Abstract
Although epidemiology studies have associated maternal trichloroethylene (TCE) exposure with decreased birth weight and preterm birth, mechanistic explanations for these associations are currently lacking. We hypothesized that TCE targets the placenta with adverse consequences for pregnancy outcomes. Pregnant Wistar rats were exposed orally to vehicle or 480 mg TCE/kg body weight from gestational days (gd) 6-16, and tissues were collected on gd16. Exposure to TCE significantly decreased average fetal weight without reducing maternal weight. In placenta, TCE significantly increased 8-hydroxy-deoxyguanosine, global 5-hydroxymethylcytosine, and mRNA expression of Tet3, which codes for an enzyme involved in 5-hydroxymethylcytosine formation. Furthermore, glutathione S-transferase activity and immunohistochemical staining were increased in placentas of TCE-exposed rats. The present study provides the first evidence that TCE increases markers of oxidative stress in placenta in a fetal growth restriction rat model, providing new insight into the placenta as a potentially relevant target for TCE-induced adverse pregnancy outcomes.
Keywords: Trichloroethylene, fetal growth restriction, oxidative stress, Wistar rats, glutathione S-transferase, 8-hydroxy-deoxyguanosine, 5-hydroxymethylcytosine
1. Introduction
Trichloroethylene (TCE) is a chlorinated organic solvent that has been used in commercial applications such as metal degreasing, paint stripping, and dry cleaning. In the United States, 2.5 million pounds per year of TCE were used in 2011, while the global usage was 945 million pounds per year [1]. TCE is a widespread environmental pollutant due to improper disposal [2], and is among the most frequently detected United States Environmental Protection Agency (US EPA) regulated drinking water contaminants, detected in 4.5% (groundwater source) to 15% (surface water source) of US public water supplies [3]. In addition, TCE exposure can occur in occupational settings, with an estimated 3.5 million workers in the United States exposed to TCE with short-term exposure levels in air ranging from 1.3 mg/m3 to 1,084 mg/m3 (the highest mean concentration was reported for a degreasing operation) [4].
Metabolism of TCE plays an important role in its toxicity, with liver and kidneys being key sites of TCE bioactivation and target organs of TCE toxicity [5-7]. TCE is metabolized via two main pathways: cytochrome P450-mediated oxidation with trichloroacetic acid and dichloroacetic acid as major bioactive metabolites, and glutathione conjugation with subsequent metabolism to form S-(1,2-dichlorovinyl)-L-cysteine (DCVC) as a major bioactive metabolite (Lash 2000). The placenta expresses key TCE metabolizing enzymes including CYP2E1 and glutathione-S-transferase (GST) (Lash 2000). TCE is classified as a known human carcinogen by the International Agency for Research on Cancer (IARC) and the National Toxicology Program (NTP), based on strength of evidence of TCE as a kidney carcinogen [8-10]. TCE exposure increases cellular generation of reactive oxygen species (ROS), with evidence linking TCE-induced oxidative stress with toxicity in rat kidney [11], rat and mouse liver [12, 13], mouse brain [14, 15], and mouse immune cells [14, 16, 17]. Moreover, the nephrotoxic TCE metabolite S-(1,2-dichlorovinyl)-L-cysteine (DCVC) increases indicators of oxidative stress in kidney [18-21] and placental cells [22, 23].
Recent epidemiology reports indicate significant associations between exposure to TCE and adverse pregnancy outcomes that include decreased birth weight and preterm birth [24, 25]. TCE has been shown to cross the placenta in humans [26, 27]. Although the placenta plays key roles in fetal growth, development, and pregnancy [28], investigation of TCE-induced toxicity in placental cells has been limited to the human placental HTR-8/SVneo cell line in vitro [22, 23, 29]. Furthermore, the potential for in vivo TCE exposure to induce oxidative stress in the placenta has not been previosuly reported, to our knowledge, although the TCE metabolite DCVC stimulates generation of ROS in placental HTR-8/SVneo cells [22, 29].
Because the placenta is the essential interface between mother and fetus, changes in its function can significantly impact pregnancy outcomes [30]. The goal of the current study was to identify effects of TCE exposure during pregnancy on pregnancy outcomes and biomarkers of oxidative stress in the placenta of Wistar rats.
2. Materials and methods
2.1. Materials
Trichloroethylene (99% pure) was purchased from Sigma Chemical Company (St. Louis, MO, USA). RNAlater, RNeasy Plus Mini Kit and QIAamp DNA Mini Kit were purchased from SABiosciences (Valencia, CA, USA). The Colorimetric 8-OHdG DNA Damage Quantification Direct kit and MethylFlash Hydroxymethylated DNA Quantification kit were purchased from Epigentek (Farmingdale, NY, USA).
2.2. Animals
Animal experiments were approved by the University of Michigan Institutional Animal Care and Use Committee (Protocol #PRO00006721) and performed in accordance with all state and federal regulations for use of vertebrate animals. Timed-pregnant Wistar rats between 60-90 days of age weighing 200-250 grams were obtained from Charles River (Portage, MI, USA). The day after copulation was designated as day 0 of pregnancy. Rats were shipped at gestational day (gd) 2 and individually housed in a controlled environment with a 12-hour light/dark cycle. Dams were fed standard rat chow (Purina 5001) and water ad libitum.
2.3. Exposure and Experimental Design
Rats were administered TCE daily from gd 6-16 using vanilla miniwafers [31]. We chose this gestational age range to include TCE exposure of placenta from its earliest stages, and we terminated the experiment on gd 16 to allow us to examine placenta without potential confounding from rats going into early labor. Oral exposure was chosen as a route relevant for human environmental TCE exposure, and wafers were chosen instead of gavage to minimize stress. Rats were fed wafer only (vehicle controls) or wafer with 480 mg TCE/kg body weight between 8:00-9:00 am daily. The TCE dose was selected because 400-500 mg TCE/kg/d was previously shown to stimulate oxidative stress in rat liver [12]. Dams were weighed daily and the amount of TCE added to the wafer was adjusted based on the individual daily body weight of the dam. To ensure that the wafers were eaten quickly, rats were trained to eat the wafer without TCE over three consecutive days preceding TCE exposure. Prior to administration, rats were housed for 1 h without food, then TCE (undiluted) was pipetted onto a miniwafer that was immediately offered to the rats and readily consumed. Exposure on gd 16 occurred approximately 2 hours prior to euthanasia. Exposures were conducted in two blocks. The first block included eight TCE-exposed rats and seven controls (it was discovered that one rat in the control group was not pregnant). The second block included three rats in the control group and two rats in the TCE treatment group for a total N=10 in each treatment group.
2.4. Dissections
Data were collected from all dams for measures of litter size and fetal body, maternal body, liver, and kidney weights. Rats were euthanized at gd 16 with carbon dioxide followed by cardiac exsanguination.
Dams were euthanized alternating between controls and TCE-treated rats, conducted within a 4-h period in the morning. The uterine horn was removed and examined for resorbed or dead fetuses. Fetal weights and litter size were recorded as well as maternal body, kidney, and liver weights. Placentas were snap frozen in liquid nitrogen and then stored in the −80°C freezer or in RNAlater reagent for future analysis.
2.5. Assessment of DNA base modifications
Genomic DNA was extracted from placental tissue of dams in the first exposure block (7 control dams and 8 TCE-exposed dams; 3 placentas per litter). Each tissue sample was weighed and then homogenized using a FastPrep-24 tissue and cell lyser (MP Biomedicals, Solon, OH, USA). DNA was extracted from the homogenized tissue using a QIAmp DNA Mini kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. Concentration and purity of the extracted DNA was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE, USA). The samples were stored at −80°C until assayed for levels of 8-hydroxy-deoxyguanosine (8-OHdG), 5-hydroxymethylcytosine (5-hmC), and 5-methylcytosine (5-mC).
Levels of 8-OHdG, 5-mC and 5-hmC were assessed in placental samples using the EpiQuik 8-OHdG DNA Damage Quantification Direct Colorimetric kit, MethylFlash Methylated DNA Quantification kit (Colorimetric) and MethylFlash Hydroxymethylated DNA Quantification kit (Epigentek, Farmingdale, NY), respectively, following the manufacture’s protocols. For the 5-mC assay, placental DNA was pooled on a litter basis prior to assay. For the 8-OHdG and 5-hmC assay, DNA of each placenta was analyzed and then the data were averaged per litter. The same 3 placentas used in the 5-mC assay were also used in 5-hmC assay. Briefly, genomic DNA was added to strip wells that were pretreated to have a high affinity for DNA. Then, capture and detection antibodies were used to determine 8-OHdG or the methylated and hydroxymethylated fractions of the DNA. The absorbance was read at 450 nm on a Molecular Devices SpectraMax Gemini M2e spectrophotometer. The experiment was carried out in triplicate. Relative quantities of 5-mC and 5-hmC were calculated based on the manufacturer’s guidelines. In addition, the same DNA samples used with the MethylFlash Hydroxymethylated DNA Quantification kit were also used to conduct a LUminometric global methylation assay (LUMA) according to Sant et al. [32] (data not shown).
2.6. Immunohistochemical detection of GST in rat placenta
Placentas were fixed in 10% formalin (Fisher) for a minimum of 48 h. Immunohistochemistry was performed by the University to Michigan Histology Core. Briefly, unstained 5-μm sections were cut from paraffin-embedded, formalin-fixed tissue using a rotary microtome and mounted on glass slides. Placenta GST detection was performed using a commercially available primary rabbit polyclonal antibody against GST-pi (GWB-BBP465, GenWay Biotech). We analyzed for GST-pi because it is the GST isoform that is predominantly expressed in the placenta; the other GST isoforms are either not expressed in the placenta (GST-alpha) or are expressed at low concentrations (GST-theta and GST-mu) [33]. To analyze for protein expression of GST-pi in the placenta, heat-induced antigen retrieval was performed with citrate buffer (pH 6) for 10 min. Immunoperoxidase staining was completed on a Dako AutoStainer at room temperature using a LSAB2 visualization kit (Agilent Dako). Briefly, peroxidase block was followed by a 30-min incubation with primary antibody at a dilution of 1:1500 rabbit polyclonal. Samples were then incubated sequentially with a biotinylated link antibody (produced in goat) at a dilution of 1:500 for 30 min, streptavidin-HRP conjugate incubation for 20 min, and 3,3′-diaminobenzidine chromogen solution for 10 min. As an antibody control, placental sections were incubated with secondary antibody only. Microscopy imaging was conducted using Nikon Elements. One placenta per litter was harvested from dams in the first exposure block (2 control and 3 TCE-exposed rats) and second exposure block (3 control and 2 TCE-exposed rats). For each placenta, 5-6 images were analyzed, with one image selected in each of the four quadrants and one or two in the middle of the image field.
2.7. Glutathione S-transferase (GST) and γ-glutamyltransferase (GGT) enzyme activity assays
Activity levels of GST and GGT were measured in a subset of rat placentas collected from dams in the first exposure block (1 control and 2 TCE-exposed rats) and second exposure block (3 control and 2 TCE-exposed rats), 3 placentas per litter. Additional litters were not included due to limitation of tissue availability. The placentas were rinsed in phosphate-buffered saline (PBS), pooled, and homogenized in 2 mL cold PBS with 2 mM EDTA. The homogenized tissues were then centrifuged at 10,000 × g for 15 min at 4°C. The tissue extracts were collected and stored at −80°C and assayed the next day.
GST enzyme activity was assayed according to Habig et al. [34], as modified by the Glutathione S-Transferase Assay kit (Cayman Chemical; Ann Arbor, MI, USA) for 96-well plates. GST activity was determined by adding 10 μl of 1-chloro-2,4-dinitrobenzene, 20 μL GSH, and 20 μL placental tissue extract to 150 μL GST buffer (containing 100 mM potassium phosphate and 0.1% Triton X-100). A SpectraMax M2e Multi-Mode Microplate Reader (Molecular Devices) was used to quantify formation of 2,4-dinitrophenylglutathione kinetically at 340 nm (ε = 9.6 mM−1 cm−1). GST activity was calculated according to the manufacturer’s guidelines. One unit of enzyme activity is defined as the amount of enzyme that forms 1.0 nmol of S-2,4-dinitrophenyl GSH/min at 25 °C. The level of protein was determined by the bicinchoninic acid (BCA) protein assay (Thermo Scientific, Waltham, MA, USA), using bovine serum albumin as a standard.
GGT enzyme activity was measured according to the method by Orlowski and Meister [35] as modified for the GGT Activity Colorimetric Assay kit (GenWay Biotech, San Diego, CA, USA) for 96-well plates. Briefly, 90 μL of γ-glutamyl-p-nitroanilide substrate was added to 10 μL of supernatant from placental tissue homogenates in a 96-well plate. The plate was then incubated at 37°C and absorbance was measured kinetically using a spectrofluorometer at 410 nm for appearance of p-nitroanilide (molar extinction =8800 M−1cm−1). GGT activity was calculated according to the manufacturer’s guidelines. One unit of GGT was defined as the amount of enzyme that generated 1.0 μmole of p-nitroanilide per minute at 37°C. Protein amount was quantified using the BCA assay (Thermo Scientific), with bovine serum albumin as a standard.
2.8. RNA extraction and sample preparation
RNA was extracted from the same placentas used for DNA base modification assays. Placentas were dissected from the uterus, weighed, and a 25-mg sample was homogenized using a FastPrep-24 tissue and cell lyser (MP Biomedicals, Solon, OH, USA). The RNA was extracted using an RNeasy kit (SABiosicence) according to the manufacturer’s protocol. RNA concentration and purity were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE, USA).
2.9. Analysis of placental mRNA expression of Tet2 and Tet3 genes
Placental expression of Tet2 and Tet3 genes was assessed using real time quantitative reverse transcription PCR (qRT-PCR). Aliquots of 1 μg of mRNA were used for cDNA synthesis using the RT2 First Strand kit (SABiosciences) following the manufacturer’s protocol. qRT-PCR was performed using 12.5 μL of RT2SYBR Green qPCR Master Mix, 1 μL of gene-specific primer target for Tet2 and Tet3 (SABiosciences, CA), 4 μL of cDNA template and 7.5 μL of nuclease-free H2O, for a total volume of 25 μL. Samples were analysed using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The qRT-PCR was run with an initial denaturation step of 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, and 5 s at 60°C. The ΔΔCt method [36] was used to quantify gene expression. To minimize error due to normalization [37], the target gene was normalized to the geometric mean of three non-differentially expressed housekeeping genes β2-microglobulin 5′ CGTGCTTGCCATTCAGAAAACT and 5′ GGTGGGTGGAACTGAGACAC 3′, β-actin 5′AAGCCGGCCTTGCACAT 3′ and 5′ CGCCACCAGTTCGCCA 3′, and T binding protein (Tbp) 5′GAATAAGAGAGCCACGAACAACTG 3′ and 5 ′ATTGTTCTTCACTCTTGGCTCCT 3′, using CFX manager software (Bio-Rad Laboratories), consistent with recommendations of the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines [38]. Tet2 and Tet3 mRNA analyses were conducted on placenta collected from all dams in the first exposure block (7 controls and 8 TCE-treated; 3 pooled placentas per litter). All samples were analyzed in triplicate by qRT-PCR (technical replicates).
2.10. Statistical analysis
For all analyses, the dam was used as the statistical unit. Analysis for differences in average fetal weight used mixed model ANOVA in SPSS software (SPSS Inc., Chicago, IL, USA), with treatment as the fixed effect and litter as the random effect. Other data were analysed by t-tests using Graphpad Prism 5.0 (LaJolla, CA, USA). All data were expressed as mean ± SEM.
3. Results
3.1. Reproductive effects of TCE
All exposed rats were pregnant on the day of euthanasia except for one rat in the control group that was discovered earlier to not be pregnant. TCE exposure during pregnancy decreased average fetal body weight by 10% (Fig. 1A; p<0.05) with no significant change in litter size (Fig 1B). The average maternal body weight of TCE-treated rats was not significantly different from controls (Fig 1C). In addition, maternal liver and kidney weights did not change with TCE exposure compared with controls (Supplemental Data, Fig. S1). No clinical signs of toxicity were observed over the course of the experiment.
Fig. 1.
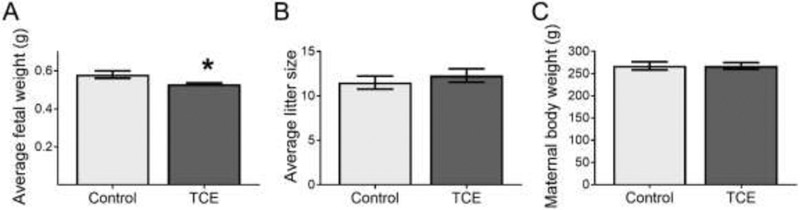
Indicators of fetal and maternal health on gd 16 of rats exposed to 0 (control) or 480 mg TCE/kg-day from gestational day 6-16. N=10 rats per group. The bars indicate means ± SEM. A) Average fetal weight per litter. Fetal weights were averaged per litter and a grand mean was calculated for statistical comparison. B) Litter size (number of fetuses per litter). C) Maternal body weight. *Statistically significantly different (p<0.05).
3.2. TCE effects on placental DNA base oxidation and methylation
Levels of 8-OHdG in the placentas of TCE-treated rats were significantly increased by 42.2% compared with controls (Fig. 2; p=0.02). Likewise, TCE exposure increased global DNA levels of 5-hmC in placentas of TCE-exposed rats by 53.9% compared with controls (Fig. 2; p=0.005). However, global placental 5-mC levels were not significantly modified by TCE exposure (Fig. 2; p=0.84, not significant) using the commercial immunoassay kit, a finding corroborated by the LUminometric methylation assay (data not shown).
Fig. 2.

Placental DNA modification by TCE: changes in 8-hydroxy-deoxyguanosine (8-OHdG), 5-hydroxymethylcytosine (5-hmC), and 5-methylcytosine (5-mC). Placentas were analyzed on gd 16 of control rats (N=7) and rats exposed to 480 mg TCE/kg-d (N=8) from gd 6-16. Samples from 3 placentas per litter were assayed. The data were statistically analyzed on a per litter basis and are shown as mean ± SEM. * The horizontal dashed line indicates control value = 1. Significantly increased compared with control (p=0.02). **Significantly increased compared with control (p=0.005).
3.3. TCE effects on placental Tet gene
Because 5-hmC can be formed by Tet enzyme conversion of 5-mC to 5-hmC as well as by oxidation of DNA bases, we also assessed mRNA expression of the Tet2 and Tet3 genes. A statistically significant increase in mRNA expression for Tet3 (p=0.02), but not Tet2, was observed (Fig. 3).
Fig. 3.
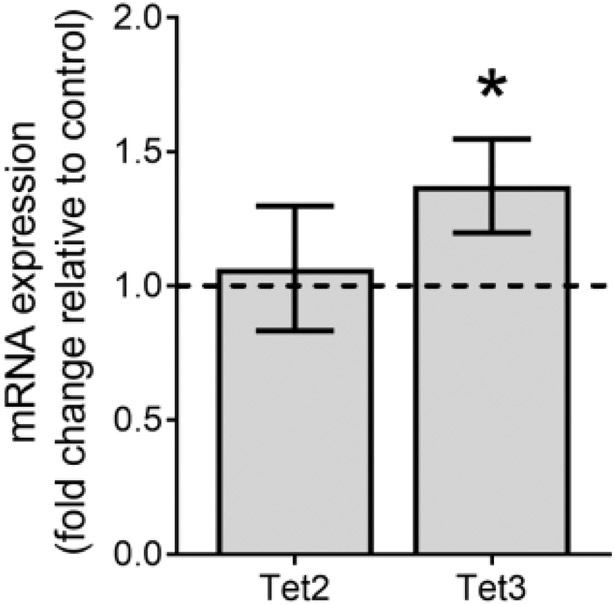
TCE-induced change of ten eleven translocation (Tet) mRNA expression levels in placenta. Placentas were analyzed on gd 16 of control rats (N=7) and rats exposed to 480 mg TCE/kg-d (N=8) from gd 6-16. Samples from 3 placentas were pooled within litter for analysis. The horizontal dashed line indicates control value = 1. *Significantly increased compared with control (p<0.05).
3.4. Assessment of placental GST and GGT
Exposure to TCE increased GST activity in rat placenta tissue 1.8-fold compared with controls (p<0.05, Fig 4A). Moreover, immunohistochemical staining indicated increased protein expression of GST-pi in TCE-treated rat placenta. In representative images showing the left basal and labyrinth zones on the fetal side of the placenta (Fig. 4), GST-pi antibody stained throughout the tissues in both the labyrinth and the basal zones of the placenta. However, the staining was visibly increased and darker within the labyrinth as well as the basal zones in placenta of TCE-treated rats (Fig. 4C) compared with control rats (Fig. 4B). In contrast to GST, activity for GGT was not significantly increased (Supplemental Data, Fig. S2).
Fig. 4.
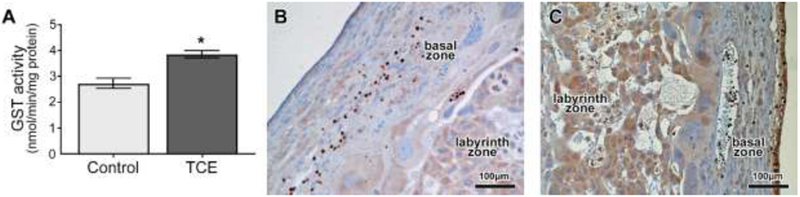
TCE effects on glutathione S-transferase (GST) in gd 16 placentas of control rats or rats exposed from gd 6-16 to 480 mg/kg TCE/kg-day. A) Activity of placental GST. Data are shown as means ± SEM. N=4 rats per group, with 3 placentas/litter. *Significantly increased compared with vehicle control (p=0.02). B and C) Immunohistochemical staining for GST-pi in representative images of left labyrinth and basal zones on the fetal side of placenta from control (B) and TCE-exposed (C) rats, showing increased intensity of staining throughout the labyrinth and basal zones of the placenta in the TCE-exposed placenta.
4. Discussion
Despite reductions of release into the environment over recent decades, TCE exposure remains significant due to exposures in the workplace and contamination of water, soil, and air [2]. In 2014, TCE was reclassified by the International Agency for Research on Cancer (IARC) and the National Toxicology Program (NTP) as a known human carcinogen [8-10]. Although less studied than cancer risk, TCE is also linked to adverse pregnancy outcomes in humans [39].
Our finding that TCE exposure during pregnancy decreased fetal weight in Wistar rats is consistent with previous epidemiology studies that associated TCE exposure with decreased birth weight. Specifically, a study of pregnant women exposed to TCE-contaminated drinking water at Camp LeJeune found increased odds for small for gestational age (SGA) and decreased birth weight associated with TCE exposure [25, 40]. Similarly, Forand et al. found that maternal exposure to TCE from home vapor intrusion increased risk for fetal growth restriction, low birth weight, and preterm birth in a New York State cohort [24]. Our results showing fetal growth restriction in rats exposed during pregnancy to TCE without overt toxicity to the dam, as indicated by lack of significant changes in maternal body and organ (liver and kidney) weights, support use of this animal model for studying placental TCE responses of potential relevance to fetal growth restriction. The reduction in average fetal weight we observed approximates the clinical definition of fetal growth restriction for human infants born below the 10th percentile for gestational age [41].
Our finding of decreased fetal weight following oral exposure of pregnant Wistar rats to 480 mg TCE/kgd on gd 6-16 differs from a prior study with Sprague-Dawley rats that found no significant reduction in fetal weight on gd 21 following exposure to 500 mg TCE/kg/d delivered by gavage from gd 6-15 [42]. It is possible that differences between the strain of rat, gestational age of fetal assessment, and form of oral exposure (TCE on a wafer treat in our study versus gavage of TCE diluted in soybean oil) contributed to the different results for the effect of TCE on fetal weight. Although a prior study by Healy and colleagues reported that inhalation exposure of Wistar rats to 100 ppm TCE from gd 8-21 for 4 hours per day significantly reduced fetal weight at term by approximately 9% [43], differences in route of exposure, dose, and gestational age of fetal assessment limit further comparisons with our results.
In the rat placenta, TCE increased levels of 8-OHdG, consistent with prior studies in animals and humans. For example, levels of 8-OHdG were significantly higher in urine of TCE-exposed workers compared with controls [44, 45]. In a subchronic mouse study, exposure to 800 or 1000 mg TCE/kg/d by gavage increased liver thiobarbituric acid-reactive substances during the first 2 weeks only, and at the highest concentration of 1000 mg TCE/kg/d increased liver 8-OHdG concentrations at multiple time points throughout the 8-week study [13]. Toraason and coworkers showed that TCE exposure elevated levels of 8-OHdG and thiobarbituric acid-reactive substances in rat liver 24 h after a single i.p. injection of 500 mg/kg (lowest effective dose) [12]. Similarly, a single oral TCE dose of 2000 mg/kg increased thiobarbituric acid-reactive substances in rat liver, peaking 6 h after dose administration [46, 47]. Despite these reports that exposure to TCE increases 8-OHdG and other markers of oxidative stress, to our knowledge, no prior studies have explored TCE-induced markers of oxidative stress in placenta in vivo.
Glutathione S-transferases (GSTs) are a family of enzymes that catalyze conjugation of a variety of substrates with GSH, and increased GST expression can be part of the cellular antioxidant response to ROS [48]. As such, our observations that TCE exposure increased GST activity and GST-pi protein in the rat placenta are consistent with increased placental ROS, as well as with a prior report that TCE increased GST activity in the liver and lung of rats [49]. Our detection of the GST-pi protein in rat placenta is consistent with previous studies that found GST-pi in human placenta, as well as in kidney and the digestive tract [50]. Furthermore, our finding of increased expression of GST-pi protein in placenta of TCE-treated rats is consistent with a report that occupational exposure to TCE was associated with increased levels of GST-pi in the urine [51], although Bruning and colleagues found a positive association between exposure to TCE and urinary concentrations of GST-alpha but not GST-pi in a retrospective study [52]. In rat kidney cells, GST-alpha is the primary isoform responsible for GST conjugation of TCE [53]: however, GST-alpha is not abundantly expressed in the placenta [33].
In addition to having a role in antioxidant response, GST is important for initiating glutathione-dependent metabolism of TCE [7]. Studies have linked the TCE glutathione pathway metabolite DCVC to oxidative stress as a mechanism of toxicity in kidney cells [18-21] and a human placental cell line [22]. Although there is a paucity of research regarding TCE metabolism by the glutathione conjugation pathway in placenta, studies have reported the ability of human placental GST to metabolize other toxicants [54, 55]. After conjugation by GST, subsequent biotransformation by GGT is necessary to produce DCVC. In contrast to prior reports that TCE increases GGT activity in kidneys of mice, rats, and humans [56], we failed to detect statistically significantly increased GGT activity in placenta of TCE-exposed rats. One possibility is that variability and small sample size (N=4) contributed to the lack of statistical significance for GGT in our experiment. Regardless, we detected placental GGT activity and its presence, along with TCE-stimulated placental GST activity, implies that the placenta has the capability to bioactivate TCE via the glutathione pathway. Given that DCVC induces oxidative stress in human placental cells [22], we suggest that this TCE metabolite may play a key role in stimulating oxidative stress in placenta. However, further studies are warranted to validate a link between TCE metabolism by the glutathione activation pathway and placental toxicity.
Our findings of increased 5-hmC and Tet3 gene expression along with indicators of oxidative stress in placenta of TCE-exposed rats are consistent with the hypothesis proposed by Chia and co-workers that TET enzyme activation could be due to oxidative stress [57]. Rakoczy and co-workers previously reported modest but significant increases in Tet mRNA expression in mouse placenta [58]. However, unlike Rakoczy et al. who found increased Tet1, Tet2, and Tet3, we observed a significant increase in Tet3 only. Because Tet3 is involved in DNA demethylation in mouse zygotes [59, 60] and the placenta includes tissue of embryonic origin, it is interesting that TCE exposure induced placental Tet3. In contrast, Tet2, which is linked most strongly to cancer [61], was unchanged in placenta of our TCE-exposed rats. Although TCE exposure decreases DNA methylation in mouse liver [62], mouse cerebellum [63], and human hepatic L-02 cells [64], our study found no significant changes in DNA methylation (5-mC) in placentas of TCE-exposed rats. However, Jiang et al. found no TCE effect on global DNA methylation in mouse liver, similar to our result in rat placenta, but they did find DNA hypomethylation and hypermethylation of specific genes [65]. Future experiments could explore possible linkages between TCE exposure, ROS, 5-hmC, and gene expression, including validation of the 5-hmC results, to identify specific hydroxy-methylation changes of DNA in placental DNA. However, such experiments are beyond the scope of the current study.
Studies with autoimmune-prone MRL +/+ mice reported increased oxidative stress using TCE exposure at lower doses but longer exposure periods than in our study. Blossom et al. found decreased levels of GSH in cerebellum of MRL +/+ mice exposed to 0.1 mg/ml TCE in drinking water (~31 mg/kg/day) from gd 1 until postnatal day 42 [14]. A study by Wang et al. reported increased levels of oxidative stress lipid peroxidation markers including malondialdehyde (MDA) and 4-hydroxynonenal (HNE) in MRL +/+ mice exposed i.p. once per week for 6 or 12 weeks with 10 mmol TCE /kg [66]. In another study, Wang et al. demonstrated significantly increased serum levels of MDA and HNE in female MRL +/+ mice exposed to 0.5 mg/ml TCE in drinking water for 48 weeks [67].
Oxidative stress in gestational tissues has been associated with several pathologies of human pregnancy, including preterm labor, preeclampsia, and fetal growth restriction [68, 69]. Furthermore, increased levels of the urinary oxidative stress markers 8-isoprostane and 8-OHdG in early or mid-gestation were associated with increased risk for preeclampsia and decreased gestational length, respectively, in a mostly Hispanic and African-American population of New Jersey [70]. Moreover, in a predominantly White Boston cohort with repeated measures of 8-isoprostane and 8-OHdG across gestation, average levels of urinary 8-isoprostane were associated with increased preterm birth, although increased urinary 8-OHdG was associated with decreased preterm birth [71]. Of particular interest, further statistical analysis of the latter Boston cohort found evidence for oxidative stress, as indicated by urinary 8-isoprostane, mediating associations between maternal phthalate exposure and preterm birth [72]. Because oxidative stress and inflammation are closely inter-related, we measured the pro-inflammatory cytokine IL-6 in maternal blood: there was considerable variability among the measures and differences between TCE-exposed and control rats were not statistically significantly different (Supplement Figure S3). There was lack of neutrophil infiltration in both the control and TCE-treated rat placental tissues (data not shown).
Although both rats and humans form hemochorial placentas in pregnancy, there are significant species differences. Whereas the yolk sac in women disappears by the end of the first trimester, rats develop an inverted yolk sac placenta early in pregnancy that provides critical nutrient transport until it ruptures and disappears closer to term around gd 17-18 [73]. With implantation in the rat occurring around gd 5, our TCE exposure period of gd 6-16 includes placentation from the early yolk sac placenta through establishment of the chorioallantoic placenta that begins around gd 11-12 [74]. Our observed effects of TCE on fetal growth and placental responses may have been due to directs effects on the fetus and placenta or indirect results of impacts on the yolk sac. Nonetheless, our exposure regimen provided sub-chronic TCE exposure over the period of critical placental development.
Inhalation predominates as the TCE exposure route in the workplace and can be an exposure route for the general population due to vapor intrusion [75]. TCE exposure via drinking water is an additional concern for the general public due to contamination from prior waste disposal practices [3]. TCE is among the most frequently detected contaminants of US public water supplies and US EPA National Priority List (commonly referred to as Superfund) sites [3], and is readily absorbed from the gastrointestinal tract of mice [76] and rats [77]. The US EPA Maximum Contaminant Level (MCL) for drinking water is 5 μg TCE/L [78], although exceedance of the MCL has been reported [3]. The TCE dose used in the present study is within an order of magnitude higher than would occur with occupational exposure at the US OSHA 100 ppm TCE permissible exposure limit (PEL) over an 8-h workday, but is several orders of magnitude higher than would be expected with environmental exposures from oral ingestion.
By administering TCE orally to rats on a wafer, our study has the advantage of using ingestion as a route of exposure relevant to humans. Although exposure via wafer may have introduced dose variability due to TCE evaporation, we minimized time for volatilization by presenting the wafer without TCE in a three-day training period, which allowed the rats to recognize the wafer as a treat and eat the wafer more quickly. Moreover, the low variability in maternal weights, fetal body weights, and maternal liver and kidney weights suggests that TCE evaporation from the wafer had minimal contribution to internal dose variation. Likewise, the low variability suggests that the statistically significant mean differences observed between TCE-exposed and control rats, though modest for some parameters, are meaningful indices of effect. Although measurement of TCE or its metabolites in serum and/or placenta was beyond the scope of the present investigation, collection of such data would have provided internal exposure verification and facilitated possible future comparisons with human studies. Furthermore, inclusion of fetal sex in future analyses of TCE effects on placenta is needed because of known sex-related differences in rat placental development [79]. It is possible that combining sexes contributed to the modest magnitude of some of the effects we observed. Finally, although our study provides a lowest observable effect level for TCE toxicity in the rat placenta and a potential underlying mechanism for adverse birth outcomes, future studies are needed to characterize the full dose-response relationship for TCE toxicity in placenta of pregnant rats including doses relevant to human environmental exposures.
5. Conclusions
In summary, TCE decreased fetal weight and increased biomarkers of oxidative stress that have been associated with adverse pregnancy outcomes in humans, including fetal growth restriction. Although oxidative stress is implicated as a mechanism involved in TCE-induced kidney and liver intoxication, to our knowledge, this is the first study that explores TCE-induced oxidative stress in the placenta in vivo. Results of the current study suggest that oxidative stress in the placenta is a possible mechanism relevant for epidemiological associations of TCE exposure and adverse birth outcomes [24, 39, 80]. Additionally, findings of increased levels of Tet3 enzyme and 5-hmC suggest that exposure to TCE could modify DNA in placenta through an oxidative stress mechanism. However, because it is likely that oxidative stress is not unique to the placenta given prior study observations in non-gestational tissues, other factors may contribute to fetal growth deficits and further studies are needed to validate this and other possible mechanisms by which TCE exposure may lead to fetal growth restriction in humans. Nonetheless, our findings contribute to the weight of evidence for TCE-induced adverse pregnancy outcomes, providing new insight into TCE effects on the placenta.
Highlights.
Trichloroethylene exposure during pregnancy decreased fetal weight in rats
Trichloroethylene exposure increased markers of oxidative stress in rat placenta
Trichloroethylene exposure increased rat placental mRNA expression of Tet3
Acknowledgements
The authors acknowledge the University of Michigan’s Immunology Core for its assistance with cytokine ELISA analysis and University of Michigan DNA Sequencing Core for assistance with the RNA microarray analysis. We thank Anthony Su for helpful discussions, Kevin Sun for technical assistance with the rat experiments, and Drs. Kirsten Herold and Ingrid Bergin for their comments and assistance with editing this manuscript. We also thank Drs. Lauren M. Tetz, Kelly A. Hogan, and Erica J. Boldenow, for demonstrating techniques and for helpful discussions. This work was supported by the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH) to RL-C, (P42ES017198), with fellowship support to IH from the NIEHS, NIH (T32ES007062; F31ES021238) and to SMH from the NIEHS, NIH (T32ES007062). Additional support was provided by the Integrated Health Sciences Core of the Michigan Center for Lifestage Environmental Exposure and Disease through a center grant from the NIEHS, NIH (P30ES017885).
Abbreviations
- BCA
bicinchoninic acid
- CYP
cytochrome
- DCVC
S-(1,2-dichlorovinyl)-L-cysteine
- ELISA
enzyme-linked immunosorbant assay
- gd
gestational day
- GGT
γ-glutamyltransferase
- GSH
glutathione
- GST
glutathione S-transferase
- 5-hmC
5-hydroxymethylcytosine
- 8-OHdG
8-hydroxy-deoxyguanosine
- IL
interleukin
- 5-mC
5-methylcytosine
- PBS
phosphate-buffered saline
- qRT-PCR
real time quantitative reverse transcription PCR
- TCE
trichloroethylene
- TET
ten-eleven translocation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- [1].Glauser J, Funda C, CEH marketing research report; C2 chlorinated solvents., SRI International, Menlo Park, CA: 2012. [Google Scholar]
- [2].US EPA, Toxicological Review of Trichloroethylene (CAS No. 79-01-6), National Center for Environmental Assessment, Washington, DC, 2011. [Google Scholar]
- [3].ATSDR (Agency for Toxic Substances and Disease Registry), Toxicological profile for Trichloroethylene (TCE) (Draft for Public Comment), U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, 2014. [Google Scholar]
- [4].NTP, Toxicology and Carcinogenesis Studies of Trichloroethylene (CAS No. 79-01-6) in Four Strains of Rats (Aci, August, Marshall, Osborne-Mendel) (Gavage Studies), Natl Toxicol Program Tech Rep Ser 273 (1988) 1–299. [PubMed] [Google Scholar]
- [5].Forkert PG, Millen B, Lash LH, Putt DA, Ghanayem BI, Pulmonary bronchiolar cytotoxicity and formation of dichloroacetyl lysine protein adducts in mice treated with trichloroethylene, J Pharmacol Exp Ther 316(2) (2006) 520–9. [DOI] [PubMed] [Google Scholar]
- [6].Rhomberg LR, Dose-response analyses of the carcinogenic effects of trichloroethylene in experimental animals, Environmental health perspectives 108 Suppl 2 (2000) 343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lash LH, Chiu WA, Guyton KZ, Rusyn I, Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target organ toxicity, Mutation research. Reviews in mutation research 762 (2014) 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guha N, Loomis D, Grosse Y, Lauby-Secretan B, Ghissassi FE, Bouvard V, Benbrahim-Tallaa L, Baan R, Mattock H, Straif K, Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites, The lancet oncology 13(12) (2012) 1192–1193. [DOI] [PubMed] [Google Scholar]
- [9].IARC, Carcinogenicity of trichloroethylene tetrachloroethylene, and other chlorinated agents, International Agency for Research on Cancer, Lyon, France, 2014. [Google Scholar]
- [10].NTP, Report on Carcinogens, Fourteenth Edition, U.S. Department of Health and Human Services, Public Health Service, Research Triangle Park, NC, 2016. [Google Scholar]
- [11].Siddiqi A, Nafees S, Rashid S, Sultana S, Saidullah B, Hesperidin ameliorates trichloroethylene-induced nephrotoxicity by abrogation of oxidative stress and apoptosis in wistar rats, Mol Cell Biochem 406(1-2) (2015) 9–20. [DOI] [PubMed] [Google Scholar]
- [12].Toraason M, Clark J, Dankovic D, Mathias P, Skaggs S, Walker C, Werren D, Oxidative stress and DNA damage in Fischer rats following acute exposure to trichloroethylene or perchloroethylene, Toxicology 138(1) (1999) 43–53. [DOI] [PubMed] [Google Scholar]
- [13].Channel SR, Latendresse JR, Kidney JK, Grabau JH, Lane JW, Steel-Goodwin L, Gothaus MC, A subchronic exposure to trichloroethylene causes lipid peroxidation and hepatocellular proliferation in male B6C3F1 mouse liver, Toxicological sciences : an official journal of the Society of Toxicology 43(2) (1998) 145–54. [DOI] [PubMed] [Google Scholar]
- [14].Blossom SJ, Doss JC, Hennings LJ, Jernigan S, Melnyk S, James SJ, Developmental exposure to trichloroethylene promotes CD4+ T cell differentiation and hyperactivity in association with oxidative stress and neurobehavioral deficits in MRL+/+ mice, Toxicol Appl Pharmacol 231(3) (2008) 344–53. [DOI] [PubMed] [Google Scholar]
- [15].Blossom SJ, Melnyk S, Cooney CA, Gilbert KM, James SJ, Postnatal exposure to trichloroethylene alters glutathione redox homeostasis, methylation potential, and neurotrophin expression in the mouse hippocampus, Neurotoxicology 33(6) (2012) 1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Blossom SJ, Gilbert KM, Exposure to a metabolite of the environmental toxicant, trichloroethylene, attenuates CD4+ T cell activation-induced cell death by metalloproteinase-dependent FasL shedding, Toxicol Sci 92(1) (2006) 103–14. [DOI] [PubMed] [Google Scholar]
- [17].Blossom SJ, Pumford NR, Gilbert KM, Activation and attenuation of apoptosis of CD4+ T cells following in vivo exposure to two common environmental toxicants, trichloroacetaldehyde hydrate and trichloroacetic acid, J Autoimmun 23(3) (2004) 211–20. [DOI] [PubMed] [Google Scholar]
- [18].van de Water B, Zoetewey JP, de Bont HJ, Mulder GJ, Nagelkerke JF, The relationship between intracellular Ca2+ and the mitochondrial membrane potential in isolated proximal tubular cells from rat kidney exposed to the nephrotoxin 1,2-dichlorovinyl-cysteine, Biochem Pharmacol 45(11) (1993) 2259–67. [DOI] [PubMed] [Google Scholar]
- [19].van de Water B, Zoeteweij JP, de Bont HJ, Mulder GJ, Nagelkerke JF, Role of mitochondrial Ca2+ in the oxidative stress-induced dissipation of the mitochondrial membrane potential. Studies in isolated proximal tubular cells using the nephrotoxin 1,2-dichlorovinyl-L-cysteine, The Journal of biological chemistry 269(20) (1994) 14546–52. [PubMed] [Google Scholar]
- [20].Xu F, Papanayotou I, Putt DA, Wang J, Lash LH, Role of mitochondrial dysfunction in cellular responses to S-(1,2-dichlorovinyl)-L-cysteine in primary cultures of human proximal tubular cells, Biochem Pharmacol 76(4) (2008) 552–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen Q, Jones TW, Brown PC, Stevens JL, The mechanism of cysteine conjugate cytotoxicity in renal epithelial cells. Covalent binding leads to thiol depletion and lipid peroxidation, J Biol Chem 265(35) (1990) 21603–11. [PubMed] [Google Scholar]
- [22].Hassan I, Kumar AM, Park HR, Lash LH, Loch-Caruso R, Reactive oxygen stimulation of interleukin-6 release in the human trophoblast cell Line HTR-8/SVneo by the trichlorethylene metabolite s-(1,2-dichlorovinyl)-l-cysteine, Biol Reprod 95(3) (2016) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Elkin ER, Harris SM, Loch-Caruso R, Trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine induces lipid peroxidation-associated apoptosis via the intrinsic and extrinsic apoptosis pathways in a first-trimester placental cell line, Toxicol Appl Pharmacol 338 (2018) 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Forand SP, Lewis-Michl EL, Gomez MI, Adverse birth outcomes and maternal exposure to trichloroethylene and tetrachloroethylene through soil vapor intrusion in New York State, Environmental health perspectives 120(4) (2012) 616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ruckart PZ, Bove FJ, Maslia M, Evaluation of contaminated drinking water and preterm birth, small for gestational age, and birth weight at Marine Corps Base Camp Lejeune, North Carolina: a cross-sectional study, Environ Health 13 (2014) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beppu K, Transmission of the anesthetic agents through the placenta in painless delivery and their effects on newborn infants, The Keio journal of medicine 17(2) (1968) 81–107. [DOI] [PubMed] [Google Scholar]
- [27].Laham S, Studies on placental transfer. Trichlorethylene, IMS, Industrial medicine and surgery 39(1) (1970) 46–9. [PubMed] [Google Scholar]
- [28].Koukoura O, Sifakis S, Spandidos DA, DNA methylation in the human placenta and fetal growth (review), Molecular medicine reports 5(4) (2012) 883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boldenow E, Hassan I, Chames MC, Xi C, Loch-Caruso R, The trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine but not trichloroacetate inhibits pathogen-stimulated TNF-alpha in human extraplacental membranes in vitro, Reprod Toxicol 52 (2015) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G, Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Am J Obstet Gynecol 215(1 Suppl) (2016) S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seegal RF, Brosch KO, Okoniewski RJ, Effects of in utero and lactational exposure of the laboratory rat to 2,4,2',4'- and 3,4,3',4'-tetrachlorobiphenyl on dopamine function, Toxicol Appl Pharmacol 146(1) (1997) 95–103. [DOI] [PubMed] [Google Scholar]
- [32].Sant KE, Nahar MS, Dolinoy DC, DNA methylation screening and analysis, Methods in molecular biology (Clifton, N.J.) 889 (2012) 385–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Raijmakers MT, Steegers EA, Peters WH, Glutathione S-transferases and thiol concentrations in embryonic and early fetal tissues, Hum Reprod 16(11) (2001) 2445–50. [DOI] [PubMed] [Google Scholar]
- [34].Habig WH, Pabst MJ, Jakoby WB, Glutathione S-transferases. The first enzymatic step in mercapturic acid formation, J Biol Chem 249(22) (1974) 7130–9. [PubMed] [Google Scholar]
- [35].Orlowski M, Meister A, Gamma-glutamyl-p-nitroanilide: A new convenient substrate for determination and study of L- and D-gamma-glutamyl transpeptidase activities, Biochim Biophys Acta 73 (1963) 679–81. [DOI] [PubMed] [Google Scholar]
- [36].Yuan JS, Reed A, Chen F, Stewart CN Jr., Statistical analysis of real-time PCR data, BMC Bioinformatics 7 (2006) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes, Genome Biol 3(7) (2002) RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT, The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments, Clin Chem 55(4) (2009) 611–22. [DOI] [PubMed] [Google Scholar]
- [39].Chiu WA, Jinot J, Scott CS, Makris SL, Cooper GS, Dzubow RC, Bale AS, Evans MV, Guyton KZ, Keshava N, Lipscomb JC, Barone S, Fox JF, Gwinn MR, Schaum J, Caldwell JC, Human health effects of trichloroethylene: key findings and scientific issues, Environmental health perspectives 121(3) (2013) 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bove F, Shim Y, Zeitz P, Drinking water contaminants and adverse pregnancy outcomes: a review, Environmental health perspectives 110 Suppl 1 (2002) 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Divon MY, Fetal growth restriction: Diagnosis, 2018. http://www.uptodate.com. (Accessed Aug 8 2018).
- [42].Fisher JW, Channel SR, Eggers JS, Johnson PD, MacMahon KL, Goodyear CD, Sudberry GL, Warren DA, Latendresse JR, Graeter LJ, Trichloroethylene, trichloroacetic acid, and dichloroacetic acid: do they affect fetal rat heart development?, Int J Toxicol 20(5) (2001) 257–67. [DOI] [PubMed] [Google Scholar]
- [43].Healy TE, Poole TR, Hopper A, Rat fetal development and maternal exposure to trichloroethylene 100 p.p.m, Br J Anaesth 54(3) (1982) 337–41. [DOI] [PubMed] [Google Scholar]
- [44].Abusoglu S, Celik HT, Tutkun E, Yilmaz H, Serdar MA, Bal CD, Yildirimkaya M, Avcikucuk M, 8-hydroxydeoxyguanosine as a useful marker for determining the severity of trichloroethylene exposure, Archives of environmental & occupational health 69(3) (2014) 180–6. [DOI] [PubMed] [Google Scholar]
- [45].Hu C, Jiang L, Geng C, Zhang X, Cao J, Zhong L, Possible involvement of oxidative stress in trichloroethylene-induced genotoxicity in human HepG2 cells, Mutat Res 652(1) (2008) 88–94. [DOI] [PubMed] [Google Scholar]
- [46].Cojocel C, Beuter W, Muller W, Mayer D, Lipid peroxidation: a possible mechanism of trichloroethylene-induced nephrotoxicity, Toxicology 55(1-2) (1989) 131–41. [DOI] [PubMed] [Google Scholar]
- [47].Ogino K, Hobara T, Kobayashi H, Ishiyama H, Gotoh M, Imamura A, Egami N, Lipid peroxidation induced by trichloroethylene in rat liver, Bull Environ Contam Toxicol 46(3) (1991) 417–21. [DOI] [PubMed] [Google Scholar]
- [48].Hayes JD, McLellan LI, Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress, Free Radic Res 31(4) (1999) 273–300. [DOI] [PubMed] [Google Scholar]
- [49].Kumar P, Prasad AK, Mani U, Maji BK, Dutta KK, Effect of trichloroethylene (TCE) inhalation on biotransformation enzymes of rat lung and liver, Journal of environmental biology / Academy of Environmental Biology, India 23(1) (2002) 1–6. [PubMed] [Google Scholar]
- [50].Terrier P, Townsend AJ, Coindre JM, Triche TJ, Cowan KH, An immunohistochemical study of pi class glutathione S-transferase expression in normal human tissue, Am J Pathol 137(4) (1990) 845–53. [PMC free article] [PubMed] [Google Scholar]
- [51].Vermeulen R, Zhang L, Spierenburg A, Tang X, Bonventre JV, Reiss B, Shen M, Smith MT, Qiu C, Ge Y, Ji Z, Xiong J, He J, Hao Z, Liu S, Xie Y, Yue F, Guo W, Purdue M, Beane Freeman LE, Sabbisetti V, Li L, Huang H, Rothman N, Lan Q, Elevated urinary levels of kidney injury molecule-1 among Chinese factory workers exposed to trichloroethylene, Carcinogenesis 33(8) (2012) 1538–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bruning T, Sundberg AG, Birner G, Lammert M, Bolt HM, Appelkvist EL, Nilsson R, Dallner G, Glutathione transferase alpha as a marker for tubular damage after trichloroethylene exposure, Archives of toxicology 73(4-5) (1999) 246–54. [DOI] [PubMed] [Google Scholar]
- [53].Cummings BS, Parker JC, Lash LH, Role of cytochrome P450 and glutathione S-transferase alpha in the metabolism and cytotoxicity of trichloroethylene in rat kidney, Biochemical pharmacology 59(5) (2000) 531–43. [DOI] [PubMed] [Google Scholar]
- [54].Obolenskaya MY, Teplyuk NM, Divi RL, Poirier MC, Filimonova NB, Zadrozna M, Pasanen MJ, Human placental glutathione S-transferase activity and polycyclic aromatic hydrocarbon DNA adducts as biomarkers for environmental oxidative stress in placentas from pregnant women living in radioactivity- and chemically-polluted regions, Toxicol Lett 196(2) (2010) 80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pacifici GM, Rane A, Glutathione S-epoxidetransferase in the human placenta at different stages of pregnancy, Drug metabolism and disposition: the biological fate of chemicals 9(5) (1981) 472–5. [PubMed] [Google Scholar]
- [56].Lash LH, Qian W, Putt DA, Jacobs K, Elfarra AA, Krause RJ, Parker JC, Glutathione conjugation of trichloroethylene in rats and mice: sex-, species-, and tissue-dependent differences, Drug metabolism and disposition: the biological fate of chemicals 26(1) (1998) 12–9. [PubMed] [Google Scholar]
- [57].Chia N, Wang L, Lu X, Senut MC, Brenner C, Ruden DM, Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress, Epigenetics 6(7) (2011) 853–6. [DOI] [PubMed] [Google Scholar]
- [58].Rakoczy J, Padmanabhan N, Krzak AM, Kieckbusch J, Cindrova-Davies T, Watson ED, Dynamic expression of TET1, TET2, and TET3 dioxygenases in mouse and human placentas throughout gestation, Placenta 59 (2017) 46–56. [DOI] [PubMed] [Google Scholar]
- [59].Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabo PE, Pfeifer GP, Li J, Xu GL, The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes, Nature 477(7366) (2011) 606–10. [DOI] [PubMed] [Google Scholar]
- [60].Shen L, Inoue A, He J, Liu Y, Lu F, Zhang Y, Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes, Cell stem cell 15(4) (2014) 459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huang Y, Rao A, Connections between TET proteins and aberrant DNA modification in cancer, Trends in genetics : TIG 30(10) (2014) 464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tao L, Kramer PM, Ge R, Pereira MA, Effect of dichloroacetic acid and trichloroacetic acid on DNA methylation in liver and tumors of female B6C3F1 mice, Toxicological sciences : an official journal of the Society of Toxicology 43(2) (1998) 139–44. [DOI] [PubMed] [Google Scholar]
- [63].Blossom SJ, Cooney CA, Melnyk SB, Rau JL, Swearingen CJ, Wessinger WD, Metabolic changes and DNA hypomethylation in cerebellum are associated with behavioral alterations in mice exposed to trichloroethylene postnatally, Toxicol Appl Pharmacol 269(3) (2013) 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang H, Hong WX, Ye J, Yang X, Ren X, Huang A, Yang L, Zhou L, Huang H, Wu D, Huang X, Zhuang Z, Liu J, Analysis of trichloroethylene-induced global DNA hypomethylation in hepatic L-02 cells by liquid chromatography-electrospray ionization tandem mass spectrometry, Biochem Biophys Res Commun 446(2) (2014) 590–5. [DOI] [PubMed] [Google Scholar]
- [65].Jiang Y, Chen J, Tong J, Chen T, Trichloroethylene-induced gene expression and DNA methylation changes in B6C3F1 mouse liver, PLoS One 9(12) (2014) e116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang G, Konig R, Ansari GA, Khan MF, Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells, Free Radic Biol Med 44(7) (2008) 1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang G, Cai P, Ansari GA, Khan MF, Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response, Toxicology 229(3) (2007) 186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I, Role of oxidative stress in intrauterine growth restriction, Gynecol Obstet Invest 64(4) (2007) 187–92. [DOI] [PubMed] [Google Scholar]
- [69].Al-Gubory KH, Fowler PA, Garrel C, The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes, Int J Biochem Cell Biol 42(10) (2010) 1634–50. [DOI] [PubMed] [Google Scholar]
- [70].Peter Stein T, Scholl TO, Schluter MD, Leskiw MJ, Chen X, Spur BW, Rodriguez A, Oxidative stress early in pregnancy and pregnancy outcome, Free Radic Res 42(10) (2008) 841–8. [DOI] [PubMed] [Google Scholar]
- [71].Ferguson KK, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B, Meeker JD, Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth, Am J Obstet Gynecol 212(2) (2015) 208.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ferguson KK, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B, Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy, Environ Health Perspect (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wooding P, Burton G, Comparative Placentation: Structures, Functions and Evolution, Springer-Verlag Berlin, Heidelberg: 2008. [Google Scholar]
- [74].Fonseca BM, Correia-da-Silva G, Teixeira NA, The rat as an animal model for fetoplacental development: a reappraisal of the post-implantation period, Reprod Biol 12(2) (2012) 97–118. [DOI] [PubMed] [Google Scholar]
- [75].Wu C, Schaum J, Exposure assessment of trichloroethylene, Environ Health Perspect 108 Suppl 2 (2000) 359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Withey JR, Collins BT, Collins PG, Effect of vehicle on the pharmacokinetics and uptake of four halogenated hydrocarbons from the gastrointestinal tract of the rat, J Appl Toxicol 3(5) (1983) 249–53. [DOI] [PubMed] [Google Scholar]
- [77].Kim S, Kim D, Pollack GM, Collins LB, Rusyn I, Pharmacokinetic analysis of trichloroethylene metabolism in male B6C3F1 mice: Formation and disposition of trichloroacetic acid, dichloroacetic acid, S-(1,2-dichlorovinyl)glutathione and S-(1,2-dichlorovinyl)-L-cysteine, Toxicol Appl Pharmacol 238(1) (2009) 90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].US EPA, Exposure Factors Handbook 2011 Edition (Final Report), U.S. Environmental Protection Agency, Washington, DC, 2011. [Google Scholar]
- [79].Kalisch-Smith JI, Simmons DG, Pantaleon M, Moritz KM, Sex differences in rat placental development: from pre-implantation to late gestation, Biology of Sex Differences 8(1) (2017) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bove F, Shim Y, Zeitz P, Drinking water contaminants and adverse pregnancy outcomes: a review, Environ Health Perspect 110 Suppl 1 (2002) 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


