Abstract
Background
Ultrasound with developing line may by suitable for medical personnel who are inexperienced in the use of ultrasound-guided radial artery puncture. In this trial, we assessed whether this technology could increase the success rate of radial artery puncture performed by interns.
Material/Methods
Seventy-seven patients undergoing general anesthesia were enrolled and randomly divided into 2 groups: an ultrasound with developing line group and a traditional ultrasound group. All radial artery punctures were performed by interns who received theoretical explanation (including video demonstration of puncture) and on-site guidance puncture once. The primary end-point was the success rate of cannulation at the first attempt and the secondary end-point was cannulation failure rate.
Results
The success rate of cannulation at the first attempt in ultrasound in the developing line group was significantly higher than that in the traditional ultrasound group (proportion difference: 34.21%, 95% confidence interval [CI], −0.5483 to −0.1334; P=0.0025). However, no significant between-group difference was observed with respect to failure rate (mean difference 95% CI, (−0.0084 to 0.2743; P=0.0866). The ultrasonic location time in the ultrasound with developing line group was significantly lower than that in the traditional ultrasound group (mean difference −12.4 seconds, 95% CI, 10.64 to 13.98 s; P<0.0000).
Conclusions
Use of ultrasound with developing line significantly improved the success rate of radial artery puncture performed by interns as compared to that with use of traditional dynamic ultrasound guidance technology.
MeSH Keywords: Internship and Residency, Radial Artery, Ultrasonography
Background
Radial artery puncture is commonly used in the operating room, intensive care unit, and emergency room for monitoring the arterial pressure and obtaining arterial blood samples for gas analysis [1–3]. The technique of radial artery puncture is challenging. Many studies have shown that the success rate of ultrasound-guided radial artery puncture is higher than that achieved with blinded method [4–7]. However, some studies have also shown that ultrasound guidance does not improve the success rate of radial artery puncture [8–12], which may be related to the operator’s ultrasound training and clinical experience [13–16]. For beginners, such as interns, who have insufficient experience in ultrasound and puncture, it is difficult to use ultrasound to guide radial artery puncture [17]. Therefore, this study investigated methods to improve the success rate of arterial puncture by interns. Our research team has achieved good results with use of ultrasound with developing line. Although this technology also requires ultrasound, it does not require a skilled ultrasound operator to identify the site of puncture [18]. Therefore, this technology is theoretically suitable for personnel who are inexperienced in the use of ultrasound. However, it is still unclear whether the optimized ultrasonic localization system improves the success rate of radial artery puncture. Therefore, in this trial, we compared the success rate of interns in radial artery puncture using the traditional method and optimized ultrasonic localization technology.
Material and Methods
This study was approved by the Institutional Review Board of Beijing You’an Hospital, Capital Medical University (Institutional Review Board #201706). Written informed consent was obtained from all participants prior to their enrolment. This trial was registered prior to patient enrolment at clinicaltrials.gov (ChiCTR1800015337, Principal Investigator: Zhefeng Quan, Date of Registration: March 24, 2018). This manuscript adheres to the applicable CONSORT guidelines.
The sample size of this study was estimated on the basis of our preliminary experiment, in which the success rates of first puncture in the ultrasound and control groups were 76.7% and 43.3%, respectively. The test level α was taken as 0.05, Z 0.05/2=1.96, and the power level 1-β was taken as 0.8, Z 0.2=0.84. The sample size for each group was calculated as 32. Taking into account the withdrawal rate (approximately 20%), a total sample size of 76 was used with 38 patients in each group.
Patients scheduled to undergo elective hepatectomy or splenectomy with general anesthesia between March and May 2018 at the Beijing You’an Hospital and the Beijing Friendship Hospital of Capital Medical University were recruited in the trial. Inclusion criteria were: age between 18 and 60 years, body weight between 50 and 85 kg, and American Society of Anesthesiologists Grade II or III. Exclusion criteria were: negative Allen’s test, peripheral vascular disease, ulnar artery occlusion, hemorrhagic shock, atherosclerosis, morbid obesity, unstable angina, Raynaud’s disease, cardiogenic shock, diabetes, hypertension, and patients who had previously undergone multiple arterial punctures.
Patients were randomly assigned to traditional ultrasound or ultrasound with developing line groups using a sealed envelope. All radial artery punctures were performed using the short-axis out-of-plane (SA-OOP) procedure. Radial artery puncture in the traditional ultrasound group was made under the guidance of conventional dynamic ultrasound, while radial artery puncture in the ultrasound with developing line group was made with the developing line. The developing line is connected to the ultrasound probe and remains perpendicular to the long axis of the probe (Figure 1) [18].
Figure 1.
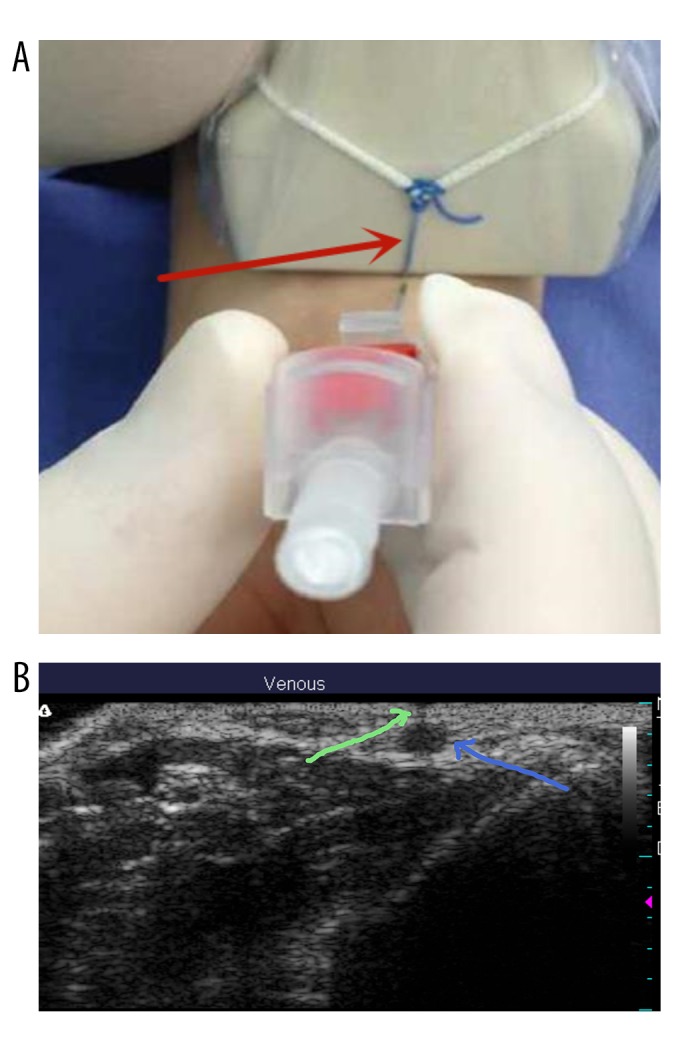
A developing line was tied on the midpoint of the ultrasound probe and perpendicular to the long axis (red arrow). (A) The developing line was directed at the beating radial artery by adjusting the ultrasonic probe. The needle of the arterial cannula was placed at the contact point of the developing line and skin using the operator’s right hand. (B) Ultrasonic image of the radial artery (blue arrow) and the low-density shadow of the developing line (green arrow).
First, the developing line was aligned with the center of the radial artery projection point on the skin through the ultrasound screen. This line produces a visible mark on the ultrasound screen and improves the accuracy of needle placement. Then, the puncture needle was placed at the intersection of the developing line and the skin (puncture point), and the direction of the puncture needle was kept perpendicular to the probe plane (puncture direction). The puncture process does not use dynamic ultrasound technology. All patients were treated with the same radial artery puncture technique. Needles in both groups were inserted at a 45° angle and the entry of them into the artery was confirmed by observing blood backflow into the end of the needle. After entering the radial artery, the needle was put down from 45° to 15° and the needle was slowly pushed forward for 2 to 3 mm. After pulling out the core, the cannula was introduced into the radial artery. Then, the puncture needle was fixed and the blood pressure was monitored with a pressure transducer. All radial artery punctures were performed by interns who were in the anesthesiology rotation. They had not performed radial artery puncture or received relevant training before. They were randomly assigned to the traditional ultrasound group or the ultrasound with developing line groups. All interns received the corresponding ultrasound-guided radial artery puncture training in their respective groups. The interns received a theoretical explanation (including a demonstration video of the puncture) and on-site guidance puncture once.
Electrocardiogram, non-invasive arterial blood pressure, and peripheral oxygen saturation of all patients were monitored using the IntelliVue MP70 device (Philips, the Netherlands). Patients were placed in supine position and administrated 0.05 mg/kg midazolam and 0.1 μg/kg sufentanil for analgesia and sedation. The right median cubital vein was chosen to establish venous access. The left arm was used for the puncture. The left wrist was extended over a rolled towel and the hand was positioned in dorsiflexion. The skin at the insertion site was cleaned with povidone iodine, and 2% lidocaine was used for local anesthesia. The ultrasonic probe was connected to the ultrasound system (Terason2000+, Terason, Burlington, MA). When the arterial image was located, parameters were set at a frequency of 6 MHz and a depth of 2 cm, and the probe was adjusted to optimize the image.
The patient’s general condition was recorded and the distance between the skin and the radial artery and the internal diameter of the radial artery were recorded. The primary end-point was to compare the success rate of cannulation at first attempt, and the secondary end-points were ultrasound localization time, puncture time, and cannulation time, and these were compared between the 2 groups.
Cannulation was considered as successful when the cannula was smoothly inserted into the radial artery. Puncture failure was defined when the cannulation into the radial artery was not achieved after 3 or more attempts. In case of failure, the puncture was performed by the instructor. The success rate and failure rate of the first puncture were recorded. Ultrasound localization time was defined as the time from the beginning of the ultrasound probe to the skin to the penetration of the puncture needle into the skin. Puncture time was defined as the time between the penetration of the puncture needle into the skin until the puncture needle penetrated the radial artery, Cannulation time was defined as the time from the penetration of the needle into the skin to the insertion of cannula into the radial artery. Vascular complications, including hematoma, thrombosis, vasospasm, and edema, were recorded.
Statistical analysis was performed using SAS software (Version 9.3.1, SAS Institute, Cary, NC). The normality of data was assessed by Kolmogorov-Smirnov test. Normally distributed variables are presented as mean ± standard deviation and between-group differences assessed by t test. Between-group differences with respect to success rate and incidence of complication were assessed using the chi-squared test or Fisher exact test. P<0.05 was considered statistically significant.
Results
Seventy-seven patients were randomly divided into 2 groups (traditional ultrasound group, n=38; ultrasound with developing line group, n=39). There were no drop-outs during the trial. The flow chart is shown in Figure 1. Baseline characteristics of patients are shown in Table 1.
Table 1.
Baseline characteristics of study subjects (n=77).
| Parameter | Traditional ultrasound (n=38) | Ultrasound with developing line (n=39) |
|---|---|---|
| Age (years) | 49.1±8.2 | 46.2±8.4 |
| Weight (kg) | 65.4±9.9 | 67.1±9.0 |
| Height (cm) | 167.2±7.3 | 166.1±7.1 |
| ASA II/III | 21/17 | 24/15 |
| Male/Female (n) | 26/12 | 29/10 |
| Mean arterial pressure (mmHg) | 77.5±6.9 | 76.5±9.7 |
No significant between-group differences were observed with respect to distance from skin to radial artery [Mean difference: 0.0758 (95% CI, −0.0997 to 0.2513), P=0.3919, Table 2] and inner diameter of radial artery [Mean difference: −0.2043 (95% CI, −0.6483 to 0.2397), P=0.3621, Table 2].
Table 2.
Comparison of the depth from skin to radial artery and inner diameter of radial artery between the 2 groups.
| Parameter | Traditional ultrasound (n=38) | Ultrasound with developing line (n=39) | P | Mean difference (95% CI) |
|---|---|---|---|---|
| Depth from skin to radial artery (mm) | 2.4±0.9 | 2.6±1.0 | 0.3919 | 0.0758 (−0.0997, 0.2513) |
| Inner diameter of radial artery (mm) | 2.5±0.4 | 2.4±0.3 | 0.3621 | −0.2043 (−0.6483, 0.2397) |
CI – confidence interval.
In the ultrasound with developing line group, the success rate of cannulation at the first attempt (71.79%), which was significantly higher than that in the traditional ultrasound group (34.21%) [proportion difference: −0.3408 (95% CI, −0.5483 to −0.1334), P=0.0025, Table 3]. Cannula insertion failure rates in the 2 groups were 5.13% and 18.42%, respectively, and the difference was not statistically significant [proportion difference: 0.1329 (95% CI, −0.0084 to 0.2743), P=0.0866; Table 3].
Table 3.
Comparison of success rate of cannula insertion at first attempt and failure rate between the 2 groups.
| Parameter | Traditional ultrasound (n=38) | Ultrasound with developing line (n=39) | P | Proportion difference (95% CI) |
|---|---|---|---|---|
| Success rate of cannula insertion at first attempt | 13 (34.21%) | 28 (71.79%) | 0.0025 | −0.3408 (−0.5483, −0.1334) |
| Failure rate of cannula insertion | 7 (18.42%) | 2 (5.13%) | 0.0866 | 0.1329 (−0.0084, 0.2743) |
CI – confidence interval.
The average ultrasonic location time in the ultrasound with developing line group was 18.6±4.8 seconds (s) as against 6.2±1.6 s in the traditional ultrasound group. The mean difference of 12.4 s (95% CI, 10.64 to 13.98 s) was statistically significant (P<0.0000, Table 4). However, no significant between-group difference was observed with respect to cannulation time [mean difference: −4.90 s (95% CI, −11.85 to 2.04 s), P=0.1637; Table 4].
Table 4.
Comparison of ultrasonic location time and cannulation time between the 2 groups.
| Parameter | Traditional ultrasound (n=38) | Ultrasound with developing line (n=39) | P | Mean difference (95% CI) |
|---|---|---|---|---|
| Ultrasonic location time (s) | 18.6±4.8 | 6.2±1.6 | <0.0000 | 12.30 (10.64, 13.98) |
| Cannulation time (s) | 27.6±12.6 | 31.1±17.9 | 0.1637 | −4.90 (−11.85, 2.04) |
CI – confidence interval.
No instances of thrombosis, occlusion, or aneurysm occurred in this study. No significant between-group difference was observed with respect to incidence of vasospasm [proportion difference: −0.0963 (95% CI, −0.3316 to 0.1391), P=0.4361] or hematoma formation [proportion difference: −0.0963% (95% CI, −0.1391 to 0.3316), P=0.4361; Table 5].
Table 5.
Vascular complications during radial artery puncture.
| Parameter | Traditional ultrasound (n=38) | Ultrasound with developing line (n=39) | P | Proportion difference (95% CI) |
|---|---|---|---|---|
| Vasospasm | 23 (60.52%) | 17 (43.59%) | 0.4361 | −0.0963 (−0.3316, 0.1391) |
| Hematoma | 11 (28.95%) | 5 (12.82%) | 0.4361 | 0.0963 (−0.1391, 0.3316) |
| Thrombosis | 0 | 0 | ||
| Occlusion | 0 | 0 | ||
| Aneurysm | 0 | 0 |
CI – confidence interval.
Discussion
Every year many interns enter teaching hospitals for clinical training. Radial artery puncture is a very important clinical procedure. Reported success rates for radial artery puncture at first attempt by interns range between 34% and 38% [19,20]. The relatively low success rates are largely attributable to difficulty in accurate localization of the artery by interns. The procedure of radial artery puncture can be divided into 3 steps. The first step is to locate the puncture site, the second step is the puncture, and the last step entails insertion of the cannula into the radial artery. The first step is particularly important because appropriate localization facilitates the success of the puncture and insertion. The first difficulty encountered by interns during radial artery puncture is the exact positioning of the puncture point. The purpose of this study was to find ways to improve the ability of interns to accurately locate the radial artery in a short period of time. Ultrasound with developing line has achieved a good effect among experienced anesthesiologists [18]. In this method the puncture point is determined by the ultrasound with developing line; subsequently, the puncture is guided by dynamic ultrasound.
This study showed that the success rate of radial artery puncture at the first attempt under ultrasound with developing line by interns increased from 34.21% to 71.79% and the location time was reduced by an average of 12.4 s. The developing line technique can locate the projection point of radial artery so as to accurately locate the puncture site and improve the initial success rate of radial artery puncture, while in the traditional dynamic ultrasound technique, the artery was placed in the middle of the ultrasound screen, the puncture needle was inserted through the center of the ultrasound probe surface so that it can theoretically puncture the center of the blood vessel. However, this method requires selection of the median point of the ultrasound plane on visual observation. It is difficult to accurately associate the radial artery in the ultrasound screen with the radial artery on the forearm of the patient based on visual observation, and it is also difficult to visually find the right projection point of the radial artery that has an average internal diameter of 2.4 mm on the surface. Therefore, in clinical settings, the puncture route is constantly corrected through dynamic observation of the puncture needle. However, this kind of dynamic ultrasound technology is difficult to master in a short time by interns who lack clinical experience. In particular, interns cannot determine whether the white point on the ultrasound screen represents the tip or the body of the needle, which makes it difficult for them to take advantage of dynamic ultrasound. Moreover, their inexperience prolongs the ultrasound location time and puncture time. The location technology of ultrasound with developing line does not require extensive training. It is easy for beginners to use a developing line with a thickness equivalent to 0.07 mm to locate the midpoint of radial artery that has an inner diameter of 2.4 mm on the ultrasound screen. The developing line is only used for localization and the insertion needs to be done blindly, which does not extend the puncture time. However, interns in the dynamic ultrasound group lacked experience in radial arterial puncture technique. In our study, we used the traditional wired ultrasound, which limits the location of the ultrasound imaging monitor. The interns were required to pay attention to both the imaging monitor and the puncture site at the same time. Another study used wireless remote display system with a tablet computer, which facilitates the ultrasound-guided radial artery catheterization [21], showing no significant between-group difference in incidence of adverse effects. However, the incidence of vasospasm and hematoma in the ultrasound with developing line group were reduced by 16.93% and 16.13%, respectively, when compared with that in the traditional ultrasound group. This may be related to the improved success rate of puncture in the ultrasound with developing line group. If the sample size is large enough, this difference may even be statistically significant. Other adverse effects such as thrombosis, occlusion, and aneurysms were not found in either group.
The limitations of this study include the lack of a double-blind study design and the fact that instructions for radial artery puncture were provided by different teachers. However, all the teachers are experienced attending physicians who had performed more than 300 radial arterial punctures.
Conclusions
In this study, use of ultrasound with developing line improved the success rate of interns in achieving radial artery puncture as compared to that with the traditional dynamic ultrasound guidance technology. After gaining experience with this technique, the interns will be better equipped to use the dynamic ultrasound to perform radial artery puncture.
Footnotes
Source of support: Supported by Beijing Municipal Science and Technology Commission (no. Z171100001017036)
Conflicts of interests
None.
References
- 1.Anantasit N, Cheeptinnakorntaworn P, Khositseth A, et al. Ultrasound versus traditional palpation to guide radial artery cannulation in critically ill children: A randomized trial. J Ultrasound Med. 2017;36(12):2495–501. doi: 10.1002/jum.14291. [DOI] [PubMed] [Google Scholar]
- 2.Kherad B, Köhncke C, Spillmann F, et al. Postprocedural radial artery occlusion rate using a sheathless guiding catheter for left ventricular endomyocardial biopsy performed by transradial approach. BMC Cardiovasc Disord. 2016;16(1):253. doi: 10.1186/s12872-016-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aouad-Maroun M, Raphael CK, Sayyid SK, et al. Ultrasound-guided arterial cannulation for paediatrics. Cochrane Database Syst Rev. 2016;9:CD011364. doi: 10.1002/14651858.CD011364.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiberenge RK, Ueda K, Rosauer B. Ultrasound-guided dynamic needle tip positioning technique versus palpation technique for radial arterial cannulation in adult surgical patients: a randomized controlled trial. Anesth Analg. 2018;126(1):120–26. doi: 10.1213/ANE.0000000000002261. [DOI] [PubMed] [Google Scholar]
- 5.Hansen MA, Juhl-Olsen P, Thorn S, et al. Ultrasonography-guided radial artery catheterization is superior compared with the traditional palpation technique: A prospective, randomized, blinded, crossover study. Acta Anaesthesiol Scand. 2014;58(4):446–52. doi: 10.1111/aas.12299. [DOI] [PubMed] [Google Scholar]
- 6.Gopalasingam N, Hansen MA, Thorn S, et al. Ultrasound-guided radial artery catheterisation increases the success rate among anaesthesiology residents: A randomised study. J Vasc Access. 2017;18(6):546–51. doi: 10.5301/jva.5000702. [DOI] [PubMed] [Google Scholar]
- 7.Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (radial artery access with ultrasound trial) JACC Cardiovasc Interv. 2015;8(2):283–91. doi: 10.1016/j.jcin.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Peters C, Schwarz SK, Yarnold CH, et al. Ultrasound guidance versus direct palpation for radial artery catheterization by expert operators: A randomized trial among Canadian cardiac anesthesiologists. Can J Anaesth. 2015;62(11):1161–68. doi: 10.1007/s12630-015-0426-8. [DOI] [PubMed] [Google Scholar]
- 9.Zaremski L, Quesada R, Kovacs M, et al. Prospective comparison of palpation versus ultrasound-guided radial access for cardiac catheterization. J Invasive Cardiol. 2013;25(10):538–42. [PubMed] [Google Scholar]
- 10.Tangwiwat S, Pankla W, Rushatamukayanunt P, et al. Comparing the success rate of radial artery cannulation under ultrasound guidance and palpation technique in adults. J Med Assoc Thai. 2016;99(5):505–10. [PubMed] [Google Scholar]
- 11.Laursen CB, Pedersen RL, Lassen AT. Ultrasonographically guided puncture of the radial artery for blood gas analysis: A prospective, randomized controlled trial. Ann Emerg Med. 2015;65(5):618–19. doi: 10.1016/j.annemergmed.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Peters C, Schwarz SK, Yarnold CH, et al. Ultrasound guidance versus direct palpation for radial artery catheterization by expert operators: A randomized trial among Canadian cardiac anesthesiologists. Can J Anaesth. 2015;62(11):1161–68. doi: 10.1007/s12630-015-0426-8. [DOI] [PubMed] [Google Scholar]
- 13.Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: An updated meta-analysis of randomized controlled trials. PLoS One. 2014;9:e111527. doi: 10.1371/journal.pone.0111527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tangwiwat S, Pankla W, Rushatamukayanunt P, et al. Comparing the success rate of radial artery cannulation under ultrasound guidance and palpation technique in adults. J Med Assoc Thai. 2016;99(5):505–10. [PubMed] [Google Scholar]
- 15.Zaremski L, Quesada R, Kovacs M, et al. Prospective comparison of palpation versus ultrasound-guided radial access for cardiac catheterization. J Invasive Cardiol. 2013;25(10):538–42. [PubMed] [Google Scholar]
- 16.Nakayama Y, Inagaki Y, Nakajima Y, et al. A practical training program for peripheral radial artery catheterization in adult patients: A prospective, randomized controlled trial. Anesthesiology. 2016;125(4):716–23. doi: 10.1097/ALN.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 17.Bobbia X, Grandpierre RG, Claret PG, et al. Ultrasound guidance for radial arterial puncture: A randomized controlled trial. Am J Emerg Med. 2013;31(5):810–15. doi: 10.1016/j.ajem.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Quan Z, Tian M, Chi P, et al. Modified short-axis out-of-plane ultrasound versus conventional long-axis in-plane ultrasound to guide radial artery cannulation: A randomized controlled trial. Anesth Analg. 2014;119(1):163–69. doi: 10.1213/ANE.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 19.Li YH, Wang YQ, Zeng L, et al. Use of 1-ml hollow tube-assisted radial artery catheterization in clinical anesthesiology. Int J Clin Exp Med. 2014;7(9):2740–43. [PMC free article] [PubMed] [Google Scholar]
- 20.Fuyan L, Dong Y, Zhiwei L, et al. Use of ultrasound guidance in the insertion of radial artery catheters. International Journal of Anesthesiology and Resuscitation. 2014;3(35):238–40. [Google Scholar]
- 21.Tsuchiya M, Mizutani K, Funai Y, Nakamoto T. In-line positioning of ultrasound images using wireless remote display system with tablet computer facilitates ultrasound-guided radial artery catheterization. J Clin Monit Comput. 2016;30(1):101–6. doi: 10.1007/s10877-015-9692-9. [DOI] [PubMed] [Google Scholar]


