Abstract
Background
This study aimed to investigate cognitive function, hippocampal neuronal changes, and the expression of inflammatory cytokines in a mouse model of hepatic ischemia-reperfusion injury.
Material/Methods
Sixty mice were divided into the sham group, which underwent surgery without vascular occlusion; the I/R1 group, with occlusion of the left hepatic artery and portal vein for 20 min, and reperfusion for 30 min; and the I/R2 group, with occlusion of the left hepatic artery and portal vein for 40 min, and reperfusion for 30 min. At postoperative day 4 and 11, ten mice from each group underwent the Morris water maze (MWM) task. Hippocampal tissues were stained for Nissl bodies. Expression of nuclear factor-κB (NF-κB) and choline acetyltransferase (ChAT) were quantified by immunohistochemistry. Serum tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were measured by enzyme-linked immunosorbent assay (ELISA).
Results
Groups I/R1 and I/R2 showed a significantly increased latency in the MWM test between days 5–9, compared with the sham group (P<0.05), with no difference by day 11; the I/R2 group had an initial lower crossing frequency (P<0.05), with no difference by day 18. The I/R2 group showed reduced numbers of Nissl bodies in hippocampal neurons. The I/R1 and I/R2 groups had increased expression of NF-κB, TNF-α, and IL-1β and decreased ChAT. No differences between the groups were found in levels of NF-κB, TNF-α, IL-1β, or ChAT by day 18.
Conclusions
A mouse model of hepatic ischemia-reperfusion injury showed transient and reversible cognitive dysfunction, changes in hippocampal neurons, and expression of inflammatory cytokines.
MeSH Keywords: Cognitive Science, Hippocampus, Reperfusion
Background
Postoperative cognitive dysfunction (POCD) varies in severity and includes memory deficit, personality changes, impaired orientation, and social impairment [1]. Although recent progress in medical and surgical techniques have improved the safety of anesthesia and surgery, POCD is not uncommon and may persist for several weeks or months [2]. POCD can have serious effects on postoperative recovery and long-term quality of life, results in an economic burden for the family and society, and can increase postoperative mortality [2]. The pathogenesis of POCD remains unclear but is associated with dysfunction of the central nervous system, endocrine, or immune system. Currently, POCD is considered to result from several factors, including the effects of anesthesia and surgical trauma [3,4]. POCD most commonly occurs following major surgery, such as cardiothoracic surgery. Due to its complex pathogenesis and its effects on postoperative recovery, POCD has recently become an area of clinical research interest [5].
Hepatic ischemia-reperfusion injury is a frequent complication of liver transplantation surgery and general anesthesia [6]. In addition to systemic organ damage, hepatic ischemia-reperfusion injury may also impair patient cognitive function [6,7]. Hepatic ischemia-reperfusion injury is associated with an acute inflammatory response induced by cytokines that include tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6, which are under the regulation of nuclear factor- κB (NF-κB) [8]. Acetylcholine is an important neurotransmitter in the central cholinergic system that has important functions in learning and memory, and choline acetyltransferase (ChAT) is an enzyme that has a role in the synthesis of acetylcholine [9–12].
Therefore, this study aimed to investigate cognitive function, hippocampal neuronal changes, and the expression of inflammatory cytokines in a mouse model of hepatic ischemia-reperfusion injury. The Morris water maze (MWM) task was chosen as the method to evaluate cognitive function. The morphological neuronal changes in the mouse hippocampus were studied, with the expression of tissue and serum factors that may be involved in the pathogenesis of postoperative cognitive impairment, including NF-κB and ChAT.
Material and Methods
Experimental animals and grouping
A total of 60 male Kunming specific pathogen-free (SPF) mice aged 2 months with a body weight of 20–25 g were provided by the Laboratory Animal Center, Anhui University. Mice were provided with food and water ad libitum with normal 12 hr light and dark cycles, and were kept at 20–24°C with 50–70% relative humidity. Mice were randomly assigned into three groups: the sham group (N=20), which underwent surgery without vascular occlusion; the I/R1 group (N=20), with occlusion of the left hepatic artery and portal vein for 20 min, and reperfusion for 30 min; and the I/R2 group (N=20), with occlusion of the left hepatic artery and portal vein for 40 min, and reperfusion for 30 min.
Reagents and equipment
Pentobarbital (F20030816) and paraformaldehyde (F2002083) were purchased from Shanghai Chemical Reagent Co. Ltd. (Shanghai, China). A rabbit polyclonal antibody to choline acetyltransferase (ChAT) (JC1653278) was purchased from Merck Millipore (Burlington, MA, USA). A rabbit polyclonal antibody to nuclear factor-κB (NF-κB) (F20090218) and immunohistochemistry (IHC) staining kit (SP-9001) were purchased from Zhoangshan Jinqiao Bio (Beijing, China). The Morris water maze (model XR-XM101) was purchased from the Pharmaceutical Institute, Chinese Medical Academy. A high-speed homogenizer (FSH-2A) was provided by Rongti Instruments (China). A tissue microtome (RM2235) was purchased from Leica (Wetzlar, Germany). The CX23 light microscope was purchased from Olympus (Tokyo, Japan). A fully automated ultracentrifuge (model H-1600A) was purchased from Hunan Instruments, China.
Preparation of the mouse model of hepatic ischemia-reperfusion injury
Mice were fasted for 12 h before surgery but had access to water ad libitum. Mice were anesthetized using an intraperitoneal injection of 3% pentobarbital (30 mg/kg), placed on the operation table, and the skin was sterilized. A 3 cm midline incision was made on the abdominal wall to expose the hepatic portal system. The sham group mice (N=20) underwent dissection of the hepatic artery and hepatic vein without occlusion. The I/R1 and I/R2 groups, which were the hepatic ischemia-reperfusion injury groups were prepared according to the method previously described [8]. Briefly, an artery clamp was used to occlude left hepatic artery and portal vein for 20 min or 40 min. The clamp was then removed for 30 min of reperfusion, followed by abdominal wall closure. Liver tissues were collected to confirm the model preparation. Tail artery blood pressure was monitored during surgery and the rectal temperature was also continuously maintained within 37–38.5°C using a heating light. After surgery, the mice were kept in a warm chamber and received penicillin for 3 days.
Liver tissue histopathology and transmission electron microscopy
Mouse liver tissue samples were collected from the middle lobe and were fixed in 4% paraformaldehyde, dehydrated and embedded in paraffin wax. Serial tissue sections were cut onto glass slides at 5 μm, stained with hematoxylin and eosin (H&E) and imaged under light microscopy. Also, liver tissue samples were immediately fixed in 2.5% glutaraldehyde followed by 1% osmic acid. After dehydration and resin embedding, 2 μm ultra-thin tissue sections were prepared for staining with uranyl acetate and lead citrate. Images were captured by transmission electron microscopy.
Morris water maze (MWM) task
The Morris water maze (MWM) apparatus consisted of a circular water tank measuring 120 cm in diameter, and 30 cm in height, with a non-reflective inner wall. The tank was filled with water to a level at 10 cm below the top. Four equidistant points were used to divide the tank into four quadrants. A transparent platform, 10 cm in diameter, was fixed in quadrant III, one cm below the water surface, with the central point of the platform 30 cm from the tank wall.
The MWM navigation session evaluated the learning function of the mice. The water temperature was maintained at 20–22°C during the experiment. Mice were separately placed in each of four quadrants, with their heads facing towards the wall. An automatic system monitored the swimming path of the mice, which was less than 90 s for each trial. The time that the mice needed to locate the fixed platform was recorded as the latency period. Mice that could not locate the fixed platform were manually guided to the platform and stayed for 10 s, and were recorded as having 90 s latency. For each daily session, the average latency from four trials was recorded, and the test was performed for 7 days.
The spatial memory of the mice was tested on day 8 of training. Following removal of the platform, the mice were re-tested. The number of crossings of the original platform location, the averaged velocity, and the central region locomotor duration and movement distance were measured during 90 s.
Hippocampal sample preparation
Following the MWM assay, the mice were anesthetized by intraperitoneal injection of 3% pentobarbital (30 mg/kg), and underwent perfusion with fixative on the operation table. The heart was exposed after opening the thoracic cavity. The perfusion needle was inserted into the left ventricle for saline infusion. The right auricle was removed to drain out the blood.
The fixation buffer consisted of 4% paraformaldehyde in 0.01M PBS buffer (pH 7.4). Fixation buffer was added when the liver became pale. Brain tissues were extracted after the disappearance of limb twitching and hardening of the body organs and were kept in fixation buffer. Based on the use of a mouse brain atlas, hippocampal tissues were localized for preparing 3 mm coronal sections, which were embedded in paraffin wax for further use.
Nissl body staining of hippocampal neurons
From the tissue blocks of the mouse brain, 10 μm tissue sections were de-waxed and immersed in staining buffer for 30 min. After rinsing in water, tissue sections were dehydrated in 95% and absolute ethanol, permeabilized in xylene, and stained with Nissl’s stain for Nissl bodies, or rough endoplasmic reticulum (RER), seen as violet staining. The sections were mounted in neutral resin. IPP version 6.0 software was used to calculate the number of Nissl bodies.
Expression levels of NF-κB and ChAT in the hippocampal CA3 region
Immunohistochemical staining was performed using a three-stage streptavidin-peroxidase method following the instructions of the test kit. Briefly, 5 μm tissue sections were heated at 60°C followed by de-waxing treatment and permeabilization. Antigen retrieval was performed using citrate salt buffer for 25 min, followed by incubation with 3% H2O2 for 10 min at room temperature. After washing three times with PBS, goat serum was added for 20 min to block nonspecific antigen binding.
The rabbit anti-NF-κB and rabbit anti-ChAT primary antibodies were added and incubated overnight at 4°C. After washing in PBS, the tissue sections were incubated for 30 min in horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG secondary antibody. Tissue sections were washed three times in PBS, for 5 min each time, and the chromogenic substrate was added, followed by rinsing with tap water. Hematoxylin counterstaining was followed by rinsing with 0.1% HCl-ethanol, followed by dehydration in graded ethanols and mounting in resin. Counting of positive cells was performed by light microscopy under ×100 objective. Four separate fields were selected within the hippocampal CA3 region followed by analysis of the integrated optical density (IOD) by IPP version 6.0 software. The average value was calculated for the number of positive cells.
Serum levels of inflammatory cytokines
Blood was drawn from mice in each group in the day 11 and day 18 following surgery, followed by isolation of serum for analysis of the levels of TNF-α and IL-1β using an enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions.
Statistical analysis
Data analysis was performed using SPSS version 18.0 software. Measurement data were presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare the difference between the three study groups, followed by the Student-Newman-Keuls (SNK) method for paired comparisons. Statistical significance was represented by P<0.05.
Results
Histology of liver hepatocytes in the mouse model of hepatic ischemia-reperfusion injury
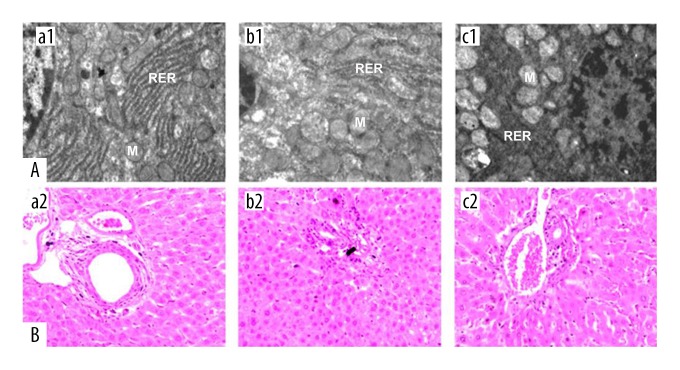
Liver histology from liver samples in the sham group showed widely distributed rough endoplasmic reticulum (RER) in the hepatocytes, which was regularly arranged, with normal lysosomes (Figure 1A). Mitochondria were normally distributed with a round or oval shape, without swelling (Figure 1B). Liver tissue histology from liver tissue samples from mice in the I/R1 group had hepatocytes with abundant RER with mitochondrial swelling but with few breakages. Liver tissue histology from liver tissue samples from mice in the I/R2 group showed prominent mitochondrial swelling with disruption of mitochondrial membranes, vacuoles, and relatively fewer RER, lysosomes, or ribosomes.
Figure 1.
Cell morphology of the mouse hepatocytes in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group. (A) Electron microscopy shows the subcellular morphology of the mouse hepatocytes. Magnification ×10,000. (B) Photomicrographs of the light microscopy of the liver tissues in the three mouse study groups. Hematoxylin and eosin (H&E). Magnification ×100. (a1, a2) The sham group, consisting of mice that underwent anesthesia, surgery, and separation of the hepatic artery and vein, but without occlusion. (b1, b2) The I/R1 group, in which the left hepatic artery and portal vein were clamped for 20 min, followed by reperfusion for 30 min. (c1, c2) The I/R2 group, in which the left hepatic artery and portal vein were clamped for 40 min followed by reperfusion for 30 min. M – mitochondria; RER – rough endoplasmic reticulum.
Spatial navigation findings from the Morris water maze (MWM) task
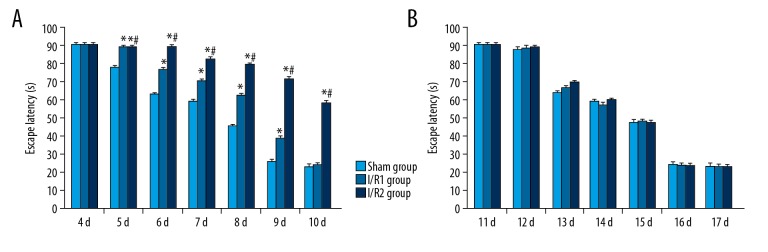
Mice in the I/R1 group showed significantly increased latency in navigation at 5–9 days following surgery, when compared with the sham group (P<0.05). The I/R2 group also showed s significantly increased latency period at 5–10 days following surgery, when compared with the sham group (P<0.05). However, at 11 days following surgery, no significant difference in latency in navigation was found between all groups (P>0.05) (Figure 2A). At day 6 and day 7 following surgery, the navigation latency period was increased with increasing duration of ischemia duration, representing a time-dependent effect. With repeated training sessions, all three study groups showed a reduced latency period (Figure 2B).
Figure 2.
(A, B) Latency in navigation assay of in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group. The three mouse study groups included: the sham group, consisting of mice that underwent anesthesia, surgery, and separation of the hepatic artery and vein, but without occlusion; the I/R1 group, in which the left hepatic artery and portal vein were clamped for 20 min, followed by reperfusion for 30 min; and the I/R2 group, in which the left hepatic artery and portal vein were clamped for 40 min followed by reperfusion for 30 min. Comparison with the sham group (* P<0.05). Comparison with the I/R1 group (# P<0.05).
Spatial evaluation from the MWM task
At 11 days following surgery, mice in the I/R2 group showed significantly reduced crossing times, a lower average speed, and reduced central zone distance and duration compared with the Sham group and the I/R1 group (P<0.05) (Table 1). At 18 days following surgery, a second evaluation found no significant difference between all the indices studied (Table 2).
Table 1.
Comparison of the spatial assay indices at 11 days postoperatively in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group (mean ±SD) (n=10).
| Group | Crossing times | Average speed (cm/sec) | Central distance (cm) | Central duration (sec) |
|---|---|---|---|---|
| Sham | 6.20±1.13 | 11.36±1.51 | 176.84±30.82 | 13.15±1.92 |
| I/R1 | 5.90±0.96# | 11.41±1.07# | 188.20±20.46# | 13.16±1.36# |
| I/R2 | 3.20±1.91* | 9.60±1.48* | 124.13±38.51* | 8.80±3.29* |
The sham group consisted of mice that underwent anesthesia, surgery, and separation of the hepatic artery and vein, but without occlusion. The I/R1 group consisted of mice in which the left hepatic artery and portal vein were clamped for 20 min, followed by reperfusion for 30 min. The I/R2 group consisted of mice in which the left hepatic artery and portal vein were clamped for 40 min, followed by reperfusion for 30 min.
P<0.05 compared with the sham group;
P<0.05 compared with the I/R2 group.
Table 2.
Comparison of spatial probe assay indices at 18 days postoperatively in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group (mean ±SD) (n=10).
| Group | Crossing times | Average speed (cm/sec) | Central distance (cm) | Central duration (sec) |
|---|---|---|---|---|
| Sham | 5.80±1.22 | 11.56±3.78 | 185.63±20.17 | 13.93±1.77 |
| I/R1 | 5.80±1.13 | 12.21±0.90 | 195.50±16.62 | 14.21±1.47 |
| I/R2 | 5.80±.12 | 12.27±1.00 | 191.25±16.51 | 13.83±1.53 |
The sham group consisted of mice that underwent anesthesia, surgery, and separation of the hepatic artery and vein, but without occlusion. The I/R1 group consisted of mice in which the left hepatic artery and portal vein were clamped for 20 min, followed by reperfusion for 30 min. The I/R2 group consisted of mice in which the left hepatic artery and portal vein were clamped for 40 min, followed by reperfusion for 30 min.
Staining for Nissl bodies in hippocampal neurons
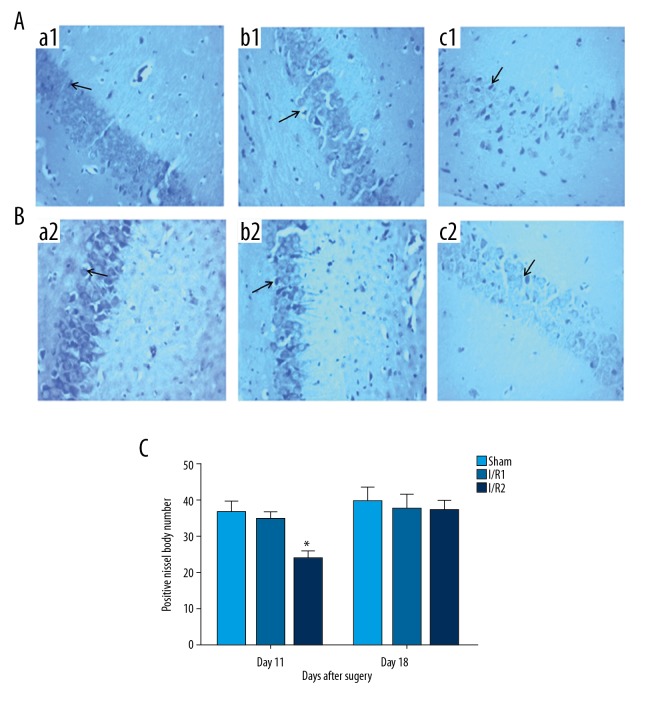
At 11 days after surgery, mice in the I/R2 group showed a significant reduction in the number of Nissl bodies in the hippocampal neurons identified by light microscopy compared with the other groups, plus mild cytoplasmic edema (P<0.05) (Figure 3A, 3C). The sham group and the I/R1 group showed a regular arrangement of hippocampal neurons, with clear Nissl bodies, transparent cytoplasm, round or oval-shaped nuclei without major abnormality (Figure 3A). At 18 days following surgery, the number of Nissl bodies in the I/R2 group was significantly increased compared with that at day 11 following surgery (P<0.05) (Figure 3C). However, no significant abnormality was found in hippocampal neurons among all groups at day 18 following surgery (P>0.05) (Figure 3B, 3C).
Figure 3.
Photomicrographs of the Nissl histochemical stain for Nissl bodies (blue/violet), or rough endoplasmic reticulum (RER), in the motor neurons of the hippocampus in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group. (A) Photomicrograph of the light microscopy shows hippocampal Nissl bodies at 11 days postoperatively. Nissl stain. Magnification ×100. (B) Photomicrograph of the light microscopy shows hippocampal Nissl bodies at 18 days postoperatively. Nissl stain. Magnification ×100. (a1, a2) The sham group, consisting of mice that underwent anesthesia, surgery, and separation of the hepatic artery and vein, but without occlusion. (b1, b2) The I/R1 group, in which the left hepatic artery and portal vein were clamped for 20 min, followed by reperfusion for 30 min. (c1, c2) The I/R2 group, in which the left hepatic artery and portal vein were clamped for 40 min followed by reperfusion for 30 min. Data are presented as the mean ±SD (n=10). (C) Comparison between the sham group at day 11 after surgery, and the I/R2 group at day 18 after surgery (* P<0.05). Arrows show the Nissl bodies.
Choline acetyltransferase (ChAT) expression in the hippocampal CA3 region in all groups
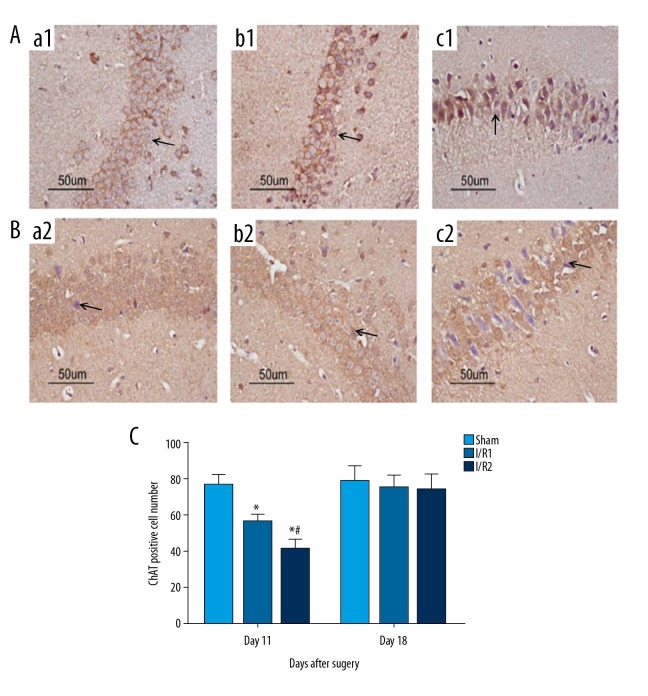
When compared with the sham group at day 11 following surgery, the I/R1 group and the I/R2 group showed significantly reduced levels of choline acetyltransferase (ChAT) protein expression (P<0.05), with lower expression in I/R2 group (Figure 4A, 4C). At day 18 following surgery, ChAT protein expression was significantly increased compared with that at day 11 following surgery (P<0.05) (Figure 4C). However, no significant difference was found in ChAT expression by day 18 following surgery (P>0.05) (Figure 4B, 4C).
Figure 4.
Photomicrographs of the immunohistochemical staining for the expression of choline acetyltransferase (ChAT) in the mouse hippocampal CA3 region in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group. (A) Light microscopy of the immunohistochemical staining for choline acetyltransferase (ChAT) in tissue sections of the mouse hippocampal CA3 region at 11 days postoperatively. Magnification ×100. (B) Light microscopy of the immunohistochemical staining for ChAT in tissue sections of the mouse hippocampal CA3 region at 18 days postoperatively. Magnification ×100. (a1, a2) The sham group, consisting of mice that underwent anesthesia, surgery, and separation of the hepatic artery and vein, but without occlusion. (b1, b2) The I/R1 group, in which the left hepatic artery and portal vein were clamped for 20 min, followed by reperfusion for 30 min. (c1, c2) The I/R2 group, in which the left hepatic artery and portal vein were clamped for 40 min followed by reperfusion for 30 min. Data are presented as the mean ±SD (n=10). (C) Comparison between with sham group at day 11 with day 18 after surgery (* P<0.05). Comparison between the I/R1 group at day 11 after surgery with day 18 after surgery (# P<0.05). Arrows indicate the positively stained cells.
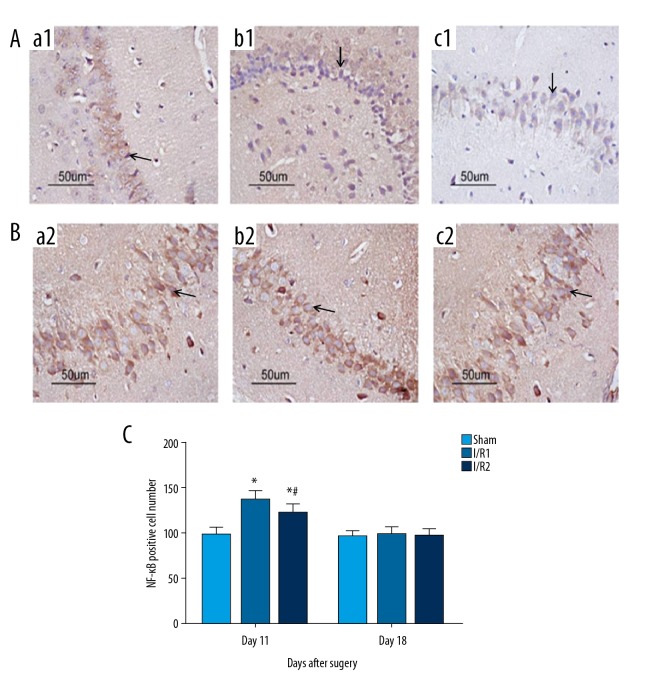
Nuclear factor-κB (NF-κB) protein expression in the hippocampal CA3 region of all groups of mice
When compared with the sham group at day 11 following surgery, the I/R1 and the I/R2 group had significantly increased expression levels of nuclear factor-κB (NF-κB) (P<0.05). Mice in the I/R21 group showed a greater increase in NF-κB expression than the mice in the I/R1 group (Figure 5A, 5C). At day 18 following surgery, NF-κB protein expression was significantly reduced compared with that at day 11 following surgery (P<0.05) (Figure 5C). However, no significant difference was found in NF-κB expression of the hippocampal CA3 region between all groups at 18 days after surgery (P>0.05) (Figure 5B, 5C).
Figure 5.
Photomicrographs of the immunohistochemical staining for the expression of nuclear factor-κB (NF-κB) in the mouse hippocampal CA3 region in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group. (A) Light microscopy of the immunohistochemical staining for nuclear factor-κB (NF-κB) in tissue sections of the mouse hippocampal CA3 region at 11 days postoperatively. Magnification ×100. (B) Light microscopy of the immunohistochemical staining for NF-κB in tissue sections of the mouse hippocampal CA3 region at 18 days postoperatively. Magnification ×100. (a1, a2) The sham group, consisting of mice that underwent anesthesia, surgery, and separation of the hepatic artery and vein, but without occlusion. (b1, b2) The I/R1 group, in which the left hepatic artery and portal vein were clamped for 20 min, followed by reperfusion for 30 min. (c1, c2) The I/R2 group, in which the left hepatic artery and portal vein were clamped for 40 min followed by reperfusion for 30 min. Data are presented as the mean ±SD (n=10). (C) Comparison between with sham group at day 11 with day 18 after surgery (* P<0.05). Comparison between the I/R1 group at day 11 after surgery with day 18 after surgery (# P<0.05). Arrows indicate the positive cells.
Serum levels of the inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), in the three groups of mice
Compared with the sham group at day 11 following surgery, the I/R1 group and the I/R2 group had significantly increased serum levels of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) (P<0.05), with more increased serum levels in the I/R21 group (Figure 6). At day 18 following surgery, TNF-α and IL-1β levels were significantly decreased compared with day 11 following surgery (P<0.05) (Figure 5C). However, no significant difference was found between all three groups at 18 days after surgery (P>0.05) (Figure 6).
Figure 6.
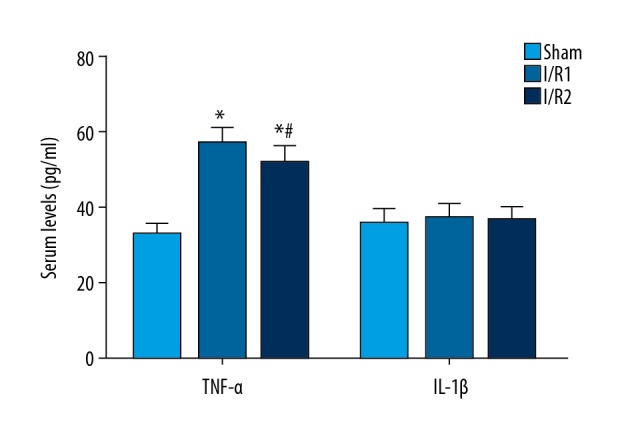
Serum levels of the inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), were determined using an enzyme-linked immunosorbent assay (ELISA) in the mouse model groups with hepatic ischemia-reperfusion injury and the sham group. Serum was isolated from mice in each of the three study groups, followed by measuring the levels of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) using an enzyme-linked immunosorbent assay (ELISA) kit. Data are presented as the mean ±SD (n=10). Comparison of the cytokine levels in the sham group at day 11 after surgery with day 18 after surgery (* P<0.05). Comparison between the I/R1 group at day 11 after surgery with day 18 after surgery (# P<0.05).
Discussion
In a mouse model of hepatic ischemia-reperfusion injury, the left hepatic artery and portal vein can be clamped to generate ischemia in 70% of the liver tissue, making it a popular animal model for studying liver ischemia and reperfusion injury [13]. In this study, adult mice, aged between 2~3 months, were studied in three groups. The three groups of rats studied included the sham group, which underwent surgery without vascular occlusion; the I/R1 group, with occlusion of the left hepatic artery and portal vein for 20 minutes, and reperfusion for 30 minutes; and the I/R2 group, with occlusion of the left hepatic artery and portal vein for 40 minutes, and reperfusion for 30 minutes.
The mouse model of hepatic ischemia-reperfusion injury was used with histology and electron microscopy used to evaluate the cell and tissue changes in the liver. The findings of the present study showed that the sham group had large amounts of mitochondria with round or oval-shaped nuclei, without swelling, and prominent rough endoplasmic reticulum (RER) (Nissl bodies) with a regular arrangement, and an increased number of lysosomes. The mice in the I/R1 group showed mild swelling of mitochondria in the hepatocytes with few defects and large amounts of RER. The mice in the I/R2 group showed mitochondrial swelling, defects, vacuoles, reduced numbers of RER and lysosomes indicating the successful preparation of the hepatic ischemia-reperfusion model.
In the Morris water maze (MWM) task, mice were tested for the presence of a certain type of reference memory [12,14,15] that belongs to declarative or explicit memory [16,17], which is similar to deficits of declarative or explicit memory in clinical patients with cognitive dysfunction [18]. This study used the MWM task in mice to represent the evaluation of cognitive function. The findings of this study showed a significant increase in the latency period in the navigation sessions of the MWM task of mice in the I/R1 and I/R2 groups between days 5–9 following surgery, compared with the sham group (P<0.05). The I/R2 group also had longer latency at 5–10 days after surgery compared with the sham group (P<0.05). No significant difference between the three groups was found at day 11 following surgery (P>0.05). At day 11 following surgery, mice in the I/R2 group had significantly lower crossing times, average speed, central duration, and central time during the assay when compared with the sham group (P<0.05). No significant difference was found in all indices at day 18 after surgery in the assay (P>0.05). These data suggest that hepatic ischemia-reperfusion injury can lead to short-term cognitive dysfunction, which can recover with time, as there was no deficit found in terms of long-term memory in the mice in this study.
The hippocampus is closely associated with neurophysiological functions, including learning and memory. Structural changes in the hippocampus can reflect cognitive functional status in patients, including in postoperative cognitive dysfunction (POCD). A previously published study found that stress-induced inflammation could induce neuroinflammation resulting in cognitive dysfunction associated with abnormal hippocampal long-term potentiation (LTP) and abnormal electrophysiological activities associated with learning and memory [19,20]. Previous studies have shown a close relationship between abnormalities in the hippocampus and the occurrence of cognitive dysfunction [21]. In this study, the morphology of hippocampal neurons was studied in the mouse model of hepatic ischemia-reperfusion injury. Light microscopy showed that mice in the I/R2 group showed decreased numbers of Nissl bodies in the hippocampal neurons at 11 days after surgery, and both the sham and I/R1 groups showed a regular and dense distribution of hippocampal neurons, with clear and sharp Nissl bodies, clear cytoplasm, oval or round nuclei, evenly distributed chromatin, and no significant structural abnormalities. No significant morphological abnormalities were found in hippocampal neurons at day 18 following surgery. These results showed that hepatic ischemia-reperfusion injury induced short-term cognitive dysfunction that might be associated with structural injury of the hippocampus, and that these changes might be reversible.
Acetylcholine (Ach) is a regulatory neurotransmitter that is closely associated with memory and learning. Choline acetyltransferase (ChAT) is the rate-limiting enzyme of Ach biosynthesis and its activity represents the level of Ach synthesis. Lu et al. found relatively higher ChAT activity and choline intake in rats during the MWM task [22]. Zhang et al. performed anesthesia and surgery on rats and found that memory deficit was associated with defects in the central cholinergic system [23]. Therefore, in this study, the expression of ChAT in the mouse hippocampus was studied to determine whether its expression could reflect the effect of hepatic ischemia-reperfusion injury on cognitive function.
Nuclear factor-κB (NF-κB) plays crucial roles in hepatic ischemia-reperfusion injury [24]. Matsui et al. showed that NF-κB activation in rat hepatic ischemia-reperfusion injury was closely associated with reperfusion injury [25]. The findings of this study showed that NF-κB inhibition effectively alleviated hepatic ischemia-reperfusion injury. NF-κB has previously been shown to induce the expression of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and can directly damage hepatocytes, and participates in neurodevelopment and brain injury repair, which affect cognitive function [26]. Therefore, as this study showed, it is possible that hepatic ischemia-reperfusion injury can induce short-term cognitive dysfunction, associated with expression of inflammatory factors induced by NF-κB in the brain tissue. In this study, mice presented significant cognitive impairment at 11 days after surgery for partial hepatic ischemia-reperfusion injury, and both NF-κB and ChAT showed delayed expression at day 11 following surgery in the hippocampal CA3 region, consistent with the behavioral phenotypes of mice at day 6 and day 7 following surgery. These findings were most likely related to the delayed expression of NF-κB and ChAT in mouse hippocampal tissues. Currently, few studies have been undertaken on cognitive dysfunction caused by hepatic ischemia-reperfusion injury. The findings of this study showed that the upregulation of NF-κB in hepatic ischemia-reperfusion injury induced short-term cognitive dysfunction. However, the key inflammatory mediators downstream of NF-κB activation that are involved in inducing cognitive dysfunction following surgery remain unclear and require further investigation.
Conclusions
The findings of this study showed that in a mouse model of hepatic ischemia-reperfusion injury, short-term cognitive dysfunction occurred in a time-dependent manner, which also recovered in a time-dependent manner. The study findings showed that the mechanism for postoperative cognitive dysfunction (POCD) in this model might be associated with the effects of nuclear factor-κB (NF-κB) on the induction of expression of the inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), in the hippocampal region of the brain. Further studies are required to determine the long-term effects of hepatic ischemia-reperfusion injury on learning or memory deficit using this animal model.
Footnotes
Source of support: This study was supported by the Anhui Public Welfare Technology Application Research Linkage Project in 2017 (No. 1704f0804021: Prevention of Postoperative Cognitive Dysfunction in Elderly Patients by Multimodal Anesthetic Management)
Conflict of interest
None.
References
- 1.Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127(2):496–505. doi: 10.1213/ANE.0000000000003514. [DOI] [PubMed] [Google Scholar]
- 2.Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int. 2017;114(7):110–17. doi: 10.3238/arztebl.2017.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umholtz M, Nader ND. Anesthetic immunomodulation of the neuroinflammation in postoperative cognitive dysfunction. Immunol Invest. 2017;46(8):805–15. doi: 10.1080/08820139.2017.1373898. [DOI] [PubMed] [Google Scholar]
- 4.Qiao Y, Feng H, Zhao T, et al. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: The influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. doi: 10.1186/s12871-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Martensson J, Gogenur I, Asghar MS. Exploring postoperative cognitive dysfunction and delirium in noncardiac surgery using MRI: A systematic review. Neural Plast. 2018;2018 doi: 10.1155/2018/1281657. 1281657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Yang Y, Feng Y, et al. A review of melatonin in hepatic ischemia-reperfusion injury and clinical liver disease. Ann Med. 2014;46(7):503–11. doi: 10.3109/07853890.2014.934275. [DOI] [PubMed] [Google Scholar]
- 7.Saidi RF, Kenari SK. Liver ischemia-reperfusion injury: An overview. J Invest Surg. 2014;27(6):366–79. doi: 10.3109/08941939.2014.932473. [DOI] [PubMed] [Google Scholar]
- 8.Gendy AM, Abdallah DM, El-Abhar HS. The potential curative effect of rebamipide in hepatic ischemia-reperfusion injury. Naunyn Schmiedebergs Arch Pharmacol. 2017;390(7):691–700. doi: 10.1007/s00210-017-1370-7. [DOI] [PubMed] [Google Scholar]
- 9.Pehrson AL, Hillhouse TM, Haddjeri N, et al. Task- and treatment length-dependent effects of vortioxetine on scopolamine-induced cognitive dysfunction and hippocampal extracellular acetylcholine in rats. J Pharmacol Exp Ther. 2016;358(3):472–82. doi: 10.1124/jpet.116.233924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabri O, Meyer PM, Graf S, et al. Cognitive correlates of α4β2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain. 2018;141(6):1840–54. doi: 10.1093/brain/awy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones C. alpha7 Nicotinic acetylcholine receptor: A potential target in treating cognitive decline in schizophrenia. J Clin Psychopharmacol. 2018;38(3):247–49. doi: 10.1097/JCP.0000000000000859. [DOI] [PubMed] [Google Scholar]
- 12.Dam K, Fuchtemeier M, Farr TD, et al. Increased homocysteine levels impair reference memory and reduce cortical levels of acetylcholine in a mouse model of vascular cognitive impairment. Behav Brain Res. 2017;321:201–8. doi: 10.1016/j.bbr.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Lei X, Li W, et al. TNIP1 alleviates hepatic ischemia/reperfusion injury via the TLR2-Myd88 pathway. Biochem Biophys Res Commun. 2018;501(1):186–92. doi: 10.1016/j.bbrc.2018.04.209. [DOI] [PubMed] [Google Scholar]
- 14.Brombacher TM, De Gouveia KS, Cruywagen L, et al. Nippostrongylus brasiliensis infection leads to impaired reference memory and myeloid cell interference. Sci Rep. 2018;8(1):2958. doi: 10.1038/s41598-018-20770-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au JL, Weishaupt N, Nell HJ, et al. Motor and hippocampal dependent spatial learning and reference memory assessment in a transgenic rat model of Alzheimer’s disease with stroke. J Vis Exp. 2016;(109) doi: 10.3791/53089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson AJ, Dygacz A, Miles C. Hebb repetition effects for non-verbal visual sequences: Determinants of sequence acquisition. Memory. 2017;25(9):1279–93. doi: 10.1080/09658211.2017.1293692. [DOI] [PubMed] [Google Scholar]
- 17.Davis SW, Wing EA, Cabeza R. Contributions of the ventral parietal cortex to declarative memory. Handb Clin Neurol. 2018;151:525–53. doi: 10.1016/B978-0-444-63622-5.00027-9. [DOI] [PubMed] [Google Scholar]
- 18.Sapkota S, Wiebe SA, Small BJ, Dixon RA. Apolipoprotein E and Clusterin can magnify effects of personality vulnerability on declarative memory performance in non-demented older adults. Int J Geriatr Psychiatry. 2016;31(5):502–9. doi: 10.1002/gps.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titus DJ, Wilson NM, Freund JE, et al. Chronic cognitive dysfunction after traumatic brain injury is improved with a phosphodiesterase 4B inhibitor. J Neurosci. 2016;36(27):7095–108. doi: 10.1523/JNEUROSCI.3212-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Yan F, Feng J, et al. Dexmedetomidine inhibits inflammatory reaction in the hippocampus of septic rats by suppressing NF-kappaB pathway. PLoS One. 2018;13(5):e0196897. doi: 10.1371/journal.pone.0196897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Yuan L, Zhang L, et al. Prophylactic use of troxerutin can delay the development of diabetic cognitive dysfunction and improve the expression of Nrf2 in the hippocampus on STZ diabetic rats. Behav Neurol. 2018;2018 doi: 10.1155/2018/8678539. 8678539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu L, Li Z, Zuo Y, et al. Radioprotective activity of glutathione on cognitive ability in X-ray radiated tumor-bearing mice. Neurol Res. 2018;40(9):758–66. doi: 10.1080/01616412.2018.1476080. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Jiang X, Huang L, et al. Central cholinergic system mediates working memory deficit induced by anesthesia/surgery in adult mice. Brain Behav. 2018;8(5):e00957. doi: 10.1002/brb3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao XQ, Liang B, Liu Y, Huang XQ. Agaricoglycerides protect against hepatic ischemia/reperfusion injury by attenuating inflammatory response, oxidative stress, and expression of NF-kappaB. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/142736. 142736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui N, Kasajima K, Hada M, et al. Inhibiton of NF-kappaB activation during ischemia reduces hepatic ischemia/reperfusion injury in rats. J Toxicol Sci. 2005;30(2):103–10. doi: 10.2131/jts.30.103. [DOI] [PubMed] [Google Scholar]
- 26.Wei P, Zheng Q, Liu H, et al. Nicotine-induced neuroprotection against cognitive dysfunction after partial hepatectomy involves activation of BDNF/TrkB signaling pathway and inhibition of NF-kappaB signaling pathway in aged rats. Nicotine Tob Res. 2018;20(4):515–22. doi: 10.1093/ntr/ntx157. [DOI] [PubMed] [Google Scholar]