Abstract
A variety of mouse strains and sexes are used in studies of corneal wound healing and nerve regeneration. However, there is a gap of knowledge about corneal nerve density and its function in different mouse strains and sexes. In this study, we report a strain divergence of total and substance P (SP) sensory corneal nerves in uninjured mice. The BALB/c mouse showed the highest nerve density, corneal sensitivity, and tear volume followed by CFW and then C57BL/6. No differences were found in total nerves and SP-positive nerves between sexes. After injury damaged the corneal nerves, an important role for mouse strains, biologic sex, and their association to corneal nerve regeneration was identified. All female mice have a faster nerve regeneration rate than males. The molecular mechanism of this sexual divergence involves higher secretion neurotrophic factors in tears, which in turn modulate gene expression in trigeminal ganglion neurons. An important upstream signaling regulator was β-estradiol, and topical treatment with β-estradiol confirmed its function in corneal nerve regeneration. In conclusion, our study shows that the strain and sex of laboratory mice significantly affect the different indicators of corneal innervation and nerve regeneration. Researchers investigating corneal diseases should carefully consider these factors.—Pham, T. L., Kakazu, A., He, J., Bazan, H. E. P. Mouse strains and sexual divergence in corneal innervation and nerve regeneration.
Keywords: β-estradiol, trigeminal ganglia, docosanoids, neurotrophic factors, dry eye
Corneal nerves are vital to maintain the homeostasis of the ocular surface and tissue clarity (1–3). Many diseases that affect the cornea can compromise corneal innervation, leading to a decrease in tear production and blink reflex as well as impaired epithelial wound healing (4–6). Therefore, better knowledge about corneal nerve function and repair will increase therapeutic strategies for pathologies that affect corneal innervation. The use of animal models to study corneal nerves plays an important role in increasing our understanding of nerve damage and repair.
Among the animal species currently used, including mouse (7), rat (8), rabbit (9), guinea pig (10), cat (11), and dog (12), mouse shows several advantages such as a well-described corneal nerve anatomy and chemistry (7), the availability of genetically modified mice (13), and finding compatible research material resources such as specific antibodies, quantitative PCR primers (14), etc. Comparison of different mouse strains on their ability to repair corneal wounds and to dry eye environment exposure has been reported (15, 16); however, there are no studies on how different strains can affect mouse corneal innervation after injury.
Another recognizable variable is sex. Wang et al. (17) have demonstrated that estrogen regulates corneal epithelial wound healing in the BALB/c mouse, and the effect of sex has also been reported in human corneal transplantation procedures (18). Moreover, sexual differences have been described in a model of peripheral nerve regeneration (19). Neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) and the Semaphorin 7A (Sema7A), play a pivotal role in enhancing corneal nerve reoutgrowth (20–22), and increase in corneal nerve regeneration stimulated by pigment epithelium-derived factor (PEDF) plus docosahexaenoic acid (DHA) correlates with increased release of these 3 compounds in tears (23). Sexual divergence of BNDF and Sema7A has been reported in human platelets and in mouse olfactory bulb neurogenesis (24, 25); however, the mouse strain and sexual differences of these factors have not been studied in mouse tears. Herein, we investigate the effect of strain and sex on corneal nerve regeneration after injury and tear secretion of NGF, BDNF, and Sema7A using 3 mouse strains: BALB/C, CFW, and C57BL/6.
Most of the neurons that innervate the cornea originate in the trigeminal ganglia (TG). To understand the molecular mechanism of nerve regeneration on the 3 strains and the effect of sex better, we also investigate the gene expression in the TG of genes involved in repair and inflammation.
In this study, we report, for the first time, strain and sexual divergence of corneal innervation in normal and postcorneal injury conditions.
MATERIALS AND METHODS
Animals
All animals were handled in compliance with the guidelines of the Association for Research in Vision and Ophthalmology Statement for the use of Animals in Ophthalmology and Vision Research, and the experimental protocols were approved by the Institutional Animal Care and Use Committee at Louisiana State University Health New Orleans. Ten-week-old male and female C57BL/6, BALB/c, and CFW mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Ten-week-old male CFW mice (for the estrogen treatment experiments) were kindly provided by Dr. Nicolas Bazan (Louisiana State University Health New Orleans Neuroscience Center).
Corneal injury
Mice were anesthetized with a mix of ketamine (200 mg/kg) and xylazine (50 mg/kg) injected intraperitoneally, and 1 drop of proparacaine hydrochloride solution (0.5%) was applied to the eye subjected to injury. As previously described (26), the central part of the cornea was demarcated with a 2-mm trephine, and the epithelium and the anterior stroma were gently removed under a surgical microscope using a corneal rust ring remover (Algerbrush II; Alger Equipment Co., Lago Vista, TX, USA). One drop of tobramycin ophthalmic solution (0.3%) was applied to the eye to prevent postoperative infection. All surgeries were performed by the same investigator (J.H.).
Corneal wound healing analysis
Mice were euthanized 24 h after injury, and corneas were stained with 0.5% methylene blue for 20 s and then washed with PBS for 2 min. Photographs were taken with a dissecting microscope (SMZ 1500; Nikon, Tokyo, Japan) through an attached digital camera (DXM 1200; Nikon). The images were quantified using Photoshop CC 2014 software (Adobe, San Jose, CA, USA). Additional male and female C57BL/6 mice were injured, and the corneal wound healing was assessed 32 h after.
Corneal sensitivity
Corneal sensitivity was measured on the central cornea, inside the surgical area, using a Cochet-Bonnet aesthesiometer (Luneau Ophtalmologie, Chartres Cedex, France) as previously described (6). Briefly, the length of the monofilament was varied from 6.0 to 0.5 cm in 0.5-cm fractions until the corneal touch threshold was found. Corneas were tested 4 times at each filament length. A positive response was considered when the animal blinked 50% or more of the times tested. If no blink response could be elicited at a monofilament length of 0.5 cm, corneal sensitivity was recorded as 0. The same examiner (T.L.P.) performed all the measurements.
Measurement of tear volume (Schirmer’s test)
Tear production was assessed as previously described (6) with the phenol red thread test (Zone-Quick; FCI Ophthalmics, Pembroke, MA, USA). The thread was placed on the lower eyelid for 15 s. The yellow thread changed to a red color when it came into contact with tears. The entire length of the red portion of the thread is measured in millimeters. The same examiner (T.L.P.) performed all of the measurements.
Immunostaining and imaging of corneal nerves
At 2 or 4 wk after injury, mice were euthanized and the eyes enucleated and fixed with Zamboni’s fixative (American Master Tech Scientific, Lodi, CA, USA) for 45 min at room temperature. The corneas were then carefully excised and fixed for an additional 15 min, followed by 3 washes with PBS. To block nonspecific binding, corneas were incubated with 10% normal goat serum plus 0.5% Triton X-100 in PBS for 1 h at room temperature. Then corneas were incubated with primary antibodies rabbit monoclonal anti-PGP9.5 (1:500), ab108986 (Abcam, Cambridge, MA, USA), and rat monoclonal anti-substance-P (SP; 1:100) (sc-21715; Santa Cruz Biotechnology, Dallas, TX, USA) for 24 h at room temperature with constant shaking. After being washed with PBS, the corneas were incubated with the corresponding secondary antibody (anti-rabbit Alexa-Fluor 488 and goat anti-rat Alexa-Fluor 488 from Thermo Fisher Scientific, Waltham, MA, USA) for 24 h at 4°C. Four radial cuts were performed on each cornea, flatly mounted on a slide with the endothelium side up, and examined with a fluorescent microscope (Deconvolution microscope DP80; Olympus, Tokyo, Japan); the images were merged together to build the entire view of the corneal nerve network. The corneal nerve density was measured using Photoshop CC 2014 (Adobe) as previously described (6, 7). Briefly, the subbasal nerve fibers in each image were drawn with 4-pixel lines following the course of each fiber by using the brush tool. The nerve area and the total area of the image were obtained by using the histogram tool. The percentage of total nerve area was quantified for each image.
Quantitative analysis of gene expression in TG
TG were harvested and kept in RNAlater solution (Thermo Fisher Scientific) until homogenized on ice using a Dounce homogenizer. Total mRNA was extracted using an RNeasy mini kit (Qiagen, Germantown, MD, USA) as described by the manufacturer. Purity and concentration of RNA were determined with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Afterward, RNA samples were stored at −80°C until used. Quantitative PCR was performed with the Biomark HD system (Fluidigm, San Francisco, CA, USA). Two hundred nanograms of RNA was reverse-transcribed using iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA, USA), and the cDNA was preamplified using the PreAmp Master Mix (PN 100-5580; Fluidigm) and pool δ gene assays of 96 primer pairs for 1) neuropeptides and related genes, 2) neurotrophins and related genes, 3) regeneration-asociated genes (RAGs), and 4) proresolution and related inflammation genes. Data was collected and analyzed using Fluidigm real-time PCR analysis software. The gene expression data at 2 wk postinjury was normalized to specific strain and gender baseline. The average of normalized gene expression of female and male mice in the same strain was calculated and subjected to Venn diagram to show the common elements between 3 mouse strains with the same ≤−2 and ≥2 cutoff.
Tear collection
Five microliters of sterile PBS was instilled in the inferior cul-de-sac of the eye, and 30 s later the tears were collected. Tears from 5 eyes were pooled and immediately centrifuged at 10,000 g for 3 min. Proteins in the supernatant were determined using Quick Start Bradford Protein Assay (Bio-Rad). The samples were stored at −80°C until Western blot analysis.
Topical treatment with E2
Male CFW mice were treated topically with E2 (Cayman Chemicals, Ann Arbor, MI, USA) dissolved in DMSO and then diluted to a concentration of 1 μM in sterile PBS (<0.1% ethanol). Five microliters was administered topically to each eye 3 times a day. The control group received 5 μl of sterile PBS containing <0.1% ethanol. The aqueous solutions were prepared daily. The topical dose was selected based on previously published studies (17, 27). For the determination of neurotrophins and Sema7A, tears were analyzed by Western blot every 3 d until significant changes in secretion of BDNF, NGF, and Sema7A were detected (12 d). Afterward, the mouse right cornea was injured and the treatment was continued in the same way for an additional 14 d.
Western blot analysis
Seven micrograms of proteins from tears were subjected to SDS-PAGE (NuPage Novex 4–12% gel; Thermo Fisher Scientific) and then transfer to a PVDF membrane (Bio-Rad). The membranes were blocked with 5% nonfat milk in PBS plus 0.1% Tween 20 and then probed with the specific primary antibodies (rabbit polyclonal anti-BDNF, rabbit polyclonal anti-NGF, and rabbit polyclonal anti-Sema7A from Santa Cruz Biotechnology) all at 1:100 dilution. The membranes were washed with PBS plus 0.1% Tween 20 and further incubated with goat anti-rabbit horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology). Protein bands were visualized using chemiluminescent detection reagent (Thermo Fisher Scientific) and quantified with LAS-4000 Imaging System (GE Healthcare, Waukesha, WI, USA).
Statistical analysis
Data are expressed as mean ± sd of ≥3 independent experiments. The data were analyzed by 1-way ANOVA followed by Tukey honest significant difference post hoc test at 95% confidence level to compare the different groups and considered significant when P < 0.05. The Pearson relation analysis was used to determine the relationship between factors including corneal nerve density, corneal sensitivity, tear volume, and corneal wound healing. All statistical analyses were performed using the Stata 14 (StataCorp, College Station, TX, USA). Graphs were made using Prism 7 software (GraphPad Software, La Jolla, CA, USA). The Venn diagram was made using Bio Vinci (BioTuring, San Diego, CA, USA).
RESULTS
Strain-related corneal nerve density in the mouse
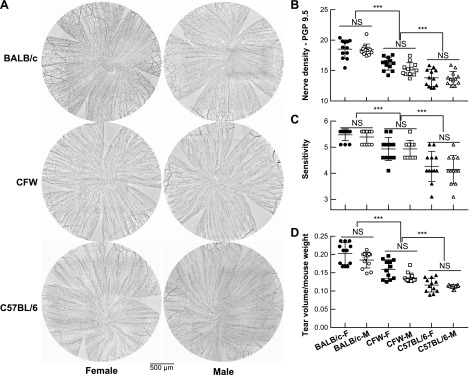
First, we compared corneal nerve density and sex between the BALB/c, C57BL/6, and CFW mice. To accomplish this, 12 corneas/strain from 10-wk-old mice of both sexes were dissected and stained with PGP9.5 and epithelial nerve density from whole-mount images was calculated (Fig. 1A, B). Corneal nerve density was significantly different from strain to strain. The highest corneal epithelial nerve density was found in the BALB/c mouse, followed by the CFW and then C57BL/6 mouse. There was no sex-related difference in all observed strains. These results were supported by distinct corneal physiologic responses such as sensitivity and tear volume (Fig. 1C, D). BALB/c mice show the greatest score of sensitivity as well as tear volume, whereas the CFW mice were ranked second and C57BL/6 was the lowest one. A correlation analysis showed a strong positive relationship of corneal nerve–sensitivity and corneal nerve–tear volume regardless of the mouse strains and sexes (Supplemental Fig. S1).
Figure 1.
Difference of corneal nerve density, sensitivity, and tear volume in 3 common use mouse strains. A) Representative images of corneal innervation in BALB/c, CFW, and C57BL/6 strains and gender for each strain. B) Corneal nerve density from 12 corneas in each of the 6 groups (strains and genders) was analyzed. There was no significant difference of sex in all studied mouse strains but a significant divergence of corneal nerve density between strains. The BALB/c mouse has the highest innervated corneas followed by CFW and C57BL/6. ***P < 0.001, 1-way ANOVA, followed by Tukey’s honest significant difference [Tukey’s honest significant difference (HSD)] multiple pairwise comparisons. C, D) Sensitivity (C) and tear volume (D) in different strains and gender. ***P < 0.001, 1-way ANOVA, followed by Tukey’s HSD multiple pairwise comparisons. All correlation association analyses are present in the Supplemental Fig. S1. NS, not significant.
Corneal wound healing is semidependent on nerve density
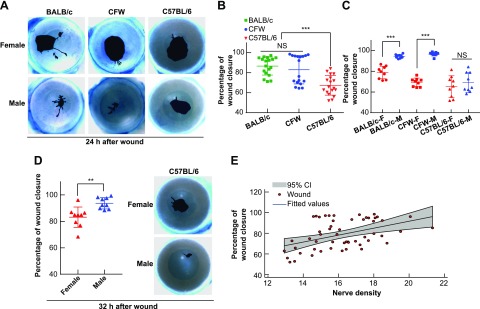
Although the effect of sexes (17) and strains (15) on corneal wound healing in the mouse has been reported separately, the relationship of corneal wound healing and corneal nerve density has not been documented. The same strains, sexes, and ages of mice, as described in the previous experiments, were used and subjected to corneal injury. The epithelial wound closure was evaluated at 24 h after damage by corneal staining with methylene blue. The BALB/c and CFW mice corneas healed faster than the C57BL/6 mouse regardless of sex (Fig. 2B), and the corneas of the male mice consistently healed faster than the female ones in all studied mouse strains (Fig. 2C, D). At 24 h after injury, both BALB/c and CFW mice showed a significant difference in the percentage of cornea wound closure between the male and female mouse (Fig. 2A, C). In the C57BL/6 mouse strain at 24 h, there was no difference between sexes with 69.32 ± 9.49% in the male and 65.42 ± 6.73% in the female (Fig. 2C). In this strain, sexual divergence was detected at 32 h after injury when the wound closure values were 93.49 ± 2.77% for male and 83.02 ± 5.01% for female (Fig. 2D). A correlation of wound healing (right eye, injured) and nerve density (left eye, uninjured) in the same mouse performed at 24 h after wound showed a strong positive correlation to support the premise that the higher the nerve density, the faster wound healing (Fig. 2E). These results are consistent with the observation that the 2 highly innervated-cornea mice BALB/c and CFW (Fig. 1B) showed a higher rate of wound closure (Fig. 2B). However, the interference of a sexual-related factor exists because the corneas of male mice consistently healed faster than the female ones (Fig. 2A–D) despite no significant difference of corneal nerve density between sexes in all studied mouse strains (Fig. 1B). These results suggest that corneal wound healing depends on other factors in addition to nerve density.
Figure 2.
Mouse strain and gender alter corneal wound healing in a corneal nerve density semidependent manner. A) Representative images of corneal wounds at 24 h after injury. The wounded areas were stained with methylene blue. B) Comparison of wound closure in the mouse corneas of the 3 mouse strains ***P < 0.001, 1-way ANOVA, followed by Tukey’s honest significant difference (HSD) multiple pairwise comparisons. C) Comparison of wound healing between female (F) and male (M) in the different strains. ***P < 0.001, 1-way ANOVA, followed by Tukey’s HSD multiple pairwise comparisons. NS, not significant. D) Sexual divergence is found in BALB/c and CFW mice at 24 h, and in C57BL/6 mice at 32 h. **P < 0.01, Student’s t test. E) Pearson correlation coefficient of corneal wound closure on the right injured eye with corneal nerve density on the left uninjured eye (R = 0.45, P < 0.001, Pearson’s pairwise correlation). Corneal wound healing is positively related to corneal nerve density regardless of gender on the wound healing efficiency.
Corneal nerve regeneration is both sex- and strain dependent
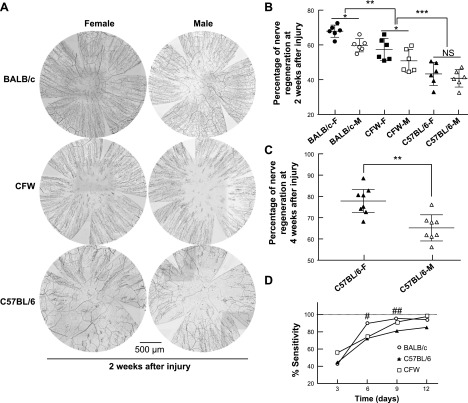
Our previous results show that density of corneal nerves is characteristic of the mouse strain; we then investigated the regeneration of corneal nerves after 2 wk of injury considering both mouse strains and sexes (Fig. 3A). In these experiments, we normalized corneal nerve density to the basal values of each gender-strain specific group and expressed as percentage of regeneration. The data showed that both sex (P = 0.0037, n = 36) and strain (P < 0.001, n = 36) affected the regeneration of corneal innervation after injury. The BALB/c mouse showed the higher regeneration speed (63.09 ± 5.32%) followed by CFW (53.67 ± 6.81%) and C57BL/6 (41.94 ± 5.61%) (Fig. 3A, B). At 2 wk after injury, the female mice showed a significantly higher nerve density than the male in both the BALB/c and CFW strains, whereas in the C57BL/6, similar sexual divergence was detected at 4 wk after the wound (Fig. 3C and Supplemental Fig. S2). To determine the functionality of the regenerated corneal subbasal nerve plexus, sensitivity was measure at 3, 6, 9, and 12 d after injury. Three days after injury all mice show a dramatic drop in corneal sensitivity (Fig. 3D); afterward, corneal sensitivity responded to regenerated nerve density in a strain-dependent manner with the BALB/c mice showing the fastest recovery of sensitivity at 6 d after injury, CFW mice at 9 d, and C57BL/6 not completely recovered at 12 d after wound. Similar to the results without injury (Fig. 1D), there was not sexual divergence in corneal sensitivity after injury.
Figure 3.
Mouse strain and gender divergence in corneal nerve regeneration. A) Representative whole-mount images of corneal nerve plexus of 3 mouse strains and different genders 2 wk after injury. The subbasal nerves were stained with the pan-neuronal marker PGP9.5. For better visualization, the images were inverted to a white background. B) Percentage of corneal nerve density was calculated based on the specific mouse-gender corneal nerve density without injury from Fig. 1B. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA, followed by Tukey’s honest significant difference (HSD) multiple pairwise comparisons. NS, not significant. C) Sexual difference in corneal nerve regeneration was found in C57BL/6 at 4 wk after injury. **P < 0.01, Student’s t test. D) Different speeds of corneal nerve regeneration affected the recovery of corneal sensitivity. Sensitivity was measured at 3, 6, 9, and 12 d after wound and calculated by using the values of the same cornea before injury as 100%. Sensitivity is shown as mouse strain difference only because we did not find any association of gender in the recuperation of corneal sensitivity. After 3 d of injury, all mice show a dramatical drop in corneal sensitivity. #P < 0.05 (shows higher sensitivity of BALB/c mouse with respect to CFW and C57BL/6 strain at 6 d after injury). ##P < 0.05 (shows higher sensitivity of BALB/c and CFW than C57BL/6 at 9 d after injury), 1-way ANOVA, followed by Tukey’s HSD multiple pairwise comparisons.
Cornea sensitivity is correlated with a higher density of substance P–positive nerves
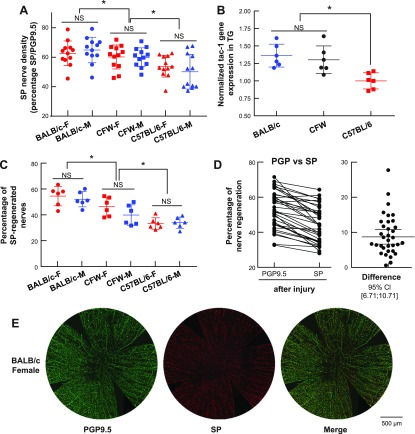
Previous studies have shown that calcitonin gene-related peptide and substance P (SP) are the higher sensory peptides expressed in the cornea (1) and that SP-positive nerves comprise 60% of total subbasal nerve density in the CFW mouse (7). We assessed the SP-positive nerves in the 3 mouse strains to determine whether there is a correlation between SP nerve density and sensitivity. The BALB/c mouse had the highest percentage of SP-positive nerves compared with total nerves labeled with PGP9.5 (Fig. 4A), followed by CFW and C57BL/6. There were no sexual differences in the density of SP-positive nerves. The differences found in the strain could be because of differences in the expression level of the SP encoded gene tac-1 in the TG (Fig. 4B). Although there were no significant differences in the gene expression between BALB/c and CFW, there was lower expression of tac-1 in the C57BL/c mice.
Figure 4.
The recovery of SP-positive nerves is mouse-strain specific. A) Percentage of SP neuropeptide in mice of different strain and gender without injury. Corneas were double-stained with PGP9.5 and SP antibodies, and the whole-mount images were used to measure the nerve density. The SP relative nerve density was calculated as percentage of the total nerves labeled with PGP9.5. B) Gene expression of SP (tac-1 gene) in the TG was measured using quantitative PCR. The gene expression of SP in the TG of the same animal was normalized to C57BL/6 mouse; each data point represents a pool of 3 mice using both genders. *P < 0.05, n.s., not significant, 1-way ANOVA, followed by Tukey’s honest significant difference (HSD) multiple pairwise comparisons. C) Percentage of regeneration of SP-positive nerves 2 wk after injury. The nerve density was calculated in reference to the SP content in the noninjured corneas. *P < 0.05, 1-way ANOVA, followed by Tukey’s HSD multiple pairwise comparisons. NS, not significant. D) Difference in nerve regeneration of total nerves vs. positive SP nerves. Each point represents the same double-stained cornea at 2 wk after injury. Data are collected from all mouse strains and genders (left). The difference between PGP9.5- and SP-positive corneal nerve density is plotted with the mean and 95 confident interval (right). The positive difference ranges from 6.71 to 10.71 showing that nerve density labeled with PGP9.5 is higher than SP. P < 0.001, paired Student’s t test with same PGP9.5 and SP double-stained cornea. E) Representative images of PGP9.5, SP, and merged color in the BALB/c female cornea at 2 wk after injury.
Next, we measured the percentage of SP-positive nerves regenerated after 2 wk of corneal injury. Unlike the total nerve density, which showed a difference in regeneration of both mouse strains and sexes (Fig. 3B), the SP-positive nerves regeneration depended on only the strain with a divergence (P < 0.001, n = 36), whereas the sex did not show any difference (P = 0.206, n = 36) (Fig. 4D). This observation coincided with the measure of corneal sensitivity where we did not find sexual divergence (Fig. 1C). In addition, our data showed a strong correlation between corneal sensitivity and SP concentration. The higher the density of corneal SP-positive nerve density, the more sensitive the corneas.
An interesting observation was the different speed of regeneration between total nerves and SP-positive nerves. In the same corneas double-stained with SP and PGP9.5, we found that PGP9.5 positive nerves had higher regeneration (52.9 ± 10.51%) than SP-contained nerves (44.19 ± 10.3%) (Fig. 4E). This observation coincides with our previous study where the SP nerves regenerate slower than PGP9.5 total nerves with/without PEDF plus DHA treatment (23).
Nerve regeneration depends on tear-neurotrophins releasing divergence
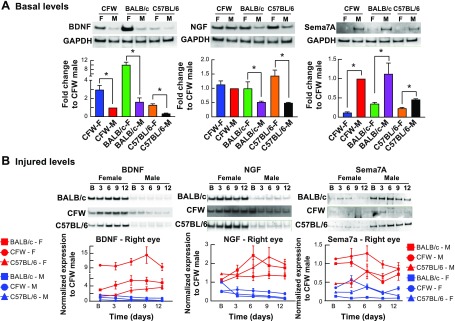
A previous study has shown that in the male C57BL/6 mice neurotrophins, NGF and BDNF, and Sema7A secreted in mouse tears by the cornea contributed to corneal nerve regeneration (23). We then measured by Western blot the amount of those 3 compounds in tears of the 3 different strains and both sex of mice before and after injury (Fig. 5). In the 3 strains, the content of BDNF and NGF were highly expressed in the female mice, whereas Sema7A was more abundant in the male mice (Fig. 5A). To compare the different gels, we normalized the densitometry of the Western blot band to the male CFW mouse because this animal was used in the following experiment. This baseline weight was used to calculate the secreted amount of BDNF, NGF, and Sema7A after injury (Fig. 5B). Tear samples were analyzed at 3, 6, 9, and 12 d after the wound with the basal values (1 d before injury) as the control for each mouse strain and sex (Fig. 5B). The density of the bands was calculated, normalized to the basal control, and multiplied by its own baseline weight values calculated from the previous experiment (Fig. 5A). The graph of BDNF, NGF, and Sema7A content in the tears (Fig. 5B, bottom panel) compares the secreted levels at times after injury. The amount of BDNF and NGF were significantly higher in female mice than male, and the amount of BDNF in tears was higher in the BALB/c mouse. All mouse strains showed an increase of BDNF and NGF in the female individuals after injury, whereas the secretion of these 2 proteins in the male mice was stable or decreased (Fig. 5B). In the C57BL/6 mice, BDNF was increased at 6 d after injury and plateaued up to 12 d, whereas NGF peaked at 9 d in this strain. Sema7A secretion was higher in the male than female (Fig. 5B). The results suggested that at least in the first phase of wound healing (24–48 h) in which there is migration of epithelial cells to cover the wound, Sema7A seems to be an important factor that can contribute to stimulating wound closure. Additionally, the two faster regenerated male mice, BALB/c and CFW (Fig. 2A), had a greater amount of this protein in tears than the C57BL/6 mouse strain (Fig. 5A).
Figure 5.
Divergence of secreted neurotrophins and Sema7A from tears. A) Differences of mouse strain and gender in secreting BDNF, NGF, and Sema7A in the tear film at basal levels. The densitometry of the Western blot bands divided by GAPDH was normalized to the CFW male. *P < 0.05, Student’s t test. B) Changes of BDNF, NGF, and Sema7A in mouse tears after different times of corneal injury are strongly gender-dependent with higher expression of BDNF and NGF in females and higher expression of Sema7A in males. Seven micrograms of protein from pooled tear samples (n = 3 mice/group) was used for Western blot. Data are the mean ± sd of 3 experiments.
Gene expression analysis in the TG after 2 wk of corneal injury
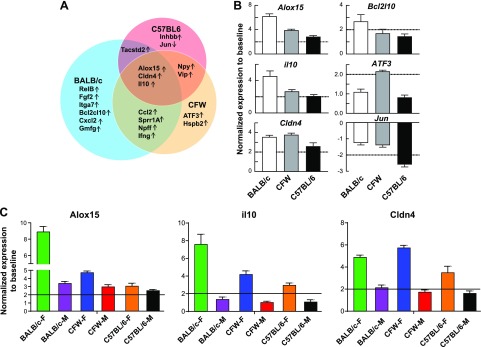
Neurons located in the TG innervate the corneal and terminate in nerve endings in the epithelium. To uncover whether changes in expression of specific genes in the TG explain the divergence in mouse strains and sexes in corneal nerve regeneration after injury, we performed a gene expression profile of each mouse strain and sex. The targeted genes were randomly chosen from 1) neuropeptides and related genes, 2) neurotrophins and related genes, 3) RAGs, and 4) proresolution and related inflammation genes. First, we conducted a Venn diagram analysis of the gene expression to show the similarities and differences between mouse strains regardless of sex (Fig. 6A). The regeneration of corneal nerves is accompanied by changes in the expression of some common genes in the TG for the 3 strains such as alox15, il-10, and cldn4 or specific mouse strain genes such as Bcl2l10 for BALB/c, atf3 for CFW, and jun for C57BL/6 (Fig. 6). When we separated the male and female gene expression of the common genes for the 3 strains, female mice showed higher expression than male mice in all the strains, especially in the 2 fastest regeneration mice, BALB/c and CFW (Fig. 6C).
Figure 6.
Gene expression analysis of TG at 2 wk after corneal injury. A) Venn diagram showing the common activated genes between the 3 mouse strains. Genes with at least 2-fold of activation/inhibition were select. For each shared or specific area, the gene names are shown with up and down arrows to indicate up- or down-regulated genes. B) The expression between male and female of 3 common genes between strains (alox15, il-10, and cldn4) and of 3 specific mouse strain genes (bcl2l10 for BALB/c, atf3 for CFW, and jun for C57BL/6) are shown. Data presented as mean ± sd of 3 experiments. C) The gene activation of alox15, il-10, and cldn4 in the different sex of every mouse strain. Data presented as mean ± sd of 3 experiments.
The effect of estrogen on the neurotrophins secretion and corneal nerve regeneration
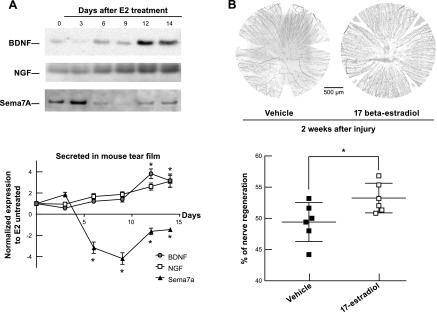
To investigate whether estrogen can change the secretion of neurotrophins and Sema7A and in nerve regeneration of a female phenotype, the male CFW mouse was used. E2 was administrated topically because the receptors of E2 are expressed in mouse and human corneas (28, 29). For the neurotrophins analysis, the mice were treated with E2 (1 µM) 3 times a day for 14 d. At d 3, 6, 9, and 12, mouse tears were collected and subjected to Western blot analysis. After 14 d of E2 treatment, the right mouse corneas were injured, and the E2 topical treatment was continued for the next 14 d. The corneal subbasal nerves were stained with PGP9.5 antibody, and its density was measured as described. E2 treatment stimulates the secretion of NGF and BDNF, and decreases the secretion of Sema7A in mouse tears after injury (Fig. 7A). The amount of NGF and BDNF in tears started to become elevated with E2 drops at d 12, whereas Sema7A secretion was inhibited at d 6. This result reflected the same natural sexual divergence described in the previous experiment (Fig. 5A). As a result, the continuous treatment of E2 in the next 14 d after injury fostered the regenerative process of corneal nerves producing an increase in subbasal nerve density in the E2-treated mice (n = 6, P < 0.05) (Fig. 7B).
Figure 7.
The effect of β-estradiol on corneal nerve regeneration in male mice. A) Secretion of BDNF, NGF, and Sema7A in tears after uninjured CFW male mice were topically treated (3×/d) with E2 for different times. A great decrease of Sema7A was observed at d 6, whereas a strong activation of BDNF and NGF was shown at d 12. Data are mean ± sd of 3 experiments with pooled tears of 6 eyes/sample. *P < 0.05, Student’s t test compared with nontreated corneas. B) Corneal nerve density at 2 wk after injury and treatment. The same CFW male mice after 2 wk of E2 topical treatment from A were injured on the right eye and treated with E2 for 2 additional weeks. Corneal nerves were stained with PGP9.5 antibodies and measured as the percentage of regeneration with respect to corneal nerve density of noninjured CFW male mice. Significant enhancement of corneal nerve recovery after E2 treatment is shown. *P < 0.05, Student’s t test.
DISCUSSION
Our results clearly show that there are strain- and sex-related differences in the density of mouse corneal nerves in both normal and regenerated conditions. In uninjured corneas, the densest innervation in the BALB/c mouse makes this strain more sensitive and humidified with more abundant tear secretion than the C57BL/6, which had less sensitivity and less tear volume (Fig. 1B). This is in agreement with a previous report showing that C57BL/6 mice have reduced tear secretion than the BALB/c mouse after exposure to a dry environment (16). Our findings, in combination with the differences of SP-sensory positive nerves in the 3 strains (Fig. 4), support a strong association between corneal nerve density, sensitivity, and tear volume. Several studies have shown that corneal nerves are important for maintaining corneal homeostasis (2). Because the concept that higher nerve density equals higher wound healing ability is generally accepted, we investigated the role of the nerve density of the different strains and sexes in the healing ability of the mouse corneas. Our results showed that the BALB/c and CFW mouse with the higher density of corneal nerve plexus have the faster wound closure in comparison with C57BL/6. Although this finding is consistent with the premise described above, it contradicts a previous report which shows that the C57BL/6 mouse heals a corneal epithelial debridement wound faster than BALB/c (15). One explanation for this difference could be the size of the wound (we used 2.0 mm diameter corneal wounds, whereas in the previous work the authors used 1.5 mm) or the injury technique (we used rotating burr that also damage the nerves of the anterior stroma, whereas the previous authors used a dulled blade to scrape the trephine-marked epithelium).
An important result was the association with sexes. In all 3 mouse strains, the faster wound closure was detected in the males (Fig. 2B, C), although there is no significant difference in the nerve density as well as sensitivity and tear volume between sexes (Fig. 1B–D). Therefore, we termed the corneal wound healing process as a nerve density–semidependent mechanism. A negative role of estrogen in corneal wound healing has been previously demonstrated in a BALB/c male mouse (17). Previous studies from our laboratory have shown that corneal nerve regeneration plays an important role in normal and diseased corneas and that treatment with PEDF plus DHA accelerates the corneal nerve regeneration when the corneal wound healing is compromised (6, 9, 30). In the present study, we investigate the effects of strain and sex in the corneal nerve regenerative ability after injury. Two weeks after injury, the quickest nerve regenerative strain was BALB/c followed by CFW and C57BL/6 (Fig. 3B); all female mice showed faster nerve regeneration than males (Fig. 3B, C), demonstrating that sexual differences strongly affect corneal homeostasis, wound healing, and nerve regeneration. Although the male mice had an advantage in short-time corneal wound healing (24-48 h) (Fig. 2), possibly due to an increase in the release of Sema7A, the female mice showed a faster speed in corneal nerve regeneration at longer periods of time (2 wk) (Fig. 3). These results are different from previous work using a sciatic nerve injury model, which showed that males, not females, had faster nerve regeneration (19, 31). However, our findings support a review of data from human clinical trials that describes faster sensory recovery after upper limb periphery nerve injuries in female patients (32).
The mechanism of this sexual divergence in corneal nerve regeneration involves higher secretion of BDNF and NGF and lower secretion of Sema7A in the female (Fig. 5B). We found more BDNF in the female tears, which result in faster nerve regeneration, whereas Wood et al. (31) found higher amounts of BDNF in the male lumbar spinal cord resulting in the faster axon growing in the male rat model of sciatic nerve injury. This variance could be because of a different kind of tissue. The cornea is mostly innervated by trigeminal sensory fibers (1), and the systemic interaction of the cornea–TG axis plays a vital role in regulating the regenerative machinery that fosters axon outgrowth in the cornea. Recently, we revealed the molecular mechanism by which PEDF + DHA-treated corneas secrete neurotrophins, such as BDNF and NGF, and Sema7A, to trigger their receptors located on the TG axon (23). Our present results suggest that, in the female, E2 plays a key role in enhancing corneal nerve regeneration through promoting the secretion of BDNF and NGF from tears and increasing corneal nerve density (Figs. 3 and 5). This mechanism can be stimulated in the E2-treated male mice (Fig. 7). In agreement with our results, previous findings have shown that E2 administration enhances nerve regeneration in male or ovariectomized female animals (33–36). Moreover, clinical observations of females who had refractive surgery, in which nerves are damaged, showed worse complications in the postmenopausal patients (37).
The BALB/c female mouse showed the highest nerve density, SP content, and sensitivity in both basal (Figs. 1B, C and 4A) and regenerated condition (Figs. 3B and 4D). Besides, this mouse showed the largest secretion of neurotrophins BDNF and NGF in the tears (Fig. 5A). An interesting observation was the greater activation rate of the alox15 gene in the TG of female mice than the males (Fig. 6C), although higher expression of alox15 was found in corneas of the BALB/c male (17). The product of this gene, 15-lipoxygenase, is a key enzyme in the synthetic pathways of DHA derivatives, docosanoids such as neuroprotectin D1, resolvins, and maresins important in the resolution of inflammation (38–40), and we have shown that neuroprotection D1 is a mediator of corneal nerve regeneration (41, 42). One possibility is that the higher gene activation of alox15 in the TG of the female mouse allows the synthesis of docosanoids to enhance the nerve regeneration. This suggestion is supported by previous studies that showed the benefit of lipoxin A4, a 15-LOX-related derivative of arachidonic acid for female mice in the immune-driven dry eye model (43, 44). Moreover, alox15 was shown to be a shared activated gene between the 3 mouse strains.
The il-10 gene encodes IL-10, an inhibitor of proinflammatory cytokines such as TNF-α and IL-1, and regulates the influx of macrophages protecting the tissues from damage. Previous studies have shown that IL-10 treatment after sciatic nerve injury stimulates axon regeneration (45, 46). Therefore, the upregulation of the il-10 gene in the TG could be a positive response to corneal injury.
The other common gene to the 3 strains was cldn4 that encode Claudin 4, a tight junction membrane protein expressed in corneal epithelial cells (47) and important in maintaining the barrier function of corneal epithelium. To our knowledge, this is the first report of the gene expression of cldn4 in the TG. It had been reported that in lesion fibers of the optic nerve in fish, the interconnection of astrocytes with tight junction proteins is needed for regeneration of the axons (48).
In summary, this study provides detailed information about corneal nerve innervation and regeneration after injury. Researchers should be aware of the differences between sexes and strains in laboratory mice when they select the animals for experiments in which corneal nerves are affected. In addition, consideration about the use of both sexes needs to be taken into account because variance in sex may produce different results. Moreover, masculinized/feminized mice could differ in their sexual dimorphism compared with either male or female, and understanding these differences could have important implications for transitioning patients or for those receiving hormone replacement therapy.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Nicolas Bazan [Louisiana State University (LSU) Health] for support with the Fluidigm technology, to Dr. Marie-Audrey Kautzmann (LSU Health) for assistance with running the Fluidigm system, and to Kenneth Terry II and Scott Sullivan (Neuroscience Center, Louisiana State University Health) for valuable help in calculating nerve density. This work was supported by the Vietnam Education Foundation (to T.L.P.) and U.S. National Institutes of Health, National Eye Institute Grant R01 EY19465 (to H.E.P.B.). The authors declare no conflicts of interest.
Glossary
- BDNF
brain-derived neurotrophic factor
- DHA
docosahexaenoic acid
- NGF
nerve growth factor
- NPD1
neuroprotectin D1
- PEDF
pigment epithelium-derived factor
- PGP9.5
Protein Gene Product 9.5
- RAG
regeneration-associated gene
- Sema7A
Semaphorin 7A
- SP
Substance P
- TG
trigeminal ganglion
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. E. P. Bazan is the guarantor of this work and, as such, has full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; T. L. Pham, J. He, and H. E. P. Bazan designed the experiments; T. L. Pham helped acquire and analyze data, and draft and critically review the manuscript; J. He performed the surgery and tissues extraction and helped with the immunohistochemistry; A. Kakazu assisted in the animal assays such as wound healing, corneal sensitivity, and tear volume; A. Kakazu and J. He critically reviewed the manuscript; and H. E. P. Bazan supervised the study, and wrote and reviewed the manuscript.
REFERENCES
- 1.Müller L. J., Marfurt C. F., Kruse F., Tervo T. M. T. (2003) Corneal nerves: structure, contents and function. Exp. Eye Res. 76, 521–542 [DOI] [PubMed] [Google Scholar]
- 2.Shaheen B. S., Bakir M., Jain S. (2014) Corneal nerves in health and disease. Surv. Ophthalmol. 59, 263–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J., Bazan N. G., Bazan H. E. P. (2010) Mapping the entire human corneal nerve architecture. Exp. Eye Res. 91, 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruzat A., Witkin D., Baniasadi N., Zheng L., Ciolino J. B., Jurkunas U. V., Chodosh J., Pavan-Langston D., Dana R., Hamrah P. (2011) Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest. Ophthalmol. Vis. Sci. 52, 5136–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J., Bazan H. E. P. (2013) Corneal nerve architecture in a donor with unilateral epithelial basement membrane dystrophy. Ophthalmic Res. 49, 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J., Pham T. L., Kakazu A., Bazan H. E. P. (2017) Recovery of corneal sensitivity and increase in nerve density and wound healing in diabetic mice after PEDF plus DHA treatment. Diabetes 66, 2511–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J., Bazan H. E. P. (2016) Neuroanatomy and neurochemistry of mouse cornea. Invest. Ophthalmol. Vis. Sci. 57, 664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorscak L., Marfurt C. F. (2008) Age-related changes in rat corneal epithelial nerve density. Invest. Ophthalmol. Vis. Sci. 49, 910–916 [DOI] [PubMed] [Google Scholar]
- 9.He J., Cosby R., Hill J. M., Bazan H. E. P. (2017) Changes in corneal innervation after HSV-1 latency established with different reactivation phenotypes. Curr. Eye Res. 42, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brock J. A., McLachlan E. M., Belmonte C. (1998) Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J. Physiol. 512, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J., Pham T. L., Bazan H. E. P. (2018) Mapping the entire nerve architecture of the cat cornea. Vet. Ophthalmol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marfurt C. F., Murphy C. J., Florczak J. L. (2001) Morphology and neurochemistry of canine corneal innervation. Invest. Ophthalmol. Vis. Sci. 42, 2242–2251 [PubMed] [Google Scholar]
- 13.Blake J. A., Eppig J. T., Kadin J. A., Richardson J. E., Smith C. L., Bult C. J.; the Mouse Genome Database Group (2017) Mouse Genome Database (MGD)-2017: community knowledge resource for the laboratory mouse. Nucleic Acids Res. 45, D723–D729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spandidos A., Wang X., Wang H., Seed B. (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38, D792–D799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal-Ghosh S., Tadvalkar G., Jurjus R. A., Zieske J. D., Stepp M. A. (2008) BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp. Eye Res. 87, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barabino S., Rolando M., Chen L., Dana M. R. (2007) Exposure to a dry environment induces strain-specific responses in mice. Exp. Eye Res. 84, 973–977 [DOI] [PubMed] [Google Scholar]
- 17.Wang S. B., Hu K. M., Seamon K. J., Mani V., Chen Y., Gronert K. (2012) Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 26, 1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkinson C. L., Romano V., Kaye R. A., Steger B., Stewart R. M. K., Tsagkataki M., Jones M. N. A., Larkin D. F. P., Kaye S. B.; National Health Service Blood Transplant Ocular Tissue Advisory Group and Contributing Ophthalmologists (OTAG Study 20) (2017) The influence of donor and recipient gender incompatibility on corneal transplant rejection and failure. Am. J. Transplant. 17, 210–217 [DOI] [PubMed] [Google Scholar]
- 19.Stenberg L., Dahlin L. B. (2014) Gender differences in nerve regeneration after sciatic nerve injury and repair in healthy and in type 2 diabetic Goto-Kakizaki rats. BMC Neurosci. 15, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsay R. M. (1988) Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci. 8, 2394–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esquenazi S., Bazan H. E. P., Bui V., He J., Kim D. B., Bazan N. G. (2005) Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 46, 3121–3127 [DOI] [PubMed] [Google Scholar]
- 22.Pasterkamp R. J., Peschon J. J., Spriggs M. K., Kolodkin A. L. (2003) Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424, 398–405 [DOI] [PubMed] [Google Scholar]
- 23.Pham T. L., He J., Kakazu A. H., Jun B., Bazan N. G., Bazan H. E. P. (2017) Defining a mechanistic link between pigment epithelium-derived factor, docosahexaenoic acid, and corneal nerve regeneration. J. Biol. Chem. 292, 18486–18499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., Virchow J. C. (2005) The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 26, 115–123 [DOI] [PubMed] [Google Scholar]
- 25.Schellino R., Trova S., Cimino I., Farinetti A., Jongbloets B. C., Pasterkamp R. J., Panzica G., Giacobini P., De Marchis S., Peretto P. (2016) Opposite-sex attraction in male mice requires testosterone-dependent regulation of adult olfactory bulb neurogenesis. Sci. Rep. 6, 36063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakazu A., He J., Kenchegowda S., Bazan H. E. P. (2012) Lipoxin A4 inhibits platelet-activating factor inflammatory response and stimulates corneal wound healing of injuries that compromise the stroma. Exp. Eye Res. 103, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sator M. O., Joura E. A., Golaszewski T., Gruber D., Frigo P., Metka M., Hommer A., Huber J. C. (1998) Treatment of menopausal keratoconjunctivitis sicca with topical oestradiol. Br. J. Obstet. Gynaecol. 105, 100–102 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T., Kinoshita Y., Tachibana M., Matsushima Y., Kobayashi Y., Adachi W., Sotozono C., Kinoshita S. (2001) Expression of sex steroid hormone receptors in human cornea. Curr. Eye Res. 22, 28–33 [DOI] [PubMed] [Google Scholar]
- 29.Tachibana M., Kasukabe T., Kobayashi Y., Suzuki T., Kinoshita S., Matsushima Y. (2000) Expression of estrogen receptor alpha and beta in the mouse cornea. Invest. Ophthalmol. Vis. Sci. 41, 668–670 [PubMed] [Google Scholar]
- 30.Cortina M. S., He J., Li N., Bazan N. G., Bazan H. E. P. (2012) Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch. Ophthalmol. 130, 76–83 [DOI] [PubMed] [Google Scholar]
- 31.Wood K., Wilhelm J. C., Sabatier M. J., Liu K., Gu J., English A. W. (2012) Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev. Neurobiol. 72, 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He B., Zhu Z., Zhu Q., Zhou X., Zheng C., Li P., Zhu S., Liu X., Zhu J. (2014) Factors predicting sensory and motor recovery after the repair of upper limb peripheral nerve injuries. Neural Regen. Res. 9, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vacca V., Marinelli S., Pieroni L., Urbani A., Luvisetto S., Pavone F. (2016) 17beta-estradiol counteracts neuropathic pain: a behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Sci. Rep. 6, 18980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanda N., Watanabe S. (2003) 17β-estradiol enhances the production of nerve growth factor in THP-1-derived macrophages or peripheral blood monocyte-derived macrophages. J. Invest. Dermatol. 121, 771–780 [DOI] [PubMed] [Google Scholar]
- 35.Islamov R. R., Hendricks W. A., Jones R. J., Lyall G. J., Spanier N. S., Murashov A. K. (2002) 17β-estradiol stimulates regeneration of sciatic nerve in female mice. Brain Res. 943, 283–286 [DOI] [PubMed] [Google Scholar]
- 36.Nobakhti-Afshar A., Najafpour A., Mohammadi R., Zarei L. (2016) Assessment of neuroprotective effects of local administration of 17- beta- estradiol on peripheral nerve regeneration in ovariectomized female rats. Bull. Emerg. Trauma 4, 141–149 [PMC free article] [PubMed] [Google Scholar]
- 37.McCarty C. A., Ng I., Waldron B., Garrett S. K., Downie J. A., Aldred G. F., Wolfe R. J., Taylor H. R.; Melbourne Excimer Laser Group (1996) Relation of hormone and menopausal status to outcomes following excimer laser photorefractive keratectomy in women. Aust. N. Z. J. Ophthalmol. 24, 215–222 [DOI] [PubMed] [Google Scholar]
- 38.Serhan C. N., Dalli J., Colas R. A., Winkler J. W., Chiang N. (2015) Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 1851, 397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan C. N., Fredman G., Yang R., Karamnov S., Belayev L. S., Bazan N. G., Zhu M., Winkler J. W., Petasis N. A. (2011) Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 18, 976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knott E. J., Gordon W. C., Jun B., Do K., Bazan N. G. (2018) Retinal pigment epithelium and photoreceptor preconditioning protection requires docosanoid signaling. Cell. Mol. Neurobiol. 38, 901–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortina M. S., He J., Li N., Bazan N. G., Bazan H. E. P. (2010) Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest. Ophthalmol. Vis. Sci. 51, 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortina M. S., He J., Russ T., Bazan N. G., Bazan H. E. P. (2013) Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest. Ophthalmol. Vis. Sci. 54, 4109–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Y., Min K., Zhang Y., Su J., Greenwood M., Gronert K. (2015) Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J. Immunol. 195, 3086–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y., Su J., Zhang Y., Chan A., Sin J. H., Wu D., Min K., Gronert K. (2018) Dietary DHA amplifies LXA4 circuits in tissues and lymph node PMN and is protective in immune-driven dry eye disease. [E-pub ahead of print] Mucosal Immunol. 11, 1674–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siqueira Mietto B., Kroner A., Girolami E. I., Santos-Nogueira E., Zhang J., David S. (2015) Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. J. Neurosci. 35, 16431–16442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakalidou M., Leibig N., Boyle V., Koulaxouzidis G., Penna V. (2011) Interleukin-10 and regeneration in an end-to-side nerve repair model of the rat. J. Peripher. Nerv. Syst. 16, 334–340 [DOI] [PubMed] [Google Scholar]
- 47.Yoshida Y., Ban Y., Kinoshita S. (2009) Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Invest. Ophthalmol. Vis. Sci. 50, 2103–2108 [DOI] [PubMed] [Google Scholar]
- 48.Mack A. F., Wolburg H. (2006) Growing axons in fish optic nerve are accompanied by astrocytes interconnected by tight junctions. Brain Res. 1103, 25–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.