Abstract
Background
This article is a meta-analysis aiming to systematically assess the efficacy and safety profiles of PD-1/PD-L1 inhibitors in patients with advanced or metastatic bladder cancer.
Methods
We extracted and examined data from phase I, II, and III clinical trials from the Medline, Embase, and the Cochrane Library, which included patients with metastatic bladder cancer who were treated with PD-1/PD-L1 inhibitors. We performed a meta-analysis to investigate several indexes of efficacy and safety, including the objective response rate (ORR), 1-year overall survival (OS) rate, 1-year progression-free survival (PFS) rate, and adverse event (AE) rate of immune checkpoint inhibitors. The material data were calculated and pooled using The R Project for Statistical Computing and Review Manager 5.3.
Results
After excluding ineligible records, 14 clinical trials were included in our analysis. The pooled frequencies of all-grade AEs and grade ≥3 AEs were 0.63 (95% CI 0.61–0.65, P=0.34) and 0.14 (95% CI 0.11–0.17, P=0.0072), respectively. The summary ORR was 0.21 (95% CI 0.18–0.24 P=0.07), and the 1-year OS and 1-year PFS rates were 0.48 (95% CI 0.42–0.54 P=0.0013) and 0.21 (95% CI 0.16–0.26 P=0.04), respectively. The OR of ORR between the PD-L1-positive and -negative groups was 3.09 (95% CI 2.01–4.75, P=0.08).
Conclusion
The PD-1/PD-L1 therapy showed appropriate efficacy and acceptable incidence of treatment-related AEs. In addition, the level of discrimination of PD-L1 expression might be related to the effect of the PD-1/PD-L1 inhibitors, and patients displaying positive expression might experience a better curative effect than patients displaying negative expression.
Keywords: PD-1 inhibitor, PD-L1 inhibitor, immunotherapy, metastatic bladder cancer, meta-analysis, bladder cancer, oncology
Introduction
Bladder cancer is the fourth most common cancer in males and the 11th most common cancer in females, with 79,030 new cases and 19,870 deaths estimated to occur in the USA in 2017. The incidence and death rates are approximately four times higher in males than females.1 Currently, systemic platinum-based chemotherapy (PBCT) is the standard of care for patients with metastatic and locally advanced urothelial carcinoma, with a median overall survival (OS) of ~14 months. However, many patients are either ineligible for or cannot tolerate the toxicities associated with PBCT. Despite advances in treatment and survival over the past 30 years, treatment regimens for metastatic urothelial carcinoma remained relatively unchanged until the emergence of PD-1 and PD-L1 immune checkpoint therapies.2–4 Immunotherapy is emerging as a viable salvage treatment for patients in whom first-line chemotherapy did not control the disease. In the past 5 years, the success of immune checkpoint inhibition has led to a resurgence of enthusiasm for immunotherapy as a treatment for solid tumors.5 The PD-1 (CD279) receptor and its ligand PD-L1 (CD274, B7-H1) comprise one of the main immune checkpoint pathways that downregulates immune activity.6 PD-1 is expressed at high levels on activated T cells, myeloid dendritic cells, B cells, thymocytes, natural killer cells, and monocytes within the tumor microenvironment in many different tumor types.7 PD-L1 is widely expressed on a multitude of immune cells (ICs) and might be upregulated on TCs.8 Anti-PD-1 and anti-PD-L1 monoclonal antibodies have displayed good activity in several clinical trials of patients with different types of cancer.9–11 However, an evidence-based systematic review and summary data for treatment indicators of the safety and efficacy of PD-1/PD-L1 inhibitors as treatments for metastatic bladder carcinoma are not available. Preliminary reports of clinical trials showed a difference in the treatment efficacy of PD-1 and PD-L1 inhibitors in patients with bladder cancer. Results from previous studies must be analyzed to offer evidence-based guidelines for clinicians. This article is a meta-analysis focusing on the further evaluation of the efficacy and safety of anti-PD-1/PD-L1 agents in patients with advanced bladder cancer, and subgroup analyses were also performed to evaluate the efficacy among patients with different PD-L1 expression levels.
Methods
Search strategy
A literature review of major computerized bibliographic databases, including Medline, Embase, and the Cochrane Library, was conducted using the following comprehensive search terms: “Urinary Bladder Neoplasms [Mesh]” OR “Bladder Cancer” OR “metastatic urothelial carcinoma” OR “metastatic bladder cancer” OR “bladder tumor” AND “immunotherapy [Mesh]” OR “programmed cell death 1” OR “programmed cell death ligand 1” OR “PD-L1” OR “PD-1” OR “immune checkpoint inhibitor” OR “Atezolizumab” OR “Pembrolizumab” OR “Durvalumab” OR “Nivolumab” OR “Avelumab”. Two authors independently screened the studies for eligibility, and disagreements were judicially resolved by a third reviewer.
Selection criteria
Inclusion articles satisfied the following criteria: 1) single-arm or randomized clinical trials evaluated anti-PD-1/PD-L1 inhibitors as treatments for patients with metastatic bladder cancer; 2) articles with or without reports of PD-L1 expression levels; and 3) data were available for at least one or all the following outcomes: objective response rate (ORR), 1-year OS rate, 1-year progression-free survival (PFS) rate, and rates of all grades of drug-related adverse events (AEs) and grade 3–4 AEs.
Exclusion criteria for articles included letters, editorials, case reports, reviews, and studies that lacked necessary data, were not related to our research topics, or were not clinical trials.
Data extraction
Table 1 summarizes the baseline characteristics of the patients. Three reviewers independently screened reports and extracted data from the included studies. The following data were collected: first author, publication year, number of patients, study phase, intervention methods, doses of drugs, rates of any grade and grade 3 or higher AEs, ORR, 1-year OS rate, 1-year PFS rate, and age (Table 1).
Table 1.
Baseline characteristics and data of included studies using PD-1/PD-L1 inhibitors
| Study | Year | Journal | No. | Phase | Intervention | Dose | Mean age (range) | Cancer type | All AE rate (%) | 3–4 AE rate | ORR (%) | 1-year OS rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balar et al13 | 2017 | The Lancet | 119 | phase II | Atezolizumab | 1,200 mg IV q3 weeks | 73 (51–92) | Metastatic/advanced BC | 66 | 16 | 23 | 57 |
| Rosenberg et al14 | 2016 | The Lancet | 310 | phase II | Atezolizumab | 1,200 mg IV q3 weeks | 65 (36–86) | Metastatic/advanced BC | 69 | 16 | 15 | 37 |
| Chen et al15 | 2014 | Nature | 205 | phase I | Atezolizumab | 1,200 mg IV q3 weeks | NR | Metastatic BC | 57.40 | 4.40 | NR | NR |
| Loriot et al16 | 2016 | Annals of Oncology | 310 | phase II | Atezolizumab | 1,200 mg IV q3 weeks | 66.5 (42–86) | Metastatic/advanced BC | 70 | 16 | 16 | NR |
| Powles et al17 | 2014 | Annals of Oncology | 65 | phase I | Atezolizumab | 15 mg/kg IV q3 weeks | 66 (32–91) | Metastatic BC | 57 | 4 | 26 | NR |
| Powles et al18 | 2017 | JAMA Oncology | 191 | phase I/II | Durvalumab | 10 mg/kg IV q2 weeks | 57 (26–82) | Metastatic/advanced BC | 60.70 | 6.80 | 17.80 | 55 |
| Massard et al19 | 2016 | Journal of Clinical Oncology | 61 | phase I/II | Durvalumab | 10 mg/kg IV q2 weeks | 66.0 (34–81) | Advanced BC | 63.90 | 4.90 | 31 | NR |
| Levy et al20 | 2016 | European Journal of Cancer | 23 | phase I/II | Durvalumab | 10 mg/kg IV q2 weeks | 67.0 (34–88) | Metastatic/advanced BC | 50 | 0 | 60 | 44 |
| Sharma et al21 | 2016 | The Lancet Oncology | 78 | phase I/II | Nivolumab | 3 mg/kg IV q2 weeks | 65.5 (31–85) | Metastatic BC | 59 | 22 | 24.40 | 46 |
| Sharma et al22 | 2017 | The Lancet Oncology | 265 | phase II | Nivolumab | 3 mg/kg IV q2 weeks | 66 (38–90) | Metastatic BC | 64 | 18 | 19.60 | NR |
| Bellmunt et al23 | 2017 | The New England Journal of Medicine | 270 | phase III | Pembrolizumab | 200 mg IV q3 weeks | 67 (NR) | Advanced BC | 60.90 | 15 | 21.10 | 43.90 |
| Plimack et al24 | 2017 | The Lancet Oncology | 25 | phase IB | Pembrolizumab | 10 mg/kg IV q2 weeks | 70 (44–85) | Metastatic/advanced BC | 45 | 15 | 26 | 50 |
| Balar et al25 | 2017 | The Lancet Oncology | 265 | phase II | Pembrolizumab | 200 mg IV q3 weeks | 74 (34–94) | Metastatic/advanced BC | 62 | 16 | 24 | NR |
| Apolo et al26 | 2017 | Journal of Clinical Oncology | 37 | phase IB | Avelumab | 10 mg/kg IV q2 weeks | 68 (63–73) | Metastatic BC | 65.90 | 6.80 | 18.20 | 54.30 |
Abbreviations: AE, adverse event; BC, bladder cancer; IV, intravenous; NR, not related; ORR, objective response rate; OS, overall survival.
Outcome measures
The outcome measures were ORR, PFS, OS, and AEs. This meta-analysis follows the guidelines provided by the PRISMA report (statement).12
Data analyses
The data analyses were performed using Computer Program Review Manager 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) and The R Project for Statistical Computing. Some degree of heterogeneity was expected, and analyses were performed using random-effects models. Safety was assessed by summarizing the risk of any-grade AEs and grade ≥3 AEs. The efficacy of PD-1/PD-L1 inhibitors was evaluated by calculating the overall ORR, pooled 1-year OS rate, and 1-year PFS rate with corresponding 95% CIs. Included patient tumor samples were centrally assessed for PD-L1 expression by immunohistochemistry (IHC). The PD-L1 tumor-infiltrating IC status was defined by the percentage of ICs: IC0 (<1%), IC1 (≥1% but <5%), and IC2/3 (≥5%). Considering that Review Manager 5.3 cannot analyze single-rate samples, the frequency rates among studies were determined using The R Project for Statistical Computing. Heterogeneity was assessed using the χ2 test and I2 statistics, and we performed subgroup analyses to evaluate heterogeneity. Publication bias was assessed using funnel plots or Egger’s funnel plots.
Results
Search results and characteristics of patients in the included studies
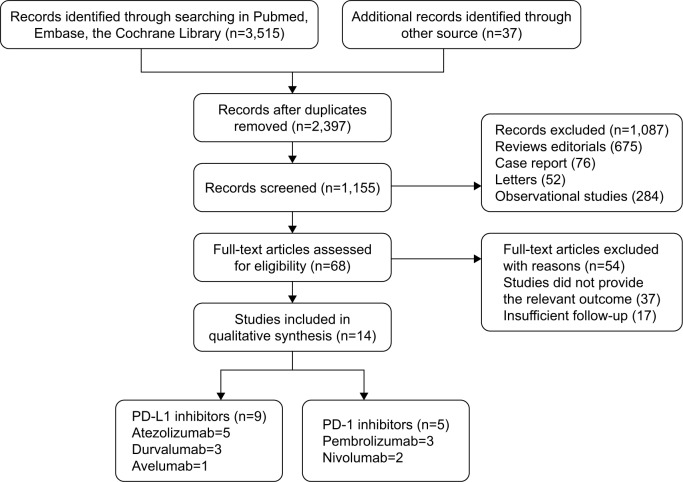
The PRISMA diagram of the study selection process and the reasons for exclusion is shown in Figure 1. Our search retrieved 3,552 publications. In all, 2,397 studies were excluded as duplicates and 1,087 were excluded because they did not meet the eligibility criteria in the initial selection. After reviewing the abstracts and full articles, 14 distinct trials were included in our analysis after removing the articles lacking necessary data and those utilizing insufficient followup periods. All studies were published in the last 4 years. Nine trials assessed PD-L1 inhibitors (atezolizumab=5, durvalumab=3, and avelumab=1), and five trials assessed PD-1 inhibitors (pembrolizumab=3 and nivolumab=2). A total of 2,224 patients were included in this analysis (Figure 1).
Figure 1.
Flowchart of study selection procedure.
Efficacy outcomes of PD-1/PD-L1 agents
Pooled 1-year OS rate, 1-year PFS rate, and overall ORR
The overall ORR, pooled 1-year OS rate, and 1-year PFS rate were used to measure the efficacy of PD-1/PD-L1 inhibitors in treating metastatic bladder cancer.
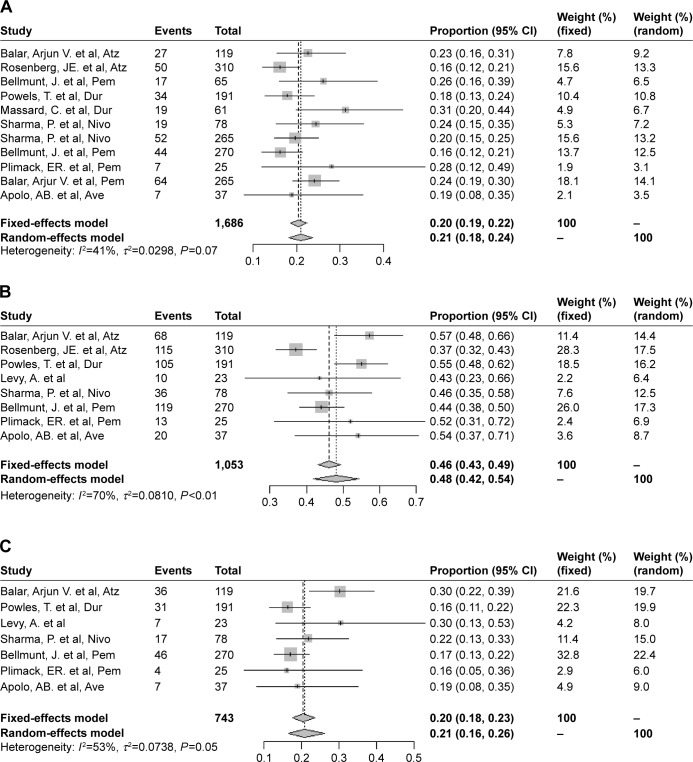
In all, 11 trials were used to analyze the ORRs, eight trials were assessed for the 1-year OS rate, and seven trials were used to assess the 1-year PFS rate. The pooled ORR, OS rate, and PFS rate were 0.21 (95% CI 0.18–0.24, I2=41%, P=0.07), 0.48 (95% CI 0.42–0.54, I2=70%, P=0.0013), and 0.20 (95% CI 0.16–0.26, I2=53.0%, P=0.05), respectively (Figure 2). We conducted asymmetry tests using Egger’s funnel plots to investigate publication bias for the overall ORR. Egger’s funnel plots did not reveal evidence of publication bias (Figure 3).
Figure 2.
(A) Forest plot for pooled ORR for patients receiving immune checkpoint inhibitors. (B) Forest plot for pooled 1-year OS rate. (C) Forest plot for pooled 1-year PFS rate.
Abbreviations: Atz, atezolizumab; Ave, avelumab; Dur, durvalumab; Nivo, nivolumab; ORR, objective response rate; Pem, pembrolizumab; OS, overall survival; PFS, progression-free survival.
Figure 3.
Asymmetry test using Egger’s funnel plots to investigate publication bias for the overall ORR.
Abbreviations: ORR, objective response rate; SE, standard error.
Different PD-L1 expression levels affect the benefits patients receive from anti-PD-1/PD-L1 inhibitors
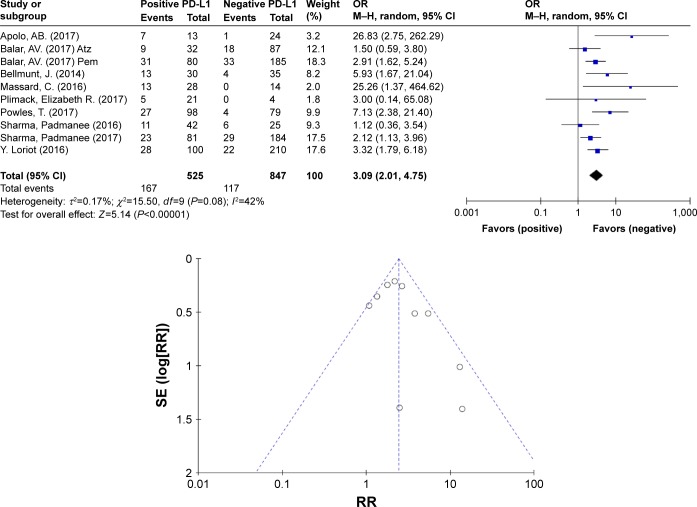
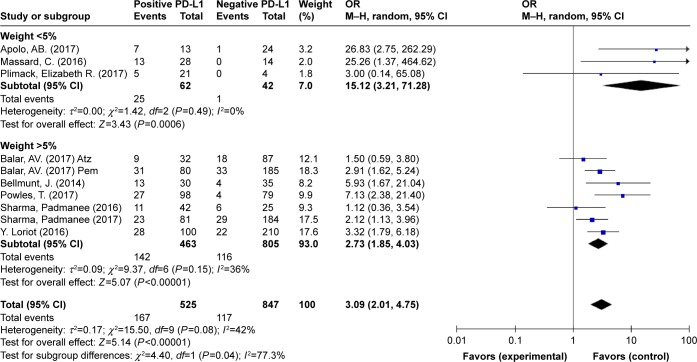
The ORR of patients with different PD-L1 expression levels was examined in 10 trials. PD-L1 positivity was defined as ≥5% of tumor-infiltrating ICs staining for PD-L1 by IHC. Patients expressing PD-L1 at levels <5% were included in the negative expression group. The pooled OR of ORRs between the PD-L1-positive and PD-L1-negative expression groups was 3.09 (95% CI 2.01–4.75, I2=42%, P=0.08), indicating a better efficacy in the PD-L1-positive group (Figure 4). Based on the outcome, heterogeneity might have affected the results; therefore, a further subgroup analysis was performed to assess if the low-weight studies (3) affected the results. The results of this analysis did not yield any significant differences (2.73 vs 3.09) in the pooled ORR between the included studies with the highest weight (>5%) and overall studies (Figure 5). Thus, PD-1/PD-L1 inhibitors displayed an encouraging survival rate and efficacy.
Figure 4.
Forest plot and funnel plot for ORs of ORR between the PD-L1-positive and PD-L1-negative expression groups.
Abbreviations: Atz, atezolizumab; M–H, Mantel–Haenszel; ORR, objective response rate; Pem, pembrolizumab; SE, standard error.
Figure 5.
Forest plot for subgroup analysis for ORs of ORR between the included studies with the highest weight (>5%) and overall studies.
Abbreviations: Atz, atezolizumab; M–H, Mantel–Haenszel; ORR, objective response rate; Pem, pembrolizumab.
Safety assessment
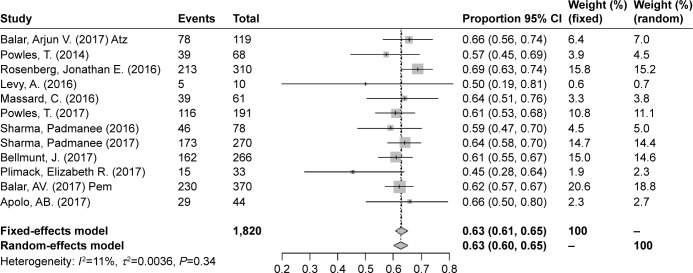
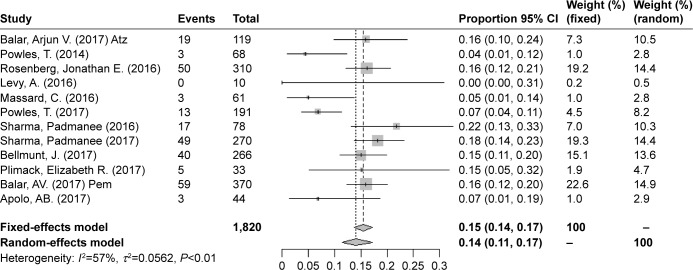
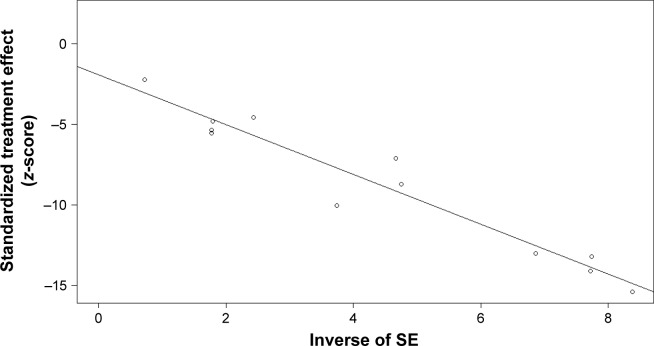
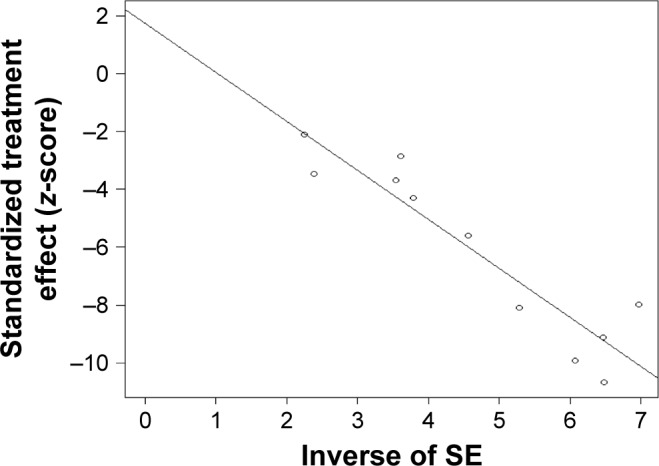
The overall risks of all-grade AEs and grade ≥3 AEs were calculated to evaluate the safety of PD-1/PD-L1 inhibitors as treatments for bladder cancers. In all, 12 studies were used to determine the pooled rate of all-grade AEs, and the same 12 studies were used to calculate the grade ≥3 AE rate. Considering the heterogeneity between included studies, we used a random-effects model to assess the summarized rate of AEs. The pooled rates of any grade AEs and grade ≥3 AEs were 0.63 (95% CI 0.60–0.65, I2=11%, P=0.34) and 0.14 (95% CI 0.11–0.17, I2=57%, P=0.0072), respectively (Figures 6 and 7). An additional funnel plot and Egger’s funnel plot asymmetry test of the grade ≥3 AEs rate did not reveal apparent evidence of publication bias (Figures 8 and 9). Based on the AE data, PD-1/PD-L1 inhibitors had an acceptable side effect profile and an acceptable safety level.
Figure 6.
Forest plot for the pooled rates of any-grade AEs.
Abbreviations: AE, adverse event; Atz, atezolizumab; Pem, pembrolizumab.
Figure 7.
Forest plot for the pooled rates of grade ≥3 AEs.
Abbreviations: AE, adverse event; Atz, atezolizumab; Pem, pembrolizumab.
Figure 8.
Funnel plot to investigate publication bias for the grade ≥3 AE rate.
Abbreviations: AE, adverse event; SE, standard error.
Figure 9.
Asymmetry test using Egger’s funnel plots to investigate publication bias for the grade ≥3 AE rate.
Abbreviations: AE, adverse event; SE, standard error.
Discussion
Patients with advanced or metastatic bladder cancer have few treatment choices and low survival rates, particularly after standard PBCT fails. Immunotherapy remains an evolving treatment modality for metastatic bladder cancer. Both the unmet need for second-line therapies for bladder cancer and a resurgence of immunotherapy as a treatment for other solid tumors coincided to spur the use of immunotherapy for bladder cancer. Several types of tumor cells have been shown to evade immune recognition by expressing PD-L1, which represents an emerging antitumor method. Recently, pembrolizumab, a PD-1 inhibitor, has been granted full the US Food and Drug Administration (FDA) approval based on its high antitumor activity, tolerability, and efficacy, as well as its notable prolonged and durable responses in the second-line setting.27
Nivolumab (a PD-1 inhibitor), atezolizumab, durvalumab, and avelumab (PD-L1 inhibitors) have also gained accelerated drug approval in the second-line setting.28 The efficacy and safety of each of these PD-1/PD-L1 inhibitors have been examined in well-organized phase I/II/III clinical trials, but data from summary studies are insufficient or lacking. To our knowledge, this study represents the first meta-analysis to systematically investigate the efficacy and AEs of five recently approved and popular PD-1/PD-L1 inhibitors drugs as treatments for metastatic or advanced bladder cancer. In addition, we also reported differences in the treatment effects on patients with distinct PD-L1 expression levels.
Since the development of immunological checkpoints, the field of immunotherapy has dramatically changed the prospects for cancer treatment. The FDA has approved mAbs for immune checkpoint ligands and receptors (such as PD-L1 and PD-1) for the treatment of >25 cancers.29 Our meta-analysis included 14 eligible single-arm and randomized controlled trial (RCT) clinical studies with 2,224 patients. Based on the data of results, the efficacy of PD-1/PD-L1 inhibitors is relatively satisfactory and encouraging, and the results also confirmed that the results of previously published phase I/II/III trials of single PD-1/PD-L1 drugs are clinically significant. However, in order to further develop the biological efficacy of immunotargeting drugs, further research support at the biomolecular level of bladder cancer is essential, especially for the microscopic classification of bladder cancer. This can play a role in selecting the patients most likely to respond to treatment with immunotherapeutic agents. The basal-squamous subtype is characterized by higher incidence in women, squamous differentiation, basal keratin expression, and a high expression of PD-L1. The luminal-infiltrated subtype contained 23 of 24 tumors, which was reported to benefit most from anti-PD-L1 treatment and had an intermediate 5-year survival comparable to basal-squamous and luminal subtypes. These tumors had increased expression of several immune markers, including PD-L1 and PD-1.30 Currently, IHC is often used to assess the expression of PD-1/PD-L1. From the data analysis result, high PD-L1 expression levels have been shown to increase the effects of anti-PD-1/PD-L1 treatments, and PD-L1 is the key target of the PD-1/PD-L1 therapy; therefore, we analyzed the ORR between the PD-L1-positive and -negative groups. The pooled OR showed that the PD-L1-positive group experienced an obvious improvement in the ORR compared with the PD-L1-negative group. Based on the heterogeneity shown in the funnel plot, we conducted additional subgroup analyses. After excluding articles with a weight <5%, we compared the OR for the remaining articles with the original OR. The results showed an acceptable difference between the total included trials and the subgroup analysis, suggesting that in patients with metastatic bladder cancer who are positive for PD-L1, the efficacy of the inhibitors will be better.
Knowledge of the physiological brake function of immune checkpoints has led to the development of PD-1/PD-L1 agents that inhibit these checkpoints and promote T-cell function. This undoubtedly increases the effectiveness of the immune system against tumor cells. However, as a side effect, this produces a series of immune-related AEs. Moreover, we systematically assessed AEs to evaluate the safety of PD-1/PD-L1 inhibitors. The reported treatment-related AEs included decreased appetite, fatigue, nausea, rash, diarrhea, asthenia, stomatitis, anemia, alopecia, and neutropenia.31 The overall rate of any-grade AEs was 63%. However, the risk of grade ≥3 AEs only reached 14% in all included patients. The outcomes obtained from the summarized data implied that PD-L1 drugs produce a wide range of AEs that should not be ignored in patients with advanced bladder cancer, although the incidence of advanced AEs (grade >3) is acceptable. Based on these data, PD-1/PD-L1 inhibitors possess a latent treatment potential for metastatic bladder cancer with an acceptable risk profile.
By scanning the outcome data, we observed moderate heterogeneity in the 14 articles included in the present meta-analysis. Heterogeneity existed in some of the analysis, with I2 >50%. Potential sources of this heterogeneity might arise from the use of different doses of PD-1/PD-L1 agents or might be attributed to patients’ underlying stable diseases. Therefore, we used random-effect models in our meta-analysis to ensure the objectivity of the results. Meanwhile, we generated Egger’s funnel plots to assess publication bias among the included studies. After our critical analysis of Egger’s funnel plot, we did not observe publication bias in the studies investigated in the meta-analysis, and therefore, publication bias was not a factor contributing to heterogeneity.
Limitations
Critically, several limitations exist in our systematic analysis. First, because a larger number of RCTs of PD-1/PD-L1 drugs targeting bladder carcinoma have not been conducted, most of the included studies were completed phase I/II/III randomized single-arm trials, and potential performance bias might exist in these studies, which causes some inevitable bias. Second, due to the scarcity of control studies on bladder cancer, the trials included in our meta-analysis lacked data showing comparisons of the PD-1/PD-L1 inhibitors with chemotherapy drugs. In one of our inclusion studies, pembrolizumab resulted in significantly longer OS than the choice of paclitaxel, docetaxel, or vinflunine (10.3 months vs 7.4 months) and was associated with a higher rate of objective response (21.1%–11.4%) and a lower rate of treatment-related AEs (60.9%–90.2%) than chemotherapy.23 Although there is almost no direct comparison with chemotherapy or other treatments in our study, included data of RCTs show that our analysis results are relatively consistent with the results of the currently finished RCTs of PD-1/PD-L1 inhibitors to metastatic bladder cancer. In addition to comparing the differences in efficacy between patients with different PD-L1 expression levels, we performed a single-rate meta-analysis to summarize the pooled precise indicators of efficacy and safety of PD-1/PD-L1 drugs and provide statistical references for clinicians. Currently, large-scale clinical trials and RCTs are ongoing in several medical experimental centers worldwide (Table 2), and we are anxiously awaiting their experimental results to further analyze the outcomes of PD-1/PD-L1 inhibitors in patients with metastatic bladder cancer.
Table 2.
Ongoing clinical trials and RCTs in locally advanced or metastatic bladder cancer
| Agent | NCT no. | Phase | No. of patients | Arms | Patients | End point | Follow-up | Completion of study |
|---|---|---|---|---|---|---|---|---|
| Nivolumab | 2632409 | III | 640 | Nivolumab vs placebo | Bladder or upper urinary tract cancer after radical surgery | DFS | 5 years | May 2020 |
| Atezolizumab | 2807636 | III | 1,200 | Atezolizumab ± chemotherapy vs chemotherapy | Locally advanced or metastatic urothelial carcinoma | PFS, OS, AE | 44 months | December 2018 |
| Pembrolizumab | 2256436 | III | 542 | Pembrolizumab vs chemotherapy | Metastatic/unresectable locally advanced BC | PFS, OS | 30 months | March 2019 |
| Avelumab | 2603432 | III | 668 | Best supportive care ± avelumab | Locally advanced/metastatic urothelial | OS | 40 months | July 2020 |
| Durvalumab | 2516241 | III | 74 | Durvalumab vs SoC | Metastatic advanced BC | PFS | 50 months | January 2024 |
| Pembrolizumab | 2500121 | II | 200 | Pembrolizumab vs placebo | Metastatic urothelial cancer after the first-line chemo as maintenance | PFS | 6 months | November 2019 |
Abbreviations: AE, adverse event; BC, bladder cancer; DFS, disease-free survival; OS, overall survival; PFS, progression-free survival; RCT, randomized controlled trial; SoC, standard of care.
Conclusion
In this meta-analysis, PD-1/PD-L1 immune checkpoint inhibitors showed durable outcomes on clinical efficacy and acceptable safety in patients with advanced and metastatic bladder cancer. Additional data from ongoing RCTs of bladder carcinoma are urgently needed to confirm that PD-1/PD-L1 drugs are tolerable and reliable. These exciting advances will provide further hope and promise to patients with urothelial bladder cancer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers: 81772713, 81372752, 81472411, and 81101932); Science and Technology Project of Qingdao, China (grant number: 15-9-1-105-jch); and Higher Educational Science and Technology Program of Shandong, China (grant number: J16LL04).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2015;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bellmunt J, Orsola A, Wiegel T, et al. Bladder cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi45–vi49. doi: 10.1093/annonc/mdr376. [DOI] [PubMed] [Google Scholar]
- 3.Milowsky MI, Rumble RB, Booth CM, et al. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology Guideline): American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34(16):1945–1952. doi: 10.1200/JCO.2015.65.9797. [DOI] [PubMed] [Google Scholar]
- 4.Ghasemzadeh A, Bivalacqua TJ, Hahn NM, Drake CG. New strategies in bladder cancer: a second coming for immunotherapy. Clin Cancer Res. 2016;22(4):793–801. doi: 10.1158/1078-0432.CCR-15-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong YNS, Joshi K, Pule M, et al. Evolving adoptive cellular therapies in urological malignancies. Lancet Oncol. 2017;18(6):e341. doi: 10.1016/S1470-2045(17)30327-3. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67. doi: 10.1016/j.ctrv.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou TC, Sankin AI, Porcelli SA, et al. A review of the PD-1/PD-L1 checkpoint in bladder cancer: from mediator of immune escape to target for treatment. Urol Oncol. 2017;35(1):14–20. doi: 10.1016/j.urolonc.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Lee-Gabel L, Nadeau MC, Ferencz TM, Soefje SA. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Pract. 2015;21(6):451–467. doi: 10.1177/1078155214538087. [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 12.Moher D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Revista Española De Nutrición Humana Y Dietética. 2009;18(3):e123. [PMC free article] [PubMed] [Google Scholar]
- 13.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. The Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Vogelzang JMJ, Shen X, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;194(4):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 16.Loriot Y, Rosenberg JE, Powles TB, et al. Atezolizumab (atezo) in platinum (plat)-treated locally advanced/metastatic urothelial carcinoma (mUC): Updated OS, safety and biomarkers from the Ph II IMvigor210 study. Ann Oncol. 2016;27(Suppl 6):266–295. [Google Scholar]
- 17.Powles T, Fine GD, Eder JP, et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in PTS with metastatic urothelial bladder cancer (UBC) Ann Oncol. 2014;25(Suppl 4):280. [Google Scholar]
- 18.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of Durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of Durvalumab (MEDI4736), an Anti-Programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119–3125. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy A, Massard C, Soria JC, Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single centre subset analysis from a phase 1/2 trial. Eur J Cancer. 2016;68:156–162. doi: 10.1016/j.ejca.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590–1598. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 23.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a nonrandomised, open-label, phase 1B study. Lancet Oncol. 2017;18(2):212–220. doi: 10.1016/S1470-2045(17)30007-4. [DOI] [PubMed] [Google Scholar]
- 25.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 26.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an Anti-Programmed Death-Ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117–2124. doi: 10.1200/JCO.2016.71.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in urothelial cancer: Recent results and future perspectives. Drugs. 2017;77(10):1077–1089. doi: 10.1007/s40265-017-0748-7. [DOI] [PubMed] [Google Scholar]
- 28.Chism DD. Urothelial carcinoma of the bladder and the rise of immunotherapy. J Natl Compr Canc Netw. 2017;15(10):1277–1284. doi: 10.6004/jnccn.2017.7036. [DOI] [PubMed] [Google Scholar]
- 29.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26. doi: 10.1186/s12929-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–556. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]