Abstract
Excessive iron increases the incidence of diabetes and worsens diabetic complications. Reciprocally, diabetes induces iron loading, partially attributable to elevated intestinal iron export according to a recent report. Herein, we show that iron uptake and the mRNA expression of iron importer divalent metal transporter 1 (DMT1) were significantly increased in the duodenum of streptozotocin-induced diabetic mice. Immunofluorescence staining of human intestinal biopsies revealed increased brush border membrane (BBM) and decreased cytoplasmic DMT1 expression in patients with diabetes, suggesting translocation of DMT1. This pattern of DMT1 regulation was corroborated by immunoblotting results in diabetic mice showing that BBM DMT1 expression was increased by 210%, in contrast to a 60% increase in total DMT1. PKC mediates many diabetic complications, and PKCα activity was increased in diabetic mouse intestine. Intriguingly, diabetic mice with PKCα deficiency did not show increases in iron uptake and BBM DMT1 expression. High-glucose treatment increased plasma membrane DMT1 expression via the activation of PKCα in cultured IECs. Inhibition of PKCα potentiated the ubiquitination and degradation of DMT1 protein. We further showed that high glucose suppressed membrane DMT1 internalization. These findings demonstrate that PKCα promotes microvillus membrane DMT1 expression and intestinal iron uptake, contributing to diabetic iron loading.—Zhao, L., Bartnikas, T., Chu, X., Klein, J., Yun, C., Srinivasan, S., He, P. Hyperglycemia promotes microvillus membrane expression of DMT1 in intestinal epithelial cells in a PKCα-dependent manner.
Keywords: diabetes, iron uptake, HIF2α ubiquitination
The worldwide prevalence of diabetes has been rising drastically in the past 30 yr, with a prevalence of 8.5% reported in 2014 (1). Iron in excess is a strong risk factor for diabetes. Increased prevalence of diabetes was initially observed in patients with hereditary hemochromatosis (2). Thereafter, accumulating evidence from meta-analyses demonstrated a positive relationship between excessive body iron and diabetic incidence in patients with non–hereditary hemochromatosis (3–5). An increased pool of labile iron causes pancreatic β-cell death and desensitizes insulin receptor activity causing type 1 and 2 diabetes (4, 6, 7). Moreover, iron accumulation potentially worsens diabetic complications in the heart, vasculature, and kidney (8, 9), the critical causes of diabetes-associated mortality. Clinical evidence has shown that iron reduction by phlebotomy or dietary iron restriction improves insulin sensitivity (10), vascular reactivity (11), hypertriglyceridemia (12), and renal function (13) in patients with diabetes. In experimental animals, dietary iron restriction improves insulin sensitivity and glucose tolerance in ob/ob (Lepob) mice (7) and ameliorates diabetic nephropathy in type 2 diabetic db/db (Leprdb) mice (14). Therefore, tight control of body iron in diabetic subjects becomes important.
Previous studies have shown increased iron loading in diabetic animals, manifested as elevations in serum iron and iron content in the liver (15, 16), heart (17), kidney (16), pancreas (16), and vasculature (18), but the mechanisms are not well understood. Increased intestinal iron absorption is a major mechanism that causes systemic iron loading. Dietary iron is primarily absorbed by duodenal epithelial cells through apical-localized divalent metal transporter 1 (DMT1, SLC11A2) and basolateral ferroportin (SLC40A1) that mediate iron import and export, respectively (19–21). Ferroportin expression and activity is inhibited by liver-derived hepcidin to prevent systemic iron overload (22). Therefore, hepcidin deficiency is a common cause of iron overload (23). Wang et al. (15) showed that diabetes decreases STAT3-dependent hepatic production of hepcidin leading to increased intestinal ferroportin expression and dietary iron absorption. Yet, it remains unknown whether iron uptake is altered in diabetes.
DMT1 in humans has 4 isoforms that differ in the N terminus through the use of exon 1A or 1B (DMT1A and DMT1B) and the C terminus, depending on whether there is an iron-responsive element (DMT1-IRE and DMT1–non-IRE) at the 3′ UTR of the mRNA (24). The DMT1A-IRE isoform, which localizes primarily at the plasma membrane in the enterocytes, has a dominant role in dietary iron absorption (24, 25). Unlike humans, mice express DMT1-IRE and DMT1–non-IRE with the same promoter (24). The expression of DMT1 is regulated at multiple levels. Dmt1 gene transcription is primarily regulated by hypoxia inducible factor 2α (HIF2α) (26, 27). Posttranscriptionally, the DMT1-IRE mRNA is stabilized by iron regulatory protein 1/2 (IRP1/2) (28). At the posttranslational level, DMT1 protein is subject to Nedd4 family-interacting protein 1/2 (Ndfip1/2)-mediated ubiquitination and proteasome-mediated degradation (29). Multiple studies have shown that elevation in intestinal DMT1 expression can lead to systemic iron loading. Anderson et al. (30) demonstrated that HIF2α and DMT1 are activated early in the pathogenesis of β-thalassemia causing excess iron accumulation in mouse models of β-thalassemia. Increased intestinal DMT1 expression because of the loss of Ndfip1 contributes to the increases in hepatic and splenic iron deposition (31). Thus, the current study was undertaken to evaluate whether intestinal DMT1 expression is up-regulated and potentially contributes to diabetes-associated iron loading.
The PKC family is group of serine/threonine-related protein kinases that have important roles in many cellular functions. PKCs are often hyperactivated in diabetes and have key roles in the pathogenesis of diabetic retinopathy, nephropathy and cardiovascular diseases by increasing permeability, contractility, angiogenesis, cell growth and apoptosis, and leukocyte adhesion (32, 33). In the present study, we show that intestinal DMT1 expression and iron uptake were increased in streptozotocin (STZ)-induced diabetic mice. We further demonstrated that PKCα, which was stimulated in diabetic mouse intestine, facilitated microvillus membrane expression of DMT1 by suppressing its ubiquitination, internalization, and degradation.
MATERIALS AND METHODS
Reagents and antibodies
RhoNox-1, a fluorescent probe that specifically binds Fe2+ (34), was purchased from Goryo Chemical (Sapporo, Japan). Rabbit anti-HIF2α (NB100) and mouse anti-human DMT1 (H00004891) antibodies were purchased from Novus Biologicals (Littleton, CO, USA). Mouse anti-PKCα antibody was obtained from BD Biosciences (San Jose, CA, USA). Rabbit anti-hemagglutinin (anti-HA; C29F4), anti-PKCδ, anti–phospho-PKCα/βII (Thr638/641), and anti–phospho-PKCδ (Thr505) antibodies were from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-rat DMT1-IRE (NRAMP21-A) antibody was from Alpha Diagnostics (San Antonio, TX, USA), mouse anti-ubiquitin (P4D1) was from Santa Cruz Biotechnology (Dallas, TX, USA), and mouse anti–β-actin (A1978) was from MilliporeSigma (Burlington, MA, USA). Alexa Fluor 568 phalloidin, Alexa Fluor 488 goat anti-mouse, and anti-rabbit IgG were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Gö6976, deferoxamine, phorbol ester (PMA), cycloheximide, d-glucose and other chemicals of molecular biology grade were from MilliporeSigma.
Animals
Transgenic mice expressing human DMT1A-IRE in intestinal epithelial cells (IECs)—DMT1IEC mice—were generated by the Emory Transgenic Core facility (Atlanta, GA, USA). In brief, template construct was purchased from OriGene Technologies (RC230318; Rockville, MD, USA), and the human DMT1A-IRE with an HA-tag fused at the C terminus (HA-DMT1) was subcloned into the MluI/BsiWI sites of pBS‑KS–villin–2‑(N‑morpholino)ethane sulfonic acid–Simian virus 40–poly A vector. The transgene DNA was excised with SalI, purified, and injected into the pronuclei of fertilized eggs of C57BL/6 (B6) mice. Transgenic founder (F0) mice were identified by PCR analysis of tail genomic DNA with the following primer pair: forward, 5′‑CTGCTTGTTGCTGTCTTCCA‑3′, and reverse, 5′‑CGCAAGCTCGTAAATGTGAG‑3′. DMT1IEC stain was maintained by breeding transgenic mice with wild-type (WT) B6 mice. DMT1IEC mice used in this study were on, or after, generation F5. PKCα−/− strain (B6;129 mixed) was bred into B6 background for >6 generations. PKCα−/− mice were genotyped with the following primers: 5′‑CCAAGTGTGAAGTGTGTGAG‑3′, 5′‑AGCTAGGTCCTGTTGGTAAC‑3′, and 5′‑GCGCATCGCCTTCTATCGC‑3′. PKCα−/− mice were crossed with DMT1IEC mice to obtain DMT1IEC;PKCα−/−mice. WT, DMT1IEC, and DMT1IEC;PKCα−/− mice at 6–8 wk old were administered STZ (50 mg/kg body weight, i.p.) for a consecutive 5 d to induce type 1 diabetes. Blood glucose levels were measured every 2 wk, and mice with fasting glucose levels between 300 and 400 mg/dl were chosen for study. Mice were studied 2 mo after the last injection of STZ. All mouse strains were fed on Laboratory Rodent Diet 5001 (LabDiet, St. Louis, MO, USA), which contains 240 ppm iron. All animal experiments were performed under approval by the Institutional Animal Care and Use Committee of Emory University and in accordance of the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Iron measurements
Blood was collected from diabetic and control mice. Serum was prepared, and iron concentration was measured with a QuantiChrom Iron Assay kit (BioAssay Systems, Hayward, CA, USA). Nonheme iron content in the liver was measured, as previously described (35). To measure intestinal iron uptake, the first 1-cm segment of the duodenum was isolated, cut longitudinally, and incubated for 5 min in PBS, which was supplemented with 100 µM FeSO4. Tissues were then embedded in OCT compound and snap-frozen in liquid nitrogen. Frozen sections were washed for 2 × 10 min with PBS, followed by incubation with RhoNox-1 (5 µM) and DAPI for 30 min. After 3 washes, the specimens were mounted with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific) and observed under a fluorescence microscope (Nikon, Tokyo, Japan). Images were taken, and fluorescent intensity in IECs was measured by using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Six representative villi from each mouse were chosen for the quantification of mean values.
Total membrane and brush border membrane preparation
Mice were sacrificed, and the duodena (∼4 cm length) were quickly removed and flushed with ice-cold PBS (without Ca2+ and Mg2+). The segment was then opened longitudinally, and the villous part was gently scraped off with a prechilled glass slide. The scraped epithelial cells were centrifuged (750 g, 5 min), washed twice with ice-cold buffer 1 (300 mM mannitol, 5 mM EGTA, 12 mM Tris, pH 7.4), and then resuspended in 4 ml buffer 2 (50 mM mannitol, 1 mM EGTA, 2 mM Tris, pH 7.4) supplemented with proteinase inhibitors (F. Hoffmann-La Roche, Basel, Switzerland). Cell suspensions were homogenized on ice for 5 min (10 times for 30 s at setting 3 with a 30-s interval) with a homogenizer PRO 200 (Pro Scientific, Oxford, CT, USA). Total homogenates were then centrifuged at 1000 g for 5 min. A fraction of the supernatant was then centrifuged at 40,000 g for 30 min with Avanti Ultracentrifuge (Beckman Coulter, Brea, CA, USA), and the resulting pellet was retained, representing the total membrane fraction. The rest of the supernatant had MgCl2 added to a final concentration of 10 mM. Homogenates were then gently mixed for 20 min at 4°C and centrifuged for 20 min at 2500 g. The supernatant was collected and centrifuged for 30 min at 40,000 g, and the resulting pellet was resuspended in buffer 2, and the MgCl2 precipitation was repeated. The final pellet harvested by ultracentrifugation represents the brush border membrane (BBM) fraction. The quality of the purified BBMs was validated as previously described (36).
Human biopsies
Institutional review board approval was obtained for all samples used in this study. After informed consent from the subjects, jejunal biopsies were obtained from patients with type 1 diabetes and from healthy controls (Table 1). Colonic biopsies were also obtained from patients with type 2 diabetes and from healthy controls (Table 2).
TABLE 1.
Medical information on human patients with type 1 diabetes and on healthy controls
| Parameters | Control 1 | Control 2 | Control 3 | T1DM 1 | T1DM 2 | T1DM 3 |
|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Male | Male |
| Age, yr | 36 | 53 | 66 | 63 | 58 | 31 |
| Years of T1DM | N/A | N/A | N/A | 4 | 47 | 14 |
| Insulin therapy | N/A | N/A | N/A | yes | yes | yes |
| HbA1c (%) | <5.6 | <5.6 | <5.6 | 7.4 | 7.3 | 8.8 |
| Fasting blood glucose (mg/dl) | <130 | <130 | <130 | 208 | 206 | 332 |
Cont, healthy control; T1DM, type 1 diabetes.
TABLE 2.
Medical information on human patients with type 2 diabetes and on healthy controls
| Parameters | Control 1 | Control 2 | Control 3 | T2DM 1 | T2DM 2 | T2DM 3 |
|---|---|---|---|---|---|---|
| Age, yr | 58 | 50 | 57 | 50 | 52 | 60 |
| Years of T2DM | N/A | N/A | N/A | 6 | 2 | 4 |
| HbA1c (%) | 5.9 | 5.4 | 5.8 | 6.6 | 8.1 | 7.3 |
| Fasting blood glucose (mg/dl) | 84 | 101 | 93 | 116 | 114 | 173 |
Cont, healthy control; T2DM, type 2 diabetes.
Confocal fluorescence microscopy
Frozen duodenal tissue sections were processed for the staining of HIF2α. Formalin-fixed paraffin sections of the duodena were prepared from DMT1IEC mice for HA-DMT1 staining, and paraffin sections of the human biopsies were analyzed for native DMT1 expression. Paraffin-embedded sections were deparaffinized and dehydrated in a graded series of xylene and ethanol, followed by an antigen-unmasking procedure via a pressure cooker and sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). Immunofluorescence staining was then performed as previously described (37). Briefly, tissue sections were permeated with PBS containing 0.2% Triton X-100 for 20 min, followed by washes. Then tissue sections were blocked with 5% goat serum before they were incubated with the indicated primary antibodies for 1 h at room temperature. After 3 washes with PBS, sections were incubated with Alexa Fluor–conjugated secondary antibody, Hoechst 33342, and/or phalloidin for 30 min at room temperature. After 3 washes with PBS, the specimens were mounted with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific) and visualized under a Nikon (Tokyo, Japan) fluorescence microscope.
Cell culture, transduction, and treatment
Human intestinal epithelial T84 cells were grown in DMEM:F12 (1:1 mixture) supplemented with 2.5 mM l-glutamine and 5% fetal bovine serum in a 5% CO2 humidified incubator at 37°C. Human intestinal epithelial Caco-2 cells were cultured in DMEM (high-glucose) supplemented with 1 mM sodium pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin, and 10% fetal bovine serum. Caco-2 and T84 cells were infected with pCDH/HA-DMT1 lentiviral particles, and stable cell lines were generated by puromycin (10 μg/ml) selection for 3 passages. For all experiments, IECs were grown on transwell filters for 2–3 wk after confluence to ensure polarization.
Quantitative RT-PCR
Duodenal mucosal scrapes, liver, and cultured cells were harvested. Total RNA was extracted with the RNeasy Mini kit (Qiagen, Hilden, Germany); 3 μg of total RNA was used for cDNA synthesis using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s instruction. Quantitative PCR was performed with iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) on the Mastercycler Realplex (Eppendorf, Hamburg, Germany). PCR primer sequences are listed in Table 3.
TABLE 3.
Primers used for real-time PCR
| Gene | Primer sequence, 5′–3′ |
|
|---|---|---|
| Forward | Reverse | |
| mDMT1-IRE | TGGGTCTGTCTTTCCTGGAC | TGCAACAGCACATACTTGTGG |
| mDMT1-non-IRE | TGGGTCTGTCTTTCCTGGAC | GAACAAGCTCACCTCCGAAC |
| mDMT1 (IRE and non-IRE) | ATCGCCATCAATCTCCTGTC | AAACGTGAGGGCCATGATAG |
| mDCytb | CAATGGAAACGCCCTAGAGA | CGACATTTCACATGGTGGAC |
| mFerroportin | TGGAATTTGGTGTCCATGTG | TCAAGTTCACGGATGTTGGA |
| mHephaestin | CTGGCATGGAGACCATCTTT | AGCAATGAGCAACAGAAGCA |
| mFerritin-H | GCCAGAACTACCACCAGGAC | TGGTTCTGCAGCTTCATCAG |
| mFerritin-L | GGGCCTCCTACACCTACCTC | CTCCTGGGTTTTACCCCATT |
| mHepcidin-1 | TTGCGATACCAATGCAGAAGA | GATGTGGCTCTAGGCTATGTT |
| mβ-actin | AGCCATGTACGTAGCCATCC | TCTCAGCTGTGGTGGTGAAG |
| hDMT1 (all isoforms) | CCAGGTACTCAAGGGCATGT | GTGCAATGCAGGATTCAATG |
| hβ-actin | GGACTTCGAGCAAGAGATGG | AGCACTGTGTTGGCGTACAG |
m, mouse; h, human.
Western blotting
Total membrane, BBMs, and cultured cells were all lysed in lysis buffer (Cell Signaling Technology) containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM Na2-EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 μg/ml leupeptin, 1% Triton X-100, and protease inhibitor mixture tablets (F. Hoffmann-La Roche). The crude lysates were sonicated for 2 × 15 s and spun at 14,000 g for 15 min. Protein concentration was determined by bicinchoninic acid assay (MilliporeSigma). Protein lysates were heated at 37°C for 30 min in 1× Laemmli buffer, loaded on SDS-PAGE gel, separated, and transferred to nitrocellulose membrane for immunoblotting with the corresponding antibodies. Densitometric analysis was performed by using ImageJ software.
Surface biotinylation
Surface biotinylation of DMT1 was performed as previously described (38). In brief, T84 cells grown on transwell filters were rinsed twice in cold PBS and incubated for 10 min in borate buffer (154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, and 10 mM H3BO3, pH 9.0). Cells were then incubated for 40 min with 0.5 mg/ml NHS-SS-biotin (Pierce Biotechnology, Rockford, IL, USA) in borate buffer. Unbound NHS-SS-biotin was quenched with Tris buffer (20 mM Tris, 120 mM NaCl, pH 7.4). Cells were then rinsed with PBS, scraped, and lysed in the lysis buffer described above. An aliquot of supernatant was retained as the total fraction representing the total cellular DMT1, and 200 µg of lysate was then incubated with streptavidin-agarose beads (Pierce Biotechnology) for 2 h. The streptavidin-agarose beads were washed 3 times in lysis buffer and twice in PBS. All the above procedures were performed at 4°C or on ice. Biotinylated surface proteins were then eluted by boiling the beads in 2× Laemmli buffer at 95°C for 5 min. Dilutions of the total and surface DMT1 were resolved by SDS-PAGE and immunoblotted with mouse anti-human DMT1 antibody.
DMT1 internalization
DMT1 protein internalization was measured as previously described (39). Filter-grown T84 cells were pretreated from the basolateral side with or without 20 mM glucose for 2 h. Cells were then biotinylated at the apical side with 0.15 mg/ml sulfo-NHS-SS-biotin (Pierce Biotechnology) in PBS for 10 min on ice, followed by an ice-cold PBS wash to remove unlabeled biotin. Cells were then overlaid with preheated complete medium and placed in the cell culture incubator to initiate internalization. At each selected time point, the cells were moved to 4°C to halt internalization, and the remaining biotin on the cell surface was stripped with glutathione (GSH) buffer containing the membrane-impermeable reducing agent GSH (50 mm GSH, 75 mm NaOH, 75 mm NaCl, 1 mm EDTA, 0.1% BSA, pH 9) 2 times for 20 min each at 4°C. GSH was neutralized with 10 mM iodoacetamide in PBS. Cells were rinsed, scraped, and lysed in the lysis buffer as previously described. Supernatants containing equal amounts of protein were incubated with streptavidin beads to pull down the remaining biotinylated proteins. Proteins were eluted from the beads, and DMT1 expression was analyzed by blotting with anti-human DMT1 antibody.
DMT1 half-life
Polarized T84 cells were treated with or without 20 mM d-glucose for 24 h in the presence of 50 μM cycloheximide. Caco-2/HA-DMT1 cells were pretreated with or without 1 µM Gö6976 for 30 min before the addition of cycloheximide for the indicated times. Protein lysates were prepared, and the expression of native DMT1 and exogenous HA-DMT1 was determined by immunoblotting with anti-human DMT1 and anti-HA antibodies, respectively.
Detection of DMT1 ubiquitination
Caco-2/HA-DMT1 cells were treated with 1 µM Gö6976 or 100 nM PMA for 1 h. Cells were lysed in the above-described lysis buffer, and 200 μg protein was used for immunoprecipitation with anti-HA antibody-conjugated magnetic protein G beads (Pierce Biotechnology). Protein complexes were then eluted from the beads and resolved by SDS-PAGE. HA-DMT1 ubiquitination was analyzed by immunoblotting with mouse anti-ubiquitin antibody.
Statistical analysis
Statistical significance was determined by a paired t test. Data are presented as the means ± se. A value of P < 0.05 was considered significant.
RESULTS
DMT1 mRNA expression and iron uptake are increased in diabetic mouse duodenum
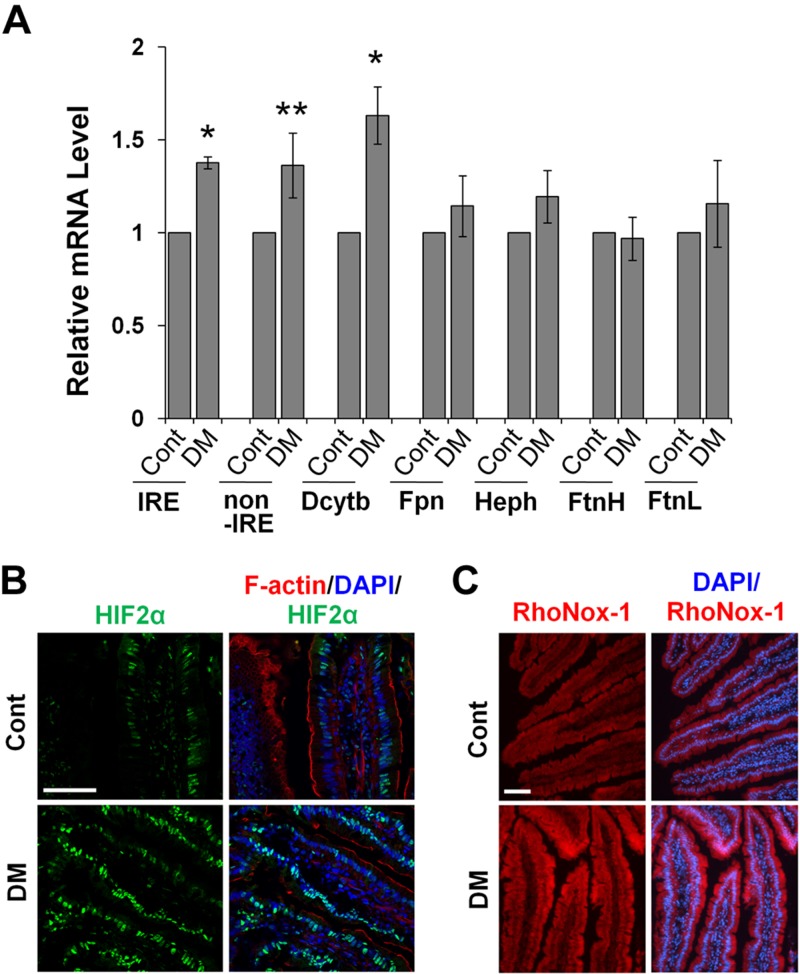
Multiple studies have shown increased iron content in the serum and tissues, including the liver, heart, kidney, and vasculatures, in diabetic animals (15, 17, 18). In this study, we first examined whether increased iron loading occurs in diabetic B6 mice. Consistent with previous reports, iron contents in the serum (Fig. 1A) and liver (Fig. 1B) were significantly increased in the diabetic group as compared with the control. A recent study (15) showed that diabetes is associated with increased iron absorption through the up-regulation of ferroportin in IECs. DMT1 has an essential role in dietary iron uptake in the gut (20, 21), and changes in DMT1 mRNA expression is an important mechanism regulating iron absorption (26, 28). We observed that, compared with the control, diabetic mice displayed a significant increase in the expression of DMT1-IRE, DMT1–non-IRE, and ferric reductase duodenal cytochrome b (Dcytb) in the duodenum (Fig. 2A). In contrast, no significant change was found in the mRNA expression of ferroportin, hephaestin, and the heavy/light chain of ferritin, which are known to regulate iron absorption and/or storage (40). A common regulator for Dmt1 and Dcytb gene transcription is the transcription factor HIF2α (26, 27). Confocal microscopic analysis revealed stronger nuclear expression of HIF2α (green) in diabetic mice (Fig. 2B). Because increased DMT1 expression potentially leads to elevated iron uptake, we next determined Fe2+ uptake using RhoNox-1, a fluorescent probe that specifically recognizes Fe2+ (34, 41). We showed that duodena treated with FeSO4 showed a stronger signal in IECs by RhoNox-1 staining (Supplemental Fig. S1A), whereas incubation of duodena (Supplemental Fig. S1A) or duodenal frozen sections (Supplemental Fig. S1B) with deferoxamine markedly reduced the staining intensity, verifying the specificity of RhoNox-1 in Fe2+ labeling. Importantly, we found that the intensity of the RhoNox-1-Fe2+ complex-mediated fluorescence was much greater in the enterocytes of diabetic mouse intestine (Fig. 2C). These findings demonstrate that dietary iron uptake is elevated in a diabetic condition, at least in part, attributable to up-regulated Dmt1 gene expression by HIF2α.
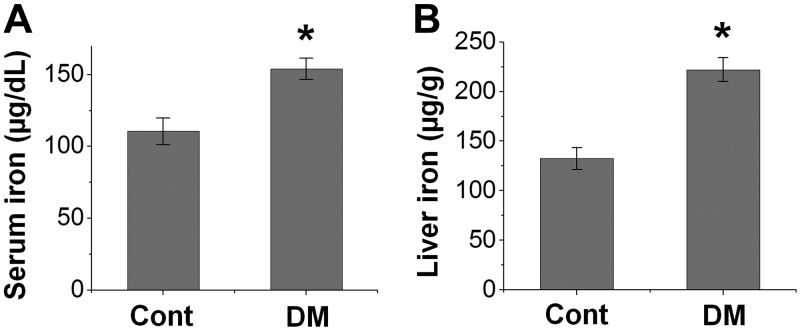
Figure 1.
Diabetes promotes body iron content in mice. Diabetic (DM) mice were induced by STZ treatment. DM and control (Cont) mice were sacrificed 2 mo later for the analysis of serum (A) and liver (B) iron content. *P < 0.01 compared with Cont. n = 6.
Figure 2.
DMT1 expression and iron uptake are elevated in the duodenum of diabetic mice. A) The mRNA expression of iron metabolism genes in control (Cont) and diabetic (DM) mouse duodenum was measured by qRT-PCR. IRE, DMT1-IRE; after IRE and non-IRE, DMT1–non-IRE. *P < 0.01, **P < 0.05 compared with Cont. n = 5. B) Representative images show the expression of HIF2α (green) with F-actin (red) by phalloidin and nuclei (blue) by DAPI in duodenal epithelial cells of Cont and DM mice. n = 5. Scale bar, 50 μm. C) Duodena dissected from Cont and DM mice were exposed to 100 µM FeSO4, and intracellular Fe2+ content was determined by labeling with 5 μM RhoNox-1 (red). Scale bar, 50 μm.
Diabetes promotes luminal membrane expression of DMT1 in humans and mice
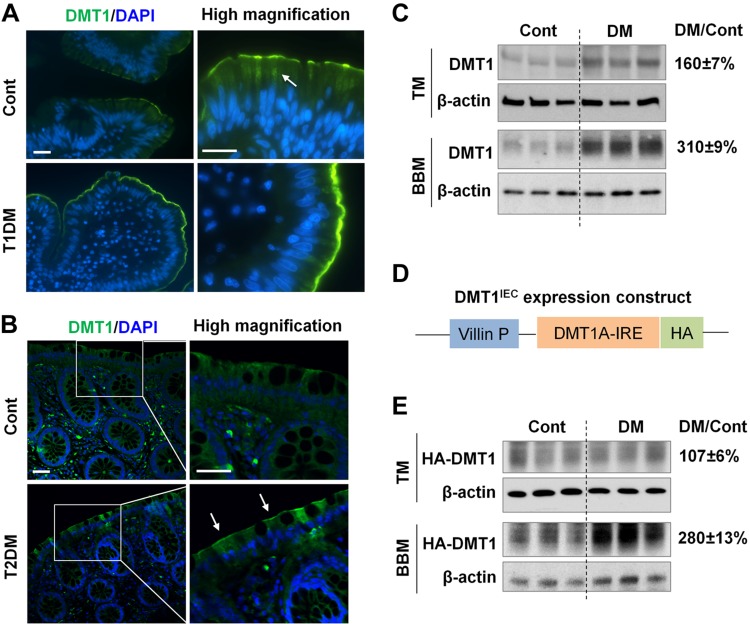
We next assessed whether intestinal DMT1 expression is altered in human patients with diabetes. We obtained jejunal biopsies from patients with type 1 diabetes and from the healthy controls (Table 1). Immunofluorescence staining with an anti-human DMT1 antibody that recognizes all 4 DMT1 isoforms revealed that DMT1 protein (Fig. 3A, green) was expressed at the luminal membrane as well as in the cytoplasm (Fig. 3A, arrow) in healthy humans (Fig. 3A, Cont), consistent with previous findings in mouse duodenum (42, 43). In contrast, DMT1 protein was primarily found at the luminal site and barely visible intracellularly in diabetic samples (Fig. 3A, T1DM), suggesting changes of DMT1 localization. We also obtained colonic biopsies from patients with type 2 diabetes (HbA1c level > 6.5%) and from healthy controls (Table 2). Consistently, luminal membrane expression of DMT1 was apparently stronger in the colonocytes of the patients with type 2 diabetes compared with the controls (Fig. 3B). This novel mechanism of regulation was further validated in diabetic mice by comparing total and microvillus membrane DMT1 expression. Western blotting showed that total expression of DMT1-IRE, the primary isoform in mouse intestine, was increased by ∼60% in diabetic mice relative to the control (Fig. 3C, TM), whereas BBM DMT1 expression was increased by 210% (Fig. 3C, BBM). We recently generated transgenic DMTIEC mice, which express HA-tagged DMT1-IRE (HA-DMT1) in IECs driven by the Villin gene promoter (Fig. 3D). Compared with WT mice, DMTIEC mice (F2 generation) showed a 4.5-fold increase in liver iron content (Supplemental Fig. S2A) and a 2.2-fold increase in hepatic hepcidin-1 mRNA expression (Supplemental Fig. S2B). The elevation in liver iron loading and the duodenal epithelial iron retention were displayed by Prussian blue staining (Supplemental Fig. S2C). These results demonstrated that the HA-DMT1 transgene was physiologically functional, and DMTIEC mice represent a novel model of dietary iron loading. With the DMTIEC transgenic strain, we further showed that BBM HA-DMT1 abundance in diabetic mice was 180% higher than it was in the control, without a significant difference in total HA-DMT1 expression (Fig. 3E). These data thus indicate that changes in DMT1 localization likely have a predominant role in the up-regulation of DMT1 activity and iron uptake in the diabetic state.
Figure 3.
Microvillus membrane expression of DMT1 is increased in diabetic humans and mice. A) Human jejunal biopsies were obtained from patients with type 1 diabetes (T1DM) and from healthy controls (Cont). DMT1 expression (green) was assessed by immunofluorescence staining with anti-human DMT1 antibody. Nuclei (blue) were stained with DAPI. Arrow, cytoplasmic localization of DMT1. Scale bar, 20 μm. B) Representative images showing DMT1 expression in human colonic biopsies from patients with T2DM and from Cont. Arrow, luminal membrane expression. Scale bar, 20 μm. C) DMT1-IRE expression in the duodenal total membrane (TM) and BBM of Cont and diabetic WT mice was determined by Western blotting with anti-rat DMT1 antibody. D) Transgenic DMT1IEC mice express HA-tagged human DMT1A-IRE isoform specifically in IECs using villin gene promoter. E) The expression of HA-DMT1A-IRE (HA-DMT1) in the duodenal TM and BBM of Cont and diabetic (DM) DMT1IEC mice was determined by immunoblotting with anti-HA antibody. β-actin was used as a loading control. The relative fold changes (DM/Cont) of DMT1 or HA-DMT1 expression in DM vs. Cont mice are shown on the right. Data are expressed as means ± se (n = 6).
Deficiency in PKCα mitigates diabetes-increased BBM DMT1 expression and iron uptake
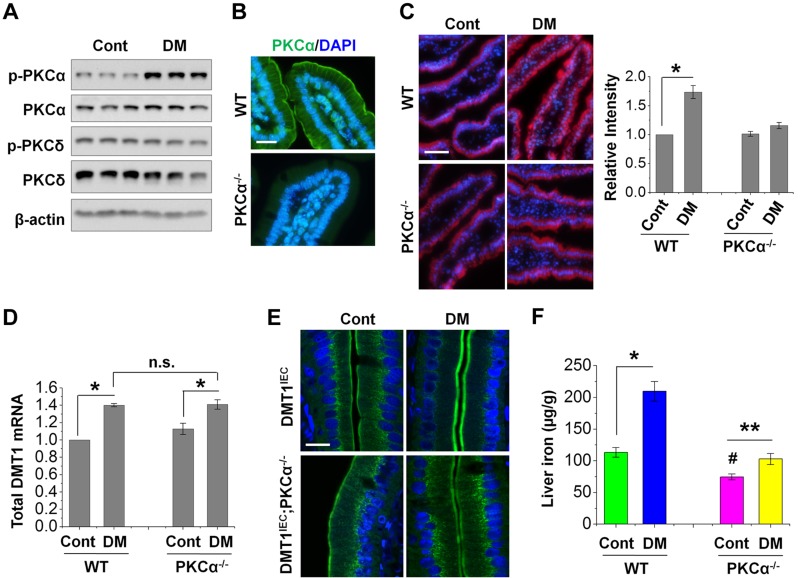
PKCs, which are often hyperactivated in diabetes, have important roles in the pathogenesis of a myriad of diabetic complications (32, 33). PKCα and PKCδ are the abundant isoforms in intestinal epithelium, and our recent study (44) showed a marked increase in PKCα association with intestinal epithelial BBM in diabetic mice. Consistently, we observed herein that the expression of the phosphorylated (active) form of PKCα, but not PKCδ, was markedly increased in diabetic mice (Fig. 4A). Microscopic analysis using PKCα−/− mice as a negative control revealed PKCα expression on the BBM as well as the basolateral membrane in the IECs of WT mice (Fig. 4B), further pointing to a potential role of PKCα in the regulation of iron uptake. Indeed, RhoNox-1 labeling revealed a ∼70% in WT diabetic mice over the WT control, whereas no significant difference was found in diabetic PKCα−/− mice (Fig. 4C). Nevertheless, deficiency in PKCα expression did not alter diabetes-induced DMT1 (IRE and non-IRE) mRNA expression (Fig. 4D). We then assessed whether PKCα regulated DMT1 localization with DMT1IEC;PKCα−/− and control DMT1IEC mice. Similar to patients with diabetes, diabetic DMT1IEC mice manifested a prominent increase in BBM expression of HA-DMT1 (Fig. 4E). By contrast, diabetes did not induce a visible change in DMT1 localization in DMT1IEC;PKCα−/− mice. These results together suggest that hyperactive PKCα signaling associated with diabetes has a key role in promoting microvillus membrane DMT1 expression and dietary iron uptake. We speculated that knockout of PKCα should attenuate hepatic iron loading in diabetic mice. Compared with diabetic WT mice, wherein a ∼90% increase in the liver iron content was observed, diabetic PKCα−/− mice elicited an increase of only ∼30% (Fig. 4F). Intriguingly, control PKCα−/− mice showed a significant decrease (∼30%) in basal level liver iron compared with the WT controls. The underlying mechanisms are not understood, but our additional data showed that PKCα regulates ferroportin expression (data not shown). In this study, we focused on the mechanisms of DMT1 regulation.
Figure 4.
PKCα mediates increased membrane DMT1 expression and iron uptake in diabetic mice. A) The expression of total and phosphorylated (p) form of PKCα and PKCδ in control (Cont) and diabetic (DM) mouse duodena was determined by immunoblotting with the corresponding antibodies. B) PKCα localization in IECs was determined by microscopic analysis using PKCα−/− mice as a negative control. Scale bar, 10 μm. C) Representative images show the labeling of Fe2+ by RhoNox-1 (5 µM) in duodenal epithelium of WT and PKCα−/− mice. The fluorescent intensity was quantified from 6 villi of each mouse (n = 5 mice/group), and the relative changes are shown on the right with the values of the WT-Cont mice set at 1. Scale bar, 50 μm. D) Total DMT1 mRNA expression in WT and PKCα−/− mouse duodenum was determined by qRT-PCR. Data are shown as means ± se (n = 6). E) Representative confocal images show the localization of HA-DMT1 in DMT1IEC and DMT1IEC;PKCα−/− mice as stained by anti-HA antibody. n = 5. Scale bar, 10 μm. F) Nonheme iron content was determined in the liver of WT and PKCα−/− mice with or without diabetes. Data are means ± se (n = 5). *P < 0.01, **P < 0.05, #P < 0.01 compared with the WT-Cont; N.s., not significant.
High glucose increases surface DMT1 expression dependent on the activation of PKCα in cultured IECs
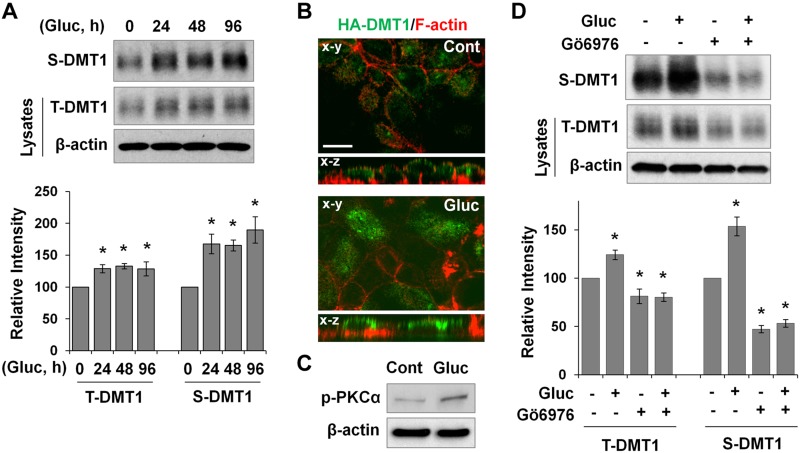
Hyperglycemia is the key factor that induces PKC activation in diabetes (32). We next examined whether high-glucose treatment increases DMT1 membrane expression using a cultured IEC model. T84 cells are polarized human IECs and abundantly express DMT1A-IRE. Compared with other polarized IECs, which require 25 mM d-glucose to grow, T84 cells require a lower concentration of 17.5 mM and were chosen for high-glucose studies. Fully polarized T84 cells were treated for 24, 48, or 96 h at the basolateral side with 20 mM d-glucose, a concentration that is often used to represent high-glucose level in cell culture studies (45, 46). Surface biotinylation showed that high-glucose treatment significantly increased surface DMT1 expression by >65% at any of the time points compared with the untreated control (Fig. 5A, S-DMT1). Total DMT1 expression was also elevated by ∼30% (Fig. 5A, T-DMT1). To further confirm the stimulatory effects of high glucose on DMT1 membrane expression, we generated T84/HA-DMT1 cells for microscopic analysis of DMT1 localization with an anti-HA antibody. F-actin costaining was performed to outline the plasma membrane as we have previously described (44). Figure. 5B shows that staining of HA-DMT1 (green) at the apical surface was much greater in glucose-treated cells compared with the untreated control, as is revealed by both horizontal (x–y) and cross-sectional (x–z) images.
Figure 5.
High glucose induces PKCα-dependent membrane accumulation of DMT1 in cultured intestinal epithelial cells. A) Filter-grown T84 cells were treated with 20 mM d-glucose, and plasma membrane DMT1 expression was determined by cell-surface biotinylation assay. Surface (S) and total (T) DMT1 expression was determined by Western blotting with anti-human DMT1 antibody. Relative changes in DMT1 expression are expressed as means ± se (n = 3 independent experiments). B) Representative confocal images of the x–y and x–z planes show localization of HA-DMT1 (green) in glucose-treated (Gluc) and untreated (Cont) T84/HA-DMT1 cells. F-actin (red) was stained with phalloidin to outline the plasma membranes. Scale bar, 10 μm. C) p-PKCα expression in Gluc-treated and Cont T84 cells was determined by immunoblotting. D) T84 cells were pretreated for 30 min with Gö 6976 (1 μM) before glucose treatment for 24 h. T- and S-DMT1 expression was evaluated by immunoblotting, and the relative changes in their expression are shown as means ± se (n = 3). *P < 0.01 compared with the untreated control (set at 100).
Consistent with findings in other cell types (45, 46), we found that PKCα phosphorylation was significantly increased in T84 cells that were treated for 24 h with high glucose (Fig. 5C). We next sought to determine whether PKCα mediates high-glucose–induced up-regulation of DMT1 expression. We did not try to generate T84 cells with stable knockdown of PKCα because multiple studies (47, 48) have shown that chronic changes in PKCα activity significantly alter colorectal cancer cell growth, complicating the data analysis. As such, PKCα was acutely inhibited by Gö 6976, a nonselective inhibitor of PKCα (49, 50). As shown in Fig. 5D, Gö 6976 treatment (24 h) mitigated the stimulatory effect of high glucose on both total and membrane expression of DMT1. It is noteworthy that Gö 6976 significantly decreased (53%) baseline membrane DMT1 expression (Fig. 5D, S-DMT1). Total DMT1 expression was also significantly decreased (19%) by Gö 6976 treatment, albeit at a lower magnitude (Fig. 5D, T-DMT1). Together, the above findings demonstrate that high glucose enhances DMT1 membrane expression in cultured IECs, likely in a PKCα-dependent manner.
PKCα enhances DMT1 protein stability through the inhibition of DMT1 ubiquitination and degradation
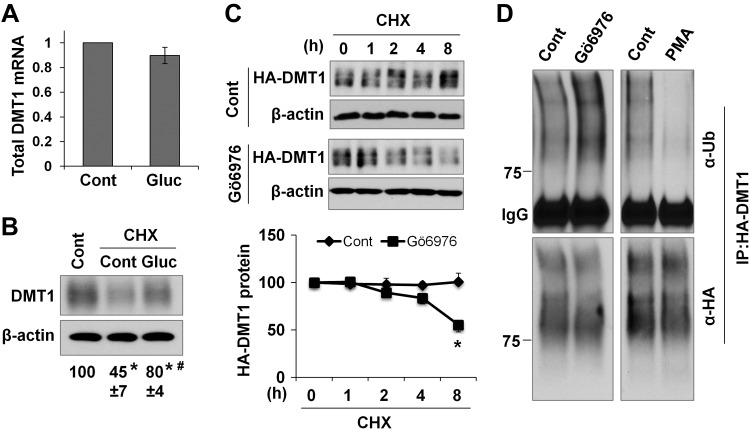
We next asked how high glucose up-regulates DMT1 protein expression using T84 cells. Because DMT1 transcript level was increased in diabetic mice, we reasoned that high glucose may up-regulate DMT1 mRNA expression. Surprisingly, quantitative PCR analysis with a primer pair that amplifies all human DMT1 isoforms did not show a significant change in DMT1 transcript level in response to high-glucose treatment (Fig. 6A). The mechanism underlying the differential regulation of Dmt1 mRNA expression by high glucose in T84 cells and in diabetic mice is not yet known. The lack of changes in mRNA expression rendered us suspect that high glucose probably regulates DMT1 protein stability. To that end, T84 cells were treated with or without glucose for 24 h in the presence of cycloheximide, an inhibitor of de novo protein synthesis. Compared with the untreated cells, cycloheximide-treated cells showed a 55% decrease in DMT1 expression, whereas cotreatment by glucose resulted in only a 20% reduction (Fig. 6B), suggesting that high glucose imposes an inhibitory effect on DMT1 degradation.
Figure 6.
High glucose stabilizes DMT1 protein, likely through PKCα-dependent inhibition of DMT1 ubiquitination. A) Total DMT1 mRNA expression was examined by qRT-PCR in 24-h glucose-treated (Gluc) and in untreated control (Cont) T84 cells. n = 3 independent experiments. B) T84 cells were treated for 24 h with or without cycloheximide (CHX, 50 μM) and glucose (Gluc, 20 mM). DMT1 expression was then determined by immunoblotting, and the relative changes are shown below the blots. *P < 0.01 compared with the untreated Cont (set at 100). #P < 0.01 compared with CHX-Cont. C) Caco-2/HA-DMT1 cells were pretreated for 30 min with Gö 6976 (1 μM) before treatment by CHX for the indicated times. The relative expression of HA-DMT1 protein was compared with its corresponding control (set at 100). Data are presented as means ± se (n = 3). *P < 0.01 compared with the 8-h time point of the Cont cells. D) Caco-2/HA-DMT1 cells were treated for 1 h with Gö 6976 (1 μM) or PMA (100 nM). HA-DMT1 was then immunoprecipitated by anti-HA antibody, and its ubiquitination was analyzed by blotting with anti-ubiquitin (α-Ub) antibody. The total amount of immunoprecipitated HA-DMT1 was detected by anti-HA (α-HA). Blots are representatives of 3 independent experiments.
The role of PKCα in the regulation of DMT1 protein stability was then assessed with HA-DMT1-IRE–expressing Caco-2 cells, another line of polarized IECs. Consistent with a previous report (51), cells treated by cycloheximide for ≤8 h did not show a significant change in HA-DMT1 expression. In contrast, cells pretreated with Gö 6976 manifested a 45% decrease in HA-DMT1 expression after 8 h of cycloheximide treatment (Fig. 6C). Degradation of DMT1 in proteasomes is preceded by ubiquitination. We thus examined whether PKCα modulates the ubiquitination of DMT1. HA-DMT1 protein was pulled down with anti-HA antibody, and its ubiquitination level was determined with anti-ubiquitin antibody. Intriguingly, inhibition of PKCα with Gö 6976 markedly increased the ubiquitination level of HA-DMT1 (Fig. 6D, left). In contrast, activation of pan-PKC by PMA caused a prominent decrease in HA-DMT1 ubiquitination (Fig. 6D, right). These results thus indicate that PKCα stabilizes DMT1 from degradation by suppressing its ubiquitination, which probably underlies high-glucose–induced up-regulation of DMT1 expression.
High glucose suppresses DMT1 internalization in cultured IECs
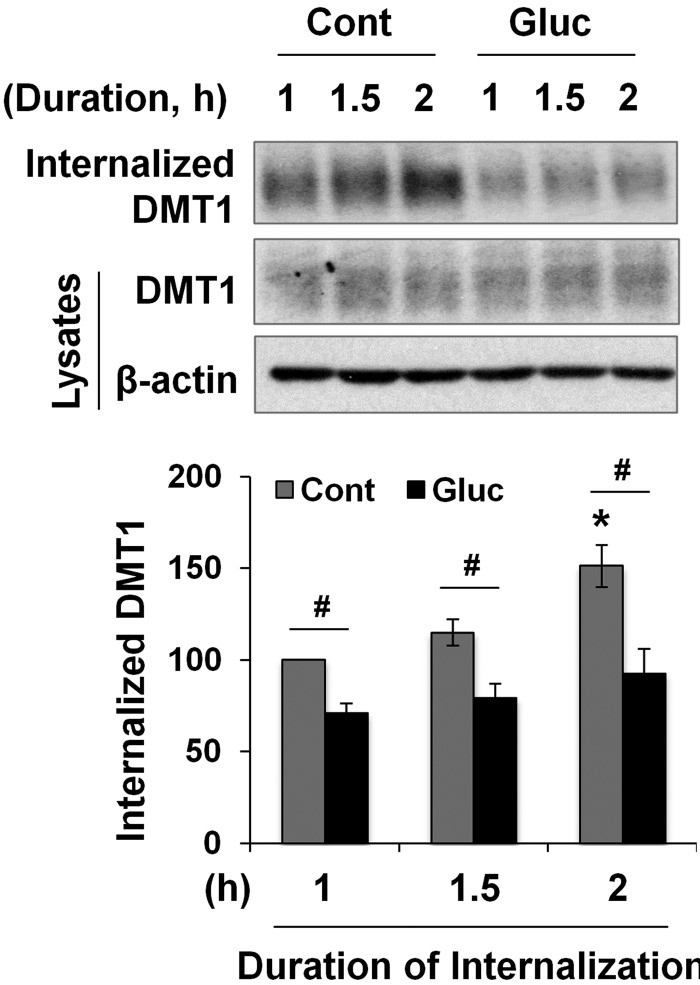
Ubiquitination not only mediates protein degradation but also induces membrane protein endocytosis (52). Our results showed that high glucose activates PKCα (Fig. 5C) and that PKCα regulates the ubiquitination of DMT1 (Fig. 6D). We thus wondered whether high-glucose treatment modulates the internalization rate of membrane DMT1. To that end, T84 cells were pretreated with or without high glucose for 2 h before biotin labeling. In untreated control cells, a 50% increase was seen in intracellular DMT1 abundance between the 1–2 h period after the initialization of internalization (Fig. 7, gray bars). In contrast, there was only a 20% increase in DMT1 endocytosis in glucose-treated cells (Fig. 7, black bars). At all time points, the amount of internalized DMT1 in glucose-treated cells was significantly less than that of the control cells. Of note, unlike that in the 24-h glucose-treated cells (Fig. 5A), total DMT1 expression did not change in the 2-h glucose-treated cells. We presumed that change in DMT1 expression from high-glucose–induced decrease in protein degradation had not been manifested within the 2-h duration because DMT1 protein has a half-life of ∼15 h (53). These findings indicate that the inhibition of DMT1 endocytosis accounts, at least in part, for high-glucose–induced up-regulation of plasma membrane expression of DMT1.
Figure 7.
The DMT1 internalization is decreased by high-glucose treatment. T84 cells were pretreated with (Gluc) or without (Cont) 20 mM d-glucose for 2 h, followed by biotinylation and the initialization of internalization. The internalization process was ended at the indicated times, and the amount of internalized DMT1, as well as the total DMT1, was quantified. Data are expressed as means ± se (n = 3). #P < 0.01, *P < 0.01 compared with the 1-h time point of the Cont (set at 100).
DISCUSSION
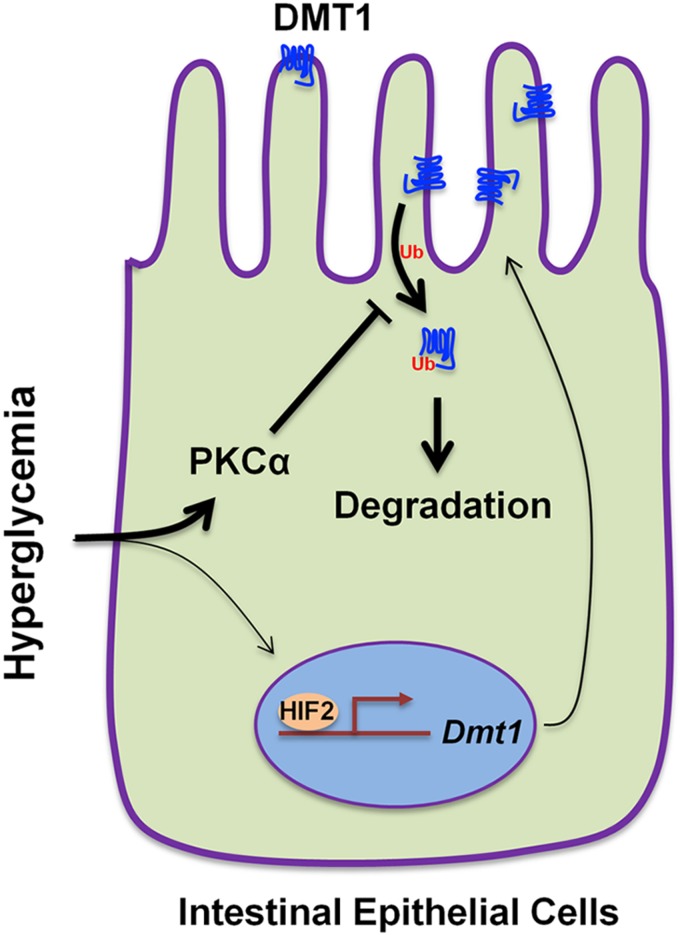
We present findings in this study that intestinal iron uptake and DMT1 expression are enhanced in diabetic mice. Increased iron uptake was largely attributed to PKCα-mediated elevation of DMT1 expression at the microvillus membrane of IECs and was partly due to HIF2α-dependent up-regulation of Dmt1 gene transcription. By using cultured IECs, we uncovered that high glucose promotes surface DMT1 expression through PKCα-dependent suppression of DMT1 ubiquitination and internalization (Fig. 8).
Figure 8.
Putative model of hyperglycemia-induced up-regulation of DMT1 expression in intestinal epithelial cells. Hyperglycemia induces elevation in microvillus membrane DMT1 expression largely (thicker lines) through PKCα-dependent inhibition of the ubiquitination, internalization, and degradation of DMT1. Increased DMT1 expression also partly (thinner lines) results from the HIF2α-dependent transactivation of Dmt1 gene transcription.
Compelling evidence has shown that iron overload increases the prevalence of diabetes by impairing pancreatic β-cell viability and insulin receptor activity through reactive oxygen species (ROS) production (4, 6). However, the potential effects of diabetes on iron transport are less clear. Multiple studies have shown increased serum iron and the elevation of iron content in the liver, heart, kidney, and vasculatures. Hepcidin deficiency is a common cause of iron overload (23), but constant increases in intestinal DMT1 expression can also lead to systemic iron loading. It was previously shown that HIF2α-mediated up-regulation of DMT1 is essential for excess iron accumulation in mouse models of β-thalassemia (30). Consistently, our transgenic DMT1IEC mice show a marked increase of iron loading in the liver. Wang et al. (15) showed that enhanced ferroportin expression in intestinal epithelial cells contributes to diabetic iron loading. Our current finding of increased DMT1 expression in diabetic mouse intestine suggests that the up-regulation of dietary iron uptake and export are together responsible for diabetic iron loading. DMT1-mediated iron uptake is the first step of iron absorption; thus, much effort has been taken to develop DMT1 inhibitors for preventing iron overload (54, 55). A potent membrane-impermeable DMT1 inhibitor XEN602 has recently been developed and successfully used to inhibit DMT1 activity in animal and cell culture systems (56–58). Nevertheless, direct evidence that pharmacologic inhibition of intestinal DMT1 prevents systemic iron overload is still lacking.
Transcriptional and posttranscriptional regulations are 2 important mechanisms in DMT1 regulation. HIF2α is an important transcription factor that transcribes the Dmt1 gene into DMT1-IRE and DMT1–non-IRE in mice (26, 27). Posttranscriptionally, iron regulatory protein 1/2 (IRP1/2) stabilizes DMT1-IRE by binding to the IRE element in the 3′ UTR of the mRNA (28). We found, in this study, that DMT1-IRE and DMT1–non-IRE mRNA were both increased in diabetic mouse duodenum, suggesting a role for HIF2α. Indeed, increased nuclear expression of HIF2α was revealed in the enterocytes of diabetic mice. In line with our findings, Zhang et al. (59) reported that DMT1 expression is increased in bone tissues of type 2 diabetic rats, although the specific mechanism was not specified. Another study showed the up-regulation of HIF2α expression in the retina of diabetic rats (60). We did not attempt to elucidate the mechanisms underlying the elevated HIF2α expression. That up-regulation is, however, less likely dependent on PKCα because Dmt1 gene transcription is not altered in PKCα−/− mice under either basal or diabetic conditions. It was previously shown that NADPH oxidase NOX4 increases HIF2α expression in renal proximal tubular cells via ROS generation (61). It remains to be determined whether ROS is responsible for the up-regulation of HIF2α in the intestine.
We have shown a novel mechanism of DMT1A-IRE regulation in the intestine. Humans express 4 different DMT1 isoforms that differ in their cellular localization. DMT1B forms are mainly localized at the endosomal and lysosomal membranes (25). DMT1A-IRE is primarily expressed at the plasma membrane (25) and is responsible for iron uptake in the intestine (21), kidney (24), and placenta (62). The current study aimed to determine the regulation of DMT1A-IRE using DMT1IEC transgenic mice and transduced IECs. We demonstrated that, in addition to transcriptional control, DMT1 was regulated posttranslationally through changes in subcellular localization as the primary mechanism in the diabetic condition. It has previously been shown that Ndfip1/2 mediates the ubiquitination of DMT1 (29) and that mice with Ndfip1 deletion show increased DMT1 expression and iron deposition in the liver (31). The same study showed that both DMT1A-IRE and DMT1B–non-IRE are colocalized with Ndfip1/2 in the endosomes in Chinese hamster ovary cells. Although only direct evidence of DMT1B-IRE ubiquitination was shown, a subsequent report found that Ndfip1−/− mice develop increased DMT1 expression in the duodenum (31), suggesting that Ndfip1 might also regulate the protein stability of DMT1A-IRE (the predominant isoform in the duodenum). Further studies are warranted to assess whether the ubiquitination and subcellular localization of intestinal DMT1A-IRE are modulated by Ndfip1/2.
A key finding of the present study is that PKCα is necessary for diabetes-induced microvillus membrane expression of DMT1. We showed that intestinal epithelial PKCα is also stimulated by hyperglycemia. To our knowledge, this is the first direct evidence showing that IEC absorptive function is modulated by hyperglycemia-induced activation of PKC. The precise mechanisms by which PKCα regulates the ubiquitination of DMT1A-IRE are not known. It is possible that PKCα changes the tertiary structure of DMT1A-IRE protein, causing decreased interaction with Ndfip1/2 or yet to be identified molecule or molecules that mediate DMT1A-IRE ubiquitination. In fact, a recent study showed that the Ser43 residue in DMT1B–non-IRE (Ser73 in DMT1A-IRE) is a potential phosphorylation site by PKC (63). That study showed that treatment by staurosporine, a potent inhibitor of PKC, reduced DMT1B–non-IRE transport activity. The interchange between the plasma membrane and intracellular proteins are counterbalanced by endocytosis and exocytosis processes. Despite our in vitro findings of a decreased rate of DMT1 internalization in response to high glucose, we cannot exclude the possibility that hyperglycemia potentiates the exocytosis of DMT1. An earlier study showed that PI3K activity is required for the recycling of internalized DMT1B to the plasma membrane (64). In contrast, the signaling pathways and scaffold proteins that regulate DMT1-IRE trafficking and recycling are not understood. The current findings implicate DMT1A-IRE localization is dynamically regulated by PKCα signaling. Our newly generated DMT1IEC transgenic mice may be a useful tool for identifying scaffold proteins that potentially regulate human DMT1A-IRE in the native intestines in mice.
In summary, we have demonstrated that diabetes induces the up-regulation of DMT1 expression and iron uptake in IECs primarily dependent on accumulative DMT1 expression at the microvillus membrane through PKCα-dependent suppression of the ubiquitination and internalization of DMT1. In line with previous reports, moderate elevation in iron stores was herein observed after 2 mo of hyperglycemia. We anticipate the magnitude of iron loading increases along with the duration of the diabetic state. Whether or not moderately increased iron aggravates diabetic complications remains poorly understood. Chaudhary et al. (65) recently showed that neuronal cell loss in the retina of diabetic Hfe−/− mice was significantly more accelerated than in nondiabetic Hfe−/− and diabetic WT mice. Of note, diabetic Hfe−/− mice showed an ∼2-fold increase in retinal iron content. Further studies are needed to elucidate whether diabetes promotes DMT1 expression and iron absorption in other tissues and to uncover how increases in iron content combining hyperglycemia affects diabetic complications.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the American Heart Association Scientist Development Grant 13SDG1623001 (to P.H.) and in part by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK107719 (to C.Y.). L.Z. is a recipient of the National Science Fund of China (NSFC Grant 81503514). The authors declare no conflicts of interest.
Glossary
- BBM
brush border membrane
- DMT1
divalent metal transporter 1
- GSH
glutathione
- HA
hemagglutinin
- HIF2α
hypoxia inducible factor 2α
- IEC
intestinal epithelial cell
- IRE
iron-responsive element
- Ndfip
Nedd4 family-interacting protein
- PMA
phorbol ester
- qRT-PCR
quantitative RT-PCR
- ROS
reactive oxygen species
- STZ
streptozotocin
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. He conceived the study; L. Zhao and P. He designed and performed the research; L. Zhao, T. Bartnikas, X. Chu, and P. He analyzed the data and interpreted the results; J. Klein provided PKCα−/− mice; S. Srinivasan provided human intestinal biopsies; P. He and C. Yun wrote the manuscript; and all authors had final approval of the submitted versions.
REFERENCES
- 1.Mathers C. D., Loncar D. (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3, e442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClain D. A., Abraham D., Rogers J., Brady R., Gault P., Ajioka R., Kushner J. P. (2006) High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia 49, 1661–1669 [DOI] [PubMed] [Google Scholar]
- 3.Eshak E. S., Iso H., Maruyama K., Muraki I., Tamakoshi A. (2017) Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: a large population-based prospective cohort study. Clin. Nutr. 37:667–674 [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Real J. M., McClain D., Manco M. (2015) Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care 38, 2169–2176 [DOI] [PubMed] [Google Scholar]
- 5.Zheng X., Jiang T., Wu H., Zhu D., Wang L., Qi R., Li M., Ling C. (2011) Hepatic iron stores are increased as assessed by magnetic resonance imaging in a Chinese population with altered glucose homeostasis. Am. J. Clin. Nutr. 94, 1012–1019 [DOI] [PubMed] [Google Scholar]
- 6.Angelopoulos N. G., Zervas A., Livadas S., Adamopoulos I., Giannopoulos D., Goula A., Tolis G. (2006) Reduced insulin secretion in normoglycaemic patients with beta-thalassaemia major. Diabet. Med. 23, 1327–1331 [DOI] [PubMed] [Google Scholar]
- 7.Cooksey R. C., Jones D., Gabrielsen S., Huang J., Simcox J. A., Luo B., Soesanto Y., Rienhoff H., Abel E. D., McClain D. A. (2010) Dietary iron restriction or iron chelation protects from diabetes and loss of β-cell function in the obese (ob/ob lep−/−) mouse. Am. J. Physiol. Endocrinol. Metab. 298, E1236–E1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nankivell B. J., Chen J., Boadle R. A., Harris D. C. (1994) The role of tubular iron accumulation in the remnant kidney. J. Am. Soc. Nephrol. 4, 1598–1607 [DOI] [PubMed] [Google Scholar]
- 9.Schafer A. I., Cheron R. G., Dluhy R., Cooper B., Gleason R. E., Soeldner J. S., Bunn H. F. (1981) Clinical consequences of acquired transfusional iron overload in adults. N. Engl. J. Med. 304, 319–324 [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Real J. M., Peñarroja G., Castro A., García-Bragado F., Hernández-Aguado I., Ricart W. (2002) Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and β-cell function. Diabetes 51, 1000–1004 [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Real J. M., Peñarroja G., Castro A., García-Bragado F., López-Bermejo A., Ricart W. (2002) Blood letting in high-ferritin type 2 diabetes: effects on vascular reactivity. Diabetes Care 25, 2249–2255 [DOI] [PubMed] [Google Scholar]
- 12.Bofill C., Joven J., Bages J., Vilella E., Sans T., Cavallé P., Miralles R., Llobet J., Camps J. (1994) Response to repeated phlebotomies in patients with non–insulin-dependent diabetes mellitus. Metabolism 43, 614–620 [DOI] [PubMed] [Google Scholar]
- 13.De Mello V. D., Zelmanovitz T., Perassolo M. S., Azevedo M. J., Gross J. L. (2006) Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am. J. Clin. Nutr. 83, 1032–1038 [DOI] [PubMed] [Google Scholar]
- 14.Ikeda Y., Enomoto H., Tajima S., Izawa-Ishizawa Y., Kihira Y., Ishizawa K., Tomita S., Tsuchiya K., Tamaki T. (2013) Dietary iron restriction inhibits progression of diabetic nephropathy in db/db mice. Am. J. Physiol. Renal Physiol. 304, F1028–F1036 [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Li H., Jiang X., Shi W., Shen Z., Li M. (2014) Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes 63, 1506–1518 [DOI] [PubMed] [Google Scholar]
- 16.Johnson W. T., Evans G. W. (1984) Effects of the interrelationship between dietary protein and minerals on tissue content of trace metals in streptozotocin-diabetic rats. J. Nutr. 114, 180–190 [DOI] [PubMed] [Google Scholar]
- 17.Singh J., Chonkar A., Bracken N., Adeghate E., Latt Z., Hussain M. (2006) Effect of streptozotocin-induced type 1 diabetes mellitus on contraction, calcium transient, and cation contents in the isolated rat heart. Ann. N. Y. Acad. Sci. 1084, 178–190 [DOI] [PubMed] [Google Scholar]
- 18.Rowe P. A., Kavanagh K., Zhang L., Harwood H. J., Jr., Wagner J. D. (2011) Short-term hyperglycemia increases arterial superoxide production and iron dysregulation in atherosclerotic monkeys. Metabolism 60, 1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donovan A., Lima C. A., Pinkus J. L., Pinkus G. S., Zon L. I., Robine S., Andrews N. C. (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200 [DOI] [PubMed] [Google Scholar]
- 20.Gunshin H., Fujiwara Y., Custodio A. O., Direnzo C., Robine S., Andrews N. C. (2005) Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Invest. 115, 1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shawki A., Anthony S. R., Nose Y., Engevik M. A., Niespodzany E. J., Barrientos T., Öhrvik H., Worrell R. T., Thiele D. J., Mackenzie B. (2015) Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G635–G647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 [DOI] [PubMed] [Google Scholar]
- 23.Fleming R. E., Ponka P. (2012) Iron overload in human disease. N. Engl. J. Med. 366, 348–359 [DOI] [PubMed] [Google Scholar]
- 24.Hubert N., Hentze M. W. (2002) Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc. Natl. Acad. Sci. USA 99, 12345–12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanatori I., Tabuchi M., Kawai Y., Yasui Y., Akagi R., Kishi F. (2010) Heme and non-heme iron transporters in non-polarized and polarized cells. BMC Cell Biol. 11, 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah Y. M., Matsubara T., Ito S., Yim S. H., Gonzalez F. J. (2009) Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9, 152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastrogiannaki M., Matak P., Keith B., Simon M. C., Vaulont S., Peyssonnaux C. (2009) HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Invest. 119, 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galy B., Ferring-Appel D., Kaden S., Gröne H. J., Hentze M. W. (2008) Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab. 7, 79–85 [DOI] [PubMed] [Google Scholar]
- 29.Foot N. J., Dalton H. E., Shearwin-Whyatt L. M., Dorstyn L., Tan S. S., Yang B., Kumar S. (2008) Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood 112, 4268–4275 [DOI] [PubMed] [Google Scholar]
- 30.Anderson E. R., Taylor M., Xue X., Ramakrishnan S. K., Martin A., Xie L., Bredell B. X., Gardenghi S., Rivella S., Shah Y. M. (2013) Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc. Natl. Acad. Sci. USA 110, E4922–E4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foot N. J., Leong Y. A., Dorstyn L. E., Dalton H. E., Ho K., Zhao L., Garrick M. D., Yang B., Hiwase D., Kumar S. (2011) Ndfip1-deficient mice have impaired DMT1 regulation and iron homeostasis. Blood 117, 638–646 [DOI] [PubMed] [Google Scholar]
- 32.Geraldes P., King G. L. (2010) Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 106, 1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koya D., King G. L. (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47, 859–866 [DOI] [PubMed] [Google Scholar]
- 34.Niwa M., Hirayama T., Okuda K., Nagasawa H. (2014) A new class of high-contrast Fe(II) selective fluorescent probes based on spirocyclized scaffolds for visualization of intracellular labile iron delivered by transferrin. Org. Biomol. Chem. 12, 6590–6597 [DOI] [PubMed] [Google Scholar]
- 35.Wang C. Y., Knutson M. D. (2013) Hepatocyte divalent metal-ion transporter-1 is dispensable for hepatic iron accumulation and non-transferrin-bound iron uptake in mice. Hepatology 58, 788–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He P., Zhao L., No Y. R., Karvar S., Yun C. C. (2016) The NHERF1 PDZ1 domain and IRBIT interact and mediate the activation of Na+/H+ exchanger 3 by ANG II. Am. J. Physiol. Renal Physiol. 311, F343–F351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He P., Klein J., Yun C. C. (2010) Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 285, 27869–27878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He P., Zhang H., Yun C. C. (2008) IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J. Biol. Chem. 283, 33544–33553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.No Y. R., He P., Yoo B. K., Yun C. C. (2014) Unique regulation of human Na+/H+ exchanger 3 (NHE3) by Nedd4-2 ligase that differs from non-primate NHE3s. J. Biol. Chem. 289, 18360–18372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soares M. P., Hamza I. (2016) Macrophages and iron metabolism. Immunity 44, 492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikeda Y., Horinouchi Y., Hamano H., Hirayama T., Kishi S., Izawa-Ishizawa Y., Imanishi M., Zamami Y., Takechi K., Miyamoto L., Ishizawa K., Aihara K. I., Nagasawa H., Tsuchiya K., Tamaki T. (2017) Dietary iron restriction alleviates renal tubulointerstitial injury induced by protein overload in mice. Sci. Rep. 7, 10621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinder D., Oates P. S., Thomas C., Sadleir J., Morgan E. H. (2000) Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut 46, 270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canonne-Hergaux F., Gruenheid S., Ponka P., Gros P. (1999) Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 93, 4406–4417 [PubMed] [Google Scholar]
- 44.He P., Zhao L., Zhu L., Weinman E. J., De Giorgio R., Koval M., Srinivasan S., Yun C. C. (2015) Restoration of Na+/H+ exchanger NHE3-containing macrocomplexes ameliorates diabetes-associated fluid loss. J. Clin. Invest. 125, 3519–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S. H., Choi H. J., Lee J. H., Woo C. H., Kim J. H., Han H. J. (2001) High glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress, and TGF-β 1. Kidney Int. 59, 1695–1705 [DOI] [PubMed] [Google Scholar]
- 46.Hempel A., Maasch C., Heintze U., Lindschau C., Dietz R., Luft F. C., Haller H. (1997) High glucose concentrations increase endothelial cell permeability via activation of protein kinase C α. Circ. Res. 81, 363–371 [DOI] [PubMed] [Google Scholar]
- 47.Gwak J., Jung S. J., Kang D. I., Kim E. Y., Kim D. E., Chung Y. H., Shin J. G., Oh S. (2009) Stimulation of protein kinase C-alpha suppresses colon cancer cell proliferation by down-regulation of β-catenin. J. Cell. Mol. Med. 13(8B)2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pysz M. A., Leontieva O. V., Bateman N. W., Uronis J. M., Curry K. J., Threadgill D. W., Janssen K. P., Robine S., Velcich A., Augenlicht L. H., Black A. R., Black J. D. (2009) PKCα tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp. Cell Res. 315, 1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. (1993) Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J. Biol. Chem. 268, 9194–9197 [PubMed] [Google Scholar]
- 50.Chang Q., Tepperman B. L. (2003) Effect of selective PKC isoform activation and inhibition on TNF-α-induced injury and apoptosis in human intestinal epithelial cells. Br. J. Pharmacol. 140, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Touret N., Martin-Orozco N., Paroutis P., Furuya W., Lam-Yuk-Tseung S., Forbes J., Gros P., Grinstein S. (2004) Molecular and cellular mechanisms underlying iron transport deficiency in microcytic anemia. Blood 104, 1526–1533 [DOI] [PubMed] [Google Scholar]
- 52.Hicke L., Dunn R. (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19, 141–172 [DOI] [PubMed] [Google Scholar]
- 53.Paradkar P. N., Roth J. A. (2006) Post-translational and transcriptional regulation of DMT1 during P19 embryonic carcinoma cell differentiation by retinoic acid. Biochem. J. 394, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montalbetti N., Simonin A., Simonin C., Awale M., Reymond J. L., Hediger M. A. (2015) Discovery and characterization of a novel non-competitive inhibitor of the divalent metal transporter DMT1/SLC11A2. Biochem. Pharmacol. 96, 216–224 [DOI] [PubMed] [Google Scholar]
- 55.Buckett P. D., Wessling-Resnick M. (2009) Small molecule inhibitors of divalent metal transporter-1. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G798–G804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z., Kodumuru V., Sviridov S., Liu S., Chafeev M., Chowdhury S., Chakka N., Sun J., Gauthier S. J., Mattice M., Ratkay L. G., Kwan R., Thompson J., Cutts A. B., Fu J., Kamboj R., Goldberg Y. P., Cadieux J. A. (2012) Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg. Med. Chem. Lett. 22, 5108–5113 [DOI] [PubMed] [Google Scholar]
- 57.Wolff N. A., Garrick M. D., Zhao L., Garrick L. M., Ghio A. J., Thévenod F. (2018) A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 8, 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue X., Ramakrishnan S. K., Weisz K., Triner D., Xie L., Attili D., Pant A., Győrffy B., Zhan M., Carter-Su C., Hardiman K. M., Wang T. D., Dame M. K., Varani J., Brenner D., Fearon E. R., Shah Y. M. (2016) Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 24, 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W. L., Meng H. Z., Yang M. W. (2015) Regulation of DMT1 on bone microstructure in type 2 diabetes. Int. J. Med. Sci. 12, 441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright W. S., McElhatten R. M., Messina J. E., Harris N. R. (2010) Hypoxia and the expression of HIF-α and HIF-2α in the retina of streptozotocin-injected mice and rats. Exp. Eye Res. 90, 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diebold I., Flügel D., Becht S., Belaiba R. S., Bonello S., Hess J., Kietzmann T., Görlach A. (2010) The hypoxia-inducible factor-2α is stabilized by oxidative stress involving NOX4. Antioxid. Redox Signal. 13, 425–436 [DOI] [PubMed] [Google Scholar]
- 62.Chong W. S., Kwan P. C., Chan L. Y., Chiu P. Y., Cheung T. K., Lau T. K. (2005) Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues. Hum. Reprod. 20, 3532–3538 [DOI] [PubMed] [Google Scholar]
- 63.Seo Y. A., Kumara R., Wetli H., Wessling-Resnick M. (2016) Regulation of divalent metal transporter-1 by serine phosphorylation. Biochem. J. 473, 4243–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Touret N., Furuya W., Forbes J., Gros P., Grinstein S. (2003) Dynamic traffic through the recycling compartment couples the metal transporter Nramp2 (DMT1) with the transferrin receptor. J. Biol. Chem. 278, 25548–25557 [DOI] [PubMed] [Google Scholar]
- 65.Chaudhary K., Promsote W., Ananth S., Veeranan-Karmegam R., Tawfik A., Arjunan P., Martin P., Smith S. B., Thangaraju M., Kisselev O., Ganapathy V., Gnana-Prakasam J. P. (2018) Iron overload accelerates the progression of diabetic retinopathy in association with increased retinal renin expression. Sci. Rep. 8, 3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.