Abstract
Type V, or “autotransporter,” secretion is a term used to refer to several simple protein export pathways that are found in a wide range of Gram-negative bacteria. Autotransporters are generally single polypeptides that consist of an extracellular (“passenger”) domain and a β barrel domain that anchors the protein to the outer membrane (OM). Although it was originally proposed that the passenger domain is secreted through a channel formed solely by the covalently linked β barrel domain, experiments performed primarily on the type Va, or “classical,” autotransporter pathway have challenged this hypothesis. Several lines of evidence strongly suggest that both the secretion of the passenger domain and the membrane integration of the β barrel domain are catalyzed by the barrel assembly machinery (Bam) complex, a conserved hetero-oligomer that plays an essential role in the assembly of most integral OM proteins. The secretion reaction appears to be driven at least in part by the folding of the passenger domain in the extracellular space. Although many aspects of autotransporter biogenesis remain to be elucidated, it will be especially interesting to determine whether the different classes of proteins that fall under the type V rubric—most of which have not been examined in detail—are assembled by the same basic mechanism as classical autotransporters.
INTRODUCTION
Type V, or “autotransporter,” secretion is an umbrella term that is often used to refer to a group of distinct but conceptually related protein export pathways that are widely distributed in Gram-negative bacteria. Autotransporters are generally single polypeptides that contain a signal peptide that promotes translocation across the inner membrane (IM) via the Sec pathway, an extracellular (“passenger”) domain, and a domain that anchors the protein to the outer membrane (OM). Passenger domains have a wide variety of functions, but they often promote virulence (1). In the archetypical, or “classical” (type Va), autotransporter pathway, which was discovered in 1987, the passenger domain is located at the N terminus of the protein adjacent to the signal peptide (2). Although passenger domains range in size from ∼20 to 300 kDa and are highly diverse in sequence (3), X-ray crystallographic and in silico studies predict that they usually fold into a repetitive structure known as a β helix (4–8) (Fig. 1). The membrane anchor domains are ∼30 kDa and are also highly diverse in sequence but contain short conserved sequence motifs (3, 9). Like most membrane-spanning segments associated with OM proteins (OMPs), these domains fold into a closed, amphipathic β sheet or “β barrel” structure. The C-terminal domains that have been crystallized to date all form nearly superimposable 12-stranded β barrels (10–15). The two domains are connected by a short α-helical “linker” that is embedded inside the β barrel domain (10, 12, 13, 16). Many passenger domains are released from the cell surface by a proteolytic cleavage following their secretion (17).
Figure 1.
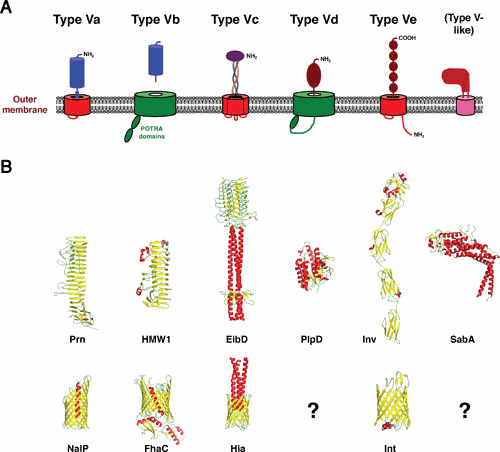
Illustration of type V secretion pathways. (A) Proteins in type V (and type V-like) secretion pathways consist of a 12-stranded (red), 16-stranded (green), or predicted 8-stranded (pink) β barrel domain and an extracellular (“passenger”) domain that typically folds into a β-helical (blue), mixed coiled-coil/β roll/β prism (purple) or globular (brown) structure. The 16-stranded β barrel domains are members of the Omp85 superfamily and contain periplasmic POTRA domains. In most cases the β barrel and passenger domains are covalently linked, but in the type Vb pathway the β barrel domain and the extracellular component (“exoprotein”) are separate polypeptides. In the type Vc pathway both domains are formed through the assembly of three identical subunits. The passenger domain is located at the N terminus of the protein in the type Va, Vb, Vc, and Vd pathways, but it is found at the C terminus in the type Ve pathway. In the type V-like pathway the extracellular domain is located in a loop that connects the first two β strands of the β barrel domain. (B) Crystal structures of representative polypeptides from each pathway are shown. α-helical segments are colored red and β strands are colored yellow. The structures include the pertactin (Prn) passenger domain (4) (PDB code 1DAB), a fragment of the HMW1 exoprotein (98) (PDB code 2ODL), a fragment of the EibD passenger domain (24) (PDB code 2XQH), the phospholipase D (PlpD) passenger domain (34) (PDB code 5FYA), the invasin (Inv) passenger domain (28) (1CWV), the SabA extracellular domain (36) (PDB code 4O5J), and the NalP, FhaC, Hia, and intimin (Int) β barrel domains (PDB codes 1UYO, 4QKY, 2GR7, and 4E1S) (10, 18, 29, 99). The helix inside the FhaC β barrel was generated from a neighboring asymmetric unit in the crystal lattice. No structures of β barrel domains of type Vd or type V-like proteins have been reported. Modified from Molecular Microbiology (100), with permission.
Several other pathways have been described that appear to be variations on the same theme (Fig. 1). Trimeric autotransporters (type Vc pathway) are comprised of three identical subunits that each contain an N-terminal passenger domain that can exceed 4,000 residues in length and an ∼80-residue C-terminal segment that contributes four β strands to a single 12-stranded β barrel. Although the structure of the β barrel domains is very similar to those of classical autotransporters (18, 19), the three passenger domains assemble into a long coiled-coil “stalk” that emerges from the β barrel domain. The stalk is interspersed with and/or terminated by globular β-roll or β-prism “head” domains that function as adhesins (20–27). In the intimin/invasin (type Ve) pathway, the order of the domains is reversed. These “inverted autotransporters” contain a 12-stranded β barrel domain at (or near) the N terminus and a passenger domain comprised of multiple immunoglobulin (Ig)-like repeats at the C terminus (28–30). Although the structure of the β barrel domain resembles that of classical and trimeric autotransporters, the linker does not form an α helix (29). In the type Vb, or two-partner secretion (TPS), pathway, a single “exoprotein” is secreted by a coordinately expressed OM transporter. While exoproteins have the same β-helical architecture as the passenger domains of classical autotransporters, the transporters are members of the Omp85 superfamily, a group of proteins that have 16-stranded β barrel domains and 1 to 7 periplasmic POTRA (polypeptide transport-associated) domains that are believed to mediate protein-protein interactions (31, 32). The TPS pathway is the only type V pathway in which a β barrel protein secretes a noncovalently linked polypeptide (for details, see reference 101). The type Vd pathway is related to the type Vb pathway in that the C-terminal domains are similar to TpsB proteins, but the covalently linked passenger domains are patatin-like lipases that are released into the environment (33–35). Finally, a family of Helicobacter pylori proteins (at least some of which are adhesins) has been described in which an extracellular α-helical domain of up to ∼1,000 amino acids is situated between β strands 1 and 2 of a putative 8-stranded β barrel (36–40). These proteins have been proposed to represent a “type V-like” pathway based on their modular organization (40), but they do not have a clear phylogenetic relationship to other autotransporters and their structure is unique.
AUTOTRANSPORTER ASSEMBLY AND THE MECHANISM OF PASSENGER DOMAIN SECRETION
Although the first classical autotransporter was discovered over 30 years ago (2), the mechanism(s) by which passenger domains are translocated across the OM through the type V pathways is still not well understood. It was originally proposed that passenger domains are secreted through a channel formed solely by the covalently linked β barrel domain (hence the name “autotransporter”) (2). Indeed, it is easy to imagine how translocation in the type Va pathway, which proceeds in a C- to N-terminal direction (41, 42), might involve the insertion of a C-terminal hairpin into the β barrel pore followed by the progressive secretion of more distal segments. The resolution of the hairpin following the completion of translocation would explain why the two domains are connected by an intrabarrel linker. It should be noted, however, that the self-transport model was proposed before significant insights into the biogenesis of bacterial OMPs had emerged. Our view of autotransporter secretion has evolved considerably in recent years and has been strongly influenced both by new experimental data (that focus primarily on the type Va pathway) and by the identification and characterization of the machinery that catalyzes OMP assembly.
Based on all of the available evidence, it now appears that the β barrel domain does play a role in translocation but that the process by which passenger domains are transported across the OM is more complex than originally envisioned. On a fundamental level, the finding that translocation is abolished by the replacement of the C terminus of an autotransporter with the β barrel of another OMP suggests that the native β barrel domain does not simply target the passenger domain to an unlinked transporter (43). Furthermore, the finding that mutations that slow the folding and/or membrane integration of the β barrel domain concomitantly delay the initiation of passenger domain translocation also suggests that the β barrel domain promotes the transport reaction (9, 44). The idea that autotransporters are completely autonomous secretion systems, however, was first challenged by two contradictory lines of evidence. Crystal structures revealed that the β barrel pore of classical autotransporters is only ∼10 Å in diameter and therefore only wide enough to accommodate a single α helix or a hairpin in an extended conformation (10–15). Molecular dynamic simulations also indicated that β barrel domains are relatively rigid and are unlikely to expand significantly without an input of energy (45, 46). Paradoxically, considerable evidence has emerged that polypeptides that have local tertiary structures can be secreted by the type Va pathway. A subset of native type Va and type Ve passenger domains undergo disulfide bonding in the periplasm, and at least some ∼10- to 20-kDa heterologous polypeptides that fold in the periplasm are secreted effectively when they are fused to passenger domains (29, 47–51). An analysis of the secretion of peptides that vary in length and structural complexity also suggests that the translocation channel is ∼17 to 20 Å wide (52). Furthermore, evidence that the linker is already embedded inside the β barrel in an α-helical conformation during translocation strongly suggests that the active transport channel contains at least an α helix and an extended polypeptide (53, 54). Finally, several studies have indicated that the β barrel domain reaches its native state only after the passenger domain is completely secreted (55–57). Taken together, the results imply that during translocation the β barrel domain is in an open or distorted conformation that would be incompatible with stable integration into a lipid bilayer.
A plausible alternative to the self-transport hypothesis that accounts for the secretion of folded polypeptides arose from an analysis of stalled translocation intermediates. One study exploited the fortuitous discovery that the insertion of a peptide linker near the middle of the passenger domain of a classical autotransporter (the Escherichia coli O157:H7 EspP protein) did not affect the initiation of translocation but transiently stalled translocation when the inserted peptide was in the vicinity of the transport channel (41). Site-specific photo-cross-linking experiments showed that passenger domain residues located near the site of stalling are in close proximity to BamA, a member of the Omp85 superfamily. BamA is an essential component of the barrel assembly machinery (Bam) complex, a hetero-oligomer that catalyzes the membrane insertion of essentially all β barrel proteins, including autotransporters (58–61). In a second study, chemical cross-linking experiments showed that a related autotransporter (Hbp) was close to the Bam complex when the secretion of the passenger domain was stalled by a different method (55). Interestingly, the crystal structure of BamA together with molecular dynamics simulations strongly suggests that the BamA β barrel can open laterally (62). Although the function of the BamA lateral gate is still unclear, the results raise the intriguing possibility that passenger domains are secreted through a hybrid channel composed of open forms of both the linked β barrel domain and the BamA β barrel. Such a channel would presumably be wide enough to accommodate the transport of polypeptides that have local tertiary structures.
The analysis of these and other assembly intermediates has led to a detailed model for the biogenesis of classical autotransporters. The finding that the EspP linker becomes protected from proteolysis and chemical modification (53) prior to the initiation of passenger domain translocation suggests that the β barrel domain begins to fold in the periplasm (Fig. 2, step I). Consistent with this idea, a recent study indicated that the trimeric β barrel of a type Vc autotransporter begins to assemble in the periplasm (63). The observation that the linker is required for the membrane integration of the EspP β barrel domain in vivo (53) and accelerates assembly in an in vitro assay (64) suggests that it nucleates early folding events. Photo-cross-linking experiments (44, 56) have shown that at this stage the EspP β barrel domain interacts with the periplasmic chaperone Skp, a jellyfish-like homotrimer that binds to both small and large β barrels in a 1:1 or 2:1 ratio (65, 66). Subsequently, the β barrel domain is targeted to the Bam complex (Fig. 2, step II). Cross-links between specific residues of the EspP β barrel domain and two lipoprotein subunits of the Bam complex, BamB and BamD, can be detected at this step (44). Interestingly, a map generated by projecting the molecular interactions implied by the photo-cross-linking experiments onto the crystal structure of the Bam holocomplex supports the idea that the β barrel domain is already folded into a cylinder-like structure (67). The initiation of passenger domain translocation requires an additional assembly step that appears to correspond to the movement (but not full integration) of the β barrel domain into the OM (44, 68) (Fig. 2, step III). As suggested above, the passenger domain might be transported through a hybrid channel that contains the BamA β barrel in an open conformation. Available evidence indicates that translocation involves a stepwise transfer of passenger domain segments from the chaperone SurA, which binds to the first POTRA domain (69), to membrane-proximal POTRA domains and then to the transport channel (44) (Fig. 2, step IV). In the TPS pathway, exoproteins use a similar path to traverse the cognate transporter (70). Following the completion of translocation (Fig. 2, step V), a surface-exposed basic or large polar residue stimulates a final step in the folding of at least some classical autotransporters that may correspond to the closing of the β barrel domain (57) (Fig. 2, step VI). Ultimately, the β barrel domain is released from the Bam complex and the passenger domain is cleaved (Fig. 2, step VII).
Figure 2.
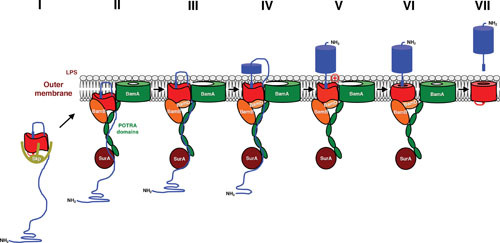
Model for the assembly of a classical autotransporter. Available evidence suggests that the β barrel domain (red) begins to fold in the periplasm (step I) and incorporates the C terminus of the passenger domain (blue) in a hairpin conformation. At this stage the β barrel domain interacts with the molecular chaperone Skp. The partially folded β barrel domain is then targeted to the OM, where it binds to BamA, BamB, and BamD in a stereospecific fashion (step II). The surface exposure of the passenger domain and the initiation of translocation require an additional assembly step in which the β barrel domain moves into the membrane (step III). Both autotransporter and BamA β barrels are in an open conformation at this stage. Translocation involves the progressive movement of passenger domain segments from the chaperone SurA to the POTRA domains of BamA to the transport channel and is driven at least in part by vectorial folding (step IV). Following the completion of translocation the hairpin is resolved (step V), and an unusual lipid-facing basic or large polar residue found in at least a subset of autotransporters facilitates the completion of β barrel domain assembly (step VI). The β barrel domain is then released from the Bam complex, and, in some cases, the two domains are separated by an intrabarrel cleavage or an extrabarrel cleavage mediated by a trans-acting protease (step VII). In E. coli the Bam complex contains five subunits, but BamC and BamE have been omitted for clarity. Modified from Molecular Microbiology (57, 100), with permission.
ENERGETICS OF PASSENGER DOMAIN SECRETION
Because the periplasm is devoid of ATP and there is no electrochemical gradient across the OM, the source of energy for passenger domain translocation has remained unclear. It is possible that in some cases an interaction between the passenger domain or specialized components of the OM transport machinery and an energized IM protein drives the translocation reaction. The observation that the Bam complex and SurA are sufficient to promote passenger domain translocation into proteoliposomes, however, suggests that autotransporter assembly does not strictly require an exogenous energy source (71).
To explain the energetics of autotransporter secretion, it was proposed years ago that small segments of the passenger domain passively diffuse through the transport channel and then fold in the extracellular space (72). Folding would trap the passenger domain on the cell surface and thereby provide the driving force for translocation. This hypothesis is especially attractive given that most passenger domains are composed of modular β helices that might fold in a stepwise fashion. A subset of passenger domains contain so-called passenger-associated transport repeats that might also contribute to progressive folding (73). Indeed, even the passenger domain of a classical autotransporter that has a globular structure has been predicted to fold sequentially based on the arrangement of its secondary-structure elements (12). During the last decade the “vectorial folding” hypothesis has been supported by several observations. Studies that have analyzed the refolding or unfolding of passenger domains in vitro or the effect of mutations on passenger domain secretion in vivo have demonstrated that the folding of a conserved ∼20- to 25-kDa “stable core” segment located at the C termini of many passenger domains plays a key role in driving translocation (6, 54, 68, 74–76). The results of kinetic simulations also suggest that passenger domain secretion is driven by the free energy of folding in the extracellular milieu (77). Furthermore, an analysis of insertions and deletions in the intimin passenger domain suggested that secretion by the type Ve pathway is driven by sequential folding of the Ig-like domains (78). A recent study provided intriguing evidence that the folding of classical autotransporter passenger domains on the cell surface is not spontaneous but is nucleated by the fifth extracellular loop of the β barrel domain (79).
Despite the evidence that supports the vectorial folding model, several observations have strongly suggested that autotransporter secretion is not driven solely by passenger domain folding. It has been shown, for example, that multiple point mutations introduced into the middle of the EspP passenger domain destabilize the protein but only moderately impair translocation (80). In addition, an intrinsically disordered polypeptide fused to the C terminus of EspP was secreted as rapidly and efficiently as the native passenger domain (51). The disordered polypeptide is unusually acidic, and the neutralization of multiple acidic amino acids was shown to stall translocation. Taken together with the finding that many native passenger domains are acidic, this observation suggests that charge interactions and/or the Donnan potential across the OM (81) might help to drive translocation. Furthermore, it seems likely that the secretion of ∼10- to 20-kDa folded polypeptides that has been reported would also require the input of energy from an alternative source. In this regard, it is noteworthy that structural studies on the OM transporters associated with the chaperone/usher and type VIII secretion pathways suggest that they drive translocation by defining a low-energy pathway or using an entropy-based diffusion mechanism (82, 83).
ACCESSORY FACTORS IN AUTOTRANSPORTER ASSEMBLY
Like all OMPs, autotransporters must remain in an assembly-competent conformation in the periplasm. Consistent with this expectation, several periplasmic chaperones that play a broad role in OMP biogenesis, including DegP, FkpA, Skp, and SurA, have been shown to interact with autotransporters in vivo and/or in vitro (41, 56, 84, 85). Presumably because periplasmic chaperones have redundant or at least partially overlapping functions (86), however, the requirement for specific chaperones in autotransporter assembly appears to be protein-, organism-, and condition-dependent (41, 63, 84, 87, 88).
It is still unclear whether factors other than periplasmic chaperones and the Bam complex play a general role in autotransporter assembly. The finding that both EspP and the E. coli autotransporter Ag43 can be assembled into proteoliposomes that contain only the Bam complex in purified-protein- or spheroplast-based assays (71, 89) certainly suggests that no other factors are absolutely essential. There is evidence, however, that a member of the Omp85 superfamily (TamA) and an IM-anchored protein (TamB) that interacts with TamA facilitate the assembly of a subset of autotransporters, including the Citrobacter p1121 protein and Ag43 (90, 91). Interestingly, TamA/B has also been implicated in the assembly of inverse autotransporters (92). Although different models for the function of TamA/B have been proposed (67, 93), the observation that the Tam system facilitates the biogenesis of the fimbrial usher protein FimD (94) suggests that it is not an autotransporter-specific assembly factor. In addition, the efficient assembly of a subset of trimeric autotransporters requires the activity of a trimeric IM lipoprotein that is encoded in the same operon (95).
CONCLUDING REMARKS
Studies on type V secretion suggest that autotransporter β barrel domains are not autonomous transporters but that the assembly of the β barrel domain and the secretion of the passenger domain are catalyzed by the Bam complex in a concerted reaction. Although it is possible that BamA promotes passenger domain translocation indirectly by keeping the autotransporter β barrel domain in an open conformation, the fact that some members of the Omp85 superfamily catalyze secretion reactions suggests that BamA might play a direct role in translocation. In any case, to obtain a better understanding of autotransporter biogenesis, it will likely be necessary to elucidate the function of the Bam complex, which so far has remained elusive (96). Of course the degree to which the assembly of type Vc-Ve and “autotransporter-like” proteins resembles the assembly of the better-characterized classical autotransporters also remains to be investigated. Indeed, it should be interesting to determine why features of the Omp85 superfamily are found only in the type Vd pathway and if the folding of predominantly α-helical passenger domains drives their secretion. Given that the loops of most OMPs are relatively short (<75 residues) and that at least in some cases only small insertions are tolerated (97), the presence of the large loop structures in “autotransporter-like” proteins is intriguing. Based on the discovery of these proteins, the structural similarity of the β barrel domains found in multiple type V pathways, and the unique structures of passenger domains, it is tempting to speculate that the Bam complex evolved to facilitate the efficient export of a range of specialized polypeptides that are paired with specific types of β barrels.
ACKNOWLEDGMENTS
I thank Matt Doyle for helping to generate Fig. 1.
Work conducted in my laboratory is supported by the NIDDK Intramural Research Program (Z01-DK052037).
Contributor Information
Harris D. Bernstein, Genetics and Biochemistry Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892
Maria Sandkvist, Department of Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan.
Eric Cascales, CNRS Aix-Marseille Université, Mediterranean Institute of Microbiology, Marseille, France.
Peter J. Christie, Department of Microbiology and Molecular Genetics, McGovern Medical School, Houston, Texas
REFERENCES
- 1.Henderson IR, Nataro JP. 2001. Virulence functions of autotransporter proteins. Infect Immun 69:1231–1243. 10.1128/IAI.69.3.1231-1243.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pohlner J, Halter R, Beyreuther K, Meyer TF. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458–462. 10.1038/325458a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Celik N, Webb CT, Leyton DL, Holt KE, Heinz E, Gorrell R, Kwok T, Naderer T, Strugnell RA, Speed TP, Teasdale RD, Likić VA, Lithgow T. 2012. A bioinformatic strategy for the detection, classification and analysis of bacterial autotransporters. PLoS One 7:e43245. 10.1371/journal.pone.0043245. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emsley P, Charles IG, Fairweather NF, Isaacs NW. 1996. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381:90–92. 10.1038/381090a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Otto BR, Sijbrandi R, Luirink J, Oudega B, Heddle JG, Mizutani K, Park SY, Tame JR. 2005. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J Biol Chem 280:17339–17345. 10.1074/jbc.M412885200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Junker M, Schuster CC, McDonnell AV, Sorg KA, Finn MC, Berger B, Clark PL. 2006. Pertactin beta-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc Natl Acad Sci U S A 103:4918–4923. 10.1073/pnas.0507923103. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangwer KA, Mushrush DJ, Stauff DL, Spiller B, McClain MS, Cover TL, Lacy DB. 2007. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc Natl Acad Sci U S A 104:16293–16298. 10.1073/pnas.0707447104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heras B, Totsika M, Peters KM, Paxman JJ, Gee CL, Jarrott RJ, Perugini MA, Whitten AE, Schembri MA. 2014. The antigen 43 structure reveals a molecular Velcro-like mechanism of autotransporter-mediated bacterial clumping. Proc Natl Acad Sci U S A 111:457–462. 10.1073/pnas.1311592111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leyton DL, Johnson MD, Thapa R, Huysmans GH, Dunstan RA, Celik N, Shen HH, Loo D, Belousoff MJ, Purcell AW, Henderson IR, Beddoe T, Rossjohn J, Martin LL, Strugnell RA, Lithgow T. 2014. A mortise-tenon joint in the transmembrane domain modulates autotransporter assembly into bacterial outer membranes. Nat Commun 5:4239. 10.1038/ncomms5239. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J 23:1257–1266. 10.1038/sj.emboj.7600148. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnard TJ, Dautin N, Lukacik P, Bernstein HD, Buchanan SK. 2007. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat Struct Mol Biol 14:1214–1220. 10.1038/nsmb1322. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berg B. 2010. Crystal structure of a full-length autotransporter. J Mol Biol 396:627–633. 10.1016/j.jmb.2009.12.061. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Tajima N, Kawai F, Park SY, Tame JR. 2010. A novel intein-like autoproteolytic mechanism in autotransporter proteins. J Mol Biol 402:645–656. 10.1016/j.jmb.2010.06.068. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Zhai Y, Zhang K, Huo Y, Zhu Y, Zhou Q, Lu J, Black I, Pang X, Roszak AW, Zhang X, Isaacs NW, Sun F. 2011. Autotransporter passenger domain secretion requires a hydrophobic cavity at the extracellular entrance of the β-domain pore. Biochem J 435:577–587. 10.1042/BJ20101548. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Gawarzewski I, DiMaio F, Winterer E, Tschapek B, Smits SHJ, Jose J, Schmitt L. 2014. Crystal structure of the transport unit of the autotransporter adhesin involved in diffuse adherence from Escherichia coli. J Struct Biol 187:20–29. 10.1016/j.jsb.2014.05.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Barnard TJ, Gumbart J, Peterson JH, Noinaj N, Easley NC, Dautin N, Kuszak AJ, Tajkhorshid E, Bernstein HD, Buchanan SK. 2012. Molecular basis for the activation of a catalytic asparagine residue in a self-cleaving bacterial autotransporter. J Mol Biol 415:128–142. 10.1016/j.jmb.2011.10.049. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dautin N, Bernstein HD. 2007. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol 61:89–112. 10.1146/annurev.micro.61.080706.093233. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Meng G, Surana NK, St Geme JW, III, Waksman G. 2006. Structure of the outer membrane translocator domain of the Haemophilus influenzae Hia trimeric autotransporter. EMBO J 25:2297–2304. 10.1038/sj.emboj.7601132. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahid SA, Bardiaux B, Franks WT, Krabben L, Habeck M, van Rossum BJ, Linke D. 2012. Membrane-protein structure determination by solid-state NMR spectroscopy of microcrystals. Nat Methods 9:1212–1217. 10.1038/nmeth.2248. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Nummelin H, Merckel MC, Leo JC, Lankinen H, Skurnik M,Goldman A. 2004. The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel β-roll. EMBO J 23:701–711. 10.1038/sj.emboj.7600100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szczesny P, Linke D, Ursinus A, Bär K, Schwarz H, Riess TM, Kempf VA, Lupas AN, Martin J, Zeth K. 2008. Structure of the head of the Bartonella adhesin BadA. PLoS Pathog 4:e1000119. 10.1371/journal.ppat.1000119. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards TE, Phan I, Abendroth J, Dieterich SH, Masoudi A, Guo W, Hewitt SN, Kelley A, Leibly D, Brittnacher MJ, Staker BL, Miller SI, Van Voorhis WC, Myler PJ, Stewart LJ. 2010. Structure of a Burkholderia pseudomallei trimeric autotransporter adhesin head. PLoS One 5:e12803. 10.1371/journal.pone.0012803. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agnew C, Borodina E, Zaccai NR, Conners R, Burton NM, Vicary JA, Cole DK, Antognozzi M, Virji M, Brady RL. 2011. Correlation of in situ mechanosensitive responses of the Moraxella catarrhalis adhesin UspA1 with fibronectin and receptor CEACAM1 binding. Proc Natl Acad Sci U S A 108:15174–15178. 10.1073/pnas.1106341108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leo JC, Lyskowski A, Hattula K, Hartmann MD, Schwarz H, Butcher SJ, Linke D, Lupas AN, Goldman A. 2011. The structure of E. coli IgG-binding protein D suggests a general model for bending and binding in trimeric autotransporter adhesins. Structure 19:1021–1030. 10.1016/j.str.2011.03.021. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Hartmann MD, Grin I, Dunin-Horkawicz S, Deiss S, Linke D, Lupas AN, Hernandez Alvarez B. 2012. Complete fiber structures of complex trimeric autotransporter adhesins conserved in enterobacteria. Proc Natl Acad Sci U S A 109:20907–20912. 10.1073/pnas.1211872110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malito E, Biancucci M, Faleri A, Ferlenghi I, Scarselli M, Maruggi G, Lo Surdo P, Veggi D, Liguori A, Santini L, Bertoldi I, Petracca R, Marchi S, Romagnoli G, Cartocci E, Vercellino I, Savino S, Spraggon G, Norais N, Pizza M, Rappuoli R, Masignani V, Bottomley MJ. 2014. Structure of the meningococcal vaccine antigen NadA and epitope mapping of a bactericidal antibody. Proc Natl Acad Sci U S A 111:17128–17133. 10.1073/pnas.1419686111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koiwai K, Hartmann MD, Linke D, Lupas AN, Hori K. 2016. Structural basis for toughness and flexibility in the C-terminal passenger domain of an Acinetobacter trimeric autotransporter adhesin. J Biol Chem 291:3705–3724. 10.1074/jbc.M115.701698. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamburger ZA, Brown MS, Isberg RR, Bjorkman PJ. 1999. Crystal structure of invasin: a bacterial integrin-binding protein. Science 286:291–295. 10.1126/science.286.5438.291. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Fairman JW, Dautin N, Wojtowicz D, Liu W, Noinaj N, Barnard TJ, Udho E, Przytycka TM, Cherezov V, Buchanan SK. 2012. Crystal structures of the outer membrane domain of intimin and invasin from enterohemorrhagic E. coli and enteropathogenic Y. pseudotuberculosis. Structure 20:1233–1243. 10.1016/j.str.2012.04.011. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leo JC, Oberhettinger P, Schütz M, Linke D. 2015. The inverse autotransporter family: intimin, invasin and related proteins. Int J Med Microbiol 305:276–282. 10.1016/j.ijmm.2014.12.011. [PubMed] [DOI] [PubMed] [Google Scholar]
- 31.Gentle IE, Burri L, Lithgow T. 2005. Molecular architecture and function of the Omp85 family of proteins. Mol Microbiol 58:1216–1225. 10.1111/j.1365-2958.2005.04906.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Arnold T, Zeth K, Linke D. 2010. Omp85 from the thermophilic cyanobacterium Thermosynechococcus elongatus differs from proteobacterial Omp85 in structure and domain composition. J Biol Chem 285:18003–18015. 10.1074/jbc.M110.112516. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salacha R, Kovacić F, Brochier-Armanet C, Wilhelm S, Tommassen J, Filloux A, Voulhoux R, Bleves S. 2010. The Pseudomonas aeruginosa patatin-like protein PlpD is the archetype of a novel type V secretion system. Environ Microbiol 12:1498–1512. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.da Mata Madeira PV, Zouhir S, Basso P, Neves D, Laubier A, Salacha R, Bleves S, Faudry E, Contreras-Martel C, Dessen A. 2016. Structural basis of lipid targeting and destruction by the type V secretion system of Pseudomonas aeruginosa. J Mol Biol 428(9 Part A):1790–1803. 10.1016/j.jmb.2016.03.012. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Casasanta MA, Yoo CC, Smith HB, Duncan AJ, Cochrane K, Varano AC, Allen-Vercoe E, Slade DJ. 2017. A chemical and biological toolbox for type Vd secretion: characterization of the phospholipase A1 autotransporter FplA from Fusobacterium nucleatum. J Biol Chem 292:20240–20254. 10.1074/jbc.M117.819144. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang SS, Nguyen ST, Perry AJ, Day CJ, Panjikar S, Tiralongo J, Whisstock JC, Kwok T. 2014. The three-dimensional structure of the extracellular adhesion domain of the sialic acid-binding adhesin SabA from Helicobacter pylori. J Biol Chem 289:6332–6340. 10.1074/jbc.M113.513135. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hage N, Howard T, Phillips C, Brassington C, Overman R, Debreczeni J, Gellert P, Stolnik S, Winkler GS, Falcone FH. 2015. Structural basis of Lewis(b) antigen binding by the Helicobacter pylori adhesin BabA. Sci Adv 1:e1500315. 10.1126/sciadv.1500315. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javaheri A, Kruse T, Moonens K, Mejías-Luque R, Debraekeleer A, Asche CI, Tegtmeyer N, Kalali B, Bach NC, Sieber SA, Hill DJ, Königer V, Hauck CR, Moskalenko R, Haas R, Busch DH, Klaile E, Slevogt H, Schmidt A, Backert S, Remaut H, Singer BB, Gerhard M. 2016. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat Microbiol 2:16189. 10.1038/nmicrobiol.2016.189. [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.Moonens K, Gideonsson P, Subedi S, Bugaytsova J, Romaõ E, Mendez M, Nordén J, Fallah M, Rakhimova L, Shevtsova A, Lahmann M, Castaldo G, Brännström K, Coppens F, Lo AW, Ny T, Solnick JV, Vandenbussche G, Oscarson S, Hammarström L, Arnqvist A, Berg DE, Muyldermans S, Borén T, Remaut H. 2016. Structural insights into polymorphic ABO glycan binding by Helicobacter pylori. Cell Host Microbe 19:55–66. 10.1016/j.chom.2015.12.004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppens F, Castaldo G, Debraekeleer A, Subedi S, Moonens K, Lo A, Remaut H. 2018. Hop-family Helicobacter outer membrane adhesins form a novel class of type 5-like secretion proteins with an interrupted β-barrel domain. Mol Microbiol 110:33–46. 10.1111/mmi.14075. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Ieva R, Bernstein HD. 2009. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc Natl Acad Sci U S A 106:19120–19125. 10.1073/pnas.0907912106. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Junker M, Besingi RN, Clark PL. 2009. Vectorial transport and folding of an autotransporter virulence protein during outer membrane secretion. Mol Microbiol 71:1323–1332. 10.1111/j.1365-2958.2009.06607.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Saurí A, Oreshkova N, Soprova Z, Jong WS, Sani M, Peters PJ, Luirink J, van Ulsen P. 2011. Autotransporter β-domains have a specific function in protein secretion beyond outer-membrane targeting. J Mol Biol 412:553–567. 10.1016/j.jmb.2011.07.035. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Pavlova O, Peterson JH, Ieva R, Bernstein HD. 2013. Mechanistic link between β barrel assembly and the initiation of autotransporter secretion. Proc Natl Acad Sci U S A 110:E938–E947. 10.1073/pnas.1219076110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalid S, Sansom MS. 2006. Molecular dynamics simulations of a bacterial autotransporter: NalP from Neisseria meningitidis. Mol Membr Biol 23:499–508. 10.1080/09687860600849531. [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.Tian P, Bernstein HD. 2010. Molecular basis for the structural stability of an enclosed β-barrel loop. J Mol Biol 402:475–489. 10.1016/j.jmb.2010.07.035. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veiga E, de Lorenzo V, Fernández LA. 2004. Structural tolerance of bacterial autotransporters for folded passenger protein domains. Mol Microbiol 52:1069–1080. 10.1111/j.1365-2958.2004.04014.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Skillman KM, Barnard TJ, Peterson JH, Ghirlando R, Bernstein HD. 2005. Efficient secretion of a folded protein domain by a monomeric bacterial autotransporter. Mol Microbiol 58:945–958. 10.1111/j.1365-2958.2005.04885.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.Swanson KA, Taylor LD, Frank SD, Sturdevant GL, Fischer ER, Carlson JH, Whitmire WM, Caldwell HD. 2009. Chlamydia trachomatis polymorphic membrane protein D is an oligomeric autotransporter with a higher-order structure. Infect Immun 77:508–516. 10.1128/IAI.01173-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leyton DL, Sevastsyanovich YR, Browning DF, Rossiter AE, Wells TJ, Fitzpatrick RE, Overduin M, Cunningham AF, Henderson IR. 2011. Size and conformation limits to secretion of disulfide-bonded loops in autotransporter proteins. J Biol Chem 286:42283–42291. 10.1074/jbc.M111.306118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang’ethe W, Bernstein HD. 2013. Charge-dependent secretion of an intrinsically disordered protein via the autotransporter pathway. Proc Natl Acad Sci U S A 110:E4246–E4255. 10.1073/pnas.1310345110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saurí A, Ten Hagen-Jongman CM, van Ulsen P, Luirink J. 2012. Estimating the size of the active translocation pore of an autotransporter. J Mol Biol 416:335–345. 10.1016/j.jmb.2011.12.047. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Ieva R, Skillman KM, Bernstein HD. 2008. Incorporation of a polypeptide segment into the β-domain pore during the assembly of a bacterial autotransporter. Mol Microbiol 67:188–201. 10.1111/j.1365-2958.2007.06048.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 54.Peterson JH, Tian P, Ieva R, Dautin N, Bernstein HD. 2010. Secretion of a bacterial virulence factor is driven by the folding of a C-terminal segment. Proc Natl Acad Sci U S A 107:17739–17744. 10.1073/pnas.1009491107. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sauri A, Soprova Z, Wickström D, de Gier JW, Van der Schors RC, Smit AB, Jong WS, Luirink J. 2009. The Bam (Omp85) complex is involved in secretion of the autotransporter haemoglobin protease. Microbiology 155:3982–3991. 10.1099/mic.0.034991-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 56.Ieva R, Tian P, Peterson JH, Bernstein HD. 2011. Sequential and spatially restricted interactions of assembly factors with an autotransporter β domain. Proc Natl Acad Sci U S A 108:E383–E391. 10.1073/pnas.1103827108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peterson JH, Hussain S, Bernstein HD. 2018. Identification of a novel post-insertion step in the assembly of a bacterial outer membrane protein. Mol Microbiol 110:143–159. 10.1111/mmi.14102. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262–265. 10.1126/science.1078973. [PubMed] [DOI] [PubMed] [Google Scholar]
- 59.Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235–245. 10.1016/j.cell.2005.02.015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Jain S, Goldberg MB. 2007. Requirement for YaeT in the outer membrane assembly of autotransporter proteins. J Bacteriol 189:5393–5398. 10.1128/JB.00228-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hagan CL, Kim S, Kahne D. 2010. Reconstitution of outer membrane protein assembly from purified components. Science 328:890–892. 10.1126/science.1188919. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noinaj N, Kuszak AJ, Gumbart JC, Lukacik P, Chang H, Easley NC, Lithgow T, Buchanan SK. 2013. Structural insight into the biogenesis of β-barrel membrane proteins. Nature 501:385–390. 10.1038/nature12521. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sikdar R, Peterson JH, Anderson DE, Bernstein HD. 2017. Folding of a bacterial integral outer membrane protein is initiated in the periplasm. Nat Commun 8:1309. 10.1038/s41467-017-01246-4. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussain S, Bernstein HD. 2018. The Bam complex catalyzes efficient insertion of bacterial outer membrane proteins into membrane vesicles of variable lipid composition. J Biol Chem 293:2959–2973. 10.1074/jbc.RA117.000349. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walton TA, Sandoval CM, Fowler CA, Pardi A, Sousa MC. 2009. The cavity-chaperone Skp protects its substrate from aggregation but allows independent folding of substrate domains. Proc Natl Acad Sci U S A 106:1772–1777. 10.1073/pnas.0809275106. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schiffrin B, Calabrese AN, Devine PWA, Harris SA, Ashcroft AE, Brockwell DJ, Radford SE. 2016. Skp is a multivalent chaperone of outer-membrane proteins. Nat Struct Mol Biol 23:786–793. 10.1038/nsmb.3266. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albenne C, Ieva R. 2017. Job contenders: roles of the β-barrel assembly machinery and the translocation and assembly module in autotransporter secretion. Mol Microbiol 106:505–517. 10.1111/mmi.13832. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Soprova Z, Sauri A, van Ulsen P, Tame JR, den Blaauwen T, Jong WS, Luirink J. 2010. A conserved aromatic residue in the autochaperone domain of the autotransporter Hbp is critical for initiation of outer membrane translocation. J Biol Chem 285:38224–38233. 10.1074/jbc.M110.180505. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bennion D, Charlson ES, Coon E, Misra R. 2010. Dissection of β-barrel outer membrane protein assembly pathways through characterizing BamA POTRA 1 mutants of Escherichia coli. Mol Microbiol 77:1153–1171. 10.1111/j.1365-2958.2010.07280.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baud C, Guérin J, Petit E, Lesne E, Dupré E, Locht C, Jacob-Dubuisson F. 2014. Translocation path of a substrate protein through its Omp85 transporter. Nat Commun 5:5271. 10.1038/ncomms6271. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Roman-Hernandez G, Peterson JH, Bernstein HD. 2014. Reconstitution of bacterial autotransporter assembly using purified components. eLife 3:e04234. 10.7554/eLife.04234. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klauser T, Pohlner J, Meyer TF. 1992. Selective extracellular release of cholera toxin B subunit by Escherichia coli: dissection of Neisseria Iga β-mediated outer membrane transport. EMBO J 11:2327–2335. 10.1002/j.1460-2075.1992.tb05292.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doyle MT, Tran EN, Morona R. 2015. The passenger-associated transport repeat promotes virulence factor secretion efficiency and delineates a distinct autotransporter subtype. Mol Microbiol 97:315–329. 10.1111/mmi.13027. [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.Velarde JJ, Nataro JP. 2004. Hydrophobic residues of the autotransporter EspP linker domain are important for outer membrane translocation of its passenger. J Biol Chem 279:31495–31504. 10.1074/jbc.M404424200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 75.Renn JP, Clark PL. 2008. A conserved stable core structure in the passenger domain β-helix of autotransporter virulence proteins. Biopolymers 89:420–427. 10.1002/bip.20924. [PubMed] [DOI] [PubMed] [Google Scholar]
- 76.Baclayon M, Ulsen P, Mouhib H, Shabestari MH, Verzijden T, Abeln S, Roos WH, Wuite GJ. 2016. Mechanical unfolding of an autotransporter passenger protein reveals the secretion starting point and processive transport intermediates. ACS Nano 10:5710–5719. 10.1021/acsnano.5b07072. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Besingi RN, Chaney JL, Clark PL. 2013. An alternative outer membrane secretion mechanism for an autotransporter protein lacking a C-terminal stable core. Mol Microbiol 90:1028–1045. 10.1111/mmi.12414. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leo JC, Oberhettinger P, Yoshimoto S, Udatha DB, Morth JP, Schütz M, Hori K, Linke D. 2016. Secretion of the intimin passenger domain is driven by protein folding. J Biol Chem 291:20096–20112. 10.1074/jbc.M116.731497. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan X, Johnson MD, Zhang J, Lo AW, Schembri MA, Wijeyewickrema LC, Pike RN, Huysmans GHM, Henderson IR, Leyton DL. 2018. Molecular basis for the folding of β-helical autotransporter passenger domains. Nat Commun 9:1395. 10.1038/s41467-018-03593-2. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang’ethe W, Bernstein HD. 2013. Stepwise folding of an autotransporter passenger domain is not essential for its secretion. J Biol Chem 288:35028–35038. 10.1074/jbc.M113.515635. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stock JB, Rauch B, Roseman S. 1977. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem 252:7850–7861. [PubMed] [PubMed] [Google Scholar]
- 82.Geibel S, Procko E, Hultgren SJ, Baker D, Waksman G. 2013. Structural and energetic basis of folded-protein transport by the FimD usher. Nature 496:243–246. 10.1038/nature12007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goyal P, Krasteva PV, Van Gerven N, Gubellini F, Van den Broeck I, Troupiotis-Tsaïlaki A, Jonckheere W, Péhau-Arnaudet G, Pinkner JS, Chapman MR, Hultgren SJ, Howorka S, Fronzes R, Remaut H. 2014. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature 516:250–253. 10.1038/nature13768. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruiz-Perez F, Henderson IR, Leyton DL, Rossiter AE, Zhang Y, Nataro JP. 2009. Roles of periplasmic chaperone proteins in the biogenesis of serine protease autotransporters of Enterobacteriaceae. J Bacteriol 191:6571–6583. 10.1128/JB.00754-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruiz-Perez F, Henderson IR, Nataro JP. 2010. Interaction of FkpA, a peptidyl-prolyl cis/trans isomerase with EspP autotransporter protein. Gut Microbes 1:339–344. 10.4161/gmic.1.5.13436. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rizzitello AE, Harper JR, Silhavy TJ. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J Bacteriol 183:6794–6800. 10.1128/JB.183.23.6794-6800.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Purdy GE, Fisher CR, Payne SM. 2007. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J Bacteriol 189:5566–5573. 10.1128/JB.00483-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peterson JH, Plummer AM, Fleming KG, Bernstein HD. 2017. Selective pressure for rapid membrane integration constrains the sequence of bacterial outer membrane proteins. Mol Microbiol 106:777–792. 10.1111/mmi.13845. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Norell D, Heuck A, Tran-Thi TA, Götzke H, Jacob-Dubuisson F, Clausen T, Daley DO, Braun V, Müller M, Fan E. 2014. Versatile in vitro system to study translocation and functional integration of bacterial outer membrane proteins. Nat Commun 5:5396. 10.1038/ncomms6396. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Selkrig J, Mosbahi K, Webb CT, Belousoff MJ, Perry AJ, Wells TJ, Morris F, Leyton DL, Totsika M, Phan MD, Celik N, Kelly M, Oates C, Hartland EL, Robins-Browne RM, Ramarathinam SH, Purcell AW, Schembri MA, Strugnell RA, Henderson IR, Walker D, Lithgow T. 2012. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat Struct Mol Biol 19:506–510, S1. 10.1038/nsmb.2261. [DOI] [PubMed] [Google Scholar]
- 91.Shen HH, Leyton DL, Shiota T, Belousoff MJ, Noinaj N, Lu J, Holt SA, Tan K, Selkrig J, Webb CT, Buchanan SK, Martin LL, Lithgow T. 2014. Reconstitution of a nanomachine driving the assembly of proteins into bacterial outer membranes. Nat Commun 5:5078. 10.1038/ncomms6078. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heinz E, Stubenrauch CJ, Grinter R, Croft NP, Purcell AW, Strugnell RA, Dougan G, Lithgow T. 2016. Conserved features in the structure, mechanism, and biogenesis of the inverse autotransporter protein family. Genome Biol Evol 8:1690–1705. 10.1093/gbe/evw112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bamert RS, Lundquist K, Hwang H, Webb CT, Shiota T, Stubenrauch CJ, Belousoff MJ, Goode RJA, Schittenhelm RB, Zimmerman R, Jung M, Gumbart JC, Lithgow T. 2017. Structural basis for substrate selection by the translocation and assembly module of the β-barrel assembly machinery. Mol Microbiol 106:142–156. 10.1111/mmi.13757. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stubenrauch C, Belousoff MJ, Hay ID, Shen HH, Lillington J, Tuck KL, Peters KM, Phan MD, Lo AW, Schembri MA, Strugnell RA, Waksman G, Lithgow T. 2016. Effective assembly of fimbriae in Escherichia coli depends on the translocation assembly module nanomachine. Nat Microbiol 1:16064. 10.1038/nmicrobiol.2016.64. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Grin I, Hartmann MD, Sauer G, Hernandez Alvarez B, Schütz M, Wagner S, Madlung J, Macek B, Felipe-Lopez A, Hensel M, Lupas A, Linke D. 2014. A trimeric lipoprotein assists in trimeric autotransporter biogenesis in enterobacteria. J Biol Chem 289:7388–7398. 10.1074/jbc.M113.513275. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noinaj N, Gumbart JC, Buchanan SK. 2017. The β-barrel assembly machinery in motion. Nat Rev Microbiol 15:197–204. 10.1038/nrmicro.2016.191. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janssen R, Tommassen J. 1994. PhoE protein as a carrier for foreign epitopes. Int Rev Immunol 11:113–121. 10.3109/08830189409061719. [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.Yeo HJ, Yokoyama T, Walkiewicz K, Kim Y, Grass S, Geme JW, III. 2007. The structure of the Haemophilus influenzae HMW1 pro-piece reveals a structural domain essential for bacterial two-partner secretion. J Biol Chem 282:31076–31084. 10.1074/jbc.M705750200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 99.Clantin B, Delattre AS, Rucktooa P, Saint N, Méli AC, Locht C, Jacob-Dubuisson F, Villeret V. 2007. Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter superfamily. Science 317:957–961. 10.1126/science.1143860. [PubMed] [DOI] [PubMed] [Google Scholar]
- 100.Bernstein HD. 2015. Looks can be deceiving: recent insights into the mechanism of protein secretion by the autotransporter pathway. Mol Microbiol 97:205–215. 10.1111/mmi.13031. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nash ZM, Cotter PA. 2019. Bordetella filamentous hemagglutinin, a model for the two partner secretion pathway. Microbiol Spectr 7:PSIB-0024-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


