Abstract
Introduction
Possible joint effects of subjective cognitive decline (SCD) and apolipoprotein E (APOE) ε4 genotype on incident mild cognitive impairment (MCI) were examined for men and women separately.
Methods
Cognitively normal participants with and without SCD were included from the first follow-up examination of the population-based Heinz Nixdorf Recall study. Sex-stratified logistic regression models estimated main effects and interactions (additive, multiplicative) of SCD at the first follow-up (yes+/no−) and APOE ε4 (positive+/negative−) groups for MCI 5 years later.
Results
Odds for MCI 5 years later were higher in SCD/APOE ε4 group +/+ than the sum of groups +/− and −/+ in women, with a trend for positive interaction. Odds for incident MCI in men was highest in group +/−, with no interaction effect.
Discussion
Our findings indicate that APOE ε4 may play an important role in the association of SCD and incident MCI, especially considering sex. Further studies need to examine these associations with larger sample sizes.
Keywords: APOE, mild cognitive impairment, Population-based, sex, subjective cognitive decline
1. Introduction
Identification of populations with elevated risk of developing dementia for early prevention strategies at an asymptomatic stage is of high public health relevance. The apolipoprotein E (APOE) ε4 allele is not only the main genetic risk factor for Alzheimer's disease (AD) but also for earlier stages such as mild cognitive impairment (MCI) [1], [2]. There is evidence pointing toward the APOE ε4 genotype influencing cognition even in a pre-MCI state. Subjective cognitive decline (SCD) is proposed to be such a preclinical stage defined by self-perceived cognitive worsening, while cognitive performance is not impaired. Some studies report SCD as a risk factor for memory decline, MCI, and AD [3], [4], whereas other studies report no association with cognitive decline [5]. It is well known that sex plays an important role in AD research. Studies show higher MCI and AD prevalence in women [6], [7]. Sex-specific differences are known to occur in the course of dementia [1], [8] and even male and female brains show structural differences altering dementia-related processes [9]. Nevertheless, sex-stratified analyses in AD research and especially SCD are rare and often report heterogeneous results. For sex and APOE ε4 genotype, some studies indicate a sex-dependent APOE ε4 risk for AD [10], whereas other studies report no interaction with sex [11]. For SCD and APOE ε4 genotype, one study reported positive joint effects leading to accelerated cognitive decline [12]. There are no studies examining this association regarding the risk of MCI, especially not considering sex. Therefore, we examined the joint effects of SCD and APOE ε4 genotype for the risk of incident MCI in men and women separately. We sought to characterize our analysis sample and the general cognitive performance for SCD investigation. Then, we examined the sex-stratified associations of SCD and APOE ε4 genotype groups with incident MCI after 5 years. For a comprehensive understanding, we examined the association of SCD and incident MCI in APOE ε4 carriers and noncarriers. Possible interaction effect of SCD and APOE ε4 genotype on incident MCI was examined. With the insights from these analyses, we aim to contribute to a better specification and identification of potential at risk populations that are suitable for early intervention.
2. Methods
2.1. Study population
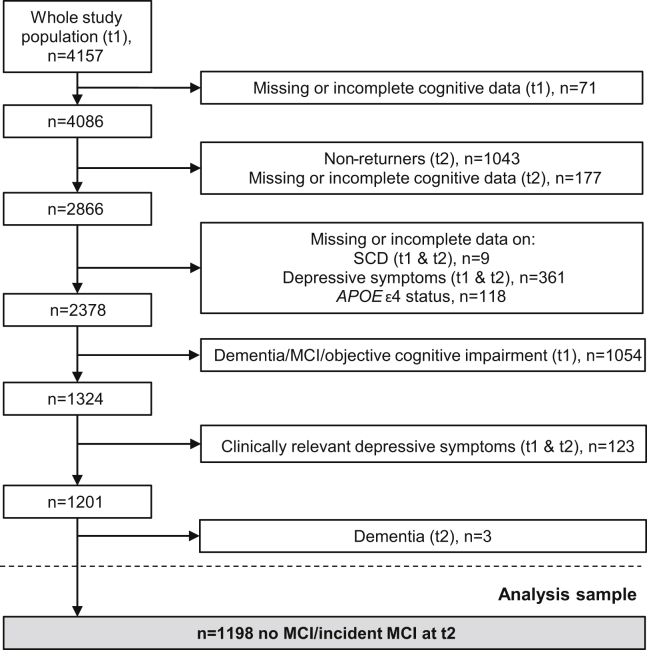
In the Heinz Nixdorf Recall (Risk Factors, Evaluation of Coronary Calcification, and Lifestyle) study, participants were randomly sampled in three cities in Germany. The study design has previously been described [13], [14]. Briefly, 4814 participants aged 45 to 75 years were enrolled between 2000 and 2003 (t0, baseline). Participants were not examined regarding their cognitive performance at t0. Participants were invited for follow-up examinations every 5 years (t1, n = 4157, 2005–2008; t2, n = 3087, 2010–2015). A standardized cognitive performance assessment was not conducted at t0 and was first introduced at t1, the first follow-up examination. The cognitive assessment was extended for the second follow-up examination, t2. The analysis sample was selected as shown in Fig. 1. The following definitions were used for exclusion of participants based on cognitive impairment, clinically relevant depressive symptoms, and dementia. Cognitive impairment at t1 was defined as a performance of one standard deviation (SD) below the age- and education-adjusted mean except for the clock-drawing test, where a performance ≥3 was rated as impaired (for a detailed description, see the study by Winkler et al. [15]). A Center for Epidemiologic Studies Depression scale score of ≥18 was defined as clinically relevant depressive symptoms (CES-D, see 2.5 [16]). Dementia diagnosis was defined as a previous physician's diagnosis of dementia, meeting the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [17]) dementia diagnosis criteria or taking cholinesterase inhibitors (anatomic-therapeutic-chemical classification issued by the World Health Organization [8], code: N06DA) or other antidementia drugs (N06DX). All participants provided written informed consent. The study was approved by the University of Duisburg-Essen Institutional Review Board and followed established guidelines of good epidemiological practice.
Fig. 1.
A sample flowchart for the present study. Abbreviations: APOE, apolipoprotein E; MCI, mild cognitive impairment; SCD, subjective cognitive decline; t1, first follow-up examination; t2, second follow-up examination.
2.2. Cognitive assessment procedures
The extended cognitive performance assessment consisted of eight subtests: (1) immediate and (2) delayed word list, (3) Labyrinth test, (4) verbal fluency “animals”, (5) clock-drawing test, (6,7) Trail Making Test A and B, (8) Color-Word test (card 1, card 2, difference card 3–card 2). For a detailed description of tests (1) to (5), see the study by Wege et al. [18] and for the extended cognitive assessment (6) to (8), see the study by Tebrügge et al. [19]. For the five subtests that have already been used at t1, we performed z-transformation of the raw data at t2 using our own defined norm-data from t1: raw data were z-transformed based on the mean and SD of the appropriate age and education group at t1 (age: 50–59 years, 60–69 years, and ≥70 years; education: ≤10 years, 11–13 years, and ≥14 years). For the subtests of the extended cognitive performance assessment, z-transformation was based on the same education groups from t1 and the following three age groups from t2: 55–64 years, 65–74 years, and ≥75 years. Except for the clock-drawing test, the age- and education-adjusted test scores were scaled to have a mean of 10 and an SD of 3 [20]. Tests were grouped into four domains: (1) attention—Trail Making Test A, Color-Word test card 1 and card 2; (2) executive function—Trail Making Test B, Labyrinth test, Color-Word test interference performance, verbal fluency; (3) verbal memory—eight-word list immediate and delayed recall; (4) visuoconstruction—clock-drawing test. Within each domain, newly scaled scores of the tests were added. To account for the differing numbers of tests in each domain, domain scores were then scaled to have a mean of 10 and a SD of 3. Cognitive impairment was defined as a performance of more than one SD below the mean (≤7) in at least one total domain score of the domains attention, executive function, verbal memory, or as a score of ≥3 in visuoconstruction [21].
2.3. Definition of SCD and MCI
Subjective cognitive decline was assessed at t1 with the question: “In comparison to two years ago, would you rate your memory function as better, same or worse?” Subjective cognitive decline was defined as present if the participant's answer was “worse”. Participants responding “better” or “same” were defined as not having SCD.
The outcome variable was MCI assessed at t2. The MCI diagnosis was based on meeting all of the following published MCI criteria [22]: (1) cognitive impairment in at least one of the above reported four domains; (2) subjective cognitive decline; (3) normal functional abilities and daily activities; (4) no dementia diagnosis. To examine incident MCI, participants with MCI at t1 were excluded as reported previously (Fig. 1). Participants at t2 not meeting MCI criteria as described above were categorized as “no MCI”.
2.4. Apolipoprotein E
Cardio-MetaboChip BeadArrays were used for genotyping of two single-nucleotide polymorphisms (rs7412 and rs429358) to discriminate between the APOE alleles ε2, ε3, and ε4. Participants defined as APOE ε4–positive had at least one allele 4 (2/4, 3/4, 4/4). All other participants were defined as APOE ε4–negative.
2.5. Assessment of covariates
Information about participants' socioeconomic status was collected by computer-assisted interviews. Education was defined according to the International Standard Classification of Education based on total years of formal education, combining school, and vocational training [23]. The four education categories were ≤10 years, 11–13 years, 14–17 years, and ≥18 years. Depressive symptoms were measured using the 15-item short assessment form of the German version of the CES-D [16], [24].
2.6. Statistical analysis
All analyses were conducted separately for men and women. Participants with and without SCD at t1 were compared regarding sociodemographic characteristics and performance on cognitive tests at t2 using Mann-Whitney U tests or Pearson's chi square tests, as appropriate.
For logistic regression analyses, we defined the following four groups to examine possible joint effects of SCD at t1 and APOE ε4 genotype on MCI 5 years later at t2: participants without SCD and APOE ε4–negative genotype (group A, reference group), participants with SCD only (group B, single-risk), participants with APOE ε4–positive genotype only (group C, single-risk) and participants with SCD and APOE ε4–positive genotype (group D, high-risk). We performed binomial logistic regression models to estimate odds ratios (ORs) and their 95% confidence interval (CI) for all groups and the risk of MCI 5 years later (unadjusted and adjusted for age [t2], education and score on CES-D [t2]). We checked for possible additive effects comparing the OR of the high-risk group to the added ORs of both single-risk groups. This was further examined calculating the relative excess risk due to interaction (RERI) using the formula:
The 95% CIs for RERI were obtained using the delta method, with RERI = 0 as reference and positive and negative scores indicating a positive or negative interaction, respectively [25].
Logistic regression models were calculated to examine the association of SCD at t1 and MCI 5 years later at t2 in APOE ε4 genotype groups (unadjusted, adjusted for age [t2], education, and score on CES-D [t2]). Odds ratios for incident MCI in APOE ε4–positive participants with SCD were compared with ORs in APOE ε4–negative participants with SCD. This was further investigated calculating the measure of interaction on a multiplicative scale based on the following logistic regression model:
p/(1−p) were the odds of the outcome and β3 was the regression coefficient of the modification effect on a multiplicative scale [26]. A score of 1 describes no interaction, a score smaller than 1 indicates a negative interaction and a score of more than 1 indicates a positive interaction.
Post hoc power analyses were performed to identify the minimum sample size per group that was needed to reach a statistically significant result with a power of 1 − β = 0.8. Level of significance was set a priori as α = 0.05. Analyses were conducted using IBM SPSS Statistics 25.0 and R Statistical Software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Table 1 shows the sociodemographic characteristics of participants without and with SCD stratified for sex and APOE ε4 status. Male APOE ε4–positive participants without SCD did not differ in any characteristic compared with male APOE ε4–positive participants with SCD. APOE ε4–negative men without SCD showed lower scores on the CES-D for both examinations and a lower percentage of incident MCI cases compared with APOE ε4–negative men with SCD. Female APOE ε4–positive participants without SCD were significantly younger, had a lower percentage of incident MCI cases and showed lower scores on the CES-D for both examinations compared with female APOE ε4–positive participants with SCD. APOE ε4–negative women without SCD showed lower scores on the CES-D for both examinations in comparison with APOE ε4–negative women with SCD.
Table 1.
Sex-stratified sociodemographic characteristics in total and for APOE ɛ4–positive and –negative SCD−/SCD+ participants
| Sociodemographic characteristics |
Total |
APOE ɛ4–positive∗ |
P value† |
APOE ɛ4–negative |
P value† | ||
|---|---|---|---|---|---|---|---|
| SCD− |
SCD+ |
SCD− |
SCD+ |
||||
| Men | n = 605 | n = 117 | n = 34 | n = 361 | n = 93 | ||
| Age (Years), t2 | 68.15 ± 6.9 | 67.26 ± 6.9 | 68.56 ± 6.7 | .28 | 68.10 ± 6.9 | 69.33 ± 7.1 | .14 |
| Education‡ | |||||||
| ≤ 10 Years | 22 (4) | 2 (2) | 3 (9) | .11 | 12 (3) | 5 (5) | .77 |
| 11–13 Years | 243 (40) | 49 (42) | 9 (27) | 146 (40) | 39 (42) | ||
| 14–17 Years | 210 (35) | 38 (33) | 12 (35) | 128 (36) | 32 (34) | ||
| ≥ 18 Years | 130 (22) | 28 (24) | 10 (29) | 75 (21) | 17 (18) | ||
| Score On Depression Scale (CES-D)§, t1 | 5.27 ± 3.7 | 5.00 ± 3.7 | 6.50 ± 4.5 | .08 | 4.86 ± 3.5 | 6.75 ± 4.0 | <.001 |
| Score On Depression Scale (CES-D)§, t2 | 5.08 ± 4.1 | 5.22 ± 4.1 | 6.00 ± 4.6 | .43 | 4.65 ± 3.8 | 6.25 ± 4.5 | .002 |
| Incident MCI, t2 |
55 (9) |
10 (9) |
5 (15) |
.29 |
23 (6) |
17 (18) |
<.001 |
| Women |
n = 593 |
n = 108 |
n = 49 |
n = 320 |
n = 116 |
||
| Age (Years), t2 | 67.45 ± 6.8 | 66.89 ± 7.5 | 69.45 ± 6.8 | .028 | 67.43 ± 6.6 | 67.18 ± 6.7 | .69 |
| Education‡ | |||||||
| ≤ 10 Years | 43 (7) | 7 (7) | 6 (12) | .38 | 24 (8) | 6 (5) | .38 |
| 11–13 Years | 390 (66) | 66 (61) | 29 (59) | 221 (69) | 74 (64) | ||
| 14–17 Years | 81 (14) | 22 (20) | 6 (12) | 35 (11) | 18 (16) | ||
| ≥ 18 Years | 79 (13) | 13 (12) | 8 (16) | 40 (13) | 18 (16) | ||
| Score On Depression Scale (CES-D)§, t1 | 6.09 ± 4.2 | 5.56 ± 4.1 | 6.92 ± 4.4 | .045 | 5.82 ± 4.2 | 6.97 ± 4.3 | .012 |
| Score On Depression Scale (CES-D)§, t2 | 5.99 ± 4.2 | 5.57 ± 3.9 | 7.61 ± 4.8 | .015 | 5.56 ± 4.1 | 6.91 ± 4.3 | .002 |
| Incident MCI, t2 | 43 (7) | 7 (7) | 10 (20) | .011 | 16 (5) | 10 (9) | .17 |
Abbreviations: APOE, apolipoprotein E; CES-D, Center for Epidemiologic Studies Depression Scale; MCI, mild cognitive impairment; SCD (+/−), with and without subjective cognitive decline; t1, first follow-up examination; t2, second follow-up examination.
NOTE. Data are presented as means (±standard deviation) or numbers (%) unless otherwise indicated.
APOE ɛ4–positive = at least one 4 allele (2/4, 3/4, 4/4).
Comparisons between SCD groups calculated using Mann-Whitney-U test and Pearson's chi square test, as appropriate.
Owing to rounding, percentages do not always total 100.
Participants with a score of ≥18 were excluded.
Table 2 shows the cognitive performance of participants without and with SCD stratified for sex and APOE ε4 status. Male APOE ε4–positive participants without SCD did not differ from male APOE ε4–positive participants with SCD in any cognitive test performed. APOE ε4–negative men without SCD performed significantly better than APOE ε4–negative men with SCD in verbal fluency. Female APOE ε4–positive participants without SCD did not differ from APOE ε4–positive female participants with SCD in any cognitive test performed. APOE ε4–negative women without SCD performed significantly better than APOE ε4–negative women with SCD in 8-word list (delayed) and Trail Making Test B.
Table 2.
Sex-stratified scores of cognitive performance at t2 in total and for APOE ε4–positive and –negative SCD−/SCD+ participants
| Cognitive domains |
Total |
APOE ε4–positive∗ |
P value† |
APOE ε4–negative |
P value† | ||
|---|---|---|---|---|---|---|---|
| SCD− |
SCD+ |
SCD− |
SCD+ |
||||
| Men | n = 605 | n = 117 | n = 34 | n = 361 | n = 93 | ||
| Verbal memory‡ | |||||||
| Immediate recall | 5.58 ± 1.1 | 5.60 ± 1.2 | 5.35 ± 1.1 | 0.29 | 5.58 ± 1.1 | 5.60 ± 1.1 | 0.82 |
| Delayed recall | 3.93 ± 1.8 | 3.85 ± 1.8 | 3.76 ± 1.7 | 0.70 | 3.99 ± 1.7 | 3.83 ± 1.8 | 0.38 |
| Executive function | |||||||
| Problem solving/speed of processing§ | 44.89 ± 20.1 | 45.22 ± 18.5 | 47.44 ± 15.8 | 0.23 | 44.18 ± 21.2 | 46.31 ± 19.1 | 0.09 |
| Verbal fluency¶ | 24.82 ± 6.3 | 25.55 ± 6.4 | 24.00 ± 7.1 | 0.21 | 25.09 ± 6.3 | 23.17 ± 5.2 | 0.009 |
| Speed of processing/visual search/mental flexibility# | 108.66 ± 56.2 | 104.76 ± 48.6 | 117.18 ± 66.9 | 0.52 | 107.62 ± 56.5 | 114.47 ± 59.7 | 0.25 |
| Selective attention/interference performance∗∗ | 24.76 ± 16.4 | 25.92 ± 20.9 | 25.24 ± 10.7 | 0.43 | 24.13 ± 15.6 | 25.55 ± 14.7 | 0.29 |
| Visuoconstruction | |||||||
| Impaired visual spatial organization†† | 70 (12) | 10 (9) | 5 (15) | 0.29 | 39 (11) | 16 (17) | 0.09 |
| Attention | |||||||
| Speed of processing‡‡ | 40.35 ± 16.2 | 38.13 ± 12.6 | 38.91 ± 13.0 | 0.79 | 40.86 ± 17.9 | 41.68 ± 13.8 | 0.19 |
| Color word reading§§ | 14.89 ± 2.8 | 14.50 ± 2.66 | 15.24 ± 2.83 | 0.17 | 14.93 ± 2.9 | 15.12 ± 2.9 | 0.52 |
| Color naming¶¶ |
22.84 ± 4.7 |
22.55 ± 4.51 |
23.32 ± 3.96 |
0.24 |
22.74 ± 4.4 |
23.44 ± 5.8 |
0.88 |
| Women |
n = 593 |
n = 108 |
n = 49 |
n = 320 |
n = 116 |
||
| Verbal memory‡ | |||||||
| Immediate recall | 5.76 ± 1.2 | 5.82 ± 1.2 | 5.78 ± 1.1 | 0.98 | 5.68 ± 1.2 | 5.92 ± 1.2 | 0.06 |
| Delayed recall | 4.33 ± 1.7 | 4.45 ± 2.0 | 4.35 ± 1.7 | 0.68 | 4.20 ± 1.7 | 4.58 ± 1.7 | 0.035 |
| Executive function | |||||||
| Problem solving/speed of processing§ | 48.80 ± 27.1 | 48.66 ± 27.3 | 45.20 ± 20.5 | 0.91 | 50.03 ± 28.7 | 47.07 ± 24.7 | 0.54 |
| Verbal fluency¶ | 25.04 ± 5.8 | 25.00 ± 5.8 | 24.65 ± 7.5 | 0.93 | 25.26 ± 5.6 | 24.64 ± 5.5 | 0.45 |
| Speed of processing/visual search/mental flexibility# | 106.30 ± 54.8 | 102.19 ± 51.3 | 124.43 ± 70.4 | 0.06 | 109.18 ± 56.8 | 94.53 ± 41.1 | 0.017 |
| Selective attention/interference performance∗∗ | 22.89 ± 13.0 | 21.83 ± 10.0 | 22.49 ± 9.5 | 0.73 | 23.39 ± 13.8 | 22.64 ± 14.3 | 0.23 |
| Visuoconstruction | |||||||
| Impaired visual spatial organization†† | 93 (16) | 12 (11) | 10 (20) | 0.12 | 55 (17) | 16 (14) | 0.40 |
| Attention | |||||||
| Speed of processing‡‡ | 39.25 ± 15.3 | 36.82 ± 15.0 | 39.59 ± 12.4 | 0.06 | 40.31 ± 16.7 | 38.44 ± 12.5 | 0.63 |
| Color word reading§§ | 14.78 ± 2.9 | 14.70 ± 2.18 | 15.59 ± 4.2 | 0.46 | 14.65 ± 3.0 | 14.84 ± 2.5 | 0.16 |
| Color naming¶¶ | 21.64 ± 4.0 | 21.78 ± 3.91 | 21.04 ± 3.2 | 0.22 | 21.56 ± 4.08 | 21.99 ± 4.0 | 0.27 |
Abbreviations: APOE, apolipoprotein E; MCI, mild cognitive impairment; SCD (+/−), with and without subjective cognitive decline; t2, second follow-up examination.
NOTE. Data are presented as means (±standard deviation) or as numbers (%) of participants unless otherwise indicated.
APOE ε4–positive = at least one 4 allele (2/4, 3/4, 4/4).
Comparisons between SCD groups calculated using Mann-Whitney-U test and Pearson's chi square test, as appropriate.
8-word list (immediate recall and delayed recall, range: 0–8, higher scores indicating better performance).
Labyrinth test (range: 14–180 s, higher scores indicating lower performance).
Semantic category “Animals” (range: 0–58, higher scores indicating better performance).
Trail Making Test B (range: 21–300 s, higher scores indicating lower performance).
Color-Word test interference (difference card 3–card 2; range: 2–263 s, higher scores indicating lower performance).
Clock-drawing test (impaired performance with a cutoff point: score ≥3 [range: 1–5, with higher scores indicating lower performance]).
Trail Making Test A (range: 14–130 s, higher scores indicating lower performance).
Color-Word test card 1 (range: 9–39 s, higher scores indicating lower performance).
Color-Word test card 2 (range: 10–40 s, higher scores indicating lower performance).
Results of binary logistic regression analyses for men are shown in Table 3. The regression model calculating ORs for incident MCI for groups B, C, and D with group A as reference group showed the highest OR for group B. The high-risk group D showed a lower OR. For group C, we observed the lowest OR. This was present in unadjusted as well as adjusted models. Odds ratio for incident MCI in the high-risk group was almost half of the sum of single-risk group ORs. No additive effects of SCD and APOE ε4–positive genotype were observed, although RERI scores showed an insignificant negative tendency with broad CIs. APOE ε4–negative men with SCD showed almost 2-fold higher ORs compared with APOE ε4–positive men with SCD (see right column, Table 3). The multiplicative scale showed the same tendency for the adjusted model.
Table 3.
Risk of incident MCI for SCD and APOE ε4 genotype groups in men
| Risk of incident MCI | SCD− |
SCD+ |
OR (95% CI) for SCD+ within APOE ε4 genotype | ||
|---|---|---|---|---|---|
| Number of no MCI/MCI | OR (95% CI) | Number of no MCI/MCI | OR (95% CI) | ||
| Unadjusted | |||||
| APOE ε4–negative | 338/23 | 1 (reference) | 76/17 | 3.29 (1.66–6.43) | 3.29 (1.66–6.43) |
| group A | group B | P < .001 | P < .001 | ||
| APOE ε4–positive∗ | 107/10 | 1.37 (0.61–2.90) | 29/5 | 2.53 (0.81–6.70) | 1.84 (0.54–5.63) |
| group C | P = .42 | group D | P = .08 | P = .30 | |
| Effect modification: RERI (95% CI) = −1.13 (−4.35 to 2.09), P = .49; multiplicative scale: ratio of ORs (95% CI) = 0.56 (0.14–2.08), P = .40. | |||||
| Adjusted | |||||
| APOE ε4–negative | 338/23 | 1 (reference) | 76/17 | 2.90 (1.43–5.81) | 2.90 (1.43–5.81) |
| group A | group B | P = .003 | P = .003 | ||
| APOE ε4–positive∗ | 107/10 | 1.43 (0.62–3.08) | 29/5 | 2.41 (0.75–6.57) | 1.69 (0.48–5.28) |
| group C | P = .38 | group D | P = .11 | P = .38 | |
| Effect modification: RERI (95% CI) = −0.92 (−4.01 to 2.16), P = .56; multiplicative scale: ratio of ORs (95% CI) = 0.58 (0.14–2.22), P = .43. ORs are adjusted for age, education and depressive symptoms at t2. | |||||
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; MCI, mild cognitive impairment at t2; OR, odds ratio; RERI, relative excess risk due to interaction; SCD (+/−), with and without subjective cognitive decline; t2, second follow-up examination.
NOTE. Risk estimates were calculated by binomial logistic regression analyses and measures of effect modification were assessed for men.
APOE ε4–positive = at least one 4 allele (2/4, 3/4, 4/4).
Results of binary logistic regression analyses for women are shown in Table 4. The regression model calculating the ORs for incident MCI for groups B, C, and D with group A as reference group showed the highest OR for the high-risk group D. For group C, we observed the lowest OR. This was present in unadjusted as well as adjusted models. The OR for incident MCI in the high-risk group was higher than the added ORs of the single-risk groups. This additive association was also represented by positive RERI scores showing a trend for a positive interaction of SCD and APOE ε4 genotype with broad CIs. This was present for the unadjusted as well as adjusted models. APOE ε4–positive women with SCD showed an almost 2-fold higher OR compared with APOE ε4–negative women with SCD (see right column, Table 4). The multiplicative scale showed the same tendency. Although we detected possible additive and multiplicative positive interaction, none of the scores reached statistical significance.
Table 4.
Risk of incident MCI for SCD and APOE ε4 genotype groups in women
| Risk of incident MCI | SCD− |
SCD+ |
OR (95% CI) for SCD+ within APOE ε4 genotype | ||
|---|---|---|---|---|---|
| Number of no MCI/MCI | OR (95% CI) | Number of no MCI/MCI | OR (95% CI) | ||
| Unadjusted | |||||
| APOE ε4–negative | 304/16 | 1 (reference) | 106/10 | 1.79 (0.76–4.02) | 1.79 (0.76–4.02) |
| group A | group B | P = .16 | P = .16 | ||
| APOE ε4–positive∗ | 101/7 | 1.32 (0.49–3.18) | 39/10 | 4.87 (2.01–11.37) | 3.70 (1.33–10.85) |
| group C | P = .56 | group D | P < .001 | P = .013 | |
| Effect modification: RERI (95% CI) = 2.76 (−1.06 to 6.59), P = .16; multiplicative scale: ratio of ORs (95% CI) = 2.06 (0.56–8.00), P = .28. | |||||
| Adjusted | |||||
| APOE ε4–negative | 304/16 | 1 (reference) | 106/10 | 1.55 (0.65–3.54) | 1.55 (0.65–3.54) |
| group A | group B | P = .31 | P = .31 | ||
| APOE ε4–positive∗ | 101/7 | 1.31 (0.49–3.21) | 39/10 | 3.70 (1.48–8.90) | 2.82 (0.98–8.50) |
| group C | P = .57 | group D | P = .004 | P = .06 | |
| Effect modification: RERI (95% CI) = 1.84 (−1.23 to 4.92), P = .24; multiplicative scale: ratio of ORs (95% CI) = 1.82 (0.48–7.25), P = .38. ORs are adjusted for age, education and depressive symptoms at t2. | |||||
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; MCI, mild cognitive impairment at t2; OR, odds ratio; RERI, relative excess risk due to interaction; SCD (+/−), with and without subjective cognitive decline; t2, second follow-up examination.
NOTE. Risk estimates were calculated by binomial logistic regression analyses and measures of effect modification were assessed for women.
APOE ε4–positive = at least one 4 allele (2/4, 3/4, 4/4).
4. Discussion
We observed a tendency for a positive interaction effect of SCD and APOE ε4 genotype on incident MCI 5 years later in women. Interestingly, men with SCD and APOE ε4–negative genotype showed an increased risk of incident MCI. Although the association of SCD and MCI has been examined thoroughly, no study has investigated the important factors SCD and APOE ε4 genotype and their possible joint effects in men and women separately. Thus, no direct comparison of our results with other studies was possible. However, we could compare our findings in some aspects and certain combinations of our group characteristics.
Regarding SCD and APOE ε4, population-based studies as well as studies with memory-clinic patients have shown higher rates of cognitive decline and worse cognitive performance in APOE ε4 carriers with SCD compared with noncarriers either with or without SCD [12], [27]. A current systematic review incorporating cross-sectional and two longitudinal studies examined the association of SCD and APOE ε4 [28]. The authors reported an additional risk of APOE ε4 in participants with SCD for objective cognitive impairment, which coincides with the results of our female but not our male participants. It is possible that positive associations found in other studies are driven by the positive interaction in women.
Regarding APOE ε4 genotype and incident MCI, APOE ε4–positive women with SCD showed strong associations with MCI 5 years later, whereas APOE ε4–negative women with SCD did not. This is in line with the literature as APOE ε4 is a genetic risk factor for AD dementia and conversion from its earlier stages [29]. APOE ε4–positive men with SCD and especially APOE ε4–negative men with SCD showed strong associations with incident MCI. This exhibits similarities to Zokaei et al. [30] reporting a memory advantage in midlife for male APOE ε4 carriers. This is in contrast to studies in old-aged participants, where APOE ε4 carriers show worse memory performance compared with noncarriers. The authors proposed this outcome to be explained by the underlying antagonistic pleiotropy [31]. This theory describes opposite effects of genes on fitness at different ages, thereby enabling the evolutionary survival of a gene despite its disadvantage later in life. This transfers to APOE ε4 and AD: later in life, carrying the ε4 allele is a risk factor for AD but an earlier beneficial effect of this genotype would explain the pronounced results we found in male APOE ε4–negative participants with SCD. We were not able to investigate this association with inclusion of age groups due to small sample sizes. Findings of cortical thickening in AD vulnerable areas in APOE ε4 carriers aged 48 to 75 years also support the possible mechanism of antagonistic pleiotropy for APOE ε4 [32], [33].
Regarding sex and APOE ε4, the literature suggests that the reported sex-specific difference may be explained by the role of sex hormones on brain function. Women undergo menopause, characterized by extensive physiological changes that ultimately lead to estrogen loss. Estrogen is thought to have a neuroprotective effect [34], [35]. Estrogen loss due to menopause might have a significant effect on cognitive decline and AD [36], [37]. Studies suggest that APOE ε4 could possibly diminish or neutralize the neuroprotective effect of estrogen [38], [39]. This could explain the selective association between SCD and MCI in female APOE ε4 carriers but not in female noncarriers. Compared with male APOE ε4 carriers, female carriers were found to have significantly reduced connectivity in the precuneus that is part of the default network, which has been shown to be altered in patients with AD [40]. Another study found female APOE ε4 carriers with MCI to show increased AD associated biomarkers, although male and female carriers were both more likely to convert to MCI or AD than noncarriers [10]. Other divergences were identified by a recent meta-analysis [41], reporting APOE ε4–positive women to be at higher risk of developing MCI than APOE ε4–positive men, specifically between the ages of 55 and 70 years.
Regarding sex and SCD, women seem to experience subjective decline more often than men. In our analysis sample, we only observed a small difference of SCD frequency between men and women (28% and 30%, respectively). Rickenbach et al. [42] conclude that men overestimate their memory function while women underestimate it. Multiple etiologies result in SCD expression, which itself could contribute to the observed differences in men and women. Concerns associated with SCD are part of the SCD plus criteria [43]. Looking at the frequencies in our sample, women with SCD reported additional concerns more often than men (women: 16% SCD and concerns, 14% SCD and no concerns, 70% no SCD; men: 10% SCD and concerns, 18% SCD and no concerns, 72% no SCD). This might indicate that the perceived decline afflicts women stronger than men, resulting in a differential meaning of SCD for men and women. Owing to insufficient sample sizes, we could not incorporate SCD-associated concerns in our analyses. Studies show that sex differences are well known in the course of AD dementia. More women than men have AD and a study also showed sex differences in MCI [44]. Tomita et al. [45] found a link between SCD and cognition in men but not in women. In a population-based study, SCD was associated with increased risk of dementia only in women [46]. In our study, SCD had a predictive value for MCI in women as well as in men. Sex-specific differences presented themselves through the APOE ε4 genotype. Differences by sex should be considered when evaluating SCD and a sex-sensitive approach in AD research is indicated.
Although extensive efforts have been made to standardize SCD assessment and thus increase comparability between studies, there is still heterogeneity across studies. Administrative aspects (e.g., brevity, availability) often influence the selection of the questionnaire or instrument used [47]. This increases the complex nature of SCD and makes it a challenging task to determine its true meaning. Subjective cognitive decline is by definition a subjective concept and identified through self-report. Additional confirmation about SCD by an informant might have strengthened SCD as a predictor for conversion to MCI in our study [48]. Subjective cognitive decline is related to a diversity of factors that may affect SCD and its expression, for example, underlying comorbidities, anxiety, character traits, and socioeconomic status [49], [50], [51]. The accuracy of cognitive complaints in our study might not only be influenced by sex but also by several of these factors. These variables themselves again might differ between men and women to a certain degree and thereby mask the full impact of SCD.
This is the first study to examine the sex-stratified joint effect of SCD and APOE ε4 genotype on incident MCI. Participants were randomly selected from mandatory registries, which reduced the selection or volunteer bias. Our cohort is comparatively young regarding preclinical AD research, which is of special interest in this context. Our study represents the general population and we cover a broad age range, including midlife. This enables us to target more people in the prodromal phase of AD.
Despite several strengths, some limitations must be acknowledged. Cognitive data originate from the first and second follow-up examination resulting in a healthier cohort. We included participants that were cognitively normal at the first follow-up, whereby potential bias should be minimized. A gold standard to operationalize SCD is still missing. We assessed SCD with a single question instead of a detailed survey because our study was designed before recommendations were published by the working group of the Subjective Cognitive Decline Initiative [43]. The question we used includes retrospective assessment over 2 years, which seems to be crucial for the classification of SCD. In our study, SCD is based on self-report only and it is probably influenced by other factors in addition to sex and APOE ε4. Interaction effects did not reach statistical significance, most likely due to small groups. Post hoc power calculations indicated that larger groups are necessary to better interpret the results (data not shown) and to increase precision of interaction measures. Our analyses focused on APOE ε4 as a genetic risk factor. Incorporating biomarkers may reflect real-life conditions more accurately.
In conclusion, our study indicates that SCD and APOE ε4 genotype might have a positive interaction effect on the risk of MCI 5 years later only in women, whereas for men, we observed at most a tendency for a negative interaction effect. These highly contrary results for men and women underline the urgent need for more studies examining this association and taking sex into account. This might help differentiate subpopulations at risk for future studies.
Research in context.
-
1.
Systematic review: We reviewed the literature using common online databases to identify previous publications about SCD in context with APOE ε4 genotype, sex, and/or MCI. Several studies focused on MCI, few studies focused on sex or APOE ε4 genotype, whereas no studies exist researching all criteria. Related studies investigating these factors have been appropriately cited.
-
2.
Interpretation: We evaluated sex-stratified associations and possible interaction of SCD and APOE ε4 genotype with incident MCI in a population-based cohort. Our results add to the existing literature as the first ever study to show that depending on sex, SCD, and APOE ε4 are differentially associated with incident MCI.
-
3.
Future directions: Future studies should implement sex and APOE ε4 status when investigating SCD and MCI. We propose using larger sample sizes to confirm our findings, as well as extending the outcome variable to dementia in longer follow-up periods.
Acknowledgments
Advisory Board: Meinertz T., Hamburg, Germany (Chair); Bode C., Freiburg, Germany; de Feyter P.J., Rotterdam, Netherlands; Güntert B., Hall LT, Austria; Gutzwiller F., Bern, Switzerland; Heinen H., Bonn, Germany; Hess O., Bern, Switzerland; Klein B., Essen, Germany; Löwel H., Neuherberg, Germany; Reiser M., Munich, Germany; Schmidt G. (†), Essen, Germany; Schwaiger M., Munich, Germany; Steinmüller C., Bonn, Germany; Theorell T., Stockholm, Sweden; Willich S.N., Berlin, Germany.
Criteria and Endpoint Committee: C. Bode, Freiburg, Germany (Chair); K. Berger, Münster, Germany; H.R. Figulla, Jena, Germany; C. Hamm, Bad Nauheim, Germany; P. Hanrath, Aachen, Germany; W. Köpcke, Münster, Germany; Ringelstein, Münster, Germany; C. Weimar, Essen, Germany; A. Zeiher, Frankfurt, Germany.
The authors thank the Heinz Nixdorf Foundation [Germany; Chairman: Martin Nixdorf; Past Chairman: Dr Jur Gerhard Schmidt (deceased)], for their generous support of this study. This study is also supported by the German Ministry of Education and Science (BMBF), and the German Aero-space Center [Deutsches Zentrum für Luft- und Raumfahrt (DLR)], Bonn, Germany. The German Research Council supported the study (DFG project: ER 155/6-2) and funded the study of psychosocial factors and neighborhood level information (DFG project SI 236/8-1 and SI 236/9-1). The sponsor of the study transferred the monitoring of the study to the German Ministry of Education and Science, Bonn using an international advisory board and quality control as well as event committee, but had no role concerning the study design, data collection, analysis, interpretation, or writing the report. Regarding the cognitive data, the authors have received a research grant from the Else Kröner-Fresenius-Stiftung (2015_A119). The corresponding authors had full access to all data in the study and final responsibility for the submission of the manuscript for publication.
The authors have nothing to disclose.
References
- 1.2018 Alzheimer's disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 2.Kryscio R.J., Schmitt F.A., Salazar J.C., Mendiondo M.S., Markesbery W.R. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66:828–832. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 3.Luck T., Luppa M., Briel S., Matschinger H., Konig H.H., Bleich S. Mild cognitive impairment: Incidence and risk factors: Results of the leipzig longitudinal study of the aged. J Am Geriatr Soc. 2010;58:1903–1910. doi: 10.1111/j.1532-5415.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 4.Reisberg B., Shulman M.B., Torossian C., Leng L., Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessen E., Eckerstrom M., Nordlund A., Selseth Almdahl I., Stalhammar J., Bjerke M. Subjective Cognitive Impairment Is a Predominantly Benign Condition in Memory Clinic Patients Followed for 6 Years: The Gothenburg-Oslo MCI Study. Dement Geriatr Cogn Dis Extra. 2017;7:1–14. doi: 10.1159/000454676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hooren S.A., Valentijn A.M., Bosma H., Ponds R.W., van Boxtel M.P., Jolles J. Cognitive functioning in healthy older adults aged 64-81: A cohort study into the effects of age, sex, and education. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- 7.Di Carlo A., Lamassa M., Baldereschi M., Inzitari M., Scafato E., Farchi G. CIND and MCI in the Italian elderly: Frequency, vascular risk factors, progression to dementia. Neurology. 2007;68:1909–1916. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 8.Mielke M.M., Vemuri P., Rocca W.A. Clinical epidemiology of Alzheimer's disease: Assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skup M., Zhu H., Wang Y., Giovanello K.S., Lin J.A., Shen D. Sex differences in grey matter atrophy patterns among AD and aMCI patients: Results from ADNI. Neuroimage. 2011;56:890–906. doi: 10.1016/j.neuroimage.2011.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmann A., Tian L., Henderson V.W., Greicius M.D., Alzheimer's Disease Neuroimaging Initiative I Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beydoun M.A., Boueiz A., Abougergi M.S., Kitner-Triolo M.H., Beydoun H.A., Resnick S.M. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33:720–731.e4. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dik M.G., Jonker C., Comijs H.C., Bouter L.M., Twisk J.W., van Kamp G.J. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57:2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- 13.Schmermund A., Möhlenkamp S., Stang A., Grönemeyer D., Seibel R., Hirche H. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: Rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J. 2002;144:212–218. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 14.Stang A., Moebus S., Dragano N., Beck E.M., Möhlenkamp S., Schmermund A. Baseline recruitment and analyses of nonresponse of the Heinz Nixdorf Recall Study: Identifiability of phone numbers as the major determinant of response. Eur J Epidemiol. 2005;20:489–496. doi: 10.1007/s10654-005-5529-z. [DOI] [PubMed] [Google Scholar]
- 15.Winkler A., Dlugaj M., Weimar C., Jockel K.H., Erbel R., Dragano N. Association of diabetes mellitus and mild cognitive impairment in middle-aged men and women. J Alzheimers Dis. 2014;42:1269–1277. doi: 10.3233/JAD-140696. [DOI] [PubMed] [Google Scholar]
- 16.Hautzinger M., Bailer M. Beltz-Test-GmbH; Göttingen: 1993. Allgemeine Depressions-Skala: ADS; Manual. [Google Scholar]
- 17.American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 1994. Diagnostic and statistical manual of mental disorders (DSM-IV) [Google Scholar]
- 18.Wege N., Dlugaj M., Siegrist J., Dragano N., Erbel R., Jöckel K.H. Population-based distribution and psychometric properties of a short cognitive performance measure in the population-based Heinz Nixdorf Recall Study. Neuroepidemiology. 2011;37:13–20. doi: 10.1159/000328262. [DOI] [PubMed] [Google Scholar]
- 19.Tebrügge S., Winkler A., Gerards D., Weimar C., Moebus S., Jöckel K.H. Olfactory function is associated with cognitive performance: Results of the Heinz Nixdorf Recall Study. J Alzheimers Dis. 2018;63:319–329. doi: 10.3233/JAD-170863. [DOI] [PubMed] [Google Scholar]
- 20.Ivnik R.J., Malec J.F., Smith G.E., Tangalos E.G., Petersen R.C., Kokmen E. Mayo’s Older Americans‘ Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–104. [Google Scholar]
- 21.Shulman K.I. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–561. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 23.UNESCO . 1997. International standard classification of education (ISCED) https://unesdoc.unesco.org/ark:/48223/pf0000111387. [Google Scholar]
- 24.Radloff L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Hosmer D.W., Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 26.de Mutsert R., Jager K.J., Zoccali C., Dekker F.W. The effect of joint exposures: Examining the presence of interaction. Kidney Int. 2009;75:677–681. doi: 10.1038/ki.2008.645. [DOI] [PubMed] [Google Scholar]
- 27.Striepens N., Scheef L., Wind A., Meiberth D., Popp J., Spottke A. Interaction effects of subjective memory impairment and ApoE4 genotype on episodic memory and hippocampal volume. Psychol Med. 2011;41:1997–2006. doi: 10.1017/S0033291711000067. [DOI] [PubMed] [Google Scholar]
- 28.Ali J.I., Smart C.M., Gawryluk J.R. Subjective Cognitive Decline and APOE varepsilon4: A Systematic Review. J Alzheimers Dis. 2018;65:303–320. doi: 10.3233/JAD-180248. [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Grau S., Ruiz A. Genome research in pre-dementia stages of Alzheimer's disease. Expert Rev Mol Med. 2016;18:e11. doi: 10.1017/erm.2016.12. [DOI] [PubMed] [Google Scholar]
- 30.Zokaei N., Giehl K., Sillence A., Neville M.J., Karpe F., Nobre A.C. Sex and APOE: A memory advantage in male APOE epsilon4 carriers in midlife. Cortex. 2017;88:98–105. doi: 10.1016/j.cortex.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams G.C. Pleiotropy, natural-selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 32.Espeseth T., Westlye L.T., Fjell A.M., Walhovd K.B., Rootwelt H., Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Espeseth T., Westlye L.T., Walhovd K.B., Fjell A.M., Endestad T., Rootwelt H. Apolipoprotein E epsilon4-related thickening of the cerebral cortex modulates selective attention. Neurobiol Aging. 2012;33:304–322.e1. doi: 10.1016/j.neurobiolaging.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 34.Yue X., Lu M., Lancaster T., Cao P., Honda S., Staufenbiel M. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishunina T.A., Fischer D.F., Swaab D.F. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28:1670–1681. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Henderson V.W. Estrogen-containing hormone therapy and Alzheimer's disease risk: Understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Brinton R.D. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe K., Haan M., Byers A., Tangen C., Kuller L. Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 39.Burkhardt M.S., Foster J.K., Laws S.M., Baker L.D., Craft S., Gandy S.E. Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. J Alzheimers Dis. 2004;6:221–228. doi: 10.3233/jad-2004-6302. [DOI] [PubMed] [Google Scholar]
- 40.Damoiseaux J.S., Seeley W.W., Zhou J., Shirer W.R., Coppola G., Karydas A. Gender modulates the APOE epsilon4 effect in healthy older adults: Convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neu S.C., Pa J., Kukull W., Beekly D., Kuzma A., Gangadharan P. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017;74:1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickenbach E.H., Agrigoroaei S., Lachman M.E. Awareness of Memory Ability and Change: (In)Accuracy of Memory Self-Assessments in Relation to Performance. J Popul Ageing. 2015;8:71–99. doi: 10.1007/s12062-014-9108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Au B., Dale-McGrath S., Tierney M.C. Sex differences in the prevalence and incidence of mild cognitive impairment: A meta-analysis. Ageing Res Rev. 2017;35:176–199. doi: 10.1016/j.arr.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Tomita T., Sugawara N., Kaneda A., Okubo N., Iwane K., Takahashi I. Sex-specific effects of subjective memory complaints with respect to cognitive impairment or depressive symptoms. Psychiatry Clin Neurosci. 2014;68:176–181. doi: 10.1111/pcn.12102. [DOI] [PubMed] [Google Scholar]
- 46.Peres K., Helmer C., Amieva H., Matharan F., Carcaillon L., Jacqmin-Gadda H. Gender differences in the prodromal signs of dementia: memory complaint and IADL-restriction. a prospective population-based cohort. J Alzheimers Dis. 2011;27:39–47. doi: 10.3233/JAD-2011-110428. [DOI] [PubMed] [Google Scholar]
- 47.Rabin L.A., Smart C.M., Crane P.K., Amariglio R.E., Berman L.M., Boada M. Subjective Cognitive Decline in Older Adults: An Overview of Self-Report Measures Used Across 19 International Research Studies. J Alzheimers Dis. 2015;48:S63–S86. doi: 10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gifford K.A., Liu D., Lu Z., Tripodis Y., Cantwell N.G., Palmisano J. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimers Dement. 2014;10:319–327. doi: 10.1016/j.jalz.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Oijen M., de Jong F.J., Hofman A., Koudstaal P.J., Breteler M.M. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimers Dement. 2007;3:92–97. doi: 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Snitz B.E., Weissfeld L.A., Cohen A.D., Lopez O.L., Nebes R.D., Aizenstein H.J. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry. 2015;23:985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson J.D., Rentz D.M., Aghjayan S.L., Buckley R.F., Meneide T.F., Sperling R.A. Subjective cognitive concerns are associated with objective memory performance in Caucasian but not African-American persons. Age Ageing. 2017;46:988–993. doi: 10.1093/ageing/afx077. [DOI] [PMC free article] [PubMed] [Google Scholar]