Abstract
Accurate staging and response assessment is vital in the management of childhood malignancies. Fluorine-18 fluorodeoxyglucose positron emission tomography/CT (FDG PET-CT) provides complimentary anatomical and functional information. Oncological applications of FDG PET-CT are not as well-established within the paediatric population compared to adults. This article will comprehensively review established oncological PET-CT applications in paediatric oncology and provide an overview of emerging and future developments in this domain.
Introduction
As access to positron emission tomography/CT (PET-CT) has widened, this technology has been incorporated into many adult oncology patient pathways. Use of PET-CT in paediatric and adolescent patients has lagged behind. Reasons include relative rarity of paediatric cancers, lack of robust trial data and fears over long-term radiation effects.
Imaging guidelines for paediatric patients are often extrapolated from adult guidance, which may not be wholly appropriate due to different patterns of disease and tumour biology. Trial protocols, used on an institution-by-institution basis, add to the heterogeneity of imaging and treatment strategies seen in the paediatric literature. A summary of current indications for paediatric oncological PET-CT as recommended in UK and European guidelines1–4 are summarised in Table 1.
Table 1.
Indications for FDG PET-CT in paediatric malignancies as per current published guidelines
| Malignancy | Disease | Published guidelines | |||
| EANM 20081 | EANM 20092 | RCR 20143 | RCR 20164 | ||
| Haematological | Hodgkin's lymphoma | ✓ | ✓ | # | |
| Non-Hodgkin's lymphoma | ✓ | ✓ | # | ||
| Extra medullary leukaemia | ✓ | ||||
| Langerhans cell histiocytosis | ✓ | ✓ | |||
| Nervous system | Neuroblastoma (MIBG negative) | ✓ | ✓ | ✓ | |
| Selected brain tumours | ✓ | ✓ | ✓ | # | |
| Sarcoma | Osteosarcoma | ✓ | ✓ | ✓ | |
| Ewing's sarcoma | ✓ | ✓ | ✓ | ||
| Rhabdomyosarcoma | ✓ | ✓ | ✓ | ||
| MPNST | ✓ | ✓ | ✓ | ||
| Solid organ | Germ cell tumour | ✓ | ✓ | # | |
| Hepatoblastoma | ✓ | ✓ | ✓ | ||
| Wilms tumour | ✓ | ✓ | ✓ | ||
| Other | Malignancy of unknown origin | ✓ | ✓ | ✓ | |
| Biopsy target | ✓ | # | |||
| Relapse | ✓ | ✓ | ✓ | # | |
EANM, European Association of Nuclear Medicine; FDG PET-CT, fluorodeoxyglucose positron emission tomography/CT; MIBG, metaiodobenzylguanidine; MPNST, malignant peripheral nerve sheath tumour; RCR, Royal College of Radiologists.
✓denotes paediatric-specific indication in guidance.
# denotes extrapolated indication from adult guidelines.
The aim of this article is to provide an up-to-date overview of established and emerging indications for fluorine-18 fluorodeoxyglucose (FDG) PET-CT in paediatric and adolescent oncology patients for radiologists and paediatricians involved in the requesting, reporting and management of these conditions.
Technique
Children are more radiosensitive and have a longer post-exposure life expectancy than adults. The major drawback for PET-CT is the additional ionising radiation dose from the CT component. In recent years, integrated PET-MRI has emerged as a viable alternative, with the benefit of no ionising radiation for the MR component. However, due to issues around cost, scan time and scanner availability, PET-CT remains the workhorse in paediatric oncology. A full review of the advantages and disadvantages is beyond the scope of the article, but the interested reader is directed to a recent review article.5
In most clinical situations the benefits of PET-CT outweigh associated radiation risks. However, there are a number of optimisation techniques which can be utilised to reduce dose and need for repeat imaging. These include: tailored CT exposure factors (decreased mAs and kVp) with increased pitch of the scan; personalised CT protocols (non-contrast vs contrast-enhanced, low-dose CT for attenuation correction vs full diagnostic CT); reducing injected tracer dose and increasing bed position time; and use of iterative reconstruction techniques to improve signal-to-noise ratio.6 Furthermore, patient factors should be considered (Table 2).
Table 2. a.
Common pitfalls encountered in paediatric PET-CT
| Pitfall | Recommendation |
| Non-compliance of patient | Information leaflet Detailed information for parent Pre-scan visit to the department and scanner Scan patient with cuddly toy (Figure 1) Consider GA in young patients |
| Bladder uptake | Pre-scan voiding Consider catheterisation/furosemidea |
| Brown fat uptake | Warm the room Reduce stress Pre-scan propranolol |
PET, positron emission tomography.
Catheterisation and furosemide administration are not performed at our institution.
Figure 1.

A young child undergoing PET-CT scan. Sagittal (a) and axial (b) slices show the patient’s cuddly toy (*) that was taken into the scanner for reassurance. PET, positron emission tomography.
The European Association of Nuclear Medicine (EANM) dose calculator7 helps ensure the optimal balance between radiation dose and image quality. However, these are largely regarded as upper limits and reflect two-dimensional PET techniques rather than newer three-dimensional acquisitions. Examples of injected activity and effective dose for different age/weight is given in Table 3 using the International Commission of RadiologicalProtection 128 and three-dimensional acquisition data.8
Table 3.
The injected activity and effective dose by age/weights of paediatric patients undergoing body PET imaging based on International Commission of Radiological Protection 1288
| Weight (kg) | 10 | 20 | 30 | 56 | 70 |
| Approximate age (years) | 1 | 5 | 10 | 15 | Adult |
| FDG administered activity (MBq) | 38 | 68 | 96 | 311 | 370 |
| Effective dose (mSv) | 3.61 | 3.81 | 3.55 | 7.46 | 7.00 |
FDG, fluorodeoxyglucose; MBq, megabecquerel; PET, positron emission tomography;mSV, millisievert.
Children should fast for 4–6 h prior to imaging, including any enteral or parenteral nutrition, because caloric intake stimulates insulin release, which increases cardiac and skeletal muscle FDG uptake. Serum glucose <6.6 mmol l−1 is typically deemed optimal for scanning.
PET-CT applications in paediatric oncology
Hodgkin’s Lymphoma
Lymphoma is the third commonest childhood malignancy after leukaemia and brain tumours. Hodgkin’s Lymphoma (HL) accounts for 45% of cases and is currently staged according to Lugano criteria.9 FDG PET-CT is highly sensitive for disease staging and routinely used for response assessment and treatment adaption in adults.
Given the effectiveness of modern chemo-radiotherapy regimes, patients are living longer and are at greater risk of secondary malignancies and cardiovascular disease, primarily related to involved-field radiotherapy (IFRT). Therefore, current efforts are focused on reducing the number of patients receiving consolidation RT, without compromising long-term survival.
Staging
FDG PET-CT has increased sensitivity and specificity for staging compared to CT alone.9 It is the technique of choice in HL and avoids under treatment.10 Stage changes in 15–50% of patients have been reported in multiple studies with management changes in approximately half of these.11–13
Standard work-up for HL has previously included bone marrow biopsy (BMB) to establish bone marrow involvement (BMI), which indicates Stage IV disease (Figure 2). FDG PET-CT is now established as a reliable tool for evaluating BMI in adults with HL, and BMB is no longer routinely performed if the patient has a negative PET-CT. This approach is not established in paediatric practice, but there is emerging evidence to suggest that BMB can be safely excluded in the presence of normal marrow on PET-CT, in the absence of high-risk factors.14 Hassan et al reported a high sensitivity, specificity and negative predictive value (NPV) for BMI on PET-CT in paediatric HL.15 Positive predictive value (PPV) was limited by inadequate sampling and diffuse patterns of uptake. Despite the growing evidence base for BMB omission in children, given the relatively low spatial resolution of PET and the heterogeneous nature of early marrow infiltration, some researchers remain understandably cautious.16 In our institution, patients with low-risk HL and negative PET may not undergo BMB after multidisciplinary meeting discussion.
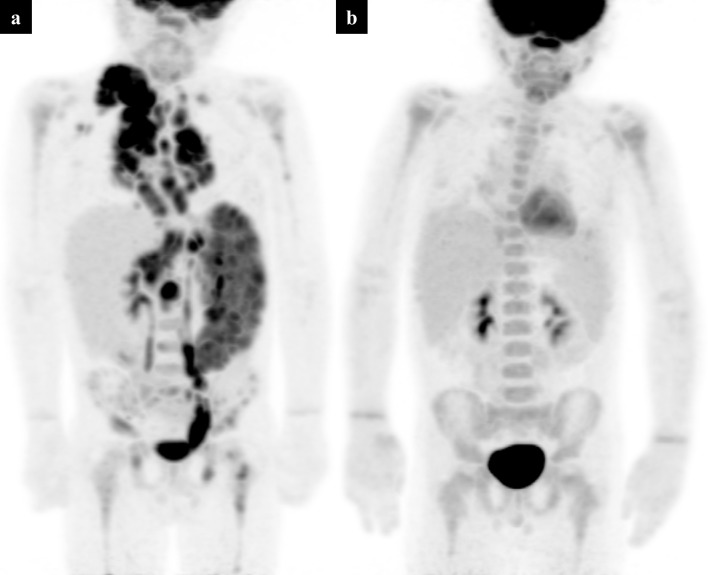
Figure 2.
Baseline staging PET-CT in a 15-year-old girl with biopsy-proven Hodgkin’s lymphoma. PET MIP (a) demonstrates supra- and infra- diaphragmatic disease with diffuse FDG uptake in an enlarged spleen and throughout the bones, in keeping with Stage IV disease. Axial PET slice through the pelvis (b) shows heterogeneous tracer uptake in the pelvic bones. The corresponding low-dose CT component (c) and fused PET-CT (d) allow localisation of the bone involvement. FDG, fluorodeoxyglucose; MIP, maximum intensity projection; PET, positron emission tomography.
Interim scanning and response-adapted therapy
Standard UK practice has been based on the EuroNet PHL-C1 trial, where patients receive two cycles of OEPA chemotherapy (vincristine, etoposide, prednisolone and doxyrubicin) after baseline PET-CT.17 Interim PET-CT is performed after two cycles and further treatment is then adapted to response. However, the EuroNet PHL-C2 trial has begun recruiting and an updated protocol will soon be implemented.
In the C1 trial, patients with a complete metabolic and morphological response to chemotherapy receive no additional radiotherapy (Figure 3). If there was persistent metabolically active disease on interim PET-CT (Figure 4a and b), IFRT was administered.18 The main goal of EuroNet-PHL-C1 was to reduce radiotherapy given to low-risk patients with chemo-sensitive disease, reducing long-term sequelae without compromising overall survival (OS).19 Final results are awaited, but interim analysis in 201620 demonstrated IFRT could be omitted in approximately 50% of patients without a significant drop in event-free survival (EFS). Important changes in the EuroNet-PHL-C2 trial include randomisation to further chemotherapy regimens in intermediate and high-risk groups, as well as the addition of a “late response assessment” PET-CT. Multiple chemotherapy/radiotherapy arms will be introduced for patients with positive ERA and “late response assessment” PET-CT.21 Metabolic response is evaluated using the Deauville score (DS) - a 5-point visual scale comparing lesion uptake to background mediastinal and liver activity (Table 4).20,22 A final major protocol change between EuroNet C1 and C2 is the classification of DS 3 as complete metabolic response (i.e. negative), which was previously considered positive. This aims to further reduce the amount of administered IFRT to this cohort of patients.
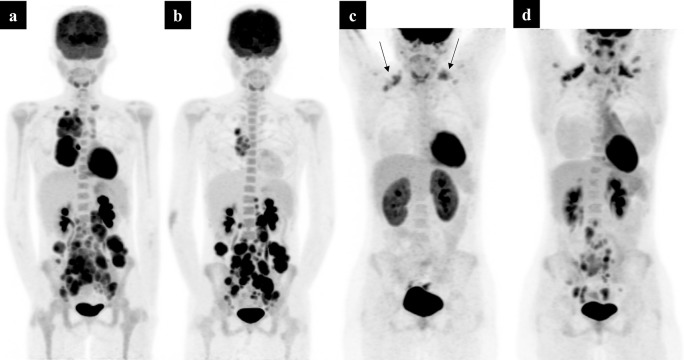
Figure 3.
A 6-year-old boy with biopsy-proved Hodgkin’s lymphoma. Baseline PET MIP (a) shows Stage IV disease with multifocal splenic deposits. Interim PET MIP after two cycles of OEPA chemotherapy (b) shows an excellent response to treatment with residual low-grade activity equal to that to the mediastinal blood pool in the mediastinum (Deauville Scale 2). This represents a complete metabolic response. Note the low-grade homogeneous uptake in the bone marrow (b). This is indicative of bone marrow hyperplasia secondary to chemotherapy, not to be confused with bone marrow disease. MIP, maximum intensity projection; PET, positron emission tomography.
Figure 4.
A 14-year-old with biopsy-proven Hodgkin’s lymphoma. Initial baseline PET MIP (a) shows disease above and below the diaphragm – Stage III disease. Interim PET MIP imaging (b) after 2 cycles of OEPA chemotherapy, shows a partial metabolic response, but with residual disease markedly above liver activity (Deauville Scale 5). The patient had further standard OEPA therapy but continued to show partial metabolic response on PET-CT (not shown), so was switched to alternative chemotherapy. The subsequent PET imaging (c) shows a complete metabolic response – note the brown fat activity within the supra clavicular fossae (arrows). The patient went on to routine follow-up and was found to have recurrent lymphadenopathy on an ultrasound abdomen, 6 months later. A PET-CT (d) confirmed relapse within the abdomen and pelvis. MIP, maximum intensity projection; PET, positron emission tomography.
Table 4.
The Deauville scoring system for defining disease response as used in the EuroNet-PHL-C2 trial
| Deauville score | Imaging features | Interpretation |
| 1 | No residual uptake | CMR |
| 2 | Slight uptake, below mediastinal blood pool | CMR |
| 3 | Uptake above mediastinal blood pool but below liver uptake | CMR |
| 4 | Uptake slightly above liver uptake | IR/PD |
| 5 | Marked increased in uptake or any new lesion | IR/PD |
CMR, complete metabolic response; IR, inadequate response; PD, progressive disease.
DS is more reliable than other scoring systems for evaluating HL at interim and end-of-treatment scans23,24 but κ scores for interobserver agreement are lower in the paediatric population than in adults (0.56 vs 0.85).25,26 In paediatric HL it is unclear if interim PET-CT has a prognostic role.23 HL is chemo- and radio-sensitive and has a good response to standard therapies. Therefore, an interim negative PET-CT is expected to have a high NPV. However, a proportion of patients with negative interim PET-CT go on to relapse and conversely many PET-positive patients achieve long-term remission.27,28 Despite investigation of multiple PET-based criteria, the PPV of PET-CT for predicting relapse at interim assessment is suboptimal, limited by a lack of specificity distinguishing between post-treatment inflammatory uptake and treatment-resistant disease.28–32
End-of-treatment and follow-up
End-of-treatment PET-CT is not widely used in the UK as it does not alter treatment escalation or IFRT, although reports suggest end-of-treatment PET-CT may allow better prediction of disease relapse.23 There is no evidence to support use of surveillance PET-CT post-treatment but it does have a role in re-staging patients with histologically confirmed relapse (Figure 4c and d).19
Non-Hodgkin’s Lymphoma
Paediatric non-Hodgkin’s Lymphoma (pNHL) covers multiple histological subtypes with varying natural histories, imaging findings and responses to treatment.
The major histological pNHL subtypes are of mature B-cell lineage: Burkitt Lymphoma predominates in young children, diffuse large B-cell lymphoma in mid-to-late teens and primary mediastinal B-cell lymphoma in late adolescence and young adults.33,34 T-cell lymphomas are much rarer34 but important subtypes include: anaplastic large cell lymphoma and lymphoblastic lymphoma. The behaviours of these lymphomas overlap with leukaemia and this should be borne in mind when considering imaging options.33
Staging
The international paediatric NHL staging system revised in 2015, modifying the St Jude (Murphy) staging classification35 developed in the 1980s, does not explicitly advocate the use of PET-CT over CT or MRI.
Use of PET-CT in pNHL is currently reserved for cases where there is diagnostic uncertainty and refined staging will alter management. Due to the heterogeneous nature and low prevalence of pNHL, large multicentre prospective trials are lacking, as is evidence supporting routine baseline PET-CT. Furthermore, due to the rapid doubling times of some tumours e.g. BL (24–48 h), patients may have already started treatment (steroids, chemotherapy or surgery) because of systemic illness, airway compromise or bowel obstruction. In these situations, it is not advisable to delay management for PET-CT.
pNHLs which are high-grade would be expected to show marked FDG avidity, verging on 100% sensitivity with improved NPV compared to CT alone pre- and post-treatment.18,36 However, while increasing diagnostic accuracy is always desirable, upstaging patients does not necessarily alter risk stratification, on which current treatment regimens are based.37
Response assessment and relapse
In pNHL there is no robust prognostic data available. However, in adult patients with high-grade NHL the OS and progression-free survival (PFS) are only reliably assessed at the end of treatment, rather than the interim time-point.20 The NPV of end-of-treatment PET-CT in these patients remains high although with a lower PPV. Due to the excellent outcomes from chemotherapy, routine end-of-treatment PET-CT is currently not advocated in pNHL and morphological imaging is the mainstay of follow-up.36
PET-CT can be used as a problem-solving tool where there are residual masses to distinguish functionally active disease from fibrosis. However, this relies on an adequate baseline study with which to compare. This is especially important in low-grade lymphomas, where baseline FDG uptake can be less avid and persistent low-grade uptake at follow-up does not necessarily imply adequate treatment response.
Brain tumours
Brain tumours are the most common paediatric solid tumour and the use of FDG PET-CT in imaging these tumours is discussed in both UK and European guidelines. However, due to the high background uptake of FDG in normal brain parenchyma and the inability to differentiate tumour and inflammatory response, the indications discussed are only rarely used in clinical practice as a problem solving tool.
FDG PET-CT can be useful in targeting metabolically active areas within a tumour to improve diagnostic yield from biopsy and in assessment of histological grade; aggressive tumours displaying higher uptake.38–40 However, this can be a limitation when trying to identify low-grade gliomas as they can demonstrate activity similar to background brain uptake.41 Glioblastomas and medulloblastomas generally demonstrate intense uniform enhancement, whereas brain stem gliomas have low-grade uptake in less than 50% of the tumour volume. Ependymomas have low-grade uptake throughout.38
This information, if used to correlate biopsy site, can lead to improved tumour delineation and sampling.42 Hip et al reported that both FDG PET-CT and MR spectroscopy were able to detect areas of increased metabolism within a tumour.43 However, there was poor correlation between the two modalities and tumour metabolism was better demonstrated on MR spectroscopy.
A further use for FDG PET-CT is to determine if there is residual disease or recurrence, which can often be problematic when using conventional imaging (CI). FDG PET-CT has sub-optimal efficacy following treatment as FDG activity can be seen due to an inflammatory response.44 There is increasing evidence of the superior accuracy of amino-acid analogue PET-CT [e.g. choline, L-dihydroxyphenylalanine (DOPA)] with a higher tumour-to-background ratio than FDG, but these tracers are only available at limited centres currently.45
Leukaemia
Leukaemia is the commonest childhood malignancy and is almost always associated with the acute form of the disease - acute lymphoblastic leukaemia being five times more common than acute myeloid leukaemia (AML).46 Conventional imaging is only performed when there is clinical suspicion of extra medullary disease (EMD). Around 20 to 40% of patients with AML have EMD at diagnosis and this is associated with high relapse rates.47
The use of PET-CT in patients with leukaemia can aid in the detection of EMD, especially in detecting subclinical, multifocal disease.48 However, its use in clinical practice is limited due to the lack of definitive treatment options. Chemotherapy, local radiotherapy and surgical options are discussed in the literature but there is no current consensus on best consolidation therapy. If this were established, PET-CT may have a crucial role in patient stratification. The sensitivity for the detection of EMD has been reported to be 93.3%, the specificity being 71.4%.49 The false positives are reportedly due to inflammatory/infective pathology and the false negatives affecting the meninges or skin.
Cunningham et al48 also reported inconclusive results in the ability of FDG PET-CT to predict complete response: 18/35 patients with negative PET-CT studies continued to be disease-free at follow-up (3 months to 6 years), whereas 16 patients relapsed during follow-up (all within 13 months). Work in adults using fluorine-18 fluorodeoxythymidine PET-CT to assess bone marrow response in AML patients who subsequently relapsed, may offer an alternative to FDG in the future.50 These preliminary studies highlight a potential role for baseline PET-CT to identify EMD before commencing therapy and avoid under treatment; however, there needs to be a consensus on the best local treatment options to justify the use of PET-CT in the patient cohort.
Osteosarcoma
The commonest primary bone tumour in children and adolescents is osteosarcoma. Although 5-year survival reaches ~70% in localised disease, a much poorer prognosis (around 30%) is seen in metastatic disease.51 Aggressive surgical management following neoadjuvant chemotherapy is the mainstay of local control, with adjuvant chemotherapy to treat systemic spread. Patients with metastatic disease limited to the lungs have a better prognosis than those with other sites of distant disease.52 Accurate staging is of great importance to guide optimal treatment and stratify aggressive metastatectomy.
Staging
FDG PET-CT is the most accurate imaging technique for staging.53–55 This is of particular importance for detection of osseous metastases close to physes, which may be obscured by physiological uptake on bone scintigraphy (Figure 5).56 Due to the inherent high cerebral metabolism of FDG, PET-CT has the potential to miss metastatic lesions around the skull base.
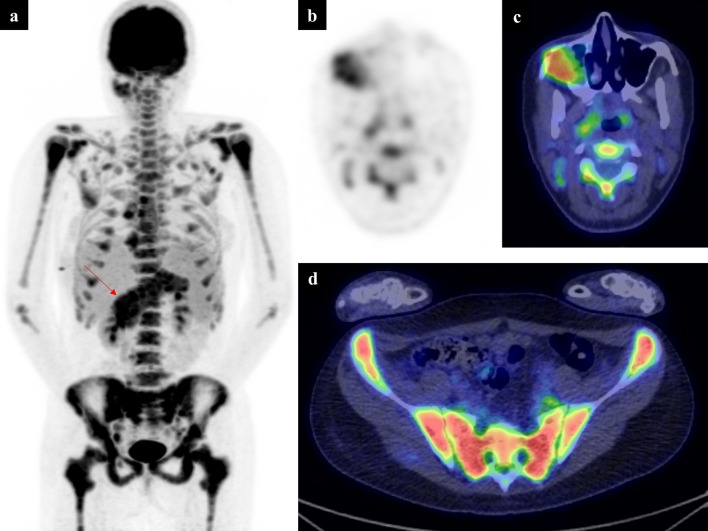
Figure 5.
A 15-year-old with a left distal femoral osteosarcoma. Following initial chemotherapy, a new suspicious lesion was demonstrated within the left tibial epiphysis on response CT and MRI (not shown). Due to the proximity to the proximal tibial physis, and the possibility to significantly alter management, a full body PET-CT was performed. A selected lower limb image from the PET MIP (a) shows low-grade uptake in the lateral distal left femur, at the site of the known osteosarcoma (b) and a new focus of uptake in the proximal tibial region. Low-dose CT (c) and fused axial PET-CT slices through the proximal tibia (d) show a sclerotic, FDG avid deposit in the left tibial epiphysis. Involvement of both sides of the left knee joint led to an above knee amputation. FDG, fluorodeoxyglucose; MIP, maximum intensity projection; PET, positron emission tomography.
Similarly, due to the spatial resolution limitations of PET-CT, uptake in small nodules (<7 mm) may not be appreciated. Furthermore, due to the CT component being acquired in gentle respiration, a thin-section thoracic CT in full inspiration remains a key part of baseline staging (Figure 6), in order not to miss these. Despite evidence supporting the superior accuracy of FDG PET-CT for detection of bone metastases there is a lack of consensus and this is compounded by guidelines only recommending, but not mandating, use of FDG PET-CT and clinical trials often including this technique as optional at the discretion of the local clinician.57 Efforts should be made to update clinical guidelines to reflect recent literature supporting more widespread use of FDG PET-CT.
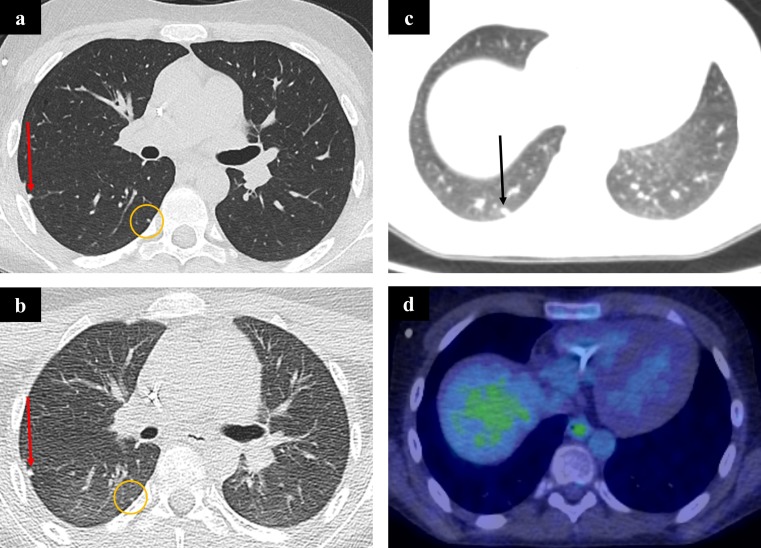
Figure 6.
A 13-year-old boy with metastatic osteosarcoma. Compare the single breath-hold staging chest CT (a) with the CT component of the PET-CT during quiet respiration (b) at the same window level. Two small metastases are shown in both images. The first (red arrows) is visible on both scans, but the second (circled) is more difficult to discern on the PET-CT due to poor lung expansion, breathing artefact and noise. A further metastasis (arrow) in the same patient (c) does not show any FDG activity (d). This is likely due to the small size of the metastasis and limited PET resolution. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Response to therapy
Neoadjuvant chemotherapy to reduce local disease burden allows more complete surgical resection. Degree of pathological tumour necrosis correlates with prognosis and effectiveness of chemotherapy; research in adult58 and paediatric59 bone sarcoma has shown that an end-of-treatment decrease in primary bone tumour FDG avidity correlates with histological response.
Interim FDG PET-CT response has not been comprehensively studied in paediatric OSa. Two small studies reported that metabolic tumour volume (MTV) and total lesion glycolysis (TLG) were predictive of histological response after a single cycle of chemotherapy.60,61 In patients with a poor response to initial chemotherapy, this could help avoid further suboptimal therapy and reduce time to surgery. A prospective study has shown reduction in tumour maximum standardised uptake value (SUVmax) on interim (5 weeks) and end-of-treatment (10 weeks) FDG PET-CT after neoadjuvant therapy correlated with good response (>90% tumour necrosis), but did not predict EFS.62
The value of interim PET-CT has not yet been conclusively proven. The ability to detect poorly responding tumours at interim assessment is of uncertain benefit, as no reliable alternative chemotherapy regimen has been found to significantly affect outcome in poorly responding OSa.63
Predicting outcome
Multiple studies have shown that PET-CT metrics such as SUVmax (both pre- and post-chemotherapy), TLG and MTV reliably predict patients with poorer outcomes. A comprehensive review of this literature is beyond the scope of this article but further information is available in a recent review article.64
Others have not demonstrated the same findings in more homogeneous paediatric populations65 raising the important question of the impact of different tumour biology in children and older adolescents, and how this may alter FDG uptake. A more recent prospective hypothesis-generating study, has shown that MTV, TLG and SUVpeak have the potential to predict EFS and OS at baseline, interim and post-therapy.61 Their work also corroborates the above assertions that these PET-parameters could predict histological response to neoadjuvant chemotherapy.59,66–68
Relapse
Conventional imaging is used in follow-up, tailored by clinical suspicion. PET-CT may have a role in patients with clinical relapse to define extent. A small retrospective study by Angelini et al69 reported that when there was clinical suspicion of local recurrence only, PET-CT showed systemic disease (lung, loco-regional lymph nodes and distant disease) in over one third of patients. A further advantage of PET-CT is in the detection of periprosthetic recurrence. PET is only minimally affected by metallic artefact from endoprostheses and may more accurately depict new tumour sites compared to CT alone.70 However, radiologists should be aware of this potential pitfall when reporting.
Ewing sarcoma
Ewing sarcoma is the second commonest paediatric bone tumour. The presence and pattern of metastases, size of primary and poor treatment response all adversely affect prognosis, in a similar way to OSa.71
Staging
FDG PET-CT is more sensitive in detection of metastases in sarcoma patients (including ES) with a sensitivity of 90% for metastatic bone lesions, compared to 75% with conventional imaging.72 London et al55 studied 314 lesions from varying sarcomas, including ES, finding that FDG PET-CT was more sensitive and specific at all metastatic sites apart from the lungs, with a sensitivity of 83% and specificity of 98% vs 78% and 97% for CI. Clinical guidelines have not been updated for several years and consequently the superior efficacy of FDG PET-CT may not be widely appreciated by the community. This should be addressed in the next iteration of clinical guidelines.57
Predicting response
There have been conflicting results on the use of PET-CT in response assessment in cohorts of mixed sarcoma patients. Two groups demonstrated that persistent high SUVmax following induction chemotherapy conveyed a worse prognosis.73,74 Another group reported that change in SUVmax did not predict histological response following chemotherapy but that 90% reduction in MTV was associated with favourable response.67 A further study showed that multiple PET-derived metrics did not significantly predict response in 21 patients with ES.65
Soft tissue sarcoma
Soft tissue sarcomas encompass a heterogeneous group of primitive mesenchymal malignancies, of which rhabdomyosarcoma (RMS) makes up over half. The RMS classification alone includes four histological subtypes, with varying presentations, tumour behaviour and treatment strategies, making generalisations about conventional and functional imaging difficult. This problem is further exacerbated by the relative rarity of these tumours (fewer than 60 cases per year in the UK), leading to guidelines based largely on expert consensus opinion.
Staging
Primary tumours are almost universally FDG avid.75 Sites of spread in order of decreasing frequency include lungs, loco-regional lymph nodes (LN), bone marrow and cortical bone.76 Outcome is strongly linked to site and number of metastases; the EFS in metastatic RMS drops to around 25% in children.77 Identification of LN involvement in RMS patients can be challenging, with over estimation of disease in reactive nodes and under estimation of disease in morphologically normal nodes, which can have major effects on morbidity and mortality. The only published systematic review of FDG PET-CT in RMS to date78 reported a higher sensitivity and specificity (80–100% and 89–100% respectively) for LN metastases compared to CI (67–86% and 90–100% respectively), fewer indeterminate results and more true-negative results. Routine PET-CT for nodal staging has recently been advocated by an international paediatric oncology group consensus paper.79 Conventionally, bone scintigraphy has been used for exclusion of osseous metastases. PET-CT is more accurate and of prognostic value in RMS.75,78 Some groups have replaced bone scintigraphy with FDG PET-CT for baseline RMS work-up.79,80 Unlike other paediatric malignancy protocols, PET-CT scan coverage should include the extremities, due to the high prevalence of clinically occult extremity metastases (Figure 7).81 The application of PET-CT to date has been variable and on a centre-by-centre basis. The above consensus recommendations may go some way to homogenising imaging at initial presentation.
Figure 7.
A 16-year-old girl with lethargy and raised inflammatory markers. Ultrasound and MRI abdomen (not shown) showed a diffusely enlarged pancreas. The working diagnosis of haematological malignancy led to a bone marrow aspirate, which suggested possible rhabdomyosarcoma. A PET-CT was performed to assess for primary and distant disease. The PET MIP (a) showed diffuse FDG uptake within the pancreas (arrow), but also diffuse abnormal uptake within the appendicular and axial skeleton. A focal area of abnormality centred on the right maxilla (b and c) was suggested as the likely primary site. Fused axial slice through the pelvis (d) showed marked FDG uptake within the pelvic bones compatible with bone marrow infiltration. FDG, fluorodeoxyglucose; MIP, maximum intensity projection; PET, positron emission tomography.
Predicting outcome and relapse
Clinical and pathological risk factors that predict outcome in RMS include age, tumour size and invasion, primary site, histological differentiation and metastatic disease.82 As yet, no metabolic parameters are routinely used in prognostication, and predicting relapse remains difficult. A small study has shown PET-CT can predict local relapse after radiation therapy.83 Baum et al84 determined that visual scoring of tumour intensity was predictive of OS, as was a ratio of Tumour SUVmax/Liver SUVmax of >4.6. EFS in this cohort was also predicted by tumour intensity. Multivariate analysis showed no significant parametric PET factors, but LN positivity and distant disease were predictive, affirming that accurate staging at baseline is invaluable. More recently, Casey et al85 demonstrated that baseline primary tumour SUVmax >9.0 was predictive of poorer OS and 3-year PFS. Furthermore, a negative end-of-treatment PET was predictive of 3-year PFS but not significant for OS. This remains an area for further research.51
Malignant peripheral nerve sheath tumours
These affect neurofibromatosis Type 1 patients with malignant transformation occurring in previously benign plexiform neurofibromata.51 Malignant peripheral nerve sheath tumours (MPNST) are not limited to children and most PET-CT studies report heterogeneous groups of all ages.86 Management includes tumour excision, usually guided by pre-operative biopsy. Mean SUVmax of MPNST in children is higher than benign nerve sheath tumours.87,88 Whilst Tsai et al87 reported a cut-off SUVmax >4.0 allowed differentiation between benign and malignant tumours, and Azizi et al88 reported all malignant tumours had an SUVmax >3.15, it is recognised that a wide overlap in SUVmax values exists.86 This equates to a high NPV (up to 100%) but a low specificity for avid tumours. Therefore, there is a strong reliance on histological sampling when clinical symptoms of malignant transformation are suspected (Figure 8). Brahmi et al89 published a small retrospective analysis demonstrating the utility of PET-CT guided biopsy, reporting no indeterminate results and a diagnostic accuracy of 96%.
Figure 8.
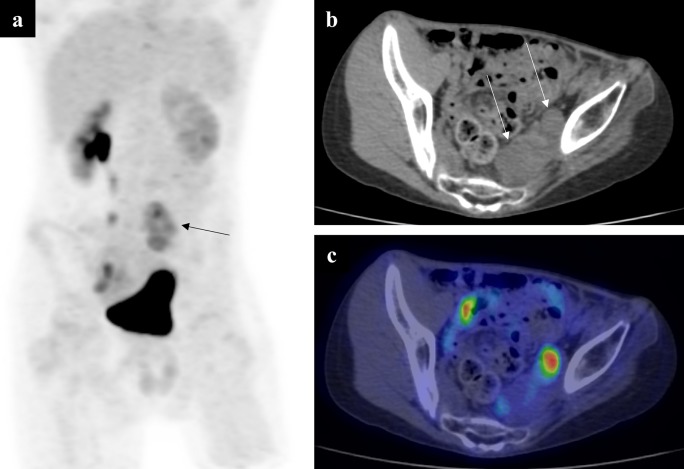
A 13-year-old boy with a pelvic neurofibroma. The neurofibroma had previously shown malignant transformation and was treated. New symptoms of pain and left-sided lower limb neurology prompted an MR scan, which showed increase in the size of the residual neurofibroma (not shown). A PET MIP (a) showed a left-sided region of increased FDG uptake (arrow). Note the two discreet soft tissue masses on the low-dose CT component (b) at this level (arrows). On the fused axial PET-CT slice (c), the medial lesion has little-to-no FDG uptake, but the lateral mass shows high avidity. This imaging allowed a targeted biopsy to be performed, which showed sarcomatous transformation on histology. FDG, fluorodeoxyglucose; MIP, maximum intensity projection; PET, positron emission tomography.
Recently, focus has switched to PET-CT’s utility in predicting malignant change in asymptomatic patients, for earlier diagnosis and improved OS. This may be important in children who have difficulty in verbally expressing symptomatology. Azizi et al88 performed a prospective study of 41 patients undergoing 104 PET(-CT) examinations, reporting that 4 of 8 patients with malignant lesions were asymptomatic. None of the asymptomatic patients died during follow-up (median 4.2 years), whereas 2 of 4 symptomatic patients died within a year of imaging. This not only highlights the poor outcome in MPNSTs but that there may be benefit in early detection before clinical symptoms are present. However, the specificity was as low as 45.1% for the reasons discussed above.
Neuroblastoma
Neuroblastoma (NB) most commonly affects children under 5 years old. Metastatic disease at presentation is reported in around 50% of cases and prognosis varies from good in those younger than 12 months of age, to demonstrably poorer in those over 2 years old.90
Staging
Most NB tumour cells take up and concentrate the catecholamine precursor analogue metaiodobenzylguanidine (MIBG), consequently the mainstay of NB imaging is Iodine-123 MIBG scintigraphy. However, 10% of tumours do not concentrate this substrate and will appear MIBG negative. FDG PET-CT has a valuable role in these cases, as even poorly differentiated tumours will accumulate FDG. A recent meta-analysis of 1134 patients91 showed FDG PET-CT has a higher sensitivity for lesion detection than MIBG scintigraphy but lower specificity, which may lead to the need for biopsy in soft tissue lesions. However, 39/40 studies included were retrospective in nature, reflecting that good quality prospective trials in this area are lacking. High physiological uptake of FDG in the brain obscuring skull base metastases is a known limitation.92 Neither technique reliably detects small-volume bone marrow involvement and international consensus is to perform bone marrow sampling routinely.93
Recently, the prognostic role of FDG PET-CT akin to that seen in lymphoma has been evaluated. As MIBG uptake is related to transporter expression rather than cellular differentiation, MIBG cannot reliably be used to infer differentiation and therefore prognosis. A small retrospective cohort study reported that FDG PET-CT was a better predictor of PFS.94
Relapse
As FDG PET-CT is less sensitive and specific for bone and bone marrow disease, which is the most common site of relapse, MIBG scintigraphy (alongside CI) remains the gold standard in follow-up. However, PET-CT can be used first line in the follow-up of known MIBG-negative tumours. It is recognised that MIBG-positive tumours can become negative at relapse and FDG PET-CT has a problem-solving role in these cases (Figure 9).95
Figure 9.
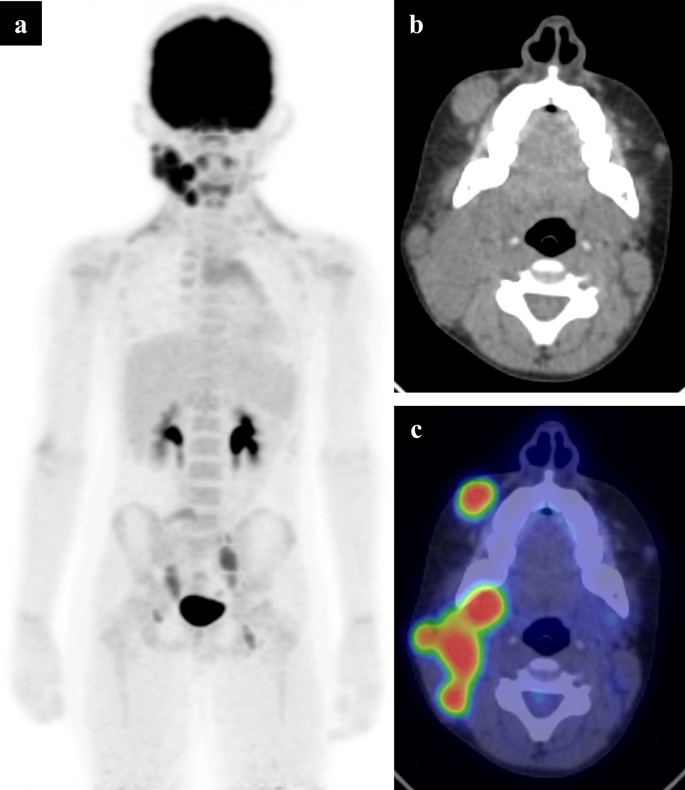
11-year-old boy with known Rosai-Dorfman disease. The patient experienced rapid increase in a right neck lump and was shown to have avid FDG uptake in the right cervical chain (a). The low dose CT component (b) showed a conglomerate mass at the angle of the mandible, which showed high uptake on the fused axial PET-CT (c) as well as a separate mass in the soft tissues overlying the right maxilla. This is compatible with histiocytosis with lymphomatous transformation. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Future developments
Fluorine-18 fluorophenylalanine (F-DOPA) and Gallium-68 Somatostatin Receptor (SSR) are alternative PET tracers which are not yet widely available but have higher reported sensitivities compared to FDG PET-CT and MIBG scintigraphy.95 A newer tracer 18F-Meta-Fluorobenzylguanidine (MFBG) is also in development and shows early promise.96 An in-depth review of these tracers is beyond the scope of this article but a recent review provides more details for interested readers.95
Wilms tumour
Current UK and European protocols do not advocate use of FDG PET-CT in patient management. Diagnosis and staging is with ultrasound and MRI, despite the fact that Wilms’ tumours are FDG-avid and correlate well with histology.97,98 Limited data suggest that SUVmax after neoadjuvant chemotherapy may predict tumour viability and FDG PET-CT may detect more sites of disease at relapse than MRI, but further work is required to confirm initial findings.99 In the meantime, FDG PET-CT has a limited problem-solving role for restaging relapsed patients and guiding biopsy.
Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a group of rare disorders characterised by proliferation of Langerhans-type cells.100,101 LCH can present as a single lesion (sometimes termed as an eosinophilic granuloma) or several lesions involving a single or multiple body systems (Figure 10).102 Although diagnosis requires histology and immunophenotyping, prognosis is determined by organ involvement and treatment response.
Figure 10.
A 9-year-old with relapsed neuroblastoma. An anterior planar I-123-MIBG scan (a) shows a site of avid disease at the left costophrenic angle (arrow), but a recent MR (not shown) revealed multiple new areas of concern that were not MIBG avid. A PET MIP (b) and fused axial slice (c) show this corresponding area to be FDG avid (straight arrows), but reveal more extensive sites of disease in the mediastinum (*), right-side of the sacrum (curved arrow) and left chest wall (arrow in d). These sites likely represent MIBG-negative disease, which is more common in neuroblastoma relapse, but this figure also demonstrates PET’s superior spatial resolution. I-123-MIBG, Iodine-123-metaiodobenzylguanidine; FDG, fluorodeoxyglucose; PET, positron emission tomography.
A few small retrospective studies suggest a potential role for FDG PET-CT in LCH. Kaste et al103 reviewed five children with bone involvement and reported that PET-CT was beneficial for staging when compared to bone scans. Mueller et al104 evaluated patients who underwent both FDG PET-CT and whole body MRI within 30 days of each other with no tumour specific treatment. The sensitivity and specificity of PET-CT was 67 and 76%, compared to 81 and 47% for MRI. Lower PET-CT sensitivity was attributed to false-negatives where lesions in the brain or head and neck were obscured by physiological uptake, or small bone lesions were missed. MRI should be considered for evaluation of the brain. Another group compared FDG PET-CT and conventional imaging for lesion detection, therapy response and disease recurrence.105 PET-CT was complementary in lesion detection of 236/256 (92%) lesions, superior in 90/256 (35%) lesions, but inferior at evaluating skull or vertebral column lesions.
Germ cell tumour
The largest reported series includes 11 scans in 9 patients, over an 11-year period.106 FDG PET-CT was used in staging, biopsy guidance, assessment of residual metabolic activity and recurrence detection with a change in management in 3 patients. Other smaller studies have confirmed germ cell tumours are metabolically active on FDG PET-CT.107,108 Clearly, extrapolating from this data alone is not feasible and a larger multicentre study is required before the role of PET-CT is firmly understood in this setting.
Hepatoblastoma
Hepatoblastoma is rare but makes up almost three-quarters of all paediatric hepatic primary malignancies. An international cross-society consensus (PRETEXT) has recently been published to standardise radiological assessment of paediatric patients with hepatobiliary malignancies.109 These standards will be used in an upcoming international multicentre Paediatric Hepatic International Tumour Trial. According to PRETEXT standards, ultrasound and MRI are first-line investigations in diagnosis and staging of primary hepatic malignancies. FDG PET-CT has a limited role in detection of suspected tumour relapse with negative CI, in the context of rising blood serum alpha-fetoprotein.
This is a pragmatic approach in view of the lack of published trial data, with only a small collection of case reports pertaining to relapse.110–112 A small retrospective study showed the potential utility of FDG PET-CT in the setting of clinical relapse.113 In 8/9 patients, PET-CT correctly demonstrated biopsy-proven recurrence, whereas CI only detected 4 cases. In the PET-CT negative case, recurrence was not detected, and remission was confirmed. PET-CT can also potentially be used in problem-solving when there is a question about the presence or extent of metastatic disease prior to any surgery with curative intent. However, more data is required before routine use. The Paediatric Hepatic International Tumour Trial may provide a structured framework within which PET-CT can be studied in a larger population across the spectrum of disease and thus more clearly define its role.
Table 5 summarises the evidence discussed in this paper for paediatric PET-CT in oncology.
Table 5.
Indications for FDG PET-CT by paediatric tumour type, and key supporting in-text referenced evidence
| Use of PET-CT | |||||
| Pathology | Biopsy targeting | Staging | Interim | Response assessment | Recurrence |
| Haematological | |||||
| Hodgkin’s | 6, 12–17 | 10–21 | 23 | 20 | |
| Non-Hodgkin’s | 19, 36 | 21, 36 | |||
| Leukaemia | 48 | 48 | 49 | ||
| Sarcoma | |||||
| Osteosarcoma | 52–54 | 59–61 | 58, 60, 65–67 | 61, 68 | |
| Ewing’s sarcoma | 54, 71 | 72, 73 | 64, 66 | ||
| Rhabdmyosarcoma | 75–81 | 82 | 83 | ||
| MPNST | 89 | 87, 88 | |||
| CNS tumours | 38–40, 42, 43 | ||||
| Neuroblastoma | 90, 91, 93 | 94 | |||
FDG, fluorodeoxyglucose ; PET, positron emission tomography.
Conclusion
FDG PET-CT has an established role in staging paediatric malignancies such as HL, RMS and non-MIBG avid NB. Current data supports increased use in bone sarcoma and NHL, but larger prospective multicentre trials are needed to clearly outline the utility. The role of PET-CT in Wilms’, hepatoblastoma, LCH and germ cell tumours is limited to problem solving at present.
Contributor Information
Greg Chambers, Email: g.chambers@nhs.net.
Russell Frood, Email: russell.frood@doctors.org.uk.
Chirag Patel, Email: chirag.patel13@nhs.net.
Andrew Scarsbrook, Email: a.scarsbrook@nhs.net.
REFERENCES
- 1.Stauss J, Franzius C, Pfluger T, Juergens KU, Biassoni L, Begent J, et al. . Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging 2008; 35: 1581–8. doi: 10.1007/s00259-008-0826-x [DOI] [PubMed] [Google Scholar]
- 2.Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, et al. . EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging 2009; 36: 2103–10. doi: 10.1007/s00259-009-1264-0 [DOI] [PubMed] [Google Scholar]
- 3. The Royal College of Radiologists Guidelines for the use of PET-CT in children. Second edition [Internet] London; 2014. www.rcr.ac.uk. [Google Scholar]
- 4.London RC of P. Glasgow RC of P. Glasgow RC of S. Royal College of Physicians and Surgeons. Edinburgh TRC of R. Society BNM. Committee A of RSA Evidence-based indications for theuse of PET-CT in the United Kingdom 2016 [Internet]. Royal College of Physicians (RCP);RoyalCollege of Radiologists.London. 2012; 71: e171–88. doi: 10.1016/j.crad.2016.05.001. [DOI] [Google Scholar]
- 5.Gatidis S, Bender B, Reimold M, Schäfer JF. PET/MRI in children. Eur J Radiol 2017; 94: A64–A70. doi: 10.1016/j.ejrad.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 6.Parisi MT, Bermo MS, Alessio AM, Sharp SE, Gelfand MJ, Shulkin BL. Optimization of Pediatric PET/CT. Semin Nucl Med 2017; 47: 258–74. doi: 10.1053/j.semnuclmed.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 7.EANM dose calculator [Internet] Dosage Calculator. 2017. Available from: http://www.eanm.org/publications/dosage-calculator [cited 2018 Jun 14].
- 8.Mattsson S, Johansson L, Leide Svegborn S, Liniecki J, Noßke D, Riklund K.Å., et al. . ICRP publication 128: radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP 2015; 44(2 suppl): 7–321. doi: 10.1177/0146645314558019 [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. . Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol 2014; 32: 3059–67. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu L, Chen Y, Wu J. The role of 18F-FDG PET and 18F-FDG PET/CT in the evaluation of pediatric Hodgkin’s lymphoma and non-Hodgkin’s lymphoma. Hell J Nucl Med 2013; 16: 230–6. [DOI] [PubMed] [Google Scholar]
- 11.Riad R, Omar W, Kotb M, Hafez M, Sidhom I, Zamzam M, et al. . Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging 2010; 37: 319–29. doi: 10.1007/s00259-009-1276-9 [DOI] [PubMed] [Google Scholar]
- 12.Robertson VL, Anderson CS, Keller FG, Halkar R, Goodman M, Marcus RB, et al. . Role of FDG-PET in the definition of involved-field radiation therapy and management for pediatric hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys 2011; 80: 324–32. doi: 10.1016/j.ijrobp.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Kabickova E, Sumerauer D, Cumlivska E, Drahokoupilova E, Nekolna M, Chanova M, et al. . Comparison of 18F-FDG-PET and standard procedures for the pretreatment staging of children and adolescents with Hodgkin’s disease. Eur J Nucl Med Mol Imaging 2006; 33: 1025–31. doi: 10.1007/s00259-005-0019-9 [DOI] [PubMed] [Google Scholar]
- 14.Agrawal K, Mittal BR, Bansal D, Varma N, Srinivasan R, Trehan A, et al. . Role of F-18 FDG PET/CT in assessing bone marrow involvement in pediatric Hodgkin’s lymphoma. Ann Nucl Med 2013; 27: 146–51. doi: 10.1007/s12149-012-0665-5 [DOI] [PubMed] [Google Scholar]
- 15.Hassan A, Siddique M, Bashir H, Riaz S, Wali R, Mahreen A, et al. . 18F-FDG PET-CT imaging versus bone marrow biopsy in pediatric Hodgkin’s lymphoma: a quantitative assessment of marrow uptake and novel insights into clinical implications of marrow involvement. Eur J Nucl Med Mol Imaging 2017; 44: 1198–206. doi: 10.1007/s00259-017-3647-y [DOI] [PubMed] [Google Scholar]
- 16.Adams HJA, Nievelstein RAJ, Kwee TC. Opportunities and limitations of bone marrow biopsy and bone marrow FDG-PET in lymphoma. Blood Rev 2015; 29: 417–25. doi: 10.1016/j.blre.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 17.Euronet PHL C1 [Internet] EU Clinical Trials Register. 2018. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-004053-88/DE [cited 2018 Jun 14].
- 18.Kluge R, Kurch L, Montravers F, Mauz-Körholz C. FDG PET/CT in children and adolescents with lymphoma. Pediatr Radiol 2013; 43: 406–17. doi: 10.1007/s00247-012-2559-z [DOI] [PubMed] [Google Scholar]
- 19.Kluge R, Kurch L, Georgi T, Metzger M. Current role of FDG-PET in pediatric hodgkin’s lymphoma. Semin Nucl Med 2017; 47: 242–57. doi: 10.1053/j.semnuclmed.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 20.Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging 2017; 44(Suppl 1): 97–110. doi: 10.1007/s00259-017-3690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Second international inter-group study for classical hodgkin lymphoma in children and adolescents [Internet]. clinicaltrials.gov.. 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT02684708.
- 22.Mauz-Körholz C, Metzger ML, Kelly KM, Schwartz CL, Castellanos ME, Dieckmann K, et al. . Pediatric hodgkin lymphoma. Journal of Clinical Oncology 2015; 33: 2975–85. doi: 10.1200/JCO.2014.59.4853 [DOI] [PubMed] [Google Scholar]
- 23.Bakhshi S, Bhethanabhotla S, Kumar R, Agarwal K, Sharma P, Thulkar S, et al. . Posttreatment PET/CT rather than interim PET/CT using deauville criteria predicts outcome in pediatric hodgkin lymphoma: A prospective study comparing PET/CT with conventional imaging. J Nucl Med 2017; 58: 577–83. doi: 10.2967/jnumed.116.176511 [DOI] [PubMed] [Google Scholar]
- 24.Isik EG, Kuyumcu S, Kebudi R, Sanli Y, Karakas Z, Cakir FB, et al. . Prediction of outcome in pediatric Hodgkin lymphoma based on interpretation of 18FDG-PET/CT according to ΔSUVmax, Deauville 5-point scale and IHP criteria. Ann Nucl Med 2017; 31: 660–8. doi: 10.1007/s12149-017-1196-x [DOI] [PubMed] [Google Scholar]
- 25.Kluge R, Chavdarova L, Hoffmann M, Kobe C, Malkowski B, Montravers F, et al. . Inter-reader reliability of early FDG-PET/CT response assessment using the Deauville scale after 2 cycles of intensive chemotherapy (OEPA) in Hodgkin’s lymphoma. PLoS One 2016; 11: e0149072. doi: 10.1371/journal.pone.0149072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrington SF, Qian W, Somer EJ, Franceschetto A, Bagni B, Brun E, et al. . Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 2010; 37: 1824–33. doi: 10.1007/s00259-010-1490-5 [DOI] [PubMed] [Google Scholar]
- 27.Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, Neuberg D, et al. . End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin's lymphoma. Annals of Oncology 2011; 22: 910–5. doi: 10.1093/annonc/mdq549 [DOI] [PubMed] [Google Scholar]
- 28.Furth C, Steffen IG, Amthauer H, Ruf J, Misch D, Schönberger S, et al. . Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin’s lymphoma: analysis of a prospective multicenter trial. J Clin Oncol 2009; 27: 4385–91. doi: 10.1200/JCO.2008.19.7814 [DOI] [PubMed] [Google Scholar]
- 29.Hussien A, Furth C, Schönberger S, Hundsdoerfer P, Steffen I, Amthauer H, et al. . FDG-PET response prediction in pediatric Hodgkin’s lymphoma: impact of metabolically defined tumor volumes and individualized SUV measurements on the positive predictive value. Cancers 2015; 7: 287–304. doi: 10.3390/cancers7010287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilivitzki A, Radan L, Ben-Arush M, Israel O, Ben-Barak A. Early interim FDG PET/CT prediction of treatment response and prognosis in pediatric Hodgkin disease—added value of low-dose CT. Pediatr Radiol 2013; 43: 86–92. doi: 10.1007/s00247-012-2517-9 [DOI] [PubMed] [Google Scholar]
- 31.Ferrari C, Niccoli Asabella A, Merenda N, Altini C, Fanelli M, Muggeo P, et al. . Pediatric Hodgkin lymphoma: predictive value of interim 18 F-FDG PET/CT in therapy response assessment. Med 2017; 96: e5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu HJ, Halkar R, Alavi A, Goris ML. An evaluation of the predictive value of mid-treatment 18F-FDG-PET/CT scans in pediatric lymphomas and undefined criteria of abnormality in quantitative analysis. Hell J Nucl Med 2013; 16: 169–74. [DOI] [PubMed] [Google Scholar]
- 33.Sandlund JT. Non-Hodgkin lymphoma in children. Curr Hematol Malig Rep 2015; 10: 237–43. doi: 10.1007/s11899-015-0277-y [DOI] [PubMed] [Google Scholar]
- 34.Hochberg J, El-Mallawany NK, Abla O. Adolescent and young adult non-Hodgkin lymphoma. Br J Haematol 2016; 173: 637–50. doi: 10.1111/bjh.14074 [DOI] [PubMed] [Google Scholar]
- 35.Rosolen A, Perkins SL, Pinkerton CR, Guillerman RP, Sandlund JT, Patte C, et al. . Revised international pediatric non-Hodgkin lymphoma staging system. J Clin Oncol 2015; 33: 2112–8. doi: 10.1200/JCO.2014.59.7203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel Rahman H, Sedky M, Hamoda A, Raafat T, Youssef A, Omar W, et al. . Role of FDG-PET scan in the management of pediatric mature B cell non-Hodgkin’s lymphoma. CCHE experience. J Egypt Natl Canc Inst 2016; 28: 95–9. doi: 10.1016/j.jnci.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 37.Bakhshi S, Radhakrishnan V, Sharma P, Kumar R, Thulkar S, Vishnubhatla S, et al. . Pediatric nonlymphoblastic non-Hodgkin lymphoma: baseline, interim, and posttreatment PET/CT versus contrast-enhanced CT for evaluation—a prospective study. Radiology 2012; 262: 956–68. doi: 10.1148/radiol.11110936 [DOI] [PubMed] [Google Scholar]
- 38.Zukotynski K, Fahey F, Kocak M, Kun L, Boyett J, Fouladi M, et al. . 18F-FDG PET and MR imaging associations across a spectrum of pediatric brain tumors: A report from the pediatric brain tumor consortium. J Nucl Med 2014; 55: 1473–80. doi: 10.2967/jnumed.114.139626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utriainen M, Metsahonkala L, Salmi T, Utriainen T, Kalimo H, Pihko H, et al. . Metabolic characterization of childhood brain tumors: comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer 2002; 95: 1376–86. [DOI] [PubMed] [Google Scholar]
- 40.Kruer MC, Kaplan AM, Etzl MM, Carpentieri DF, Dickman PS, Chen K, et al. . The value of positron emission tomography and proliferation index in predicting progression in low-grade astrocytomas of childhood. J Neurooncol 2009; 95: 239–45. doi: 10.1007/s11060-009-9922-4 [DOI] [PubMed] [Google Scholar]
- 41.La Fougère C, Suchorska B, Bartenstein P, Kreth F-W, Tonn J-C. Molecular imaging of gliomas with PET: Opportunities and limitations. Neuro Oncol 2011; 13: 806–19. doi: 10.1093/neuonc/nor054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pirotte BJM, Lubansu A, Massager N, Wikler D, Van Bogaert P, Levivier M, et al. . Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg 2010; 5: 486–99. doi: 10.3171/2010.1.PEDS09481 [DOI] [PubMed] [Google Scholar]
- 43.Hipp SJ, Steffen-Smith EA, Patronas N, Herscovitch P, Solomon JM, Bent RS, et al. . Molecular imaging of pediatric brain tumors: comparison of tumor metabolism using 18F-FDG-PET and MRSI. J Neurooncol 2012; 109: 521–7. doi: 10.1007/s11060-012-0918-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryken TC, Aygun N, Morris J, Schweizer M, Nair R, Spracklen C, et al. . The role of imaging in the management of progressive glioblastoma: A systematic review and evidence-based clinical practice guideline. J Neurooncol 2014; 118: 435–60. [DOI] [PubMed] [Google Scholar]
- 44.Juhász C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S. Comparison of Amino Acid Positron Emission Tomographic Radiotracers for Molecular Imaging of Primary and Metastatic Brain Tumors. Mol Imaging 2010; 13. doi: 10.2310/7290.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belson M, Kingsley B, Holmes A. Risk Factors for Acute Leukemia in Children: A Review. Environ Health Perspect 2007; 115: 138–45. doi: 10.1289/ehp.9023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Støve HK, Sandahl JD, Abrahamsson J, Asdahl PH, Forestier E, Ha S-Y, et al. . Extramedullary leukemia in children with acute myeloid leukemia: A population-based cohort study from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Pediatr Blood Cancer 2017; 64: e26520. doi: 10.1002/pbc.26520 [DOI] [PubMed] [Google Scholar]
- 48.Cunningham I, Kohno B. 18 FDG-PET/CT: 21st century approach to leukemic tumors in 124 cases. Am J Hematol 2016; 91: 379–84. doi: 10.1002/ajh.24287 [DOI] [PubMed] [Google Scholar]
- 49.Zhou W-lan, Wu H-bing, Wang L-juan, Tian Y, Dong Y, Wang Q-shi. Usefulness and pitfalls of F-18-FDG PET/CT for diagnosing extramedullary acute leukemia. Eur J Radiol 2016; 85: 205–10. doi: 10.1016/j.ejrad.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 50.Han EJ, Lee B-hee, Kim J-A, Park YH, Choi WH. Early assessment of response to induction therapy in acute myeloid leukemia using 18F-FLT PET/CT. EJNMMI Res 2017; 7: 75. doi: 10.1186/s13550-017-0326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison DJ, Parisi MT, Shulkin BL. The role of 18 F-FDG-PET/CT in pediatric sarcoma. Semin Nucl Med 2017; 47: 229–41. doi: 10.1053/j.semnuclmed.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 52.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. . Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. Journal of Clinical Oncology 2002; 20: 776–90. doi: 10.1200/JCO.2002.20.3.776 [DOI] [PubMed] [Google Scholar]
- 53.Hurley C, McCarville MB, Shulkin BL, Mao S, Wu J, Navid F, et al. . CComparison of (18) F-FDGPET-CT and bone scintigraphy for evaluation of osseous metastases in newly diagnosed andrecurrent osteosarcoma. Pediatr Blood Cancer 2016; 63: 1381–6. doi: 10.1002/pbc.26014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quartuccio N, Fox J, Kuk D, Wexler LH, Baldari S, Cistaro A, et al. . Pediatric bone sarcoma: diagnostic performance of ¹⁸F-FDG PET/CT versus conventional imaging for initial staging and follow-up. AJR Am J Roentgenol 2015; 204: 153–60. doi: 10.2214/AJR.14.12932 [DOI] [PubMed] [Google Scholar]
- 55.London K, Stege C, Cross S, Onikul E, Graf N, Kaspers G, et al. . 18F-FDG PET/CT compared to conventional imaging modalities in pediatric primary bone tumors. Pediatr Radiol 2012; 42: 418–30. doi: 10.1007/s00247-011-2278-x [DOI] [PubMed] [Google Scholar]
- 56.Byun BH, Kong C-B, Lim I, Kim BI, Choi CW, Song WS, et al. . Comparison of (18)F-FDG PET/CT and (99 m)Tc-MDP bone scintigraphy for detection of bone metastasis in osteosarcoma. Skeletal Radiol 2013; 42: 1673–81. doi: 10.1007/s00256-013-1714-4 [DOI] [PubMed] [Google Scholar]
- 57.Meyer JS, Nadel HR, Marina N, Womer RB, Brown KLB, Eary JF, et al. . Imaging guidelines for children with Ewing sarcoma and osteosarcoma: A report from the Children’s Oncology Group Bone Tumor Committee. Pediatr Blood Cancer 2008; 51: 163–70. doi: 10.1002/pbc.21596 [DOI] [PubMed] [Google Scholar]
- 58.Benz MR, Czernin J, Tap WD, Eckardt JJ, Seeger LL, Allen-Auerbach MS, et al. . FDG-PET/CT imaging predicts histopathologic treatment responses after neoadjuvant therapy in adult primary bone sarcomas. Sarcoma 2010; 2010: 143540. doi: 10.1155/2010/143540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denecke T, Hundsdörfer P, Misch D, Steffen IG, Schönberger S, Furth C, et al. . Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging 2010; 37: 1842–53. doi: 10.1007/s00259-010-1484-3 [DOI] [PubMed] [Google Scholar]
- 60.Hyun B, Kong C-B, Lim I, Kim B, Choi C, Song W, et al. . Early response monitoring to neoadjuvant chemotherapy in osteosarcoma using sequential 18 F-FDG PET/CT and MRI. Eur J Nucl Med Mol Imaging 2014; 41: 1553–62. [DOI] [PubMed] [Google Scholar]
- 61.Im HJ, Kim TS, Park S-Y, Min HS, Kim JH, Kang HG, et al. . Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging 2012; 39: 39–49. doi: 10.1007/s00259-011-1936-4 [DOI] [PubMed] [Google Scholar]
- 61.Davis JC, Daw NC, Navid F, Billups CA, Wu J, Bahrami A, et al. . 18F-FDG uptake during early adjuvant chemotherapy predicts histologic response in pediatric and young adult patients with osteosarcoma. J Nucl Med 2018; 59: 25–30. doi: 10.2967/jnumed.117.190595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. . Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 2016; 17: 1396–408. doi: 10.1016/S1470-2045(16)30214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costelloe CM, Chuang HH, Madewell JE. FDG PET/CT of primary bone tumors. AJR Am J Roentgenol 2014; 202: W521–W531. doi: 10.2214/AJR.13.11833 [DOI] [PubMed] [Google Scholar]
- 65.Bailly C, Leforestier R, Campion L, Thebaud E, Moreau A, Kraeber-Bodere F, et al. . Prognostic value of FDG-PET indices for the assessment of histological response to neoadjuvant chemotherapy and outcome in pediatric patients with Ewing sarcoma and osteosarcoma. PLoS One 2017; 12: e0183841. doi: 10.1371/journal.pone.0183841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byun BH, Kim SH, Lim SM, Lim I, Kong C-B, Song WS, et al. . Prediction of response to neoadjuvant chemotherapy in osteosarcoma using dual-phase 18 F-FDG PET/CT. Eur Radiol 20152015; 25: 2015–24. doi: 10.1007/s00330-015-3609-3 [DOI] [PubMed] [Google Scholar]
- 67.Gaston LL, Di Bella C, Slavin J, Hicks RJ, Choong PFM. 18F-FDG PET response to neoadjuvant chemotherapy for Ewing sarcoma and osteosarcoma are different. Skeletal Radiol 2011; 40: 1007–15. doi: 10.1007/s00256-011-1096-4 [DOI] [PubMed] [Google Scholar]
- 68.Byun BH, Kong CB, Park J, Seo Y, Lim I, Choi CW, et al. . Initial metabolic tumor volume measured by 18F-FDG PET/CT can predict the outcome of osteosarcoma of the extremities. J Nucl Med 2013; 54: 1725–32. doi: 10.2967/jnumed.112.117697 [DOI] [PubMed] [Google Scholar]
- 69.Angelini A, Ceci F, Castellucci P, Graziani T, Polverari G, Trovarelli G, et al. . The role of 18F-FDG PET/CT in the detection of osteosarcoma recurrence. Eur J Nucl Med Mol Imaging 2017; 44: 1712–20. doi: 10.1007/s00259-017-3698-0 [DOI] [PubMed] [Google Scholar]
- 70.Sharp SE, Shulkin BL, Gelfand MJ, McCarville MB. FDG PET/CT appearance of local osteosarcoma recurrences in pediatric patients. Pediatr Radiol 2017; 47: 1800–8. doi: 10.1007/s00247-017-3963-1 [DOI] [PubMed] [Google Scholar]
- 71.Rodríguez-Galindo C, Liu T, Krasin MJ, Wu J, Billups CA, Daw NC, et al. . Analysis of prognostic factors in ewing sarcoma family of tumors: review of St. Jude Children's Research Hospital studies. Cancer 2007; 110: 375–84. doi: 10.1002/cncr.22821 [DOI] [PubMed] [Google Scholar]
- 72.Völker T, Denecke T, Steffen I, Misch D, Schönberger S, Plotkin M, et al. . Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol 2007; 25: 5435–41. doi: 10.1200/JCO.2007.12.2473 [DOI] [PubMed] [Google Scholar]
- 73.Palmerini E, Colangeli M, Nanni C, Fanti S, Marchesi E, Paioli A, et al. . The role of FDG PET/CT in patients treated with neoadjuvant chemotherapy for localized bone sarcomas. Eur J Nucl Med Mol Imaging 2017; 44: 215–23. doi: 10.1007/s00259-016-3509-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salem U, Amini B, Chuang HH, Daw NC, Wei W, Haygood TM, et al. . 18 F-FDG PET/CT as an Indicator of Survival in Ewing Sarcoma of Bone. J Cancer 2017; 8: 2892–8. doi: 10.7150/jca.20077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Federico SM, Spunt SL, Krasin MJ, Billup CA, Wu J, Shulkin B, et al. . Comparison of PET-CT and conventional imaging in staging pediatric rhabdomyosarcoma. Pediatr Blood Cancer 2013; 60: 1128–34. doi: 10.1002/pbc.24430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol 2009; 27: 3391–7. doi: 10.1200/JCO.2008.19.7483 [DOI] [PubMed] [Google Scholar]
- 77.Panda SP, Chinnaswamy G, Vora T, Prasad M, Bansal D, Kapoor G, et al. . Diagnosis and Management of Rhabdomyosarcoma in Children and Adolescents: ICMR Consensus Document. Indian J Pediatr 2017; 84: 393–402. doi: 10.1007/s12098-017-2315-3 [DOI] [PubMed] [Google Scholar]
- 78.Norman G, Fayter D, Lewis-Light K, Chisholm J, McHugh K, Levine D, et al. . An emerging evidence base for PET-CT in the management of childhood rhabdomyosarcoma: systematic review. BMJ Open 2015; 5: e006030. doi: 10.1136/bmjopen-2014-006030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borinstein SC, Steppan D, Hayashi M, Loeb DM, Isakoff MS, Binitie O, et al. . Consensus and controversies regarding the treatment of rhabdomyosarcoma. Pediatr Blood Cancer 2018; 65: e26809. doi: 10.1002/pbc.26809 [DOI] [PubMed] [Google Scholar]
- 80.Reilly BK, Kim A, Peña MT, Dong TA, Rossi C, Murnick JG, et al. . Rhabdomyosarcoma of the head and neck in children: Review and update. Int J Pediatr Otorhinolaryngol 2015; 79: 1477–83. doi: 10.1016/j.ijporl.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 81.Scheer M, Dantonello T, Brossart P, Dilloo D, Schweigerer L, Feuchtgruber S, et al. . Importance of whole-body imaging with complete coverage of hands and feet in alveolar rhabdomyosarcoma staging. Pediatr Radiol 2018; 48: 648–57. doi: 10.1007/s00247-017-4066-8 [DOI] [PubMed] [Google Scholar]
- 82.Yang L, Takimoto T, Fujimoto J. Prognostic model for predicting overall survival in children and adolescents with rhabdomyosarcoma. BMC Cancer 2014; 14: 654. doi: 10.1186/1471-2407-14-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dharmarajan KV, Wexler LH, Gavane S, Fox JJ, Schoder H, Tom AK, et al. . Positron emission tomography (PET) evaluation after initial chemotherapy and radiation therapy predicts local control in rhabdomyosarcoma. Int J Radiat Oncol Biol Phys 2012; 84: 996–1002. doi: 10.1016/j.ijrobp.2012.01.077 [DOI] [PubMed] [Google Scholar]
- 84.Baum SH, Frühwald M, Rahbar K, Wessling J, Schober O, Weckesser M. Contribution of PET/CT to prediction of outcome in children and young adults with rhabdomyosarcoma. J Nucl Med 2011; 52: 1535–40. doi: 10.2967/jnumed.110.082511 [DOI] [PubMed] [Google Scholar]
- 85.Casey DL, Wexler LH, Fox JJ, Dharmarajan KV, Schoder H, Price AN, et al. . Predicting outcome in patients with rhabdomyosarcoma: Role of [18F]fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys 2014; 90: 1136–42. doi: 10.1016/j.ijrobp.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 86.Tovmassian D, Abdul Razak M, London K. The Role of [18F]FDG-PET/CT in predicting malignant transformation of plexiform neurofibromas in neurofibromatosis-1. Int J Surg Oncol 2016; 2016: 1–7. doi: 10.1155/2016/6162182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai LL, Drubach L, Fahey F, Irons M, Voss S, Ullrich NJ. 18F]-Fluorodeoxyglucose positron emission tomography in children with neurofibromatosis type 1 and plexiform neurofibromas: correlation with malignant transformation. J Neurooncol 2012; 108: 469–75. doi: 10.1007/s11060-012-0840-5 [DOI] [PubMed] [Google Scholar]
- 88.Azizi AA, Slavc I, Theisen BE, Rausch I, Weber M, Happak W, et al. . Monitoring of plexiform neurofibroma in children and adolescents with neurofibromatosis type 1 by [18 F]FDG-PET imaging. Is it of value in asymptomatic patients? Pediatr Blood Cancer 2018; 65: e26733. doi: 10.1002/pbc.26733 [DOI] [PubMed] [Google Scholar]
- 89.Brahmi M, Thiesse P, Ranchere D, Mognetti T, Pinson S, Renard C, et al. . Diagnostic accuracy of PET/CT-guided percutaneous biopsies for malignant peripheral nerve sheath tumors in neurofibromatosis type 1 patients. PLoS One 2015; 10: e0138386. doi: 10.1371/journal.pone.0138386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Howman-Giles R, Shaw PJ, Uren RF, Chung DKV. Neuroblastoma and other neuroendocrine tumors. Semin Nucl Med 2007; 37: 286–302. doi: 10.1053/j.semnuclmed.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 91.Xia J, Zhang H, Hu Q, you LS. Zhang L qing, Zhang A, et al. Comparison of diagnosing and staging accuracy of PET (CT) and MIBG on patients with neuroblastoma: systemic review and meta-analysis. J Huazhong Univ Sci Technol Med Sci 2017; 37: 649–60. [DOI] [PubMed] [Google Scholar]
- 92.Taggart DR, Han MM, Quach A, Groshen S, Ye W, Villablanca JG, et al. . Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and [18F]fluorodeoxyglucose positron emission tomography to evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J Clin Oncol 2009; 27: 5343–9. doi: 10.1200/JCO.2008.20.5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park JR, Bagatell R, Cohn SL, Pearson AD, Villablanca JG, Berthold F, et al. . Revisions to the international neuroblastoma response criteria: A consensus statement from the National Cancer Institute clinical trials planning meeting. J Clin Oncol 2017; 35: 2580–7. doi: 10.1200/JCO.2016.72.0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang SY, Rahim MK, Kim Y-il, Cheon GJ, Kang HJ, Shin HY, et al. . Clinical significance of pretreatment FDG PET/CT in MIBG-avid pediatric neuroblastoma. Nucl Med Mol Imaging 2017; 51: 154–60. doi: 10.1007/s13139-016-0451-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pfluger T, Piccardo A. Neuroblastoma: MIBG Imaging and New Tracers. Semin Nucl Med 2017; 47: 143–57. doi: 10.1053/j.semnuclmed.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 96.Pandit-Taskar N, Zanzonico P, Staton KD, Carrasquillo JA, Reidy-Lagunes D, Lyashchenko S, et al. . Biodistribution and dosimetry of 18F-meta-fluorobenzylguanidine: a first-in-human PET/CT imaging study of patients with neuroendocrine malignancies. J Nucl Med 2018; 59: 147–53. doi: 10.2967/jnumed.117.193169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Begent J, Sebire NJ, Levitt G, Brock P, Jones KP, Ell P, et al. . Pilot study of F18-Fluorodeoxyglucose Positron Emission Tomography/computerised tomography in Wilms’ tumour: Correlation with conventional imaging, pathology and immunohistochemistry. Eur J Cancer 2011; 47: 389–96. doi: 10.1016/j.ejca.2010.09.039 [DOI] [PubMed] [Google Scholar]
- 98.Moinul Hossain AKM, Shulkin BL, Gelfand MJ, Bashir H, Daw NC, Sharp SE, et al. . FDG positron emission tomography/computed tomography studies of Wilms’ tumor. Eur J Nucl Med Mol Imaging 2010; 37: 1300–8. doi: 10.1007/s00259-010-1396-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Misch D, Steffen IG, Schönberger S, Voelker T, Furth C, Stöver B, et al. . Use of positron emission tomography for staging, preoperative response assessment and posttherapeutic evaluation in children with Wilms tumour. Eur J Nucl Med Mol Imaging 2008; 35: 1642–50. doi: 10.1007/s00259-008-0819-9 [DOI] [PubMed] [Google Scholar]
- 100.Allen CE, Li L, Peters TL, Leung HC, Yu A, Man TK, et al. . Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol 2010; 184: 4557–67. doi: 10.4049/jimmunol.0902336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salotti JA, Nanduri V, Pearce MS, Parker L, Lynn R, Windebank KP. Incidence and clinical features of Langerhans cell histiocytosis in the UK and Ireland. Arch Dis Child 2009; 94: 376–80. doi: 10.1136/adc.2008.144527 [DOI] [PubMed] [Google Scholar]
- 102.Maarten Egeler R, van Halteren AGS, Hogendoorn PCW, Laman JD, Leenen PJM. Langerhans cell histiocytosis: fascinating dynamics of the dendritic cell-macrophage lineage. Immunol Rev 2010; 234: 213–32. doi: 10.1111/j.0105-2896.2009.00883.x [DOI] [PubMed] [Google Scholar]
- 103.Kaste SC, Rodriguez-Galindo C, McCarville ME, Shulkin BL. PET-CT in pediatric Langerhans cell histiocytosis. Pediatr Radiol 2007; 37: 615–22. doi: 10.1007/s00247-007-0467-4 [DOI] [PubMed] [Google Scholar]
- 104.Mueller WP, Melzer HI, Schmid I, Coppenrath E, Bartenstein P, Pfluger T. The diagnostic value of 18F-FDG PET and MRI in paediatric histiocytosis. Eur J Nucl Med Mol Imaging 2013; 40: 356–63. doi: 10.1007/s00259-012-2278-6 [DOI] [PubMed] [Google Scholar]
- 105.Phillips M, Allen C, Gerson P, McClain K. Comparison of FDG-PET scans to conventional radiography and bone scans in management of Langerhans cell histiocytosis. Pediatr Blood Cancer 2009; 52: 97–101. doi: 10.1002/pbc.21782 [DOI] [PubMed] [Google Scholar]
- 106.Hart A, Vali R, Marie E, Shaikh F, Shammas A. The clinical impact of 18F-FDG PET/CT in extracranial pediatric germ cell tumors. Pediatr Radiol 2017; 47: 1508–13. doi: 10.1007/s00247-017-3899-5 [DOI] [PubMed] [Google Scholar]
- 107.Murphy JJ, Tawfeeq M, Chang B, Nadel H. Early experience with PET/CT scan in the evaluation of pediatric abdominal neoplasms. J Pediatr Surg 2008; 43: 2186–92. doi: 10.1016/j.jpedsurg.2008.08.064 [DOI] [PubMed] [Google Scholar]
- 108.Kleis M, Daldrup-Link H, Matthay K, Goldsby R, Lu Y, Schuster T, et al. . Diagnostic value of PET/CT for the staging and restaging of pediatric tumors. Eur J Nucl Med Mol Imaging 2009; 36: 23–36. doi: 10.1007/s00259-008-0911-1 [DOI] [PubMed] [Google Scholar]
- 109.Towbin AJ, Meyers RL, Woodley H, Miyazaki O, Weldon CB, Morland B, et al. . 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol 20182018; 48: 536–54. doi: 10.1007/s00247-018-4078-z [DOI] [PubMed] [Google Scholar]
- 110.Philip I, Shun A, McCowage G, Howman-Giles R. Positron emission tomography in recurrent hepatoblastoma. Pediatr Surg Int 2005; 21: 341–5. doi: 10.1007/s00383-005-1406-9 [DOI] [PubMed] [Google Scholar]
- 111.Sironi S, Messa C, Cistaro A, Landoni C, Provenzi M, Giraldi E, et al. . FDG PET of hepatoblastoma in transplanted liver. AJR 2004; 182: 1214–6. [DOI] [PubMed] [Google Scholar]
- 112.Figarola MS, McQuiston SA, Wilson F, Powell R. Recurrent hepatoblastoma with localization by PET-CT. Pediatr Radiol 2005; 35: 1254–8. doi: 10.1007/s00247-005-1568-6 [DOI] [PubMed] [Google Scholar]
- 113.Cistaro A, Treglia G, Pagano M, Fania P, Bova V, Basso ME, et al. . A comparison between ¹⁸F-FDG PET/CT imaging and biological and radiological findings in restaging of hepatoblastoma patients. Biomed Res Int 2013; 2013: 709037. doi: 10.1155/2013/709037 [DOI] [PMC free article] [PubMed] [Google Scholar]