Abstract
We studied monogenean communities of 11 populations of Astyanax aeneus (Günther) separated by small geographical distances along 60 km of the Lacantún river in Chiapas, Mexico, in February and August 2012. We found 12 monogenean taxa. Amongst these, five species specialist for Astyanax were widely distributed regionally, constituting 90% of the total collected monogeneans, with one of these species dominating most component communities. The high similarities in terms of composition between the component communities (SJaccard > 60%) as well as in terms of the abundance and composition between infracommunities (SBray Curtis > 40%), provide empirical evidence that transmission, both between hosts at the same location and between component communities, is high and effective. No resemblance pattern was detected between locations in terms of their spatial distribution. The composition of these communities was spatially and temporally consistent over the two very different weather periods sampled. These communities were not saturated. Our analysis suggests that the potential richness of the infracommunities is proportional to the number of monogenean species available in the component community. We found aggregation in the populations and between monogenean species. Intraspecific aggregation is density dependent, suggesting that intraspecific competition for space is not a limiting factor for the development of the population. We evaluated the associations for each species pair and detected 77% negative interactions (134/177 associations), suggesting that interspecific competition plays an important role in shaping these communities. The negative correlations of abundance between pairs of species contributes to confirmation of competition. Intraspecific aggregation increased relative to interspecific aggregation with richness in the component community, facilitating coexistence of the species. Our results suggest that these are interactive communities, where monogeneans disperse efficiently from a common source, colonize patches (hosts) together, and compete with other species even at low population densities. Finally, the coexistence of these species is favored by the unpredictable recruitment and aggregated use of fragmented resources.
Keywords: Metacommunity, Transmission, Species richness, Saturation, Competition
Graphical abstract

Highlights
-
•
We explore richness and coexistence of monogeneans of a small fish in a large river.
-
•
Empirical evidence that transmission between hosts/locations is efficient is provided.
-
•
We provide empirical evidence of unsaturated/low density but interactive communities.
-
•
Monogeneans show intra and interspecific aggregation both density-dependent.
-
•
They establish regionally consistent negative interactions repeated between locations.
1. Introduction
Understanding how local communities are configured and their interactions within a region are among the basic objectives of metacommunity studies (Logue et al., 2011; Sarremejane et al., 2017). In this context, parasitic systems constitute excellent study models to explore essential aspects of ecology (Price and Clancy, 1983; Holmes and Price, 1986; Rohde, 1991; Gotelli and Rohde, 2002).
The populations of host fish distributed along a river constitute patches of resources for parasites. Within each population, each fish constitutes a patch and is a habitable island for parasites (Price and Clancy, 1983; Holmes and Price, 1986). Fish movements potentially affect the distribution and abundance of parasites in the system. Hence, along the river, parasite communities of fish may vary depending on the continuity or separation of the habitats (Janovy et al., 1997; Weichman and Janovy, 2000; Knipes and Janovy, 2009). Some studies focused on the spatial variation of parasites of riverine fish have shown that the composition of parasite communities is relatively persistent (Knipes and Janovy, 2009; Ferrari-Hoeinghaus et al., 2006; Ferreira-Sobrinho and Tavares-Dias, 2016). By contrast, other studies report considerable changes in the community structure across such a spatial gradient (Weichman and Janovy, 2000; Bellay et al., 2012; Acosta et al., 2015; Córdova and Pariselle, 2007; Espinal-Carrión and López-López, 2010). Hence, doubts remain as to what extent fish parasite communities in a river are independent between host populations that inhabit that river.
The biology of monogeneans (Platyhelminthes: Monogenea) allows examination of the connectivity both between the patches of habitat that constitute the populations of their hosts (component community sensu Holmes and Price, 1986), and between monogeneans that infect each fish (infracommunity). A basic question is whether or not the dispersion of monogeneans is limited when the population of hosts are separated on a scale of kilometers along a river. In a system like this, the dispersion of these parasites between populations must be passive. In other words, the monogeneans will be transported by fish that migrate from one population to another; within each host population, the contact or proximity between fish will facilitate the process of parasite transmission between hosts. Determining whether the dispersion of the species is limited or not, is important because it will allow exploration of various assembly mechanisms of the communities to explain the local coexistence of species in a metacommunity (Worthen and Rohde, 1996; Logue et al., 2011).
Currently it is accepted that communities of parasites are present in an isolationist-interactive continuum, depending on whether or not the interaction between species occurs (Holmes, 1973, 1990; Rohde, 1979, 1991; Holmes and Price, 1980, 1986; Kennedy et al., 1986; Kennedy, 1990, 1995; Worthen and Rohde, 1996; Morand et al., 1999; Poulin and Luque, 2003). Caswell (1976) has suggested that non-interactive communities lack saturation and species can coexist in the community because space is not limited by the number of individuals. Rohde (1977, 1979, 1991) argues that many communities of parasites exist with low population densities, and that in such cases, interactions between species do not occur. Rohde suggests that intraspecific interactions explain niche restriction because they facilitate reproduction, enabling cross-linking.
Moreover, it has been demonstrated that aggregation is an important factor to determine local richness of monogeneans in fish populations (Morand et al., 1999; Simková et al., 2000, 2001; Agrawal et al., 2017). The host population represents a collection of patches of resources amongst which the parasites are heterogeneously distributed. Some patches (i.e. hosts) have many individuals (parasites) while others have only a few. Aggregation then refers to the degree to which individuals are added between the patches (Ives, 1991). Generally, parasite populations are distributed in an aggregated manner between individual hosts, regardless of whether they are from poor or rich communities (Poulin, 1998a). This means that the majority of hosts have a few parasites, while most parasites are concentrated in a few hosts (Poulin and Morand, 2004). Intraspecific aggregation allows the coexistence of species that would otherwise be excluded (Ives, 1988, 1991). More parasite species can coexist in the same host population when their distributions between individual hosts are aggregated. Now, the number of individuals of a parasite species in a host may depend on the presence or the number of a second species. Accordingly, intensity data of the infections in a sample can be used to identify positive or negative associations between pairs of helminth species within hosts. In general, consistently negative associations constitute strong evidence of competitive interactions between species (Dezfuli et al., 2001; Poulin, 2001, 2007).
With this in mind, the goal of this study was to explore the factors that might contribute to the richness and coexistence of monogenean parasite species of Astyanax aeneus (Günther) (Teleostei: Characidae) in a large neotropical river in southern Mexico, using separation on a scale of kilometers between locations that are inhabited by these fish populations (distance range from 780 m to 60 kms between locations). On this small geographical scale, we assumed that the isolation of parasite communities depends on the size of oncomiradicia or propagules with respect to the distance between patches, and the size of the fish host itself (<10 cm) that would be sufficient to isolate the communities of parasites. By applying an observational empirical study, we intended to indirectly assess the dispersion of the species as a determining factor for the assembly of these communities, and to describe patterns of local coexistence of monogenean species inside a metacommunity. Two hypotheses were explored: 1, ecological conditions of each location will offer distinct opportunities for transmission. If fish movements are restricted, the differences between each parasite population could reveal ecological differences. However, if fish are moving freely along the river, the communities of parasites in the different locations should be indistinguishable due to the mixing of hosts. We expect a low rate of dispersion between populations of monogeneans when there is poor connectivity between habitat patches. This would be observed as high variability in the composition and structure of the communities. By contrast, homogeneity in the composition and structure of communities would indicate a high rate of dispersion of the species that would mean high connectivity between patches. In this context, we expect that the closest patches would have greater similarity in their communities, and that this similarity would decline with distance between patches of host populations.
Our model of study consisted of many patches, identical in resources (hosts), that sustain several populations of monogeneans. The level of competition that a monogenean experiences would depend on the number and species of monogeneans that cohabit the same patch (host), and the distribution of monogeneans in these patches. In the second hypothesis, we assessed aggregation levels of populations of monogeneans to test whether this was important in determining the local richness of parasites within a host population; whether the coexistence between species was improved, and whether intraspecific aggregation exceeded interspecific aggregation. To assess the importance of interspecific interactions in the structure of these communities, we also determined co-occurrence patterns of monogeneans among A. aeneus populations, to identify consistent negative correlations.
2. Materials and methods
2.1. Host species and sampling
The characid A. aeneus is one of the most ubiquitous fish in Central America; it is distributed from lowland areas to altitudes as high as 1000 m. In the Gulf of Mexico and the Caribbean, it is found from the Papaloapan river in Mexico to Costa Rica. On the Pacific-side, it occurs from the Almeria river in Mexico to Colombia. It is a tolerant species that lives in rivers, creeks, lakes and coastal lagoons. It forms shoals, is omnivorous and attains a maximum length of 140 mm (Bussing, 1998; Miller et al., 2005).
We examined 15 A. aeneus from each of 11 locations (Fig. 1), situated at the mouth of the streams opening into the main body of the Lacantún river, at the Montes Azules Biosphere Reserve in the Lacandon forest, Chiapas in southern Mexico. This river belongs to the Usumacinta river watershed. The area of study is located ∼800 km from the mouth of the Usumacinta river in the Gulf of Mexico. This geographic area receives torrential rain (average annual precipitation 199 cm). Between June and October, the average monthly precipitation is > 20 cm/month, whilst between the months of February and April, average precipitation is < 8 cm/month (Hudson et al., 2005; Rodiles-Hernández, 2005). Hence, water volume in the main river and its tributaries, the width of the main river and streams, stream velocity, turbidity and many other environmental factors are completely distinct between these periods. To obtain a general view of the parasitism in both seasons, we examined 158 fish during February and 150 during August 2012; in total we examined 308 specimens of A. aeneus.
Fig. 1.
Eleven sample locations situated on the opening of streams tributaries to the main Rio Lacantún in the Biosphere Reserve Montes Azules (RBMA), Chiapas, México: (1) Río Tzendales (16°17′ 10.8″ N; 90°53′12.6″ W), (2) Río Manzanares (16°10′14.6″ N; 90°50′36.2″ W), (3) Arroyo Miranda (16°08′08.1″ N; 90°55′14.9″ W), (4) Río Danta (16°09′08.1″ N; 90°54′06.3″ W), (5) Arroyo Lagarto (16°08′14.0″ N; 90°54′24.4″ W), (6) Embarcadero Estación Chajul (16°06′38.4″ N; 90°56′ 23.6″ W), (7) Arroyo José (16°06′50″ N; 90°56′03.3″ W), (8) Río Chajul (16°05′58.2″ N; 90°57′30.1″ W), (9) Río San Pablo (16°06′ 10.0″ N; 91°00′52.2″ W), (10) Río Puerto Rico (16°05′04.4″ N; 91°01′11.2″ W), (11) Río Ixcan (16°07′17.5″ N; 91°05′11.3″ W).
Fish were collected using gill nets, transferred to the laboratory and kept alive in aerated containers until they were examined for monogeneans, performed within 8 h of capture. Each fish was measured (standard length) and examined under a stereo microscope in Petri dishes with river water. Skin, scales, mouth, branchial cavity, anus, and fins of each host were examined. Fish were euthanized and the branchial arches were removed, separated from the brachial cavity and evaluated individually (protocol for the use of fish in research based on the NORM – 019 – STPS – 1993 established by the Instituto de Ecología, Pesquerías y Oceanografía del Golfo de México EPOMEX, Campeche, Mexico; specimens collected under the Cartilla Nacional de Colector Científico FAUT-0105 issued by the Secretaría del Medio Ambiente y Recursos Naturales [SEMARNAT] to GSM). All monogeneans found were fixed in 4% hot formaldehyde, stained with Gomori's triple stain and mounted on Canada balsam. Taxonomic identification was performed based on morphometric analysis of the specimens (see Mendoza-Franco et al., 2013).
The prevalence and abundance of infections were calculated according to Bush et al. (1997). Analysis was conducted at two hierarchical levels of the community (Holmes and Price, 1986): infracommunity, including the monogenean parasites of each fish examined, and the component community, referring to the monogeneans of all the hosts examined for each location and date.
2.2. Data analysis
2.2.1. Richness
To assess the effectiveness of our sampling, all component communities were evaluated using cumulative species curves employing the EstimateS program (8.0 RK version Coldwell http://viceroy.eeb.unconn.edu/estimates). For each component, asymptotic richness was tested based on the Clench model (Soberón and Llorrente, 1993), as well as the final slope of the cumulative species curve (Jiménez-Valverde and Hortal, 2003), that is, the gradient between two sampling endpoints. The criterion for sufficient sampling was that the slope of the cumulative species curve was not more than 0.1 species per sample. Empirically, this final value of the slope of the curve indicates that at least 70% of the species have been recorded in the component community (Jiménez-Valverde and Hortal, 2003). The Clench model is described by the function,
| V2 = (a * V1) / (1 + (b*V1)) |
Where V2 is the observed richness, V1 is the number of examined hosts, a and b are curve parameters; a is the aggregation rate of new species and b is a parameter related to the shape of the curve. We iteratively calculated these values utilizing EstimateS and Statistica (Jiménez-Valverde and Hortal, 2003). The slope of the species accumulation curve was calculated as follows:
| a / (1 + b*n)2 |
where a and b are the parameters defined above and n is the number of hosts examined in a given component community.
Furthermore, to answer the question of how many species we had not detected through sampling, we estimated the number of rare species that were not detected in each component community by calculating the non-parametric Jackknife estimator according to the following function (Poulin, 1998b, 2007; Magurran, 2004):
| Sj = SO + a(H – 1) / H |
Where SO is the observed richness, H is the number of hosts examined and a is the number of monogenean taxa found in only one host of the sample.
2.2.2. Classification of communities
We quantified the similarity in the composition between component communities by calculating the Jaccard index. The similarity between infracommunities of the same component community was quantified using the Bray-Curtis index (the complement, 1 – B, is used), whose value is based on the abundance of the most frequent species, underestimating the contribution of the moderately common and rare species.
2.2.3. Saturation
To determine the saturation, we plotted the mean infracommunity parasite richness (local richness) against richness in the component community (regional richness) and calculated the function that best fit the data (Kennedy and Guégan, 1994; Cornell, 1996; Morand et al., 1999; Poulin, 2007). According to Morand et al. (1999) the dependence of infracommunity richness on component community richness, meaning no limit to infracommunity parasite species richness with increasing component community richness, indicates non-saturation.
2.2.4. Intraspecific aggregation
We quantified the monogeneans’ intraspecific aggregation and interspecific aggregation following Ives (1988, 1991) as it has been applied to the analysis of fish ectoparasite communities by Morand et al. (1999); Šimková et al., 2000, 2001, and Agrawal et al. (2017). We calculated the parameter J for each monogenean taxa as an intraspecific aggregation measurement that quantifies the relative increase of conspecific competitors above the average number that a monogenean will find when it infects a new host (Ives, 1988), i.e., J is a measure of the proportional increase in the number of conspecific competitors that an individual monogenean experiences in a random distribution:
Where n1i, is the number of monogeneans of species 1 in the host i; m1 is the mean number of monogenean species 1 per host, and V1 is the variance in the number of monogenean species 1. A value of J = 0 indicates that individual monogeneans are randomly distributed, while a value of J = 0.5 indicates a 50% increase in the average number of conspecific monogeneans expected in a patch (host) above what would be found if the individuals were randomly distributed (Ives, 1988). In other words, J = 0.5 indicates a 50% increase in the aggregation of individuals of the same species (Šimková et al., 2001).
2.2.5. Interspecific aggregation
To measure the association between two species within each of the infracommunities, we calculated the C1,2 index (Ives, 1988, 1991), a measure of a proportional increase in the number of heterospecific monogenean competitors with respect to a random association. Thus, C1,2 is the relative change in the average number of heterospecific monogeneans with which the monogeneans of species 1 have to compete in a situation where the species are not independently distributed (Ives, 1988), through the following function:
Where n1i and n2i are the numbers of monogeneans of species 1 and 2 in the host i; m1i and m2i are the mean number of monogeneans per host of species 1 and 2; P is the number of hosts and Cov is the co-variability between a pair of species. When C > 0, the two species are positively correlated, therefore, they are aggregated in the host (Ives, 1988). If C < 0, the species are negatively correlated and there is segregation between species. As for the value of J, if C1,2 = 0.5, there are 50% of the expected number of heterospecific competitors in the host, above what one would expect if the monogenean species 1 and 2 were randomly distributed (Šimková et al., 2001).
To evaluate the decrease in competition due to the intraspecific aggregation, we compared the relative intensity of intraspecific aggregation versus interspecific aggregation in a pair of species, 1 and 2. We calculated A1,2 following Ives (1988), Jaenike and James (1991), Stevenster (1996), Morand et al. (1999), Šimková et al. (2000), according to the following function:
If A1,2 > 1, the intraspecific aggregation is greater than the interspecific aggregation.
2.2.6. Correlations
In all our analyses, we used the distribution of abundance of monogenean species, i.e. the number of monogeneans of each species per host. Because the observed frequency distribution of the number of monogeneans per fish host were almost invariably aggregated, i. e., the variance of the number of individual monogeneans per host was much greater than the mean (see results section) and because histograms visually indicate that our data were not normally distributed, therefore for the different correlation analysis performed, we used the Spearman's rank correlation coefficient. To evaluate the correlations between the intensities of two monogenean species across hosts, we removed fish that were not infected by either of the two parasite species. In all cases, we indicate statistical significance of values of Spearman's coefficient, r, with an asterisk symbol: *p < 0.05; **p < 0.01, ***p < 0.001.
3. Results
3.1. Taxonomic composition of the communities
We recorded 12 taxa of monogeneans of two families and seven genera; abundance and prevalence data are annotated in Table 1. Taxonomic classification of these monogeneans was reported by Mendoza-Franco et al. (2009, 2013).
Table 1.
Monogenea taxa parasites of Astyanax aeneus collected in 2012 from 11 localities at the río Lacantún, Reserva de la Biósfera Montes Azules, Chiapas, México. Data are prevalence/and abundance of infections; total no. of monogeneans collected/J (aggregation) values.
| TZENDALE |
MANZANA |
MIRANDA |
DANTA |
LAGARTO |
EMBARCAD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feb | Ags | Feb | Ags | Feb | Ags | Feb | Ags | Feb | Ags | Feb | |
| Gyrodactylidae | |||||||||||
| A. anacanthocotyle | 7/0.1 ± 0.3; 1/-1 |
7/1.1 ± 1.0; 17/-0.1 |
40/1.1 ± 1.6; 17/0.9 |
73/1.5 ± 1.6; 22/0.4 |
33/1.3 ± 2.1; 19/1.7 |
13/0.4 ± 1.3; 6/7.3 | 7/0.1 ± 0.3; 1/-1 |
13/0.1 ± 0.4; 2/-1 |
40/0.8 ± 1.1; 12/0.6 |
20/0.3 ± 0.6; 4/0.8 |
|
| Anacanthocotyle sp. | |||||||||||
| G. neotropicalis | |||||||||||
| Gyrodactylus sp. | |||||||||||
| Dactylogyridae | |||||||||||
| C. chajuli | 13/0.1 ± 0.4; 2/-1 |
7/0.1 ± 0.3; 1/-1 |
33/0.6 ± 1.0; 9/1.2 |
13/0.3 ± 0.8; 4/4.6 |
13/0.2 ± 0.6; 3/2.3 |
7/0.1 ± 0.5; 2/6.5 |
|||||
| C. exiguum | 7/0.1 ± 0.5; 2/6.5 |
13/0.1 ± 0.3; 2/-0.8 |
7/0.1 ± 0.2; 1/-1 |
7/0.1 ± 0.3; 1/-1 |
|||||||
| Cacatuocotyle sp. | |||||||||||
| C. costaricensis | 60/2.9 ± 7.0; 41/4.9 |
47/2.2 ± 3.4; 33/1.8 |
53/2.9 ± 4.8; 44/2.2 |
40/0.6 ± 0.8; 9/0.1 |
53/1.7 ± 2.3; 26/1.1 |
67/1.7 ± 1.9; 26/0.6 |
80/2.9 ± 3.5; 44/0.9 |
33/0.5 ± 0.8; 8/0.4 |
33/0.5 ± 0.9; 8/0.8 |
60/0.7 ± 0.7; 11/-0.5 |
|
| D. kabatai | 7/0.1 ± 0.3; 1/-1 |
33/0.4 ± 0.6; 6/-0.1 |
7/0.1 ± 0.3; 1/-1.5 |
7/0.1 ± 0.3; 1/-1 |
20/0.6 ± 1.5; 9/3.8 |
7/0.1 ± 0.3; 1/-1 |
13/0.1 ± 0.4; 2/-1 |
||||
| P. heteroancistrium | 20/0.2 ± 0.4; 3/-1 |
47/1.5 ± 2.6; 22/2.3 |
7/0.1 ± 0.3; 1/-1 |
7/0.1 ± 0.3; 1/-1 |
47/1.1 ± 1.6; 17/0.9 |
33/1.7 ± 2.9; 25/2.2 |
27/0.5 ± 0.9; 7/1.4 |
7/0.1 ± 0.3; 1/-1 |
|||
| U. strombicirrus | |||||||||||
| Dactylogyridae gen. sp. | 7/0.1 ± 0.3; 1/-1 |
||||||||||
| JOSÉ |
CHAJUL |
SN PABLO |
PTO RICO |
IXCÁN |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feb | Ags | Feb | Ags | Feb | Ags | Feb | Ags | Feb | Ags | |
| Gyrodactylidae | ||||||||||
| A. anacanthocotyle | 25/0.4 ± 0.7; 3/0.8 | 7/0.1 ± 0.3; 1/-1 |
20/0.2 ± 0.4; 3/-1 |
7/0.1 ± 0.3; 1/-1 |
47/0.6 ± 0.7; 10/-0.2 |
20/0.2 ± 0.4; 3/-1 |
13/0.2 ± 0.6; 3/2.3 |
|||
| Anacanthocotyle sp. | 20/0.2 ± 0.4; 3/-1 |
|||||||||
| G. neotropicalis | 13/0.3 ± 0.8; 4/4.6 |
7/0.1 ± 0.3; 1/-1 |
||||||||
| Gyrodactylus sp. | 7/0.1 ± 0.3; 1/-1 |
7/0.1 ± 0.3; 1/-1 |
||||||||
| Dactylogyridae | ||||||||||
| C. chajuli | 13/0.4 ± 1.1; 6/4.8 |
13/0.1 ± 0.4; 1/-1 |
20/0.3 ± 0.7; 5/1.4 |
7/0.1 ± 0.3; 1/-1 |
27/0.3 ± 0.6; 5/0.2 |
|||||
| C. exiguum | 7/0.1 ± 0.3; 1/-1 |
7/0.1 ± 0.3; 1/-1 |
||||||||
| Cacatuocotyle sp. | 7/0.1 ± 0.3; 1/-1 |
|||||||||
| C. costaricensis | 33/0.6 ± 0.9; 9/0.5 |
25/0.5 ± 1.1; 4/2 |
73/1.7 ± 1.8; 26/0.4 |
27/0.4 ± 0.8; 6/1.5 |
40/0.9 ± 1.3; 13/0.9 |
60/1.4 ± 1.7; 21/0.6 |
53/1.6 ± 2.2; 24/1.1 |
60/1.9 ± 2.0; 29/0.5 |
60/2.7 ± 3.7; 40/1.4 |
33/1.1 ± 1.6; 16/1.2 |
| D. kabatai | 7/0.1 ± 0.3; 1/-1.5 |
20/0.2 ± 0.4; 3/-1 |
7/0.1 ± 0.3; 1/-1 |
13/0.1 ± 0.4; 2/-1 |
20/0.3 ± 0.6; 4/0.8 |
13/0.8 ± 2.8; 12/10.4 |
||||
| P. heteroancistrium | 13/0.2 ± 0.6; 3/2.3 |
33/0.7 ± 1.3; 11/1.4 |
27/0.3 ± 0.6; 5/0.2 |
40/0.8 ± 1.3; 12/1.0 |
13/0.1 ± 0.4; 2/-1 |
33/0.6 ± 1.0; 9/0.8 |
||||
| U. strombicirrus | 7/0.1 ± 0.3; 1/-1 |
13/0.3 ± 0.8; 4/4.6 |
||||||||
| Dactylogyridae gen. sp. | 7/0.1 ± 0.3; 1/-1 |
13/0.1 ± 0.4; 2/-1 |
||||||||
Five species of Astyanax-specialist monogeneans were well distributed regionally and were frequent and abundant. Three of these: Characithecium costaricensis (Price and Bussing, 1967), Palombitrema heteroancistrium (Price and Bussing, 1968) and Anacanthocotyle anacanthocotyle Kritsky and Fritts, 1970 were the most prevalent and abundant, and were collected at most of the sampling locations (Table 1). Together, they accounted for 86% of the monogeneans (682 monogeneans of the three above-mentioned species of the 793 collected in total) collected during this study (Table 3). To a lesser extent, Diaphorocleidus kabatai (Molnar, Hanek and Fernando, 1974) and Cacatuocotyle chajuli Mendoza-Franco et al. (2013) were also widely distributed, but in general, were registered with a prevalence < 10% (Table 1). Cacatuocotyle exiguum Mendoza-Franco et al. (2013) was registered in five locations with low prevalence. The remaining registered taxa (Table 1) were rare species from which a few specimens were collected from a few locations. These included Anacanthocotyle sp., Gyrodactylus neotropicalis Kritsky and Fritts, 1970, Gyrodactylus sp., Cacatuocotyle sp., Urocleidoides strombicirrus (Price and Bussing, 1967), and Dactylogyridae gen. sp.
Table 3.
Data for the six monogenean species more frequent and abundant in the samples and its intraspecific aggregation J, values.
| Number of component communities from which was recorded (total number of specimens, and range min – max in component community) | Correlation values between prevalence vs abundance | Mean J ± Sd value (number, and range min – max of J > 0 values recorded) | Correlation values between J vs | |
|---|---|---|---|---|
| C. costaricensis | 20 (438, 4–44) | r = 0.75*** | 1.1 ± 1.1 (19, 0.1–4.9) | Mean intensity r = 0.62** Total monogeneas r = 0.44* Maximum no. monogeneans in an infracommunity r = 0.51* Mean no. monogeneans per host r = 0.48* |
| A. anacanthocotyle | 18 (125, 1–22) | r = 0.90*** | 0.37 ± 2.0 (8, 0.5–7.3) | No. of individuals of A. anacanthocotyle r = 0.66** Mean intensity r = 0.89*** Abundance r = 0.67*** |
| P. heteroancisthrium | 14 (119, 1–25) | r = 0.94*** | 0.5 ± 1.3 (9, 0.2–2.3) | Prevalence r = 0.94*** No. of individuals of P. heteroancisthrium r = 0.77*** Abundance r = 0.77*** Mean intensity r = 0.84*** Mean no. of species r = 0.53* |
| D. kabatai | 13 (44, 1–12) | r = 0.89*** | 0.36 ± 3.3 (3, 0.8–10) | Prevalence r = 0.54* No. of individuals of D. kabatai r = 0.84*** Mean intensity r 0.89*** Abundance r = 0.70*** No. of species r = 0.54* |
| C. chajuli | 11 (39, 1–9) | r = 0.79*** | 1.5 ± 2.7 (7, 0.2–6.5) | No. of individuals of C. chajuli r = 0.55 p = 0.07 NS Abundance r = 0.55 p = 0.07 NS Mean no. of monogeneans per host r = - 0.58 p = 0.06 NS |
| C. exiguum | 6 (8, 1–2) | r = 0.46, p < 0.33 N.S. | 0.27 ± 3.0 (1, 6.5) | No. of individuals of C. exiguum r = 0.97 p = 0.06 NS Abundance r = 1 p = 0.06 NS |
*p < 0.05; **p < 0.01; ***p < 0.001.
3.2. Richness
Monogeneans were registered in all locations. The sampling effort was large enough to obtain an almost complete inventory. The proportion of observed species with respect to the total number of estimated species, SO/SJ > 0.7 (Table 2), indicates that in the majority of the locations, we recovered more than 70% of the monogenean taxa that composed the community.
Table 2.
Parameters of Astyanax aeneus and monogenean communities sampled in 2012 from 11 localities along the Río Lacantún, Chiapas, México. Fifteen hosts were examined from each locality/date, except from José in February, when only 8 hosts were examined.
| TZENDALES |
MANZANARES |
MIRANDA |
DANTA |
LAGARTOS |
EMBARCA |
JOSÉ |
CHAJUL |
SAN PABLO |
PUERTO RICO |
IXCAN |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feb | Aug | Feb | Aug | Feb | Aug | Feb | Aug | Feb | Aug | Feb | Feb | Aug | Feb | Aug | Feb | Aug | Feb | Aug | Feb | Aug | |
| Mean standard length of hosts ± Sd | 43.8 ±6.0 |
52.1 ±12.1 |
43.3 ±18.9 |
48.7 ±17.6 |
37.7 ±8.5 |
46.1 ±24 |
49.5 ±8.2 |
74.1 ±17.9 |
33.0 ±3.0 |
54.5 ±7.4 |
34.8 ±4.0 |
40.8 ±9.7 |
47.6 ±3.2 |
31.5 ±2.2 |
43.7 ±8.3 |
43.4 ±8.4 |
44.6 ±9.0 |
35.7 ±6.3 |
47.5 ±4.5 |
34.8 ±4.0 |
45.3 ±6.9 |
| Total no. of Monogenean species found (SO) | 4 | 6 | 5 | 4 | 3 | 3 | 3 | 5 | 3 | 5 | 5 | 3 | 3 | 7 | 2 | 3 | 4 | 5 | 6 | 6 | 7 |
| No. of monogenean species estimated (Jackknife SJ) | 6.8 | 7.8 | 5.9 | 5.8 | 6.6 | 3 | 3 | 6.8 | 3 | 6.8 | 7.8 | 4 | 3.8 | 11.6 | 2 | 6.6 | 4.9 | 5.9 | 7.8 | 6.2 | 7.9 |
| Proportion of species of monogeneans recovered (SO/SJ) | 0.58 | 0.76 | 0.84 | 0.68 | 0.45 | 1 | 1 | 0.73 | 1 | 0.73 | 0.64 | 0.75 | 0.78 | 0.6 | 1 | 0.45 | 1 | 0.84 | 0.76 | 1 | 0.88 |
| Total no. of individual monogeneans | 7 | 89 | 62 | 68 | 11 | 62 | 36 | 80 | 13 | 29 | 20 | 16 | 8 | 40 | 17 | 15 | 30 | 35 | 55 | 54 | 46 |
| Berger-Parker Index | 0.42 | 0.46 | 0.53 | 0.64 | 0.81 | 0.41 | 0.72 | 0.55 | 0.61 | 0.41 | 0.55 | 0.56 | 0.5 | 0.65 | 0.64 | 0.86 | 0.7 | 0.68 | 0.53 | 0.74 | 0.34 |
| Dominant species | P. het | C. cos | C. cos | C. cos | C. cos | C. cos | C. cos | C. cos | C. cos | A.ana | C. cos | C. cos | C. cos | C. cos | P. het | C. cos | C. cos | C. cos | C. cos | C. cos | C. cos |
| Mean no. of species per host | 0.47 | 2.2 | 1.4 | 1.4 | 0.53 | 1.33 | 0.93 | 1.47 | 0.6 | 1.1 | 1.07 | 0.53 | 0.6 | 1.4 | 0.6 | 0.53 | 1.1 | 1.07 | 1.73 | 1.33 | 1.3 |
| Maximum no. of species in a host | 3 | 5 | 5 | 3 | 2 | 3 | 2 | 4 | 2 | 3 | 2 | 1 | 3 | 3 | 2 | 2 | 3 | 2 | 4 | 4 | 3 |
|
| |||||||||||||||||||||
| % of host with 0 species | 66.6 | 0 | 20 | 0 | 60 | 26.6 | 20 | 20 | 46.6 | 20 | 13.3 | 46.6 | 62.5 | 13.3 | 46.6 | 53.3 | 20 | 13.3 | 0.06 | 20 | 20 |
| Mean no. of individual monogeneans per host | 0.47 | 5.9 | 4.13 | 4.53 | 0.73 | 4.13 | 2.4 | 5.33 | 0.87 | 1.9 | 1.33 | 1.07 | 1 | 2.6 | 1.1 | 1 | 2 | 2.33 | 3.7 | 3.6 | 3.1 |
| Maximum number of monogeneans in a host | 3 | 40 | 13 | 17 | 3 | 16 | 6 | 26 | 4 | 4 | 3 | 4 | 6 | 10 | 4 | 4 | 7 | 8 | 10 | 16 | 12 |
| Mean Bray Curtis similarity index ± Sd between infracommunities | 0.46 | 0.35 | 0.22 | 0.31 | 0.43 | 0.24 | 0.28 | 0.31 | 0.27 | 0.17 | 0.29 | 0.29 | 0.38 | 0.29 | 0.29 | 0.34 | 0.25 | 0.18 | 0.26 | 0.23 | 0.14 |
The richness in each of the 11 component communities (Table 2) did not correlate with the number of fish examined (SO, r = - 0.21; SJ, r = 0.21), nor with the mean size of the hosts (SO, r = - 0.005; SJ, r = - 0.001). The total abundance of monogeneans did not correlate with the mean size of the hosts (total samples r = 0.38; February r = - 0.19, August r = 0.47).
The component communities showed spatial and temporal consistency in their taxonomic composition, with some temporal variability in richness and abundance. Greater richness and abundance were registered in August in most of the component communities, suggesting that the transfer between components increases during the rainy season. However, we did not detect directionality or a defined spatial-temporal pattern (Table 1, Table 2). The maximum number of monogenean taxa in a host varied from one to five (Table 2). However, hosts were infected mostly with two or three monogenean taxa. Some infections were highly abundant (>10 monogeneans per host and up to 44 individuals of a single species); however, hosts were normally found parasitized by three to ten monogeneans.
3.3. Monogenean transmission and similarity intra and inter locations
Our results suggest that monogenean transmission within each component community is intense and effective. In most of the studied locations, all the examined fish presented monogeneans, or at least only a few were not infected (Table 2). However, in some locations up to half, and in some cases more than half of the examined fish were not parasitized by monogeneans. The similarity of the infracommunities within each component community was normally high, >20% (Table 2).
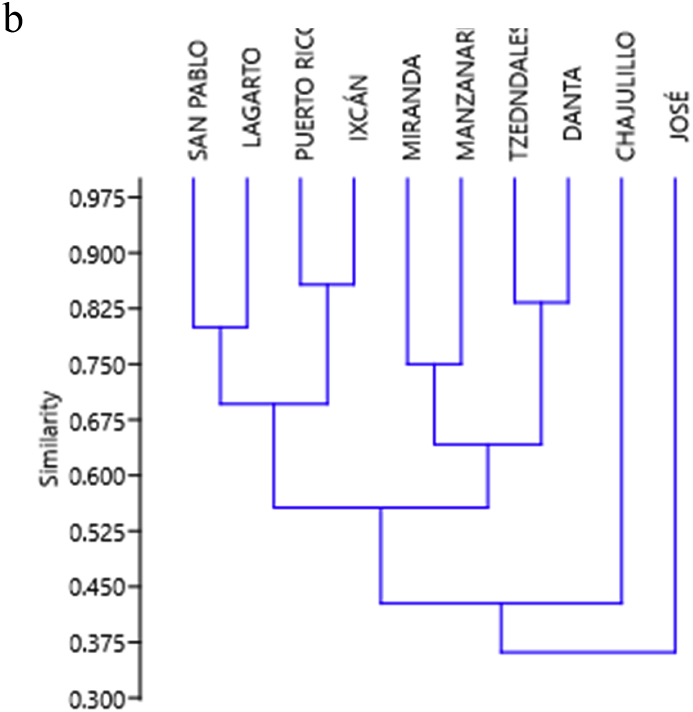
All component communities examined were characterized by low diversity and high dominance (Table 2). The value of the Berger-Parker index was >0.5 for most of the component communities. In 18 out of 21, C. costaricensis was dominant, while P. heteroancistrium prevailed in two component communities and A. anacanthocotyle dominated in one (Table 2). The presence of the five species of wide regional distribution and the dominance described (Table 1, Table 2) contributed to the high similarity observed between the component communities that in February was >50% and in August was >60%. We did not detect any higher similarity pattern between neighboring locations. All communities had a similar composition, no matter who their closest neighbors were (Fig. 1, Fig. 2a, Fig. 2b).
Fig. 2a.
Resemblance (Jaccard index) between components of community (February).
Fig. 2b.
Resemblance (Jaccard index) between components of community (August).
3.4. Host saturation
No convincing evidence of a curvilinear relationship was found when plotting the mean richness recorded in an infracommunity versus the richness in the component communities (Fig. 3). The proportion of variance of the distribution of observations that could explain a curvilinear relationship was similar to the one that could explain a linear relationship (Fig. 3). This indicates that we could not find an upper limit of local species richness in the individual hosts with respect to the size of the regional pool of species. The maximum richness of the infracommunities, in most cases, was kept below that of the component community (Table 2). This means that empty niches were observed. We found a positive correlation between the observed richness (SO) in the component community and the mean richness of infracommunities (considering total sample r = 0.70***; separated by sampling months: February r = 0.77**; August r = 0.63*), and also with the maximum richness recorded in an infracommunity (total sample r = 0.68**; February r = 0.68**; August r = 0.66*); this suggests that, as the richness of component communities increased, there were more species in the infracommunities. Our data also showed that the increase in individual monogenean numbers positively correlated with a rise in species richness: SO vs total number of monogeneans in the component community (total sample r = 0.56**; February r = 0.64*; August r = 0.46 NS), vs average of individual monogeneans per infracommunity (total sample r = 0.56**; February r = 0.64*; August r = 0.46 NS). Our data demonstrated also that the number of species in infracommunities increased with the total number of monogeneans in the component communities. Hence, mean richness in infracommunities showed positive correlations with the total abundance of monogeneans in the component community (total sample r = 0.92***; February r = 0.90***; August r = 0.92***), with the mean number of monogeneans per infracommunity (total sample r = 0.93***; February r = 0.90***; August r = 0.92***), as well as with the maximum number of monogeneans recorded in an infracommunity (total sample r = 0.86***; February r = 0.77**; August r = 0.83***). This proportional relationship between the richness in the component community and the richness in the infracommunities suggests that a maximum level of richness does not exist and is consistent with the absence of saturation in the communities.
Fig. 3.
Relationship between component community monogenean species richness and mean infracommunity species richness; A) total samples; B) samples of February; C) samples of August.
3.5. Intraspecific aggregation of monogeneans
The data show that the populations of these monogeneans are aggregated. We quantified the aggregation of each identified taxon in each component community, calculating a total of 92 values of J. Amongst these, 49 were positive values, where J > 0, varying from 0.1 to 10 (Table 1, Table 3). These records corresponded to the most frequently occurring species that were widely distributed (Table 3). The most frequent species with greatest regional distribution, C. costaricensis, showed aggregation in 95% of its records (J > 0 in 19 out of 20 components where it was recorded) (Table 3). Furthermore, P. heteroancistrium and C. chajuli were aggregated in more than 60% of the registered component communities (Table 3). Rare species did not show aggregation because they were found in very low numbers. Hence, 43 of the 92 calculated J values were negative J < 0. In the majority of these cases (38/43) J = −1 corresponded to component communities where one to three monogeneans of a given species were quantified.
The value of J is density-dependent. In general, the aggregation of each species increases with the number of monogenean taxa (Table 3). At low population densities, J values of a given species were negative; however, when the number of monogenean species increased, positive J values were obtained (Table 3). P. heteroancistrium and D. kabatai showed correlations between J and the observed richness or mean richness of the infracommunities, suggesting that they are sensitive to the presence of another species, whilst intraspecific aggregation level is independent of the presence of another species for C. costarricenses, A. anacanthocotyle and C. chajuli.
3.6. Interspecific aggregation of monogeneans
We calculated 175 values of interspecific aggregation, C1,2, between 48 pairs of species, including all of the registered monogenean taxa in each location and for each date (Table 4). A total of 74% (131/175) of these associations were negative, with a value of C1,2 < 0, 14 values of C1,2 = 0, while 17% (30/175) gave positive values of C1,2 > 0 (Table 4). The interactions between the five most abundant species of monogeneans amongst themselves, as well as the interactions of these abundant species vs the least abundant taxa were mostly negative (Table 4) and constituted 56% of the negative interactions (74/131). Negative interactions between pairs of species showed a clear numerical dominance for one of them; C. costaricensis was always the most abundant species in the associations where it participated. Negative associations (57/131) among the less frequent monogenean taxa involved just a few individuals. Hence, these species are rarely found in or co-habit the same host. The recorded values of C1,2 in these cases indicate the presence of both species in the same component community, but the absence of co-infections in the infracommunities or at most, in rare cases, co-infections of both monogenean taxa in one to three infracommunities.
Table 4.
Values of C1,2 index that measure the association between two species, and A1,2 index that compare the relative intensity of intraspecific aggregation versus interspecific aggregation in a pair of species. Number of positive and negative values of C1,2 (±) within fish infected by both species of monogenean pair. Below diagonal number of values of Ai,j > 1 within fish infected by both species of monogenean pair, [and range min – max of A1,2 > 1 values]. Abbreviations: Ana, A. anacanthocotyle; Ans, Anacanthocotyle sp.; Cac, C. chajuli; Cas, Cacatuocotyle sp.; Cex, C. exiguum; Cha, C. costaricensis; Dac, Dactylogyridae gen. sp.; Dia, D. kabatai; Gyn, G. neotropicalis; Gys, Gyrodactylus sp.; Pal, P. heteroancistrium; Uro, U. strombicirrus. (*) species more frequent and abundant in our samplings.
| *Cha | *Pal | *Ana | *Cac | *Dia | Ans | Cex | Cas | Gyn | Gys | Uro | Dac | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *Cha | 6/7 | 4/12 | 3/7 | 5/7 | 0/1 | 3/3 | 1/0 | 0/2 | 0/2 | 0/2 | 0/3 | |
| *Pal | 9 | 0/12 | 2/2 | 2/9 | 0/1 | 2/1 | 0/1 | 0/1 | 0/2 | 0/2 | 0/3 | |
| *Ana | 9 | 5 | 2/7 | 2/8 | 0/1 | 1/4 | 0/1 | 1/1 | 0/2 | 0/2 | 2/1 | |
| *Cac | 4 | 1 | 1 | 3/3 | 1/2 | 0/1 | 0/1 | 0/1 | ||||
| *Dia | 3 | 3 | 1 | 0 | 0/1 | 2/1 | 1/0 | 0/1 | 0/2 | 0/2 | 0/3 | |
| Ans | 0 | 0 | 0 | 0 | 1/0 | 0/1 | ||||||
| Cex | 1 | 1 | 0 | 0 | 1 | 0/1 | ||||||
| Cas | 0 | 0 | 0 | 0 | 0 | 0/1 | ||||||
| Gyn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/1 | ||||
| Gys | 0 | 0 | 0 | 0 | 0 | 0/2 | ||||||
| Uro | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Dac | 0 | 0 | 0 | 0 | 0 | 0 |
Our results suggest that the C1,2 values are density-dependent: the values increase with the number of species and with monogenean abundance in the component communities and in infracommunities. Therefore, considering the 175 calculated values of C1,2, we found significant positive correlations with the total number of registered monogeneans in the component community (r = 0.26***), with the mean number of monogeneans per host (r = 0.27***), with the maximum number of taxa of monogeneans recorded in a host (r = 0.32***), with the average richness of monogenean taxa per host (r = 0.22***).
3.7. Interspecific association and negative interactions
Our data show that associations between pairs of species of monogeneans are consistent in space, because they were recorded in several locations. We calculated the correlations between 106 pairs of the five most abundant species in the component communities; 68% (73/106) of these values showed negative correlations in the intensity of the monogenean species, and 15 of these comparisons were significant (Table 5). Significant negative interactions were detected between the five frequent species that were repeated in different locations (Table 5). For example, the interaction between A. anacanthocotyle/C. costaricensis was recorded in 16 component communities, 13 of which were negative and five correlations were significant. A. anacanthocotyle/P. heteroancistrium was recorded in 12 component communities, all of which were negative and four of which were significant. A. anacanthocotyle/C. chajuli, C. costaricensis/C. chajuli and C. costaricensis/P. heteroancistrium showed significant negative correlations in two components each.
Table 5.
Spearman's rank correlation coefficients of pairwise associations between the intensity of infection of the five monogenean species commonly found in A. eneus from 11 locations at Río Lacantún, Chiapas, Mexico. Samples taken from February and August are distinguished by an F or an A respectively. A double hyphen indicates insufficient data for calculation. Below the diagonal are the number of fish harbouring at least one of the two species in a pair, i.e. actual sample sizes, fish not harbouring worms from either species in a pairwise association (double zeros) were excluded. Values bold marked correspond to associations where A1,2 > 1; note 9 values marked with an § identifiying negative combinations where C1,2 <0, values of Spearman's correlation significantly negative and A1,2 > 1.
| C. costaricensis | A. anacanthocotyle | P. heteroancistrium | D. kabatai | C. chajuli | |
|---|---|---|---|---|---|
| Tzendales | |||||
| C. costaricensis | A - 0.65**§ | A 0.48 | A - 0.01 | A 0.58 | |
| A. anacanthocotyle | A 14 | F-1; A - 0.71**§ | F --; A - 0.29 | F --; A - 0.53* | |
| P. heteroancistrium | A 10 | F 4; A 14 | F −1; A - 0.34 | F – 1; A 0.67 | |
| D. kabatai | A 11 | F 1; A 12 | F 4; A 10 | F --; A - 0.25 | |
| C. chajuli | A 9 | F 2; A 11 | F 5; A 7 | F 2; A 5 | |
| Manzanares | |||||
| C. costaricensis | F - 0.54*§; A - 0.39 | A - 0.55 | F - 0.32 | F - 0.42 | |
| A. anacanthocotyle | F 11; A 15 | A - 0.51* | F - 0.67 | F - 0.72*§ | |
| P. heteroancistrium | A 9 | ||||
| D. kabatai | F 7 | F 6 | F 0.61 | ||
| C. chajuli | F 9 | F 9 | F 5 | ||
| Miranda | |||||
| C. costaricensis | A - 0.11 | F - 0.44; A 0.03 | F 0.44 | ||
| A. anacanthocotyle | A 9 | A - 0.49 | |||
| P. heteroancistrium | F 6; A10 | A 11 | F -- | ||
| D. kabatai | F 6 | F 2 | |||
| C. chajuli | |||||
| Danta | |||||
| C. costaricensis | F - 0.62*§; A - 0.40 | A 0.64*§ | A 0.51 | F - 0.30 | |
| A. anacanthocotyle | F 11: A 12 | A - 0.35 | A - 0.77 | F - 0.88 | |
| P. heteroancistrium | A 12 | A 5 | A - 0.03 | ||
| D. kabatai | A 12 | A 4 | A 6 | ||
| C. chajuli | F 11 | F 4 | |||
| Lagarto | |||||
| C. costaricensis | F −0.70; A - 0.86***§ |
A - 0.59 | A - 0.39 | F - 0.07 | |
| A. anacanthocotyle | F 6; A 10 | A - 0.59 | A - 0.54 | F - 0.94 | |
| P. heteroancistrium | A 8 | A 9 | A - 0.72 | ||
| D. kabatai | A 5 | A 6 | A 5 | ||
| C. chajuli | F 6 | F 4 | |||
| Embarcadero | |||||
| C. costaricensis | F - 0.84*** | F 0.66 | F - 0.53 | F - 0.64* | |
| A. anacanthocotyle | F12 | F - 0.84 | F - 0.70 | F - 0.81 | |
| P. heteroancistrium | F 9 | F 4 | F -1 | F -- | |
| D. kabatai | F 10 | F 4 | F 3 | F -- | |
| C. chajuli | F 10 | F 4 | F 2 | F 3 | |
| José | |||||
| C. costaricensis | A 0.50 | F - 0.77 | F - 0.86*; A - | ||
| A. anacanthocotyle | A 3 | A -- | |||
| P. heteroancistrium | |||||
| D. kabatai | F 6 | F -- | |||
| C. chajuli | F 7; A 2 | A 2 | F 3 | ||
| Chajul | |||||
| C. costaricensis | F - 0.21 | F 0.06; A - 0.69*§ | F 0.06 | F - 0.12 | |
| A. anacanthocotyle | F 11 | F - 0.86 | F -- | F - 0.81 | |
| P. heteroancistrium | F 12; A 8 | F 3 | F - 0.96 | F 0.05 | |
| D. kabatai | F 11 | F 3 | F 5 | F - 0.94 | |
| C. chajuli | F 12 | F 4 | F 4 | F 6 | |
| San Pablo | |||||
| C. costaricensis | A - 0.07 | A - 0.42 | A 0.35 | F - 0.62 | |
| A. anacanthocotyle | A 10 | A - 0.93* | A -- | ||
| P. heteroancistrium | A 11 | A 7 | A - 0.79 | ||
| D. kabatai | A 9 | A 3 | |||
| C. chajuli | F 7 | ||||
| Puerto Rico | |||||
| C. costaricensis | F 0.59; A - 0.01 | A - 0.17 | A - 0.04 | F - 0.24 | |
| A. anacanthocotyle | F 8; A 11 | A - 0.59*§ | A - 0.58 | F - 0.79 | |
| P. heteroancistrium | A 12 | A 10 | A -- | ||
| D. kabatai | A 10 | A 8 | A 1 | ||
| C. chajuli | F 10 | F 5 | |||
| Ixcán | |||||
| C. costaricensis | F - 0.05; A 0.05 | F 0.68; A - 0.48 |
F - 0.40; A - 0.80 |
||
| A. anacanthocotyle | F 9; A 6 | F - 0.57; A - 0.19 | F - 0.76; A - 0.86 |
||
| P. heteroancistrium | F 9; A 9 | F 4; A 6 | F - 0.70; A - 0.41 |
||
| D. kabatai | F 11; A 7 | F 5; A 4 | F 4; A 5 | ||
| C. chajuli | |||||
*p < 0.05; **p < 0.01; ***p < 0.00.
Monogenean species that interacted with two and up to three species in the same component community were detected. For example A. anacanthocotyle was found in Tzendales in August, interacting with C. costaricensis, P. heteroancistrium and C. chajuli. In all three cases, the negative interactions were significant. In Embarcadero, in February, C. costaricensis significantly negatively interacted with A. anacanthocotyle and C. chajuli (Table 5).
In total we calculated 99 values of A1,2, one per species pair that we recorded at each location and date. For 40 pairs of species A1,2 > 1 (Table 4, Table 5), indicating that intraspecific aggregations were stronger than interspecific aggregations. Positive values of A1,2 >1 were recorded amongst the five most frequent species of monogeneans. Only eight positive values of A1,2 involved rare species (Table 4). We did not find any correlation between the values of A1,2 and richness or abundance parameters (number of observed species in the community component, mean of observed species per host, total number of individual monogeneans in the component community, mean number of monogeneans per host). This suggests that the increase in diversity does not correlate with an increase in the intraspecific aggregation compared to the interspecific aggregation. Twelve combinations of species with significant values of negative correlation (Table 5) showed positive values of A1,2 >1 in conjunction with values of C1,2 <0, indicating significant negative interactions between these species associated with stronger intraspecific aggregations than interspecific ones.
4. Discussion
The general pattern emerging from our results is that of monogenean metacommunities (sensu Logue et al., 2011) with efficient transmission, made up of a limited group of specialist species that are regionally well distributed, occur frequently, dominate but do not saturate these communities, and provide temporal and spatial consistency. These frequent species show aggregation at the population level, as well as displaying interspecific aggregation, both of which are density-dependent. They establish regionally consistent negative interactions that are repeated between locations. Coexistence of these species is facilitated by intraspecific aggregation that is comparatively stronger than interspecific aggregation.
4.1. Transmission
We indirectly measured monogenean transmission by quantifying response variables, including the composition and abundance of species. Nevertheless, our data provides unquestionable empirical evidence that transmission between hosts (infracommunities) from the same location (component community), as well as between component communities, is efficient. The variation in richness and abundance of monogenean species recorded suggest that transmission, colonization and establishment are continuous processes between infracommunities of a single component and between component communities. This is surprising, considering the nature of these communities, because we should expect component communities to be isolated by the size of the host fish (<10 cm), the scale of distances between the locations (from <1 to 60 km of separation between locations), the powerful main river (a tropical river with >15 m width, and average depth of > 2 m), and by torrential rains (annual average precipitation of 199 cm). However, our data strongly suggest the opposite: that these component communities are not isolated but rather communicate through the wide and free movement of the fish, combined with the gregariousness of the fish species and their population densities (Bussing, 1998; Miller et al., 2005), promoting the dispersion of the monogeneans. Nevertheless, to obtain conclusive data regarding the dispersion and transmission processes in these metacommunities, it is necessary to apply experimental methodologies.
High similarity between infracommunities within the same component community and between component communities were recorded, even without the presence of any visible resemblance pattern associated with the distances that separate them. This similarity is given by the presence of five species of wide regional distribution and by their high prevalences and abundances. These communities are spatially consistent. Furthermore, our data suggest that this consistency is also maintained throughout the year, across very different climatic conditions. The observed variations in composition and abundance can be associated with the local conditions of each component community.
4.2. Saturation
We did not find evidence of saturation in these communities. Our analysis suggested that the potential richness of infracommunities is proportional to the number of monogenean species available in the component community. In other words, the richness of the infracommunities is a reflection of the availability of species for recruitment. Therefore, in our study, the saturation of species is not a major obstacle that prevents the invasion of a new species; the registration of six rare species in our samples contributes to this explanation. According to Rohde et al. (1995) and Worthen and Rohde (1996), communities of fish ectoparasites are not saturated assemblages. Rohde (1991) argues that most parasites in the gills live in low density populations and in habitats with abundant resources, and many potential niches for these parasites are empty. Our results agree with these concepts and are very similar to the ones presented by Morand et al. (1999), where 36 communities of ectoparasites of marine fish were examined and found a similar richness (S = 5) to the one analyzed in the present work that also did not find saturation either.
4.3. Intraspecific aggregation
We found that monogeneans aggregate both at the population level and between species. These patterns have been previously described in ectoparasite communities of fish (Rohde et al., 1995; Morand et al., 1999; Simková et al., 2000, 2001; Agrawal et al., 2017). We observed that intraspecific aggregation (J) of the most abundant species increase with the intensity of the infection. This positive correlation suggests that conspecific individuals aggregate in the absence of intraspecific competition. Therefore, we may hypothesize that intraspecific competition for space is not a limiting factor for the development of the population. This would facilitate conspecific contacts as suggested by Rohde and is also supported by the arguments of Stock and Holmes (1988), Geets et al. (1997) and Šimková et al. (2001). Therefore, intraspecific competition may be low when the niches are not saturated. A similar pattern of density-dependency was described by Šimková et al. (2001), where communities of nine species of Dactylogyrus parasites of Rutilus rutilus in the Czech Republic were analyzed.
Our data suggest that aggregation increases with the number of monogeneans in each patch (hosts, infracommunity); however, it does not correlate with the number of patches occupied. In fact, we recorded a positive correlation between the aggregation of monogeneans with the mean intensity, abundance and total number of a given monogenean species, but not with prevalence. This observation is consistent with the increase in the number of monogeneans in each infected patch. We can suppose that fish become infected and the population of monogeneans increases within each colonized fish. The infection of new patches (infracommunities within the same component or between components) will be the result of fortuitous encounters between parasitized hosts with non-parasitized hosts, and the development of the population in a given host, as suggested by Parrish and Edelstein-Keshet (1999).
4.4. Interspecific aggregation
We evaluated interspecific aggregation within infracommunities by considering the number of positive and negative associations for each species pair (a total of 175 associations). Our analysis allowed the detection of 77% of negative interactions between species pairs, and a proportion of comparatively low positive associations (16%). Positive associations are common among native populations of ectoparasites in the gills of fish (Morand et al., 1999; Simková et al., 2000, 2001). Abundant negative interspecific associations between pairs of some monogenean species suggest that competition plays an important role in the formation of these communities (Dezfuli et al., 2001; Poulin, 2001; Poulin and Morand, 2004). These co-occurrences between pairs of species, positive or negative, are the strongest indication that interactions between species can determine the richness and composition of communities (Dezfuli et al., 2001; Poulin, 2001, 2007).
Dezfuli et al. (2001) found negative interactions between pairs of intestinal helminth species of Salmo trutta in rivers of Northern Italy. However, none of these negative associations were found in more than one fish population. We found 12 pairs of species with significant negative associations that were repeated in several locations, suggesting that competition between these species is a factor that contributes to community diversity. The reproducibility of negative interspecific interactions in these communities of monogeneans is low due to a reduction in sample size, i.e., the number of infracommunities where the interaction is developed could be decisive to obtain statistical significance in other community components where these negative interactions were detected but were not significant. This variability of associations of parasites across A. aeneus populations suggests that abundance and dispersion of parasite species between individual hosts can have an impact on the extent of interspecific competition, as Dobson (1985) has argued.
The coexistence of potentially competing species can be facilitated by reducing the intensity of the competition through the aggregated use of hosts as they are patches of resources (Jaenike and James, 1991; Simková et al., 2000). Calculated values of A1,2 in our data suggest that the reduction of interspecific aggregation with respect to intraspecific aggregation occurs in comparatively fewer cases than the opposite, which according with Simková et al. (2000), could indicate that our communities are interactive. Morand et al. (1999) found that intraspecific aggregation increased with respect to interspecific aggregation with an increase in richness of ectoparasites. They argued that this causes interspecific interactions to decrease, facilitating coexistence. Simková et al. (2000) did not find negative interactions, and based on the absence of negative correlations between the abundances among species pairs, argued that only when high population densities were achieved could competition play an important role. Agrawal et al. (2017) evaluated 10 combinations of five species of congeneric monogeneans in 72 Wallago attu freshwater sharks in India, from which 10,920 monogeneans were recovered at an average of 151 individuals per fish examined. Populations of Thaparocleidus were aggregated (J > 0); they found that J positively correlated with the intensity of the infection. In other words, when they found more monogeneans, their populations were more aggregated. Therefore, in all species combinations, they found that intraspecific aggregation was greater than the interspecific aggregation (A1,2 > 1), facilitating the coexistence of species.
In this context, our results suggest that an increase in the diversity of monogeneans promotes major interspecific aggregation for some pairs of abundant species. Species richness of infracommunities and in the community component positively correlated with the levels of intraspecific aggregation versus interspecific aggregation. This allows us to point out that intraspecific aggregation increases relative to interspecific aggregation when richness in the community component increases. Consequently, interspecific interactions are reduced with respect to intraspecific interactions and this facilitates the coexistence of species.
In particular, the five dominant species are good dispersers, had similar biological features (fertility, longevity, lifecycle) and exploited the same resources where food and space were not limited. However, our results suggest that when they disperse so effectively and colonize free patches, they compete with one another (see Slatkin, 1974; Ives, 1988). In our system of study, the densities of monogeneans were rather low. Therefore, the level of competition that each oncomiracidium or juvenile will experience when they arrive at a new patch host will be low, and will depend on the number and types of the other monogenean species that are either already present in the same host, or those that arrive at the same time. Given the consistency in the distribution of individuals of different species that we recorded, we can assume that the transmission of some species of monogeneans may be combined (monogeneans coming in “clumps” in a single fish) such that colonization of new infracommunities within the same component or between components faces the problem of the arrival of two or more heterospecific individuals at the same time. This spreading from a common source and joint colonization, suggest that an initial interspecific interaction will work to build these communities. This is because when the transmission of propagules is multiple or linked, as we assumed, these species will have to compete even at low population densities (Ives, 1988). The first need of the individuals of both species will be to settle down/establish themselves (Poulin, 2007). The abundance of space and the availability of unlimited food (as long as the host survives) will be favorable factors that influence their development post-colonization. Our data provide empirical evidence that high aggregation levels of these species of monogeneans can contribute to the richness of species within a population of hosts, because with population growth, intraspecific and interspecific aggregations will facilitate contact between individuals and the coexistence of the most frequent species. Our results suggest these communities of monogeneans can be interactive, with the coexistence of species favored by unpredictable recruitment and the aggregated use of fragmented resources (Cornell, 1996). The extent to which intraspecific aggregation will be high enough for interspecific aggregation to be important for coexistence can only be determined with planned experiments on particular communities (Ives, 1988). Nevertheless, finally, these communities can only be understood by examining the way in which new monogeneans are recruited, as this is the way in which monogeneans are acquired by hosts.
Conflicts of interest
None.
Acknowledgements
This work was supported by Natura and Ecosistemas Mexicanos, A. C. though financial support by Alianza WWF – Fundación Carlos Slim and Petroleos Mexicanos (PEMEX) Mexico. Thanks are due to Roberto Moreno Ceballos, Universidad de Ciencias y Artes de Chiapas, Tuxtla Gutiérrez Chiapas, México for his help in preparing the map.
References
- Acosta A.A., Queiroz J., Brandão H., Silva R.J. Helminth fauna of Astyanax fasciatus Cuvier, 1819, in two distinct sites of the Taquari river, São Paulo state, Brazil. Braz. J. Biol. 2015;75:242–250. doi: 10.1590/1519-6984.15113. [DOI] [PubMed] [Google Scholar]
- Agrawal N., Rajvanshi S., Asthana A. Intraguild interactions between five congeneric species of Thaparocleidus (Monogenoidea) from the freshwater shark Wallago attu, Lucknow, India. J. Helminthol. 2017;91:718–725. doi: 10.1017/S0022149X17000049. [DOI] [PubMed] [Google Scholar]
- Bellay S., Takemoto R.M., Oliveira E.F. Is the community of fish gill parasites structured in a neotropical floodplain? Acta Parasitol. 2012;57:53–60. doi: 10.2478/s11686-012-0011-z. [DOI] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: margolis et al. revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Bussing W.A. Universidad de Costa Rica; San José de Costa Rica: 1998. Peces de las aguas continentales de Costa Rica; p. 468. [Google Scholar]
- Caswell H.L. Community structure: a neutral model analysis. Ecol. Monogr. 1976;46:327–354. [Google Scholar]
- Córdova L., Pariselle A. Monogeneoidea en Serrasalmus rhombeus (Linnaeus, 1766) de la Cuenca Amazónica Boliviana. Rev. Peru. Biol. 2007;11:14–16. [Google Scholar]
- Cornell H.V. Unsaturated patterns in species assemblages: the role of regional processes in setting local species richness. In: Ricklefs R.E., Schluter D., editors. Species Diversity in Ecological Communities. University of Chicago Press; Chicago: 1996. pp. 243–252. [Google Scholar]
- Dezfuli B.S., Giari L., De Biaggi S., Poulin R. Associations and interactions among intestinal helminths of the brown trout, Salmo trutta, in northern Italy. J. Helminthol. 2001;331:336. [PubMed] [Google Scholar]
- Dobson A.P. The population dynamics of competition between parasites. Parasitology. 1985;91:317–347. doi: 10.1017/s0031182000057401. [DOI] [PubMed] [Google Scholar]
- Espinal-Carrión T., López-López E. Helminths and lipid peroxidation in Astyanax aeneus (Pisces: Characidae) from a river in the humid subtropics of southeastern Mexico. Dis. Aquat. Org. 2010;88:215–224. doi: 10.3354/dao02144. [DOI] [PubMed] [Google Scholar]
- Ferrari-Hoeinghaus A.P., Takemoto R.M., Oliveira L.C., Makrakis M.C., Baumgartner G. Host-parasite relationships of monogeneans in gills of Astyanax altiparanae and Rhamdia quelen of the São Francisco Verdadeiro river, Brazil. Parasite. 2006;13:315–320. doi: 10.1051/parasite/2006134315. [DOI] [PubMed] [Google Scholar]
- Ferreira-Sobrinho A., Tavares-Dias M. A study on monogenean parasites from the gills of some cichlids (Pisces: Cichlidae) from the Brazilian Amazon. Rev. Max. Biodiv. 2016;87:1002–1009. [Google Scholar]
- Geets A., Coene H., Ollevier F. Ectoparasites of the whitespotted rabbitfish, Siganus sutor (Valenciennes, 1835) of the Kenyan coast: distribution within the host population and site selection on the gills. Parasitology. 1997;115:69–79. doi: 10.1017/s0031182097001054. [DOI] [PubMed] [Google Scholar]
- Gotelli N.J., Rohde K. Co-occurrence of ectoparasites of marine fishes: a null model analysis. Ecol. Lett. 2002;5:86–94. [Google Scholar]
- Holmes J.C. Site selection by parasitic helminths: interspecific interactions, site segregation and their importance to the development of helminth commnunities. Can. J. Zool. 1973;51:333–347. doi: 10.1139/z73-047. [DOI] [PubMed] [Google Scholar]
- Holmes J.C. Competition, contacts and other factors restricting niches of parasite helminths. Ann. Parasitol. Hum. Comp. 1990;65:69–72. doi: 10.1051/parasite/1990651069. [DOI] [PubMed] [Google Scholar]
- Holmes J.C., Price P.W. Parasite ommunities: the roles of phylogeny and ecology. Syst. Zool. 1980;29:203–213. [Google Scholar]
- Holmes J.C., Price P.W. Communities of parasites. In: Kikkawa J., Anderson D.J., editors. Community Ecology: Pattern and Process. Blackwell Sc Publ; 1986. pp. 187–213. [Google Scholar]
- Hudson P.F., Hendrickson D.A., Benke A.C., Varela-Romero A., Rodiles-Hernández R., Minckley W.L. Rivers of Mexico. In: Benke C., Cushing C.E., editors. Rivers of Mexico. Rivers of North America. Elsevier, Inc.; 2005. pp. 1031–1084. [Google Scholar]
- Ives A.R. Aggregation and the co-existence of competitors. Ann. Zool. Fenn. 1988;25:75–88. [Google Scholar]
- Ives A.R. Aggregation and co-existence in a carrion fly community. Ecol. Monogr. 1991;61:75–94. [Google Scholar]
- Jaenike J., James A.C. Aggregation and the coexistence of mycophagous Drosophila. J. Anim. Ecol. 1991;60:913–928. [Google Scholar]
- Janovy J., Jr., Snyder S.D., Clopton R.E. Evolutionary constrains on population structure: the parasites of Fundulus zebrinus (Pisces: Cyprinodontidae) in the South plate river of Nebraska. J. Parasitol. 1997;83:584–592. [PubMed] [Google Scholar]
- Jiménez-Valverde A., Hortal J. Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Rev. Ibér. Arac. 2003;8:151–161. [Google Scholar]
- Kennedy C.R. Helminth communities in freshwater fish: structured communities or stochastic assemblages? In: Esch G.W., Bush A.O., Aho J.M., editors. Parasite Communities: Patterns and Processes. Chapman and Hall; London New York: 1990. pp. 131–156. [Google Scholar]
- Kennedy C.R. Richness and diversity of microparasite communities in tropical eels Anguilla reinhardtii in Queensland, Autralia. Parasitology. 1995;111:233–245. [Google Scholar]
- Kennedy C.R., Bush A.O., Aho J.M. Patterns in helminth communities: why are birds and fish different? Parasitology. 1986;93:205–215. doi: 10.1017/s0031182000049945. [DOI] [PubMed] [Google Scholar]
- Kennedy C.R., Guégan J.F. Regional versus local helminth parasite richness in British freshwater fish: saturated or unsaturated parasite communities? Parasitology. 1994;109:175–185. doi: 10.1017/s0031182000076289. [DOI] [PubMed] [Google Scholar]
- Knipes A.K., Janovy J., Jr. Community structure and seasonal dynamics of Dactylogyrus spp. (Monogenea) on the fathead minnow (Pimephales promelas) from the Salt valley Watershed, Lancaster county, Nebraska. J. Parasitol. 2009;95:1295–1305. doi: 10.1645/GE-2166.1. [DOI] [PubMed] [Google Scholar]
- Logue J.B., Mouquet N., Peter H., Hillebrand H. Empirical approaches to metacommunities: a review and comparison with theory. Trends Ecol. Evol. 2011;26:482–491. doi: 10.1016/j.tree.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Magurran A.E. Blackwell Publishing; Oxford, UK: 2004. Measuring Biological Diversity; p. 256. [Google Scholar]
- Mendoza-Franco E.F., Caspeta-Mandujano J.M., Salgado-Maldonado G. New species of Cacatuocotyle (monogenoidea, Dactylogyridae) parasitizing the anus and the gill lamellae of Astyanax aeneus (Pisces, Ostariophysi: Characidae) from the Rio Lacantún basin in the Biosphere Reserve of Montes Azules, Chiapas, Mexico. Parasitol. Res. 2013;112:199–205. doi: 10.1007/s00436-012-3126-0. [DOI] [PubMed] [Google Scholar]
- Mendoza-Franco E.F., Reina R.G., Torchin M.E. Dactylogyrids (Monogenoidea) parasitizing the gills of Astyanax spp. (Characidae) from Panama and southeast Mexico, a new species of Diaphorocleidus and a proposal for Characithecium N. Gen. J. Parasitol. 2009;95:46–55. doi: 10.1645/GE-1592.1. [DOI] [PubMed] [Google Scholar]
- Miller R.R., Minckley W.L., Norris S.M. University of Chicago Press; Chicago: 2005. Freshwater Fishes of Mexico; p. 496. [Google Scholar]
- Morand S., Poulin R., Rohde K., Hayward C. Aggregation and species coexistence of ectoparasites of marine fishes. Int. J. Parasitol. 1999;29:663–672. doi: 10.1016/s0020-7519(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Parrish J.K., Edelstein-Keshet L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science. 1999;284:99–101. doi: 10.1126/science.284.5411.99. [DOI] [PubMed] [Google Scholar]
- Poulin R. Large-scale patterns of host use by parasites of freshwater fishes. Ecol. Lett. 1998;1:118–128. [Google Scholar]
- Poulin R. Comparison of three estimators of species richness in parasite component communities. J. Parasitol. 1998;84:485–490. [PubMed] [Google Scholar]
- Poulin R. Interactions between species and the structure of helminth communities. Parasitology Suppl. 2001;122:S3–S11. doi: 10.1017/s0031182000016991. [DOI] [PubMed] [Google Scholar]
- Poulin R. Princeton University Press; Princeton: 2007. Evolutionary Ecology of Parasites; p. 332. [Google Scholar]
- Poulin R., Luque J.L. A general test of the interactive-isolationist continuum in gastrointestinal parasite communities of fish. Int. J. Parasitol. 2003;33:1623–1630. doi: 10.1016/j.ijpara.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Poulin R., Morand S. Smithsonian Books; Washington: 2004. Parasite Biodiversity; p. 216. [Google Scholar]
- Price P.W., Clancy K.M. Patterns in number of helminth parasite species in freshwater fishes. J. Parasitol. 1983;69:449–454. [Google Scholar]
- Rodiles-Hernández R. Diversidad de peces continentales en Chiapas. In: González-Espinosa M., Ramírez-Marcial N., Ruiz-Montoya L., editors. Diversidad Biológica de Chiapas. Plaza y Valdés, ECOSUR. COCYTECH; México: 2005. pp. 195–220. [Google Scholar]
- Rohde K. A non-competitive mechanismresponsible for restricting niches. Zool. Anz. 1977;99:164–172. [Google Scholar]
- Rohde K. A critical evaluation of intrinsic and extrinsic factors responsible for niche restriction in parasites. Am. Nat. 1979;114:648–671. [Google Scholar]
- Rohde K. Intra- and interspecific interactions in low density populations in resource-rich habitats. Oikos. 1991;60:91–104. [Google Scholar]
- Rohde K., Hayward C., Heap M. Aspects of the ecology of metazoan ectoparasites of marine fishes. Int. J. Parasitol. 1995;25:945–970. doi: 10.1016/0020-7519(95)00015-t. [DOI] [PubMed] [Google Scholar]
- Sarremejane R., Canedo-Argüelles M., Prat N., Mykra H., Muotka T., Bonada N. Do metacommunities vary through time? Intermitent rivers as model systems. J. Biogeogr. 2017;44:2752–2763. [Google Scholar]
- Simková A., Desdevises Y., Gelnar M., Morand S. Co-existence of nine gill ectoparasites (Dactylogyrus: Monogenea) parasitising the roach (Rutilus rutilus L.): history and present ecology. Int. J. Parasitol. 2000;30:1077–1088. doi: 10.1016/s0020-7519(00)00098-9. [DOI] [PubMed] [Google Scholar]
- Šimková A., Gelnar M., Sasal P. Aggregation of congeneric parasites (Monogenea: Dactylogyrus) among gill microhabitats within one host species (Rutilus rutilus L.) Parasitology. 2001;123:599–607. doi: 10.1017/s0031182001008782. [DOI] [PubMed] [Google Scholar]
- Slatkin M. Competition and regional coexistence. Ecology. 1974;55:128–134. [Google Scholar]
- Soberón J., Llorrente J. The use of species accumulation functions for the prediction of species richness. Conserv. Biol. 1993;7:480–488. [Google Scholar]
- Stevenster J.G. Aggregation and co-existence I. Theory and analysis. J. Anim. Ecol. 1996;65:297–307. [Google Scholar]
- Stock T.M., Holmes J.C. Functional relationship and microhabitats distribution on enteric helminths of grebes (Podicipedidae): the evidence for interactive communities. J. Parasitol. 1988;74:214–227. [PubMed] [Google Scholar]
- Weichman M.A., Janovy J., Jr. Parasite community structure in Pimephales promelas (Pisces: Cyprinidae) from two converging streams. J. Parasitol. 2000;86:654–656. doi: 10.1645/0022-3395(2000)086[0654:PCSIPP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Worthen W.B., Rohde K. Nested subset analyses of colonization-dominated communities: metazoan ectoparasites of marine fishes. Oikos. 1996;75:471–478. [Google Scholar]