Abstract
Objective:
To evaluate the efficacy and learning curve of ultrasoundguided vacuum-assisted excision (US-VAE) of benign breast lesions, and to assess characteristics associated with residual lesion.
Methods:
This was a retrospective study with institutional review board-approval. Sonographic and clinical follow-up were performed 6 months after intervention. Effectiveness and safety of the technique were analyzed. The cumulative summation (CUSUM) graphs were used to evaluate learning curves concerning complete excision and hematoma.
Results:
152 ultrasound-VAEs in 143 patients were included. Initial complete resection was achieved in 90.8 % (138 of 152). 6-month follow-up was completed for 143 (94%) of cases and complete resection was observed in 72 % (100 of 143). Mean maximum size without residual tumor was 16.9 mm, while with residual lesion it was 21.9 mm (p = < 0.001), with a volume of 1.53 and 3.39 cm3, respectively (p = < 0.001). Increase in lesion size and volume was associated with less effectiveness (p = 0.05), clinical control (p = 0.05), and higher risk of clinically significant hematoma (p = 0.05). Receiver operating characteristic analysis demonstrate a volume threshold of 2.6 cm3 (r = 0.71, specificity 84.5%) for leaving no residual lesion. Cumulative summation graphs demonstrate that, on average, 11 excisions were required to acquire skills to perform complete excision in more than 80% at the end of the ultrasound-VAE and 18 excisions at 6 months.
Conclusion:
Ultrasound-VAE is an effective treatment for benign breast lesions. Breast lesion volume should be considered when assessing for percutaneous treatment.
Advances in knowledge:
A follow-up of the learning process of ultrasound-VAE will be a valuable tool to assess the efectiveness and safety of the technique i
Introduction
Ultrasound-guided vacuum assisted excision (US-VAE) is a recognized minimally invasive therapeutic alternative for treatment of benign breast lesions.1–3 Complete excision effectiveness has been reported to be in a range from 70 to 100%.4–10 Among published series, initial palpation of the mass varies between 13.8 and 88%,3,8,10–14 while others have been reported in non-palpable tumors without prior biopsy, with a subsequent large proportion of cysts and fibrocystic changes in the definitive histological report.
Regarding follow-up and residual tumor, sonographic evaluation at >6 months allows for a better assessment of complete resection and effectiveness of the treatment. However, studies reporting long-term sonographic assessment of percutaneous treatment demonstrate heterogeneous results with complete excision rates of 38–100%3,7,8,10–12,15
Advantages over conventional surgery have been shown in the meta-analysis by Ding et al16 where no difference in residual lesion or hematoma using Mammotome® were found. They found smaller scar size, bleeding, and surgical time; however, other vacuum-assisted breast biopsy (VAB) systems such as EnCor® or Vaccora® were excluded. In addition, the great heterogeneity of results, lesions, and patients between studies makes them difficult to compare.
Factors that predict complete excision have been described. Mass size is an independent predictor of initial complete excision and at 6 months post treatment, with a higher proportion of residual lesions by ultrasound in the larger masses.8,17,18 Partial excision rate increases in case of lesion greater than 2 cm.3,8
Currently, there are no well-established patient selection criteria or standards for effectiveness, safety or follow-up, to allow quality assessment of the procedure. In addition, objective criteria have not been established regarding training required to acquire US-VAE performance sufficiency or learning curves to determine skills acquisition or maintenance.
Only one study that evaluated US-VAE learning using the “moving average curves (MAC)” method.19 However, learning was not measured in terms of complete resection or effectiveness, but rather in terms of timing and adequate positioning of the needle under the mass, which do not guarantee complete excision.
Therefore, the aim of this study is to determine the efficacy of ultrasound-VAE for the treatment of benign breast lesions assessing chtaracteristics associated with partial excision, and to propose a learning curve methodology that will help to evaluate the number of cases needed to achieve effectiveness and performance according to objective quality criteria.
Methods and Materials
Study design
This is a single-center retrospective study approved by the Institutional Ethics Committee. Females with a benign breast lesion who had an indication for ultrasound-VAE and treated from March 1, 2012 to February 26, 2016, were included. Indications for treatment included palpable lump, increase in size, pain, nipple discharge and anxiety referred by the patient. All patients had evaluation by a breast surgeon and a breast radiologist with informed consent for the intervention.
Breast imaging
Lesion characteristics including shape, location, size, and distances to skin and pectoralis major muscle were determined by ultrasound assessment. Mass volume was calculated according to the equation V = 4/3 × π × (A/2 × B/2 × C/2) (A: longitudinal, B: transverse, C: anteroposterior), used by Kim et al.8
Percutaneous procedure
The MyLab™ 25 Gold ultrasound system (Esaote SpA, Genoa, Italy) was used for all interventions. All VAEs were performed with the SenoRx Encor Vacuum Aspiration Biopsy System (CR Bard, Murray Hill, NJ), using predominantly a 10 G needles and two cases with 7G. All percutaneous excisions were performed by radiologists fully dedicated to breast imaging with more than 2 years of experience in breast interventions.
Patients were placed in supine position with their arm behind their head. The mass was located with the transducer parallel to its major axis and after local anesthesia, the biopsy needle was placed under the lesion ensuring continuous ultrasound visualization of the tip.
Once the correct positioning of the needle and its aperture were confirmed, multiple biopsy cores samples were obtained under continuous ultrasound visualization. The intervention finished when complete resection was achieved or due to complication. Local compression was done for 5 min and patients were discharged with a compressive bandage. Tissue obtained was sent fresh to pathologic diagnosis. Number of cores, time of the procedure, complete excision, and complications were recorded.
Follow-up
Clinically significant hematoma (blood collection greater than 3 cm and clinically palpable) was assessed by ultrasound performed 24 h after the excision. Patients were followed at 6 months by physical exam and breast US.
Statistical analysis
Breast lesions and patient characteristics were analyzed. A univariate descriptive analysis of the variables of interest was performed and then a multivariate analysis between main outcomes (complete/incomplete mass excision, clinical control of symptoms and hematoma) was done.
Χ2 test and Fisher’s exact test were used to analyze the relationship between categorical variables. In the case of bivariate analysis between categorical and quantitative variables, the Students t-test or U-test of Mann–Whitney were applied according to the normality conditions. Additionally, ROC curves were determined to evaluate possible cut-off points to predict residual lesion.
Learning curves
The cumulative summation (CUSUM) model20–22 was used to create and to assess learning curves for Ultrasound-VAE. The CUSUM analysis is based on the sequential cumulative sum of results in a process over a period of time, evaluating performance variations according to objective standard criteria for failure or success. This method has proven to be useful for assessing processes concerning learning and health.20,23
We calculated and assessed the CUSUM curves of complete excision at the end of the intervention, complete excision at 6 months and clinically significant hematoma at 24 h post-VAE, retrospectively. Sonographic assessment was used to determine the presence or absence of residual lesion and hematoma.
The CUSUM value (s), the performance acceptable boundary (H0) and performance unacceptable boundary (H1) were calculated with a confidence level of α = 0.05 and β = 0.2, according to the formulae described in literature.20–22 We defined acceptable failure rate (p0) for complete excision as the presence of residual lesion in less than 20% of procedures and unacceptable failure rate (p1) as residual tumor in more than 40% of interventions. The acceptable failure rate for significant hematoma was defined as hematoma >30 mm in less than 15% of procedures and unacceptable failure rate as hematoma >30 mm in more than 30%.
The number of cases required to evaluate p0 and p1 were calculated. Finally, the CUSUM graphs of those radiologists who had met the total of needed cases (calculated number of cases for p0 and p1) for analysis were included.
Results
Patients
A total of 152 Ultrasound-VAE in 143 females were included in the study. Mean age was 36.9 years old (17–78 years). Mean mass size was 18.2 mm (4–45 mm) with an average volume of 2.1 cm3. Lesions were classified into three categories according to their maximum diameter: 50 (32.9%) were less than 16 mm, 88 (57.9%) between 16 and 25 mm and 14 (9.2%) greater than 25 mm. The most common indication for excision was palpable mass in 105 (69%) patients and patient´s anxiety in 90 (59%). Patients and tumor characteristics are summarized in Table 1.
Table 1.
Patients and tumor main characteristics
| Characteristics | Total | |
| n = 152 | ||
| n | % | |
| Patient | ||
| Multiple breast lesions | 62 | 40.8 |
| Previous breast surgery | 31 | 20.4 |
| Family breast cancer | 42 | 27.6 |
| Breast | ||
| Left | 79 | 52.0 |
| Right | 73 | 48.0 |
| Lesion’s shape | ||
| Oval | 89 | 58.6 |
| Round | 13 | 8.6 |
| Lobulated | 29 | 19.1 |
| Irregular | 21 | 13.8 |
| Mamographic density (ACR) | ||
| A | 2 | 1.6 |
| B | 35 | 28.4 |
| C | 59 | 48.0 |
| D | 27 | 22.0 |
| Without mammography | 29 | 19.1 |
| Clinical indication for excfision | ||
| Palpable mass (by patient) | 105 | 69.1 |
| Anxiety | 90 | 59.2 |
| Increase in size | 43 | 28.3 |
| Pain | 37 | 24.3 |
| Discharge | 8 | 5.3 |
| Re-excision | 5 | 3.3 |
| Mean | Range | |
| Ultrasound | ||
| Maximun size (mm) | 18.18 | 4.0; 45.0 |
| Volume (cm3) | 2.06 | 0.03; 27.3 |
| Distance to skin (mm) | 6.28 | 1.0; 70.0 |
| Distance to pectoral (mm) | 6.32 | 0.0; 50.0 |
| Clasification by size | ||
| <16 mm | 50 | 32.9 |
| 16–25 mm | 88 | 57.9 |
| >25 mm | 14 | 9.2 |
ACR, American College of Radiology.
Procedure ultrasound-VAE
The majority of the excisions, 150 (98.7%) were performed with a 10G needle because 7G was not available at Hospital Vall d'Hebron s supply catalog at the time of the study. We only had two patients who were treated with 7G as an exception because of the size of their lesions (32 mm and 45 mm). Mean VAB sampling time was 16.8 min with 25 cores per lesion. Complete excision was achieved in 138 (90.8%) cases, whereas in 14 (9.2%) the intervention finished with partial excision due to: 4 vasovagal reactions, 3 active bleeding, 1 skin injury and 3 cases with high risk of skin damage. Pain was reported as mild (0–3/10) in 139 (91.4%) patients and moderate (4-7/10) in 12 (7.9%) patients.
Pathology
Prior to performing the ultrasound-VAE 141 patients (92.8%) had benign diagnosis made by core needle biopsy, whereas 11 (7.2%) patients had lesions considered benign by previous radiological surveillance. Neither lesions with atypia nor carcinomas were included. Fibroadenoma was found in the final pathology report in 73.7% of the cases. Three lobular carcinoma in situ and one flat epithelium atypia were reported, all of them with sonographic and MRI assessment and follow-up at 6 months without residual tumor. Only one patient was found to have focal micro invasive lobular carcinoma (0.7% of cases) in the final pathology and she was treated with lumpectomy and sentinel node without residual tumor.
6-month follow-up
Out of 152 ultrasound-VAE, 143 (94%) had sonographic and clinical follow-up at 6 months. Complete excision was confirmed by ultrasound in 103 (72.0%) cases. Masses with partial excision at 6 months had significantly greater size and volume. The mean maximum size of masses without residual tumor was 16.9 mm, while with residual lesion were 21.9 mm (p≤ 0.001), with a volume of 1.53 and 3.39 cm3 respectively (p ≤ 0.001). In addition, a significant difference regarding complete resection by size groups was found (p = 0.003). There was no significant difference when comparing residual lesion and breast density. Radiological and clinical 6 month follow-up results comparing complete vs partial excision are shown in Table 2.
Table 2.
Radiologic and clinical follow-up after 6 months
| Characteristics before ultrasound-guided VAE | 6 months ultrasound follow-up | 6 months Clinical follow-up | ||||||||
| Complete excision (n = 103) | Residual lesion (n = 40) | p-value |
Asymptomatic (n = 126) |
Symptomatic (n = 17) |
p-value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 36.84 | (10.48) | 35.10 | (11.89) | 0.198 | 36.3 | (10.48) | 36.8 | (11.89) | 0.997 |
| Ultrasound | ||||||||||
| Maximun size (mm) | 16.85 | (6.19) | 21.85 | (7.68) | <0.001 | 17.58 | (6.19) | 23.19 | (7.68) | 0.014 |
| Volumen (cm3) | 1.53 | (1.30) | 3.39 | (4.49) | <0.001 | 1.68 | (1.30) | 4.59 | (4.49) | 0.016 |
| Distance to skin (mm) | 6.38 | (7.46) | 6.05 | (6.26) | 0.344 | 6.60 | (7.46) | 3.96 | (6.26) | 0.025 |
| Distance to pectoral (mm) | 6.07 | (6.10) | 6.64 | (6.14) | 0.864 | 6.21 | (6.10) | 6.34 | (6.14) | 0.898 |
| n | % | n | % | n | % | n | % | |||
| Clasification by size | ||||||||||
| <16 mm | 40 | (87.0) | 6 | (13.0) | 0.003 | 44 | (95.6) | 2 | (4.4) | 0.042 |
| 16–25 mm | 57 | (68.7) | 26 | (31.3) | 72 | (86.8) | 11 | (13.2) | ||
| >25 mm | 6 | (42.9) | 8 | (57.1) | 10 | (71.4) | 4 | (28.6) | ||
| Palpable lump (by surgeon) | ||||||||||
| No | 10 | (71.4) | 4 | (28.6) | 1.000 | 11 | (78.6) | 3 | (21.4) | 0.378 |
| Yes | 93 | (72.1) | 36 | (27.9) | 115 | (89.2) | 14 | (10.8) | 0.998 | |
| Mamographic density (ACR) | ||||||||||
| A | 1 | (100.0) | 0 | (0.0) | 0.534 | 1 | (100.0) | 0 | (0.0) | 0.488 |
| B | 26 | (76.5) | 8 | (23.5) | 29 | (85.3) | 5 | (14.7) | ||
| C | 41 | (73.2) | 15 | (26.8) | 52 | (92.9) | 4 | (7.1) | ||
| D | 16 | (59.3) | 11 | (40.7) | 22 | (81.5) | 5 | (18.5) | ||
| Without mammography | 19 | (76.0) | 6 | (24.0) | 22 | (88.0) | 3 | (12.0) | ||
ACR, American College of Radiology; SD, standard deviation;VAE, vacuum-assisted excision.
The correlation analysis between the lesion’s maximum size and sonographic residual at 6 months to predict complete excision showed a ROC curve with an area under the curve (AUC) of 0.698, with a Youden cut-off point of 20.0 mm (sensitivity 62.5% and specificity 68.0%). A similar analysis with mass volume showed an AUC of 0.697 with Youden cut-off of 2.6 cm3 (sensitivity 50.0% and specificity 84.5%).
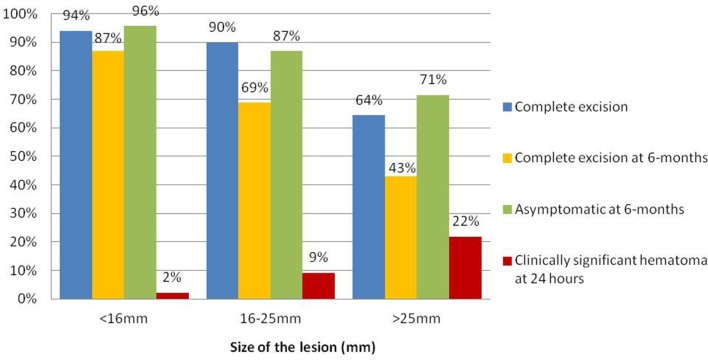
At 6 months, 128 (88.1%) patients treated were asymptomatic and without a palpable lump. Assessing by size groups, 44 (95.6%) of the patients with lesions <16 mm were asymptomatic, whereas in tumors of 16–25 mm and >25 mm the proportion was 72 (86.6%) and 10 (71.4%) patients respectively (p = 0.042). Females with persistent palpable lump after treatment had masses closer to the skin, with a mean distance of 3.4 mm vs 6.6 mm in asymptomatic females (p = 0.0245). Only 3.9% of the patients with complete excision remained symptomatic during the follow-up, while 67.5% of the patients with partial excision (n = 40) were asymptomatic despite having small residual lesions by ultrasound.Figure 1s the differences of complete resection, hematoma and clinical control according to tumor size is shown inFigure 1
Figure 1. .
Radiological and clinical effectiveness of ultrasound-VAE by tumor size
Learning curves
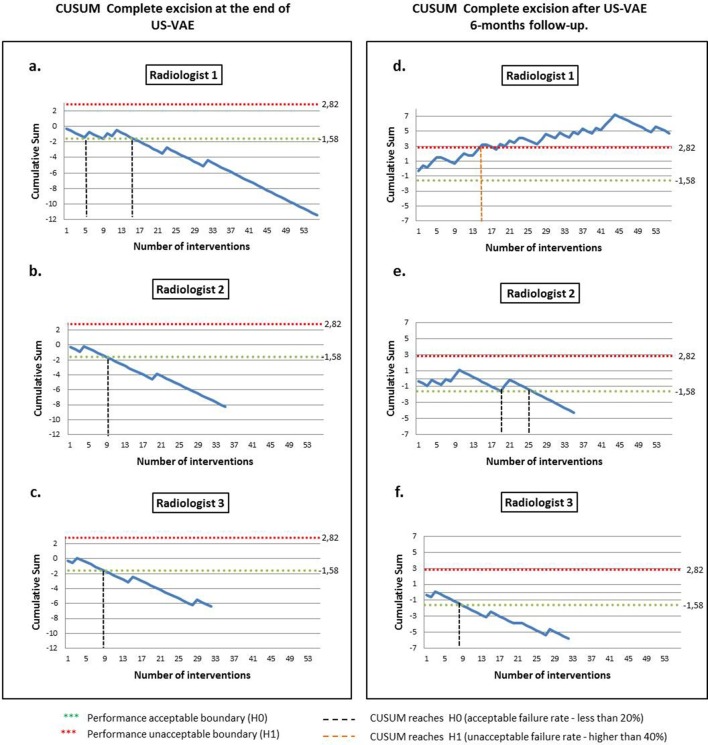
The estimated number of US-VAE calculated to assess complete excision with the CUSUM model were 18 cases for p0 and 24 cases for p1. When evaluating significant hematoma, 26 and 35 cases were estimated respectively. For that reason, we limited the analysis to three radiologists who had performed more than 30 US-VAE: R1 (56 cases), R2 (35 cases), R3 (32 cases).
Figure 2 shows the CUSUM analysis for complete excision at the end of US-VAE and at the 6 month follow-up. R1 achieved the performance acceptable boundary (H0) after 16 cases, while R2 and R 3 after 9 cases. Taken into account these results, on average 11 US-VAE were required to overcome H0, and to be able to acquire skills with more than 80% of complete excision effectiveness. None of them reached the performance unacceptable boundary (H1).
Figure 2. .
CUSUM analysis of complete excision rate of bengin breastlesions treated by ultrasound-VAE. Acceptable excision rate more then 80%with unacceptable failure rate of 40%. Type 1 error (α) of 0.05 and type 2 error (β) of 0.2. Radiologist 1 (a, d). Radiologist 2 (b, e). Radiologist 3 (c, f). CUSUM, cumulative summation; US-VAE, ultrasound-guided vacuum-assisted excision.
At 6 months R1 failed to achieve H0, but more importantly,R1 crossed the H1 after 16 interventions, which led us to conclude that his/her skills and competence performing ultrasound-VAE differs from our proposed standards, performing with less than 60% of complete excision effectiveness.
R2 approached H0 in attempt 19, but only kept acceptable performance after excision 26. R3 achieved H0 after 9 US-VAE. Both R2 and R3 kept an acceptable failure rate after crossing H0 during their remaining interventions. With this information, on average 18 excisions were required to achieve H0 at 6 months.
The CUSUM analysis for significant hematoma formation at 24 h showed that on average 14 interventions were required to reach H0, confirming skills acquisition for performing US-VAE with less than 15% significant hematoma post-intervention.
Because of the different results observed in CUSUM graphs, an analysis for each radiologist was done. We found significant differences in complete excision (p = 0.004) and clinical control (p = 0.019) at 6 months by radiologist. Radiologist’s mean VAB time expended per intervention differed significantly (p < 0.001), with R1 taking less time per VAE without differences in either mass mean size or mean VAB cores per lesion. Table 3 shows the differences between radiologists.
Table 3.
Differences in tumor and procedure characteristics discriminated by radiologists
| Benign lesion treated by ultrasound-guided VAE | Radiologist 1 | Radiologist 2 | Radiologist 3 | p-value | |||
| (n = 55) | (n = 35) | (n = 32) | |||||
| Mean | Range | Mean | Range | Mean | Range | ||
| Size (mm) | 17.5 | (4.0–35.0) | 19.1 | (7.0–45.0) | 17.3 | (6.0–34.0) | 0.576 |
| Volume (cm3) | 1.7 | (0.03–10.0) | 2.71 | (0.09–27.3) | 1.9 | (0.07–6.6) | 0.407 |
| Distance to skin (mm) | 5.6 | (1.0–20.0) | 4.3 | (1.0–14.0) | 5.9 | (1.5–15.0) | 0.111 |
| Distance to pectoralis (mm) | 7.2 | (1.0–30.0) | 5.3 | (0.0–22.0) | 4.6 | (1.0–9.0) | 0.010 |
| VAB cores number | 25 | (2–126) | 25 | (2–136) | 23 | (6–86) | 0.911 |
| VAB time (min) | 12.6 | (2–35) | 16.4 | (2–60) | 19.5 | (7–50) | <0.001 |
| Haematic colection at 24 h (mm) | 17.6 | (0.0–73.0) | 15.1 | (0.0–40.0) | 16.3 | (7.0–25.0) | 0.546 |
| n | % | n | % | n | % | ||
| Complete excision at 24 h | 49 | (87.5) | 31 | (88.6) | 30 | (93.8) | 0.699 |
| Complete excision at 6 monthsa | 31 | (60.8) | 27 | (79.4) | 27 | (90.0) | 0.004 |
| Clinically asymptomatic at 6 monthsa | 41 | (80.4) | 30 | (88.2) | 29 | (96.7) | 0.019 |
VAE, vacuum-assisted excision.
p bold are statistically significant.
Radiologist 1 (n = 52), Radiologist 2 (n = 34), Radiologist 3 (n = 30).
Discussion
Our study has shown that the US-VAE is an effective method to excise benign breast lesions. We confirm that nodule size and volume are independent predictors of complete resection, with a higher proportion of residual in larger nodules.17,18,24
Kim et al8 reported that the initial size is the only variable that correlates significantly with recurrence. Our estimated cut-off size of the initial lesion capable of predicting residuals at 6 months is 20 mm and the cut-off volume is 2.6 cm3. Both parameters have low sensitivity although volume has a greater specificity, allowing prediction of residual mass below 2.6 cm3 in 84.5% of cases. Our results are similar to those reported by Papathermelis et al who achieved 86.7 % complete excision for lesions smaller than 2.51 cm3.25
We observed significant differences in resection between size groups. There are no other studies discriminating masses between 16 25 mm, which are probably the main target lesions for this kind of treatment. Masses bigger than 25 mm are associated with higher residuals, however up to 67.5% of these patients were asymptomatic regardless of incomplete resection.
Despite a general higher complete resection rate (90.8%) our effectiveness decreased to 72% during the follow-up. This is similar to other reports with complete resection rate at 6–12 months of 38–100%.3,7,8,10–12,14,15 Our average size is comparable to the work of Kim et al8 who used a Mammotome in tumors with average size of 19 mm, achieving 61% complete resection at 6 months.
Our results are probably more comparable with those reported by Wang et al10 who use the EnCor system describing complete resection in 93% at long-term. However, they used a 7G gauge needles in 44% of cases.
Main limitations of the published data are heterogeneous results concerning effectiveness of US-VAE with lack of established criteria for treatment, training and quality evaluation criteria. Our results confirmed significant differences during follow-up according to tumor size groups and radiologists. For that reason, we also evaluated radiologist’s learning curves using de CUSUM model in order to predict skills acquisition and their maintenance within our proposed quality standard.
There were similar results at the end of US-VAE among radiologists, however significant differences were found after 6 months. This situation not only confirms, but in part explains the high variability of effectiveness rates when evaluating US-VAE. Outcomes after the procedure need to be established more than just focusing on learning how to locate the needle. This is important concerning treatment of benign lesions but could be also relevant regarding the possible application for high risk lesions.
Park et al19 evaluate learning of excision with VAB using the MAC from one subject 105, but needle positioning and less time expended do not guarantee success. Michalopoulos et al26 mention learning curves for fellows in breast imaging but they do not report percentages of success or failures. Some authors report that 5 to 15 procedures of VAB are necessary to achieve adequate learning,27 while others consider weeks to learn interventional techniques, including percutaneous excision,28 but there is no standard assessment criteria or established outcome monitoring.
To our knowledge, this is the first study that proposes standardized criteria using the CUSUM model to determine and to evaluate the learning curve of US-VAE. Our results show that the CUSUM model is a sensitive tool to identify when a radiologist reaches adequate performance. We propose that radiologists have acquired an acceptable skill if they demonstrate complete excision rate higher than 80%, requiring initially according to our results, around 11 cases. However, long-term adequate effectiveness learning might need around 18 cases.
Additionally, to apply this model we defined a critical unacceptable effectiveness boundary of complete excision rate of less than 60%, in which the learning process must be evaluated and corrected. This is probably the most important information because it allows for correction of the learning process and mistakes. The key regarding monitoring and assessment is to identify factors that may be influencing the final outcome. No differences in size, volume, biopsy cylinders or significant distances to skin were found among radiologists. We found shorter time per excision by Radiologist 1 that would be a factor to be taken into account. If we had corrected Radiologist 1’s failure tendency, our global effectiveness could have been better. Assessment of learning curves by the CUSUM model and establishment of quality standard criteria concerning US-VAE might help to improve effectiveness of this therapeutic alternative.
Our results will help us to improve the right selection of cases for percutaneuous treatment in order to avoid partial results in the long term. In addition, time spent for each case and additional core samples after considering complete removal should be evaluated. We believe that using prospective learning curves and surveillance of all radiologists in our service concerning US-VAE will allow earlier identification of possible causes of low efficacy or safety as a quality control in daily practice.
CUSUM analysis and learning curves could be applied to monitor training of US-VAE but also could be useful to assess skill acquisition in other breast interventions. Combining use of phantoms to perform repetitive training sessions with analysis of data with CUSUM can be an easier and faster way to create learning curves in a short period of time for training of residents, fellows or breast radiologists who want to learn vacuum excision. Learning curves can be followed prospectively or in real time in a personalized setting by using a mobile application which facilitates registration, analysis of data and graph construction. These would allow consistent registration of data and follow-up evolution of training in a personalized way.
There are some limitations in our study such as the number of interventions included and our limited availability of 7G needles. The learning curve analysis was done retrospectively so there was no room for improvement during the study, a prospective CUSUM analysis might contribute to improve our effectiveness. Finally, other variables such as pain, time expended, symptoms and patient satisfaction outcomes should be included when evaluating the CUSUM success or failure rate.
Conclusion
The effectiveness of US-VAE to achieve complete excision at 6 months varies according to tumor size and the learning curve. The CUSUM model and the quality criteria proposed allow assessment of skills acquisition and performance when using percutaneous excision.
Footnotes
Conflict of interest: Dr Salazar declares that he is consultant for BARD España S.L.U.
Contributor Information
Juan Pablo Salazar, Email: jpsalaza@vhebron.net.
Ignacio Miranda, Email: imiranda@vhebron.net.
Juan de Torres, Email: jtorres@vhebron.net.
Martin Espinosa-Bravo, Email: maespino@vhebron.net.
Rafael Salvador, Email: rafasalvador@imaginebarcelona.com.
Isabel T Rubio, Email: irubior@unav.es.
REFERENCES
- 1.Park HL, Kim LS. The current role of vacuum assisted breast biopsy system in breast disease. J Breast Cancer 2011; 14: 1–7. doi: 10.4048/jbc.2011.14.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson AT, Henry-Tillman RS, Smith LF, Harshfield D, Korourian S, Brown H, et al. Percutaneous excisional breast biopsy. Am J Surg 2002; 184: 550–4. doi: 10.1016/S0002-9610(02)01099-1 [DOI] [PubMed] [Google Scholar]
- 3.Yom CK, Moon BI, Choe KJ, Choi HY, Park YL. Long-term results after excision of breast mass using a vacuum-assisted biopsy device. ANZ J Surg 2009; 79: 794–8. doi: 10.1111/j.1445-2197.2009.05103.x [DOI] [PubMed] [Google Scholar]
- 4.Fine RE, Boyd BA, Whitworth PW, Kim JA, Harness JK, Burak WE. Percutaneous removal of benign breast masses using a vacuum-assisted hand-held device with ultrasound guidance. Am J Surg 2002; 184: 332–6. doi: 10.1016/S0002-9610(02)00951-0 [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Kim EK, Kim MJ, Moon HJ, Yoon JH. Vacuum-assisted breast biopsy under ultrasonographic guidance: analysis of a 10-year experience. Ultrasonography 2014; 33: 259–66. doi: 10.14366/usg.14020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyss P, Varga Z, Rössle M, Rageth CJ. Papillary lesions of the breast: outcomes of 156 patients managed without excisional biopsy. Breast J 2014; 20: 394–401. doi: 10.1111/tbj.12283 [DOI] [PubMed] [Google Scholar]
- 7.Li S, Wu J, Chen K, Jia W, Jin L, Xiao Q, et al. Clinical outcomes of 1,578 Chinese patients with breast benign diseases after ultrasound-guided vacuum-assisted excision: recurrence and the risk factors. Am J Surg 2013; 205: 39–44. doi: 10.1016/j.amjsurg.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Park BW, Kim SI, Youk JH, Kwak JY, Moon HJ, et al. Long-term follow-up results for ultrasound-guided vacuum-assisted removal of benign palpable breast mass. Am J Surg 2010; 199: 1–7. doi: 10.1016/j.amjsurg.2008.11.037 [DOI] [PubMed] [Google Scholar]
- 9.Thurley P, Evans A, Hamilton L, James J, Wilson R. Patient satisfaction and efficacy of vacuum-assisted excision biopsy of fibroadenomas. Clin Radiol 2009; 64: 381–5. doi: 10.1016/j.crad.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 10.Wang ZL, Liu G, Li JL, Ding Q, Su L, Tang J, et al. Sonographically guided percutaneous excision of clinically benign breast masses. J Clin Ultrasound 2011; 39: 1–5. doi: 10.1002/jcu.20752 [DOI] [PubMed] [Google Scholar]
- 11.Wang ZL, Liu G, Huang Y, Wan WB, Li JL. Percutaneous excisional biopsy of clinically benign breast lesions with vacuum-assisted system: comparison of three devices. Eur J Radiol 2012; 81: 725–30. doi: 10.1016/j.ejrad.2011.01.059 [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Bartolomé P, Vega-Bolívar A, Torres-Tabanera M, Ortega E, Acebal-Blanco M, Garijo-Ayensa F, et al. Sonographically guided 11-G directional vacuum-assisted breast biopsy as an alternative to surgical excision: utility and cost study in probably benign lesions. Acta Radiol 2004; 45: 390–6. doi: 10.1080/02841850410005633 [DOI] [PubMed] [Google Scholar]
- 13.Povoski SP, Jimenez RE. A comprehensive evaluation of the 8-gauge vacuum-assisted Mammotome(R) system for ultrasound-guided diagnostic biopsy and selective excision of breast lesions. World J Surg Oncol 2007; 5: 83. doi: 10.1186/1477-7819-5-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo HJ, Chen X, Tu G, Wang J, Wu CY, Yang GL. Therapeutic application of ultrasound-guided 8-gauge Mammotome system in presumed benign breast lesions. Breast J 2011; 17: 490–7. doi: 10.1111/j.1524-4741.2011.01125.x [DOI] [PubMed] [Google Scholar]
- 15.Slanetz PJ, Wu SP, Mendel JB. Percutaneous excision: a viable alternative to manage benign breast lesions. Can Assoc Radiol J 2011; 62: 265–71. doi: 10.1016/j.carj.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 16.Ding B, Chen D, Li X, Zhang H, Zhao Y. Meta analysis of efficacy and safety between mammotome vacuum-assisted breast biopsy and open excision for benign breast tumor. Gland Surg 2013; 2: 69–79. doi: 10.3978/j.issn.2227-684X.2013.05.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko EY, Bae YA, Kim MJ, Lee KS, Lee Y, Kim LS. Factors affecting the efficacy of ultrasound-guided vacuum-assisted percutaneous excision for removal of benign breast lesions. J Ultrasound Med 2008; 27: 65–73. [DOI] [PubMed] [Google Scholar]
- 18.Krainick-Strobel U, Huber B, Majer I, Bergmann A, Gall C, Gruber I, et al. Complete extirpation of benign breast lesions with an ultrasound-guided vacuum biopsy system. Ultrasound Obstet Gynecol 2007; 29: 342–6. doi: 10.1002/uog.3840 [DOI] [PubMed] [Google Scholar]
- 19.Park HS, Jeon CW. Learning curve for breast mass excision using a vacuum-assisted biopsy system. Minim Invasive Ther Allied Technol 2014; 23: 235–40. doi: 10.3109/13645706.2014.894918 [DOI] [PubMed] [Google Scholar]
- 20.Noyez L. Control charts, Cusum techniques and funnel plots. A review of methods for monitoring performance in healthcare. Interact Cardiovasc Thorac Surg 2009; 9: 494–9. doi: 10.1510/icvts.2009.204768 [DOI] [PubMed] [Google Scholar]
- 21.Yap CH, Colson ME, Watters DA. Cumulative sum techniques for surgeons: a brief review. ANZ J Surg 2007; 77: 583–6. doi: 10.1111/j.1445-2197.2007.04155.x [DOI] [PubMed] [Google Scholar]
- 22.David O, Ospina A, Á M, Medina R, Marulanda MC, María L. Revista colombiana de anestesiología colombian journal of anesthesiology investigación científicay tecnológica curvas de aprendizaje de sumatoria acumulada (CUSUM) en procedimientos básicos de anestesia. Rev Colomb Anestesiol 2014; 42: 142–53. [Google Scholar]
- 23.Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care 2000; 12: 433–8. doi: 10.1093/intqhc/12.5.433 [DOI] [PubMed] [Google Scholar]
- 24.Torres-Tabanera M, Mata-Olmo M, Sánchez-Gómez S, Tejerina-González E, Sainz-Miranda M, Lag-Asturiano E. Vacuum assisted biopsy for percutaneous treatment of fibro-epithelial breast lesions in non-elective cases: study of 111 cases In: ECR 2011/C-1414; 2011. doi: 10.1594/ecr2011/C-1414 [DOI] [Google Scholar]
- 25.Papathemelis T, Heim S, Lux MP, Erhardt I, Scharl A, Scharl S. Minimally invasive breast fibroadenoma excision using an ultrasound-guided vacuum-assisted biopsy device. Geburtshilfe Frauenheilkd 2017; 77: 176–81. doi: 10.1055/s-0043-100387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalopoulos NV, Maniou I, Zografos GC. Breast lesion excision system biopsy: the learning curve. AJR Am J Roentgenol 2012; 199: W667. doi: 10.2214/AJR.12.9154 [DOI] [PubMed] [Google Scholar]
- 27.Zografos GC, Zagouri F, Sergentanis TN. Vacuum-assisted breast biopsy: an easy-to-learn procedure? Am J Surg 2008; 196: 798. doi: 10.1016/j.amjsurg.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 28.Holmes DR, Silverstein MJ. A minimally invasive breast biopsy clinic: an innovative way to teach breast fellows how to perform breast ultrasound and ultrasound-guided breast procedures. Am J Surg 2006; 192: 439–43. doi: 10.1016/j.amjsurg.2006.06.007 [DOI] [PubMed] [Google Scholar]