Abstract
Three-dimensional (3D) printing technology in congenital cardiology and cardiac surgery has experienced a rapid development over the last decade. In presence of complex cardiac and extra-cardiac anatomies, the creation of a physical, patient-specific model is attractive to most clinicians. However, at the present time, there is still a lack of strong scientific evidence of the benefit of 3D models in clinical practice and only qualitative evaluation of the models has been used to investigate their clinical use. 3D models can be printed in rigid or flexible materials, and the original size can be augmented depending on the application the models are needed for. The most common applications of 3D models at present include procedural planning of complex surgical or interventional cases, in vitro simulation for research purposes, training and communication with patients and families. The aim of this pictorial review is to describe the basic principles of this technology and present its current and future applications.
Introduction
The possibility to create physical, patient-specific models by means of three-dimension (3D) printing is appealing when dealing with complex anatomies in paediatric and adult patients with congenital heart disease (CHD). Patients with CHD are regularly monitored with imaging, yet often a single imaging modality is not sufficient to fully appreciate the complexity of cardiac anatomy, physiology and extra cardiac spatial relationships. Moreover, these patients frequently undergo multiple surgeries with implantation of devices, increasing their complexity and resulting in unique anatomical variations. For these reasons, 3D printing has proved useful in congenital cardiology and cardiac surgery.1,2
Experts in non-invasive cardiovascular imaging have a great ability to understand cardiac anatomy from traditional cross-sectional images even in the presence of complex CHD. However, holding a physical 3D reconstruction of a patient’s anatomy can augment the understanding of spatial relationships and of the real dimensions of both cardiac and extra cardiac structures. Furthermore, 3D printing is likely to be even more helpful for physicians with less expertise in analysing and interpreting cardiovascular images, for instance cardiac surgeons and interventional cardiologists.
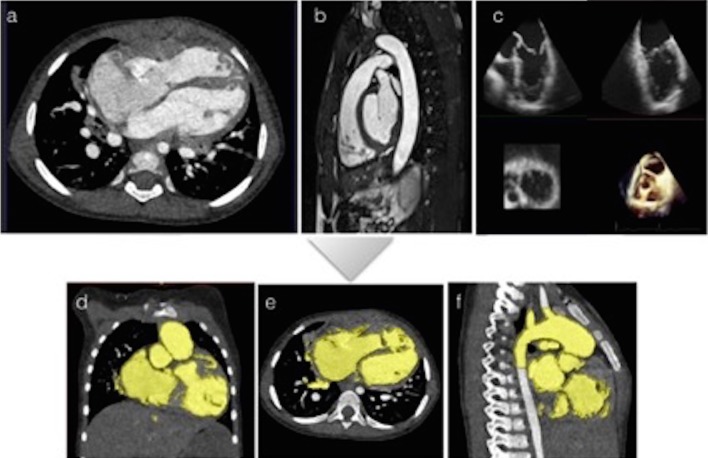
Generally, what can be imaged in 3D can be printed in 3D, so using 3D images from CT, MRI or 3D echocardiography as inputs, it is possible to produce 3D printed models of the entire heart or of a specific region of interest (Figure 1). Most commonly, CT images or 3D MRI images (whole-heart MRI or contrast-enhance MR angiography) are used to produce 3D models. Through an accurate post-processing analysis, or “segmentation” process, the images are transformed into 3D surface files (.stl) and sent to the printer (Figure 2). ECG-gated 3D balanced steady state free precession images usually have adequate quality to perform the segmentation process, however mobile structures such as valve leaflets cannot be adequately seen and segmented. Cardiac MRI has the advantage of being radiation free, however acquisition time are long and might not be well tolerated in paediatric patients. The use of current multidetector cardiac CT scans and non-ECG gated acquisitions allow to reduce the radiation exposure, maintaining a good spatial resolution. The final result will depend greatly on the quality of the input images and on the operator performing the segmentation, who needs to be able to fully understand the cardiac anatomy. This suggests either the need to train a 3D printing operator for specific cardiac applications or the importance of close liaison between bioengineers and clinicians when creating a model.
Figure 1.
All imaging modalities can be used input images for the creation of 3D models. (a) Non-gated cardiac CT images; (b) Balance SSFP with ECG gating and respiratory navigation can be used to acquire 3D images of the heart in cardiac MRI. (c) 3D echocardiography provides excellent visualization of the atrio-ventricular valves. Panels (d), (e) and (f) are examples of the segmentation process with a commercially available software (ScanIP, Synopsis, US) using tresholding and manual editing from multiplanar non-gated CT images of a patient with apical VSD. This step allows the operator to identify and separate the different cardiac and extracardiac structures: the blood pool usually present a higher signal intensity comparing to the myocardiaum and vascular walls and this automated software identifies the endocardial contours creating a colour mask (showed in yellow in panel d,e,f) that need to be carefully checked for accuracy by the operator. Only the volume included in the segmentation process, and highlighted in yellow, will be used for the creation of the model.
Figure 2.
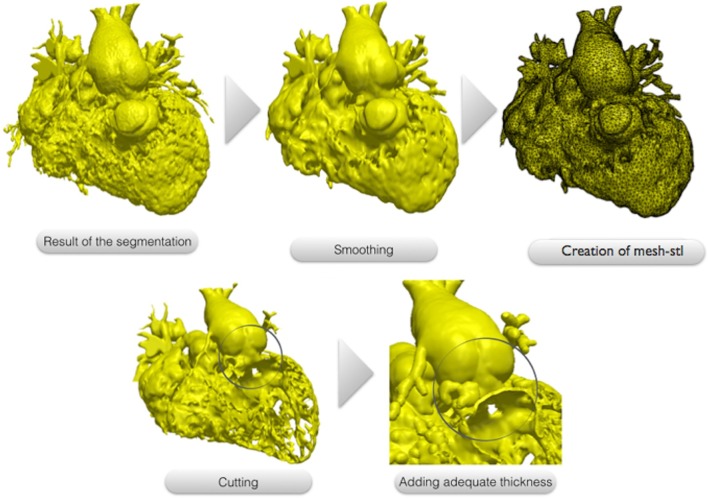
The 3D images resulting from the segmentation process usually present a dyshomogenous surface due to the presence of multiple trabeculations, mostly inside the right ventricle. This file is modified though a smoothing tool using a commercial available software (ScanIP, Synopsis, US) and converted to a surface mesh file called “.stl”. The surface can be cut exposing the region of interest for the surgeon. In our case, the right and left ventricle free walls have been removed to allow the visualization of the multiple apical VSD. Finally a 1.2 mm thickness is added to the surface to make it suitable for printing.
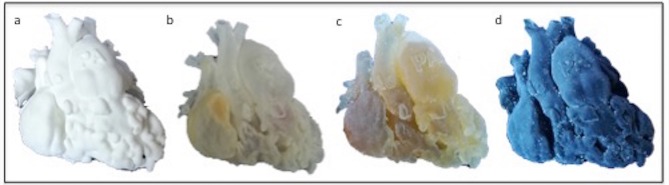
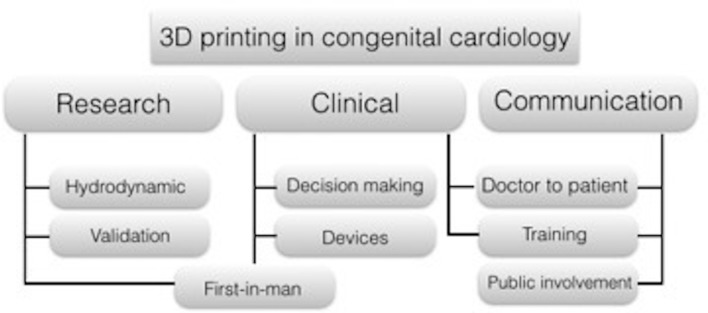
A variety of materials can be chosen for printing. Compliant rubber-like material or silicone are likely preferable for surgical practice, enabling cutting into the model, but are typically more expensive (Figure 3). The cost of the model however depends also on its size, and small 3D models are cheaper because a smaller amount of material is used for printing (Figure 4). Rigid materials (e.g. SLA resins) can be used for educational or communication purposes. Indeed, 3D models have been used for different purposes in CHD, including procedural planning, research applications, training and doctor-patient communication (Figure 5).
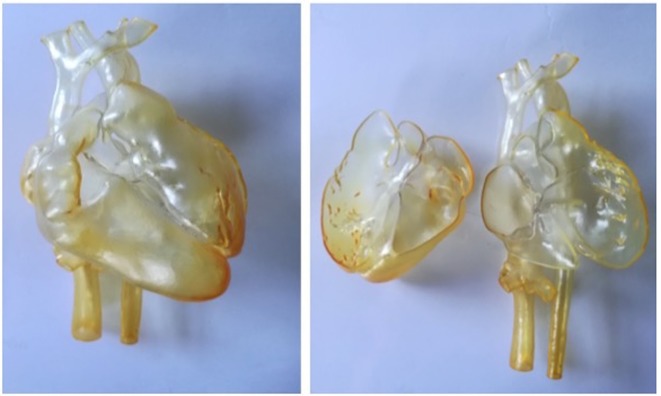
Figure 3.
Depending on the application of the model, different materials can be used. In this figure, the same heart of a 1-year-old patient with double outlet right ventricle was printed in (a) Nylon; (b) Transparent resin (c) Compliant rubber-like material and (d) Compliant rubber-like material black
Figure 4.

Foetal ultrasound images were used as input to create a 1:1 3D model of a foetal heart. Foetal heart models can be printed also in bigger scale to better appreciate small cardiac structures. In this image, a foetal heart model is compared to 1-penny coin (diameter 20.3 mm)
Figure 5.
Application of 3D models in congenital cardiology and cardiac surgery. Biomedical engineers use the physical model to create experimental set ups and test hydrodynamic conditions in patient specific settings; these setups are used also to validate computation models. Physical models are used for clinical purposes in surgical planning and decision-making of complex procedures, as well as to test new application of devices to patient specific anatomy. Medical students and trainee can benefit from the use of three-dimensional models during cardiac morphology courses as well as cardiac surgeon from practicing complex procedures. Comparing to medical images, physical models are much easier to understand for patients and parents.
Procedural planning
Patient-specific 3D printed models can be used to plan surgical and percutaneous interventions (Figures 6 and 7). Case reports and small case series have suggested that 3D printed models facilitate the decision-making process in complex cases.3 However, only qualitative analysis of the models by means of satisfaction questionnaires has so far been used to evaluate their benefit. A recent multicentre prospective study aimed to evaluate the impact of 3D printing models in planning 40 complex CHD surgeries, providing surgeons with a 3D printed model after a first multidisciplinary discussion and registering a change in surgical strategy in 19/40 cases.4 As for other smaller series, the main limitation of the study is that measurable outcomes, e.g. cross-clamp time, bypass time, days of hospital admission or mid- and long-term follow-up information, were not included. 3D printing has also been used successfully for percutaneous procedures, for instance adopting 3D printed models of right ventricular outflow tract in patients with pulmonary valve regurgitation as a tool to aid clinicians in selecting patients eligible for percutaneous pulmonary valve implantation.5 Meaningful clinical applications include:
Figure 6.
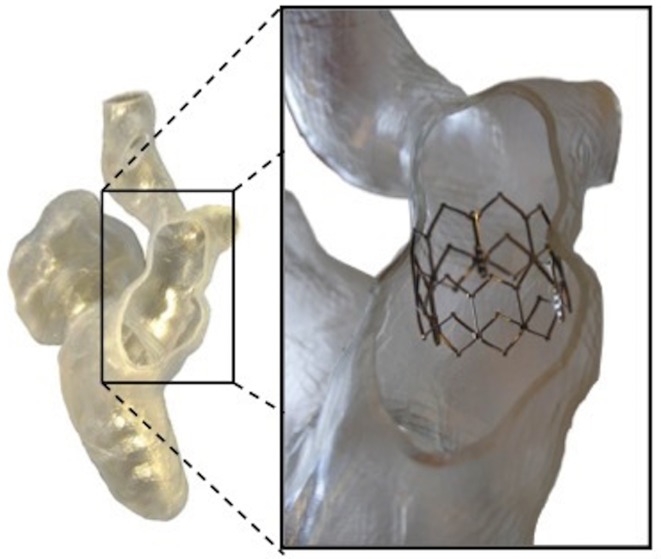
Patient specific model was used to test a range of devices potentially suitable for the patient case, checking geometrical anchoring and suitability to patient anatomy.
Figure 7.
3D model from MRI images of a 16-year-old patient with congenitally corrected transposition of the great arteries, ventricular septal defect and pulmonary stenosis who underwent multiple surgeries with interposition of a conduit between the right sided left ventricle and the main pulmonary artery. The 3D model was used to plan further intervention on the conduit.
Visualization of the size of the pathological structures in presence of rare congenital abnormalities (Figure 8).
Three-dimensional visualization of intra cardiac structures (Figure 9).
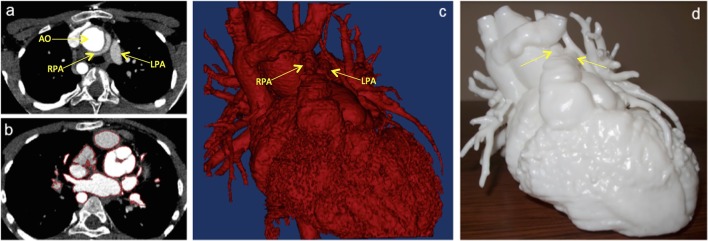
Understanding of the spatial relationship of the great vessels in cases of complex CHD, particularly in post surgical anatomies (Figure 10).6
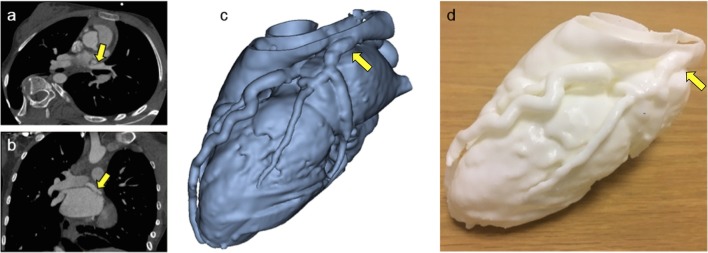
Figure 8.
A 14-year-old female patient who was referred for cardiac CT after echocardiography showing prominent coronary artery flow, suggesting presence of fistula or anomalous artery connection. CT images confirmed the diagnosis of anomalous origin of the circumflex coronary artery from right pulmonary artery (a and b). 3D model (c) was manufactured (d) for better understanding of coronary anatomy and dimensions.
Figure 9.
Complex congenital case: double outlet right ventricle with transposition of the great arteries and non-committed VSD, repaired with LV to aorta baffle presenting with symptoms of left ventricular outflow tract (LVOT) obstruction. Model manufactured on clinical request for assessment of LVOT anatomy. The yellow arrow shows the LV to aorta intracardiac baffle. (a,b) Two views from CT data, (c) 3D reconstruction, (d) 3D-printed model.
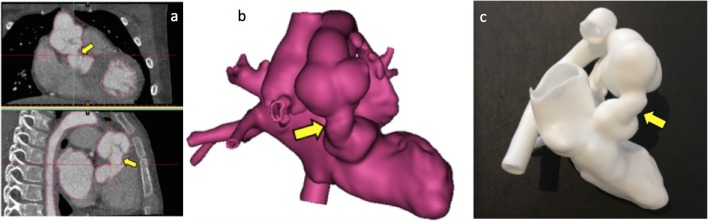
Figure 10.
Complex congenital case: A 11-year-old patient truncus arteriosus presenting with conduit and pulmonary stenosis. The model was used to assess the relationship between the aorta and the pulmonary arteries in order to plan the surgery. (a) CT images showing the position of the branch pulmonary arteries and the aorta. (b) Segmentation process to create the 3D model (highlighted in red); (c) 3D reconstruction used as input file to create the 3D model; (d) 3D model printed in white resin.
Research applications
Patient-specific 3D models can be incorporated in the context of experimental set-ups for research applications (Figure 11). Models also represent useful tools to validate computational simulations, with several studies reporting good correlations between patient-specific model simulations in vitro and in silico when assessing cardiovascular hydrodynamics in CHD,7 also accounting for the presence of devices, such as percutaneous valves.8
Figure 11.

Patient-specific model of right ventricular outflow tract is connected to a hydrodynamic circuit to simulate realistic flow and pressure conditions to be assessed with MRI acquisitions. These in vitro setups are used to simulate hydrodynamic conditions and study the complex physiology of patients with CHD, typically performing parametric studies. Mock circulatory systems incorporating patient-specific models can also be designed to be MRI compatible and thus allow for the acquisition of detailed visual information, such as 4D MRI flow sequences.
Training
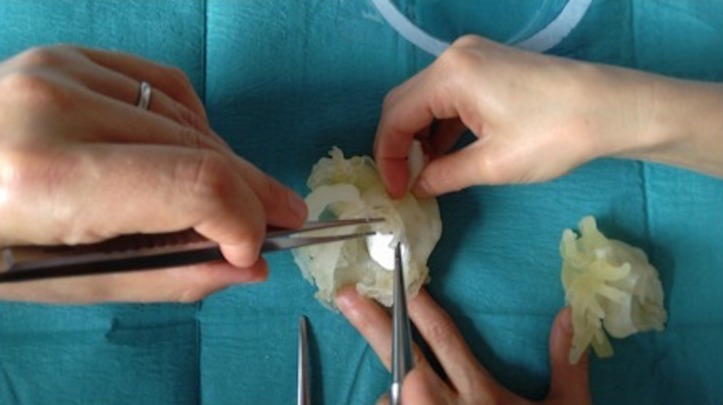
Congenital heart disease patients are particularly challenging for trainees and fellows in cardiac surgery, with limited opportunity to practice without endangering paediatric patients. 3D models printed with flexible materials have been proposed as a tool for practicing surgical procedures (Figure 12). Models were found useful although the elasticity of the material was reported as different from real myocardial tissue by the surgeons.9 Clinical staff can benefit from additional training with 3D models for increasing the appreciation of CHD anatomy after cardiac surgery, e.g. during training courses for cardiac nurses.10 Furthermore, 3D models present advantages over specimens in terms of their cost, ease of reproducibility and conservation/storage, or can be used in conjunction with specimens providing a richer training experience when studying congenital cardiac morphology (Figure 13).
Figure 12.
In this example, a cardiac surgery fellow is practicing on the creation of an intracardiac baffle from the VSD to the aorta in a patient with DORV using a 3D model printed in compliant rubber-like material.
Figure 13.
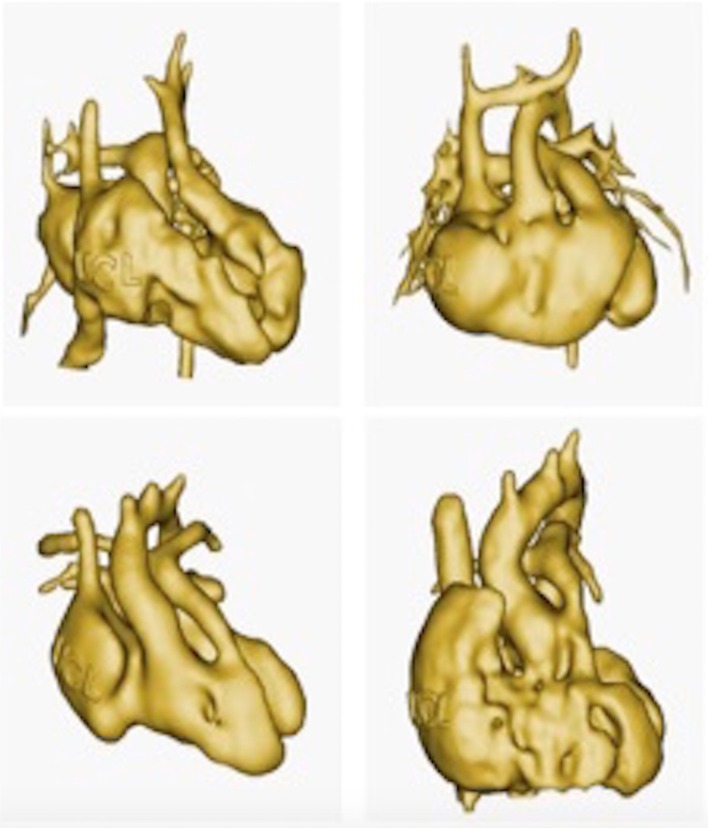
The use of 3D model is particularly valuable for education in congenital cardiology and cardiac surgery. Patient anatomies are often unique and it is difficult to capture the wide range of anatomical variations that occur in the context of the same cardiac malformation. In this example, four patient specific virtual 3D models of double outlet right ventricle are imaged. (From 3D library at http://www.ucl.ac.uk/cardiac-engineering/research/library-of-3d-anatomies),
From doctor-patient communication to public engagement
CHD’s are chronic medical conditions that can affect the patient’s lifestyle from childhood to adult age. The doctor-patient working alliance is a crucial component of patient adherence and satisfaction.11 Communication between cardiologists, cardiac surgeon and patients (and their families) is particularly challenging given the complexity of the defects and medical terminology, the need for multiple surgeries, the dynamics of a communication triad when considering clinician-patient-parent, as well as the delicate process of transition that CHD patients go through as adolescents and young adults. A study involving parents of CHD patients showed that the use of 3D models during medical consultations was enthusiastically appreciated, despite no objective evidence of substantially improved knowledge of their child’s condition after viewing the models.12 Young people with CHD also responded positively when viewing their own 3D heart models during routine clinical consultations, reporting a better experience compared to previous visits13 (Figure 14). The benefit of 3D models in this context may derive from subtle changes in communication dynamics and overall engagement of patients with their health status, rather than measurable changes in knowledge.
Figure 14.

Example of a 3D model of an adult patient with transposition of the great arteries who underwent Mustard procedure. The model was printed with a off-white plaster powder on a Projet 660 printer, and was then used during a workshop with the patient.
Beyond the clinic, 3D models have also been used to bring together art and medicine to foster patients and public involvement and engagement and explore novel stimulating ways of portraying the complexity of cardiovascular anatomy in the presence of CHD (Figure 15).
Figure 15.

Making the Invisible Visible II, Sofie Layton, 2018 (detail). Installation including 3D printed hearts of paediatric and adult patients with congenital heart disease, and with soundscape by Jules Maxwell. As part of “The Heart of the Matter” exhibition (www.insidetheheart.org)
Recommendations for use in clinical practice
There are no current recommendations or guidelines for standardised use of 3D models in congenital cardiology and cardiac surgery. Current use in clinical practice includes ad hoc application for planning or practicing complex procedures. The use of 3D models is currently limited to very complex cases with unique cardiac anatomy, e.g. patients with double outlet right ventricle with non-committed VSD. Small studies and case series have described the use of the 3D models in clinical practice; however, it is very difficult to correlate their use with clinical outcomes, because of the small populations investigated so far and the uniqueness of each patient. The outcomes of on-going research focusing on scientific evidences of 3D model’s usefulness will likely influence the development of clinical recommendations.
Future perspectives
Novel techniques that allow automatic segmentation of complex cardiac and extra cardiac structures (e.g. atlas-based segmentation methods) will reduce the post processing time, making 3D printing technology easier to translate in clinical practice. The patient-specific approach that is very often necessary in the management of patients with CHD may also be applied also in challenging cases of patients with acquired conditions. The use of the models may facilitate the development of new devices and new surgical techniques, also beyond CHD, whilst the combination of 3D models with tissue engineering could lead to bioprinting patient-specific grafts and heart valves.14,15
Conclusion
All imaging modalities (CT, MRI and echocardiography) have developed towards 3D reconstruction techniques, implicitly suggesting the clinical need of going beyond the traditional 2D evaluation of cardiac structures and better appreciating CHD anatomy. Despite its attractiveness, scientific evidence of the usefulness of 3D printing in CHD there is still lacking, and several factors still prevent its becoming widely available or embedded in clinical centres. Firstly, initial costs related to equipment capital investments are high (although smaller, more affordable 3D printers are becoming increasingly refined). Secondly, a relatively long time is required to produce a model from start to finish, with the time of segmentation depending on image quality and printing time on the technology being used, but in certain cases taking >24 h. And finally, the need for expertise in both cardiovascular imaging and engineering to create a model.
Up to now, 3D models have been proposed for complex cases where surgical planning is controversial, with subjective benefit of the use of the models. It may also be the case that practicing a surgery on a patient-specific model in simple cases might also be beneficial, leading to reduced surgical time in the theatre. Further prospective studies quantifying the impact of 3D printing technology on variables such as peri-operative and long-term outcomes, patient adherence, or educational impact on students/trainees, as well as a cost-benefit analysis, are urgently needed to prove the real impact of this technology in the management of patients with CHD.
Footnotes
Acknowledgment: The authors acknowledge the generous support of the British Heart Foundation.
Contributor Information
Elena Giulia Milano, Email: milano.elenagiulia@gmail.com.
Claudio Capelli, Email: c.capelli@ucl.ac.uk.
Jo Wray, Email: Jo.Wray@gosh.nhs.uk.
Benedetta Biffi, Email: b.biffi@ucl.ac.uk.
Sofie Layton, Email: sofie.layton@icloud.com.
Matthew Lee, Email: ml14802@my.bristol.ac.uk.
Massimo Caputo, Email: M.Caputo@bristol.ac.uk.
Andrew M Taylor, Email: a.taylor76@ucl.ac.uk.
Silvia Schievano, Email: s.schievano@ucl.ac.uk.
Giovanni Biglino, Email: g.biglino@bristol.ac.uk.
REFERENCES
- 1.Garekar S, Bharati A, Chokhandre M, Mali S, Trivedi B, Changela VP, et al. . Clinical application and multidisciplinary assessment of three dimensional printing in double outlet right ventricle with remote ventricular septal defect. World J Pediatr Congenit Heart Surg 2016; 7: 344–50. doi: 10.1177/2150135116645604 [DOI] [PubMed] [Google Scholar]
- 2.Bramlet M, Olivieri L, Farooqi K, Ripley B, Coakley M. Impact of three-dimensional printing on the study and treatment of congenital heart disease. Circ Res 2017; 120: 904–7. doi: 10.1161/CIRCRESAHA.116.310546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farooqi KM, Nielsen JC, Uppu SC, Srivastava S, Parness IA, Sanz J, et al. . Use of 3-dimensional printing to demonstrate complex intracardiac relationships in double-outlet right ventricle for surgical planning. Circ Cardiovasc Imaging 2015; 8. doi: 10.1161/CIRCIMAGING.114.003043 [DOI] [PubMed] [Google Scholar]
- 4.Valverde I, Gomez-Ciriza G, Hussain T, Suarez-Mejias C, Velasco-Forte MN, Byrne N, et al. . Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg 2017; 52: 1139–48. doi: 10.1093/ejcts/ezx208 [DOI] [PubMed] [Google Scholar]
- 5.Schievano S, Migliavacca F, Coats L, Khambadkone S, Carminati M, Wilson N, et al. . Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology 2007; 242: 490–7. doi: 10.1148/radiol.2422051994 [DOI] [PubMed] [Google Scholar]
- 6.Biglino G, Moharem-Elgamal S, Lee M, Tulloh R, Caputo M. The perception of a three-dimensional-printed heart model from the perspective of different stakeholders: a complex case of truncus arteriosus. Front Pediatr 2017; 5: 209. doi: 10.3389/fped.2017.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biglino G, Cosentino D, Steeden JA, De Nova L, Castelli M, Ntsinjana H, et al. . Using 4D cardiovascular magnetic resonance imaging to validate computational fluid dynamics: a case study. Front Pediatr 2015; 3: 107. doi: 10.3389/fped.2015.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capelli C, Biglino G, Petrini L, Migliavacca F, Cosentino D, Bonhoeffer P, et al. . Finite element strategies to satisfy clinical and engineering requirements in the field of percutaneous valves. Ann Biomed Eng 2012; 40: 2663–73. doi: 10.1007/s10439-012-0617-1 [DOI] [PubMed] [Google Scholar]
- 9.Yoo SJ, Spray T, Austin EH, Yun TJ, van Arsdell GS. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg 2017; 153: 1530–40. doi: 10.1016/j.jtcvs.2016.12.054 [DOI] [PubMed] [Google Scholar]
- 10.Biglino G, Capelli C, Koniordou D, Robertshaw D, Leaver LK, Schievano S, et al. . Use of 3D models of congenital heart disease as an education tool for cardiac nurses. Congenit Heart Dis 2017; 12: 113–8. doi: 10.1111/chd.12414 [DOI] [PubMed] [Google Scholar]
- 11.Fuertes JN, Toporovsky A, Reyes M, Osborne JB. The physician-patient working alliance: Theory, research, and future possibilities. Patient Educ Couns 2017; 100: 610–5. doi: 10.1016/j.pec.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 12.Biglino G, Capelli C, Wray J, Schievano S, Leaver LK, Khambadkone S, et al. . 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open 2015; 5: e007165. doi: 10.1136/bmjopen-2014-007165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biglino G, Koniordou D, Gasparini M, Capelli C, Leaver LK, Khambadkone S, et al. . Piloting the use of patient-specific cardiac models as a novel tool to facilitate communication during cinical consultations. Pediatr Cardiol 2017; 38: 813–8. doi: 10.1007/s00246-017-1586-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Best C, Strouse R, Hor K, Pepper V, Tipton A, Kelly J, et al. . Toward a patient-specific tissue engineered vascular graft. J Tissue Eng 2018; 9: 204173141876470. doi: 10.1177/2041731418764709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lueders C, Jastram B, Hetzer R, Schwandt H. Rapid manufacturing techniques for the tissue engineering of human heart valves. Eur J Cardiothorac Surg 2014; 46: 593–601. doi: 10.1093/ejcts/ezt510 [DOI] [PubMed] [Google Scholar]