Abstract
Amid worsening opioid overdose death rates, the nation continues to face a persistent addiction treatment gap limiting access to quality care for opioid use disorder (OUD). Three FDA-approved medications (methadone, buprenorphine, and extended-release naltrexone) have high quality evidence demonstrating reductions in drug use and overdose events, but most individuals with OUD do not receive them. The development of a unified public health framework such as a Cascade of Care could improve system level practice and treatment outcomes. In response to feedback from many stakeholders over the past year, we have expanded upon the OUD Treatment Cascade, first published in 2017, with additional attention to prevention stages and both individual-level and population-based services to better inform efforts at the state and federal level. The proposed cascade framework has attracted considerable interest from federal agencies including the CDC and NIDA along with policy makers nationwide. We have reviewed recent literature and evidence based interventions related to prevention, identification, and treatment of individuals with OUD and modeled updated figures from the 2016 National Survey on Drug Use and Health. Many currently employed interventions (prescriber guidelines, prescription monitoring programs, naloxone rescue) address prevention of OUD or downstream complications but not treatment of the underlying disorder itself. An OUD Cascade of Care framework could help structure local and national efforts to combat the opioid epidemic by identifying key targets, interventions, and quality indicators across populations and settings to achieve these ends. Improved data collection and reporting methodology will be imperative.
Introduction
Efforts to address the longstanding addiction treatment gap in the United States have taken on additional import in the face of roughly 72,000 annual drug-related overdose deaths, the majority involving opioids (1). Most compelling, the unfettered rise in opioid-related overdose deaths has increasingly led to historical comparisons to the gravity of the AIDS epidemic and the magnitude of national response required to effectively change course (2). Building on a decade of successful management of HIV as a chronic medical condition – and declining death rates - the HIV field developed a public health framework, the HIV Cascade of Care (3), to monitor progress across populations and treatment settings. Similar Cascade of Care (COC) models have also now been applied to other chronic medical disorders including diabetes (4) and Hepatitis C (5) and can be expanded to incorporate prevention services in addition to treatment and recovery stages at either the individual or population level (6,7).
Organizing efforts to respond to the opioid epidemic around an opioid use disorder (OUD) Cascade of Care framework could improve outcomes and reduce mortality (8,9). Increasingly, NIDA and the CDC are suggesting this framework as it can be applied to track progress at the individual patient level or to assess population-level outcomes. The HIV cascade framework established key stages (diagnosis, engagement in care, initiation of antiretroviral regimens, viral suppression, retention in care) through which HIV-infected persons can progress to successfully achieve sustained viral suppression, thereby improving individual health and eliminating transmission risk to others (3). The United Nations established a 90-90-90 goal aiming to successfully diagnose 90% of all HIV-infected persons worldwide, successfully engage 90% of those diagnosed in treatment, and virally suppress at least 90% of those in treatment by the year 2020 in order to maximize individual patient health and also slow or stop transmission of new infections. As of 2010 when the HIV Cascade of Care gathered national interest as a framework, the United States had only managed to shepherd an estimated 19% of infected individuals to viral suppression (3). Although a comparable 90% goal of success at each stage for individuals with OUD is not necessarily attainable across all populations and settings-- in part due to key differences between the nature of HIV and OUD-- setting ambitious targets can motivate a more robust policy response, mobilize funding at the appropriate scale, and highlight areas in greatest need of additional resources and intervention development.
Developing Key Stages in an OUD Cascade of Care
The OUD Cascade of Care model as presented below encompasses four interrelated domains regarding all persons with OUD or at risk for developing OUD: Prevention, Identification, Treatment, and Recovery (see Figure 1). Within the broader OUD Cascade of Care, the treatment cascade emphasizes progressive stages specific to those already identified as having OUD or post-overdose, including 1). Engagement in care, 2). Initiation of medications for OUD (MOUD), 3). Retention, and 4). Remission (Figure 1, far right side). The following sections detail the broader OUD Cascade of Care, encompassing prevention and identification, in addition to the treatment cascade. The manuscript intends to clarify and expand upon the model first published by Williams et al in 2017 (8) and reflect revisions following feedback from stakeholders in the field over the past year. Finally, we have attempted to map relevant interventions across the four domains in relation to one another. While the framework is currently proposed with candidate concepts, it will require further specification depending on its application, such as for monitoring population trends nationally or following patients at the individual level in a given care system.
Figure 1:
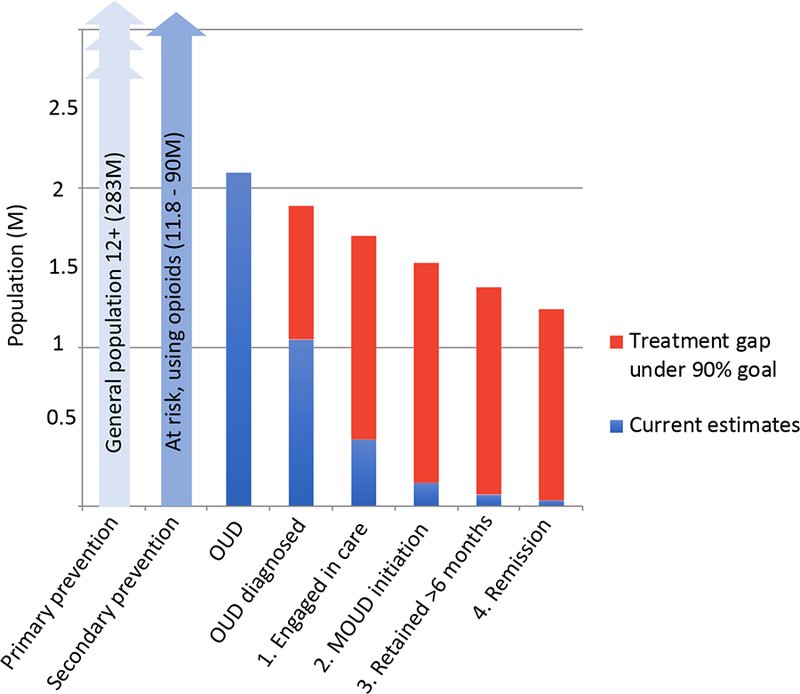
OUD Cascade of Care, 2016 Estimates Among Publicly Reporting Providers
Prevention
The broader OUD Cascade of Care framework has been expanded to reflect the domain of Prevention given its vital importance to public health strategies to contain harms from opioid use at the population level, including overdose, and reduce the incidence of opioid misuse and OUD. Figure 1 encompasses three populations: 1) Approximately 283 million individuals ages 12+ among the general population who would benefit from primary prevention services to increase awareness of the dangers of opioids and reduce initial exposure, 2) At risk populations, who warrant secondary prevention and screening services for early identification of OUD, including those being prescribed opioids for pain, or reporting non-medical use of opioids (prescribed or illicitly obtained), and those at higher risk based on characteristics such as family history and psychiatric comorbidity but who may have yet to initiate opioid use (ranging from 11.8 million to 90 million), and 3) Those with OUD and in need of treatment (2.1 million). It is worth emphasizing that the size of these populations are estimates and are largely derived from a single data source, the National Survey on Drug Use and Health (NSDUH) administered by SAMHSA annually (10). Although robust, the NSDUH does not sample incarcerated populations and the response rate has waned over recent years. Given these and other limitations, it is possible that widely used figures are underestimates of the true national burden of opioid use and those at risk for developing OUD (11,12).
Once identified with OUD or following an overdose, patients should be shepherded into treatment and toward recovery. Otherwise those individuals without OUD remain in pools of unaffected individuals and continue to benefit from prevention services, such as general health promotion or OUD awareness campaigns and prescriber guidelines regarding the cautious use of opioids.
Identification: Diagnosing OUD
Individuals who are high-risk for developing OUD include those prescribed opioids as long-term opioid therapy for non-cancer pain or those receiving more than 90 mg morphine equivalents (MEQ) daily, with a history of substance use disorders (SUDs), psychiatric comorbidity, problematic substance use (such as binge drinking or cocaine use), prior overdose, or prior criminal arrest (13,14). These risk factors are especially predictive of addiction risk among young males 18–35 years of age (14). Concomitant use or prescription of other sedating medications such as benzodiazepines or medical comorbidities that impair respiratory drive (e.g. sleep apnea, congestive health failure) also increase risk of opioid overdose. Individuals at risk for OUD can be identified through routine screening, such as under an SBIRT model, in primary care or in acute care settings (15). Additionally, surveillance through prescription drug monitoring programs and intermittent urine drug screening can help identify patients with aberrant opioid use behaviors warranting further assessment. Harm reduction approaches, such as needle exchange, naloxone distribution, and supervised injection facilities assist and identify affected individuals who have not yet engaged in treatment or in high-risk areas for outbreaks of infectious disease such as HIV or hepatitis C (16). The Centers for Disease Control and Prevention has recently identified 220 vulnerable counties across the country (17).
Medically treated overdoses, or other presentations such as infections related to drug injection, represent opportunities to identify patients with OUD who are in immediate need of specialized treatment services. In the past two years, dramatically improved outcomes have been demonstrated for patients who initiate buprenorphine to treat OUD in emergency departments (18). Such interventions should become the standard of care.
Finally, the criminal justice system interfaces with a disproportionate number of individuals with OUD and at risk for overdose (19). Although the justice system refers about half of all patients with substance use disorders to specialty treatment, court mandated treatment rarely allows for medication based treatment with methadone or buprenorphine, severely limiting treatment options for these vulnerable and high risk populations (20). Despite a 2016 presidential mandate that drug courts must allow for all medications for OUD in order to continue to receive federal funding, and despite best practice guidance from the National Association of Drug Court Professionals (21), many justice-involved individuals, whether at pre-arraignment, post-arraignment (but pre-sentencing), post-sentencing, or probation/parole stages, find that they are disallowed from starting or continuing on these lifesaving medications (20).
Important differences exist in the diagnosis and treatment of patients with HIV and OUD that complicate direct comparisons for all cascade stages. While individuals infected with HIV can go for many years before becoming symptomatic and must be diagnosed with a lab test, OUD is a clinical diagnosis. In contrast to HIV where viral suppression requires daily medication adherence, some patients with OUD can enter short-term remission without medical intervention, although subsequent relapse is likely. For these reasons, the Cascade of Care framework does not seamlessly translate from HIV to OUD. Regardless, challenges to reaching and accurately diagnosing individuals who often have complex comorbidity, behavioral risk factors, and marginalized social status span both fields.
Treatment: Engagement in Care
Among patients with OUD, successful medication (MOUD) initiation and retention are the two clinical milestones likely to have the greatest impact on lowering risk of mortality (22,23). The State Targeted Response (STR) funding for the opioid epidemic from SAMHSA via the 21st Century Cures Act now requires states to monitor population level receipt of MOUD and adherence to treatment. This is critical as studies have repeatedly shown that many patients who receive buprenorphine (24–26) or XR-naltrexone (27,28) only do so for 30–60 days, falling far short of a minimum threshold of 6–12 months thought to be necessary to confer sustained benefit (29,30).
The four stages of the treatment cascade on the right side are operationalized as follows:
Engagement in Care: Percent of individuals with OUD receiving specialty services in a given year
MOUD Initiation: Percent of individuals in care who receive MOUD at least once
Retention: Percent of individuals receiving MOUD who continue for 180+ days
Remission: Percent of retained individuals who no longer meet past-year criteria for OUD
These four successive stages build on evidence for effective treatment of OUD (31) and expert consensus on standards of care (29, 32). Competing rational arguments can be made for different definitions of treatment duration as evidence is lacking for a specific duration of treatment with MOUD known to optimize long-term outcomes. As a result, guidance tends to recommend a minimum of 1–2 years of long-term treatment without a predefined duration (29,33,34). The 180-day minimum duration specified within the retention stage corresponds to a quality measure recently endorsed by the National Quality Forum (30,35).
Treatment: MOUD Initiation and Retention Beyond 6 Months
Data are also lacking on which patients have the greatest chance of responding to a specific OUD medication and which patients require longer rather than shorter durations of treatment with MOUD. In general, published studies suggest demographic characteristics (e.g., age, race, sex) are less important than addiction severity (e.g., progression to heroin use, injection, overdose history, comorbid cocaine use) and treatment history (e.g. multiple failed attempts at tapers in the past) in predicting long-term retention and outcomes. Forthcoming data from CTN 0051 (a comparative effectiveness trial of buprenorphine and XR-naltrexone) reflect the difficulties in patient-treatment matching, suggesting structural factors, such as housing status, may be as, or more important than markers of addiction severity on treatment outcomes (36). As a result, similar to the HIV Cascade of Care, the proposed treatment cascade does not address medication choices at the individual patient level. Additional work is needed to build on new MOUD treatment models such as interim buprenorphine/methadone or low-threshold programs (37), which focus on initiating medication without delay or requirement of comprehensive evaluation, participation in therapy, or abstinence from other drugs. The OUD treatment cascade framework, coupled with standardized reporting of quality measures, could help identify which patients struggle at which stages of the cascade and thereby help to motivate development and implementation of interventions tailored to specific subpopulations and settings (12,38,39).
Many touted interventions and services for responding to the opioid epidemic (e.g., prescription drug monitoring programs and prescriber guidelines) address primary prevention (reducing risk of incidence of OUD) or secondary prevention (early identification) but do not necessarily facilitate progression through the treatment end of the cascade for individuals once identified with OUD (far right of Figure 1). Further, expanding access to short-term detoxification programs or “detox beds” cannot contribute to successful progression along the cascade unless these facilities are repurposed and entitled “medication initiation units” (40). Detoxification itself is not a treatment for OUD and when used in isolation increases risks of adverse events such as overdose (41). Similarly, although naloxone rescue can be a critical intervention for reducing death from overdose, a life-threatening medical complication from OUD, it is not a treatment for underlying OUD. Although these prevention services can also benefit patients with OUD, whether currently in treatment or not, by themselves these interventions are not effective treatments and have not been shown to reduce overdose risk at the individual level.
Remission
While abstinence may not be achievable for some patients with OUD, the complete and continuous cessation of opioid use confers the greatest protection against opioid-related overdose death and other morbidity related to OUD. However, this should not be interpreted to suggest that patients do not benefit from treatment and related services if they continue to intermittently use opioids (42,43). The final stage of the treatment cascade (in comparison with Williams et al 2017(8)) has been re-labeled “Remission” rather than “Continuous Abstinence” to reflect these important considerations. As many patients with OUD have concurrent substance use disorders with other classes of drugs, such as benzodiazepines, additional treatment services are often necessary to complement those emphasized in the treatment cascade. Recovery management under a continuing care model with recovery checkups (44) and other services to sustain beneficial outcomes under healthcare reform warrant further research (45).
The benefits of harm reduction approaches, often forms of tertiary prevention, such as needle exchange, safe drug use education, naloxone distribution, and supervised injection facilities have become more apparent, especially in geographic areas at risk for outbreaks of infectious disease such as HIV (16). Compared to just 10 years ago, many service providers nationwide now enthusiastically embrace “meeting patients where they are at” rather than making treatment and service provision contingent on complete abstinence. These efforts may help retain patients in primary care services whether they sporadically adhere to MOUD or continue using opioids or other drugs.
Applications
The HIV Cascade of Care models the many applications that a unified framework can provide to clinicians, researchers, and policymakers working with highly marginalized populations who have health conditions affected by complex environmental and behavioral interactions. For instance, because of its simplicity, the HIV Cascade of Care allows for comparisons at the population level to assess outcomes, identify critical gaps in care and resulting disparities, and develop interventions across locations and time (46–48). An OUD Cascade of Care similarly holds great promise to identify critical gaps and track progress to improve treatment outcomes and reduce disease-related mortality. Identifying the challenges encountered by patients at each stage of the Cascade can target clinical and policy interventions to help federal and state efforts achieve the greatest impact.
The OUD Cascade of Care framework can be used for several and complementary purposes. It could, for example, enhance accreditation standards for treatment programs. For instance, it may not be appropriate for programs that lack MOUD prescribers to be accredited or reimbursed as providers of OUD treatment. Additionally, the framework can inform data collection and reporting. Standardized methods with universal metrics for data collection could facilitate comparisons across time and locations to improve quality. The Cascade framework can also inform treatment planning and monitoring of key targets, and implementation strategies to improve outcomes. As an example, Belenko and colleagues (49) have demonstrated the utility of applying the cascade framework to juvenile justice populations with substance use to detect gaps in care and opportunities for improvement. Finally, such a framework could quantify current gaps in care processes for individuals and subpopulations with OUD; provide tools for goal setting, accountability, measurement of progress; identify needed treatment resources; and increase use of guideline-consistent, evidence based care processes through stewardship of quality measures (35) and related incentivized reimbursement algorithms.
Conceptual Challenges and Limitations
Important differences exist between HIV and substance use disorders that are likely to complicate implementation of a cascade of care model for OUD. A larger percentage of US adults view substance use disorders as behavioral or antisocial problems related to “bad character” rather than as neurobiological or genetic medical conditions (50) which may erode policy support for services and negatively influence legislation (51). As a result, it remains an unfortunately common practice in many treatment programs to discharge patients who do not achieve complete abstinence or remain continuously abstinent from opioids or other drugs which deprives these higher risk patients of evidence based care when they need it most. There is also considerable variation in insurance coverage for OUD treatment. For example, many state Medicaid programs do not cover all levels of care required for the effective treatment of this condition and often limit durations of treatment with medications such as buprenorphine (52). Adopting a cascade framework may help emphasize evidence based interventions over moral ideology and stigma.
A key decision in developing quality measure frameworks pertains to selection of the primary outcome. Remission (no longer meeting past-year criteria for OUD) is designated as the final stage along the treatment section of the Cascade. As even a single episode of opioid use can carry risk of acute mortality, the ideal of continuous abstinence could serve as an organizing principle for resource deployment despite the fact that many patients will receive benefit from OUD treatment even if continuous abstinence has not been achieved or is not the individual patient’s treatment goal (42,43). The same can be said in other areas of medicine, wherein patients benefit from treatment (e.g., diabetes, hypertension) but do not necessarily meet primary end points (e.g., HbA1c or blood pressure targets). Significantly, patients with OUD have lower rates of overdose and mortality while on MOUD (compared to being off of medication), irrespective of whether they intermittently use opioids (23) and all efforts should be made to successfully retain patients in care as long as possible. Although stigma, prejudice, and misinformation have historically led to premature discharge of patients from OUD treatment, the impetus to protect patients from these harms should not obscure the importance of continuous abstinence from opioids as the greatest protection against an opioid-related overdose (12). Furthermore, some patients who develop OUD have underlying chronic pain syndromes and a subset may require ongoing opioid use as part of their medical management. Additional efforts are needed to determine best practices for these complex populations. It may also be that Remission as a final stage neglects to fully address the importance of quality of life and improved level of functioning under a recovery model that also echoes the World Health Organization’s insistence that health is not merely the absence of disease. Stakeholders (including patients and families) and leaders in the field should consider which stages at the end of the Cascade can be most impactful. It may be that Remission is the penultimate stage with Recovery following. The field currently lacks validated measurement based approaches for assessing recovery specific to OUD populations and this is an area for further research.
Current Progress
Translating the 90-90-90 goal to addiction treatment generally (7) or the opioid epidemic specifically (8, 53,54) underscores tremendous gaps in care facing affected populations (Figure 1). In the United States, an estimated 2.1 million individuals meet past-year criteria for OUD according to the National Survey on Drug Use and Health (NSDUH) (10) while only 20% receive specialty care in a given year (55,56), leaving the great majority without any treatment services. Among those accessing treatment, fewer than 35% are estimated to receive evidence based treatment with one of the three FDA-approved medications (methadone, buprenorphine, or extended-release naltrexone) in a given care episode according to the National Survey of Substance Abuse Treatment Services (57–59). Further, premature treatment drop out has long plagued the field. Among the subgroup of people with OUD who initiate medication based treatment, a great majority discontinue during the first few weeks or months (23–26). Less is known about long-term recovery trajectories beyond six months (60). These estimates are at the national level and do not reflect state- or system-level models. Further, NSDUH is not designed to identify individuals in full sustained remission from OUD and as a result cannot reflect treatment responders who no longer meet criteria for OUD. As a result, Figure 1 as currently presented reflects the estimated 2.1 million individuals in need of treatment at a given time in the country and current performance along the OUD Cascade of Care in successfully shepherding these individuals to the final stage.
Future Steps
Table 1 indicates assessment settings wherein patients are likely to present and the respective prevention and screening services aligned with these populations. The populations are proposed based on known risk factors for development of OUD or overdose. Some individuals move between populations over time. These interventions have varying levels of supporting evidence with some in widespread use despite limited demonstration of effectiveness.
Table 1:
Populations at risk for OUD by risk level, setting, and related interventions
| Risk factors/Populations | Assessment settings | Interventions for prevention, identification, and bridging to specialized treatment for OUD* |
|---|---|---|
|
Acute presentations - Overdose reversals - ED complications (infection, injury) - Entering SUD treatment - Inpatient/residential/outpatient - Criminal Justice involvement |
-Emergency departments -Hospitals -Substance abuse treatment -Criminal justice settings |
-Urine drug screens -Harm reduction services -Overdose prevention training and reversals -Warm hand-offs to treatment -Supervised MOUD induction (e.g. from ED) -Police referrals to treatment (e.g. PAARI, LEAD) |
|
Patients using opioids
High Risk - >90mg MEQ daily - Receiving LTOT for non-cancer pain - Prior acute presentation - Prior SUD treatment/diagnosis - Psychiatric comorbidity At Risk - Family History of SUD - Young male adults (age 18–35) - Active risky substance use |
-Pain clinics -Physician offices -Primary care -Behavioral health clinics -Adolescent health programs -Outreach programs -Employee assistance programs -Schools |
-Frequent clinical assessments -Alternative treatments for chronic pain (e.g. non-opioid, non-pharmacologic) -Urine drug screens (random) -Prescriber guidelines -Academic detailing of physicians -Pill counts -Refill limits -PDMPs -Naloxone distribution -SBIRT -Targeted prevention programs |
|
General population -Ages 12+ |
-Public venues -Social media -Primary care -Schools |
-Resiliency enrichment programs (e.g. Lifeskills) -Health promotion and skills training -Public service announcements -Drug take-backs |
Interventions may be relevant to multiple populations but are listed once alongside the category most relevant for simplicity. MOUD= Medications for Opioid Use Disorder; OUD= Opioid Use Disorder; ED=Emergency Department; PAARI= Police Assisted Addiction and Recovery Initiative; LEAD= Law Enforcement Assisted Diversion; MEQ= Morphine Equivalents (mg); LTOT=Long term opioid therapy; SUD= Substance Use Disorder; PDMP=Prescription Drug Monitoring Program; SBIRT= Screening, Brief Intervention and Referral to Treatment
Individuals with acute presentations within emergency departments, criminal justice, or specialty addiction treatment settings may pose particularly high risks (61,62). As recently endorsed by the FDA, individuals who have experienced an opioid overdose are candidates for MOUD. We distinguish at-risk populations as individuals without characteristics known to increase risk of adverse outcomes but who nonetheless warrant closer scrutiny than the general population. Among the general population (i.e., those not prescribed or using opioids), screening for OUD should follow guidelines recommended within the Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework. In this model, all primary care patients are assessed annually for SUDs (15). Although SBIRT helps identify patients with OUD, it has yet to demonstrate any impact on meaningful reductions in opioid use on its own, likely because patients are unlikely to follow up on referrals, suggesting the need for immediate intervention (18).
A distinction between acute presentations and routine screening and clinical management emphasizes opportunities for identifying barriers and implementing services needed to develop differential treatment pathways. For instance, individuals presenting with overdose warrant same day on site inductions in the Emergency Department (18) whereas those identified through routine screening in the criminal justice system should be initiated on MOUD during incarceration and well before release to the community (61). While these are key high-risk populations, most patients with OUD are identified in general practice and specialty addiction treatment settings and benefit from other models of care delivery. As a result, different policy, funding, and clinical structures are needed in each case.
Patients identified with OUD require a comprehensive treatment plan to help them successfully progress through the stages of the treatment cascade. However, patients with OUD are difficult to identify, track, and engage in treatment due to their marginalized status. In response, community-based and low threshold outreach strategies are needed (Table 1). In addition, the healthcare system has been historically ill equipped to detect OUD and other substance use disorders due to limited training and clinical supervision related to the treatment of substance use disorders. Many patients in need of addiction treatment lack insight (62,63), struggle with ambivalence, or fear stigmatization and prejudicial responses from providers and do not proactively seek treatment (10). Those eligible for insurance coverage, whether public or commercial, often have unstable lives that compromise their ability to enroll in health insurance, much less engage in primary care or routine health services (64). As a result, innovative outreach strategies and universal non-judgmental screening efforts (i.e. outside of traditional clinical settings) are needed to identify patients with risky behaviors or undiagnosed OUD. Table 1 lists specific settings where high-risk populations can be identified along with relevant interventions.
Given that many patients with OUD fail to receive treatment, and among those that do, the majority receive care that deviates from evidence based practice, there are several pressing research questions that could help move the field forward. Table 2 lists several opportunities for investigation, at the clinical, implementation, and policy levels, into interventions that can improve rates of successful MOUD initiation and retention, the two most critical stages of the OUD Cascade of Care. Ultimately, improving data collection and reporting systems will be a vital part of any effective response at scale for tracking success along the cascade.
Table 2:
Key Research Challenges
| Clinical Research |
| - Develop clinical and systems level interventions to improve medication adherence and treatment retention - Identify clinical decision support tools and programming to better match patients at the individual level with adjunctive psychosocial and behavioral treatments - Determine optimal duration of care for treatment responders for each MOUD modality - Evaluate safe and effective methods for transitioning patients in long-term recovery off of MOUD if indicated |
| Implementation Science |
| - Identify barriers to treatment engagement and retention - Devise effective implementation strategies in real world and various settings - Characterize which patients would do better being managed in primary care v. mental health v. specialty settings |
| Policy |
| - Improve data collection and reporting methods - Assess potential benefits and risks of making OUD an (anonymously) reportable disorder at the state or federal level such as TB or HIV - Define when to require observation and related emergency stabilization protocols - Develop guidelines consistent with other areas of medicine and mental health for treatment over objection for patients with impaired capacity |
MOUD= Medications for Opioid Use Disorder; OUD= Opioid Use Disorder
Conclusion
Adoption of an OUD Cascade of Care offers opportunities to enhance outcomes through improved treatment program accreditation standards, data collection and reporting, and monitoring of key clinical targets. Developing quality measures to identify which patients struggle at which stages of the Cascade could also help target clinical and policy interventions aimed at improving patient outcomes. We have proposed broad candidate concepts to elaborate an OUD Cascade of Care to encompass major interventions across domains of prevention, identification, treatment, and recovery. Additionally, we have emphasized stages within an OUD treatment cascade specific to patients identified with OUD or post-overdose since MOUD initiation and retention are associated with reducing overdose and mortality. Millions of patients with OUD do not have access to quality care. These unidentified and untreated populations will continue to disproportionally contribute to the nation’s worsening overdose death rates until they are connected to effective treatment services. We hope that the OUD Cascade of Care framework will help to structure local and national efforts to combat the opioid epidemic by identifying key targets, interventions, and quality indicators across populations and settings to achieve these ends.
Acknowledgments
Funding provided by NIDA grant K23DA044342–01 (Williams) and the Christopher D. Smithers Foundation, Inc.
Footnotes
Conflicts of Interest: Dr. Nunes received medication or software for research studies from Alkermes and Reckitt-Benckiser. Dr. Bisaga received medication, extended-release naltrexone, for NIH funded research studies from Alkermes.
References
- 1.Centers for Disease Control and Prevention. National Vital Statistics System. Provisional counts of drug overdose deaths as of 9/5/2018. National Center for Health Statistics, Centers for Disease Control and Prevention, 2018. [Google Scholar]
- 2.Williams AR, Bisaga A. From AIDS to opioids- how to respond to an epidemic. New England Journal of Medicine. 2016; September 1;375(9):813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. HIV/AIDS. 2011:52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali MK, Bullard KM, Gregg EW, et al. A Cascade of Care for Diabetes in the United States: Visualizing the Gaps. Annals Int Med. 2014;161(10):681–689. [DOI] [PubMed] [Google Scholar]
- 5.Yehia BR, Schranz A, Umscheid CA, et al. The Treatment Cascade for Chronic Hepatitis C Virus Infection in the United States: A Systematic Review and Meta-Analysis. PLOS ONE, 9(7): e 101554, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn T, Sherwood J, Remien RH, Nash D, Auerbach JD. Towards an integrated primary and secondary HIV prevention continuum for the United States: a cyclical process model. J Int AIDS Soc. 2017; November 19:21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonsalves GS, Paltiel AD, Cleary PD, et al. A flow-based model of the HIV care continuum in the United States. J Acquir Immune Defic Syndr. 2017; 75(5):548–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams AR, Nunes EV, Olfson M. To battle the opioid overdose epidemic, deploy the “Cascade of Care” model. Health Affairs blog, March 13, 2017. [Google Scholar]
- 9.Socias EM, Volkow N, Wood E. Adopting the ‘Cascade of Care’ framework: an opportunity to close the implementation gap in addiction care? Addiction. 2016;doi: 10.1111/add.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SAMHSA Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health. HHS. SMA17–5044. Rockville, MD, 2017. [Google Scholar]
- 11.Compton WM, Dawson D, Duffy SQ, Grant B. The effect of inmate populations on estimates of DSM-IV alcohol and drug use disorders in the United States. Am J Psych. 2009;167(4):473–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AR. Performance measures and quality improvement for the opioid epidemic In: Opioid Addiction: An American Crisis (Compton M & Manseau MWeds.). APPI Press, 2018. [Google Scholar]
- 13.Cicero TJ, Ellis MS. Understanding the demand side of the prescription opioid epidemic: Does the initial source of opioids matter? Drug Alcohol Depend. 2017;173:S4–S10. [DOI] [PubMed] [Google Scholar]
- 14.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. New Eng J Med. 2016;374:154–163. [DOI] [PubMed] [Google Scholar]
- 15.Babor T, McRee B, Kassebaum P, et al. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse. 2017;28 (3), 7–30. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Quader AS, Feelemeyer J, Modi S, et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS and Behavior. 2013;17(9): 2878–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Handel MM, Rose CE, Hallisey EJ et al. County-Level Vulnerability Assessment for Rapid Dissemination of HIV or HCV Infections Among Persons Who Inject Drugs, United States. J Acquir Immune Defic Syndr. 2016;November 1;73(3):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency Department-Initiated Buprenorphine/Naloxone Treatment for Opioid Dependence: A Randomized Controlled Trial.JAMA. 2015;28;313(16)1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkelman TNA, Chang VW, Binswanger I. Health, polysubstance use, and criminal justice involvement among adults with varying levels of opioid use. JAMA Network Open. 2018. 1(3):e180558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matusow H, Dickman SL, Rich JD, et al. Medication assisted treatment in US drug courts: Results from a nationwide survey of availability, barriers and attitudes. J Subst Abuse Treat. 2013; 44:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Association of Drug Court Professionals. Adult drug court best practice standards, Volume II. NADCP; Alexandria VA, 2015. [Google Scholar]
- 22.Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2010;106:32–51. [DOI] [PubMed] [Google Scholar]
- 23.Sordo L, Barrio G, Bravo MJ et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for premature discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abus Treat 95:9–17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon AJ, Wei-Hsuan LC, Cochran G, et al. Patterns and quality of buprenorphine opioid agonist treatment in a large Medicaid program. J Addict Med. 2015;9(6):470–477. [DOI] [PubMed] [Google Scholar]
- 26.Stein B, Sorbero M, Dick AW, Pacula RL, Burns RM, Gordon AJ. Physician capacity to treat opioid use disorder with buprenorphine-assisted treatment. JAMA. 2016;316(11):1211–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvis B, Holtyn A, Subramanian S, et al. Extended-release injectable naltrexone for opioid use disorder: A systematic review. Addiction, in press, 2018. doi: 10.1111/add.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan JR, Shackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine/naloxone utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abus Treat. 2017;July 3 pii: S0740–5472(16)30413–5. doi: 10.1016/j.jsat.2017.07.001. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med. 2015;9(5): 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Quality Forum (NQF). (2017). Behavioral Health 2016–2017: Technical Report. Department of Health and Human Services,. Retrieved from https://www.qualityforum.org/Publications/2017/08/Behavioral_Health_2016-2017_Final_Report.aspx
- 31.Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorders – Exhibit 2.16. 2018:2–24. Available at: https://store.samhsa.gov/product/SMA18-5063FULLDOC. Accessed October 1, 2018.
- 32.ASAM. The ASAM performance measures for the addiction specialist physician. The American Society for Addiction Medicine; Chevy Chase, MD, 2014. [Google Scholar]
- 33.National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. JAMA. 1998;280(22), 1936–1943. [PubMed] [Google Scholar]
- 34.World Health Organization. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. WHO Management of Substance Abuse Team, Geneva, Switzerland, 2009. [PubMed] [Google Scholar]
- 35.Williams AR, Nunes EV, Bisaga A, et al. Developing an Opioid Use Disorder Treatment Cascade: A Review of Quality Measures. J Subst Abus Treat; 2018: 91:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JD, Rotrosen J, Nunes EV, Tanum L (2018). Effectiveness of XR-Naltrexone vs. Buprenorphine: Results of Two Randomized Trials. ASAM National Conference, San Diego, April 12-15, 2018. [Google Scholar]
- 37.Sigmon SC, Meyer A, Hruska B, et al. (2015). Bridging waitlist delays with interim buprenorphine treatment: Initial feasibility. Addict Behav;December;51:136–42. doi: 10.1016/j.addbeh.2015.07.030. Epub 2015 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman ML, Spaeth-Rublee B, Nowels AD, Ramanuj PP, Pincus HA. Quality measures at the interface of behavioral health and primary care. Curr Psychiatry Rep. 2016;18(39):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pincus HA, Scholle SH, Spaeth-Rublee B, Hepner KA, Brown J. Quality measures for mental health and substance use: gaps, opportunities, and challenges. Health Aff. 2016;35(6):1000–1008. [DOI] [PubMed] [Google Scholar]
- 40.Nunes EV, Gordon M, Friedmann P, et al. (2018). Relapse to opioid use disorder after inpatient treatment: Protective effect of injection naltrexone. J Subst Abuse Treat; Feb(85):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strang J, McCambridge J, Best D, et al. (2003). Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ 326:959–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volkow N, Woodcock J, Compton WM, et al. (2018). Medication development in opioid addiction: Meaningful clinical end points. Sci. Transl. Med 10, eaan2595. [DOI] [PubMed] [Google Scholar]
- 43.Williams AR. (2018). After Many Years, the FDA Announces Loosened Standards for Addiction Medication Approval. Health Affairs blog, March 23, 2018. [Google Scholar]
- 44.Dennis ML, Scott CK. Four-year outcomes from the Early Re-Intervention (ERI) experiment using Recovery Management Checkups (RMCs). Drug Alcohol Depend. 2012;February 1;121(1–2):10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tai B, Volkow ND. Treatment for Substance Use Disorder: Opportunities and Challenges under the Affordable Care Act. Soc Work Public Health. 2013; 28(3–4): 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horberg MA, Hurley LB, Klein DB, et al. The HIV Care Cascade measured over time and by age, sex, and race in large national integrated care system. AIDS Patient Care and STDs. 2015;29(11):582–590. [DOI] [PubMed] [Google Scholar]
- 47.Mugavero MJ, Amico KR, Horn T, et al. The State of Engagement in HIV Care in the United States: From Cascade to Continuum to Control. Clinical Infectious Dis. 2013;57(8): 1164–1171. [DOI] [PubMed] [Google Scholar]
- 48.Zanoni BC, Mayer KH. The Adolescent and Young Adult HIV Cascade of Care in the United States: Exaggerated Health Disparities. AIDS Patient Care and STDs. 2013;28(3):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belenko S, Knight D, Wasserman GA, et al. The Juvenile Justice Behavioral Health Services Cascade: A new framework for measuring unmet substance use treatment services needs among adolescent offenders. J Subst Abus Treat. 2017;74:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pescosodio BA, Martin JK, Long JS, Medine TR, et al. “A Disease Like Any Other”? A Decade of Change in Public Reactions to Schizophrenia, Depression, and Alcohol Dependence. Am J Psychiatry 2010;167(11):1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barry CL, McGinty EE, Pescosodio B, Goldman HH. Stigma, Discrimination, Treatment Effectiveness and Policy Support: Comparing Public Views about Drug Addiction with Mental Illness. Psychiar Serv 2014;65(10):1269–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grogan CM, Andrews C, Abraham A, Humphreys K, et al. Survey Highlights Differences in Medicaid Coverage for Substance Use Treatment and Opioid Use Disorder Medications. Health Aff (Millwood) 2016;35(12)2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chalk M, Mark T. Deploying The Cascade Of Care Framework To Address The Opioid Epidemic Means Taking A Closer Look At Quality Measures. Health Affairs, Health Policy Lab, June 21, 2017. [Google Scholar]
- 54.Barrett J, Li M, Spaeth-Rublee B, Pincus HA. Value-based payment as part of a broader strategy to address opioid addiction crisis. Health Affairs blog, December 1, 2017. [Google Scholar]
- 55.Saloner B Changes in Substance Abuse Treatment Use Among Individuals With Opioid Use Disorders in the United States, 2004–2013 During the last decade, nonmedical use. JAMA. 2015;314(14)1515–1517. [DOI] [PubMed] [Google Scholar]
- 56.Wu L, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug Alcohol Depend. 2016;169:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volkow ND, Friedan TR, Hyde PS, Cha SS. Medication-assisted therapies- tackling the opioid-overdose epidemic. New Eng J Med. 2014;370(22):2063–2066. [DOI] [PubMed] [Google Scholar]
- 58.SAMHSA Substance Abuse and Mental Health Services Administration, National Survey of Substance Abuse Treatment Services (N-SSATS): 2015. Data on Substance Abuse Treatment Facilities. BHSIS Series S-88, HHS Publication No. (SMA) 17–5031. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2017. [Google Scholar]
- 59.Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis, 35(1), 22–35. 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larochelle MR, Bernson D, Land T, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med. 2018. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Green TC, Clarke J, Brinkley-Rubenstein L, et al. Postincarceration Fatal Overdoses After Implementing Medications for Addiction Treatment in a Statewide Correctional System. JAMA Psych. 2018;75(4):405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldstein RZ, Craig AD, Bechara A, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams AR, Olfson M, Galanter M. Assessing and improving clinical insight among patients in denial. JAMA Psych. 2015;72(4):303–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Capoccia VA, Grazier KL, Toal C, Ford JH, Gustafson DH. Massachusetts’s experience suggests coverage alone is insufficient to increase addiction disorders treatment. Health Aff. 2012;31(5):1000–1008. [DOI] [PubMed] [Google Scholar]


