Abstract
Partial nephrectomy (PN), also known as nephron sparing surgery, is considered as the first-line treatment in small renal masses, especially in T1/2 tumors, and is applied as a standard treatment in advanced centers. The main expected outcomes from an ideal PN are surgical margin negativity, minimal impairment in renal function, and any surgical complications. Many authors have defined PN techniques as “zero ischemia partial nephrectomy”, where surgery is performed without clamping the main renal artery in order to protect the renal parenchyma from ischemic injury. Various PN techniques employed by surgeons include: selective or segmental renal artery clamping technique; off-clamp, clampless, or unclamped technique; preoperative superselective transarterial tumor embolization technique; sequential/modified sequential preplaced suture renorrhaphy technique, radio frequency ablation-assisted technique, and combination of these techniques. The common goal of all these techniques is to provide zero ischemia without hilar clamping. This systematic review focuses on the long-term functional outcomes of PNs performed by zero ischemia techniques.
Keywords: zero ischemia, partial nephrectomy, renal function, complication, nephron sparing surgery, renal tumor
Introduction
Partial nephrectomy (PN), also known as nephron sparing surgery, is considered as the first-line treatment for small renal masses, especially for T1a tumors, and is applied as a standard treatment in advanced centers. Recently, the indications for PN were extended to include T1b/T2 renal tumors, even if the contralateral kidney was normal.1 The main expected outcomes of an ideal PN are surgical margin negativity, minimal impairment in renal function (RF), and any surgical complications.2
Traditionally, the renal artery is clamped to interrupt the renal blood flow in order to reduce the amount of bleeding during resection and repair of the parenchymal defect.
Although RF after PN is associated with high percentage of conserved parenchyma and preoperative estimated glomerular filtration rate (eGFR),3 prevention of blood flow during PN may result in renal ischemic damage.
However, the ideal warm ischemia time (WIT) safety threshold is a debate, and studies show that WIT should be kept <20–25 minutes as far as possible,4–6 and especially the ischemia rate is shown to increase for every minute over 25 minutes causing long-term RF deterioration.7
The negative effects of warm ischemia on postoperative RF and the use of new technologies in surgery led to the development of different operation techniques that aimed at decreasing parenchymal ischemia. Recently, zero ischemia PN is widely followed by urologists due to its potential advantages on RF preservation. Many authors have defined PN techniques as “zero-ischemic partial nephrectomy” (ZIPN), which involves the use of different methods in order to protect the renal parenchyma from ischemic injury. The zero-ischemic technique was first described by Gill et al.8
Different methods are used to perform ZIPN,9 such as selective or segmental renal artery clamping technique; off-clamp, clampless, or unclamped technique; preoperative superselective transarterial tumor embolization technique (P-STE); sequential/modified sequential preplaced suture renorrhaphy (SPSR) technique; radio frequency ablation (RFA)-assisted technique, and combination of these techniques. The common goal of all these techniques is to provide zero ischemia without hilar clamping.
This systematic review focuses on the long-term functional outcomes of PNs performed by zero ischemia techniques.
Evidence acquisition and synthesis
The studies written in the English language were systematically evaluated by two independent authors (MSB and MGS) in September 2018 using the Scopus, PubMed/Medline, and Web of Sciences databases. The terms with “renal cancer”, “partial nephrectomy”, “nephron spairing surgery”, “zero ischemia” , “off-clamp”, “clampless”, “unclamped”, “selective clamp”, “segmental clamp”, “renal functional outcomes”, and their combination was used for literature search.
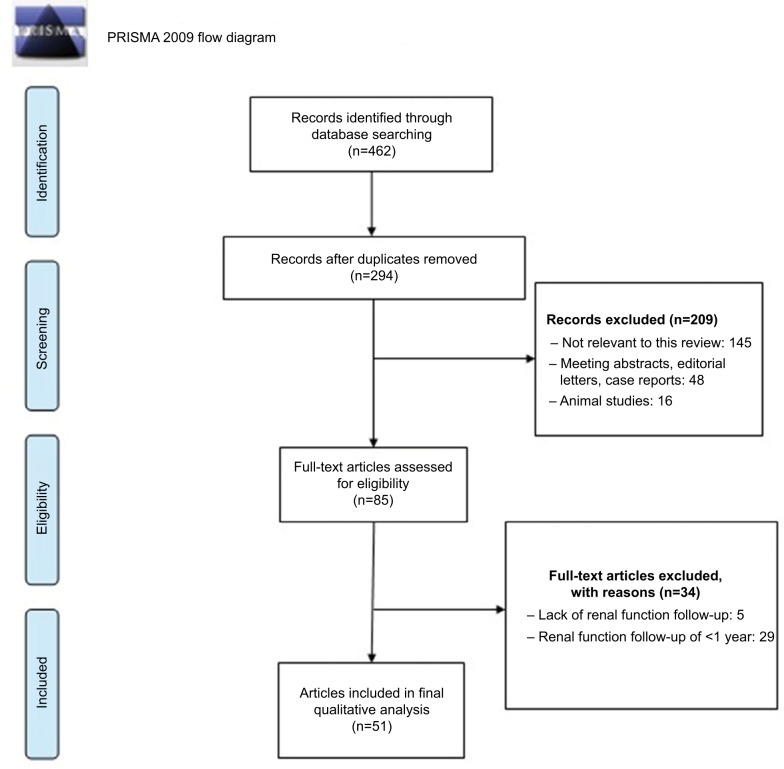
We limited our search to articles that were published in the last 10 years and restricted our search to original articles, reviews, meta-analyses, and human studies. Studies such as congress abstracts, single case reports, experimental studies, book chapters, and letter to the editor were not included in this review. Articles which had <1 year of RF follow-up were excluded from the study. Additional studies for this review were included from previous review articles and references which were cited in the selected manuscripts on this subject. Last update of search was done on November 10, 2018 (Figure 1).
Figure 1.
Flow diagram of evidence acquisition in a systematic review of long-term renal function following zero ischemia partial nephrectomy in the treatment of renal tumors.
The preoperative, perioperative, and postoperative oncological and functional data included in the studies related to zero-ischemic PNs were recorded and reviewed. In addition, perioperative bleeding is further evaluated under a separate section. This review was carried out in accordance with the PRISMA guidelines.10
Surgical techniques and effect on long-term RF
Although on-clamp (On-C) and off-clamp (Off-C) definitions were previously used for PN surgeries, the concept of zero ischemia PN for laparoscopic and robotic technique was first introduced by Gill et al.8 The technique is based on the tumor resection followed by reduction of renal blood flow by inducing controlled hypotension. The mean arterial blood pressure (MAP) was 60 mmHg during tumor resection, and this MAP was a taken as threshold to ensure maintenance of perfusion and oxygenation of tissues and vital organs. Fifteen consecutive patients underwent zero ischemia procedures: laparoscopic PN (LPN) (n=12) and robot-assisted PN (RAPN) (n=3). Outcomes of the study were favorable, with mean estimated blood loss (EBL) of 150 mL. Clavien grade 4a complications developed in one patient, all with negative margins. Median serum creatinine levels and eGFR were insignificant (0.9 mg/dL vs 0.95 mg/dL for serum creatinine and 75.3 vs 72.9 mL/min for eGFR pre- and postoperatively, respectively). Median percentage and absolute change in discharge (mean: 3 days) of eGFR and serum creatinine were 0% and 0, respectively. Although this study did not have long-term results, the results were significant as they gave the first introduction for zero ischemia in laparoscopic and robotic technique.8
Different types of zero ischemia techniques have been described, such as Off-C, segmental/selective renal artery clamping, P-STE technique, sequential/modified SPSR technique, RFA-assisted technique, and/or combination of these techniques in open (O), laparoscopic (L), and robotic (R) PNs.
Off-clamp/clampless/unclamped technique
The Off-C technique is based on the principle of cutting or enucleating the tumor with cold scissors or hemostatic instruments without placing the clamp on the main renal artery. In this technique, fingers or parenchymal clamps are used to reduce the bleeding. Renal parenchymal clamping technique is based on the principle of reducing the regional blood flow by compressing the kidney surface. Various instruments have been used to perform this technique, such as different type of modified tourniquet. Successful results of using these devices in open and laparoscopic PNs have been reported.11–13
Also, laser and hydro-jet technologies have been used to provide tumor resection, with adequate tissue resection and hemostasis of tumor base under minimal bleeding for renal tumors in Off-C PN. It has been reported that laser types such as CO2,14 diode,15 holmium,16 and potassium titanyl phosphate laser17 have been successfully used in PNs for selected series of tumors.
Hydro/water-jet assisted clampless LPN provides very high- and thin-pressure water flow for dissection and cutting of renal parenchyma.18 Hydro-jet surgery is used primarily for liver and other parenchymal organ surgeries, and successful results have been achieved in clampless PNs by providing easy bleeding control.19
Kutikov et al20 reported oncologic outcomes of Off-C open PNs, which were performed with enucleation of tumor and laser ablation of the tumor base for a series of 97RCC patients. Following the sharp incision of the parenchyma adjacent to the tumor, blunt dissection was performed between the pseudocapsule and the parenchyma, and the tumor was enucleated in this method. Argon beam and Nd:YAG laser were used for tumor bed ablation. The mean follow-up was 24.9 months. Local recurrence was observed in only one patient after 30 months, and the patient underwent radical nephrectomy. The lack of functional follow-up was the major limitation of this study.
Smith et al compared the outcomes of Off-C (n=192) and On-C (n=116, WIT mean, 23 minutes) PNs (O-L-R) to determine the safety, impact on oncological efficacy, and RF, retrospectively. In this study, after 1-year follow-up, the decrease in eGFR was significantly higher in the On-C arm (9.8% vs 12.3%, Off-C vs On-C, respectively, P=0.037). The decrease in eGFR was evident in the solitary kidney group (4.4% vs 21%, Off-C vs On-C, respectively, P=0.027). However, EBL (500 vs 200 mL, P<0.001), perioperative transfusion rate (42% vs 23%, P<0.001), and operation time (226.5 vs 192 minutes, P<0.001) were significantly higher in the Off-C technique. The rate of complications was similar in Off-C and On-C groups (9.9% vs 11.2%, P=0.72).21
Çömez et al22 reported the postoperative outcomes of 79 patients who underwent open partial nephrectomy (OPN) with Off-C (n=40) and On-C (n=33) techniques. There was no significant difference in mean age, RENAL (radius, exophytic extent, nearness to the renal sinus, anterior/posterior location, and location relative to the polar lines) nephrometry scores, comorbidity rates, length of operation, length of hospitalization, preoperative eGFR, surgical margin status, complications, and additional intervention requirement between the groups. Although the transfusion rate was statistically insignificant, it was higher in the Off-C group (0.7 vs 0.2 U, P=0.066). The follow-up time was 27 months for the Off-C and 33 months for the On-C group. Although postoperative eGFR values were similar (72.6 vs 78.3 mL/min/1.73 m2, P=0.651), differences between pre- and postoperative eGFR values were statistically significant (3.71% vs 10.21%, Off-C vs On-C, P<0.05) in these follow-up periods.
Simone et al23 reported the LPN results of 101 patients with low nephrometry score (RENAL score: 4) using sutureless technique. They used monopolar coagulation or a vessel sealing device and some hemostatic agents for tumor bed ablation. Median decreases at 3 months and 1 year postoperatively were 3% and 1.6%, respectively. Increases in serum creatinine levels after 3 months and 1 year were 0.1 mg/dL (P=0.104) and 0.09 mg/dL (P=0.157), respectively. Operation time was 60 minutes, and EBL was 100 mL. Hilar clamping and conversion to open surgery were not necessary in any patient.
In a multi-institutional study, Kaczmarek et al24 evaluated functional and perioperative outcomes of 49 patients who underwent robotic partial nephrectomy (RPN) without hilar clamping and compared with propensity score matched 283 clamped control patients. Zero-ischemic RPN group patients had a lower impairment in eGFR (2% vs 6%, Off-C vs On-C, respectively, P=0.008) in a mean follow-up of 21 months. In the zero-ischemic RPN group, the operation time was significantly shorter (156 vs 185 minutes, P<0.001), but EBL was higher (228 vs 157 mL, P=0.009). After propensity score matching and multivariable analysis, the Off-C group was associated with significantly higher EBL, shorter operative time, smaller decrease in eGFR, and higher last eGFR. There were no differences in the transfusion or complication rates between the groups.
Acar et al25 compared the functional and oncologic outcomes of RAPNs with On-C (n=14) and off-C (n=30) cases retrospectively in a mean follow-up of 18.9 months. In this study, tumors were enucleoresected with cold scissors, leaving a minimal rim of normal parenchyma. Bleeding was controlled by bipolar or monopolar electrocautery. The difference was insignificant as mean tumor size, patient age, RENAL nephrometry scores, mean EBL amount, operative time, and mean length of hospitalization were similar between the groups. Complication and open conversion rates were similar. Although the mean postoperative change in eGFR was statistically insignificant, in contrast to other studies, the mean drop in eGFR was marginally higher in the off-C group (6.7 vs 10.8 mL/min, On-C vs Off-C, P=0.13), which may partly be explained by relatively higher mean American Society of Anesthesiologists (ASA) score. This score is a global score that assesses the physical status of patients before surgery which in turn gives predictivity for operative risk.26
Abdel Raheem et al27 evaluated 62 Off-C RPN patients in their study to determine the cutoff value of the amount of bleeding and related chronic kidney disease (CKD) development according to the tumor size and the Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) score. PADUA is an anatomical scoring system according to tumor size and location. It shows the predictivity of complications in patients who are planning to undergo PN.28 The median follow-up period was 20 months. Mean tumor size was 2.6 cm, and baseline eGFR value was 90 mL/min/1.73 m2 in both the groups (tumor size ≤3.2 cm and >3.2 cm). Postoperative eGFRs were 86 vs 87 mL/min/1.73 m2 for the groups (tumor size ≤3.2 cm and >3.2 cm, respectively, P=0.012). The mean amount of EBL was 200 mL. The receiver operating characteristics analysis determined the cutoff value as 3.2 for the EBL >400 mL. Operation time (116 vs 163 minutes, P=0.002), EBL (150 vs 575 mL, P <0.001), and transfusion rate (0% vs 18.8%, P=0 .015) were higher in patients with tumor size >3.2 cm. The rate of return to radical nephrectomy was also higher in those with tumor size >3.2 cm compared with the patients with tumor size ≤3.2 cm.
During follow-up, CKD upstaging was observed in 22 (35.4%) patients. Multivariate logistic regression analysis showed that EBL >400 mL was a predictive value only for CKD upstaging (OR: 6.704, P=0.009). This study demonstrated that patients with PADUA score ≥9 and tumor size >3.2 cm are associated with an increased risk of intraoperative bleeding and perioperative blood transfusion. In addition, EBL volume of >400 mL is associated with CKD upstaging despite the application of zero ischemia.27
Pansadoro et al29 reported a prospective study of 54 consecutive patients with T1a-T1b renal tumor who had undergone retroperitoneal laparoscopic enucleation with controlled selective hypotension on demand. This is a zero ischemia technique that provides the isolation of renal artery and the placement of vessel loop around it in order to perform a controlled selective local hypotension on demand by a traction of the loop. The aim of this technique was to obtain zero ischemia without inducing a systemic hypotension. Mean tumor size was 3 cm, and mean renal nephrometry score was 7. Median EBL volume was 210 mL, and mean operative time was 125 minutes. Positive margins were observed in 5.5% of the cases. Grade IIIa and IIIb postoperative complications were seen in 5.5% and 11% of the cases, respectively. Postoperative eGFR change was 1.2 mL/min. Although only 3 months of renal function results were given, they reported that they had a safe and feasible zero-ischemic LPN in terms of surgical margin and complication rates, with no recurrence in the 20-month follow-up period. This method also provides a direct and fast access to the renal artery due to its retro-peritoneal approach, and it is also seems to be advantageous in patients with a history of prior transperitoneal operation and in obese patients.
Segmental/selective renal artery clamping technique
In this technique, the branches of the renal artery are anatomically isolated by microdissection, via inserting a micro-surgical bulldog30,31 or conventional laparoscopic bulldog32 clamping just into the the segmental/selective artery providing tumor-specific devascularization and ischemia.
This technique was first described by Nohara et al in 2008 for OPN.33 Gill et al reported the successful implementation of this technique in LPN.8,31 The first step involves the identification of tumor location and subsequent detection of the vascular structures, by angiography34,35 or Doppler ultrasonography,31 that feed this tumor. This is followed by microdissection and clamping of these tumors. These imaging techniques ensure the exact detection of the vascular branches feeding the tumor, thus minimizing the risk of bleeding and parenchymal ischemia.35,36 This technique may not be appropriate, as it may increase the risk of bleeding and intact tissue injury in patients with perirenal tissue adhesions and/or short segmental arteries.
Komninos et al37 compared the RFs of RAPNs retrospectively. RAPNs evaluated Off-C (n=23), selective clamp (SC, n=25), and main artery/On-C (n=114) groups for a mean follow-up period of >1 year. Off-C and selective clamp groups showed significantly less decrease in eGFR levels at 3 months postoperatively (eGFR decreases were 1.5%, 2%, and 8% for Off-C, SC, and On-C groups, respectively, P=0.04); however, this beneficial result was not observed after 6 months or for the last eGFR evaluation (eGFR decreases were 3%, 6%, and 3.5% for Off-C, SC, and On-C groups, respectively, P=0.48). Multiple logistic regression analysis showed that preoperative eGFR, low RENAL score, and the type of clamping procedure were the only variables predicting normal eGFR 7 days after surgery in patients who have normal RF before surgery, while only preoperative eGFR and age were correlated with normal eGFR 1 year after surgery, as long as the warm ischemia time was within 20–30 minutes.
Martin et al38 reviewed PNs by using different types of clamping techniques such as On-C, SC, progressive clamping (PC) from segmental to main renal artery clamping, or resection without hilar clamping in both LPN and RPNs. A total of 57 patients were analyzed (4 Off-C, 13 SC, 8 PC, and 32 On-C). The mean postoperative change in eGFR was 2.5, 6.6, 7.8, and 6.8 mL/min/1.73 m2, and percentage change in renal scan were 2.5, 4.9, 5.5, and 4.8 for Off-C, SC, PC, and On-C groups, respectively. No significant difference in GFR values was found between all the groups at a mean follow-up of 411 days.
Preoperative superselective transarterial tumor embolization technique
It is based on the principle of embolization of the artery feeding the tumor with preoperative angiography and subsequent resection of the tumor. This technique was first introduced with the purpose of decreasing the risk of bleeding.39
Simone et al reported oncologic and functional outcomes of the first 210 patients treated with P-STE followed by Off-C LPN. Although the tumors were relatively complex with respect to size and RENAL scoring (median size 4.2 cm, nephrometry scores ≥6), the median EBL was 150 mL, median operative time was 62 minutes, and the complication rate was 6%. Postoperative first year scintigraphic evaluation showed a 5% mean reduction in split RF. There was no new-onset stage 3 CKD in a 46-month median follow-up.40
Sequential/modified sequential preplaced suture renorrhaphy technique
This technique was described by Rizkala et al for 14 selected patients. SPSR PN technique is based on preplaced renal parenchymal sutures before resection of the tumor. Parenchymal sutures are placed to prevent bleeding by leaving a safety margin of 2 mm around the tumor, and then the fat on the tumor is removed along with the margin of safety.41,42 This technique was carried out by Lu et al, and zero ischemia was achieved in 13 of the14 patients. The amount of EBL was 60 mL, and the mean operation time was 75 minutes. None of the patients had major complications and no local recurrences or metastases were observed. Although this study did not include long-term follow-up results, the authors concluded that this method could be a feasible surgical option for the treatment of small, exophytic, and peripheral renal tumors.43
Recently, Sönmez et al42 reported modified SPSR technique with a mean follow-up results of 16.2 months. The results of this study showed that this technique was a successful method in terms of safe oncologic results and long-term RFs in selected patients. The mean tumor size was 2.72 cm, mean operation time was 126 minutes, and mean EBL was 244 mL. Creatinine and eGFR changes at 12 months postoperatively were insignificant (0.77 mg/dL and 99.7 mL/min vs 0.83 mg/dL and 95.7 mL/min, preoperatively and postoperatively, respectively, P=0.09, P=0.065). They also evaluated global functional recovery as 92.5% on day 1 and 95.9% at 12 months. None of the patients had surgical margin positivity and Clavien–Dindo grade 3–4 complications. No patient had local recurrence or metastasis during the mean follow-up of 16.2 months.
RFA-assisted technique
This technique involves creation of a margin around the tumor before excision with a bipolar RFA-assisted laparoscopic device.44 RFA treatment is mostly used in the treatment of patients who are not eligible for surgery due to age and comorbidity.45
Rimar et al46 compared the oncologic and functional outcomes of RFA-assisted zero ischemia RPN group (n=49) vs conventional RPN group (n=36). During the procedure, the bleeding areas are controlled by repuncturing and coagulated with RFA. Then, the tumor is resected with robotic scissors in the coagulated margin. Mean follow-up was 54 months vs 68.4 months for the comparison group. The mean decrease in GFR for the RFA-RPN group was −14.8 vs −16.5 mL/min/1.73 m2 for the RPN comparison group. There was no significant difference between the two groups regarding change in GFR (P=0.67). Mean operative time was longer in the RFA-RPN group (370 minutes vs 293 minutes, P<0.001). There were no significant differences in mean EBL (231 cc vs 250 cc, P=0.42). Two patients in the RFA-RPN (4.1%) and one (2.7%) patient in the comparison group had a positive surgical margin (P=0.75). Seventeen (34.7%) patients had a postoperative urine leak in the RFA-RPN group vs two (5.6%) patients in the comparison group (P=0.001). There were three recurrences (6.1%) in the RFA-RPN group and zero recurrences in the RPN group (P=0.23). This technique is associated with a similar degree of renal preservation but higher rates of postoperative urine leak and possibly higher rates of recurrence.
Zhao et al47 evaluated the safety and efficacy of zero ischemia RFA-assisted laparoscopic tumor enucleation for renal cell carcinoma. Median follow-up was 37.5 months. Median GFR was 68.6 mL/min/1.73 m2 before surgery and 65.4 mL/min/1.73 m2 1 year after surgery (P=0.09). In this study, two subgroups with GFR changes <4 cm and between 4 and 7 cm were evaluated separately. The GFR change was 3% in the group <4 cm, while it was 4.67% in the other group. Three patients (two patients in smaller group and one patient in other group) required stent insertion due to prolonged urinary leakage postoperatively (Clavien grade 3).
Zhang et al48 evaluated the perioperative outcomes of 182 patients who underwent zero ischemia RFA-assisted open (n=12) or laparoscopic (n=170) tumor enucleation. Median follow-up was 55.5 months for this study. The tumor was enucleated following the RFA treatment. Median tumor size was 3.2 cm, operative time was 100 minutes, EBL was 80 mL, and hospital stay was 7 days. The relationship of the obtained data with PADUA, RENAL, and centrality index (C-index) score systems was evaluated. All the three scoring systems correlated with duration of operation, EBL, and discharge time. RENAL score system played the most significant role (P<0.001) for operative time, EBL, and hospital stay but not for eGFR change. The mean eGFR change was 64.3 vs 60.8 mL/min/1.73 m2, preoperative and postoperative, respectively. However, correlation parameter of eGFR change was <0.2, suggesting a weak correlation with score systems. None of the patients had surgical margin positivity. Additionally, the complexities of PADUA, RENAL, and C-index scores were significantly correlated with complication and grades (for all three scores P<0.001).
Combination of techniques
After identifying different techniques, some authors reported on the use of a combination of techniques. Kopp et al49 analyzed factors impacting postoperative RF after OPN using the On-C (n=164) and Off-C (n=64) techniques retrospectively in a mean follow-up of 12 months. They used hilar occlusion before resection in the On-C PN group. They performed focal radio frequency coagulation to aid in hemostasis before tumor excision, and performed a non-ischemic resection with a sharp resection or hydro-dissection after focal compression in the clampless PN group. They used de novo eGFR <60 as a predicting factor. There was no difference between the groups for the risk of de novo eGFR <60 (P=0.135) after correcting for hypertension, diabetes mellitus, body mass index, nephrometry score, EBL, pathology, and high-grade complications. There was no significant difference with respect to preoperative eGFR <60 (13.5% vs 9.4%, On-C PN and Off-C PN, respectively, P=0.50); however, with at least 12 months of follow-up, significantly less cases of CKD were observed in the Off-C PN than in the On-C group (12.5% vs 24.4%, P=0.049).
Zero ischemia and hemorrhage
Zero ischemia PN is a surgically demanding procedure associated with the risk of increased intraoperative bleeding.24,27 Colli et al50 reported that postoperative decrease in hematocrit is associated with impaired RF at day 1 and 6 months postoperatively.
In this study, the authors concluded that prolonged hilar clamp times and prolongation of anesthesia had a negative effect on early postoperative RF but did not affect late RF. Perioperative bleeding adversely affects both early- and late-stage RF. Preventing perioperative hemorrhage may be more important than clamp time in terms of preservation of RFs after PN. Similar to this study, Wiener et al51 reported that EBL >200 mL was a relevant potential factor in estimating eGFR following RPN. Studies related to zero ischemia are generally in the form of short-term follow-up studies with a low number of patients and retrospective match control.
More recently, Abdel Raheem et al27 reported a study that evaluated Off-C RPN patients to determine the cutoff value for the amount of bleeding and related CKD development according to the tumor size and PADUA score. The median follow-up period was 20 months. Study showed that tumors >3.2 cm and PADUA score ≥9 are associated with the risk of increased perioperative blood loss and transfusion. In addition, EBL volume of >400 mL is associated with CKD upstaging despite the application of zero ischemia.
As a result, in this review, a total of 14 studies were evaluated in terms of the long-term functional results of zero ischemia followed up for at least 1 year. Compared to conventional methods, almost all of the studies performed with zero ischemia method showed that the decrease in RF was less than observed for the conventional method (Table 1). Only one study reported that the mean drop in eGFR was marginally higher in the off-C group (6.7 vs 10.8, On-C vs Off-C, P=0.13) in contrast to other studies. They explained the higher drop in the Off-C group by the relatively higher mean ASA score in this group of patients. In addition, there were no standard criteria for zero ischemia surgery selection, and the surgeon decided on the type of surgery according to the tumor size and location and patient’s comorbidity condition.25
Table 1.
Preoperative demographic data and postoperative oncologic and functional outcomes of studies
| Study | Groups | Number of patients | Tumor size (cm) | RENAL score | Operation time (minutes) | EBL (mL) | Complication rate (%), CG >3 | Positive margins (%) | Change in last eGFR (%) | Mean follow-up, (months) | WIT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaczmarek et al, 201324 | On-C/Off-C (RPN) | 283/49 | NA/2.5 | 5.6/5.3 | 185/156 | 157/228 | 0–0 | NA/3 | 6.2/1.6 | 21 | 18.5/0 |
| Acar et al, 201425 | On-C/Off-C (OPN) | 14/30 | 3.6/3.8 | 6.6/5.9 | 149.2/142.3 | 170.3/201.6 | 21.4–6.7 | 0/0 | 6.7/10.8 | 18.9 | 22.5/0 |
| Çömez et al, 201622 | On-C/Off-C (OPN) | 33/40 | 3.48/3.76 | 6.6/6.6 | 150.9/157.5 | – | 3.03–7.5 | 15.2/20 | 10.21/3.71 | 27 | 18.3/0 |
| Komninos et al, 201537 | On-C/SC/Off-C (RPN) | 114/25/23 | 3.3/3.5/1.7 | 8/8/6 | 175/163/120 | 200/500/100 | 2.6/4/0 | 6.5/8.6/0 | 3.5/6/3 | 25.42 | 24.8/18/0 |
| Zhao et al, 201247 | RFA-assisted LPN | 42 | 3.4 | NA | 120 | 82.5 | 7.14 | 0 | 4.7 | 37.5 | 0 |
| Simone et al, 201223 | Sutureless LPN | 101 | 2.4 | 4 | 60 | 100 | 0 | 0 | 1.6 | 57 | 0 |
| Simone et al, 201140 | P-STE followed by Off-C (LPN) | 210 | 4.2 | 7 | 62 | 150 | 6 | 0 | 5 | 46 | 0 |
| Sönmez et al, 201742 | Modified SPSR-PN (LPN) | 16 | 2.72 | 5.3 | 126 | 244 | 0 | 0 | 4.01 | 16.2 | 0 |
| Raheem et al, 201827 | Off-C RPN (<3.2 vs >3.2) | 46/16 | 1.7/4.8 | 7.7/9.8 (PADUA) | 116/163 | 150/575 | 4.3/0 | 6.9/11.1 | 4.4/3.3 | 20 | 0 |
| Zhang et al, 201848 | RFA-assisted PN (O-L) | 182 (12–170) | 3.2 | NA | 100 | 80 | 3.3 | NA | 5.44 | 55.5 | 0 |
| Smith et al, 201121 | On-C (L-O-R)/Off-C (L) | 116(57-51-8)/192 | 2.8/3 | NA | 192/226.5 | 200/500 | 11.2/9.9 | 6/4.7 | 12.3/9.8 | 12 | 23/0 |
| Martin et al, 201238 | On-C/PC/SC/Off-C (LPN-RPN) | 32/8/13/4 | 2.9/2.7/2.9/2.1 | NA | 196/193/184/182 | 150/250/192/288 | 13/25/15/0 | 1.7/0/0/0 | 6.8/7.8/6.6/2.5 | 13.5 | 32/18/ 0/0 |
| Kopp et al, 201249 | On-C/Off-C (OPN) | 164/64 | 3.5/4 | 6.9/6.4 | NA | 300/200 | 0.6/3.2 | 1.2/0 | 13.5/9.4 | 24.7 | 24.5/0 |
| Rimar et al, 201646 | RFA RPN / RPN | 36/49 | 2/2.6 | NA/5.7 | 293/370 | 250/231 | 16.3/11.1 | 3.3/4.1 | 16.5/14.8 | 54 | 31.1/0 |
Abbreviations: CG, Clavien grade; EBL, estimated blood loss; eGFR, estimated glomerular filtration rate; L, laparoscopic; LPN, laparoscopic partial nephrectomy; O, Open; Off-C, off-clamp; On-C, on-clamp; OPN, open partial nephrectomy; NA, not available; PADUA, Preoperative Aspects and Dimensions Used for an Anatomical; PC, progressive clamping; P-STE, preoperative superselective transarterial embolization; R, robotic; RENAL, radius, exophytic extent, nearness to the renal sinus, anterior/posterior location, and location relative to the polar lines; RFA, radio frequency ablation; RPN, robotic partial nephrectomy; SC, selective clamp. SPSR-PN, sequential preplaced suture renorrhaphy partial nephrectomy; WIT, warm ischemia time.
Also in this study, similar results were observed with similar oncologic outcomes in terms of long-term functional results in different zero ischemia PN techniques. In addition, there are not enough numbers of groups for statistical comparison. It was determined that complication rates (≥ Clavien grade 3) were higher in the PN series of zero ischemia with RFA-assisted technique compared to the other techniques.
Conclusion
Recently, zero ischemia PN is becoming widespread among urologists due to its potential advantages in RF preservation. Zero ischemia PN can contribute positively to the protection of RFs by preventing ischemic damage. However, it is still uncertain whether zero-ischemic PN adversely affects long-term RFs by increasing the potential of bleeding. Long-term functional results indicate that zero ischemia PN is a feasible surgical method for selected cases, and further prospective, randomized studies with longer follow-up are needed to elucidate the impact of zero ischemia on long-term RF.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mir MC, Ercole C, Takagi T, et al. Decline in renal function after partial nephrectomy: etiology and prevention. J Urol. 2015;193(6):1889–1898. doi: 10.1016/j.juro.2015.01.093. [DOI] [PubMed] [Google Scholar]
- 2.Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in partial nephrectomy. J Urol. 2013;189(1):36–42. doi: 10.1016/j.juro.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 3.Lane BR, Gill IS, Fergany AF, Larson BT, Campbell SC. Limited warm ischemia during elective partial nephrectomy has only a marginal impact on renal functional outcomes. J Urol. 2011;185(5):1598–1603. doi: 10.1016/j.juro.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RH, Frank I, Lohse CM, et al. The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol. 2007;177(2):471–476. doi: 10.1016/j.juro.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RH, Lane BR, Lohse CM, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012;79(2):356–360. doi: 10.1016/j.urology.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Porpiglia F, Fiori C, Bertolo R, et al. Long-term functional evaluation of the treated kidney in a prospective series of patients who underwent laparoscopic partial nephrectomy for small renal tumors. Eur Urol. 2012;62(1):130–135. doi: 10.1016/j.eururo.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RH, Lane BR, Lohse CM, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58(3):340–345. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Gill IS, Eisenberg MS, Aron M, et al. “Zero ischemia” partial nephrectomy: novel laparoscopic and robotic technique. European Urology. 2011;59(1):128–134. doi: 10.1016/j.eururo.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Hou W, Ji Z. Achieving zero ischemia in minimally invasive partial nephrectomy surgery. Int J Surg. 2015;18:48–54. doi: 10.1016/j.ijsu.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill IS, Munch LC, Clayman RV, et al. A new renal tourniquet for open and laparoscopic partial nephrectomy. J Urol. 1995;154(3):1113–1116. [PubMed] [Google Scholar]
- 12.Cadeddu JA, Corwin TS. Cable tie compression to facilitate laparoscopic partial nephrectomy. J Urol. 2001;165(1):177–178. doi: 10.1097/00005392-200101000-00043. [DOI] [PubMed] [Google Scholar]
- 13.Cadeddu JA, Corwin TS, Traxer O, et al. Hemostatic laparoscopic partial nephrectomy: cable-tie compression. Urology. 2001;57(3):562–566. doi: 10.1016/s0090-4295(00)01009-8. [DOI] [PubMed] [Google Scholar]
- 14.Gofrit ON, Khalaileh A, Ponomarenko O, et al. Laparoscopic partial nephrectomy using a flexible CO2 laser fiber. JSLS. 2012;16(4):588–591. doi: 10.4293/108680812X13462882737258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knezevic N, Kulis T, Maric M, et al. Laparoscopic partial nephrectomy with diode laser: a promising technique. Photomed Laser Surg. 2014;32(2):101–105. doi: 10.1089/pho.2013.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotan Y, Gettman MT, Ogan K, Baker LA, Cadeddu JA. Clinical use of the holmium: YAG laser in laparoscopic partial nephrectomy. J Endourol. 2002;16(5):289–292. doi: 10.1089/089277902760102767. [DOI] [PubMed] [Google Scholar]
- 17.Moinzadeh A, Gill IS, Rubenstein M, et al. Potassium-titanyl-phosphate laser laparoscopic partial nephrectomy without hilar clamping in the survival calf model. J Urol. 2005;174(3):1110–1114. doi: 10.1097/01.ju.0000168620.36893.6c. [DOI] [PubMed] [Google Scholar]
- 18.Shekarriz B. Hydro-Jet technology in urologic surgery. Expert Rev Med Devices. 2005;2(3):287–291. doi: 10.1586/17434440.2.3.287. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Chen L, Ning Y, et al. Hydro-Jet-assisted laparoscopic partial nephrectomy with no renal arterial clamping: a preliminary study in a single center. Int Urol Nephrol. 2014;46(7):1289–1293. doi: 10.1007/s11255-014-0670-9. [DOI] [PubMed] [Google Scholar]
- 20.Kutikov A, Vanarsdalen KN, Gershman B, et al. Enucleation of renal cell carcinoma with ablation of the tumour base. BJU Int. 2008;102(6):688–691. doi: 10.1111/j.1464-410X.2008.07661.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith GL, Kenney PA, Lee Y, Libertino JA. Non-clamped partial nephrectomy: techniques and surgical outcomes. BJU Int. 2011;107(7):1054–1058. doi: 10.1111/j.1464-410X.2010.09798.x. [DOI] [PubMed] [Google Scholar]
- 22.Çömez K, Çelik S, Bozkurt O, Demir Ö, Aslan G, Esen A. Partial nephrectomy for stage I renal cell carcinoma:on-clamp or off-clamp? J Urol Surg. 2016;2:38–41. [Google Scholar]
- 23.Simone G, Papalia R, Guaglianone S, Gallucci M. “Zero ischaemia”, sutureless laparoscopic partial nephrectomy for renal tumours with a low nephrometry score. BJU Int. 2012;110(1):124–130. doi: 10.1111/j.1464-410X.2011.10782.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaczmarek BF, Tanagho YS, Hillyer SP, et al. Off-clamp robot-assisted partial nephrectomy preserves renal function: a multi-institutional propensity score analysis. Eur Urol. 2013;64(6):988–993. doi: 10.1016/j.eururo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Acar Ö, Esen T, Musaoğlu A, Vural M. Do we need to clamp the renal hilum liberally during the initial phase of the learning curve of robot-assisted nephron-sparing surgery? ScientificWorldJournal. 2014;2014:498917. doi: 10.1155/2014/498917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dripps RD. New classification of physical status. Anesthesiol. 1963;24:111. [Google Scholar]
- 27.Abdel Raheem A, Santok GD, Kim LHC, et al. Off-clamp robot-assisted partial nephrectomy: how far shall we proceed? J Laparoendosc Adv Surg Tech A. 2018;28(5):579–585. doi: 10.1089/lap.2017.0464. [DOI] [PubMed] [Google Scholar]
- 28.Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (Padua) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56(5):786–793. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 29.Pansadoro A, Cochetti G, D’amico F, Barillaro F, del Zingaro M, Mearini E. Retroperitoneal laparoscopic renal tumour enucleation with local hypotension on demand. World J Urol. 2015;33(3):427–432. doi: 10.1007/s00345-014-1325-2. [DOI] [PubMed] [Google Scholar]
- 30.Dev HS, Sooriakumaran P, Stolzenburg JU, Anderson CJ. Is robotic technology facilitating the minimally invasive approach to partial nephrectomy? BJU Int. 2012;109(5):760–768. doi: 10.1111/j.1464-410X.2011.10549.x. [DOI] [PubMed] [Google Scholar]
- 31.Gill IS, Patil MB, Abreu AL, et al. Zero ischemia anatomical partial nephrectomy: a novel approach. J Urol. 2012;187(3):807–815. doi: 10.1016/j.juro.2011.10.146. [DOI] [PubMed] [Google Scholar]
- 32.Shin TY, Choi KH, Lim SK, et al. Simplified zero ischemia in robot assisted partial nephrectomy: initial yonsei experience. Korean J Urol. 2013;54(2):78–e84. doi: 10.4111/kju.2013.54.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nohara T, Fujita H, Yamamoto K, Kitagawa Y, Gabata T, Namiki M. Modified anatrophic partial nephrectomy with selective renal segmental artery clamping to preserve renal function: a preliminary report. Int J Urol. 2008;15(11):961–966. doi: 10.1111/j.1442-2042.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 34.Thiex R, Norbash AM, Frerichs KU. The safety of dedicated-team catheter-based diagnostic cerebral angiography in the era of advanced noninvasive imaging. AJNR Am J Neuroradiol. 2010;31(2):230–234. doi: 10.3174/ajnr.A1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ukimura O, Nakamoto M, Gill IS. Three-dimensional reconstruction of renovasculartumor anatomy to facilitate zero-ischemia partial nephrectomy. Eur Urol. 2012;61(1):211–217. doi: 10.1016/j.eururo.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 36.Shao P, Tang L, Li P, et al. Precise segmental renal artery clamping under the guidance of dual-source computed tomography angiography during laparoscopic partial nephrectomy. Eur Urol. 2012;62(6):1001–1008. doi: 10.1016/j.eururo.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 37.Komninos C, Shin TY, Tuliao P, et al. Renal function is the same 6 months after robot-assisted partial nephrectomy regardless of clamp technique: analysis of outcomes for off-clamp, selective arterial clamp and main artery clamp techniques, with a minimum follow-up of 1 year. BJU Int. 2015;115(6):921–928. doi: 10.1111/bju.12975. [DOI] [PubMed] [Google Scholar]
- 38.Martin GL, Warner JN, Nateras RN, Andrews PE, Humphreys MR, Castle EP. Comparison of total, selective, and nonarterial clamping techniques during laparoscopic and robot-assisted partial nephrectomy. J Endourol. 2012;26(2):152–156. doi: 10.1089/end.2011.0304. [DOI] [PubMed] [Google Scholar]
- 39.Gallucci M, Guaglianone S, Carpanese L, et al. Superselective embolization as first step of laparoscopic partial nephrectomy. Urology. 2007;69(4):642–645. doi: 10.1016/j.urology.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 40.Simone G, Papalia R, Guaglianone S, Carpanese L, Gallucci M. Zero ischemia laparoscopic partial nephrectomy after superselective transarterial tumor embolization for tumors with moderate nephrometry score: long-term results of a single-center experience. J Endourol. 2011;25(9):1443–1446. doi: 10.1089/end.2010.0684. [DOI] [PubMed] [Google Scholar]
- 41.Rizkala ER, Khalifeh A, Autorino R, et al. Zero ischemia robotic partial nephrectomy: sequential preplaced suture renorrhaphy technique. Urology. 2013;82(1):100–104. doi: 10.1016/j.urology.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 42.Sönmez MG, Kara C. The effect of zero-ischaemia laparoscopic minimally invasive partial nephrectomy using the modified sequential preplaced suture renorrhaphy technique on long-term renal functions. Wideochir Inne Tech Maloinwazyjne. 2017;3(3):257–263. doi: 10.5114/wiitm.2017.67136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J, Zu Q, du Q, et al. Zero ischaemia laparoscopic nephronsparing surgery by re-suturing. Contemp Oncol. 2014;18:355–358. doi: 10.5114/wo.2014.41385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castro A, Jenkins LC, Salas N, Lorber G, Leveillee RJ. Ablative therapies for small renal tumours. Nat Rev Urol. 2013;10(5):284–291. doi: 10.1038/nrurol.2013.68. [DOI] [PubMed] [Google Scholar]
- 45.Wen CC, Nakada SY. Energy ablative techniques for treatment of small renal tumors. Curr Opin Urol. 2006;16(5):321–326. doi: 10.1097/01.mou.0000240302.11379.5b. [DOI] [PubMed] [Google Scholar]
- 46.Rimar K, Khambati A, McGuire BB, Rebuck DA, Perry KT, Nadler RB. Radiofrequency ablation-assisted zero-ischemia robotic laparoscopic partial nephrectomy: oncologic and functional outcomes in 49 patients. Adv Urol. 2016(2016):8045210. doi: 10.1155/2016/8045210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X, Zhang S, Liu G, et al. Zero ischemia laparoscopic radio frequency ablation assisted enucleation of renal cell carcinoma: experience with 42 patients. J Urol. 2012;188(4):1095–1101. doi: 10.1016/j.juro.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Zhao X, Guo S, Ji C, Wang W, Guo H. Perioperative outcomes of zero ischemia radiofrequency ablation-assisted tumor enucleation for renal cell carcinoma: results of 182 patients. BMC Urol. 2018;18(1):41. doi: 10.1186/s12894-018-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopp RP, Mehrazin R, Palazzi K, Bazzi WM, Patterson AL, Derweesh IH. Factors affecting renal function after open partial nephrectomy-a comparison of clampless and clamped warm ischemic technique. Urology. 2012;80(4):865–871. doi: 10.1016/j.urology.2012.04.079. [DOI] [PubMed] [Google Scholar]
- 50.Colli J, Martin B, Purcell M, Kim YI, Busby EJ. Surgical factors affecting return of renal function after partial nephrectomy. Int Urol Nephrol. 2011;43(1):131–137. doi: 10.1007/s11255-010-9764-1. [DOI] [PubMed] [Google Scholar]
- 51.Wiener S, Kiziloz H, Dorin RP, Finnegan K, Shichman SS, Meraney A. Predictors of postoperative decline in estimated glomerular filtration rate in patients undergoing robotic partial nephrectomy. J Endourol. 2014;28(7):807–813. doi: 10.1089/end.2013.0640. [DOI] [PubMed] [Google Scholar]