Abstract
The placenta plays a pivotal role in the development of the fetal brain and also influences maternal brain function, but our understanding of communication between the placenta and brain remains limited. Using a gene expression and network analysis approach, we provide evidence that the placenta transcriptome is tightly interconnected with the maternal brain and fetal brain in d 15 pregnant C57BL/6J mice. Activation of serotonergic synapse signaling and inhibition of neurotrophin signaling were identified as potential mediators of crosstalk between the placenta and maternal brain and fetal brain, respectively. Genes encoding specific receptors and ligands were predicted to affect functional interactions between the placenta and brain. Paralogous genes, such as sex comb on midleg homolog 1/scm-like with 4 mbt domains 2 and polycomb group ring finger (Pcgf) 2/Pcgf5, displayed antagonistic regulation between the placenta and brain. Additionally, conditional ablation of forkhead box a2 (Foxa2) in the glands of the uterus altered the transcriptome of the d 15 placenta, which provides novel evidence of crosstalk between the uterine glands and placenta. Furthermore, expression of cathepsin 6 and monocyte to macrophage differentiation associated 2 was significantly different in the fetal brain of Foxa2 conditional knockout mice compared with control mice. These findings provide a better understanding of the intricacies of uterus-placenta-brain interactions during pregnancy and provide a foundation and model system for their exploration.—Behura, S. K., Kelleher, A. M., Spencer, T. E. Evidence for functional interactions between the placenta and brain in pregnant mice.
Keywords: fetus, paralog, Foxa2, regulation, uterine
The establishment of pregnancy requires effective molecular crosstalk between the uterus and embryo, and the maintenance of pregnancy to term requires embryo implantation, stromal cell decidualization, and placental development and function. In mice, blastocysts enter the uterus early on gestational day (GD) 4 (GD 1 is observation of a postcoital vaginal plug), and implantation into the endometrium commences around midnight on GD4 (1, 2). Decidualization of stromal cells commences on the morning of GD5 near the attached and implanting blastocyst and eventually spreads toward the mesometrial area of the uterus (3). By GD6, the trophectoderm of the developing placenta begins to directly contact the decidualized stroma. The placenta is fully formed and functional by GD15 (4). Asynchronous embryo-uterine interactions and defective stromal cell decidualization can result in pregnancy loss and miscarriage as well as later pregnancy complications such as preeclampsia and fetal growth retardation (2, 5).
Understanding the biologic and evolutionary links between the brain and reproductive system is important to obtain better insight into the molecular complexity of pregnancy (6, 7). The maternal brain (MB), uterus, and placenta interact during pregnancy and mediate maternal adaptations to support pregnancy and lactation after parturition (8, 9). The hormones estrogen, progesterone, prolactin (Prl), and oxytocin play key roles during pregnancy, and the brain robustly regulates their production via the hypothalamic-pituitary-gonadal axis (10). In mice, changes in the MB during pregnancy regulate maternal behaviors such as pup retrieval and nest building (11). In humans, pregnancy effects changes in the MB that last at least 2 yr after birth (12) and include enhancement of key hippocampal functions such as spatial memory (13, 14). In mice, changes in gene expression have been evaluated in specific regions of the MB during pregnancy (15, 16) as well as in virgin, pregnant, and postpartum periods (17). However, our understanding of how the placenta and brain communicate remains very limited.
The placenta plays a major role in development of the fetus (18). Recent evidence supports the idea that placental metabolic pathways modulate fetal brain (FB) development and suggest an important role for maternal-placental-fetal interactions and serotonin (5-hydroxytryptamine) in the fetal programming of adult mental disorders (19–21). Maternal stress factors during pregnancy, including body weight, nutrition, anemia, smoking, and substance abuse, can negatively affect and program fetal development with persistent life-long effects on offspring (22, 23). A primary regulator of placental development and function is the decidua of the uterus, which is formed by differentiation of stromal cells in the endometrium in response to progesterone and embryo implantation (24, 25). Recent studies suggest that stromal cell decidualization can be modulated by glands of the uterus (26, 27).
Here, we first used a gene expression and network analysis approach to investigate global crosstalk of genes between the mouse placenta, FB, and MB on GD15. Next, we examined the effect of the conditional ablation of forkhead box a2 (Foxa2), a key transcription factor for uterine gland function during pregnancy establishment (26), on gene expression changes in the placenta and FB. The findings provide new evidence for and insights into how the brain, uterus, and placenta interact during pregnancy.
MATERIALS AND METHODS
Animal breeding and sample collection
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri-Columbia and conducted according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) Adult (9 wk old) C57BL/6J wild-type (WT) female mice were mated with males of proven fertility to induce pregnancy. Pregnant WT mice (n = 4) were euthanized on GD15. The entire MB was quickly collected along with the FB and placenta from all implantation sites. Care was taken to isolate the placenta from the decidua by microdissection using a stereomicroscope. Each organ was washed in sterile PBS and snap frozen in liquid nitrogen. Adult female Foxa2 conditional knockout (cKO) mice were generated by breeding C57BL/6J lactotransferrin Cre (Ltf-iCre) knock-in mice with C57BL/6J floxed Foxa2 mice as described by our laboratory (26). Adult Foxa2 cKO (LtfiCre/+Foxa2f/f) mice are infertile because of defects in embryo implantation caused by an absence of leukemia inhibitory factor (LIF) expression by the glands of the uterus (27). To rescue implantation and pregnancy establishment, adult female Foxa2 cKO mice (n = 4) received intraperitoneal injections of 10 μg recombinant mouse LIF (BioLegend, San Diego, CA, USA) in sterile PBS at 10:00 am and 6:00 pm on GD4 as previously described (27). The mice were necropsied on GD15, and the placenta and FB from each implantation site were isolated, washed, and frozen as previously described.
RNA extraction and sequencing
Total RNA was isolated from the MB, FB, and placenta (n = 4 biologic replicates for each organ) using a standard Trizol-based (Thermo Fisher Scientific, Waltham, MA, USA) protocol. For placental RNA, 3 placentas were pooled from each of the 4 mice for RNA extraction. To eliminate genomic DNA contamination, extracted RNA was treated with DNase I and purified using Direct-zol MiniPrep Plus Kit according to the manufacturer’s instructions (Zymo Research, Irvine, CA, USA). RNA concentration was determined using a Qubit RNA Assay (Thermo Fisher Scientific), and quality was determined by a Fragment Analyzer instrument (Agilent, Santa Clara, CA, USA). Total RNA was submitted to the University of Missouri DNA Core Facility for RNA-Seq library construction using the Illumina TruSeq Stranded mRNA Sample Preparation Kit (Illumina, San Diego, CA, USA). Libraries were sequenced (paired-end, 75 bp) using a NextSeq500 instrument (Illumina). The mean read count generated from the RNA-Seq experiment was 27.4 million per sample. All the raw and processed data were deposited in the Gene Expression Omnibus database (GSE121799; https://www.ncbi.nlm.nih.gov/geo/).
Differential gene expression analysis
Raw sequences (FastQ files) were subjected to quality check by FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The program fqtrim (https://ccb.jhu.edu/software/fqtrim/) was used to remove adapters, perform quality trimming (Phred score >30) by a sliding window scan (6 nt), and select read length of 30 nt or longer. The reads obtained from the quality control were mapped to the Mus musculus reference genome (GRCm38.p5) by the Hisat2 aligner (28). The program featureCounts (29) was then used to quantify read counts that mapped to the genes. The differentially expressed (DE) genes between sample groups were determined by edgeR-robust (30). The false discovery rate <0.05 was used as a significance threshold.
Cluster and network analysis
A finite normal mixture model based on the Bayesian information criterion was employed to predict gene expression clusters from the RNA-Seq data using the R package mclust v.5 (31). To construct a gene expression network, an information theoretical approach (32) was adopted. In this method, mutual information (MI) of expression variation was calculated in pairwise manner between genes across the samples. The MI is the summation of log ratios of joint probability:marginal probability and measures the information content between 2 variables (genes in this case). It determines how much knowing 1 variable would predict variability of the other. The MI values were then used to generate a weighted adjacency matrix by the maximum relevance–minimum redundancy method (33) to construct gene expression networks. To compare gene expression patterns among MB, FB, and placenta samples, MI scores were generated between samples in a pairwise manner. The distance matrix of MI scores was then plotted using R (circlize package; https://CRAN.R-project.org/package=circlize) to infer links within and between the samples.
Analysis of paralogs and gene families
Paralogous genes and gene families annotated from mouse genome annotation (GRCm38.p5) were downloaded from Ensembl using the BioMart (http://useast.ensembl.org/biomart/martview/667e0bf1998bb8e993e82e8517871448) tool. The information, along with the RNA-Seq data, was used to compare expression variation of genes that had one or more paralogs with expression variation of genes that had no paralog (single copy genes). As the number of genes expressed in the MB, FB, and placenta varied, we normalized the variation of gene expression by sampling an equal number of genes (n = 1000 randomly) from the MB, FB, and placenta separately. The sampling was repeated 100 times, and the mean variance of expression was calculated for both gene types (with or without paralogs) separately for the MB, FB, and the placenta.
Functional annotation of differentially regulated genes
Gene ontology (GO) analysis was conducted by Panther tools (http://www.pantherdb.org) using Fisher’s exact test. The cluster analysis of fold enrichment of the GO terms was performed by mclust v.5 (31). The signaling pathway impact analysis (SPIA) method (34) was used to perform topology-based pathway analysis using the R and Bioconductor package ToPASeq (35). The DE genes were used as a query to predict receptor and corresponding ligands from the FANTOM5 database of receptors and ligands (Riken, Tokyo, Japan) (36). Transcription factors as regulators of correlated expression changes of genes among the MB, FB, and placenta were predicted by iRegulon (37). iRegulon is a gene set analysis tool to infer gene regulatory networks based on mapping incidences of transcription factors binding to motifs.
Quantitative RT-PCR
For quantitative RT-PCR assays, total RNA (1 μg) from the FB of GD 15 WT and Foxa2 cKO mice was reverse transcribed in a 20 μl reaction volume using iScript RT Supermix (Bio-Rad, Hercules, CA, USA) using methods previously described (38). PCR assays were performed using Bio-Rad Universal SYBR Green Supermix on a Bio-Rad CFX384 Touch Real-Time System with glyceraldehyde-3-phosphate dehydrogenase as a reference gene. All genes were assayed using predesigned and validated Bio-Rad PrimePCR primers specific to the desired mouse gene.
RESULTS
Gene expression patterns in the brain and placenta
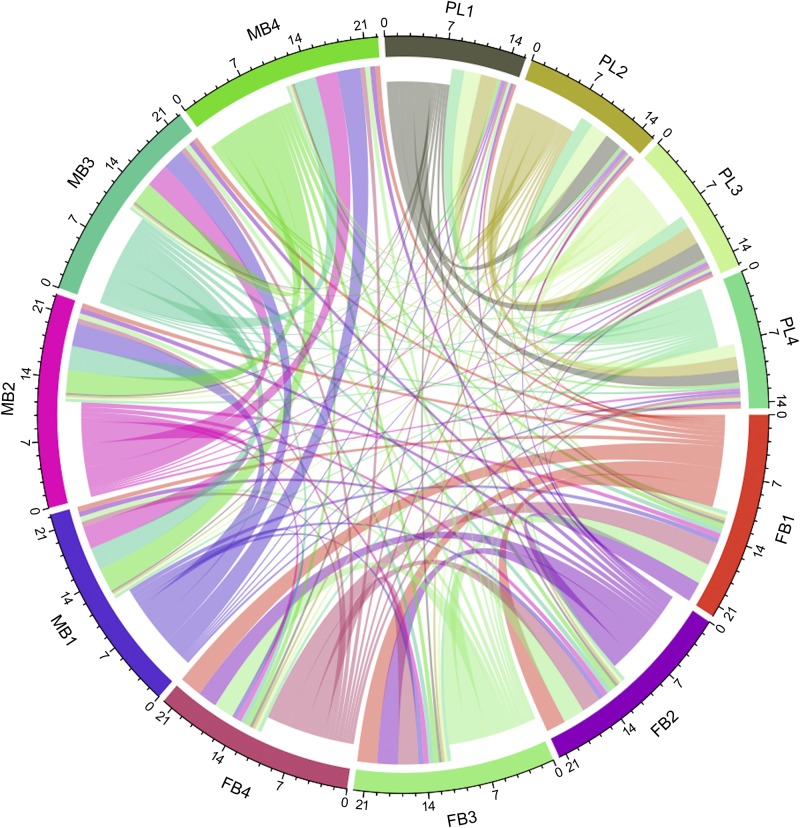
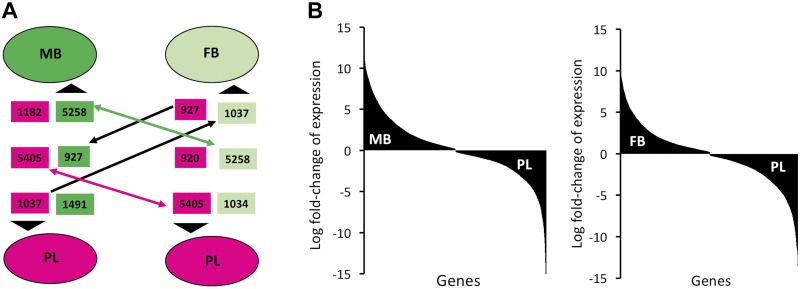
The transcriptome of the MB, FB, and placenta from C57BL/6J mice was determined on GD15, which was selected because the placenta is fully mature and functional at this timepoint of pregnancy (4). The pattern of gene expression correlations among MB, FB, and placenta samples is shown in Fig. 1. This represents a generalized pattern, inclusive of positive, negative, and nonlinear correlations, inferred by calculating the MI of gene expression variation between samples in pairwise manner. The links connecting 1 sample to another, shown as colored curves of varying width, represent quantitative measurement for correlated expression changes of genes between samples. As expected, samples within group (MB, FB, or placenta) are well connected with each other. In contrast, the links connecting samples between groups (placenta vs. FB, placenta vs. MB, or MB vs. FB) are quantitatively much less than within-group links. Thus, gene expression patterns of the MB, FB, and placenta are highly distinct. The DE gene analysis revealed thousands of genes whose expression was altered in MB and/or FB in a coordinate manner relative to the placenta (Fig. 2). Three patterns of differential gene expression were observed. One of the patterns is related to the expression changes due to tissue differences (placenta and brain). A total of 5258 genes were up-regulated in both the MB and FB compared with the placenta, and a comparable number of genes (5405) were down-regulated in both MB and FB relative to the placenta. The second pattern of gene expression was related to differences in developed MB as compared with the developing FB. Many genes were DE in the MB compared with the placenta (1491 increased and 1182 decreased), but such a difference in expression was not evident in the FB. Genes were also identified with different expression in the FB compared with the placenta (1034 increased and 920 decreased), but such differences in expression were not evident in MB. The third pattern represented gene expression changes that are neither tissue related nor development (brain) related; rather, they are related to differential regulation among the MB, FB, and the placenta. This pattern included genes that are either increased in the MB but decreased in the FB (927) or increased in the FB but decreased in the MB (n = 1037) relative to the placenta (Fig. 2).
Figure 1.
Chord diagram showing MI of gene expression among the MB, FB, and placenta (PL) of WT d 15 pregnant mice. The links by color-matched curves represent extent of correlated gene expression between the color-coded samples. The MI scale is shown on the circumference of each sample.
Figure 2.
Number and direction of DE genes. A) The numbers of increased and decreased genes (shown within rectangles) between the brain and placenta are shown. The DE gene sets are color coded according to the samples (dark green for MB, light green for FB, and pink for PL). The black arrows designate the direction of expression changes. Two-sided arrows indicate commonly regulated genes. The 1-sided arrows indicate genes that are increased between 1 group pair but decreased in the other group pair. B) The plots of log fold-changes illustrate nearly equal distribution of genes that are either increased or decreased in the MB or FB compared with the placenta. PL, placenta.
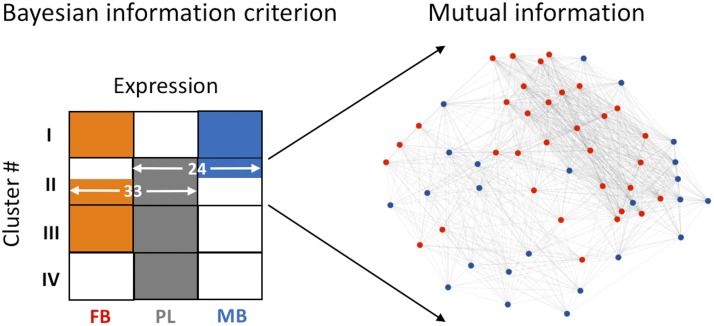
To identify genes associated with the correlated expression changes among the MB, FB, and placenta, we performed a model-based cluster analysis of gene expression using the Bayesian information criterion method (31). The analysis identified a small group of 57 genes associated with a single cluster of differential expression among the MB, FB, and placenta (Fig. 3). In this cluster, 33 genes were expressed in the FB as well as the placenta, but they were either not expressed or not very abundant in the MB (Supplemental Table S1). The other 24 genes were expressed together in the MB and placenta but either not expressed or poorly expressed in the FB. Network analysis further predicted that these genes are likely to interact with each other (Fig. 3). The property of these genes to crosstalk suggests their role in functional interaction between the placenta and brain during pregnancy.
Figure 3.
Model-based clustering of DE genes. The 4 predicted expression clusters are shown relative to gene expression patterns in the MB, FB, and placenta (PL). The color-coded boxes (orange for FB, gray for PL, and blue for MB) represent genes that are expressed in the respective sample (white box indicates that the genes are either not expressed or low in abundance in that sample). Within cluster II, 33 genes were expressed in the FB and the placenta but not in the MB, whereas the other 24 genes were expressed in the MB and the placenta but not in the FB. MI-based network analysis reveals that these genes interact among each other.
In addition, we found differential expression of specific paralogous genes in the placenta and brain during pregnancy. In the MB as well as the FB, single copy genes displayed more than a 2-fold higher variation in expression compared with gene families or genes with one or more paralogs. However, this pattern is apparently absent in the placenta (Supplemental Fig. S1). We identified several paralogs within gene families, such as Ceacam, Prl, cathepsin (Cts), Serpin, and pregnancy-specific glycoprotein (Psg), which are coordinately activated in the placenta relative to the MB and FB. Specific paralog gene pairs (n = 340) were identified when both the genes were abundantly expressed in the placenta but were either absent or poorly expressed in the brain (Supplemental Table S2). On the other hand, our data also showed paralog gene pairs (n = 8) in which both the genes were highly expression in both the MB and FB but were either absent or poorly expressed in the placenta (Supplemental Table S2). Moreover, we observed 2 genes whose paralogs seem to be differentially regulated between brain and placenta. Sex comb on midleg homolog 1 was activated in both MB and FB more than 8-fold relative to the placenta, but its paralog, scm-like with four mbt domains 2, was activated in the placenta more than 60-fold relative to both the MB and FB. Similarly, polycomb group ring finger (Pcgf) 5 was increased in the placenta more than 10-fold relative to both the MB and FB, but its paralog Pcgf2 increased more than 6-fold in the MB and FB relative to the placenta (Supplemental Table S2). Such antagonistic regulation of expression between placenta and brain was not observed with any other genes than sex comb on midleg homolog 1/scm-like with four mbt domains 2 and Pcgf2/Pcgf5, suggesting that differential regulation of these paralogs may be linked to the functional interactions between brain and placenta.
GO analysis
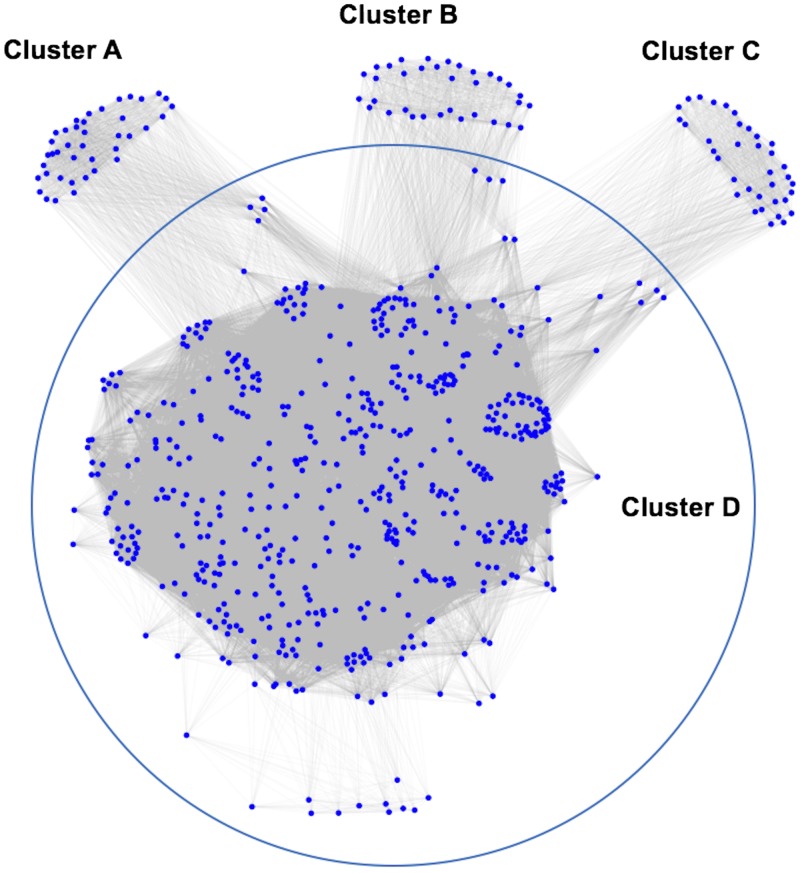
Genes that were DE between brain and placenta showed distinct enrichment clusters of specific GO terms (Supplemental Table S3). The cluster pattern is shown in Fig. 4. Cluster A represented gene functions that are exclusively associated with genes that were DE in the FB relative to the placenta. This cluster included 44 GO terms, such as multicellular organism reproduction (GO:0032504), multicellular organismal reproductive process (GO:0048609), reproduction (GO:0000003), reproductive process (GO:0022414), reproductive structure development (GO:0048608), and reproductive system development (GO:0061458). Cluster B represented 38 GO functions specific to genes that were DE in the FB relative to the MB. Among those, the cell cycle G1/S phase transition (GO:0044843), epithelial cell migration (GO:0010631), epithelium migration (GO:0090132), and response to TGF-β (GO:0071559) were most enriched. Cluster C contained 39 GO terms that represented genes that are altered in expression in the MB relative to the placenta. Of those, protein acylation (GO:0043543), rRNA metabolic process (GO:0016072), and rRNA processing (GO:0006364) are most enriched. Cluster D, on the other hand, represented a majority of the commonly enriched functions by genes that were DE among the MB, FB, and placenta. They include enzyme linked receptor protein signaling pathway (GO:0007167), regulation of response to stimulus (GO:0048585), regulation of cell morphogenesis involved in differentiation (GO:0010769), regulation of neurogenesis (GO:0050767), and regulation of neuron differentiation (GO:0045664), among others. The full list of GO terms associated with each enrichment cluster is provided in Supplemental Table S4.
Figure 4.
GO enrichment clusters. Cluster A represents biologic functions of genes differentially regulated in FB compared with the placenta. Cluster B represents biologic functions of genes differentially regulated in the FB compared with the MB. Similarly, cluster C represents biologic functions of genes that are differentially regulated in the MB compared with the placenta. Cluster D represents biologic functions of genes differentially regulated among the MB, FB, and placenta.
Signaling, receptor-ligand interaction, and cis-regulation of genes between the brain and the placenta
Significant impact of signaling pathways
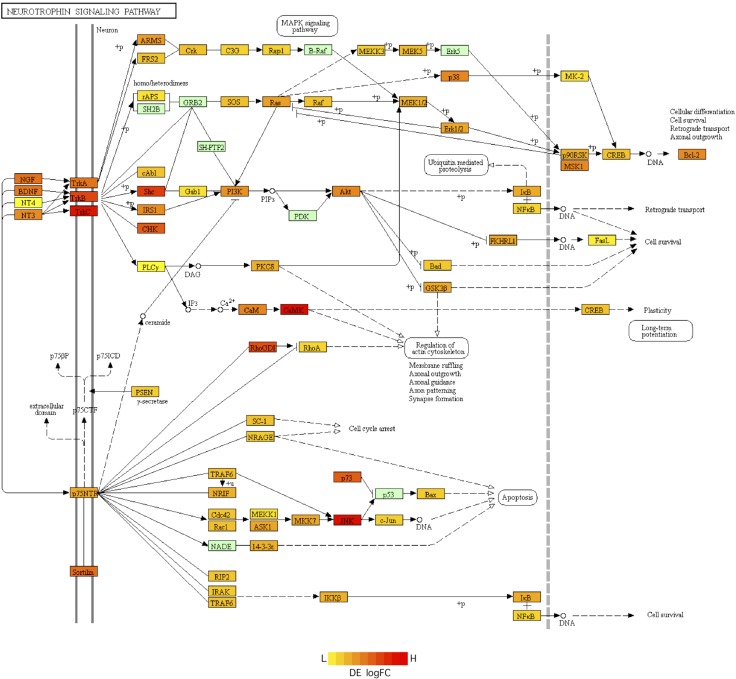
To predict which signaling pathways may be involved in the crosstalk of genes between the placenta and brain, we performed SPIA (34). This approach combines the classic enrichment analysis along with pathway topology analysis to determine how differential gene expression affects signaling pathways. Using this approach, we observed 3 signaling pathways that were significantly affected by gene expression differences between the brain and the placenta. The SPIA significance scores, as well as the individual genes of these affected pathways, are provided in Supplemental Table S5. The serotonergic synapse signaling pathway was activated, whereas the TNF signaling pathway was inhibited in the MB relative to the placenta. The neurotrophin signaling pathway was inhibited in FB relative to the placenta. Nearly 84% of the genes of the neurotrophin signaling pathway were differentially regulated in the FB relative to the placenta (Fig. 5). We did not detect any pathway that was activated in the FB relative to the placenta.
Figure 5.
Differential regulation of neurotrophin signaling pathway genes. The color of genes varies from red to yellow based on the log fold-change (logFC) of differential expression (DE) in the FB relative to the placenta (scale shown with yellow indicating low (L) expression and red indicating high (H) expression in FB relative to the placenta). Genes shown in light green do not change in expression between FB and the placenta.
Receptor-ligand interactions
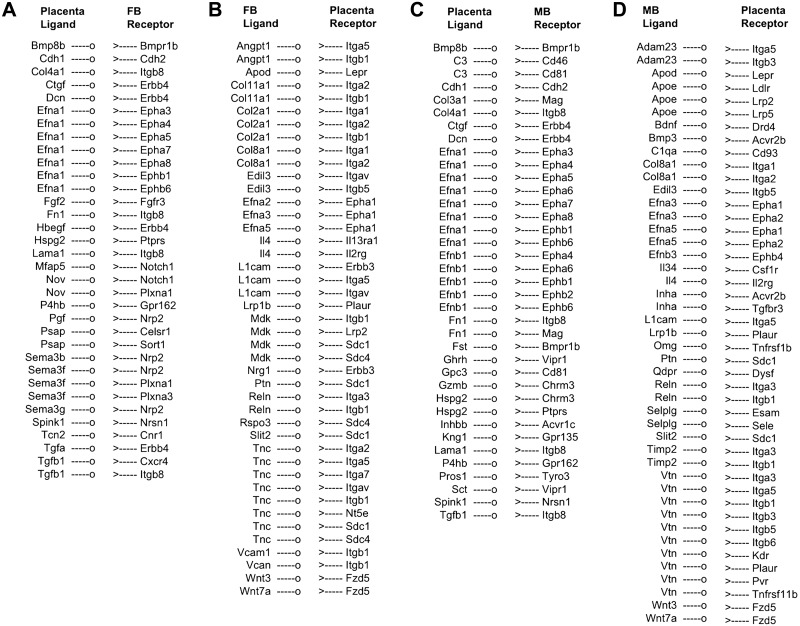
Specific ligand-encoding genes such as semaphorin 3, Tgf, and ephrin A1 were expressed in the placenta, and genes encoding corresponding receptors were expressed in the FB (Fig. 6A). We also identified ligand-encoding genes being expressed in FB and genes encoding the cognate receptors in the placenta (Fig. 6B). A similar pattern of coordinated regulation of receptor-ligand genes were observed between the MB and the placenta (Fig. 6C, D). Based on the GO analysis, the receptor-ligand genes differentially regulated between the FB and the placenta are associated with biologic functions such as brain development, fetal response to oxygen, and modulation of synaptic transmission. On the other hand, the receptor-ligand genes differentially regulated between the MB and the placenta are associated with regulation of immune system process, neuron recognition, cell surface receptor signaling, and regulation of metabolic processes. Our analysis further showed that specific functional interaction between the placenta and brain is associated with the transcriptional network of the receptor-ligand genes. For example, the receptor-ligand genes shown in Fig. 7 are differentially regulated between the FB and the placenta and are associated with the fetal response to oxygen.
Figure 6.
Receptor-ligand interactions predicted between the placenta and MB or FB. Ligands are displayed as “—o” with their corresponding receptors as “>—”. A) Placenta ligand and FB receptor interaction, B) FB ligand and placenta receptor interaction, C) Placenta ligand and MB receptor interaction, D) MB ligand and placenta receptor interaction.
Figure 7.
Transcriptional network of genes associated with GO term “fetal response to oxygen” based on expression levels in the MB, FB, and placenta.
Transcription factors and regulatory network
Our analysis predicted specific transcription factors that potentially regulated coexpression of genes between placenta and brain. The transcription factors were predicted by a ranking-and-recovery approach implemented in iRegulon tool (37) within the Cytoscape software (39). The purpose of using iRegulon was that it provided the benefit of accessing a large transcription factor motif collection from various databases. Using this approach, we predicted that the repressor element 1 silencing transcription factor is a major regulator of genes that were increased in both the MB and FB relative to the placenta (Supplemental Table S6). On the other hand, genes that were decreased in both the MB and FB relative to the placenta are likely to be regulated by a combination of transcription factors such as Finkel-Biskis-Jinkins osteosarcoma oncogene (Fos), Fosb, jun B proto-oncogene (Junb), fos-like antigen 1 (Fosl1), Jun D proto-oncogene (Jund), Jun dimerization protein 2 (Jdp2), and basic leucine zipper transcription factor, ATF-like (Batf) (Supplemental Table S6).
Uterine influences on gene expression in the placenta and FB
In this experiment, we wanted to determine if an altered uterine environment would affect placental gene expression. To test this hypothesis, we used a previously established model of uterine gland Foxa2 deficiency (26) in which Foxa2 is ablated after puberty in the developed glands using Ltf-iCre driver mice that conditionally ablates floxed genes in the epithelium of the uterus (40). The transcription factor Foxa2 is specifically expressed in the endometrial glands of the uterus and regulates production of LIF, which is expressed only by the glands on GD4 and is essential for embryo implantation (26, 27, 41, 42). The Foxa2 cKO mice are infertile because of defects in blastocyst implantation resulting from an absence of LIF expression in the uterine glands (27). Of note, intraperitoneal injections of recombinant mouse LIF on GD 4 rescues implantation and pregnancy establishment in Foxa2 cKO mice, and those mice deliver live pups at term (26). For this experiment, Foxa2 cKO mice were injected with LIF on GD4, and the placenta and FB were isolated on GD15.
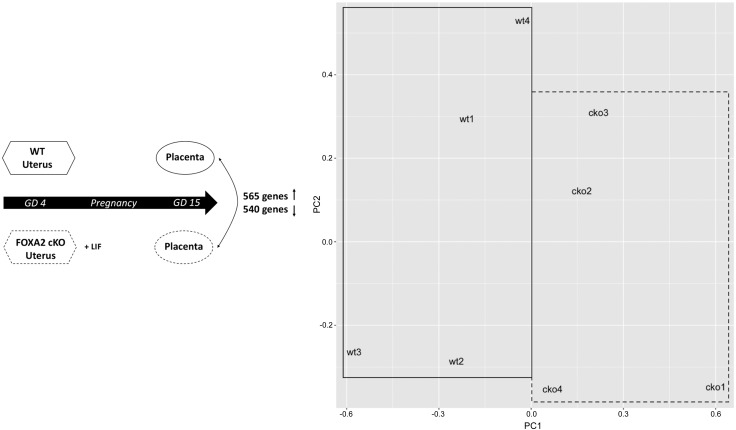
First, placentas from GD15 Foxa2 cKO and WT mice were analyzed by RNA-Seq (Fig. 8). The DE gene analysis found that 1105 genes had altered expression in the placenta of uterine Foxa2 cKO mice compared with WT mice. Of these, 565 genes were increased and 540 were decreased (Supplemental Table S7). Among the top decreased genes were deiodinase, iodothyronine type III; hemoglobin Z, β-like embryonic chain; complement component 1, q subcomponent-like 2; contactin 2; calcium/calmodulin-dependent protein kinase II, β; hemoglobin X, α-like embryonic chain in Hba complex; and pleckstrin homology like domain, family A, member 2. Among the top increased genes were Prl3a1, Prl8a6; Cts6; Psg16; glutamate receptor, ionotropic, kainate 3; and Psg26. As provided in Supplemental Table S8, GO analysis of DE genes in the Foxa2 cKO placenta identified the term “placenta development” (GO:0001890). Specific genes associated with this GO term, such as bone morphogenetic protein 7; cyclin F; E2F transcription factor 7; E2F transcription factor 8; interleukin 11 receptor, alpha chain 1; regulator of NFKB signaling; pleckstrin homology like domain, family A, member 2; polo like kinase 4; prostaglandin I2 (prostacyclin) synthase; syncytin a; testis expressed gene 19.1; and transcription factor EB, were down-regulated in the placenta of Foxa2 cKO mice. Also, Foxa2 cKO mice showed altered regulation of multiple gene families, including kinesin, Prl, solute carrier, basic helix-loop-helix, and TNF receptor gene families (Supplemental Table S8). Within the Prl gene family, specific members such as Prl3d3, Prl6a1, Prl7a1, and Prl4a1were decreased, whereas others (i.e., Prl2c1, Prl3c1, Prl8a6, and Prl3a1) were increased in the placenta of Foxa2 cKO relative to WT mice. This finding suggests that deletion of uterine gland Foxa2 triggers differential regulation of specific paralogs and gene families in the placenta and indicates direct effects of Foxa2-regulated gland products on placental development.
Figure 8.
Conditional deletion of Foxa2 in the glands of the uterus affects gene expression in the GD 15 placenta. Left: experimental design and number of increased and decreased genes in the placenta of WT mice compared with Foxa2 cKO mice.Right: principal component (PC) analysis of gene expression in placenta of WT and Foxa2 cKO mice.
Next, the expression level of 7 genes was compared in the FB of GD 15 Foxa2 cKO and WT mice by quantitative RT-PCR (Supplemental Table S9). Each of the 7 genes displayed altered expression in the FB relative to the placenta in WT mice and also in the Foxa2 cKO placenta compared with WT mice based on the RNA-Seq analysis. The quantitative RT-PCR analysis found that 2 of the 7 genes [Cts6 and monocyte to macrophage differentiation associated 2 (Mmd2)] exhibited differential expression in FB from the Foxa2 cKO as compared with WT mice (Supplemental Table S9). Notably, Cts6 expression increased about 133-fold (P < 0.03), and Mmd2 increased by 4.6-fold in the FB from Foxa2 cKO mice relative to WT mice. These data provide novel results that Foxa2-regulated genes in the uterine glands alter the transcriptome of the placenta and affect gene expression in the FB.
DISCUSSION
The current study employed a systems biology approach to examine transcriptional crosstalk of genes between the placenta and brain during pregnancy. It is known that placental function is fundamental to fetal development (6) and that the placenta safeguards the normal FB development from an adverse maternal environment (43); however, the relationship between the placenta and MB remains unclear. The present study found that genes associated with the peptidyl-tyrosine dephosphorylation (GO:0035335), olfactory bulb development (GO:0021772), and olfactory lobe development (GO:0021988) are enriched in MB relative to the placenta. Enrichment of olfactory development function suggests that the olfactory bulb of the MB may be a region of active transcriptional changes during pregnancy. As illustrated in Fig. 2, 927 genes were increased in the MB but decreased in the FB relative to the placenta. In contrast, about the same number of genes (1037) had an opposite pattern of expression in the MB and FB relative to the placenta. This pattern of gene regulation among the MB, FB, and placenta suggests that MB transcriptome may be modulated during pregnancy, likely in response to placenta and fetal development. Indeed, maternal behavior undergoes distinct transitions in response to pregnancy (44). During both pregnancy and the early postpartum period, changes in placental hormones and neurochemistry alter the neural structural plasticity of the MB (45–48). For instance, in mice, changes in MB are likely to prepare and regulate maternal behaviors such as pup retrieval and nest building. Moreover, pregnancy affects cognitive abilities such as enhancing spatial memory (13) and key hippocampal functions (14) in the brain. Indeed, modulation of olfactory information can effect behavior changes in the mother in response to pregnancy (49–52).
In hemochorial placentation, the maternal blood comes in direct contact with the fetal chorion, facilitating efficient transfer of nutrients and oxygen to the growing fetus (4, 53). Although the placenta is a transient organ, recent studies demonstrate that is highly adaptive across placental mammals (54, 55). The adaptive ability of the placenta is evident from its function that ensures development of the fetus in stressful conditions such as hypoxia and undernutrition (56). The innate adaptability of the placenta may involve duplicated genes present in virtually all mammalian genomes (57–61). Indeed, the present study found that expression variation of paralogous genes to single copy genes is relatively higher in the placenta as compared with the MB and FB. Moreover, we found an elevated number of duplicated genes contributing to the variation of gene expression of the placenta compared to the brain. Specific gene pairs, such as Prl2c2 and Prl2c3, Psg26 and Psg28, Serpinb9f and Serpinb9g, and Xlr4a and Xlr4b, are nearly identical paralogs with >98% sequence identity with each other and show abundant expression only in the placenta. In mice and humans, recently duplicated genes are utilized at later stages of placentation to meet the metabolic needs of a diverse range of pregnancy physiologies (62), and neofunctionalization of duplicated genes also contributes to adaptive genome function (63). Indeed, several Prl family paralogs expressed specifically in the mouse placenta regulate placental adaptations to the physiologic stressor of hypoxia (64–67).
Despite the adaptive nature of placental function and evolution, the placenta can be vulnerable to challenges arising from various physiologic stressors around conception and during pregnancy. Based on GO, specific functions such as glycoprotein biosynthetic process (GO:0009101), protein glycosylation (GO:0006486), and macromolecule glycosylation (GO:0043413) are significantly associated with DE genes between the FB and placenta. The enrichment of glycosylation function supports the earlier finding that glycosylation of proteins plays an important regulatory role in brain development (68). Our analysis here also predicted receptor-ligand interactions between the placenta and FB and between the placenta and MB (Fig. 6). Indeed, common interactions between the placenta and FB and the placenta and MB were found by comparing receptor-ligand interactions. A notable finding is that the Efna1 ligand is expressed in the placenta with Eph receptors (Epha3, Epha4, Epha5, Epha6, Epha7, Epha8, Ephb1, and Ephb6) expressed in both MB and FB. Similarly, Efna3 and Efna5 ligands were expressed in MB and FB with their receptor (Eph1) expressed in the placenta. The Eph receptors are protein-tyrosine kinases that mediate different developmental processes in the placenta and brain as well as erythropoiesis (69).
Pathway impact analysis suggested that differential gene expression between the placenta and FB may involve the neurotrophin signaling pathway. Neurotrophins are involved in differentiation and survival of neural cells through engagement of tropomyosin receptor kinase tyrosine kinase receptors or p75 neurotrophin receptor (70). Of note, brain-derived neurotrophic factor (BDNF) and neurotrophin-4/5 bind to the tyrosine kinase B receptor and stimulates the growth and survival of trophectoderm cells in the placenta (71). In the brain, serotonin is required for BDNF to promote differentiation of 5-hydroxytryptamine neurons (72). Here, differential gene expression between the MB and placenta positively affected the activity of serotonin synapse signaling between the MB and placenta, indicating that serotonin signaling is likely to influence maternal-fetal communication. Moreover, BDNF signaling has a biologic role in placental response to adverse maternal effects such as obesity (73) and fine particle air pollution (74). Of note, maternal BDNF can cross the placenta and reach the FB (75, 76). Finally, we found that the transcription factor REST, a key regulator of neurogenesis (77), may regulate the hundreds of genes that are increased in both the MB and FB compared with the placenta (Supplemental Table S6).
A novel finding of our second study was that the transcriptome of the GD15 placenta was altered in mice with a uterine gland–specific deletion of Foxa2 (Foxa2 cKO). Foxa2 is a pioneer transcription factor that regulates the development and function of many different organs (78). In the uterus, Foxa2 is uniquely expressed only by the glands of the endometrium and has a biologic role in their differentiation in the neonate (79) and function in the adult (26, 27, 41). During pregnancy, Foxa2 regulates expression of the critical implantation factor LIF by the glands on GD4 and also a large number of other genes that encode transporters, such as chloride channel accessory 3 and transthyretin, as well as those that encode secreted proteins, including amphiregulin; calbindin 1; Ctse; chemokine (C-X-C motif) ligand 15; insulin-like growth factor 1; Indian hedgehog; protease, serine 28; protease, serine 29; Serpina3n; and serine peptidase inhibitor, Kazal type 3 (41). Our recent work demonstrated that without LIF, implantation fails in Foxa2 cKO mice and that gland-derived Foxa2-independent factors regulate stromal cell decidualization and, in turn, pregnancy establishment and placentation (26, 27). In mice, the glands of the uterus surround the implantation site and decidualizing stromal cells and have direct access to the embryo (80, 81). Thus, the alterations in placenta gene expression observed in LIF-replaced Foxa2 cKO mice in the present study may be due to direct influence of gland secretions on the developing placenta and/or indirect via effects of gland secretions on the decidua. These findings lend further support to the idea that that secretions of the uterine glands affect stromal cell decidualization and development of the placenta (82, 83). Given that the placenta of uterine Foxa2 cKO mice displayed differential regulation of specific paralogs and gene families, including the Prl family, it is tempting to speculate that Foxa2-regulated uterine gland secretions may be an essential component of maternal adaptations to physiologic stressors during pregnancy.
In addition to alterations in the placenta, 2 genes (Cts6 and Mmd2) were DE in the FB of uterine Foxa2 cKO mice. The Cts6 gene encodes a cysteine proteinase whose expression is regulated along with cathepsin J/P in the labyrinth layer of the mouse placenta (84). Interestingly, our RNA-Seq analysis of WT mice found that the Cts6 gene was expressed only in the placenta but was absent in the GD15 MB and FB. Mmd2 is predicted to encode a receptor belonging to the progestin and adipoQ receptor family (85). RNA-Seq analysis of WT mice found that Mmd2 is abundant in the MB and FB but very low in the placenta on GD15. These differences in FB gene expression support the idea that the adaptations of the placenta to the lack of Foxa2-regulated uterine gland secretions affected FB development, which is reinforced by our first study of the GD 15 placenta and FB in WT mice. In summary, the findings here support the ideas that an intricate network of communication exists between the MB, placenta, and FB during pregnancy and that uterine glands and, by inference, their secretions or products affect placental development. These studies provide a framework for determining the cellular and molecular basis of normal and adaptive interactions between the brain, uterus, and placenta that are critical for pregnancy success.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Gregory W. Burns (Grand Rapids Research Center, Michigan State University College of Human Medicine, Grand Rapids, MI, USA) for reading the manuscript. This work was supported, in part, by the University of Missouri’s Research Council Grant (to S.K.B.), and by Grants R21 HD076347 and R01 HD096266 from the U.S. National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Development (to T.E.S.) The authors declare no conflicts of interest.
Glossary
- BDNF
brain-derived neurotrophic factor
- cKO
conditional knockout
- Cts
cathepsin
- DE
differentially expressed
- FB
fetal brain
- Foxa2
forkhead box a2
- GD
gestational day
- GO
gene ontology
- LIF
leukemia inhibitory factor
- MB
maternal brain
- MI
mutual information
- Mmd2
monocyte to macrophage differentiation associated 2
- Pcgf
polycomb group ring finger
- Prl
prolactin
- Psg
pregnancy-specific glycoprotein
- SPIA
signaling pathway impact analysis
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. K. Behura conceived the study; S. K. Behura and A. M. Kelleher designed experiments; A. M. Kelleher performed the wet-lab experiments; S. K. Behura analyzed data; and S. K. Behura, A. M. Kelleher and T. E. Spencer wrote the manuscript.
REFERENCES
- 1.Zhang S., Lin H., Kong S., Wang S., Wang H., Wang H., Armant D. R. (2013) Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 34, 939–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha J., Sun X., Dey S. K. (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. 18, 1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das S. K. (2009) Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction 137, 889–899 [DOI] [PubMed] [Google Scholar]
- 4.Watson E. D., Cross J. C. (2005) Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20, 180–193 [DOI] [PubMed] [Google Scholar]
- 5.Wang H., Dey S. K. (2006) Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199 [DOI] [PubMed] [Google Scholar]
- 6.Zeltser L. M., Leibel R. L. (2011) Roles of the placenta in fetal brain development. Proc. Natl. Acad. Sci. USA 108, 15667–15668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wessels J. M., Wu L., Leyland N. A., Wang H., Foster W. G. (2014) The brain-uterus connection: brain derived neurotrophic factor (BDNF) and its receptor (Ntrk2) are conserved in the mammalian uterus. PLoS One 9, e94036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petraglia F., Imperatore A., Challis J. R. (2010) Neuroendocrine mechanisms in pregnancy and parturition. Endocr. Rev. 31, 783–816 [DOI] [PubMed] [Google Scholar]
- 9.Napso T., Yong H. E. J., Lopez-Tello J., Sferruzzi-Perri A. N. (2018) The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front. Physiol. 9, 1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadakkadath Meethal S., Atwood C. S. (2005) The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell. Mol. Life Sci. 62, 257–270 [DOI] [PubMed] [Google Scholar]
- 11.Stolzenberg D. S., Stevens J. S., Rissman E. F. (2012) Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Horm. Behav. 62, 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoekzema E., Barba-Müller E., Pozzobon C., Picado M., Lucco F., García-García D., Soliva J. C., Tobeña A., Desco M., Crone E. A., Ballesteros A., Carmona S., Vilarroya O. (2017) Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296 [DOI] [PubMed] [Google Scholar]
- 13.Kinsley C. H., Madonia L., Gifford G. W., Tureski K., Griffin G. R., Lowry C., Williams J., Collins J., McLearie H., Lambert K. G. (1999) Motherhood improves learning and memory. Nature 402, 137–138 [DOI] [PubMed] [Google Scholar]
- 14.Galea L. A., Leuner B., Slattery D. A. (2014) Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J. Neuroendocrinol. 26, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisinger B. E., Zhao C., Driessen T. M., Saul M. C., Gammie S. C. (2013) Large scale expression changes of genes related to neuronal signaling and developmental processes found in lateral septum of postpartum outbred mice. PLoS One 8, e63824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao C., Saul M. C., Driessen T., Gammie S. C. (2012) Gene expression changes in the septum: possible implications for microRNAs in sculpting the maternal brain. PLoS One 7, e38602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray S., Tzeng R. Y., DiCarlo L. M., Bundy J. L., Vied C., Tyson G., Nowakowski R., Arbeitman M. N. (2015) An examination of dynamic gene expression changes in the mouse brain during pregnancy and the postpartum period. G3 (Bethesda) 6, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson J., Chidzanja S., Kind K., Lok F., Owens P., Owens J. (1995) Placental control of fetal growth. Reprod. Fertil. Dev. 7, 333–344 [DOI] [PubMed] [Google Scholar]
- 19.Côté F., Fligny C., Bayard E., Launay J. M., Gershon M. D., Mallet J., Vodjdani G. (2007) Maternal serotonin is crucial for murine embryonic development. Proc. Natl. Acad. Sci. USA 104, 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnin A., Levitt P. (2011) Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnin A., Goeden N., Chen K., Wilson M. L., King J., Shih J. C., Blakely R. D., Deneris E. S., Levitt P. (2011) A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods L., Perez-Garcia V., Hemberger M. (2018) Regulation of placental development and its impact on fetal growth-new insights from mouse models. Front. Endocrinol. (Lausanne) 9, 570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy V. E., Smith R., Giles W. B., Clifton V. L. (2006) Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr. Rev. 27, 141–169 [DOI] [PubMed] [Google Scholar]
- 24.Makieva S., Giacomini E., Ottolina J., Sanchez A. M., Papaleo E., Viganò P. (2018) Inside the endometrial cell signaling subway: mind the gap(s). Int. J. Mol. Sci. 19, 2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gellersen B., Brosens J. J. (2014) Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35, 851–905 [DOI] [PubMed] [Google Scholar]
- 26.Kelleher A. M., Peng W., Pru J. K., Pru C. A., DeMayo F. J., Spencer T. E. (2017) Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc. Natl. Acad. Sci. USA 114, E1018–E1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelleher A. M., Milano-Foster J., Behura S. K., Spencer T. E. (2018) Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat. Commun. 9, 2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D., Langmead B., Salzberg S. L. (2015) HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao Y., Smyth G. K., Shi W. (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 [DOI] [PubMed] [Google Scholar]
- 30.Zhou X., Lindsay H., Robinson M. D. (2014) Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 42, e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scrucca L., Fop M., Murphy T. B., Raftery A. E. (2016) Mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R J. 8, 289–317 [PMC free article] [PubMed] [Google Scholar]
- 32.Budden D. M., Crampin E. J. (2016) Information theoretic approaches for inference of biological networks from continuous-valued data. BMC Syst. Biol. 10, 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding C., Peng H. (2005) Minimum redundancy feature selection from microarray gene expression data. J. Bioinform. Comput. Biol. 3, 185–205 [DOI] [PubMed] [Google Scholar]
- 34.Tarca A. L., Draghici S., Khatri P., Hassan S. S., Mittal P., Kim J. S., Kim C. J., Kusanovic J. P., Romero R. (2009) A novel signaling pathway impact analysis. Bioinformatics 25, 75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ihnatova I., Budinska E. (2015) ToPASeq: an R package for topology-based pathway analysis of microarray and RNA-Seq data. BMC Bioinformatics 16, 350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramilowski J. A., Goldberg T., Harshbarger J., Kloppmann E., Lizio M., Satagopam V. P., Itoh M., Kawaji H., Carninci P., Rost B., Forrest A. R. (2015) A draft network of ligand-receptor-mediated multicellular signalling in human. Nat. Commun. 6, 7866; erratum: 7, 10706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janky R., Verfaillie A., Imrichová H., Van de Sande B., Standaert L., Christiaens V., Hulselmans G., Herten K., Naval Sanchez M., Potier D., Svetlichnyy D., Kalender Atak Z., Fiers M., Marine J. C., Aerts S. (2014) iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLOS Comput. Biol. 10, e1003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelleher A. M., Burns G. W., Behura S., Wu G., Spencer T. E. (2016) Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Sci. Rep. 6, 38078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daikoku T., Ogawa Y., Terakawa J., Ogawa A., DeFalco T., Dey S. K. (2014) Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology 155, 2718–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filant J., Lydon J. P., Spencer T. E. (2014) Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus. FASEB J. 28, 230–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart C. L., Kaspar P., Brunet L. J., Bhatt H., Gadi I., Köntgen F., Abbondanzo S. J. (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 [DOI] [PubMed] [Google Scholar]
- 43.Goasdoué K., Miller S. M., Colditz P. B., Björkman S. T. (2017) Review: the blood-brain barrier; protecting the developing fetal brain. Placenta 54, 111–116 [DOI] [PubMed] [Google Scholar]
- 44.Slotnick B. M., Nigrosh B. J. (1975) Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J. Comp. Physiol. Psychol. 88, 118–127 [DOI] [PubMed] [Google Scholar]
- 45.Kinsley C. H., Amory-Meyer E. (2011) Why the maternal brain? J. Neuroendocrinol. 23, 974–983 [DOI] [PubMed] [Google Scholar]
- 46.Elyada Y. M., Mizrahi A. (2015) Becoming a mother-circuit plasticity underlying maternal behavior. Curr. Opin. Neurobiol. 35, 49–56 [DOI] [PubMed] [Google Scholar]
- 47.Hillerer K. M., Jacobs V. R., Fischer T., Aigner L. (2014) The maternal brain: an organ with peripartal plasticity. Neural Plast. 2014, 574159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown C. H., Grattan D. R. (2007) Does maternal oxytocin protect the fetal brain? Trends Endocrinol. Metab. 18, 225–226 [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto S., Yamazaki C., Masumoto K. H., Nagano M., Naito M., Soga T., Hiyama H., Matsumoto M., Takasaki J., Kamohara M., Matsuo A., Ishii H., Kobori M., Katoh M., Matsushime H., Furuichi K., Shigeyoshi Y. (2006) Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc. Natl. Acad. Sci. USA 103, 4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cameron E. L. (2014) Pregnancy and olfaction: a review. Front. Psychol. 5, 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belnoue L., Malvaut S., Ladevèze E., Abrous D. N., Koehl M. (2016) Plasticity in the olfactory bulb of the maternal mouse is prevented by gestational stress. Sci. Rep. 6, 37615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lévy F., Keller M. (2009) Olfactory mediation of maternal behavior in selected mammalian species. Behav. Brain Res. 200, 336–345 [DOI] [PubMed] [Google Scholar]
- 53.Lager S., Powell T. L. (2012) Regulation of nutrient transport across the placenta. J. Pregnancy 2012, 179827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myatt L. (2006) Placental adaptive responses and fetal programming. J. Physiol. 572, 25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts R. M., Green J. A., Schulz L. C. (2016) The evolution of the placenta. Reproduction 152, R179–R189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broad K. D., Keverne E. B. (2011) Placental protection of the fetal brain during short-term food deprivation. Proc. Natl. Acad. Sci. USA 108, 15237–15241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cross J. C., Baczyk D., Dobric N., Hemberger M., Hughes M., Simmons D. G., Yamamoto H., Kingdom J. C. (2003) Genes, development and evolution of the placenta. Placenta 24, 123–130 [DOI] [PubMed] [Google Scholar]
- 58.Hou Z. C., Sterner K. N., Romero R., Than N. G., Gonzalez J. M., Weckle A., Xing J., Benirschke K., Goodman M., Wildman D. E. (2012) Elephant transcriptome provides insights into the evolution of eutherian placentation. Genome Biol. Evol. 4, 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han M. V., Demuth J. P., McGrath C. L., Casola C., Hahn M. W. (2009) Adaptive evolution of young gene duplicates in mammals. Genome Res. 19, 859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wildman D. E. (2011) Review: toward an integrated evolutionary understanding of the mammalian placenta. Placenta 32 (Suppl 2), S142–S145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guschanski K., Warnefors M., Kaessmann H. (2017) The evolution of duplicate gene expression in mammalian organs. Genome Res. 27, 1461–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knox K., Baker J. C. (2008) Genomic evolution of the placenta using co-option and duplication and divergence. Genome Res. 18, 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor J. S., Raes J. (2004) Duplication and divergence: the evolution of new genes and old ideas. Annu. Rev. Genet. 38, 615–643 [DOI] [PubMed] [Google Scholar]
- 64.Ain R., Dai G., Dunmore J. H., Godwin A. R., Soares M. J. (2004) A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc. Natl. Acad. Sci. USA 101, 16543–16548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bu P., Alam S. M., Dhakal P., Vivian J. L., Soares M. J. (2016) A prolactin family paralog regulates placental adaptations to a physiological stressor. Biol. Reprod. 94, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soares M. J., Varberg K. M., Iqbal K. (2018) Hemochorial placentation: development, function, and adaptations. Biol. Reprod. 99, 196–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soares M. J., Konno T., Alam S. M. (2007) The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol. Metab. 18, 114–121 [DOI] [PubMed] [Google Scholar]
- 68.Korpeinen T., Mononen I., Krusius T., Järnefelt J. (1982) Glycosylation of proteins in developing human brain. J. Neurochem. 39, 1737–1739 [DOI] [PubMed] [Google Scholar]
- 69.Goldman-Wohl D., Greenfield C., Haimov-Kochman R., Ariel I., Anteby E. Y., Hochner-Celnikier D., Farhat M., Yagel S. (2004) Eph and ephrin expression in normal placental development and preeclampsia. Placenta 25, 623–630 [DOI] [PubMed] [Google Scholar]
- 70.Huang E. J., Reichardt L. F. (2001) Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawamura K., Kawamura N., Sato W., Fukuda J., Kumagai J., Tanaka T. (2009) Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology 150, 3774–3782 [DOI] [PubMed] [Google Scholar]
- 72.Martinowich K., Lu B. (2008) Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 33, 73–83 [DOI] [PubMed] [Google Scholar]
- 73.Prince C. S., Maloyan A., Myatt L. (2017) Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta 49, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saenen N. D., Plusquin M., Bijnens E., Janssen B. G., Gyselaers W., Cox B., Fierens F., Molenberghs G., Penders J., Vrijens K., De Boever P., Nawrot T. S. (2015) In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an ENVIRONAGE birth cohort study. Environ. Health Perspect. 123, 834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kodomari I., Wada E., Nakamura S., Wada K. (2009) Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem. Int. 54, 95–98 [DOI] [PubMed] [Google Scholar]
- 76.Chakraborty G., Magagna-Poveda A., Parratt C., Umans J. G., MacLusky N. J., Scharfman H. E. (2012) Reduced hippocampal brain-derived neurotrophic factor (BDNF) in neonatal rats after prenatal exposure to propylthiouracil (PTU). Endocrinology 153, 1311–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mozzi A., Guerini F. R., Forni D., Costa A. S., Nemni R., Baglio F., Cabinio M., Riva S., Pontremoli C., Clerici M., Sironi M., Cagliani R. (2017) REST, a master regulator of neurogenesis, evolved under strong positive selection in humans and in non human primates. Sci. Rep. 7, 9530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Friedman J. R., Kaestner K. H. (2006) The Foxa family of transcription factors in development and metabolism. Cell. Mol. Life Sci. 63, 2317–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong J. W., Kwak I., Lee K. Y., Kim T. H., Large M. J., Stewart C. L., Kaestner K. H., Lydon J. P., DeMayo F. J. (2010) Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod. 83, 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arora R., Fries A., Oelerich K., Marchuk K., Sabeur K., Giudice L. C., Laird D. J. (2016) Insights from imaging the implanting embryo and the uterine environment in three dimensions. Development 143, 4749–4754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan J., Deng W., Cha J., Sun X., Borg J. P., Dey S. K. (2018) Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat. Commun. 9, 603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spencer T. E. (2014) Biological roles of uterine glands in pregnancy. Semin. Reprod. Med. 32, 346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burton G. J., Jauniaux E., Charnock-Jones D. S. (2010) The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 54, 303–312 [DOI] [PubMed] [Google Scholar]
- 84.Nakajima A., Kataoka K., Takata Y., Huh N. H. (2000) Cathepsin-6, a novel cysteine proteinase showing homology with and co-localized expression with cathepsin J/P in the labyrinthine layer of mouse placenta. Biochem. J. 349, 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Góñez L. J., Naselli G., Banakh I., Niwa H., Harrison L. C. (2008) Pancreatic expression and mitochondrial localization of the progestin-adipoQ receptor PAQR10. Mol. Med. 14, 697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.