Abstract
Background
HIV+ people are at increased risk of coronary artery disease, but the responsible mechanisms are incompletely understood. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is traditionally recognized for its importance in cholesterol metabolism; however, recent data suggest an additional, low‐density lipoprotein receptor–independent adverse effect on endothelial cell inflammation and function. We tested the hypotheses that PCSK9 levels are increased and that abnormal coronary endothelial function is related to PCSK9 serum levels in HIV+ individuals.
Methods and Results
Forty‐eight HIV+ participants receiving antiretroviral therapy with suppressed viral replication, without coronary artery disease, and 15 age‐ and low‐density lipoprotein cholesterol–matched healthy HIV− subjects underwent magnetic resonance imaging to measure coronary endothelial function, quantified as percentage change in coronary artery cross‐sectional area during isometric handgrip exercise, an endothelial‐dependent stressor; and blood was obtained for serum PCSK9 and systemic vascular biomarkers. Data are presented as mean±SD. Mean serum PCSK9 was 65% higher in the HIV+ subjects (302±146 ng/mL) than in the HIV− controls (183±52 ng/mL, P<0.0001). Coronary endothelial function was significantly reduced in the HIV+ versus HIV− subjects (percentage change in coronary artery cross‐sectional area, 2.9±9.6% versus 11.1±3.7%; P<0.0001) and inversely related to PCSK9 (R=−0.51, P<0.0001). Markers of endothelial activation and injury, P‐selectin and thrombomodulin, were also significantly increased in the HIV+ subjects; and P‐selectin was directly correlated with serum PCSK9 (R=0.31, P=0.0144).
Conclusions
Serum PCSK9 levels are increased in treated HIV+ individuals and are associated with abnormal coronary endothelial function, an established measure of vascular health.
Keywords: endothelial function, HIV, inflammation, magnetic resonance
Subject Categories: Atherosclerosis
Clinical Perspective
What Is New?
Although traditional coronary artery disease risk factors contribute to atherosclerosis in people living with HIV, this work demonstrates that elevated proprotein convertase subtilisin/kexin type 9 levels, independent of traditional coronary artery disease risk factors, are strongly and directly related to impaired coronary artery endothelial cell function, a driver of coronary atherosclerosis.
What Are the Clinical Implications?
The identified relationship between proprotein convertase subtilisin/kexin type 9 and coronary endothelial function in people living with HIV is of potential clinical importance, because it suggests that the effects of interventions designed to lower proprotein convertase subtilisin/kexin type 9 levels on coronary endothelial function should be evaluated in future studies.
If successful, it would provide an additional intervention to limit atherosclerosis and, potentially, lower the burden of heart disease in people living with HIV.
Introduction
Because of the increased use of highly active antiretroviral therapy, the life expectancy of people with HIV is dramatically improved.1 Today, HIV infection is viewed as a chronic manageable disease, and as the HIV+ population is aging, atherosclerotic cardiovascular disease (ASCVD) has emerged as a leading cause of morbidity and mortality in these patients.2, 3 Although traditional ASCVD risk factors and accelerated atherosclerosis are prevalent among HIV+ individuals,4, 5 people with HIV have a higher burden of ASCVD and cardiovascular event rates, even after adjustment for traditional cardiovascular risk factors,6 suggesting significant contributions from nontraditional factors.
Coronary endothelial function (CEF) reflects coronary endothelial cell NO release and, as such, can be used to probe pathophysiologic contributors to ASCVD.7 Abnormal CEF is an important contributor to the development and progression of ASCVD, an independent predictor of adverse cardiovascular events and, therefore, a potential target for medical interventions.8, 9 Although endothelial dysfunction in the peripheral circulation is reported in HIV+ people, peripheral endothelial function correlates only modestly with CEF10 and impaired CEF is more closely related to underlying atherosclerotic coronary disease.11, 12 Recent magnetic resonance imaging (MRI) advances now make it possible to noninvasively quantify NO‐mediated CEF.12 Our group previously used these noninvasive MRI approaches and reported that CEF is markedly impaired in HIV+ people even before the development of detectable coronary artery disease (CAD).13 Mechanisms linking HIV infection to impaired CEF, however, are not established.
Beyond its role in cholesterol homeostasis, proprotein convertase subtilisin/kexin type 9 (PCSK9), similar to HIV infection, is associated with the future risk of ASCVD, independent of traditional cardiovascular risk factors.14 In addition, prior work demonstrated that PCSK9 serum levels are elevated in HIV+ individuals, independent of low‐density lipoprotein cholesterol (LDL‐C).15 PCSK9 is expressed in human atherosclerotic plaque16 and in vascular smooth muscle and endothelial cells. In isolated cell preparations, PCSK9 stimulates vascular inflammation through activation of the nuclear factor‐κB and activation of proinflammatory macrophages.17 Elevated PCSK9, therefore, may be responsible for coronary endothelial dysfunction. We tested the hypotheses that PCSK9 serum levels are increased in HIV+ people without overt CAD compared with HIV− controls (HIV−) and, more important, that PCSK9 levels are related to abnormal coronary and systemic endothelial function in these patients.
Methods
The data and analytical methods that support the findings of this study are available from the corresponding author on reasonable request.
Patients
All subjects provided written informed consent to participate in the protocol, which was approved by the Johns Hopkins Medicine Institutional Review Board. Two groups of participants were recruited: first, healthy participants without HIV and without CAD, defined as those without a history of known CAD, without >1 CAD risk factor (HIV−, n=15), and, if >40 years, an Agatston coronary artery calcium score18 on a clinically indicated computed tomographic scan of 0; second, participants with a diagnosis of HIV without CAD, defined as no cardiac history (no history of myocardial infarction, angina, or CAD) and a coronary artery calcium score of 0 within the prior 5 years (HIV+, n=48). All of the HIV+ cohort were receiving highly active antiretroviral therapy. Patients were recruited from outpatient clinics at the Johns Hopkins Hospital and had no contraindications to MRI.
MRI Study Protocol
A commercial 3.0‐T whole‐body MR scanner (Achieva; Philips, Best, the Netherlands) with a 32‐element cardiac coil for signal reception was used. Detailed MR parameters were previously reported.11, 12, 19 Participants underwent MRI in the morning in the fasting state (>8 hours) before the administration of any prescribed vasoactive medications. Images were taken perpendicular to a proximal or midcoronary arterial segment that was straight over a distance of ≈20 mm.20 To ensure that slice orientation was perpendicular to the coronary artery, double oblique scout scanning was performed, as previously reported.19 Anatomical images were collected at baseline and during 4 to 7 minutes of continuous isometric handgrip exercise (IHE) using an MRI‐compatible dynamometer (Stoelting, Wood Dale, IL)21 at 30% of maximum grip strength and while being directed by a research nurse. When 2 coronary artery segments per participant could be imaged, the results for each were quantified and averaged. In a subset of subjects (n=30, 21 HIV+ and 9 HIV−), we identified the internal mammary artery (IMA) in the same imaging plane as the right coronary artery. In this subset, we simultaneously acquired IMA and right coronary artery area and flow measures before and during IHE so as to measure both systemic function and CEF simultaneously.10 Heart rate and blood pressure were measured throughout using noninvasive and MRI‐compatible electrocardiogram and calf blood pressure monitors (Invivo; Precess, Orlando, FL). The rate pressure product was calculated as follows: systolic blood pressure×heart rate.
Image Analysis
Baseline and IHE‐stress images for coronary cross‐sectional area (CSA) and IMA area were analyzed, as previously described.11, 19 Coronary flow velocity was measured in cm/s, and coronary blood flow (CBF) was calculated using the following equation: coronary artery CSA×coronary artery peak diastolic velocity×0.3, as previously described.12, 19, 22 Segments with poor image quality (blurring attributable to artifact/patient motion) on either the baseline or stress examinations were excluded from analysis. Both per‐segment and per‐patient analyses were performed. MRI interpretation was performed by two independent readers (A.H. and Y.A.) who were blinded to clinical and laboratory information at the time of analysis.
Laboratory Measurements
Venous blood samples were collected on the day of the MRI study after an overnight fast to determine the concentration of PCSK9 and a vascular activity/function panel (ie, intercellular adhesion molecules 1 and 3, vascular cell adhesion molecule‐1, E and P selectins, and thrombomodulin) in all participants. Serum samples were analyzed for the above‐mentioned markers using commercially available ELISA kits (quantikine ELISA; Bio‐Techne Corporation, Minneapolis, MN). A fasting lipid panel, CD4+ cell count, HIV RNA, and hepatitis C virus antibody measurements were obtained on the day of the study or were available in the electronic medical records within the prior 6 months.
Statistical Analysis
Statistical analysis was performed with Stata 14.2 (StataCorp LLC, College Station, TX) and Prism 7.00 (GraphPad Software, La Jolla, CA). The data were tested for normality using the Shapiro‐Wilk test. Baseline characteristics between the 2 groups were compared using the Student t test for continuous variables and Fisher's exact test for categorical variables. Parametric (Student t test) and nonparametric (Wilcoxon signed rank test for paired data and Wilcoxon rank sum test for nonpaired data) tests were used when appropriate for normally distributed and skewed data, respectively, to compare the percentage changes in CSA and CBF from rest to IHE; and to compare CEF and systemic vascular function variables between the 2 groups. Results are presented as mean±SD or as median (interquartile range) when appropriate and as noted in Results. We performed robust regression analysis to assess the linear association between serum PCSK9 levels and measures of percentage change in CSA and percentage change in CBF. Robust regression is an alternative form of regression analysis that is stable with respect to violations of assumptions for ordinary least squares regression procedures, particularly in the presence of outliers.23 Our regression analysis was adjusted for sex, smoking, hypertension, diabetes mellitus, statin use, LDL‐C, high‐density lipoprotein cholesterol, and triglyceride. Statistical significance was defined as a 2‐tailed P≤0.05.
Results
Patient Characteristics
The study cohort characteristics are presented in Table 1. The treated HIV+ participants and HIV− controls differed significantly in terms of male sex (HIV+ versus HIV−, 39 [81%] versus 7 [47%]; P=0.0169), statin use (HIV+ versus HIV−, 13 [27%] versus 0; P=0.0271), and high‐density lipoprotein cholesterol levels (HIV+ versus HIV−, 52±15 versus 63±17 mg/dL; P=0.0194). No significant differences were present in the other demographic or clinical variables, and in particular LDL‐C and menopausal status did not differ between the study groups. All HIV+ individuals were receiving highly active antiretroviral therapy with suppression of HIV RNA level (100% with HIV RNA <20 copies/mL) and a median CD4+ cell count of 665 (interquartile range, 456–933) cells/μL. None of the study participants had detectable hepatitis C virus antibodies or were intravenous drug users.
Table 1.
Demographics of the Study Cohort
| Characteristics | HIV− (n=15) | HIV+ (n=48) | P Value |
|---|---|---|---|
| Age, mean±SD, y | 49±8 | 52±12 | 0.3691 |
| Male sex, n (%) | 7 (47) | 39 (81) | 0.0169a |
| BMI, mean±SD, kg/m2 | 26±4 | 28±4 | 0.0961 |
| PCI, n (%) | 0 | 0 | ··· |
| CABG, n (%) | 0 | 0 | ··· |
| Hypertension, n (%) | 3 (20) | 12 (25) | 1.0000 |
| Diabetes mellitus, n (%) | 0 | 1 (2) | 1.0000 |
| Smoker, n (%) | 0 | 10 (21) | 0.1000 |
| ACE‐inhibitor use, n (%) | 1 (7) | 7 (15) | 0.6673 |
| Statin use, n (%) | 0 | 13 (27) | 0.0271a |
| β‐Blocker use, n (%) | 0 | 2 (4) | 1.0000 |
| Aspirin use, n (%) | 0 | 6 (13) | 0.3213 |
| LDL, mean±SD, mg/dL | 101±28 | 106±43 | 0.6745 |
| HDL, mean±SD, mg/dL | 63±17 | 52±15 | 0.0194a |
| Triglycerides, mean±SD, mg/dL | 93±50 | 121±58 | 0.0976 |
| Non‐HDL, mean±SD, mg/dL | 119±37 | 129±43 | 0.4207 |
| HAART use, n (%) | N/A | 48 (100) | ··· |
| NRTI use, n (%) | N/A | 44 (92) | ··· |
| NNRTI use, n (%) | N/A | 5 (17) | ··· |
| PI use, n (%) | N/A | 0 (0) | ··· |
| Current abacavir use, n (%) | N/A | 34 (71) | ··· |
| CD4+ cell count, median (IQR), cells/µL | N/A | 665 (456–933) | ··· |
| Viral load <20 copies/mL, n (%) | N/A | 48 (100) | ··· |
Values are expressed as number (percentage) or mean±SD, except CD4+ cell count. Student t test and Fisher's exact test were used for continuous and categorical data analysis, respectively. ACE indicates angiotensin‐converting enzyme; BMI, body mass index; CABG, coronary artery bypass grafting; CD4+, cluster of differentiation 4; HAART, highly active antiretroviral therapy; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; N/A, not applicable; NNRTI, non‐NRTI; NRTI, nucleoside reverse transcriptase inhibitor; PCI, percutaneous coronary intervention; PI, protease inhibitor.
Statistically significant difference between HIV− and HIV+.
MRI Assessment of CEF
All participants completed the MRI‐IHE protocol, and there were no significant differences in rate pressure product at rest or during IHE between the 2 groups. Representative MR images demonstrating CEF testing during IHE in an HIV+ participant are shown in Figure 1. In the HIV− group, coronary arteries dilated significantly with IHE (baseline cross‐sectional area, 11.9±4.3 mm2, with a stress‐induced area change of 11.1±3.7% [P<0.0001] versus baseline). Coronary arteries from HIV+ individuals minimally dilated with IHE (baseline area, 12.1±3.4 mm2, with a stress‐induced area change of 2.9±9.6% [P=0.0491] versus baseline; Figure 2A). In the HIV− group, CBF increased significantly with IHE (baseline flow, 41.4±15.3 mL/min, with a stress‐induced change of 37.5±10.0% [P<0.0001] versus baseline), whereas blood flow did not significantly increase in HIV+ individuals (baseline flow, 38.6±19.0 mL/min, with a stress‐induced change of 6.2±19.2% [P=0.2238]; Figure 2B). The CSA and CBF changes with IHE were significantly less in the HIV+ than in the HIV− group (P<0.0001 for both the CSA and the CBF changes between the HIV− and HIV+ groups). In an adjusted multivariable robust regression analysis, the IHE‐induced changes in CSA (P=0.0027) and in CBF (P=0.0025) remained significantly and independently reduced in the HIV+ compared with the HIV− cohort. These observations of impaired CEF in HIV+ individuals compared with risk factor–matched HIV− individuals are consistent with a prior study from our group.13
Figure 1.
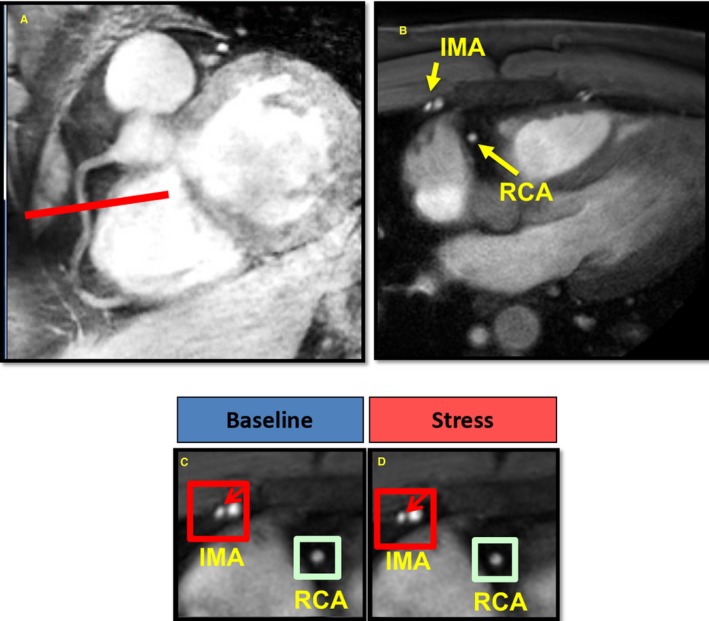
Representative magnetic resonance imaging (MRI) of the right coronary artery (RCA) and internal mammary artery (IMA) in an HIV‐infected individual. A, In this MRI scan, a scout scan obtained parallel to the RCA in a subject with HIV is shown together with the location for cross‐sectional imaging (red line). B, A view perpendicular to the RCA is shown in which the IMA is also seen (RCA and IMA labeled in yellow). The areas of the RCA and IMA are zoomed in at rest (C) and during isometric handgrip exercise (IHE; D). The red arrows point to the IMA. In this HIV+ individual, there was an abnormal response in both the IMA and RCA to IHE, with no increase in IMA or RCA area with handgrip.
Figure 2.
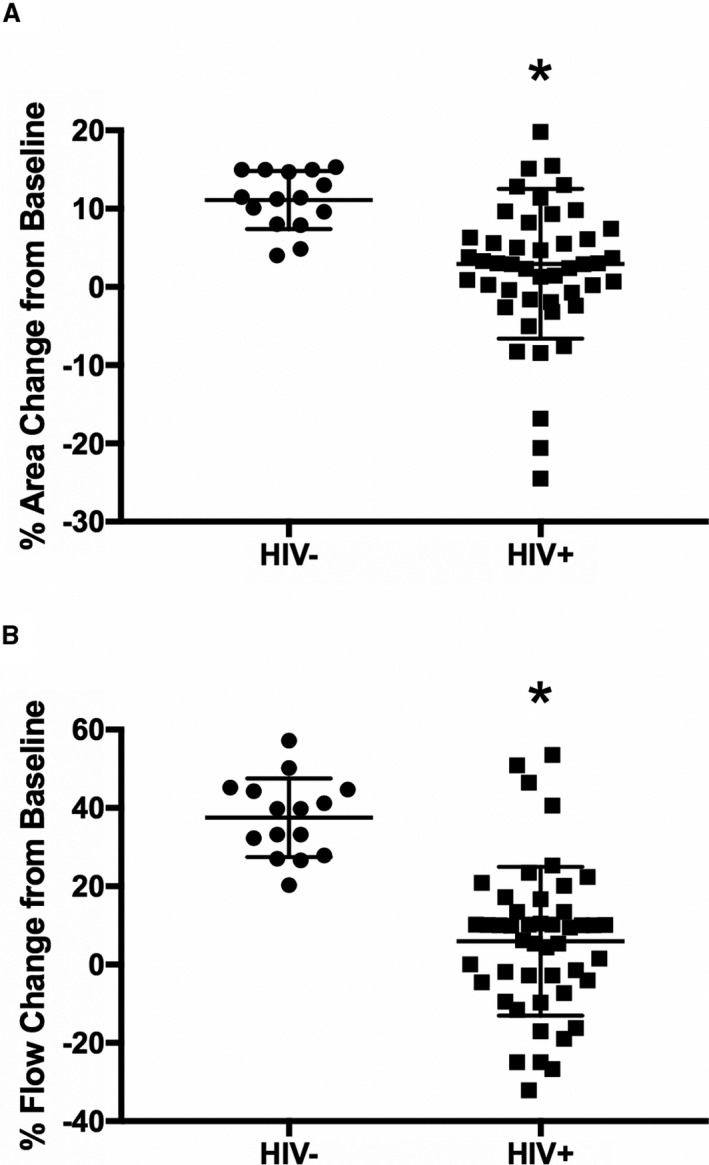
Magnetic resonance imaging assessment of coronary endothelial function in the HIV− and HIV+ cohorts. Percentage changes from baseline in coronary artery cross‐sectional area (CSA; A) and coronary blood flow (CBF; B) during isometric handgrip stress are shown for healthy subjects (HIV−, n=15) and HIV−infected individuals (HIV+, n=48). In an adjusted multivariable robust regression analysis, the isometric handgrip exercise–induced changes in CSA (P=0.0027) and in CBF (P=0.0025) remained significantly and independently reduced in the HIV+ compared with the HIV− cohort. Regression analysis was adjusted for sex, smoking, hypertension, diabetes mellitus, statin use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglyceride. Bars indicate SDs. *P<0.0001 vs HIV−.
Serum PCSK9 Levels and CEF
PCSK9 serum levels were significantly higher in the treated HIV+ than in the HIV− cohort (302±146 versus 183±52 ng/mL; P<0.0001; Figure 3A). In addition, CEF, as measured by percentage change in coronary artery CSA with IHE, was inversely related to serum PCSK9 levels in the entire study cohort (Figure 3B; R=−0.51, R 2=0.26, CEF= −0.03394× PCSK9+14.17, P<0.0001) and in the HIV+ group alone (R=−0.47, R 2=0.22, P=0.0008) but not in the HIV− group alone (R=0.33, R 2=0.10, P=0.2409). The association remained significant after correction with a robust regression analysis (P=0.0015 for PCSK9 versus % CSA); for every 100‐unit increase in serum PCSK9, there is an expected absolute 1.98% decrease in CSA with IHE (P=0.0290). PCSK9 level did not significantly correlate with CBF (R=−0.07, R 2=0.01, CSA=−0.0118×PCSK9+16.87, P=0.5617). Because statin medications can influence PCSK9 serum levels through activation of the sterol regulatory element‐binding protein‐2,24 we also assessed whether higher PCSK9 serum levels in the HIV+ individuals were related to statin use and found no significant correlation (P=0.2990).
Figure 3.
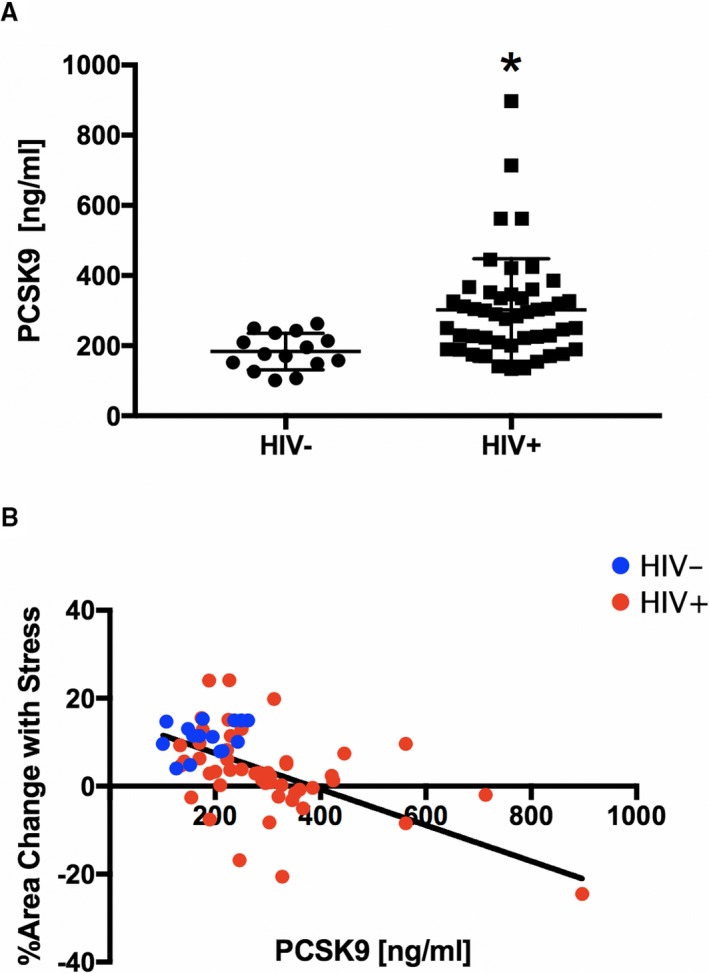
Serum proprotein convertase subtilisin/kexin type 9 (PCSK9) measures and correlation with coronary endothelial function (CEF). A, PCSK9 serum levels in healthy controls (HIV−, n=15) and HIV‐infected individuals (HIV+, n=48). B, Individual data points illustrate the inverse relationship between CEF, measured as relative change in coronary artery cross‐sectional area (CSA) in response to isometric handgrip stress, and serum PCSK9 levels (R=−0.51, R 2=0.26, CSA=−0.03394×PCSK9+14.17, P<0.0001; HIV+ alone, R=−0.47, R 2=0.22, P=0.0008; but not in HIV− alone, R=0.33, R 2=0.10, P=0.2409). The relationship between CEF and PCSK9 remains significant if the HIV+ subject with the PCSK9 value of 897 ng/mL is excluded (R=−0.37, R 2=0.14, P=0.0028). In addition, the association remained significant after correction with a robust regression analysis (P=0.0015 for PCSK9 vs % CSA). Regression analysis was adjusted for sex, smoking, hypertension, diabetes mellitus, statin use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglyceride. HIV+, red circles; and HIV− controls, blue circles. *P<0.0001 vs HIV−.
MRI Assessment of Systemic Vascular Function
In the subset of subjects (n=30) in whom the IMA was also imaged, we quantified percentage change in IMA vasoreactivity in response to IHE as a measure of systemic endothelial function, as previously reported.10 Similar to the coronary arteries, IMAs in healthy subjects (HIV−) dilated significantly with IHE (baseline CSA, 9.8±2.8 mm2, with a stress‐induced change of 10.2±7.0% [P=0.0048] versus baseline). The vasodilatory response of the IMA tended to be impaired in HIV+ individuals (baseline CSA, 9.9±2.9 mm2, with a stress‐induced change of 4.5±5.0% [P=0.0653]). Representative MR images demonstrating impaired IMA vasoreactivity during IHE in an HIV+ participant are shown in Figure 1. HIV− controls had a significantly better vasodilatory response of the IMA to IHE compared with HIV+ individuals (P=0.0003; Figure 4A).
Figure 4.
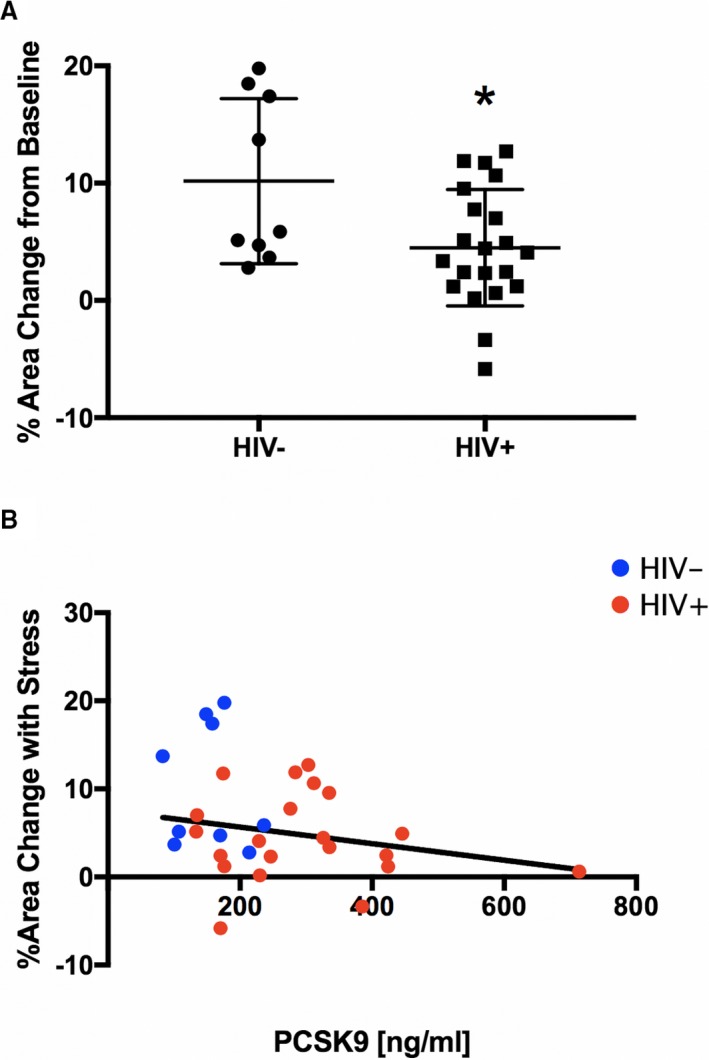
Magnetic resonance imaging assessment of systemic endothelial function. A, Percentage change from baseline in internal mammary artery (IMA) area during isometric handgrip stress shown for healthy subjects (HIV−, n=9) and HIV‐infected individuals (HIV+, n=21). B, Correlation between systemic endothelial function, measured as relative change in cross‐sectional IMA area, and serum proprotein convertase subtilisin/kexin type 9 (PCSK9; R=−0.31, R 2=0.09, CSA=−0.01408 ×PCSK9+9.778, P=0.1012). HIV+, red circles; and HIV−, blue circles. Bars indicate SDs. *P=0.0003 vs HIV−.
Relationship Between PCSK9 Serum Levels and Systemic Endothelial Function
Serum PCSK9 serum levels in the subset of subjects who had IMA imaging were significantly higher in the treated HIV+ individuals (296±135 ng/mL) than in the HIV− controls (155±52 ng/mL, P=0.0053). Although there was a trend for an inverse correlation of PCSK9 serum levels with systemic endothelial function (R=−0.31, R 2=0.09, CSA=−0.01408 ×PCSK9+9.778), this did not reach statistical significance (P=0.1012; Figure 4B).
Systemic Endothelial Activation/Injury and Relation to PCSK9 Serum Levels
Coronary endothelial dysfunction is one of the earliest, local markers of the development of coronary atherosclerosis. To determine whether PCSK9 in HIV+ individuals is also associated with established biomarkers of early systemic endothelial activation and downstream endothelial injury, we measured serum markers of vascular activation/injury, including soluble intercellular adhesion molecule‐1, soluble vascular cell adhesion molecule‐1, E‐selectin, P‐selectin, soluble intercellular adhesion molecule‐3, and thrombomodulin (Table 2). Compared with HIV− controls, both P‐selectin, a marker of early endothelial activation (80.1±28.7 versus 48.1±16.0 ng/mL; P=0.0001; Figure 5A), and thrombomodulin, a marker of endothelial injury (4.0±1.1 versus 3.2±1.1 ng/mL; P=0.0222; Figure 5B), were significantly increased in HIV+ individuals. More important, P‐selectin was significantly and directly related to serum PCSK9 (R=0.31, R 2=0.09, P‐selectin=1.446×PCSK9+168.8, P= 0.0144, Figure 5C).
Table 2.
Serum Endothelial Activation and Injury Biomarkers in the HIV+ and HIV− Cohorts
| Serum Marker | HIV− (n=15) | HIV+ (n=48) | P Value |
|---|---|---|---|
| SAA, mean±SD, pg/mL | 3.5±3.3 | 4.3±3.2 | 0.4047 |
| sICAM‐1, mean±SD, ng/mL | 603.1±188.5 | 554.9±182.9 | 0.3798 |
| sVCAM‐1, mean±SD, ng/mL | 570.4±122.6 | 605.6±171.0 | 0.4632 |
| E‐selectin, mean±SD, ng/mL | 13.8±7.9 | 17.2±7.8 | 0.1469 |
| P‐selectin, mean±SD, ng/mL | 48.1±16.0 | 80.1±28.7 | 0.0001a |
| sICAM‐3, mean±SD, ng/mL | 0.8±0.2 | 1.0±0.3 | 0.0819 |
| Thrombomodulin, mean±SD, ng/mL | 3.2±1.1 | 4.0±1.1 | 0.0222a |
Values are expressed as mean±SD. Student t test was used for continuous data analysis. SAA indicates serum amyloid A; sICAM, soluble intercellular adhesion molecule; sVCAM, soluble vascular cell adhesion molecule.
Statistically significant difference between HIV− and HIV+.
Figure 5.
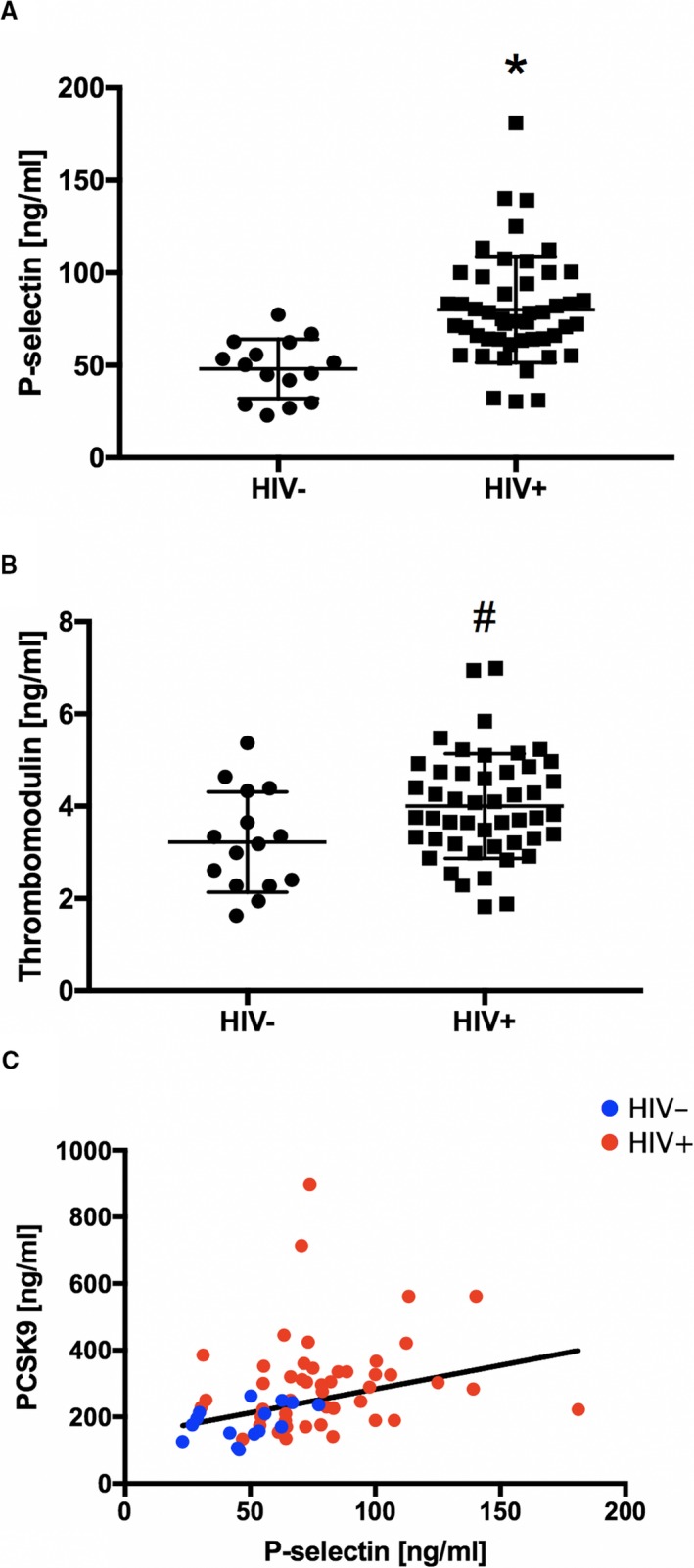
Serum proprotein convertase subtilisin/kexin type 9 (PCSK9) levels and biomarkers of systemic endothelial activation. P‐selectin (A) and thrombomodulin (B) in healthy controls (HIV−, n=15) and HIV‐ infected individuals (HIV+, n=48). C, Individual data points are shown (HIV+, red circle; HIV−, blue circles) illustrating the relationship between P‐selectin and serum PCSK9 levels (R=0.31, R 2=0.09, P‐selectin=1.446×PCSK9+168.8, P=0.0144). Bars indicate SDs. *P=0.0001, #P=0.0222 vs HIV−.
Discussion
This study demonstrates that serum PCSK9 levels are significantly higher in treated HIV+ individuals than in HIV− subjects and confirms our prior findings that CEF is significantly impaired in HIV+ people, despite adequate CD4+ counts, undetectable viral loads, and LDL‐C levels equivalent to those of an HIV− age‐matched control group. More important, we report that abnormal CEF is inversely related to serum PCSK9. In addition, MRI‐assessed systemic endothelial function, as assessed by the response of the IMA, an artery that rarely develops atherosclerosis, to IHE is impaired in treated HIV+ individuals. The IMA response is also inversely related to serum PCSK9, although to a lesser degree than is the coronary endothelial response. Finally, biomarkers of early systemic endothelial activation (P‐selectin) and endothelial injury (thrombomodulin) are elevated in HIV+ subjects, and P‐selectin is significantly and positively related to serum PCSK9 levels. Although traditional CAD risk factors contribute to atherosclerosis in HIV+ people, this work demonstrates that elevated PCSK9 levels, independent of traditional CAD risk factors, are strongly and directly related to impaired CEF, a driver of coronary atherosclerosis.
Clinical studies demonstrate an association between PCSK9 and future cardiovascular events,14 independent of traditional risk factors. Specifically, elevated serum PCSK9 levels predict adverse cardiovascular events in patients with heart failure with reduced ejection fraction,25 acute coronary syndrome,26 and stable CAD.27 However, the underlying mechanisms responsible for the relationship between PCSK9 and vascular events are not well characterized, and the relationship between PCSK9 and an early marker and driver of vascular disease, impaired endothelial vasoreactivity, was not previously reported. Therefore, our study provides new insights into the relationship between PCSK9 and accelerated vascular disease in the HIV+ patient population.
Our findings that PCSK9 levels are elevated in treated HIV+ people compared with HIV− controls are similar to those reported previously.15 The explanation for the increase is not well characterized. However, the regulatory relationship between the immunocompromised state of people living with HIV and serum PCSK9 could be bidirectional. HIV infection of CD4+ cells activates the sterol regulatory element‐binding protein‐2 in the liver and adipose tissue cell nuclei.8 In vitro experiments show that sterol regulatory element‐binding protein 1 and 2 activation can transcriptionally activate PCSK9 via a sterol‐regulatory element in its proximal promoter region.28 Furthermore, a recent study examined the regulatory effects of PCSK9 on the innate immune response. The authors demonstrated that higher PCSK9 levels were associated with acute infections, such as sepsis, and adverse mortality outcomes; the author also showed that reduced PCSK9 function was associated with increased pathogen lipid clearance via the LDL receptor and improved outcomes in HIV− patients with septic shock syndrome.29 Those studies indicate, therefore, that HIV infection increases PCSK9 levels and that increased PCSK9 may hinder an immune response to the infection, with the result that HIV infection and increased PCSK9 “reinforce” one another in the patient population with HIV.
Cardiovascular event rates remain high in HIV+ patients taking statins and after adjusting for traditional risk factors,30, 31 including LDL‐C, suggesting a role for targeting nontraditional risk factors in this population. The results of our study indicate that one such nontraditional risk factor may be elevated serum PCSK9. This is consistent with two retrospective studies that reported that PCSK9 levels in patients with acute coronary syndrome are markedly elevated compared with those in patients with stable, chronic CAD.26 In addition, multivariable logistic‐regression analysis demonstrated that increased serum PCSK9 levels were associated with a higher incidence of subsequent acute myocardial infarction in the study cohort. The impact of PCSK9 on CEF in our study was not mediated by its effects on LDL‐C because there were no differences in LDL‐C in the 2 groups. Furthermore, epidemiologic studies demonstrate that elevated serum PCSK9 levels are associated with the future risk of ASCVD independent of traditional cardiovascular risk factors, including cholesterol levels.14 Basic studies suggest an association between PCSK9 and endothelial and smooth muscle inflammatory responses through PCSK9 activation of the cellular inflammatory gatekeeper, nuclear factor‐κB.32 In addition, PCSK9 stimulates macrophage release of proinflammatory cytokines,17 which play a critical role in inducing atherogenesis by increasing the proliferation and migration of media smooth muscle cells to the intima.33 Therefore, on the basis of the results of the current study and others, we posit that HIV infection triggers PCSK9 expression, resulting in endothelial inflammation and early coronary vascular dysfunction, a critical driver of the development and progression of atherosclerosis as well as associated clinical outcomes.34
Systemic vascular dysfunction, assessed by flow‐mediated dilatation of the brachial artery, was previously described in HIV+ individuals.35 Those observations of endothelial dysfunction persist even after adjustment for traditional cardiovascular risk factors,35, 36 suggesting effects of nontraditional risk factors, the virus itself, or its treatment on systemic vascular function. Our study found that systemic endothelial function, assessed by stress‐induced vasodilatation of the IMA, is also impaired in HIV+ individuals. Although more participants in the HIV+ group were receiving angiotensin‐converting enzyme inhibitors and statins, it would be expected that these would enhance endothelial function and, therefore, this difference would not be responsible for the impaired function we observed. We observed a modest, although nonsignificant, inverse relationship between IMA endothelial dysfunction and systemic PCSK9 levels. In addition, we found that selected biomarkers of systemic endothelial activation (P‐selectin) and endothelial injury (thrombomodulin) are elevated in HIV+ subjects and that P‐selectin positively correlated with serum PCSK9 levels. Taken together, our results indicate a modest relationship between PCSK9 and systemic measures of endothelial activation, injury, and vasoactive dysfunction.
The inverse relationship between PCSK9 and CEF is strong and highly significant. Prior studies showed that peripheral arterial endothelial function correlates only modestly with CEF,10, 37 and that the latter is more closely related to underlying CAD.11, 12 Furthermore, acute plaque rupture is rare in the brachial artery, where peripheral endothelial function is usually measured. Therefore, noninvasive measures of CEF may be more relevant for understanding the underlying mechanisms for coronary disease and provide complementary information to assessments of systemic endothelial function. Finally, our observation that elevated PCSK9 serum levels are significantly related to coronary endothelial dysfunction suggests that inhibition of PCSK9 may improve impaired CEF.
Study Limitations
One limitation of the study is the cross‐sectional design; the associations between serum PCSK9 levels and depressed CEF in HIV+ individuals do not demonstrate causality. Furthermore, because all HIV+ participants were receiving highly active antiretroviral therapy, we cannot exclude the possibility that HIV treatment contributes to elevated PCSK9 levels and the associated coronary artery endothelial dysfunction. Nevertheless, these observations are novel and can be used to guide future intervention studies to evaluate causality, which are beyond the scope of the current study. In addition, there may be unknown, and therefore unmeasured, confounders that could not be adjusted. However, the 2 groups were well matched in terms of cardiovascular risk factors and our analysis did adjust for potential confounders, including sex, smoking, hypertension, diabetes mellitus, statin use, LDL‐C, high‐density lipoprotein cholesterol, and triglycerides. Although our study detected an inverse relationship between endothelial‐dependent coronary vasodilation (area change) and PCSK9, there was no significant relationship between CBF change with IHE stress and PCSK9 in participants with HIV. These findings suggest that early functional atherosclerotic changes (eg, those in coronary CSA) are more closely related to PCSK9 levels than are endothelial function measures, such as CBF, that may be more influenced by distal and downstream factors that control the microcirculation and are regulated in a different manner than are the epicardial coronary arteries. The small sample size in the HIV− group may have limited the power to detect significant differences; however, we did find a nonsignificant trend between serum PCSK9 and MRI measures of systemic endothelial function. However, the sample size was sufficient to detect a significant correlation between PCSK9 and serum markers of systemic endothelial activation.
Conclusion
This study demonstrates that serum PCSK9 levels are increased and that coronary and systemic endothelial function are impaired in treated HIV+ people compared with age‐, LDL‐C–, and risk factor–matched HIV− individuals. Furthermore, the PCSK9 levels in HIV+ people are strongly related to CEF, a physiologic indicator of vascular health, and when impaired, an early marker and driver of ASCVD and a predictor of clinical cardiovascular events.38 These findings also indicate that the association of PCSK9 with CEF is not related to the traditional role of PCSK9‐mediated changes in LDL‐C, but is independent of LDL‐C. These observations support future trials testing whether PCSK9 inhibition improves coronary endothelial dysfunction in HIV+ individuals without CAD.
Sources of Funding
This work was supported by the National Institutes of Health (5T32HL007227‐42 and HL125059), the American Heart Association (17GRNT33670943), the Johns Hopkins University Center for AIDS Research (P30AI094189 and 1704611701), the Johns Hopkins Magic That Matters Grant, the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, and the Clarence Doodeman Endowment of Johns Hopkins.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e009996 DOI: 10.1161/JAHA.118.009996.)
This work was presented as an oral abstract at the American College of Cardiology Scientific Session, March 10 to 12, 2018, in Orlando, FL.
References
- 1. Antiretroviral Therapy Cohort Collaboration . Survival of HIV‐positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non‐AIDS related morbidity. BMJ. 2009;338:a3172. [DOI] [PubMed] [Google Scholar]
- 3. Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, Schouten JT, Smieja M; Working Group 2 . Epidemiological evidence for cardiovascular disease in HIV‐infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118:e29–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lang S, Mary‐Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D; French Hospital Database on HIV‐ANRS CO4 . Increased risk of myocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. [DOI] [PubMed] [Google Scholar]
- 5. Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 8. Taylor HE, Linde ME, Khatua AK, Popik W, Hildreth JE. Sterol regulatory element‐binding protein 2 couples HIV‐1 transcription to cholesterol homeostasis and T cell activation. J Virol. 2011;85:7699–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee‐Moradie F, Umpleby AM, Green DJ, Cable NT, Jones H, Cuthbertson DJ. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307:H1298–H1306. [DOI] [PubMed] [Google Scholar]
- 10. Iantorno M, Hays AG, Schar M, Krishnaswamy R, Soleimanifard S, Steinberg A, Stuber M, Gerstenblith G, Weiss RG. Simultaneous noninvasive assessment of systemic and coronary endothelial function. Circ Cardiovasc Imaging. 2016;9:e003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schar M, Gerstenblith G, Stuber M, Weiss RG. Coronary vasomotor responses to isometric handgrip exercise are primarily mediated by nitric oxide: a noninvasive MRI test of coronary endothelial function. Am J Physiol Heart Circ Physiol. 2015;308:H1343–H1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:1657–1665. [DOI] [PubMed] [Google Scholar]
- 13. Iantorno M, Schar M, Soleimanifard S, Brown TT, Moore R, Barditch‐Crovo P, Stuber M, Lai S, Gerstenblith G, Weiss RG, Hays AG. Coronary artery endothelial dysfunction is present in HIV‐positive individuals without significant coronary artery disease. AIDS. 2017;31:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leander K, Malarstig A, Van't Hooft FM, Hyde C, Hellenius ML, Troutt JS, Konrad RJ, Ohrvik J, Hamsten A, de Faire U. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. 2016;133:1230–1239. [DOI] [PubMed] [Google Scholar]
- 15. Kohli P, Ganz P, Ma Y, Scherzer R, Hur S, Weigel B, Grunfeld C, Deeks S, Wasserman S, Scott R, Hsue PY. HIV and hepatitis C‐coinfected patients have lower low‐density lipoprotein cholesterol despite higher proprotein convertase subtilisin kexin 9 (PCSK9): an apparent “PCSK9‐lipid paradox.” J Am Heart Assoc. 2016;5:e002683 DOI: 10.1161/JAHA.115.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S, Corsini A, Catapano AL. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis. 2012;220:381–386. [DOI] [PubMed] [Google Scholar]
- 17. Ricci C, Ruscica M, Camera M, Rossetti L, Macchi C, Colciago A, Zanotti I, Lupo MG, Adorni MP, Cicero AFG, Fogacci F, Corsini A, Ferri N. PCSK9 induces a pro‐inflammatory response in macrophages. Sci Rep. 2018;8:2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 19. Hays AG, Kelle S, Hirsch GA, Soleimanifard S, Yu J, Agarwal HK, Gerstenblith G, Schar M, Stuber M, Weiss RG. Regional coronary endothelial function is closely related to local early coronary atherosclerosis in patients with mild coronary artery disease: pilot study. Circ Cardiovasc Imaging. 2012;5:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stuber M, Botnar RM, Danias PG, Sodickson DK, Kissinger KV, Van Cauteren M, De Becker J, Manning WJ. Double‐oblique free‐breathing high resolution three‐dimensional coronary magnetic resonance angiography. J Am Coll Cardiol. 1999;34:524–531. [DOI] [PubMed] [Google Scholar]
- 21. Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G. Regional myocardial metabolism of high‐energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med. 1990;323:1593–1600. [DOI] [PubMed] [Google Scholar]
- 22. Hays AG, Stuber M, Hirsch GA, Yu J, Schar M, Weiss RG, Gerstenblith G, Kelle S. Non‐invasive detection of coronary endothelial response to sequential handgrip exercise in coronary artery disease patients and healthy adults. PLoS One. 2013;8:e58047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rousseeuw PJ, Leroy AM. Robust Regression and Outlier Detection. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 24. Dong B, Wu M, Li H, Kraemer FB, Adeli K, Seidah NG, Park SW, Liu J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL‐cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res. 2010;51:1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bayes‐Genis A, Nunez J, Zannad F, Ferreira JP, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Metra M, Lupon J, Voors AA. The PCSK9‐LDL receptor axis and outcomes in heart failure: BIOSTAT‐CHF subanalysis. J Am Coll Cardiol. 2017;70:2128–2136. [DOI] [PubMed] [Google Scholar]
- 26. Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, Dandona S, Roberts R, Quyyumi AA, Chen HH, Stewart AF. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS One. 2014;9:e106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng JM. Coronary artery disease: from atherosclerosis to cardiogenic shock. 2015. Erasmus University; Rotterdam. Retrieved from http://hdl.handle.net/1765/78104. Accessed 16 June 2017.
- 28. Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D, Park SW. Sterol‐dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol‐regulatory element binding protein‐2. J Lipid Res. 2008;49:399–409. [DOI] [PubMed] [Google Scholar]
- 29. Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, Christie JD, Nakada TA, Fjell CD, Thair SA, Cirstea MS, Boyd JH. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6:258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 31. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C‐reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. [DOI] [PubMed] [Google Scholar]
- 32. Ding Z, Liu S, Wang X, Deng X, Fan Y, Shahanawaz J, Shmookler Reis RJ, Varughese KI, Sawamura T, Mehta JL. Cross‐talk between LOX‐1 and PCSK9 in vascular tissues. Cardiovasc Res. 2015;107:556–567. [DOI] [PubMed] [Google Scholar]
- 33. Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. [DOI] [PubMed] [Google Scholar]
- 35. Masia M, Padilla S, Garcia N, Jarrin I, Bernal E, Lopez N, Hernandez I, Gutierrez F. Endothelial function is impaired in HIV‐infected patients with lipodystrophy. Antivir Ther. 2010;15:101–110. [DOI] [PubMed] [Google Scholar]
- 36. Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven DE, Horsburgh CR. Endothelial function in HIV‐infected persons. Clin Infect Dis. 2006;42:1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. [DOI] [PubMed] [Google Scholar]
- 38. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long‐term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]


