Large crystals of the Fenna–Matthews–Olson protein from Prosthecochloris aestuarii were grown in a new H3 space group that permitted room-temperature neutron diffraction data collection to 2.2 Å resolution.
Keywords: neutron crystallography, photosynthesis, hydrogen, site energy, neutron diffraction, Fenna–Matthews–Olson protein, Prosthecochloris aestuarii
Abstract
The Fenna–Matthews–Olson protein from Prosthecochloris aestuarii (PaFMO) has been crystallized in a new form that is amenable to high-resolution X-ray and neutron analysis. The crystals belonged to space group H3, with unit-cell parameters a = b = 83.64, c = 294.78 Å, and diffracted X-rays to ∼1.7 Å resolution at room temperature. Large PaFMO crystals grown to volumes of 0.3–0.5 mm3 diffracted neutrons to 2.2 Å resolution on the MaNDi neutron diffractometer at the Spallation Neutron Source. The resolution of the neutron data will allow direct determination of the positions of H atoms in the structure, which are believed to be fundamentally important in tuning the individual excitation energies of bacteriochlorophylls in this archetypal photosynthetic antenna complex. This is one of the largest unit-cell systems yet studied using neutron diffraction, and will allow the first high-resolution neutron analysis of a photosynthetic antenna complex.
1. Introduction
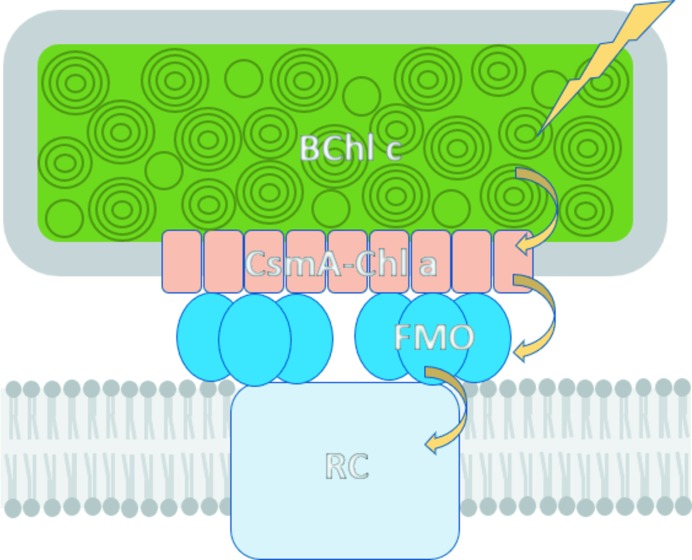
Photosynthetic antenna complexes capture photonic energy from the sun and transfer the excitation energy to the photosynthetic reaction center, driving the photochemical reactions that support most life on Earth. These complexes function by incorporating sets of specialized pigments within a protein scaffold that modulates and tunes their spectral response and energy-transfer properties. In green sulfur bacteria, the Fenna–Matthews–Olson (FMO) protein functions as a ‘wire’ that mediates energy transfer between the light-harvesting chlorosomes and the reaction center (Fig. 1 ▸; Orf & Blankenship, 2013 ▸; Blankenship, 2014 ▸). The FMO complex exists as a homotrimer, with each monomer containing seven bacteriochlorophyll (BChl) pigments embedded in a large, rigid open-face β-sandwich domain (Matthews et al., 1979 ▸). An eighth BChl is located at the interface between the subunits in the trimer (Tronrud et al., 2009 ▸). The precise organization and assembly of BChls within the array and their interactions with the surrounding protein scaffold exquisitely tune and control the energy levels of the individual pigment excitonic states, creating an energy funnel that guides the flow of energy down through the protein to the reaction center (Müh et al., 2007 ▸; Schmidt am Busch et al., 2011 ▸). While the electronic energy-transfer process in FMO has been extensively studied and is broadly understood, debate remains on the precise details of the molecular mechanisms and pathways involved (Engel et al., 2007 ▸; Panitchayangkoon et al., 2010 ▸; Duan et al., 2017 ▸).
Figure 1.
Model of the energy-transfer pathway in the photosynthetic system of P. aestuarii. Arrows show sequential energy transfer from light capture by BChl c arrays in the chlorosome, down through the CsmA protein in the baseplate and the trimeric Fenna–Matthew–Olson (FMO) complex to the photosynthetic reaction center (RC).
The X-ray structures of FMO proteins from several strains (Camara-Artigas et al., 2003 ▸; Ben-Shem et al., 2004 ▸; Larson et al., 2011 ▸) and recent mutants (Saer et al., 2016 ▸; Orf et al., 2016 ▸) show strong similarities in overall protein–pigment architectures, but the proteins exhibit differences in characteristic spectral properties that are not fully understood in terms of the available structural models. Each BChl experiences a different chemical environment within the monomer, through which interactions with the neighboring protein residues and solvent molecules distort the conformation and planarity of the tetrapyrrole ring, imparting each BChl with its own particular site energy. The local charge distribution and the hydrogen-bonding interactions with the surrounding protein and solvent are therefore thought to be important in tuning the excitation energy-transfer pathway. Although the FMO complex has been extensively characterized by X-ray crystallography, computer simulations and spectroscopic analysis, explicit information on how H atoms and water molecules may contribute to the fine-tuning of the pigment excitation energy has been lacking or inferred. Neutron diffraction is uniquely sensitive to the location of H atoms in biological structures (for a review, see O’Dell et al., 2016 ▸). Therefore, in order to better understand how individual BChl site energies are modulated by local pigment–solvent–protein interactions, we have produced a new crystal form of the FMO protein from Prosthecochloris aestuarii (PaFMO) that diffracted neutrons to a resolution of 2.2 Å at the Spallation Neutron Source (SNS) at Oak Ridge National Laboratory (ORNL). Here, we report the preparation, crystallization and preliminary X-ray and neutron analysis of this archetypal photosynthetic antenna complex.
2. Materials and methods
2.1. Macromolecule production
The PaFMO protein was purified from P. aestuarii cell cultures following established protocols (Orf et al., 2014 ▸). Briefly, the cells were disrupted by sonication, centrifuged at low speed to remove debris and centrifuged again at 186 500g for 2 h in a buffer consisting of 20 mM Tris pH 8.0. The membrane-containing pellet was resuspended in the same buffer after centrifugation, and sodium carbonate was added through dialysis to a final concentration of 0.4 M over at least 24 h. The solution was then centrifuged at 307 500g for 2 h, after which the supernatant was collected and extensively dialyzed into 20 mM Tris pH 8.0. The dialyzed material was loaded onto Q Sepharose resin (GE) and elution was carried out with an NaCl gradient, with PaFMO typically eluting at NaCl concentrations of >300 mM. Fractions containing PaFMO were pooled and concentrated before loading them onto a Superdex S75 gel-filtration column (GE) equilibrated with 20 mM Tris, 40 mM NaCl pH 8.0. The peak from the gel-filtration column was collected and concentrated before final purification on a HiTrap Q column (GE). The purified PaFMO protein was then concentrated to 12 mg ml−1 for crystallographic studies.
2.2. Crystallization
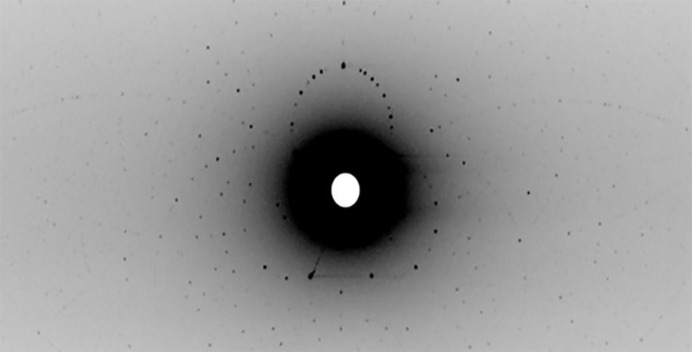
FMO crystals were grown in mother liquor consisting of 25% ammonium sulfate, 0.1 M sodium acetate pH 5.0 at 22°C by the sitting-drop vapor-diffusion method (Table 1 ▸). The crystals belonged to the trigonal space group H3, with unit-cell parameters a = b = 83.64, c = 294.78 Å. Crystals suitable for neutron diffraction analysis were grown by the sitting-drop vapor-diffusion method in a grease-sealed nine-well sandwich box with 50 ml reservoir solution. 50–1100 µl of protein solution was mixed with the same volume of reservoir solution consisting of 22.5% saturated ammonium sulfate, 0.1 M sodium acetate pH 5.0. All solutions were filtered through 0.2 µm Millipore filters. As the growth conditions were refined, the diffraction quality was assessed using the IMAGINE macromolecular neutron diffractometer at the ORNL High Flux Isotope Reactor neutron source (Meilleur et al., 2013 ▸; Schröder et al., 2018 ▸). IMAGINE uses a broad-bandpass Laue configuration (2.8 to ∼20 Å) to enable rapid (15 min exposure per frame) crystal screening (Fig. 2 ▸). Ultimately, large single crystals (with a size of about 0.3 mm3) could be reproducibly grown over two months.
Table 1. Crystallization.
| Method | Vapor diffusion |
| Plate type | Sitting drop |
| Temperature (K) | 293 |
| Protein concentration (mg ml−1) | 12 |
| Buffer composition of protein solution | 20 mM Tris, 40 mM NaCl pH 8.0 |
| Composition of reservoir solution | 22.5% saturated ammonium sulfate, 0.1 M sodium acetate pH 5.0 |
| Volume and ratio of drop | 1100 µl (1:1) |
| Volume of reservoir (ml) | 50 |
Figure 2.
A Laue neutron diffraction image recorded from a 1H/2H vapor-exchanged FMO crystal on the CG-4D IMAGINE beamline at the High Flux Isotope Reactor (HFIR), Oak Ridge National Laboratory.
2.3. Data collection and processing
Room-temperature X-ray data extending to 1.7 Å resolution were collected using a Rigaku MicroMax-007 HF rotating-anode X-ray generator at ORNL. The data were processed and scaled in HKL-3000 (Minor et al., 2006 ▸). Data-collection statistics are given in Table 2 ▸.
Table 2. X-ray and neutron data collection and processing.
Values in parentheses are for the outer shell.
| Neutron | X-ray | |
|---|---|---|
| Diffraction source | MaNDi, ORNL | Rigaku MicroMax-007 HF rotating-anode generator, ORNL |
| Wavelength (Å) | 2–4 | 1.5417 |
| Temperature (K) | 293 | 293 |
| Detector | 32 Anger cameras | R-AXIS IV |
| Crystal-to-detector distance (mm) | 420–450 | 120 |
| Rotation range per image (°) | 0† | 0.25 |
| Total rotation range (°) | 90 | 132 |
| Exposure time per image (s) | 48 h | 30 s |
| Space group | H3 | H3 |
| a, b, c ‡ (Å) | 83.16, 83.16, 294.32 | 83.64, 83.64, 294.78 |
| Resolution range (Å) | 14.63–2.20 (2.28–2.20) | 19.65–1.74 |
| Total No. of reflections | 29653 | 73470 |
| No. of unique reflections | 2344 | 3850 |
| Completeness (%) | 76.1 (60.3) | 93.3 (49.2) |
| Multiplicity | 2.5 (1.6) | 3.6 (3.3) |
| 〈I/σ(I)〉 | 5.7 (2.1) | 10.8 (2.4) |
| R merge | 0.173 (0.223) | 0.061 (0.409) |
| Overall B factor from Wilson plot (Å2) | 18.2 |
Still quasi-Laue diffraction images were collected at 10° steps in φ rotation at three unique crystal orientations and were combined into a single data set.
The absolute unit-cell parameters determined from X-ray monochromatic data were used in refinement. The axial ratios from Laue refinement scale to the absolute cell.
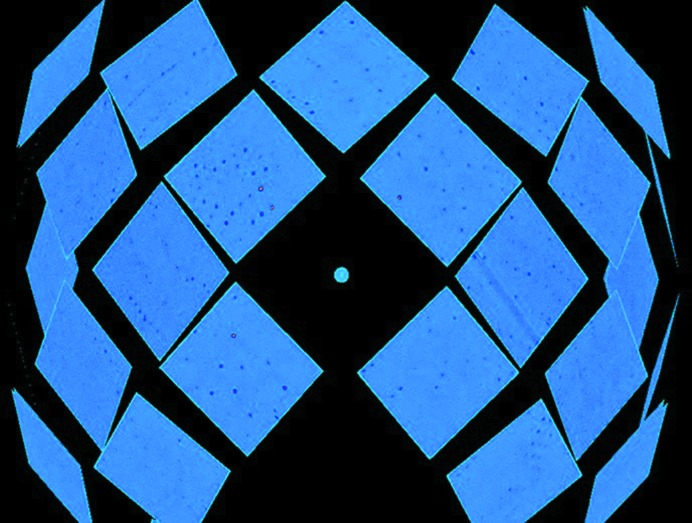
To reduce the incoherent neutron scattering background from hydrogen, exchangeable hydrogen in the crystal was replaced by deuterium through vapor-exchange equilibration against a D2O reservoir solution containing 25% saturated ammonium sulfate and 0.1 M sodium acetate (pD 5.0). Crystals were equilibrated for one month prior to neutron data collection, during which the reservoir solution was changed three times. A single PaFMO crystal of ∼0.3 mm3 in volume was mounted in a 0.8 mm diameter quartz capillary with a plug of deuterated exchange solution and sealed with wax for neutron data collection. The neutron quasi-Laue data set was collected at room temperature using the MaNDi macromolecular neutron crystallography instrument at the SNS. MaNDi is specifically designed for the analysis of large-unit-cell (>150 Å) systems (Schultz et al., 2005 ▸; Coates et al., 2015 ▸). Time-of-flight (TOF) neutron diffraction data were recorded to 2.2 Å resolution using neutrons between 2.0 and 4.0 Å wavelength. The ω angle was fixed at 90° for data collection. Nine images were collected with 48 h exposures per frame and with the crystal rotated by 10° in φ between images. A representative quasi-Laue neutron diffraction pattern from MaNDi is shown in Fig. 3 ▸. The data were processed and integrated using the Mantid package (Arnold et al., 2014 ▸) and were wavelength-normalized using LAUENORM from the LAUEGEN package (Campbell et al., 1998 ▸). LAUENORM performs a wavelength normalization of the Laue data and scaling between Laue diffraction images. Data-reduction statistics are shown in Table 2 ▸.
Figure 3.
The diffraction pattern of PaFMO from the spherical detector array of the MaNDi instrument at the Spallation Neutron Source, Oak Ridge National Laboratory.
2.4. Structure solution and refinement
The X-ray structure was solved by molecular replacement in Phaser (McCoy et al., 2007 ▸) with PDB entry 3eoj (Tronrud et al., 2009 ▸) as the starting model; the model was built in Coot (Emsley et al., 2010 ▸) and refined in PHENIX (Adams et al., 2010 ▸). Details of the X-ray structure and refinement are shown in Table 3 ▸.
Table 3. X-ray structure and refinement.
Values in parentheses are for the outer shell.
| Resolution range (Å) | 19.65–1.74 (1.804–1.740) |
| Completeness (%) | 93.4 (49.16) |
| σ Cutoff | 10.8 (2.4) |
| No. of reflections, working set | 73453 (3850) |
| No. of reflections, test set | 3693 (194) |
| Final R cryst | 0.1341 (0.1913) |
| Final R free | 0.1584 (0.2416) |
| Cruickshank DPI | 0.126 |
| No. of non-H atoms | |
| Protein | 6320 |
| Ligand | 1018 |
| Water | 474 |
| Total | 7812 |
| R.m.s. deviations | |
| Bonds (Å) | 0.011 |
| Angles (°) | 1.30 |
| Average B factors (Å2) | |
| Overall | 22.03 |
| Protein | 21.26 |
| Ligand | 20.20 |
| Water | 36.29 |
| Ramachandran plot | |
| Favored regions (%) | 98.79 |
| Additionally allowed (%) | 1.21 |
| Outliers (%) | 0 |
3. Results and discussion
PaFMO was purified from cell cultures of P. aestuarii, yielding 80 mg protein from 30 g of wet cell paste. Initial efforts to grow large crystals (>0.1 mm3) of the published P63 crystal form of PaFMO (PDB entry 3eoj), which diffract X-rays to high resolution (1.3 Å), were unsuccessful. New crystallization conditions were obtained that produced a new H3 crystal form with unit-cell parameters a = b = 83.64, c = 294.78 Å. The structure was solved at 1.7 Å resolution by molecular replacement using PDB entry 3eoj. This new crystal form has two FMO monomers in the asymmetric unit (PDB entry 6mez). Each FMO monomer contains seven BChl molecules that are held in precise orientation by the protein scaffold within a rigid ‘taco-shell’ core formed from two antiparallel β-sheets. As expected, the room-temperature structure is closely similar to the structure in PDB entry 3eoj, with a root-mean-square deviation (r.m.s.d.) of 0.240 Å for 294 Cα atoms. However, density for the eighth BChl, which is found at various occupancies in the interface between adjacent monomers in other FMO proteins (Li et al., 1997 ▸; Camara-Artigas et al., 2003 ▸, Ben-Shem et al., 2004 ▸; Larson et al., 2011 ▸), and which is modeled in two conformations in PDB entry 3eoj (Tronrud et al., 2009 ▸), was not resolved in this 1.7 Å resolution structure. We note that the eighth BChl molecule is susceptible to depletion or loss during purification of the PaFMO complex, and has been reported as lost or variably occupied in other spectroscopic and structural studies (Tronrud et al., 2009 ▸)
After extensive trials, large crystals suitable for neutron diffraction were grown from a protein concentration of 12 mg ml−1 and sitting-drop volumes of up to 2000 µl (Fig. 1 ▸). The crystals grew to a volume of about 0.3–0.5 mm3 after a month. Crystals selected for neutron diffraction were equilibrated against a deuterated reservoir solution to exchange labile H atoms with deuterium and reduce the incoherent neutron diffraction background. Ultimately, a crystal with a volume of 0.3 mm3 was selected for room-temperature neutron data collection, producing data to 2.2 Å resolution. The final data set has 29 653 reflections, which reduced to 2344 unique reflections with an R merge of 17.3%. Full processing statistics for the neutron data are reported in Table 2 ▸. We note that the R merge statistics are comparable to those reported for other neutron Laue experiments (Meilleur et al., 2013 ▸; Coates et al., 2015 ▸). The available neutron beam time (18 days) and the relatively weak diffraction from this large-unit-cell system resulted in a final data set that was 77% complete to 2.2 Å resolution, beyond which the data quality and completeness deteriorate. This study is especially notable in representing one of the largest unit-cell edge (294 Å), largest unit-cell volume (V = 1.7 × 107 Å3) and smallest crystal volume/unit cell systems yet studied using neutrons, marking a new threshold for spallation neutron protein crystallography.
Although extensively studied, the available X-ray structures of FMO lack sufficient resolution to visualize individual H atoms, and questions remain concerning the protonation states of key protein residues and how the individual site energies of each BChl may be modulated by hydrogen-bonding interactions with the protein–solvent environment. The neutron structure of PaFMO (the first neutron structure of a photosynthetic complex) will aid in directly visualizing these interactions. The initial nuclear density for the backbone amides and solvent molecules indicates extensive hydrogen-bonded protein–pigment–solvent networks. Refinement of the structure using both neutron and high-resolution X-ray data is ongoing. Thus, a fully refined structure of FMO may yield detailed insights into the specific local environment around each BChl and help to understand how the protein–pigment–solvent interactions contribute to the efficient energy-transfer dynamics.
Supplementary Material
PDB reference: Fenna–Matthews–Olson antenna complex, 6mez
Acknowledgments
This research used resources at the High Flux Isotope Reactor and the Spallation Neutron Source, which are DOE Office of Science User Facilities operated by Oak Ridge National Laboratory.
Funding Statement
This work was funded by U.S. Department of Energy, Basic Energy Sciences grant DE-SC 0001035 to Robert E. Blankenship and Dean A. A. Myles.
References
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. [DOI] [PMC free article] [PubMed]
- Arnold, O., Bilheux, J. C., Borreguero, J. M., Buts, A., Campbell, S. I., Chapon, L., Doucet, M., Draper, N., Ferraz Leal, R., Gigg, M. A., Lynch, V. E., Markvardsen, A., Mikkelson, D. J., Mikkelson, R. L., Miller, R., Palmen, K., Parker, P., Passos, G., Perring, T. G., Peterson, P. F., Ren, S., Reuter, M. A., Savici, A. T., Taylor, J. W., Taylor, R. J., Tolchenov, R., Zhou, W. & Zikovsky, J. (2014). Nucl. Instrum. Methods Phys. Res. A, 764, 156–166.
- Ben-Shem, A., Frolow, F. & Nelson, N. (2004). FEBS Lett. 564, 274–280. [DOI] [PubMed]
- Blankenship, R. E. (2014). Molecular Mechanisms of Photosynthesis, 2nd ed. Chichester: John Wiley & Sons.
- Camara-Artigas, A., Blankenship, R. E. & Allen, J. P. (2003). Photosynth. Res. 75, 49–55. [DOI] [PubMed]
- Campbell, J. W., Hao, Q., Harding, M. M., Nguti, N. D. & Wilkinson, C. (1998). J. Appl. Cryst. 31, 496–502.
- Coates, L., Cuneo, M. J., Frost, M. J., He, J., Weiss, K. L., Tomanicek, S. J., McFeeters, H., Vandavasi, V. G., Langan, P. & Iverson, E. B. (2015). J. Appl. Cryst. 48, 1302–1306.
- Duan, H.-G., Prokhorenko, V. I., Cogdell, R. J., Ashraf, K., Stevens, A. L., Thorwart, M. & Miller, R. J. D. (2017). Proc. Natl Acad. Sci. USA, 114, 8493–8498. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Engel, G. S., Calhoun, T. R., Read, E. L., Ahn, T.-K., Mancal, T., Cheng, Y.-C., Blankenship, R. E. & Fleming, G. R. (2007). Nature (London), 446, 782–786. [DOI] [PubMed]
- Larson, C. R., Seng, C. O., Lauman, L., Matthies, H. J., Wen, J., Blankenship, R. E. & Allen, J. P. (2011). Photosynth. Res. 107, 139–150. [DOI] [PubMed]
- Li, Y.-F., Zhou, W., Blankenship, R. E. & Allen, J. P. (1997). J. Mol. Biol. 271, 456–471. [DOI] [PubMed]
- Matthews, B. W., Fenna, R. E., Bolognesi, M. C., Schmid, M. F. & Olson, J. M. (1979). J. Mol. Biol. 131, 259–285. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Meilleur, F., Munshi, P., Robertson, L., Stoica, A. D., Crow, L., Kovalevsky, A., Koritsanszky, T., Chakoumakos, B. C., Blessing, R. & Myles, D. A. A. (2013). Acta Cryst. D69, 2157–2160. [DOI] [PubMed]
- Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. (2006). Acta Cryst. D62, 859–866. [DOI] [PubMed]
- Müh, F., Madjet, M. E., Adolphs, J., Abdurahman, A., Rabenstein, B., Ishikita, H., Knapp, E. W. & Renger, T. (2007). Proc. Natl Acad. Sci. USA, 104, 16862–16867. [DOI] [PMC free article] [PubMed]
- O’Dell, W. B., Bodenheimer, A. M. & Meilleur, F. (2016). Arch. Biochem. Biophys. 602, 48–60. [DOI] [PubMed]
- Orf, G. S. & Blankenship, R. E. (2013). Photosynth. Res. 116, 315–331. [DOI] [PubMed]
- Orf, G. S., Niedzwiedzki, D. M. & Blankenship, R. E. (2014). J. Phys. Chem. B, 118, 2058–2069. [DOI] [PubMed]
- Orf, G. S., Saer, R. G., Niedzwiedzki, D. M., Zhang, H., McIntosh, C. L., Schultz, J. W., Mirica, L. M. & Blankenship, R. E. (2016). Proc. Natl Acad. Sci. USA, 113, E4486–E4493. [DOI] [PMC free article] [PubMed]
- Panitchayangkoon, G., Hayes, D., Fransted, K. A., Caram, J. R., Harel, E., Wen, J., Blankenship, R. E. & Engel, G. S. (2010). Proc. Natl Acad. Sci. USA, 107, 12766–12770. [DOI] [PMC free article] [PubMed]
- Saer, R., Orf, G. S., Lu, X., Zhang, H., Cuneo, M. J., Myles, D. A. A. & Blankenship, R. E. (2016). Biochim. Biophys. Acta, 1857, 1455–1463. [DOI] [PubMed]
- Schmidt Am Busch, M., Müh, F., El-Amine Madjet, M. & Renger, T. (2011). J. Phys. Chem. Lett. 2, 93–98. [DOI] [PubMed]
- Schröder, G. C., O’Dell, W. B., Myles, D. A. A., Kovalevsky, A. & Meilleur, F. (2018). Acta Cryst. D74, 778–786. [DOI] [PubMed]
- Schultz, A. J., Thiyagarajan, P., Hodges, J. P., Rehm, C., Myles, D. A. A., Langan, P. & Mesecar, A. D. (2005). J. Appl. Cryst. 38, 964–974.
- Tronrud, D. E., Wen, J., Gay, L. & Blankenship, R. E. (2009). Photosynth. Res. 100, 79–87. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: Fenna–Matthews–Olson antenna complex, 6mez