Abstract
Osteoarthritis (OA) is a disease characterized by cartilage damage and abnormal remodeling of subchondral bone. Our previous study showed that in the early stage of OA, knee loading exerts protective effects by suppressing osteoclastogenesis through Wnt signaling, but little is known about loading effects at the late OA stage. Endoplasmic reticulum (ER) stress and autophagy are known to be involved in the late OA stage. We determined the effects of mechanical loading on ER stress and autophagy in OA mice. One hundred seventy-four mice were used for a surgery-induced OA model. In the first set of experiments, 60 mice were devoted to evaluation of the role of ER stress and autophagy in the development of OA. In the second set, 114 mice were used to assess the effect of knee loading on OA. Histologic, cellular, microcomputed tomography, and electron microscopic analyses were performed to evaluate morphologic changes, ER stress, and autophagy. Mechanical loading increased phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) and regulated expressions of autophagy markers LC3II/I and p62. Osteoarthritic mice also exhibited an elevated ratio of calcified cartilage to total articular cartilage (CC/TAC), and synovial hyperplasia with increased lining cells was found. At the early disease stage, subchondral bone plate thinning and reduced subchondral bone volume fraction (B.Ar/T.Ar) were observed. At the late disease stages, subchondral bone plate thickened concomitant with increased B.Ar/T.Ar. Mice subjected to mechanical loading exhibited resilience to cartilage destruction and a correspondingly reduced Osteoarthritis Research Society International score at 4 and 8 wk, as well as a decrease in synovitis and CC/TAC. While chondrocyte numbers in the OA group was notably decreased, mechanical loading restored chondrogenic differentiation. These results demonstrate that mechanical loading can retard the pathologic progression of OA at its early and late stages. The observed effects of loading are associated with the regulations of ER stress and autophagy.—Zheng, W., Li, X., Liu, D., Li, J., Yang, S., Gao, Z., Wang, Z., Yokota, H., Zhang, P. Mechanical loading mitigates osteoarthritis symptoms by regulating endoplasmic reticulum stress and autophagy.
Keywords: OA, eIF2alpha, P62, knee load, non-invasive physical therapy
Osteoarthritis (OA) is a chronic disease in which the articular cartilage of the joints degenerates, causing pain and physical disability (1). Over 150 million people are affected by OA worldwide, and ∼37% of the U.S. population over 60 yr of age is projected to develop OA (2, 3). Multiple risk factors, including genetic factors, age, obesity, and prior joint injuries, contribute to OA development (4). Varying therapies have been applied in an effort to limit the progression of OA, including nonpharmacologic, pharmacologic, and surgical treatments (5). Nonpharmacologic treatment may consist of weight loss, biomechanical intervention, electromagnetic stimulation, and shock wave therapy (5–8). Several medications are routinely prescribed, including nonsteroidal antiinflammatory drugs. However, nonsteroidal antiinflammatory drugs have serious adverse effects, including an increased risk of gastrointestinal disorder and cardiovascular and renal injuries (9). Currently, late stage OA is commonly addressed with surgical intervention via total joint replacement (10). In the current work, we explored the therapeutic and disease modifying potential of noninvasive physical therapy in application for early and late stage OA.
Pulsating joint loading is a lateral compressive loading modality, which applies oscillatory mechanical loads to a synovial joint (11). In our previous studies, joint loading was shown to induce bone formation and accelerate healing of injured bones (12, 13). Pulsating joint loading can be targeted to various synovial joints in a form of elbow loading, knee loading, and ankle loading. Prior works have indicated that joint loading could increase thickness of long bones, and specifically that knee loading could induce anabolic responses, lengthen the femur and tibia, accelerate healing of surgical wounds, and protect against osteonecrosis of the femoral head (12, 14, 15). Prior work also indicated that joint loading can reduce matrix metalloproteinase 13 (MMP-13) activity in the cartilage (16, 17). Joint loading is considered to change intramedullary pressure of bone cavities. The load-driven pressure may generate fluid flow in a lacuna canalicular network in bone cortex, and activate anabolic genes in bone (18, 19). Compared to whole body vibration (20) or axial/bending loading modalities (21, 22), knee loading can be directly applied to an arthritic joint. Our previous studies have also indicated that knee loading may improve remodeling of subchondral bone in OA mice (23). However, little is known about the role of knee loading in OA-induced cartilage degeneration and osteophyte formation.
The balance between bone formation and bone resorption is broken in OA. In response to OA-driven abnormal metabolism, the articular cartilage and subchondral bone undergo uncontrolled catabolic and anabolic remodeling (23, 24). The endoplasmic reticulum (ER) is a cellular organelle critical for generating, modifying, and transporting proteins (25). Under conditions of ER stress, an unfolded protein response is initiated (26, 27); prolonged and unmitigated ER stress will culminate in unfolded protein response–driven apoptosis (27, 28). At the same time, ER stress may alternatively induce antiapoptotic responses (e.g., autophagy) and help cell survival (29).
Autophagy is a major catabolic process of eukaryotic cells that degrades and recycles damaged macromolecules and organelles (29, 30). In cartilage homeostasis, its activation is reported to reduce OA severity (31, 32). Many lines of evidence indicate that ER stress and autophagy are involved in both mineralization and bone homeostasis (33, 34). During the progression of cellular injury or apoptosis, we found that knee loading regulates a protein kinase RNA-like ER kinase (PERK) pathway (35). However, the contribution of ER stress and autophagy in the progression of OA, in particular whether mechanical loading may mitigate OA-induced symptoms by regulating these processes, remains elusive.
Our hypothesis was that knee loading mitigates OA symptoms across stages through a regulatory effect on ER stress and autophagy. To test the hypothesis, we used a surgically induced mouse model of OA in which medial collateral and anterior cruciate ligaments were transected and medial meniscus was removed. To evaluate daily loading effects, we examined the role of ER stress and autophagy using transmission electron microscopy and Western blot analysis. We also determined the degree of cartilage destruction using the Osteoarthritis Research Society International (OARSI) score, and we analyzed the alteration of cartilage and subchondral bone by microcomputed tomography imaging and histology with hematoxylin and eosin (H&E) and Safranin O staining.
MATERIALS AND METHODS
Animal and material preparation
All experiments were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and were approved by the Ethics Committee of Tianjin Medical University. One hundred seventy-four 14 wk old C57BL/6 female mice (Animal Center of Academy of Military Medical Sciences, Changchun, China) were used. Four to 5 mice per cage were housed under pathogen-free conditions. They were fed standard laboratory rodent chow (Animal center of Academy of Military Medical Sciences) and water ad libitum. Mice were maintained at a constant temperature of 25°C and kept on a 12-h light/dark cycle. DMEM, MEM-α, fetal bovine serum, penicillin, streptomycin, and trypsin were purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). Other chemicals were purchased from MilliporeSigma (Burlington, MA, USA) unless otherwise stated.
Experimental design and OA surgery
Sixty mice were used to evaluate the role of ER stress and autophagy in the development of surgically induced OA. Mice were randomly assigned to a sham-treated control group (n = 6) or 1 of 9 OA surgery groups. Mice with surgically induced OA were humanely killed after induction at various experimental end points: 3, 6, and 12 h; 1, 3, 7, and 14 d; and 4 and 8 wk (n = 6 each).
To assess the effect of loading on surgically induced OA, 114 mice were divided into 3 groups (n = 38 in each group): sham-treated control group, OA group, and loading-treated OA group. Mice were humanely killed at 4 or 8 wk after establishment of the OA model or sham surgery. All animals were weighed before any treatment and at death at 4 and 8 wk.
Surgical models were performed using sterile technique and under anesthetic with 1.5% isoflurane (IsoFlo; Abbott Laboratories, North Chicago, IL, USA) at a flow rate of 1.0 L/min. The right hind limb was shaved and sterilized with 70% alcohol solution before generation of a 20-mm incision to expose the right knee joint. The medial collateral and anterior cruciate ligaments were transected and the articular cavity opened to allow for removal of the medial meniscus from its anterior attachment to the tibia. The surgery site was washed with sterile saline to remove tissue debris, and the incision was closed. For the sham-treated control animals, the surgery was performed on the right knee using the same approach without ligament transection and removal of medial meniscus. After finishing surgery on the right knee, the same procedure was conducted on the left knee. Buprenorphine hydrochloride was administered as analgesic after surgery (23).
Mechanical loading
One week after surgery, knee loading was applied to the right hind limb of OA + loading mice using a custom-made loader. Dynamic loads were sinusoidal with 1 N at 5 Hz for 5 min/d. The tip of the loader was 5 mm in diameter. The sham-treated control and OA mice were placed on the loading device without any dynamic force. After finishing the right knee loading, the same loading procedure was conducted on the left knee (23, 36). The right knee was collected for histologic analysis, and the left knee was collected for Western blot analysis at 4 or 8 wk in accordance with study group.
Histologic assay
Knee samples were fixed in 10% neutral buffered formalin for 48 h and decalcified in 14% EDTA for 2 wk. Decalcified samples were embedded in paraffin and sectioned at 5 μm thickness along the coronal plane. Tissue sections were stained with Safranin O and graded using the OARSI score to assess the histopathologic grade of cartilage (10, 37–39). Slides were stained with H&E to observe the histologic parameters of articular cartilage, subchondral bone, and synovium (40, 41). Measurements were performed on the proximal side of the growth plate. We determined subchondral bone volume fraction (B.Ar/T.Ar) (in percentages; T.Ar indicates the total tissue area and B.Ar the trabecular bone area, calculated from the total trabecular area), ratio of calcified cartilage to total articular cartilage (CC/TAC), and subchondral bone plate (SBP) thickness (23).
The synovium was evaluated by a grader unaware of the animals’ group using a modified synovitis score (6 as a maximum score), which focused on enlargement of synovial lining cell layers and cellularity. The score for the enlargement was as follows: 0 = thickness with 1–2 cells, 1 = 2–4 cells, 2 = 4–9 cells, and 3 = >9 cells. The score for cellularity was as follows: 0 = normal, 1 = slightly increased, 2 = moderately increased, and 3 = greatly increased with pannus formation and rheumatoid-like granulomas. We also evaluated osteophyte size, in which 0 = no change, 1 = small change (slight increase in thickness), 2 = medium change (≤3-fold increase), and 3 = large change (more than 3-fold increase). Furthermore, osteophyte maturity was evaluated as follows: 0 = normal, 1 = predominantly cartilaginous, 2 = mixed cartilage and bone with active vascular invasion and endochondral ossification, and 3 = predominantly bone, as previously described (10, 37, 39, 42, 43).
Microcomputed tomography
Microcomputed tomography was performed using a Scanco vivaCT 40 (Scanco Medical, Bassersdorf, Switzerland). Briefly, the excised left knee joint was scanned using an X-ray source set at 60 kV with 6 μm pixel size. Six hundred slices (1 slice = ∼10.5 µm) of a whole knee joint were used. Three-dimensional reconstruction of the image was carried out within the region of interest consisting of both trabecular and cortical areas. Bone mineral density (BMD, mg/cm3), bone volume/total volume fraction (BV/TV, %), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, µm), and trabecular bone spacing (Tb.Sp, µm) were calculated as previously described (44, 45).
Electron microscopic analysis
For electron microscopy, the knee joints were fixed for 48 h at 4°C in 4% paraformaldehyde and 2.5% glutaraldehyde solution, and decalcified in 14% EDTA for 2 wk. Decalcified samples were postfixed for 1 h in 1% osmium tetroxide. After fixation, the samples were dehydrated in graded alcohols, infiltrated in epoxypropane, and embedded in EPON812. Ultrathin ±50 nm sections were created with a Leica Ultracut Ultracut (UCT) Ultramicrotome (Leica, Wetzlar, Germany) and a Diatome 45-degree diamond knife (Hatfield, PA, USA). Sections were counterstained with uranyl acetate and lead citrate, and examined using a model 7500 electron microscope (Hitachi, Tokyo, Japan) operated at 80 kV. The images of chondrocytes of the articular cartilage were captured with a Megaview (Emsis, Muenster, Germany) camera. The percentages of the rough ER area and the number of autophagic vacuoles in the cytoplasm were determined using image analysis software (Cellsense Standard Software) (45). Using 3 fields of view from each of 3 mice, we determined the percentage of rough ER area to the total cell area as well as the number of autophagic vesicles.
Isolation of bone marrow–derived cells
After the animals were humanely killed, bone marrow–derived cells were collected by flushing the iliac, femur, and tibia with Iscove MEM (Thermo Fisher Scientific) with 2% fetal bovine serum. Cells were separated by low-density gradient centrifugation and cultured in α-MEM supplemented with 10% fetal bovine serum (15, 17).
Assays for chondrogenic differentiation
Bone marrow mononuclear cells were plated at 1 × 106 cells/ml in chondrogenic differentiation medium [MesenCult Proliferation Kit (Stemcell Technologies, Vancounver, BC, Canada) supplemented with 10−8 M dexamethasone, 50 µg/ml ascorbic acid 2-phosphate, 10 M β-glycerophosphate, and 10 ng/ml TGF-β3] in 6-well plates for 4 wk. The medium was changed every other day. Cells were fixed in 10% buffered formalin for 30 min at room temperature, washed with PBS, and incubated with 1% Alcian blue in 0.1 M HCl (pH 1.0) for at least 2 h. Chondrogenic cells were visualized as blue-stained cells (46).
Western blot analysis
Tissues were lysed in a RIPA lysis buffer containing protease inhibitors and phosphatase inhibitors (Roche Diagnostics, Rotkreuz, Switzerland). Isolated proteins were fractionated using 10% SDS-PAGE gels and electrotransferred to PVDF membranes (MilliporeSigma). Primary antibodies specific to eukaryotic translation initiation factor 2α (eIF2α), phospho-eIF2α (Cell Signaling Technology, Danvers, MA, USA), LC3, p62, (MBL, Nagoya, Japan), MMP-13 (Abcam, Cambridge, United Kingdom), and β-actin (MilliporeSigma) were used. After incubation with secondary IgG antibodies conjugated with horseradish peroxidase, signals were detected with enhanced chemiluminescence. Data are presented with reference to control intensities of β-actin (47).
Statistical analysis
Data are expressed as means ± sem. Statistical significance among groups was examined by 1-way ANOVA, and a post hoc test was conducted by the Fisher protected least significant difference test. All comparisons were 2 tailed, and statistical significance was assumed at P < 0.05.
RESULTS
All animals tolerated the procedures. No bruising or tissue damage was detected at the surgery site.
Knee loading suppressed OA-induced ER stress
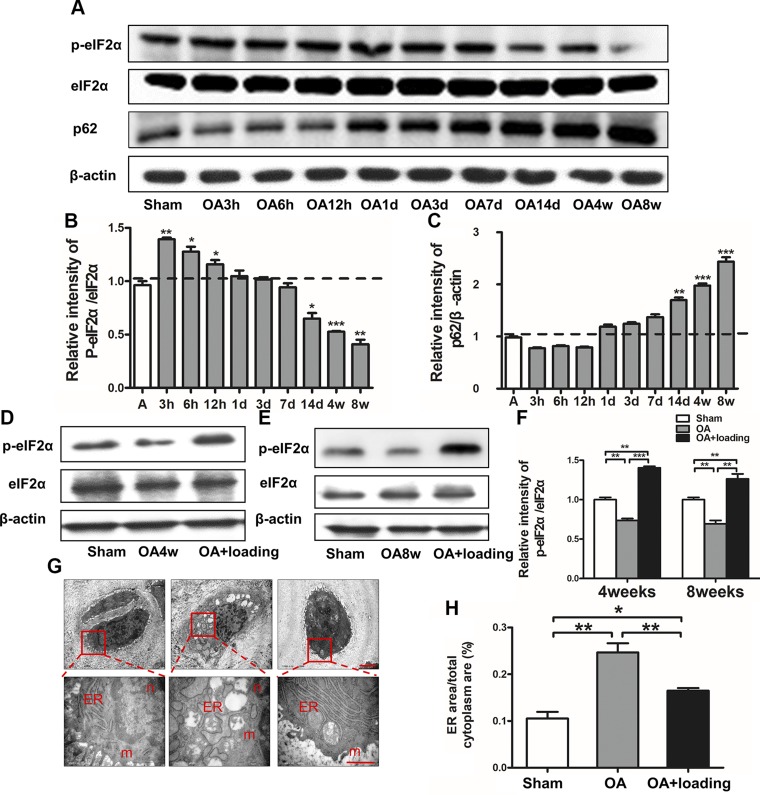
To evaluate the role of ER stress in the development of OA, the phosphorylation level of eIF2α was determined. Compared to age-matched control, the OA group exhibited a significant increase in phosphorylated eIF2α (p-eIF2α) in 3, 6, and 12 h, but the level was decreased after 14 d, 4 wk, and 8 wk (Fig. 1A, B).
Figure 1.
OA-induced ER stress and autophagy and loading-treated decreased OA-induced effects. A) To investigate role of ER stress and autophagy in pathogenesis of OA, p-eIF2α/eIF2α, and p62 were evaluated by immunoblotting. B) Compared to age-matched control animals, animals in OA group significantly increased phosphorylation of eIF2α in short term, but decreased them in a time-dependent manner in long term. C) Activation of autophagy in late stage of OA (n = 6/group). D–F) Western blot analysis of p-eIF2α/eIF2α after knee loading (D, E) and quantitative analysis of relative intensity of p-eIF2α/eIF2α (F). G, H) Electron microscopic analysis of ER stress (G) and quantitative analysis of ER area (H) (n = 3/group). N, nucleus; m, mitochondria; ER, endoplasmic reticulum. Scale bar, 2 μm. *P < 0.05, **P < 0.01, ***P < 0.001, P > 0.05. N.s., not significant.
To investigate the effect of knee loading on ER stress, the level of p-eIF2α (Fig. 1D, E) and ER images by electron microscopy (Fig. 1G) were evaluated. While the relative intensity of p-eIF2α/eIF2α in the OA group was decreased compared to that in the sham-treated control group (P < 0.01), this ratio was significantly increased by knee loading (P < 0.001 at 4 wk, P < 0.01 at 8 wk; Fig. 1F). Electron microscopy images revealed that the rough ER in the sham-treated control group was flat-saccular and regularly arranged, with ribosomes attached outside the membrane. In contrast, the rough ER in the OA group presented dilatation, partial vesiculation, and shedding and dissociation of nucleoprotein bodies. In response to knee loading, a significant recovery in ER morphology was observed (Fig. 1G). The ER area to the total cytoplasm area in the OA group was larger than that in the sham-treated control group, but this ratio was significantly decreased by knee loading (both P < 0.01; Fig. 1H).
Knee loading restored autophagy in late OA
To evaluate the role of autophagy in OA development, expression of p62 was examined after induction of OA. Compared to the age-matched control, the OA group exhibited a significant increase in p62 after 14 d, 4 wk, and 8 wk (Fig. 1A, C).
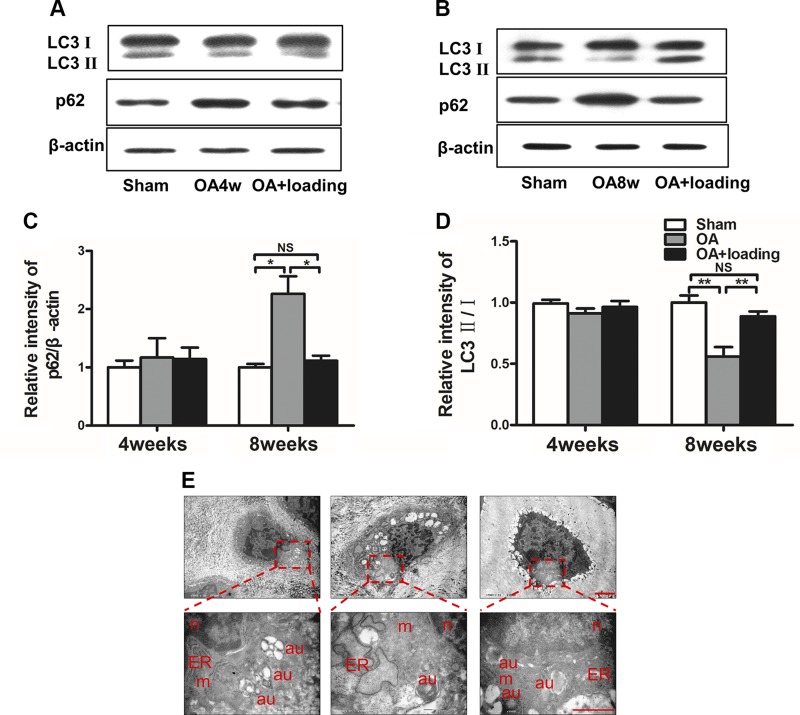
Four and 8 wk after induction of OA, we also determined expression of p62 as an index of autophagic degradation, and LC3II/I as a marker for initiation, expansion, and phagophore formation of autophagy (Fig. 2A, B). First, the relative intensity of p62/β-actin in the OA group at 8 wk was increased compared to that in the sham-treated control group. However, its expression was significantly decreased in the loaded-OA group (both P < 0.05; Fig. 2C). Second, the relative intensity of LC3II/I in the OA group at 8 wk was decreased compared to that in the sham-treated control group. However, its expression was significantly increased by knee loading (both P < 0.01; Fig. 2D). Images taken via electron microscopy revealed that the number of autophagic vacuoles was decreased in the OA group, while it was increased by knee loading (Fig. 2E).
Figure 2.
Autophagy decreased in late OA and loading restores autophagy. A–D) Immunoblot analysis (A, B) and quantitative analysis of autophagy-related factors p62 and LC3 (C, D) at 4 and 8 wk. E) Electron microscopic analysis of autophagy (n = 3/group). N, nucleus; m, mitochondria; au, autophagosome; ER, endoplasmic reticulum. Scale bar, 2 μm. *P < 0.05, **P < 0.01, and P > 0.05. N.s., not significant.
Knee loading decreased articular cartilage degradation and increased number of chondrocytes in vivo and in vitro
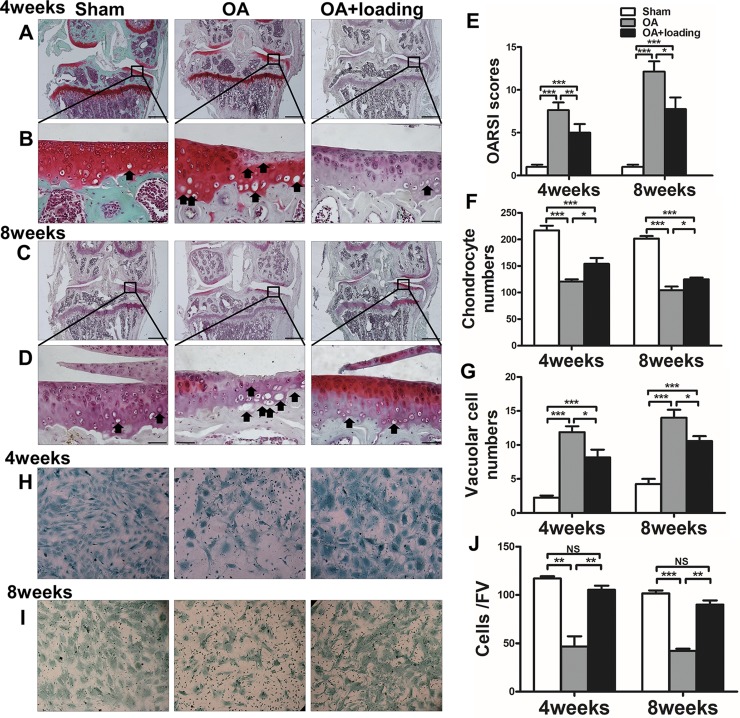
Safranin O staining showed that cartilage surface was smooth and intact in the sham-treated control group. However, the OA group exhibited massive proteoglycan loss and apparent hypocellularity at 4 wk after OA induction (Fig. 3A, B). At 8 wk, cartilage erosion was significant, with superficial fibrillation, surface discontinuity, and vertical fissure (Fig. 3C, D). At both 4 and 8 wk, OARSI scores revealed significant degeneration of the articular cartilage in the OA group compared to the sham-treated control group (both P < 0.001). However, application of knee loading significantly decreased OARSI scores at both the 4- and 8-wk end points after induction of OA (P < 0.01, P < 0.05; Fig. 3E).
Figure 3.
Knee loading protects articular cartilage from damage and increases number of chondrocytes. A–G) Representative Safranin O–stained images (A–D), quantification of OARSI scores (E), and chondrocyte (F) and vacuolar cell (G) numbers in area proximal to subchondral bone of medial tibial plateau from sham-treated control, OA, and OA + loading mice at 4 and 8 wk after surgery. Black arrows indicate vacuolar cells (B, D). Scale bars, 500 (A, C) and 50 (B, D) µm) (n = 10/group). H–J) Representative images of chondrocyte from mouse-derived bone mesenchymal cells (H, I) and quantification of chondrocyte numbers (J). FV, field of vision (J) (n = 3/group). *P < 0.05, **P < 0.01, ***P < 0.001, P > 0.05. N.s., not significant.
We counted the number of chondrocytes and vacuolar cells in the area adjacent to subchondral bone of the medial tibial plateau with Safranin O staining (Fig. 3B, D, black arrows). The number of chondrocytes in the OA group was significantly decreased compared to the sham-treated control group at 4 and 8 wk after surgery (both P < 0.001). However, after knee loading, the number of chondrocytes was significantly increased at 4 and 8 wk (both P < 0.05; Fig. 3F). The number of vacuolar cells in the OA group was significantly increased compared to the sham-treated control group at 4 and 8 wk (both P < 0.001). Quantities of these cells were decreased after application of knee loading at 4 and 8 wk (both P < 0.05; Fig. 3G).
We also performed a chondrocyte differentiation assay using bone marrow mesenchymal cells (Fig. 3H–J). We used roughly the same number of mesenchymal stromal cells in each group for chondrocyte differentiation; chondrogenic cells were visualized as blue-stained cells under the microscope. The number of differentiated chondrocytes derived from the OA group was significantly decreased compared to the sham-treated control group at 4 and 8 wk. However, the number of differentiated chondrocytes derived from loaded OA mice at the 2 time points revealed a significant restoration (both P < 0.01).
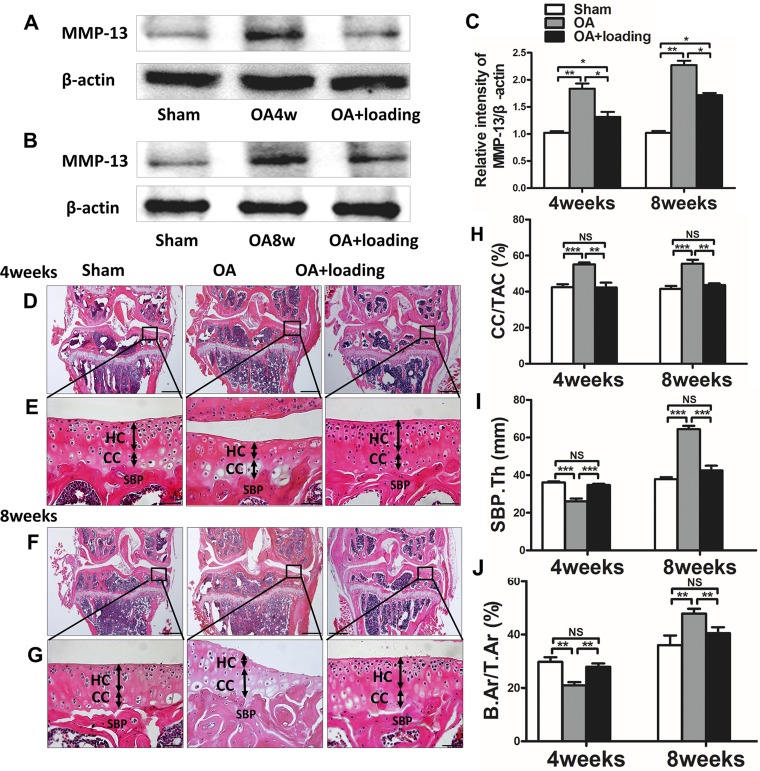
We examined expression of MMP-13 in the articular cartilage. Four and 8 wk after OA induction, the MMP-13 level in the OA group was significantly elevated compared to the control group (P < 0.01), and it was reduced by knee loading (P < 0.05; Fig. 4A–C).
Figure 4.
Knee loading decreases expression of MMP-13 and prevents abnormal changes in articular cartilage and bone mass. A–C) Western blot analysis of expression of MMP-13 (A, B) and relative intensity of MMP-13/β-actin (C). D–J) Representative H&E-stained images (D–G) and quantification of CC/TAC (H), thickness of SBP (SBP.Th) (I), and B.Ar/T.Ar (J). Double arrows indicate thickness of hyaline cartilage (HC) and calcified cartilage (CC). Scale bars, 500 (D, F) and 50 (E, G) µm (n = 10/group). *P < 0.05, **P < 0.01, ***P < 0.001, P > 0.05. N.s., not significant.
At both the at 4- and 8-wk time points, thickness of the calcified cartilage increased whereas that of hyaline cartilage decreased, with a tidemark moving toward the articular surface in the OA group. However, the calcified cartilage thickness was decreased by knee loading (Fig. 4D–H). The value of CC/TAC in the OA group was higher than that in the sham-treated control group (both P < 0.001). The value of CC/TAC was significantly decreased by knee loading at 4 and 8 wk (both P < 0.01; Fig. 4H).
Knee loading prevented bone loss at early stage and reduced bone increase at advanced stage of OA
Thickness of SBP was measured using H&E-stained sections (Fig. 4D–G) to evaluate changes in subchondral bone. Compared to the sham-treated control animals, those in the OA group showed a significantly thinner SBP at 4 wk and a thicker SBP at 8 wk (both P < 0.001). With knee loading, thickness of SBP was significantly increased at 4 wk and considerably decreased at 8 wk (both P < 0.001; Fig. 4I) relative to mice that had undergone OA induction without subsequent joint loading.
To evaluate a volume fraction of subchondral bone, B.Ar/T.Ar ratio was detected by H&E staining (Fig. 4D, F). The OA group exhibited a lower B.Ar/T.Ar ratio compared to the sham-treated control group at 4 wk (both P < 0.01). On the contrary, at 8 wk there was an opposite trend, and the B.Ar/T.Ar ratio in the OA group was higher than that of the sham-treated control group. At both 4 and 8 wk, knee loading presented significant recovery of B.Ar/T.Ar (both P < 0.01; Fig. 4J).
Knee loading prevented osteophyte formation
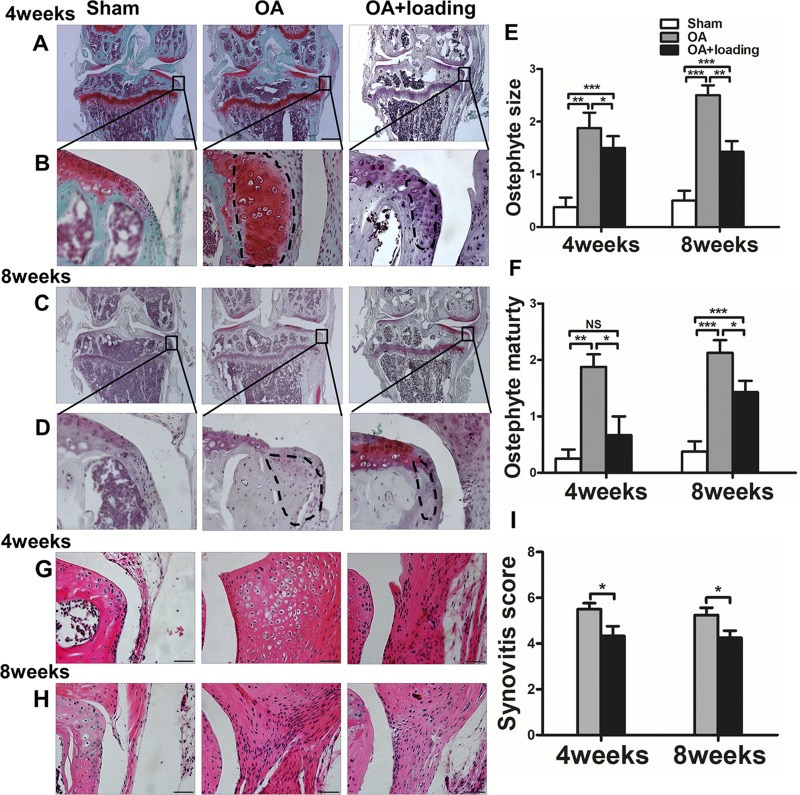
Safranin O staining showed that calcified cartilage appeared on the outer edge of the medial tibial plateau at 4 and 8 wk (Fig. 5A–D). Size and maturity of osteophytes in the OA group were noticeably higher than those in the sham-treated control group at 4 and 8 wk (P < 0.01 at 4 wk; P < 0.001 at 8 wk); However, they were significantly decreased by knee loading at 4 and 8 wk (P < 0.05 at 4 wk, P < 0.01 at 8 wk in osteophyte size, both P < 0.05 at 4 and 8 wk in osteophyte maturity; Fig. 5E, F).
Figure 5.
Knee loading reduces synovial inflammation and prevents osteophyte formation. A–F) Representative Safranin O–stained images of formation of osteophyte (A–D), and quantification of osteophyte size (E) and osteophyte maturity (F). G–I) Representative images of synovial inflammation by H&E staining (G, H) and quantification of synovitis scores (I). Scale bars, 500 (A, C) and 50 (B, D) μm (n = 10/ group). *P < 0.05, **P < 0.01, ***P < 0.001, P > 0.05. N.s., not significant.
Knee loading reduced synovial inflammation
In H&E-stained sections, we found thickening of the synovium in the OA group at 4 and 8 wk (Fig. 5G–I). Compared to the sham-treated control group without synovitis, the OA group showed significantly increased synovitis scores at 4 and 8 wk. Knee loading significantly decreased the synovitis scores at 4- and 8-wk time points (both P < 0.05; Fig. 5I).
Knee loading regulated abnormal bone remodeling
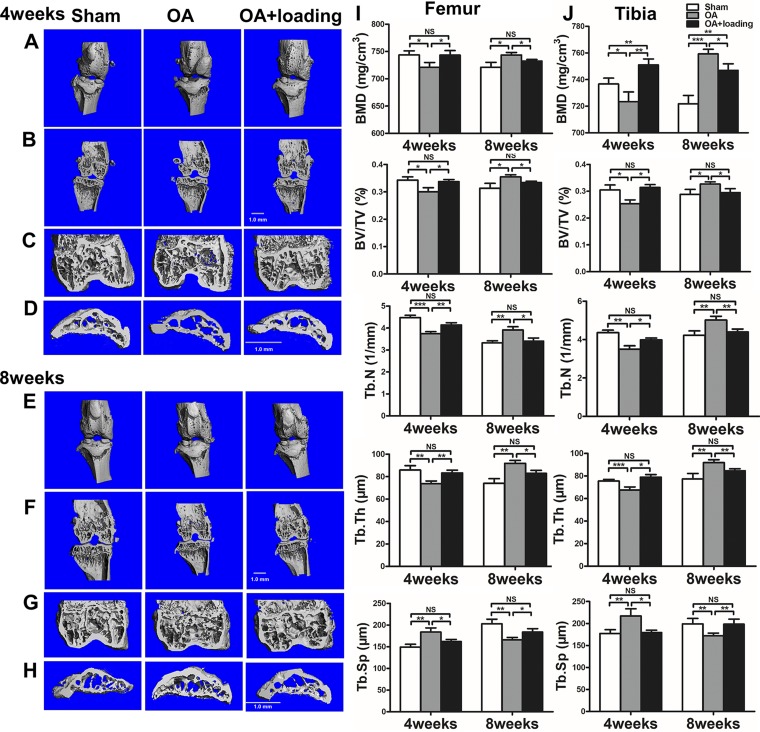
At 4 wk after induction of OA, microcomputed tomography images revealed that bone mass was significantly decreased in the distal femur and the proximal tibia. BMD, BV/TV, Tb.N, and Tb.Th were significantly reduced. However, with knee loading, both in the distal femur and proximal tibia, bone mass was significantly increased. Of note, Tb.Sp was increased (both P < 0.01) in the OA group and significantly decreased (both P < 0.05) in the knee loading group in the distal femur and proximal tibia. In contrast, by 8 wk after surgery, there was a trend toward increased bone mass in the distal femur and the proximal tibia. For example, compared to the sham-treated control group, BMD, BV/TV, Tb.N, and Tb.Th were significantly increased in the OA group. However, after loading treatment, bone mass exhibited a clear trend toward reduction. In contrast to this trend, Tb.Sp decreased in the OA group and increased in the knee loading group (Fig. 6).
Figure 6.
Knee loading prevents abnormal bone remodeling. A, B, E, F) Representative 3-dimensional images (A, E) and 2-dimensional images (B, F) of knee joint. C, D, G, H) Two-dimensional images of distal femur (C, G) and proximal tibia subchondral bone (D, H). Quantitative analysis of BMD (mg/cm3), BV/TV (%), Tb.N (1/mm), Tb.Th, and Tb.Sp (μm) in femur (I) and in tibia (J) (n = 3/group). *P < 0.05, **P < 0.01, ***P < 0.001, P > 0.05. N.s., not significant.
DISCUSSION
In response to ER stress, 3 major signaling pathways are known: a PERK-ATF4 axis, an activating transcription factor 6 (ATF6) axis, and an inositol-requiring enzyme 1a/X-box binding protein 1 (IRE1a/XBP1) axis (27, 48). While a moderate level of ER stress may assist reestablishment of cellular homeostasis, prolonged and unmitigated stress may lead to apoptosis (27, 48, 49). We have previously shown that knee loading activates PERK-ATF4 signaling in progression of cellular injury or apoptosis (35). To examine whether the PERK pathway is also involved in OA, we determined the level of p-eIF2α and evaluated morphologic changes of the rough ER in the early and late OA stages. The results showed that the OA group increased p-eIF2α in the early stage. It was decreased in the later stage, but knee loading was able to significantly suppress the OA-induced decrease in p-eIF2α. Electronic microscopy revealed that the OA group presented degeneration of the rough ER in the late OA stage, but a significant recovery was observed after application of knee loading. These results indicated that excessive ER stress was induced in the later stage of OA, and knee loading significantly improved OA symptoms by suppressing ER stress.
It has been reported that ER stress is accompanied with autophagy that may promote cellular survival (29, 50). Autophagy is a catabolic process that degrades and recycles damaged macromolecules and organelles (29, 51). Notably, our previous studies indicated that knee loading regulated ER stress in cellular injury or apoptosis (35). In this study, we evaluated linkage of autophagy to OA. At 4 wk, there was no significant change in expression of LC3 and p62. However, at 8 wk, the OA group reduced LC3 and increased p62, and these alterations in LC3 and p62 were suppressed in the loaded-OA group. Electron microscopy showed that the number of autophagosomes was decreased at 8 wk in the OA group, but knee loading restored its number. Collectively, OA induced pathologic skeletal metabolism by inducing ER stress and suppressing autophagy. These data are consistent with previous reports in which autophagy is considered important for protection of cartilage homeostasis (31, 32). Our results support a mechanism by which knee loading mitigates OA symptoms by suppressing ER stress and promoting autophagy. It is reported that joint loading regulates several signaling pathways, including Wnt, PI3K, TGFβ, and extracellular matrix receptors (18). Further analysis is needed to evaluate a potential involvement of these pathways in the observed loading effects.
The present study demonstrated that OARSI score of articular cartilage in the OA group was higher than that of the sham-treated control at 4 and 8 wk after induction of OA. In our animal model, Safranin O staining exposed destruction of articular cartilage in the tibia, including superficial fibrillation, surface discontinuity, and vertical fissures that extended into the mid zone and that lead to erosion. The number of chondrocytes in the OA group was decreased compared to the sham-treated control group at 4 and 8 wk in vivo and in vitro. Mechanical loads on the knee altered OA symptoms, and the structures of tibial articular cartilage and subchondral bone changed accordingly (52). Knee loading significantly improved OARSI scores and increased the number of chondrocytes. One possibility is that knee loading mitigated OA symptoms by stimulating differentiation and migration of chondrocytes in bone marrow to the cartilage.
The structures of tibial articular cartilage and subchondral bone were changed by induction of OA (52). Compared to the sham-treated control group, the OA group decreased BV/TV, Tb.N, Tb.Th, B.Ar/T.Ar, thickness of SBP and BMD, with an increase in CC/TAC and Tb.Sp at 4 wk. However, at 8 wk, the OA group decreased CC/TAC and Tb.Sp, and increased BV/TV, Tb.N, Tb.Th, B.Ar/T.Ar, thickness of SBP, and BMD. These results indicate that bone loss was initiated at the early stage of OA and that abnormal bone formation was induced at the later stage of OA. It is possible that instability-induced abnormal loads and friction induced degeneration of the articular cartilage and, later, its calcification. Safranin O staining showed that calcified cartilage was located on the outer edge of the medial tibial plateau, and osteophyte formation was more evident in the OA group than in the sham-treated control group. Of note, the size and maturity of osteophytes were significantly decreased with knee loading. In bone homeostasis, a balance between bone-forming osteoblasts and bone-resorbing osteoclasts is required (53, 54). Our previous and current results support the notion that knee loading improved abnormal bone remodeling by regulating activities of both osteoblasts and osteoclasts (15, 23). Collectively, abnormal bone remodeling is stimulated in the progression of OA, while knee loading corrected these abnormalities as observed during the early and late stages of OA.
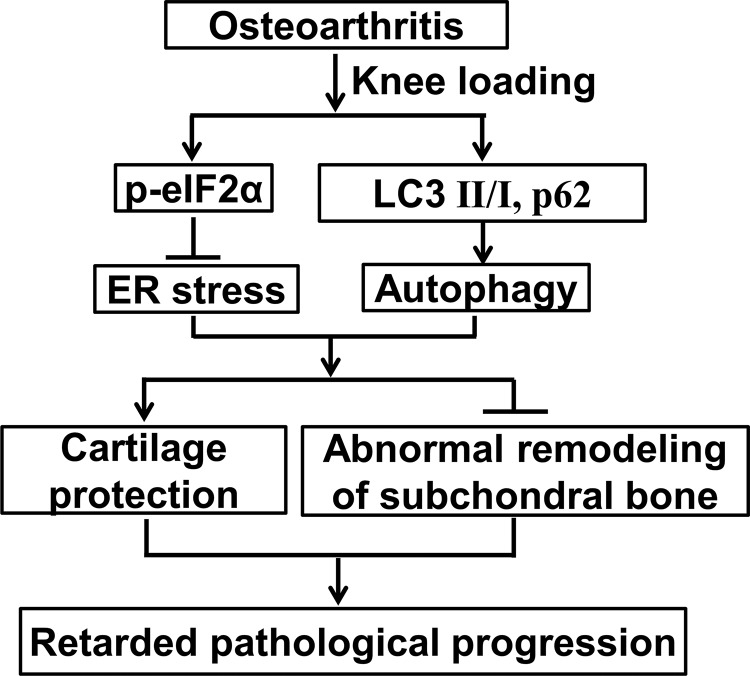
In summary, we demonstrate that ER stress and autophagy are important factors in the development of OA. Moreover, this study may provide significant clues toward identification of novel molecular biomarkers for diagnostic and prognostic evaluation of OA. Knee loading restores destruction of articular cartilage and abnormal bone remodeling in OA. Knee loading suppresses ER stress and promotes autophagy in the pathologic progress of OA (Fig. 7). Further studies should be performed to clarify the mechanism of cellular signaling underlying the role of knee loading–driven regulation of ER stress and autophagy. The present study may suggest a possibility of knee loading that could provide a novel strategy for treatment of OA.
Figure 7.
Mechanism of mechanical loading that mitigates OA symptoms by regulating ER stress and autophagy.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (81772405 and 81572100 to P.Z.; 81601863 to X.L.), China Postdoctoral Science Foundation (2016M601275 to X.L.), Tianjin Binhai New Area Health and Family Planning Commission (2016BWKL002 to P.Z.; 2016BWKZ005 to D.L.), the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (2017KJ223 to D.L.), and the U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR052144 to H.Y.). The authors declare no conflicts of interest.
Glossary
- B.Ar/T.Ar
subchondral bone volume fraction
- BMD
bone mineral density
- BV/TV
bone volume/total volume fraction
- CC/TAC
ratio of calcified cartilage to total articular cartilage
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- H&E
hematoxylin and eosin
- MMP-13
matrix metalloproteinase 13
- OA
osteoarthritis
- OARSI
Osteoarthritis Research Society International
- p-eIF2α
phosphorylated translation initiation factor 2α
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- SBP
subchondral bone plate
- Tb.N
trabecular number
- Tb.Sp
trabecular bone spacing
- Tb.Th
trabecular thickness
AUTHOR CONTRIBUTIONS
W. Zheng and P. Zhang conceived and designed the study; W. Zheng, J. Li, S. Yang, Z. Gao, and Z. Wang acquired the data; and X. Li, D. Liu, H. Yokota, and P. Zhang performed analysis and interpretation of data; P. Zhang had full access to all of the data in the study and takes responsibility for the integrity and accuracy of the data analysis; and all authors were involved in drafting the manuscript or revising it critically for important intellectual content, and approved the final manuscript.
REFERENCES
- 1.Jeon O. H., Kim C., Laberge R. M., Demaria M., Rathod S., Vasserot A. P., Chung J. W., Kim D. H., Poon Y., David N., Baker D. J., van Deursen J. M., Campisi J., Elisseeff J. H. (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson W. H., Lepus C. M., Wang Q., Raghu H., Mao R., Lindstrom T. M., Sokolove J. (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z., Kraus V. B. (2016) Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 12, 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu X., Ji X., Yang M., Fan S., Wang J., Lu M., Shi W., Mei L., Xu C., Fan X., Hussain M., Du J., Wu J., Wu X. (2018) Cdc42 is essential for both articular cartilage degeneration and subchondral bone deterioration in experimental osteoarthritis. J. Bone Miner. Res. 33, 945–958 [DOI] [PubMed] [Google Scholar]
- 5.Goldring M. B., Berenbaum F. (2015) Emerging targets in osteoarthritis therapy. Curr. Opin. Pharmacol. 22, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilz F. M., Ahrens P., Grad S., Stoddart M. J., Dahmani C., Wilken F. L., Sauerschnig M., Niemeyer P., Zwingmann J., Burgkart R., von Eisenhart-Rothe R., Südkamp N. P., Weyh T., Imhoff A. B., Alini M., Salzmann G. M. (2014) Influence of extremely low frequency, low energy electromagnetic fields and combined mechanical stimulation on chondrocytes in 3-D constructs for cartilage tissue engineering. Bioelectromagnetics 35, 116–128 [DOI] [PubMed] [Google Scholar]
- 7.Wang C. J., Weng L. H., Ko J. Y., Sun Y. C., Yang Y. J., Wang F. S. (2011) Extracorporeal shockwave therapy shows chondroprotective effects in osteoarthritic rat knee. Arch. Orthop. Trauma Surg. 131, 1153–1158 [DOI] [PubMed] [Google Scholar]
- 8.Wang C.-J., Sun Y.-C., Wong T., Hsu S.-L., Chou W.-Y., Chang H.-W. (2012) Extracorporeal shockwave therapy shows time-dependent chondroprotective effects in osteoarthritis of the knee in rats. J. Surg. Res. 178, 196–205 [DOI] [PubMed] [Google Scholar]
- 9.Lapane K. L., Yang S., Driban J. B., Liu S. H., Dubé C. E., McAlindon T. E., Eaton C. B. (2015) Effects of prescription nonsteroidal antiinflammatory drugs on symptoms and disease progression among patients with knee osteoarthritis. Arthritis Rheumatol. 67, 724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhen G., Wen C., Jia X., Li Y., Crane J. L., Mears S. C., Askin F. B., Frassica F. J., Chang W., Yao J., Carrino J. A., Cosgarea A., Artemov D., Chen Q., Zhao Z., Zhou X., Riley L., Sponseller P., Wan M., Lu W. W., Cao X. (2013) Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19, 704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P., Tanaka S. M., Jiang H., Su M., Yokota H. (2006) Diaphyseal bone formation in murine tibiae in response to knee loading. J. Appl. Physiol (1985) 100, 1452–1459 [DOI] [PubMed] [Google Scholar]
- 12.Zhang P., Yokota H. (2011) Knee loading stimulates healing of mouse bone wounds in a femur neck. Bone 49, 867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang P., Tanaka S. M., Sun Q., Turner C. H., Yokota H. (2007) Frequency-dependent enhancement of bone formation in murine tibiae and femora with knee loading. J. Bone Miner. Metab. 25, 383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P., Sun Q., Turner C. H., Yokota H. (2007) Knee loading accelerates bone healing in mice. J. Bone Miner. Res. 22, 1979–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D., Li X., Li J., Yang J., Yokota H., Zhang P. (2015) Knee loading protects against osteonecrosis of the femoral head by enhancing vessel remodeling and bone healing. Bone 81, 620–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun H. B., Zhao L., Tanaka S., Yokota H. (2012) Moderate joint loading reduces degenerative actions of matrix metalloproteinases in the articular cartilage of mouse ulnae. Connect. Tissue Res. 53, 180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamamura K., Zhang P., Zhao L., Shim J. W., Chen A., Dodge T. R., Wan Q., Shih H., Na S., Lin C. C., Sun H. B., Yokota H. (2013) Knee loading reduces MMP13 activity in the mouse cartilage. BMC Musculoskelet. Disord. 14, 312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P., Turner C. H., Yokota H. (2009) Joint loading-driven bone formation and signaling pathways predicted from genome-wide expression profiles. Bone 44, 989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P., Su M., Liu Y., Hsu A., Yokota H. (2007) Knee loading dynamically alters intramedullary pressure in mouse femora. Bone 40, 538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira L. C., Oliveira R. G., Pires-Oliveira D. A. (2016) Effects of whole body vibration on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Osteoporos. Int. 27, 2913–2933 [DOI] [PubMed] [Google Scholar]
- 21.Holguin N., Brodt M. D., Silva M. J. (2016) Activation of Wnt signaling by mechanical loading is impaired in the bone of old mice. J. Bone Miner. Res. 31, 2215–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamasaki Y., Nagira K., Osaki M., Nagashima H., Hagino H. (2015) Effects of eldecalcitol on cortical bone response to mechanical loading in rats. BMC Musculoskelet. Disord. 16, 158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Yang J., Liu D., Li J., Niu K., Feng S., Yokota H., Zhang P. (2016) Knee loading inhibits osteoclast lineage in a mouse model of osteoarthritis. Sci. Rep. 6, 24668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayasuriya C. T., Hu N., Li J., Lemme N., Terek R., Ehrlich M. G., Chen Q. (2018) Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci. Rep. 8, 7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoboue E. D., Sitia R., Simmen T. (2018) Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 9, 331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binet F., Mawambo G., Sitaras N., Tetreault N., Lapalme E., Favret S., Cerani A., Leboeuf D., Tremblay S., Rezende F., Juan A. M., Stahl A., Joyal J. S., Milot E., Kaufman R. J., Guimond M., Kennedy T. E., Sapieha P. (2013) Neuronal ER stress impedes myeloid-cell–induced vascular regeneration through IRE1α degradation of netrin-1. Cell Metab. 17, 353–371 [DOI] [PubMed] [Google Scholar]
- 27.Walter P., Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 28.Travers K. J., Patil C. K., Wodicka L., Lockhart D. J., Weissman J. S., Walter P. (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 [DOI] [PubMed] [Google Scholar]
- 29.Moretti J., Roy S., Bozec D., Martinez J., Chapman J. R., Ueberheide B., Lamming D. W., Chen Z. J., Horng T., Yeretssian G., Green D. R., Blander J. M. (2017) STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell 171, 809–823.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denny P., Feuermann M., Hill D. P., Lovering R. C., Plun-Favreau H., Roncaglia P. (2018) Exploring autophagy with gene ontology. Autophagy 14, 419–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotz M. K., Caramés B. (2011) Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat. Rev. Rheumatol. 7, 579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caramés B., Hasegawa A., Taniguchi N., Miyaki S., Blanco F. J., Lotz M. (2012) Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 71, 575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nollet M., Santucci-Darmanin S., Breuil V., Al-Sahlanee R., Cros C., Topi M., Momier D., Samson M., Pagnotta S., Cailleteau L., Battaglia S., Farlay D., Dacquin R., Barois N., Jurdic P., Boivin G., Heymann D., Lafont F., Lu S. S., Dempster D. W., Carle G. F., Pierrefite-Carle V. (2014) Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 10, 1965–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D., Zhang Y., Li X., Li J., Yang S., Xing X., Fan G., Yokota H., Zhang P. (2017) eIF2α signaling regulates ischemic osteonecrosis through endoplasmic reticulum stress. Sci. Rep. 7, 5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirasawa H., Jiang C., Zhang P., Yang F. C., Yokota H. (2010) Mechanical stimulation suppresses phosphorylation of eIF2alpha and PERK-mediated responses to stress to the endoplasmic reticulum. FEBS Lett. 584, 745–752 [DOI] [PubMed] [Google Scholar]
- 36.Tan N., Li X., Zhai L., Liu D., Li J., Yokota H., Zhang P. (2018) Effects of knee loading on obesity-related non-alcoholic fatty liver disease in an ovariectomized mouse model with high-fat diet. Hepatol. Res. 48, 839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glasson S. S., Chambers M. G., Van Den Berg W. B., Little C. B. (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18(Suppl 3), S17–S23 [DOI] [PubMed] [Google Scholar]
- 38.Pritzker K. P., Gay S., Jimenez S. A., Ostergaard K., Pelletier J. P., Revell P. A., Salter D., van den Berg W. B. (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 14, 13–29 [DOI] [PubMed] [Google Scholar]
- 39.Kim J. H., Jeon J., Shin M., Won Y., Lee M., Kwak J. S., Lee G., Rhee J., Ryu J. H., Chun C. H., Chun J. S. (2014) Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 156, 730–743 [DOI] [PubMed] [Google Scholar]
- 40.Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mankin H. J. (1992) Nontraumatic necrosis of bone (osteonecrosis). N. Engl. J. Med. 326, 1473–1479 [DOI] [PubMed] [Google Scholar]
- 42.Little C. B., Barai A., Burkhardt D., Smith S. M., Fosang A. J., Werb Z., Shah M., Thompson E. W. (2009) Matrix metalloproteinase 13–deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 60, 3723–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneko H., Ishijima M., Futami I., Tomikawa-Ichikawa N., Kosaki K., Sadatsuki R., Yamada Y., Kurosawa H., Kaneko K., Arikawa-Hirasawa E. (2013) Synovial perlecan is required for osteophyte formation in knee osteoarthritis. Matrix Biol. 32, 178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodes S. D., Wu X., He Y., Chen S., Yang H., Staser K. W., Wang J., Zhang P., Jiang C., Yokota H., Dong R., Peng X., Yang X., Murthy S., Azhar M., Mohammad K. S., Xu M., Guise T. A., Yang F. C. (2013) Hyperactive transforming growth factor-β1 signaling potentiates skeletal defects in a neurofibromatosis type 1 mouse model. J. Bone Miner. Res. 28, 2476–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Yang S., Li X., Liu D., Wang Z., Guo J., Tan N., Gao Z., Zhao X., Zhang J., Gou F., Yokota H., Zhang P. (2017) Role of endoplasmic reticulum stress in disuse osteoporosis. Bone 97, 2–14 [DOI] [PubMed] [Google Scholar]
- 46.Li C., Sunderic K., Nicoll S. B., Wang S. (2018) Downregulation of heat shock protein 70 impairs osteogenic and chondrogenic differentiation in human mesenchymal stem cells. Sci. Rep. 8, 553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H., Liu D. (2014) Baicalin inhibits high-mobility group box 1 release and improves survival in experimental sepsis. Shock 41, 324–330 [DOI] [PubMed] [Google Scholar]
- 48.Binet F., Sapieha P. (2015) ER stress and angiogenesis. Cell Metab. 22, 560–575 [DOI] [PubMed] [Google Scholar]
- 49.Hang D., Wang Q., Guo C., Chen Z., Yan Z. (2012) Treatment of osteonecrosis of the femoral head with VEGF165 transgenic bone marrow mesenchymal stem cells in mongrel dogs. Cells Tissues Organs (Print) 195, 495–506 [DOI] [PubMed] [Google Scholar]
- 50.Kishino A., Hayashi K., Hidai C., Masuda T., Nomura Y., Oshima T. (2017) XBP1-FoxO1 interaction regulates ER stress–induced autophagy in auditory cells. Sci. Rep. 7, 4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vega-Rubín-de-Celis S., Zou Z., Fernández A. F., Ci B., Kim M., Xiao G., Xie Y., Levine B. (2018) Increased autophagy blocks HER2-mediated breast tumorigenesis. Proc. Natl. Acad. Sci. USA 115, 4176–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glasson S. S., Blanchet T. J., Morris E. A. (2007) The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15, 1061–1069 [DOI] [PubMed] [Google Scholar]
- 53.Tower R. J., Campbell G. M., Müller M., Glüer C. C., Tiwari S. (2015) Utilizing time-lapse micro-CT–correlated bisphosphonate binding kinetics and soft tissue–derived input functions to differentiate site-specific changes in bone metabolism in vivo. Bone 74, 171–181 [DOI] [PubMed] [Google Scholar]
- 54.Hendrickx G., Borra V. M., Steenackers E., Yorgan T. A., Hermans C., Boudin E., Waterval J. J., Jansen I. D. C., Aydemir T. B., Kamerling N., Behets G. J., Plumeyer C., D’Haese P. C., Busse B., Everts V., Lammens M., Mortier G., Cousins R. J., Schinke T., Stokroos R. J., Manni J. J., Van Hul W. (2018) Conditional mouse models support the role of SLC39A14 (ZIP14) in hyperostosis cranialis interna and in bone homeostasis. PLoS Genet. 14, e1007321 [DOI] [PMC free article] [PubMed] [Google Scholar]