OR, USA). Colonies were transferred from De Man, Rogosa, and Sharpe medium plates to liquid De Man, Rogosa, and Sharpe medium to grow a preculture (20, 26, 27). Cell densities corresponding to 0.1 optical density of preculture were used to inoculate experimental cultures that were grown in LDM3 or LDM4.
MALDI TOF mass spectrometry analysis of L. reuteri folates
L. reuteri cell pellets were washed with PBS and water, and folates were extracted with 0.1% TFA and 50% acetonitrile. Folates were spotted in α-cyano-4-hydroxycinnamic acid (CHCA) matrix onto a MALDI target. Spectra were acquired on a Combo 200 mass spectrometer (SimulTOF, Sudbury, MA, USA) in reflector mode and annotated with MoverZ software (Proteometrics, New York, NY, USA).
NMR characterization of 5,10-EtTHF
Folates from L. reuteri grown in LDM containing [13C]2-acetate were extracted with 0.1% TFA, 50% acetonitrile and purified on a strong ion exchange solid phase extraction column (Agilent Bont Elute; Agilent Technologies, Santa Clara, CA, USA). To do so, the pH was raised to 10 with ammonia to bind polyglutamate tails to the column. Wash steps were performed with 5% ammonia, 100% methanol, and 2% formic acid in methanol. Folates were eluted with 10% formic acid and 50% acetonitrile, and the solvent was evaporated. To the purified 5, 10-EtTHF sample, 200 µl solvent containing 50% CD3CN (deuterated acetonitrile), 49.9% D2O [deuteriumoxide (2H2O)], 0.1% TFA, and 4,4-dimethyl-4-silapentane-1-sulfonic acid (125 μM) was added. The solution was transferred into a 3-mm NMR tube. An additional 1 μl [13C]2-acetate (118 mM) was added to the 5, 10-EtTHF sample for the controlled experiments. All the NMR experiments were carried out at 25°C on Bruker Ascend 700 MHz instrument equipped with TCI cryoprobe. The 1H-[13C] heteronuclear single quantum coherence (HSQC) experiments were acquired with low and high resolution along the [13C] dimension. For the low resolution 1H-[13C] HSQC, the spectrum width was 12 and 70 ppm for 1H and [13C] dimension with 2048 and 200 data points, respectively, with [13C] carrier frequency at 45 ppm. For the high resolution 1H-[13C] HSQC, the spectrum was 12 and 4.7 ppm for 1H and [13C] dimension with 2048 and 80 data points, respectively, with [13C] carrier frequency at 25 ppm. The 3-dimensional (3D) NMR experiment designed to correlate methyl proton 1H, methyl 13C, and 13CO (HMCMCO) chemical shifts through the 1J(13C-1H) and 1J(13C-13CO) coupling constants for the moiety of 13CH3-13COR was modified from the HCACO experiment and was used to correlate the methyl proton, the methyl carbon, and the carbonyl carbon of acetate or the imidine carbon of 5,10-methylmethenyl-THF. The spectrum width in 3D were 13, 8, and 14 ppm for 1H, [13C] (methyl), and [13C] (CO) with acquisition points of 2048, 20, and 34, respectively. The [13C] carrier was set to 25 and 178 ppm for methyl and CO carbon dimensions. The 2D correlation between 1H and [13C] (CO) dimension experiment using 3D HMCMCO sequence was carried out by setting the spectrum width of 1H and [13C] to 13 and 50 ppm with acquisition points of 2048 and 120, respectively. The data were processed and analyzed using Bruker topspin or NMRpipe (28, 29). The 1H and [13C] chemical shifts were referenced to 4,4-dimethyl-4-silapentane-1-sulfonic acid.
Ethionine and ethionine-d5 synthesis
Ethionine was synthesized from iodoethane or deuterated iodoethane (iodoethane-d5) and homocysteine in the presence of 1,1,3,3-tetramethylguanidine according to a protocol by Włostowski et al. (30). In brief, homocysteine was dissolved in methanol and mixed with 2 M equivalents of 1,1,3,3-tetramethylguanidine. Then 1 M equivalent of iodoethane was added, and the reaction was performed at RT for 1 h. After the methanol evaporated, 1 M equivalent of HCl was added and the precipitate was filtered and washed with water. The resulting ethionine was dissolved in 50% acetonitrile with 0.1% formic acid and analyzed with an ion trap LTQ mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
Determination of ethionine concentrations in bacterial culture supernatants
Spent L. reuteri culture supernatants were diluted with water, and d5-ethionine (100 fg/µl) was spiked into the sample. Chromatographic separation was achieved with a C18 column (Kinetex 2.6 µm particles, 100 Å pore size, 50 × 2.1 mm; Phenomenex, Torrance, CA, USA) and a buffer system consisting of aqueous 0.1% TFA and 0.1% TFA in methanol. Mass spectrometric quantitation was performed on a triplequadrupole mass spectrometer (6490; Agilent Technologies), using an optimized multiple reaction monitoring method in which the transitions m/z 169.07→151.9 (quantifier) and m/z 169.07→122.9 (qualifier) were monitored for ethionine-d5 and m/z 164.07→117.9 (quantifier) and m/z 164.07→146.9 (qualifier) for ethionine, respectively.
THP-1 cell culture
Human monocytic THP-1 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in unmodified RPMI-1640 Medium (MilliporeSigma) (31) with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% carbon dioxide, and treated with either ethionine-d5 or ethionine (see Figs. 5–7).
Histone extraction, in-gel alkylation, and digestion
THP-1 cells were treated with lysis buffer [PBS with 0.5% Triton X-100 and Halt Protease inhibitors (Thermo Fisher Scientific)]. The nuclei were collected by centrifugation and washed with lysis buffer. Histones were extracted with HCl (2 M) overnight. Nuclei were centrifuged, and the histone-containing supernatant was collected and neutralized with NaOH. Histone samples were loaded onto a SDS-polyacrylamide gel (4–12% Nupage BisTris Gel; Thermo Fisher Scientific) and stained with Coomassie (Simply Blue Safe Stain; Thermo Fisher Scientific). Bands containing histones were excised, and proteins therein were reduced and alkylated with tris(2-carboxyethyl)phosphine and iodoacetamide. Lysines were alkylated with propionic anhydride. Afterward, proteins were digested with Trypsin (Promega, Madison, WI, USA). The resulting peptides were dissolved in 0.1% formic acid (FA).
Mass spectrometry of histone peptides
Peptides were analyzed on an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific) with mass spectrometry (MS) and tandem mass spectrometry (MS/MS) measured in the orbitrap mass analyzer. Chromatography was performed using Easy Nano Spray columns (PepMap, RSLC, 25 cm; Thermo Fisher Scientific) and a 45-min gradient from 97% Buffer A (0.1% FA) and 3% Buffer B (0.1% FA in acetonitrile) to 38% Buffer B after 40 min and 85% Buffer B after 41 min. For data analysis, chromatograms were aligned with Progenesis QI (Waters, Milford, MA, USA) and features exported to Peaks 7.0 (Bioinformatics Solutions, Waterloo, ON, Canada) for protein database matching. Precursor mass tolerance was set to 10 ppm and fragment tolerance to 0.2 Da. Lysine acetylation, propionylation, and mono, di- and trimethylation as well as mono, di- and triethylation(-d5) and the possible combinations of those posttranslational modifactions were set as dynamic modifications during searches. Additionally arginine mono- and dimethylation and mono- and di-ethylation(-d5) as well as acetylation were set as dynamic modifications. Methionine oxidation was set as a dynamic modifaction. To detect ethionine(-d5) in proteins, methionine methylation was used as a search parameter. Carbamidomethylation was set as a fixed modification for cysteine. Search results were imported to Progenesis QI (Waters) and filtered for histone peptides, and relative abundances of modified peptides were calculated.
Quantitative RT-PCR
RNA was isolated from THP-1 cells by using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. Quantitation and quality control (A260/A280 ratio) were performed with a Nanodrop (Thermo Fisher Scientific) spectrophotometer. MuLV II Reverse Transcriptase (Thermo Fisher Scientific) was used for reverse transcription, and SYBR green master mix (Thermo Fisher Scientific) was used for quantitative PCR. The following amplification conditions were used: after an initial denaturation at 95°C for 10 min 40 amplification cycles of amplification were performed at 95°C for 15 s and 60°C for 1 min on a CFX Connect Real Time Detection System (Bio-Rad, Hercules, CA, USA). Primers used in this study were TNF-α: forward 5′-GCCGCATCGCCGTCTCCTAC-3′, reverse 5′-AGCGCTGAGTCGGTCACCCT-3′; GAPDH: forward 5′-TCGCCCCACTTGATTTTG-3′, reverse 5′-GAGGGATCTCGCTCCTGGAAGA-3′; and IκBα: forward 5′-CGCCCAAGCACCCGGATACA-3′, reverse 5′-AGGGCAGCTCGTCCTCTGTGA-3′. For relative quantitation the ΔΔCt method was used and mRNA levels were normalized to GAPDH mRNA levels.
RESULTS
Identification of a novel folate with a +14-Da mass shift compared with 5,10-methenyltetrahydrofolyl polyglutamate
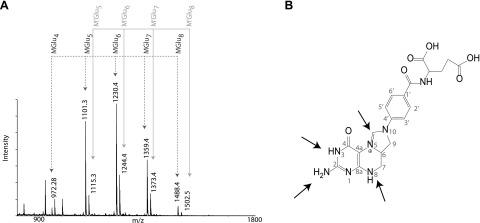
MALDI-MS spectra of acid extracted folates from L. reuteri cell pellets typically contain a series of equidistant peaks corresponding to 5,10-methenyl-THF with polyglutamate tails of variant length (MGlu). The peaks were separated by 129 Da, corresponding to the residue mass of glutamic acid (Fig. 1). Culturing of L. reuteri in LDM3 (27) led to the formation of a second series of THF polyglutamates (M’Glu), in which each ion signal differs from the corresponding 5,10-methenyl-THF-Glun peak by +14 Da, the mass of a methyl group (Fig. 1A). Methylation of THF could possibly occur at several nitrogen atoms present in the folic acid head group or by incorporation of an additional carbon into the active center of the vitamin, between N5 and N10, which would putatively result in a methylmethenyl-tetrahydrofolate or 5,10-EtTHF (Fig. 1B).
Figure 1.
L. reuteri produces novel folates. A) MALDI-TOF spectrum of L. reuteri 5,10-methenyl-THF-polyglutamates (MGlun); arrows point to signals MGlun with 4–8 glutamates. The novel folate has a 14-Da increased mass (M’Glun). B) Arrows indicate the potential localization of an additional methyl group within 5,10-methenyl-THF.
De novo synthesis of folic acid by L. reuteri
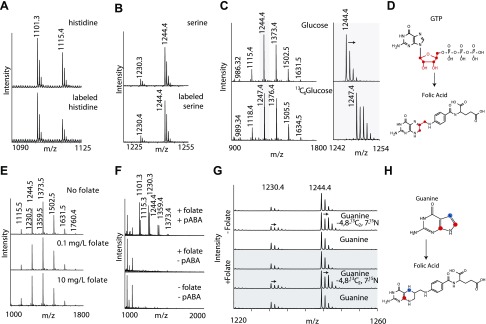
To determine how this novel folate molecule was generated by L. reuteri, we first investigated the relevant biochemical carbon sources for L. reuteri. We measured bacterial utilization of stable isotope labeled versions of known precursors of the folate metabolism. Substitution of histidine with 15N3[13C]6-histidine in the medium did not result in a mass shift from the conventional 5,10-methenyl-THF-Glu5 (at m/z 1001.3) or its methylated form (at m/z 1115.4) (Fig. 2A). Likewise, replacement of serine by [13C]3-serine did not change the mass of either folate form (Fig. 2B), demonstrating that under the present culture conditions, L. reuteri uses neither serine or histidine as substrates for 5,10-methenyl-THF or the related novel folate form.
Figure 2.
The additional methyl group in the novel folate form is not derived from histidine, serine, or glucose. A–C) Representative MALDI-TOF mass spectra of folylpolyglutamates from L. reuteri cultured in LDM containing unlabeled (top) or isotopically labeled (bottom) 15N3[13C]6-histidine (A), [13C]3-serine (B), or [13C]6-glucose (C). D) Structures representing the synthesis of folic acid from GTP. Red dots show the 5 glucose [13C] atoms that form the ribose part of GTP and are incorporated into folic acid. E–G) MALDI-TOF mass spectra of folylpolyglutamates from L. reuteri cultured in LDM containing increasing amounts of folic acid (E), with or without pABA (F), or isotopically labeled guanine (G). H) Structures representing the synthesis of folic acid from guanine. Colored dots highlight [13C] and 15N atoms in guanine and the synthesized folic acid.
We reasoned that glucose can be metabolized to formate during fermentation and be subsequently used to generate N10-formyl-THF (32). However, when providing [13C]6-glucose as the main carbon source of the medium, we observed a 3-Da mass shift (Fig. 2C), consistent with the incorporation of 3 carbons atoms into 5,10-methenyl-THF and into the novel folate form. If glucose carbon atoms were incorporated via formate into folates, a mass shift of 1 and 2 Da would be expected for 5,10-methenyl-THF and of the novel formate form, respectively. The observed 3-Da mass shift is likely because the glucose was first metabolized to ribose, which was then used to synthesize GTP. GTP in turn is a substrate of the enzyme GTP cyclohydrolase (23), which converts it into a pteridine ring system (Fig. 2D). During this reaction, 2 carbon atoms from ribose were removed; therefore, 3 carbon atoms from glucose were incorporated into the folate head group. This incorporation was observed by 3-Da mass shifts for both the 5,10-methenyl-THF-Glun and the novel form of THF polyglutamates (Fig. 2C).
L. reuteri has the capacity to synthesize THF-polyglutamates and expresses the essential enzymes folC and folC2. Mutation of folC leads to the synthesis of folic acid, which is not polyglutamylated, whereas the folC2 mutant did not produce any folates (25). Although folate deficiency leads to reduced immunomodulation, it did not affect L. reuteri’s viability. To further investigate this finding we cultured L. reuteri in media containing variable concentrations of folic acid (Fig. 2E). A change in folic acid concentrations had no effect on growth or the peak intensity, and by inference the amount of THF-Gluns retrieved from L. reuteri. Similarly, removal of para-aminobenzoic acid (pABA) from the medium formulation did not affect growth rates. However, in the absence of pABA, THFs were not detected in the cell pellet (Fig. 2F), independent of the presence of folic acid in the medium, demonstrating that L. reuteri 6475 does not import folic acid from the exterior but requires pABA to synthesize folic acid. To further confirm the de novo synthesis of folic acid, L. reuteri was cultured in a medium containing 4,8-[13C]2,7-15N-guanine. During folate biosynthesis C8 was removed and C4 and N7 were retained. Accordingly, we detected a mass shift of 2 Da in 5,10-methenyl-THF and in the novel THF form (Fig. 2G, H).
The novel folate form is 5,10-methylmethenyl-THF (5,10-EtTHF), generated by carbon uptake from acetate
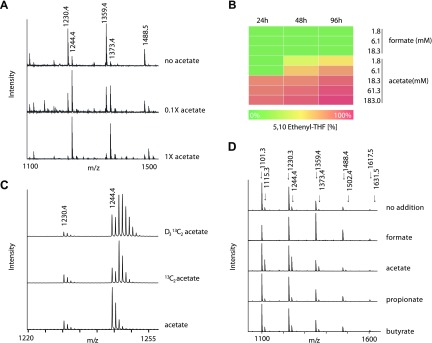
Traditionally, lactobacilli are cultured in media with high acetate concentrations to prevent the growth of (most) other bacteria, which are intolerant to acetate. Therefore, we tested whether the availability of acetate in the medium affects the ratio of the produced 5,10-methenyl-THF to the +14-Da mass shifted novel folate form. Decreasing the acetate concentration shifted the ion signal ratio toward 5,10-methenyl-THF (Fig. 3A), suggesting acetate as a substrate. The presence of formate (1.8 mM or higher) inhibited the production of the novel folate compound considerably (Fig. 3B, D).
Figure 3.
L. reuteri produces the novel folate, 5,10-ethenyltetrahydrofolyl polyglutamate, from acetate. A, C, D) MALDI-TOF mass spectrometric data of folylpolyglutamates from L. reuteri cultured in LDM containing 0 mM, 18.3 mM (0.1×), and 183 mM (1×) acetate (A), isotopically labeled acetate (C), or 183 mM of the indicated SCFAs or 18.3 mM formate (D). B) Ratios of 5,10-ethenyl-THF to 5,10-methenyl-THF after culturing in varying concentrations of formate or acetate at 3 distinct time points.
We then replaced acetate with [13C]2-acetate and D3[13C]2-acetate. The novel folate forms extracted from L. reuteri cultured with [13C]2 acetate showed a +2-Da mass shift (at m/z 1246.4) compared with the form observed in the culture with unlabeled acetate (signal at m/z 1244.4) (Fig. 3C). Most importantly, 5,10-methenyl-THF did not change its mass, demonstrating the incorporation of acetate carbons only into the novel folate form. The isotopic pattern of folates from cultures containing D3[13C]2 showed that heavy isotopes were incorporated with a mixed +3- to +4-Da shift (Fig. 3C). This mass shift can be explained by the incorporation of the 2 acetate carbon atoms together with 1 or more deuterium atoms. Additional deuterons are likely exchanged by water protons hinting a reaction mechanism that at some point increases the acidity of the α carbon atom.
Next, we investigated whether SCFAs other than formate or acetate can serve as substrates for the folate cycle in L. reuteri 6475. The bacteria were cultured in media containing propionate or butyrate. No signals were observed that would suggest the incorporation of multiple carbon atoms from propionate or butyrate, limiting the incorporation to C1 and C2 units from formate and acetate, respectively (Fig. 3D). The MALDI mass spectra of the THF-Gluns resemble those from culture conditions in LDM3 to which neither formate nor acetate were added (Fig. 3D, no addition). The ion series of such spectra contains predominantly the regular 5,10-methenyl-THF-GLun-form with a minor +14-Da peak of the novel form. We concluded that these signals carried over from the conditions of the preculture.
Structural validation of L. reuteri-produced 5,10-EtTHF
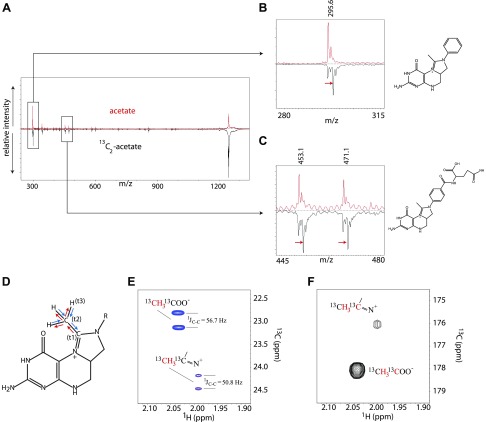
The incorporation of 2 carbons from acetate raised the possibility that 5,10-methylmethenyl-THF-Glun is formed instead of the extra methylation at a nitrogen in 5,10-methenyl-THF Glun. To localize the acetate carbons, L. reuteri was cultured in media with unlabeled acetate and with [13C]2-acetate, and extracted THF-Gluns were analyzed separately by MALDI-TOF/TOF MS (Fig. 4A). Fragment ions showed that acetate carbons were localized in the head group, ruling out any incorporation into the glutamate tail (Fig. 4B, C). However, the resulting fragments did not provide further information regarding the precise localization of the carbon atoms within the head group.
Figure 4.
L. reuteri produces 5,10-methylmethenyl (ethenyl) THF. A–C) MALDI-TOF/TOF MS/MS spectra (A) of SAX purified ethenyl THF Glu6 localizes the acetate-derived methyl group into the folate head group (B, C). D–F) NMR experiments. D) In the 3D HMCMCO experiment, the magnetization is transferred from methyl proton to methyl carbon, and then to the imidine carbon (blue arrows). After chemical shift labeling of the imidine carbon (t1), the magnetization is transferred back to methyl carbon (red arrows) followed by the chemical shift labeling (t2), and then back to methyl proton with proton detection (t3). E) Overlay of the 1H-[13C] HSQC spectra of the methyl groups from acetate with 5,10-ethenyl-THF (in blue) and 5,10-ethenyl-THF alone (in red). The signal splittings are from carbon-carbon 1-bond J couplings. F) Cross peak between methyl proton and carbonyl carbon in acetate, and imidine carbon in 5,10-ethenyl-THF. Red characters in E and F mark the 1H and [13C] nuclei from which the cross peaks were obtained.
To determine whether the 2 carbon atoms from acetate are contained within the methylmethenyl group in the THF-Glun sample, a [13C]-HSQC (33) with 70 ppm spectrum width along the [13C] dimension was carried out with carrier at 45 ppm. A cross peak at 24.34 ppm ([13C]) and 1.99 ppm (1H) was identified. These chemical shift values are very close to those of the methyl group in acetate, but were assigned unambiguously to methyl in the methylmethenyl group in N5,N10-ethylene THF (Fig. 4D, E). To exclude the unlikely presence of [13C]2-acetate in the sample giving this cross peak, [13C]2-acetate was spiked into the putative 5,10-methylmethenyl-THF-Glun sample. The methyl group of acetate was detected at 22.94 ppm ([13C]) and 2.04 ppm (1H), which proved that the cross peak at 24.34 ppm ([13C]) and 1.99 ppm (1H) was not from acetate (Fig. 4E). To query whether this methyl carbon is directly bound to another [13C]-enriched carbon, a high-resolution spectrum of [13C]-HSQC was acquired with and without acetate. The cross peak of the methyl group in N5, N10-methylmethylene THF was split along the [13C] dimension with a separation of 50.8 Hz (Fig. 4E). This value is a few hertz smaller than the average value (∼55 Hz) of 1-bond coupling between a Cα and a carbonyl carbon. The [13C]-[13C] 1-bond coupling of acetate in the same experiment is 56.7 Hz. The 50.8 Hz is also significantly larger than the [13C]-[13C] 1-bond coupling with an sp3-sp3 orbital structure (tetrahedral shapes), which is typically 30–35 Hz. Based on the observed [13C]-[13C] 1-bond coupling of 50.8 Hz, the carbon bound to the methyl group should be sp2 hybridized (34). The only carbon that is bound to a methyl group and meets the sp2 structural requirement is the carbon atom between N5 and N10 in 5,10-methylmethenyl-THF, forming a double bond with one of the nitrogen atoms. Based on the 1JC-C bond value of 50.8 Hz, and the chemical shift of 24.34 ppm ([13C]) and 1.99 ppm (1H), we can conclude that [13C]2-acetate was indeed incorporated into the methylmethenyl group of 5,10-methylmethenyl-THF-Glun. The chemical shift of carbon at a positively charged imidine could provide another piece of evidence to verify the incorporation of [13C]2-acetate into the methylmethenyl group. Accordingly, we designed a 3D HMCMCO experiment based on a 3D HCACO experiment (35) to correlate the chemical shifts of methyl proton, methyl carbon, and the carbon forming a double bond with N5 (or N10) in 5,10-ethenyl-THF. The magnetization transfer pathway (labeled as arrows) as well as the chemical shift labeling periods (labeled as t1, t2, and t3) on 3 atoms are shown in Fig. 4D. The chemical shifts of the carbonyl carbon of acetate and the imidine carbon of 5,10-methylmethenyl-THF were measured at 178.08 and 176.04 ppm, respectively, as shown in the 2D plane of the 3D HMCMCO experiment (Fig. 4F). The 176 ppm is on the high end of [13C] chemical shift range of imidine group. This experiment provides further confidence that both carbon atoms of the methylmethenyl group in 5,10- methylmethenyl-THF-Glun originated from the acetate provided in the culture medium. To our knowledge, 5,10-ethenyl-THF is a novel form of folate that has not been previously reported.
L. reuteri produces the immunomodulatory metabolite ethionine
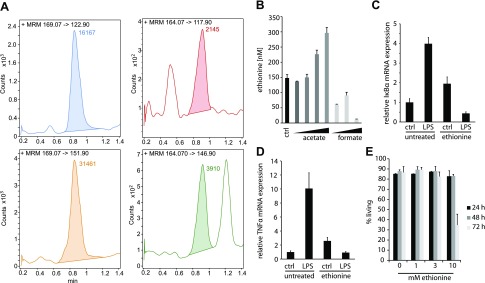
Folate metabolism is the central pathway for the transfer of C1 units. Among other biochemical reactions, the folate cycle transfers a methyl group onto homocysteine to synthesize methionine. To test whether 5,10-methylmethenyl-THF-Glun is a substrate of the same enzymatic pathway in L. reuteri and transfers 2 carbon atoms onto homocysteine, we developed an ultraperformance liquid chromatography triple quadrupole multiple reaction monitoring MS method to detect and quantify ethionine in bacterial cultures. For this we synthesized a deuterated ethionine standard and controlled its quality by ion trap MS (Supplemental Fig. S1). Chromatographic separation of spent culture supernatants was achieved by ion pairing with TFA on a C18 reverse phase column (Fig. 5A). L. reuteri was grown in media containing different amounts of acetate or formate. When L. reuteri was cultured in media with increasing acetate concentrations, a dose-dependent effect on the production of ethionine was observed. Likewise, little ethionine was detected in media containing formate (Fig. 5B).
Figure 5.
L. reuteri produces the immunomodulator ethionine. A) Triple quadrupole LC/MS multiple reaction monitoring (MRM) chromatograms of ethionine-d5 standard (left panels) and ethionine (right panels). The top row in each panel gives the m/z of the MRM transitions. Peak area counts of valid ethionine signals that have both quantifier and qualifiers are given in colored numbers. B) Quantification of ethionine in LDM3 after 7 d of culture. The LDM3 contained 0, 6.1, 18.3, 61.3, and 183 mM acetate or 6.1, 18.3, and 61.3 mM formate. C, D) mRNA levels of IκBα (C) and TNF-α (D) in THP-1 cells pretreated with 2.5 mM ethionine for 48 h and stimulated with 100 ng/ml LPS for 2 h. Expression was calculated with the ΔΔCt method and normalized to untreated samples. E) Cell viability of THP-1 cells after incubation with ethionine at given time points as determined by flow cytometry using forward scatter/side scatter exclusion.
Previous reports mention potential anti-inflammatory properties of the unconventional amino acid ethionine (36–38). Accordingly, we conducted a cell-based bio assay to assess this immunomodulatory effect. We incubated THP-1 monocytes with ethionine, stimulated with LPS, and then assessed NF-κB activation by measuring the induction of one of its target genes, IκBα (Fig. 5C). Ethionine-pretreated THP-1 cells did not upregulate IκBα after LPS stimulation, whereas untreated cells stimulated IkBa in response to LPS. Likewise, the transcription of the proinflammatory cytokine TNF-α was reduced in the presence of ethionine (Fig. 5D). Although greater ethionine concentrations (10 mM) led to reduced THP-1 viability after 72 h, these results did not explain the observed inhibitory effect of ethionine on LPS-induced immune cell activation (Fig. 5E).
Ethionine is incorporated into proteins
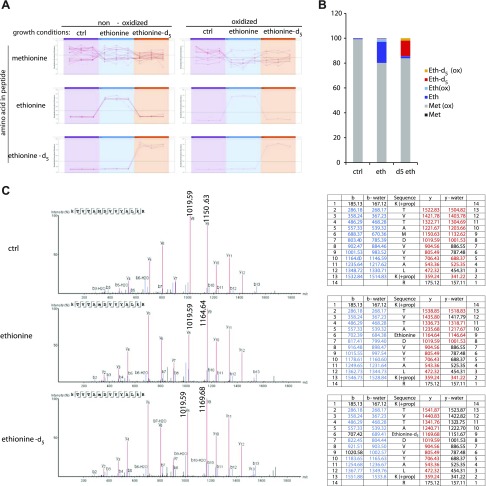
Ethionine is a homolog of the amino acid methionine, which serves as building block of proteins as well as a source of methyl group for various methylation reactions, including those that methylate proteins. However, the ethylation of proteins has never been demonstrated. Here, we analyzed histones to study ethionine incorporation and potential ethylation of histone lysine residues. THP-1 cells were cultured in the presence of ethionine or ethionine-d5-containing media, and histones were subsequently enriched by acidic extraction and SDS-PAGE. To prevent proteolysis by trypsin and to increase LC/MS retention times, we propionylated all unmodified lysine residues. Tryptic digestion peptides from histone [3H] containing methionine or oxidized methionine were detected ubiquitously in all samples. In contrast, ethionine or ethionine-d5 containing [3H] digest peptides were detected only when these uncommon amino acids were provided by the culture medium (Fig. 6A). Replacement of methionine by 2 mM ethionine or ethionine-d5 accounted for about 20% (Fig. 6B). Figure 6C shows representative spectra of an [3H] peptide in which methionine was replaced by ethionine or ethionine-d5. The series of y-ions up to y8 had identical m/z values in all 3 culture conditions. The y9 to y14 ions of the Eth-containing samples had a +14-Da mass shift, indicating replacement of methionine with ethionine. Consistently, peptides derived from ethionine-d5–treated samples had a +19-Da increase in their parent ion mass and in the m/z values of their y9 to y14 ions.
Figure 6.
Ethionine can replace methionine during protein biosynthesis. A) Relative quantitation of histone 3 peptides containing oxidized and non-oxidized methionine, ethionine, and ethionine-d5. Each line represents 1 peptide. B) Extent of incorporation of ethionine and ethionine-d5 into histone 3. C) Representative spectra and ion tables of the peptide KTVTAMDVVYALAK. B6-b13 ions and y9-y13 ions demonstrate the incorporation of ethionine (+14 Da) and ethionine-d5 (+19 Da). THP-1 cells were grown in the presence of 2.5 mM ethionine or ethionine-d5 for 48 h. Histones were isolated and ethionine incorporation was determined by LS-MS/MS. “K(+prop)” indicate propionylated lysine residues (see text).
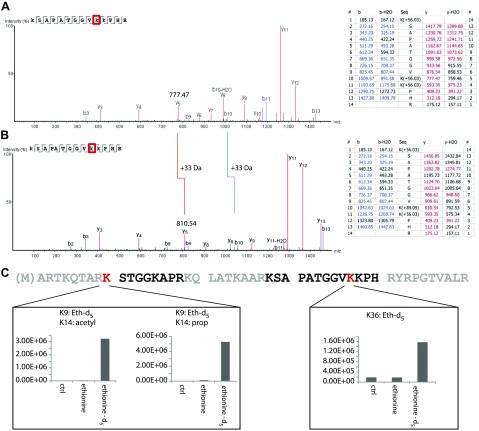
Methylation of histones is a posttranslational modification, which regulates gene expression. Previous studies showed that in vitro methylation of histones is inhibited by ethionine, and potential ethylation was not demonstrated. Because dimethylation and ethylation of lysine residues would result in the same mass shift of +28 Da, we compared untreated and ethionine-d5–treated samples. Figure 7A shows the MS/MS spectrum of an [3H] digest peptide, in which all lysine residues were propionylated (Δm = 56 Da). Y and b ion series of the same peptide (Fig. 7B) reveal a total mass shift of +89 Da (= 56 Da + 33 Da) for lysine10, which is consistent with a propyl- plus ethyl- double modification. Those modifications were also seen in the N-terminal tail of histone 3, where the regulatory lysines 9 and 36 were modified (Fig. 7C). In conclusion, ethionine is a bioactive compound capable of replacing methionine during protein translation and of inducing a novel posttranslational lysine modification such as lysine ethylation.
Figure 7.
Histone 3 lysine ethylation is a novel posttranslational modification. A, B) Representative MS/MS spectra and ion tables of the unmodified (A) and the ethylated (B) peptide KSAPATGGVKKPHR. B10-b13 ions and y5-y13 ions demonstrate the ethylation of K10 (+33 Da). C) N-terminal sequence of histone 3. Black letters mark peptides with identified lysine ethylation and red Ks mark the localization of the modified lysines. Lower panels: Quantitation of peptides containing ethionine-d5. Histone 3 peptides were from THP-1 cells grown in the presence of 2.5 mM ethionine or ethionine-d5 for 48 h.
DISCUSSION
In this study we showed that L. reuteri produces a novel folate when cultured in acetate-containing media. This novel form was identified as 5,10-methenyl-THF with an additional methyl group, as detected by NMR and MS. Other studies also described the existence of noncanonical folates (39, 40). Our results indicate that 5,10-methylmethylene-THF is produced by bacterial cells, suggesting that the mammalian microbiome may generate novel folate compounds. This novel folate is a substrate for the bacterial enzymes 5,10-methylene-THF reductase and 5,10-methylene-THF dehydrogenase (40, 41). Furthermore, our studies indicate that commensal gut microbes such as L. reuteri can generate uncommon amino acids such as ethionine, and ethionine may promote new mechanisms of protein modification such as lysine ethylation. Host protein ethylation may yield functional effects in mammals and serve as “fingerprints” of the microbiome in mammalian cell nuclei.
Whether 5,10-methylmethenyl-THF polyglutamates are also substrates of the folate cycle could not be observed directly in our experiments, because we observed only the presence of 5,10-methenyl-THF and 5,10-methylmethenyl-THF polyglutamates. However, the production of ethionine requires the interconversion of 5,10-ethenyl-THF to 5-ethyl-THF. The lack of detection of this intermediate folate, and others, is a consequence of the extraction conditions and charge stabilization. Folates are sensitive to light, oxidation, and acids (42). Therefore, most folates are converted into more stable derivates, such as the methenyl or ethenyl form.
The production of 5,10-ethenyl-THF could imply that it is either nonreactive, an inhibitor of folate-dependent enzymes, or is an alternative substrate of those enzymes. Folates transfer 1 carbon units from different sources to enable the synthesis of thymidylate, purines, and methionine (43). Here we demonstrated that 5,10-ethenyl-THF is an active compound that is used in the methionine-analogous synthesis pathway by L. reuteri to produce the amino acid ethionine. Previous studies showed ethionine biosynthesis by E. coli and other prokaryotes (44, 45). This production was later disputed, because no subsequent transfer of 2 C1 units was observed in an in vitro model that used 14C methionine (46). However, our data support the transfer of ethyl groups instead of methyl groups, resulting in ethionine biosynthesis.
Based on animal experiments, ethionine consumption was reportedly associated with pathophysiological outcomes, including pancreatitis, fatty liver disease, and hepatocellular cancer, which led to the investigation of ethionine as a carcinogen in mouse models (38). Under such circumstances, ethionine-producing L. reuteri strains would be unsuitable for probiotic interventions. However, unlike other carcinogens, the effects of ethionine can reportedly be suppressed, when sufficient methionine is present (38). Studies on the immune system of mice that were fed with an ethionine-containing diet showed reduced cell-mediated immunity, suggesting an immunosuppressive effect (47). The immunomodulatory effect was preserved when the diet contained enough methionine to prevent nonimmunologic phenotypes (36). Investigation of lymphocytes showed reduced blasting and proliferation upon stimulation with phytohemaglutinin or concavalin A, when they were pretreated with ethionine (37). Similarly, we found that in the presence of ethionine, the monocytic THP-1 cell line had a reduced expression of the inflammatory cytokines, probably via reduced activation of the NF-kB pathway.
The production of 5,10-ethenyl THF and ethionine was observed in LDM3, which contained between 6.1 and 180 mM acetate. The concentration of SCFAs, of which acetate compromises about 60%, is 70–140 mM in the human proximal colon to 20–70 mM in the distal colon. The production rate of SCFAs is 0.24–0.38/kg body weight/h (48). Lactobacillus spp. was reportedly found in the lumen of the jejunum, ileum, colon, and rectum (49). Although most of the amino acids are absorbed in the small intestine, human colonocytes are potentially capable of absorbing bacterially produced amino acids (50). For the immunomodulatory effect, local ethionine concentration should be more relevant than systemic distribution.
The mechanisms of ethionine-mediated suppression of immune responses and carcinogenesis are not fully defined. Most likely, ethionine interferes with multiple pathways, in which methionine is involved.
First, methionine is a proteinogenic amino acid. We found that THP-1 cells replace methionine with ethionine during protein expression. This finding is consistent with other studies that used radioactive ethionine to investigate incorporation into proteins (51, 52). Here, ethionine-loaded tRNA was a substrate for translation but was an inferior substrate for protein translation than methionine-loaded tRNA.
Methionine is a special amino acid because it is the first amino acid in the translation of most proteins. In vitro studies demonstrated that the ethionine can fulfill this role, albeit with lower efficiency (53). Therefore, immunomodulation by ethionine could be a consequence of reduced protein biosynthesis.
Methionine is the primary source for methyl groups used in DNA-, RNA-, and protein methylation. Methyl groups are first activated by ATP to form S-adenosylmethionine and then transferred to macromolecules. In analogy, ethionine in eukaryotic cells forms the homolog S-adenosylethionine (SAE) (54). Interestingly, S-adenosylmethionine synthases from E. coli and Salmonella do not accept ethionine as a substrate (55, 56). Therefore, ethionine does not interfere with transmethylation reactions in those bacteria, and they are protected from cytotoxic ethionine effects. However, in eukaryotes SAE is formed and has multiple effects. In general, SAE is a poor substrate for methyltransferases, leading to reduced levels of methylation of DNA and possibly changing epigenetic control on this level. Accordingly, histones showed a lower rate of methylation in the presence of ethionine/SAE. This effect is followed by an accumulation of SAE in ethionine-exposed cells and a reduction of available ATP for other reactions because it cannot readily be regenerated from SAE. Few data are present that indicate the involvement of ethionine in the ethylation of lysines. Experiments that used radioactively labeled ethionine in in vitro methylation assays remained inconclusive (37). However, MS of histones showed that ethylation of lysine residues is a novel modification.
Further investigation will be required to determine the consequences of histone ethylation. Because methylation is an epigenetic marker that is important in gene regulation, lysine ethylation could interfere with binding of proteins that exert diverse functions. In addition, it remains to be investigated whether ethyl groups are substrates of demethylases and whether ethylation is therefore reversible or a stable modification. In conclusion, we found that L. reuteri produces a novel folate form that leads to the production of the amino acid ethionine. Ethionine may suppress the activation of immune cells such as monocytes. These studies point investigators to new directions in exploring microbiome:mammalian interactions. By generating novel folate compounds and enhancing folate polyglutamylation, microbes may promote folate utilization and assimilation in mammalian cells. By fostering folate and 1-carbon metabolism, gut microbes such as L. reuteri may generate unusual amino acids such as ethionine, resulting in new types of protein (histone) modifications in mammalian cells. The intimate coordination of bacterial and mammalian biochemistry may result in profound effects on immune function and regulation of gene expression.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
D.R., A.J.C., C.N.M., J.V., and M.K. were supported by U.S. National Institutes of Health (NIH), National Cancer Institute Grant U01 CA170930 (to J.V.). The use of the City of Hope Mass Spectrometry and Proteomics core facility was partially supported by NIH, National Cancer Institute Grant P30 CA33572. The authors declare no conflicts of interest.
Glossary
- 3D
3-dimensional
- Eth
ethionine
- EtTHF
ethenyl-tetrahydrofolate
- FA
formic acid
- HMCMCO
methyl proton 1H, methyl 13C, and 13CO
- HSQC
heteronuclear single quantum coherence
- LDM
Lactobacillus defined medium
- MALDI
matrix assisted laser desorption/ionization
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- pABA
para-aminobenzoic acid
- SAE
S-adenosylethionine
- SCFA
short-chain fatty acid
- THF
tetrahydrofolate
- THF-Glun
tetrahydrofolylpolyglutamate
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. Röth designed and conducted research experiments, analyzed data, and wrote the manuscript; A. J. Chiang conducted experiments, analyzed data, and contributed to the manuscript; W. Hu conducted NMR experiments and analyzed data; G. B. Gugiu assisted with the triplequadrupole MS method development; C. N. Morra conducted experiments and provided materials; J. Versalovic designed research, provided strains, and interpreted data; M. Kalkum designed research, analyzed and interpreted data, and wrote the manuscript; and all authors contributed to writing the manuscript.
REFERENCES
- 1.Food and Agriculture Organization of the United Nations WHO (2006) Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation, Food and Agriculture Organization of the United Nations WHO, Rome, Italy [Google Scholar]
- 2.Saarela M., Lähteenmäki L., Crittenden R., Salminen S., Mattila-Sandholm T. (2002) Gut bacteria and health foods--the European perspective. Int. J. Food Microbiol. 78, 99–117 [DOI] [PubMed] [Google Scholar]
- 3.Walter J., Chagnaud P., Tannock G. W., Loach D. M., Dal Bello F., Jenkinson H. F., Hammes W. P., Hertel C. (2005) A high-molecular-mass surface protein (Lsp) and methionine sulfoxide reductase B (MsrB) contribute to the ecological performance of Lactobacillus reuteri in the murine gut. Appl. Environ. Microbiol. 71, 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuter G. (2001) The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr. Issues Intest. Microbiol. 2, 43–53 [PubMed] [Google Scholar]
- 5.Zella G. C., Hait E. J., Glavan T., Gevers D., Ward D. V., Kitts C. L., Korzenik J. R. (2011) Distinct microbiome in pouchitis compared to healthy pouches in ulcerative colitis and familial adenomatous polyposis. Inflamm. Bowel Dis. 17, 1092–1100 [DOI] [PubMed] [Google Scholar]
- 6.Vigsnæs L. K., Brynskov J., Steenholdt C., Wilcks A., Licht T. R. (2012) Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Benef. Microbes 3, 287–297 [DOI] [PubMed] [Google Scholar]
- 7.Scarpato E., Russo M., Staiano A. (2018) Probiotics in pediatric gastroenterology: emerging indications: inflammatory bowel diseases. J. Clin. Gastroenterol. 52(Suppl 1, Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10 to 12, 2017), S7–S9 [DOI] [PubMed] [Google Scholar]
- 8.Ganji-Arjenaki M., Rafieian-Kopaei M. (2018) Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J. Cell. Physiol. 233, 2091–2103 [DOI] [PubMed] [Google Scholar]
- 9.Gupta P., Andrew H., Kirschner B. S., Guandalini S. (2000) Is lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J. Pediatr. Gastroenterol. Nutr. 31, 453–457 [DOI] [PubMed] [Google Scholar]
- 10.Bibiloni R., Fedorak R. N., Tannock G. W., Madsen K. L., Gionchetti P., Campieri M., De Simone C., Sartor R. B. (2005) VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 100, 1539–1546 [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein L., Avni-Biron I., Ben-Bassat O. (2016) Probiotics and prebiotics in Crohn’s disease therapies. Best Pract. Res. Clin. Gastroenterol. 30, 81–88 [DOI] [PubMed] [Google Scholar]
- 12.Sebastián Domingo J. J. (2017) Review of the role of probiotics in gastrointestinal diseases in adults [in English, Spanish]. Gastroenterol. Hepatol. 40, 417–429 [DOI] [PubMed] [Google Scholar]
- 13.Cummings J. H., Macfarlane G. T. (1997) Role of intestinal bacteria in nutrient metabolism. JPEN J. Parenter. Enteral Nutr. 21, 357–365 [DOI] [PubMed] [Google Scholar]
- 14.Dahl W. J., Agro N. C., Eliasson Å. M., Mialki K. L., Olivera J. D., Rusch C. T., Young C. N. (2017) Health benefits of fiber fermentation. J. Am. Coll. Nutr. 36, 127–136 [DOI] [PubMed] [Google Scholar]
- 15.Topping D. L., Clifton P. M. (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064 [DOI] [PubMed] [Google Scholar]
- 16.Hollister E. B., Gao C., Versalovic J. (2014) Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146, 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas C. M., Hong T., van Pijkeren J. P., Hemarajata P., Trinh D. V., Hu W., Britton R. A., Kalkum M., Versalovic J. (2012) Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7, e31951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller M. F., Reeds P. J. (1998) Nitrogen cycling in the gut. Annu. Rev. Nutr. 18, 385–411 [DOI] [PubMed] [Google Scholar]
- 19.Gao C., Major A., Rendon D., Lugo M., Jackson V., Shi Z., Mori-Akiyama Y., Versalovic J. (2015) Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. MBio 6, e01358-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganesh B. P., Hall A., Ayyaswamy S., Nelson J. W., Fultz R., Major A., Haag A., Esparza M., Lugo M., Venable S., Whary M., Fox J. G., Versalovic J. (2018) Diacylglycerol kinase synthesized by commensal Lactobacillus reuteri diminishes protein kinase C phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. Mucosal. Immunol. 11, 380–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Mahony L., Akdis M., Akdis C. A. (2011) Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 128, 1153–1162 [DOI] [PubMed] [Google Scholar]
- 22.Santos F., Wegkamp A., de Vos W. M., Smid E. J., Hugenholtz J. (2008) High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl. Environ. Microbiol. 74, 3291–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi M., Amaretti A., Raimondi S. (2011) Folate production by probiotic bacteria. Nutrients 3, 118–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brosnan M. E., MacMillan L., Stevens J. R., Brosnan J. T. (2015) Division of labour: how does folate metabolism partition between one-carbon metabolism and amino acid oxidation? Biochem. J. 472, 135–146 [DOI] [PubMed] [Google Scholar]
- 25.Thomas C. M., Saulnier D. M. A., Spinler J. K., Hemarajata P., Gao C., Jones S. E., Grimm A., Balderas M. A., Burstein M. D., Morra C., Roeth D., Kalkum M., Versalovic J. (2016) FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. MicrobiologyOpen 5, 802–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Man J. C., Rogosa M., Sharpe M. E. (2008) A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23, 130–135 [Google Scholar]
- 27.Jones S. E., Versalovic J. (2009) Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 9, 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson B. A., Blevins R. A. (1994) NMR View: a computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 29.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 30.Włostowski M., Czarnocka S., Maciejewski P. (2010) Efficient S-alkylation of cysteine in the presence of 1,1,3,3-tetramethylguanidine. Tetrahedron Lett. 51, 5977–5979 [Google Scholar]
- 31.Moore G. E., Gerner R. E., Franklin H. A. (1967) Culture of normal human leukocytes. JAMA 199, 519–524 [PubMed] [Google Scholar]
- 32.Locasale J. W. (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodenhausen G., Ruben D. J. (1980) Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 69, 185–189 [Google Scholar]
- 34.Bystrov V. F. (1976) Spin—spin coupling and the conformational states of peptide systems. Prog. Nucl. Magn. Reson. Spectrosc. 10, 41–82 [Google Scholar]
- 35.Kay L. E., Ikura M., Tschudin R., Bax A. (2011) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. 1990. J. Magn. Reson. 213, 423–441 [DOI] [PubMed] [Google Scholar]
- 36.Aschkenasy A. (1975) Immunosuppressive effect of ethionine in rats. Resistance of this effect to methionine, tryptophan, ATP, adenosine and uridine [in French]. Ann. Nutr. Aliment. 29, 137–150 [PubMed] [Google Scholar]
- 37.Zabos P., Kyner D. A., Seide-Kehoe R., Acs G., Christman J. K. (1978) Effect of L-ethionine on macromolecular synthesis in mitogen-stimulated lymphocytes. Biochim. Biophys. Acta 520, 139–152 [DOI] [PubMed] [Google Scholar]
- 38.Alix J.-H. (1982) Molecular aspects of the in vivo and in vitro effects of ethionine, an analog of methionine. Microbiol. Rev. 46, 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaBaume L. B., Guynn R. W. (1985) Investigation into the substrate capacity of the acetaldehyde-tetrahydrofolate condensation product. Prog. Clin. Biol. Res. 183, 189–200 [PubMed] [Google Scholar]
- 40.Guynn R. W., Labaume L. B., Henkin J. (1982) Equilibrium constants under physiological conditions for the condensation of acetaldehyde with tetrahydrofolic acid. Arch. Biochem. Biophys. 217, 181–190 [DOI] [PubMed] [Google Scholar]
- 41.LaBaume L. B., Guynn R. W. (1985) Investigation into the substrate capacity of the acetaldehyde-tetrahydrofolate condensation product. Prog. Clin. Biol. Res. 183, 189–200 [PubMed] [Google Scholar]
- 42.De Paiva E. P., Costa M. M. A., de Azevedo C. A. (2015) Folate - analytical properties, bioavailability and stability in foods. Scientia Chromatographica 7, 199–222 [Google Scholar]
- 43.Bailey L. B., Gregory J. F., III (1999) Folate metabolism and requirements. J. Nutr. 129, 779–782 [DOI] [PubMed] [Google Scholar]
- 44.Fisher J. F., Mallette M. F. (1961) The natural occurrence of ethionine in bactdria. J. Gen. Physiol. 45, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loerch J. D., Mallette M. F. (1963) Ethionine biosynthesis in Escherichia coli. Arch. Biochem. Biophys. 103, 272–275 [DOI] [PubMed] [Google Scholar]
- 46.Villanueva V. R., Barbier M., Gros C., Lederer E. (1966) On the “ethionine” produced by Escherichia coli B [in French]. Biochim. Biophys. Acta. 130, 329–335 [PubMed] [Google Scholar]
- 47.Radix P. M., Walters C. S., Adkins J. A. (1983) The influence of ethionine-supplemented soy protein diet on cell-mediated and humoral immunity. J. Nutr. 113, 159–164 [DOI] [PubMed] [Google Scholar]
- 48.Den Besten G., van Eunen K., Groen A. K., Venema K., Reijngoud D.-J., Bakker B. M. (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi H., Takahashi R., Nishi T., Sakamoto M., Benno Y. (2005) Molecular analysis of jejunal, ileal, caecal and recto-sigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. J. Med. Microbiol. 54, 1093–1101 [DOI] [PubMed] [Google Scholar]
- 50.Van der Wielen N., Moughan P. J., Mensink M. (2017) Amino acid absorption in the large intestine of humans and porcine models. J. Nutr. 147, 1493–1498 [DOI] [PubMed] [Google Scholar]
- 51.Maw G. A. (1966) Incorporation and distribution of ethionine-sulfur in the protein of ethionine-sensitive and ethionine-resistant yeasts. Arch. Biochem. Biophys. 115, 291–301 [DOI] [PubMed] [Google Scholar]
- 52.Rabinovitz M., Olson M. E., Greenberg D. M. (1957) Characteristics of the inhibition by ethionine of the incorporation of methionine into proteins of the Ehrlich ascites carcinoma in vitro. J. Biol. Chem. 227, 217–224 [PubMed] [Google Scholar]
- 53.Brown J. L. (1973) The modification of the amino terminal region of Escherichia coli proteins after initiation with methionine analogues. Biochim. Biophys. Acta 294, 527–529 [DOI] [PubMed] [Google Scholar]
- 54.Cox R., Goorha S. (1984) Synthesis of S-adenosylethionine by the gamma isozyme of methionine adenosyltransferase from Friend erythroleukemic cells. Cancer Res. 44, 4938–4941 [PubMed] [Google Scholar]
- 55.Aswad D. W., Koshland D. E., Jr (1975) Evidence for an S-adenosylmethionine requirement in the chemotactic behavior of Salmonella typhimurium. J. Mol. Biol. 97, 207–223 [DOI] [PubMed] [Google Scholar]
- 56.Holloway C. T., Greene R. C., Su C. H. (1970) Regulation of S-adenosylmethionine synthetase in Escherichia coli. J. Bacteriol. 104, 734–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.