Abstract
Background
Few studies have shown that right ventricular (RV) function is independently related to adverse events regardless of left ventricular (LV) function in heart failure. We evaluated the prognostic value of global longitudinal strain (GLS) of both ventricles in patients with acute heart failure.
Methods and Results
We measured biventricular strains in 1824 randomly selected patients (973 men, aged 70±14 years) from a strain registry. A total of 799 patients (43.8%) died during the median follow‐up duration of 31.7 months. In univariate analysis, LVGLS and RVGLS were significantly associated with all‐cause mortality. We classified them into 4 strain groups according to LVGLS (≥9%) and RVGLS (≥12%). On Cox proportional hazards analysis, group 4 (<9% LVGLS and <12% RVGLS) had the worst prognosis, with a hazard ratio (HR) of 1.755 (95% confidence interval [CI], 1.473–2.091; P<0.001) compared with that of group 1 (≥9% LVGLS and ≥12% RVGLS). After multivariate analysis, both LVGLS (per 1% decrease; HR: 1.057; 95% CI, 1.029–1.086; P<0.001) and RVGLS (per 1% decrease; HR: 1.022; 95% CI, 1.004–1.040; P=0.014) were also significant. The HR of RVGLS <12% was higher in patients without pulmonary hypertension (assessed by maximal tricuspid regurgitation ≤2.8 m/s) after the adjustment of LVGLS (HR: 1.40 [95% CI, 1.11–1.77] versus 1.07 [95% CI, 0.88–1.30] with pulmonary hypertension; interaction, P=0.043).
Conclusions
In the patients with acute heart failure, RVGLS was significantly associated with all‐cause mortality regardless of LVGLS, and those with decreased biventricular GLS showed the worst prognosis. The predictive power of RVGLS was more prominent in the absence of pulmonary hypertension.
Keywords: heart failure, prognosis, strain echocardiography
Subject Categories: Heart Failure, Echocardiography
Clinical Perspective
What Is New?
In patients with acute heart failure, left and right ventricular global longitudinal strains (GLSs) were significantly associated with all‐cause mortality even after the adjustment of other clinical variables.
Patients with lower left ventricular GLS (<9%) and right ventricular GLS (<12%) had the worst prognosis.
In patients with pulmonary hypertension, the predictive power of right ventricular GLS was less prominent than that in patients without pulmonary hypertension.
What Are the Clinical Implications?
Measurement of left and right ventricular GLS can give prognostic information in admitted patients with acute heart failure.
Introduction
Along with left ventricular (LV) dysfunction, right ventricular (RV) systolic dysfunction has been considered a poor prognostic factor in patients with heart failure (HF).1, 2 RV systolic dysfunction has also been identified as a potent predictor of adverse clinical outcomes in recent studies, independent of LV function.3, 4 However, no large‐scale studies are currently being conducted on this topic.
Originally, strain measured using 2‐dimensional speckle tracking echocardiography (2DSTE) was introduced in the analysis of LV function, and strain values can reflect global and regional myocardial functions objectively.5 LV strain values can be used as prognostic indicators in patients with HF.6 Because they can represent intrinsic myocardial properties, their application has been extended recently for the analysis of the right ventricle and the left atrium. Recent echocardiographic guidelines recommended several indexes to measure RV systolic function.7 However, the objective quantification of the right ventricle has been problematic because of its complex shape. Among several echocardiographic parameters assessing RV function, global longitudinal strain (GLS) is an excellent index, and reduced RVGLS has been known to be a poor prognostic factor in several cardiovascular diseases.8, 9, 10 In this study, we evaluated the prognostic value of GLS of both ventricles and evaluated whether RVGLS can be an independent predictor of long‐term prognosis in patients with acute HF.
Methods
Study Population
The RVGLS and LVGLS values of 1824 randomly selected patients from the registry for STRATS‐AHF (Strain for Risk Assessment and Therapeutic Strategies in Patients With Acute Heart Failure; NCT: 03513653, https://clinicaltrials.gov/ct2/show/NCT03513653) were measured. STRATS‐AHF is a study of strain measurement in 4312 patients hospitalized for acute HF from 3 tertiary university hospitals in Korea from January 2009 through December 2016.11 Acute HF was defined as a rapid onset or worsening of HF symptoms and/or signs requiring urgent evaluation and treatment.12 We included all hospitalized patients with signs or symptoms of HF with either pulmonary congestion or objective findings of LV systolic dysfunction or structural heart disease in the study. We excluded patients with acute coronary syndrome or severe primary valvular disease who required surgery. All‐cause deaths and dates of deaths were identified in 100% of participants from their medical records or from the Ministry of Public Administration and Security. The study protocol was approved by the institutional review board of each hospital. The institutional review boards waved the need for written informed consent from the study patients. The study complied with the Declaration of Helsinki principles. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Calculation of the Sample Size
We estimated the sample size before the measurement of RVGLS using PASS 11 (NCSS Statistical Software). On the basis of previously reported data,13 we calculated the sample size to obtain a hazard ratio (HR) of 1.3 in both groups.14 A 2‐sided log‐rank test with an overall sample size of 1600 participants (800 in group 1 and 800 in group 2) achieved 99.1% power at a 0.05 significance level to detect an HR of 1.30 when the control group had an HR of 1.00. Considering the feasibility of RV strain measurement and the possibility of measurement errors in ≈20%, we attempted to measure RV strain in a total of 1920 randomly selected patients.
Echocardiographic Examination
We obtained all echocardiographic images using the standard echocardiographic technique suggested by the American Society of Echocardiography, using commercial echocardiographic machines and a 2.5‐MHz probe.7 The standard echocardiographic techniques included M‐mode, 2‐dimensional, and Doppler measurements. We recorded the tissue Doppler‐derived peak systolic and early and late diastolic velocities of the septal mitral annulus. LV end‐systolic and end‐diastolic volumes were measured from the apical 4‐ and 2‐chamber views, and LV ejection fraction (LVEF) was calculated using the biplane Simpson method.
Strain Analysis
We downloaded the echocardiographic images from the cardiac picture archiving and communication system in the DICOM (Digital Imaging and Communication in Medicine) format. These DICOM files were sent to the strain core laboratory. Strain analysis was conducted using a commercial software, TomTec (ImageArena 4.6), as described previously.15 TomTec software is vendor independent. For myocardial deformation analysis, the endocardial border was traced on the end‐systolic frame in the selected image. The end‐systolic frame was defined by the QRS complex or as the smallest LV volume during the cardiac cycle. The software automatically tracks speckles along the endocardial border and myocardium throughout the cardiac cycle. The peak longitudinal systolic strain was automatically defined as the peak negative value during the cardiac cycle. GLS in each view was calculated as the mean value of 6 segments of each apical view. LVGLS was measured as the average of GLS values from 3 apical views (4, 3, and 2 chambers). RVGLS was measured only in the apical 4‐chamber or focused RV view. Because it was difficult to separate the RV free wall from the interventricular septum with this version, we averaged all segmental strain values from the RV free wall and ventricular septum. GLS was analyzed on a single cardiac cycle in the patients with sinus rhythm; the GLS value was calculated as the average of 3 cardiac cycles in the patients with atrial fibrillation. The strain values were measured by a specialist who was blinded to the clinical data of the study population.
Statistical Analysis
Data were presented as mean±SD for continuous variables and numbers with frequencies for categorical variables. For comparisons among groups, we used the Student t test or 1‐way ANOVA for continuous variables and the χ2 test (or Fisher exact test if any expected count was <5 for a 2×2 table) for categorical variables. Because the GLS value was negative, we obtained the absolute value |x| for simpler interpretation. The correlation of LVGLS and RVGLS was calculated with the Pearson correlation coefficient. The NT‐proBNP (N‐terminal probrain natriuretic peptide) concentration was assessed using logarithmically transformed values (base 10, log [NT pro‐BNP]) because of its skewed distribution. Death data were collected from the medical records of the patients with regular clinical follow‐up, and all‐cause mortality and dates of deaths were identified by the Ministry of Public Administration and Security for the patients without regular follow‐up. A receiver operating characteristic curve analysis was used to evaluate the optimal cutoff values of LVGLS and RVGLS for the prediction of all‐cause deaths. A survival curve was plotted using the Kaplan–Meier method with comparison using the log‐rank test. The time to first adverse clinical event was analyzed using a multivariate Cox proportional hazards analysis to determine the independent predictors of mortality. Because we observed a sufficient number of adverse clinical events in our study, we included all significant variables in the univariate analysis as covariates in the multivariate analysis. However, in the case of a variance inflation factor >10 in the linear regression analysis, the variables with multicollinearity with others were excluded from the analysis. In the multivariate analysis, we analyzed the individual effects of LVGLS and RVGLS as continuous variables in analysis A and analyzed the grouping effect of each value in analysis B. The intra‐ and interobserver variabilities of LVGLS and RVGLS were evaluated in 20 random participants by 2 independent investigators by calculating the intraclass correlation coefficient. The data were analyzed using SPSS v20 (IBM) and MedCalc v12.3.0.0 (MedCalc Software). A 2‐sided P value of <0.05 was considered statistically significant.
Results
Patient Characteristics
Of the 1920 randomly selected patients with adequate echocardiographic images, RVGLS could be measured in 1824 patients (95.0%). We used all commercially available echocardiographic machines, and 60.3%, 21.5%, and 18.1% of the patients were examined using General Electric, Siemens, and Phillips echocardiographic machines, respectively.
The mean patient age was 70.4±13.8 years, 47% were women, and the mean LVEF was 39.3±15.2% (Table 1). Ischemic heart disease was found in 34% of the patients and atrial fibrillation in 30%. The median time interval between admission and echocardiographic examination was 1 day (interquartile range: 0–2 days). The mean LVGLS value was 9.7±4.7%, and the mean RVGLS value was 12.0±6.2%. RV systolic pressure was 45.2±15.2 mm Hg and had no correlation with RVGLS (r=0.040, P=0.162). In total, 975 (54%) patients had HF with preserved ejection fraction (<40% LVEF), 337 (18%) patients had HF with midrange ejection fraction (40–49% LVEF), and 51 (28%) patients had HF with preserved ejection fraction (≥50% LVEF).
Table 1.
Baseline Clinical, Laboratory, and Echocardiographic Findings According to the Presence of LV and RV Systolic Dysfunction
| Total (n=1824) | Group 1 (n=600) | Group 2 (n=324) | Group 3 (n=305) | Group 4 (n=595) | P Value | |
|---|---|---|---|---|---|---|
| Baseline clinical characteristics | ||||||
| Male sex, n (%) | 973 (53) | 277 (46) | 168 (52) | 177 (58) | 351 (59) | <0.001 |
| Age, y | 70.4±13.8 | 70.3±14.6 | 72.0±12.9 | 70.8±12.9 | 69.4±14.0 | 0.052 |
| Weight, kg | 60.0±12.9 | 61.1±13.7 | 60.0±12.4 | 59.6±12.3 | 59.3±12.6 | <0.001 |
| Height, cm | 160.1±9.5 | 158.6±9.7 | 159.3±9.0 | 160.7±9.4 | 161.6±9.2 | <0.001 |
| BMI, kg/m2 | 23.3±3.9 | 23.4±3.9 | 23.4±4.0 | 23.1±3.7 | 23.2±4.0 | 0.604 |
| NYHA Fc ≥IV, n (%) | 892 (49) | 240 (40) | 133 (41) | 179 (59) | 340 (57) | <0.001 |
| Physical examination | ||||||
| SBP, mm Hg | 131.7±28.5 | 133.4±28.5 | 133.2±30.5 | 133.9±27.5 | 128.1±27.6 | 0.002 |
| DBP, mm Hg | 76.0±17.7 | 74.6±17.0 | 75.5±18.0 | 78.3±17.4 | 76.6±18.5 | 0.022 |
| Heart rate, beats/min | 91.4±25.7 | 81.1±23.6 | 88.8±23.8 | 95.9±24.4 | 101.0±25.4 | <0.001 |
| Past medical history, n (%) | ||||||
| Atrial fibrillation | 566 (31) | 142 (24) | 133 (41) | 65 (21) | 226 (38) | <0.001 |
| Hypertension | 1169 (64) | 386 (64) | 219 (68) | 210 (69) | 354 (60) | 0.017 |
| Diabetes mellitus | 694 (38) | 204 (34) | 110 (34) | 140 (46) | 240 (40) | 0.001 |
| IHD | 615 (34) | 208 (35) | 106 (33) | 120 (39) | 181 (30) | 0.055 |
| Laboratory findings | ||||||
| Hemoglobin, g/dL | 12.3±2.3 | 11.9±2.3 | 12.2±2.3 | 12.2±2.2 | 12.8±2.3 | <0.001 |
| Creatinine, mg/dL | 1.61±1.83 | 1.64±2.1 | 1.49±1.44 | 1.67±1.73 | 1.62±1.76 | 0.604 |
| Total cholesterol, mg/dL | 151.7±43.4 | 154.8±44.1 | 143.7±39.1 | 158.8±44.6 | 149.5±43.4 | <0.001 |
| LogNT‐proBNP | 3.67±0.60 | 3.47±0.64 | 3.64±0.55 | 3.83±0.52 | 3.84±0.53 | <0.001 |
| Echocardiographic findings | ||||||
| LVEDD, mm | 54.2±9.7 | 51.1±8.5 | 50.6±8.0 | 57.1±9.4 | 57.6±10.0 | <0.001 |
| LVESD, mm | 41.7±11.6 | 37.0±9.8 | 37.1±9.2 | 46.0±11.1 | 47.7±11.5 | <0.001 |
| LVEDV, mL | 126.8±66.6 | 106.5±50.4 | 99.3±43.7 | 144.6±65.6 | 151.1±78.3 | <0.001 |
| LVESV, mL | 84.3±58.0 | 60.3±40.8 | 57.3±35.0 | 101.9±54.1 | 112.2±67.0 | <0.001 |
| LVEF, % | 39.3±15.2 | 48.1±13.3 | 46.9±13.2 | 32.8±11.4 | 29.7±11.9 | <0.001 |
| Mitral E‐velocity, m/s | 0.91±0.38 | 0.88±0.37 | 0.92±0.40 | 0.87±0.36 | 0.97±0.38 | <0.001 |
| Mitral A‐velocity, m/s | 0.74±0.31 | 0.81±0.30 | 0.76±0.30 | 0.74±0.29 | 0.63±0.30 | <0.001 |
| E′ velocity, cm/s | 5.2±2.3 | 5.7±2.6 | 5.4±2.1 | 4.6±1.9 | 4.8±2.0 | <0.001 |
| E/E′ ratio | 19.5±11.2 | 16.9±8.6 | 18.1±10.2 | 20.6±9.4 | 22.6±14.1 | <0.001 |
| RVSP, mm Hg | 45.6±20.2 | 45.5±28.8 | 46.5±17.3 | 44.2±15.2 | 45.9±14.1 | 0.652 |
| LVGLS, % | 9.7±4.7 | 14.0±3.9 | 12.1±2.6 | 6.7±1.5 | 5.5±1.9 | <0.001 |
| RVGLS, % | 12.0±6.2 | 17.6±4.7 | 8.2±2.6 | 15.8±3.6 | 6.6±2.9 | <0.001 |
| Definition of HF, n (%) | <0.001 | |||||
| HFrEF | 975 (54) | 161 (27) | 101 (31) | 230 (75) | 483 (81) | |
| HFmrEF | 337 (18) | 140 (23) | 72 (22) | 53 (17) | 72 (12) | |
| HFpEF | 512 (28) | 299 (50) | 151 (47) | 22 (7) | 40 (7) | |
| Medication at discharge (%) | ||||||
| RAS inhibitor | 63.0 | 63.0 | 61.4 | 65.6 | 62.5 | 0.732 |
| β‐Blocker | 52.6 | 54.2 | 53.7 | 54.4 | 49.5 | 0.327 |
| MRA | 41.4 | 37.7 | 42.6 | 41.0 | 44.6 | 0.103 |
Group 1: LVGLS (≥9%) and RVGLS (≥12%); group 2: LVGLS (≥9%) and RVGLS (<12%); group 3: LVGLS (<9%) and RVGLS (≥12%); group 4: LVGLS (<9%) and RVGLS (<12%). BMI indicates body mass index; DBP, diastolic blood pressure; HF, heart failure; HFmrEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IHD, ischemic heart disease; LV, left ventricular; LVEDD, left ventricular end‐diastolic dimension; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; LVESV, left ventricular end‐systolic volume; LVGLS, left ventricular global (peak systolic) longitudinal strain; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal probrain natriuretic peptide; NYHA Fc, New York Heart Association functional class; RAS, renin–angiotensin system; RV, right ventricular; RVGLS, right ventricular global (peak systolic) longitudinal strain; RVSP, right ventricular systolic pressure; SBP, systolic blood pressure.
Clinical Outcomes According to Biventricular GLS
A total of 799 patients (43.8%) died during the median follow‐up duration of 31.7 months (interquartile range: 11.6–54.4 months).
The cutoff value showing the highest sensitivity and specificity was assessed using the receiver operating characteristic curve analysis (9% for LVGLS and 12% for RVGLS). We classified the patients into 4 strain groups according to their LVGLS (≥9%) and RVGLS (≥12%) values. Group 1 included those with LVGLS ≥9% and RVGLS ≥12%; group 2 had LVGLS ≥9% and RVGLS <12%; group 3 had LVGLS <9% and RVGLS ≥12%; and group 4 had LVGLS <9% and RVGLS <12% (Figure 1). The patient characteristics by group are summarized in Table 1.
Figure 1.
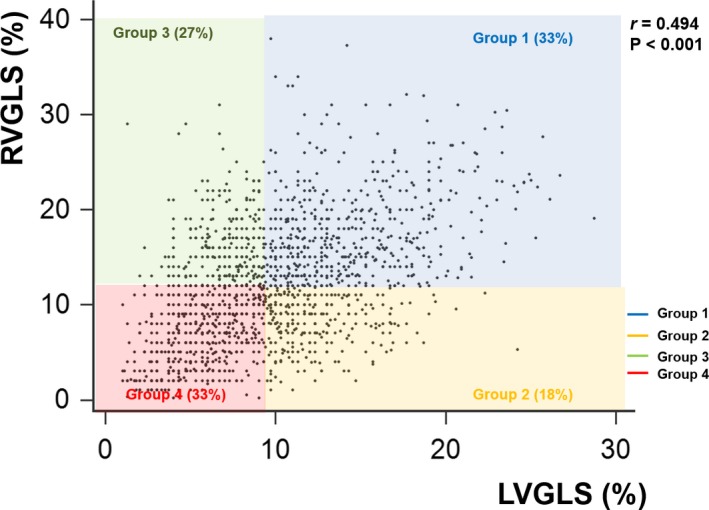
Scatter diagram according to left ventricular global longitudinal strain (LVGLS) and right ventricular global longitudinal strain (RVGLS). LVGLS shows significant correlation with RVGLS (r=0.494, P<0.001). Study patients are divided into 4 groups according to LVGLS of 9% and RVGLS of 12%.
Age and body mass index were similar among groups. Group 4 had the highest heart rate, NT‐proBNP concentration, LV dimensions, LV volumes, E/E′ ratio, and number of patients with New York Heart Association functional class IV. HF with reduced ejection fraction was the most common condition observed in group 4. However, the pattern of discharge medications was insignificant among the groups.
In the univariate analysis of all‐cause death (Table 2), age; BMI; systolic blood pressure; diastolic blood pressure; New York Heart Association functional class IV; hypertension; diabetes mellitus; ischemic heart disease; serum concentrations of hemoglobin, creatinine, total cholesterol, and NT‐proBNP; and E/E′ ratio were significant. LVGLS (per 1% decrease; HR: 1.054; 95% confidence interval [CI], 1.036–1.071; P<0.001) and RVGLS (per 1% decrease; HR: 1.033; 95% CI, 1.021–1.045; P<0.001) were also significant. Furthermore, LVGLS <9% (HR: 1.486; 95% CI, 1.291–1.709; P<0.001) and RVGLS <12% (HR: 1.405; 95% CI, 1.222–1.616; P<0.001) were significantly associated with the total mortality.
Table 2.
Univariate Analysis in the Prediction of All‐Cause Death Within 5 Years
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Age (per 1 y) | 1.048 | 1.041–1.055 | <0.001 |
| Male sex | 1.035 | 0.901–1.189 | 0.625 |
| BMI (per 1 kg/m2) | 0.925 | 0.907–0.943 | <0.001 |
| SBP (per 1 mm Hg) | 0.997 | 0.995–1.000 | 0.035 |
| DBP (per 1 mm Hg) | 0.990 | 0.986–0.994 | <0.001 |
| Heart rate (per 1 beat/min) | 1.001 | 0.998–1.004 | 0.387 |
| NYHA Fc IV | 1.452 | 1.262–1.671 | <0.001 |
| Atrial fibrillation (per 1% increase) | 1.075 | 0.927–1.246 | 0.3415 |
| Hypertension | 1.375 | 1.183–1.599 | <0.001 |
| Diabetes mellitus | 1.310 | 1.139–1.507 | <0.001 |
| IHD | 1.318 | 1.143–1.519 | <0.001 |
| Hemoglobin (per 1 g/dL) | 0.858 | 0.829–0.888 | <0.001 |
| Creatinine (per 1 mg/dL) | 1.070 | 1.041–1.099 | <0.001 |
| Total cholesterol (per 1 mg/dL) | 0.996 | 0.995–0.998 | <0.001 |
| LogNT proBNP | 2.322 | 1.943–2.774 | <0.001 |
| LVEDD (per 1 mm) | 0.992 | 0.984–0.999 | 0.033 |
| LVESD (per 1 mm) | 0.995 | 0.988–1.001 | 0.108 |
| LVEDV (per 1 mL) | 0.999 | 0.998–1.000 | 0.099 |
| LVESV (per 1 mL) | 0.999 | 0.998–1.001 | 0.342 |
| LVEF (per 1%) | 0.998 | 0.993–1.003 | 0.388 |
| E/E′ ratio (per 1) | 1.020 | 1.013–1.026 | <0.001 |
| RVSP (>31 mm Hg) | 1.344 | 1.072–1.685 | 0.010 |
| HF definition | |||
| HFrEF | Reference | 0.374 | |
| HFmrEF | 0.913 | 0.727–1.147 | 0.5241 |
| HFpEF | 1.055 | 0.895–1.243 | 0.434 |
| LVGLS (per 1% decrease) | 1.054 | 1.036–1.071 | <0.001 |
| LVGLS <9% | 1.486 | 1.291–1.709 | <0.001 |
| RVGLS (per 1% decrease) | 1.033 | 1.021–1.045 | <0.001 |
| RVGLS <12% | 1.405 | 1.222–1.616 | <0.001 |
| Strain group | <0.001 | ||
| Group 1 (LVGLS ≥9%+RVGLS ≥12%) | Reference | <0.001 | |
| Group 2 (LVGLS ≥9%+RVGLS <12%) | 1.265 | 1.019–1.569 | 0.033 |
| Group 3 (LVGLS <9%+RVGLS ≥12%) | 1.383 | 1.115–1.715 | 0.003 |
| Group 4 (LVGLS <9%+RVGLS <12%) | 1.755 | 1.473–2.091 | <0.001 |
| Use of RAS inhibitor at discharge | 0.644 | 0.560–0.741 | <0.001 |
| Use of β‐blocker at discharge | 0.596 | 0.518–0.687 | <0.001 |
| Use of MRA at discharge | 0.834 | 0.722–0.962 | 0.013 |
BMI indicates body mass index; CI, confidence interval; DBP, diastolic blood pressure; HFmrEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; IHD, ischemic heart disease; LVEDD, left ventricular end‐diastolic dimension; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; LVESV, left ventricular end‐systolic volume; LVGLS, left ventricular global (peak systolic) longitudinal strain; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal probrain natriuretic peptide; NYHA Fc, New York Heart Association functional class; RAS, renin–angiotensin system; RVGLS, right ventricular global (peak systolic) longitudinal strain; RVSP, right ventricular systolic pressure; SBP, systolic blood pressure.
Strain group, according to biventricular GLS, was a significant determinant of all‐cause death (P<0.001). In the Kaplan–Meier survival analysis, group 4 had the worst long‐term prognosis, followed by groups 3, 2, and 1 (Figure 2). In the Cox proportional hazards analysis, the HR of group 4 was 1.76 (95% CI, 1.47–2.09, P<0.001) compared with group 1; group 3 had an HR of 1.38 (95% CI, 1.12–1.72), and group 2 had an HR of 1.27 (95% CI, 1.02–1.57, P=0.033) compared with group 1.
Figure 2.
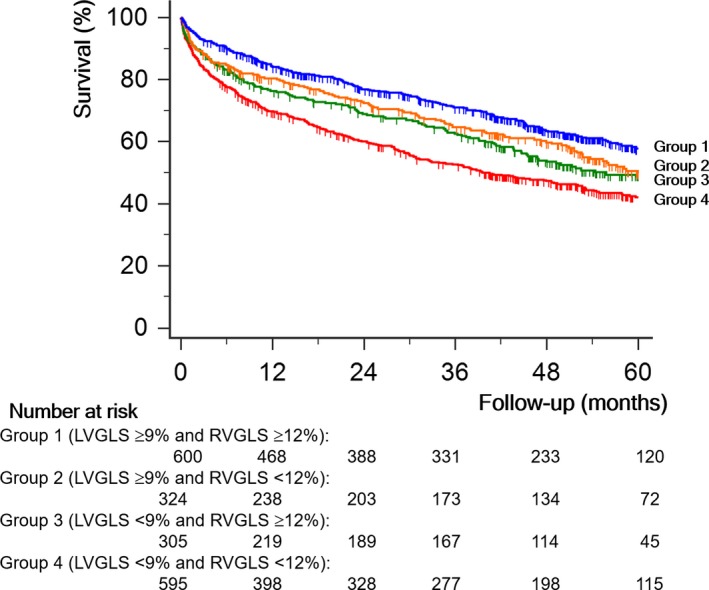
All‐cause survival curves by Kaplan–Meier analysis. Patients with impaired left ventricular global longitudinal strain (LVGLS, <9%) and impaired right ventricular global longitudinal strain (RVGLS, <12%) have the poorest all‐cause survival than other groups (P<0.001).
In the multivariate analysis (Table 3), age, male sex, systolic blood pressure, serum hemoglobin and creatinine concentrations, E/E′ ratio, and use of RAS (renin–angiotensin system) blockers and β‐blockers at discharge were significant. LVGLS (per 1% decrease; HR: 1.057; 95% CI, 1.029–1.086; P<0.001) and RVGLS (per 1% decrease; HR: 1.022; 95% CI, 1.004–1.040; P=0.014) were also significant after adjustment. In the multivariate analysis, strain was found to be significant, and group 4 had the poorest event‐free survival and all‐cause mortality (P<0.001).
Table 3.
Multivariate Analysis in the Prediction of All‐Cause Death Within 5 Years
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Analysis A | |||
| Age (per 1 y) | 1.046 | 1.036–1.056 | <0.001 |
| Male sex | 1.294 | 1.064–1.572 | 0.010 |
| SBP (per 1 mm Hg) | 0.997 | 0.993–1.000 | 0.066 |
| NYHA Fc IV | 1.150 | 0.939–1.407 | 0.177 |
| Hypertension | 1.123 | 0.898–1.405 | 0.309 |
| Diabetes mellitus | 1.060 | 0.874–1.287 | 0.553 |
| IHD | 1.080 | 0.887–1.315 | 0.443 |
| BUN (per 1 mg/dL) | 1.013 | 1.008–1.019 | <0.001 |
| Creatinine (per 1 mg/dL) | 0.997 | 0.936–1.062 | 0.924 |
| Hemoglobin, g/dL | 0.898 | 0.858–0.940 | <0.001 |
| E/E′ ratio (per 1) | 1.008 | 1.001–1.014 | 0.026 |
| LVGLS (per 1% decrease) | 1.057 | 1.029–1.086 | <0.001 |
| RVGLS (per 1% decrease) | 1.022 | 1.004–1.040 | 0.014 |
| Use of RAS inhibitor at discharge | 0.598 | 0.494–0.725 | <0.001 |
| Use of β‐blocker at discharge | 0.637 | 0.521–0.779 | <0.001 |
| Use of MRA at discharge | 1.033 | 0.848–1.258 | 0.747 |
| Analysis B | |||
| Age (per 1 y) | 1.046 | 1.036–1.056 | <0.001 |
| Male sex | 1.327 | 1.094–1.611 | 0.004 |
| SBP (per 1 mm Hg) | 0.997 | 0.993–1.000 | 0.067 |
| NYHA Fc IV | 1.148 | 0.938–1.406 | 0.180 |
| Hypertension | 1.091 | 0.879–1.363 | 0.444 |
| Diabetes mellitus | 1.043 | 0.859–1.267 | 0.669 |
| IHD | 1.087 | 0.892–1.323 | 0.408 |
| BUN (per 1 mg/dL) | 1.013 | 1.007–1.019 | <0.001 |
| Creatinine (per 1 mg/dL) | 0.998 | 0.937–1.064 | 0.958 |
| Hemoglobin, g/dL | 0.902 | 0.862–0.944 | <0.001 |
| E/E′ ratio (per 1) | 1.008 | 1.001–1.015 | 0.017 |
| Strain group | <0.001 | ||
| Group 1 (LVGLS ≥9%+RVGLS ≥12%) | Reference | <0.001 | |
| Group 2 (LVGLS ≥9%+RVGLS <12%) | 1.189 | 0.878–1.612 | 0.263 |
| Group 3 (LVGLS <9%+RVGLS ≥12%) | 1.515 | 1.147–2.000 | 0.003 |
| Group 4 (LVGLS <9%+RVGLS <12%) | 1.851 | 1.455–2.357 | <0.001 |
| Use of RAS inhibitor at discharge | 0.615 | 0.508–0.745 | <0.001 |
| Use of β‐blocker at discharge | 0.635 | 0.519–0.778 | <0.001 |
| Use of MRA at discharge | 1.035 | 0.849–1.261 | 0.734 |
BUN indicates blood urea nitrogen; CI, confidence interval; HR, hazard ratio; IHD, ischemic heart disease; LVGLS, left ventricular global (peak systolic) longitudinal strain; MRA, mineralocorticoid receptor antagonist; NYHA Fc, New York Heart Association functional class; RAS, renin–angiotensin system; RVGLS, right ventricular global (peak systolic) longitudinal strain.
Prognostic Stratification According to the Presence of Pulmonary Hypertension
When we divided our study population into 2 groups according to the presence of increased pulmonary arterial pressure (assessed by maximal velocity of tricuspid valve regurgitation >2.8 m/s). In univariate analysis, RVGLS <12% was a significant predictor of all‐cause mortality regardless of pulmonary hypertension (HR: 1.61 [95% CI, 1.30–2.00] without pulmonary hypertension versus 1.23 [95% CI, 1.03–1.48]; interaction, P=0.068). In multivariate analysis, however, RVGLS was a significant predictor of all‐cause mortality only in patients without pulmonary hypertension after adjustment LVGLS (HR: 1.40 [95% CI, 1.11–1.77] versus 1.07 [95% CI, 0.88–1.30] with pulmonary hypertension; interaction, P=0.043; Figures 3 and 4).
Figure 3.
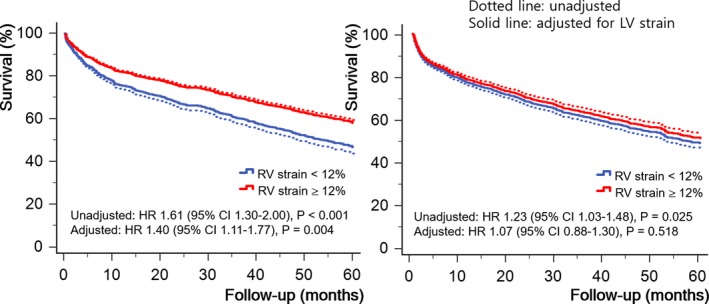
Survival curves according to the right ventricular global longitudinal strain (RVGLS) of 12%. In patients without pulmonary artery hypertension (left), RVGLS has statistical significance even after the adjustment of left ventricular global longitudinal strain (LVGLS). However, RVGLS fails to have statistical significance after the adjustment of LVGLS (right). Dotted line: unadjusted survival; solid line: adjusted survival for LVGLS. CI indicates confidence interval; HR, hazard ratio; LV, left ventricular; RV, right ventricular.
Figure 4.
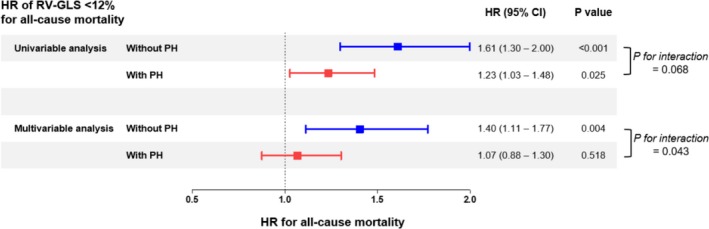
Hazard ratio (HR) of right ventricular global longitudinal strain (RVGLS) of <12% for all‐cause mortality in patients with or without pulmonary hypertension (PH). CI indicates confidence interval; HR, hazard ratio.
Variability of Strain Measurement
The intraobserver variabilities of the intraclass correlation coefficient of LVGLS and RVGLS were 0.924 (95% CI, 0.812–0.969) and 0.937 (95% CI, 0.844–0.974), respectively. The interobserver variabilities of the intraclass correlation coefficient of LVGLS and RVGLS were 0.900 (95% CI, 0.733–0.963) and 0.888 (95% CI, 0.701–0.958), respectively.
Discussion
In this study, we showed that RVGLS was significantly associated with all‐cause mortality regardless of LVGLS. Those who had decreased biventricular GLS (LVGLS <9% and RVGLS <12%) showed the worst prognosis. RVGLS has greater significance in the absence of pulmonary hypertension.
Prognostic Stratification According to Biventricular GLS
Unlike LVEF, myocardial strain values based on 2DSTE can represent myocardial deformation. These have been known to be objective and reliable markers of intrinsic myocardial contractility.5 Myocardial strain values obtained on 2DSTE, which is a simple and feasible method with good reproducibility, are strong prognostic factors among patients with HF, independent of LVEF.6, 11 In this study, LVGLS was a significant prognostic indicator of adverse clinical events (HR: 0.957; 95% CI, 0.943–0.971; P<0.001) and all‐cause death (HR: 0.949; 95% CI, 0.933–0.965; P<0.001). A cutoff value of 9% was optimal for separating patients with and without adverse clinical outcomes in our study. This result is similar to the previously reported LV cutoff value in patients with symptomatic HF.16
Along with LV dysfunction, RV dysfunction has been regarded as a poor prognostic factor in patients with HF.1, 2 Information on RV systolic function in patients with HF can provide complementary information in the stratification of patient prognosis.1 RV systolic function can be influenced by LV systolic function. Because the right ventricle is easily influenced by ventricular loading conditions, RV enlargement and RV systolic dysfunction can be caused by elevated LV end‐diastolic pressure reflected backward to the right ventricle.17 In our study, we assessed RV systolic function using RVGLS. RVGLS obtained with 2DSTE has been used as a systolic marker with considerable feasibility and reproducibility.18 The patients with a decreased RVGLS value (<12%) had an increased E/E′ ratio (21.0±13.0 versus 18.1±9.0, P<0.001), which is an echocardiographic indicator of LV end‐diastolic pressure, and left atrial diameter (47.2±9.9 mm versus 44.9±10.0 mm, P<0.001) compared with the other patients. These data suggest that the patients with decreased RVGLS values had higher LV end‐diastolic pressure.
Similar to LVGLS, RVGLS is a strong predictor of clinical outcomes in several cardiovascular diseases.8, 10, 19 In our study, the cutoff value of RVGLS in the prediction of adverse clinical outcomes was 12%. In a study of patients with advanced systolic HF awaiting heart transplantation, RVGLS showed a significant correlation with the RV systolic stroke work index, a hemodynamic parameter usually used to evaluate RV function.20 RVGLS <10.8% is the cutoff value for detection of a decreased RV systolic stroke work index (<0.25 mm Hg/L·m2). RVGLS <14.8% obtained by the velocity vector imaging algorithm was a prognostic factor in patients with HF with reduced ejection fraction (LVEF ≤35%).13
We showed that strain group based on LVGLS and RVGLS values was a significant prognostic factor in multivariate analysis. Group 1 had the best long‐term prognosis, followed by groups 2, 3, and 4. Although group 2 seemed to have a higher survival rate than group 3, there was no significant difference between them. We think this phenomenon may have originated from ventricular interdependence. The left and right ventricles have a common interventricular septum and specific myocardial fiber orientation. RV systolic function can be influenced by LV systolic function. LV contraction can account for ≈20% to 40% of RV systolic pressure, and RV contraction has been shown to influence ≈4% to 10% of LV systolic pressure in several experiments.21 The effect of RV dysfunction on long‐term prognosis may be low because a relatively healthy left ventricle can overcome RV dysfunction. Moreover, LV systolic dysfunction can activate the neurohumoral system and affect RV systolic function.22
Prognosis According to RV Function and Pulmonary Artery Systolic Pressure
As a general rule, pulmonary hypertension caused by left HF is coupled with RV systolic dysfunction.23, 24, 25 However, this relationship between pulmonary arterial pressure and RV systolic dysfunction in chronic HF is not always present because RV systolic function may adapt over time in response to an increase in RV afterload. As discussed earlier, RV enlargement and RV systolic dysfunction can be caused by elevated LV end‐diastolic pressure reflected backward to the right ventricle because the right ventricle is easily influenced by ventricular loading conditions in chronic HF.
The pathophysiology and prognosis of RV dysfunction in acute HF may differ from those in chronic HF. Consequently, pulmonary hypertension related to LV failure was the most important cause of RV dysfunction in our study. Pulmonary arterial systolic pressure was significantly higher in the patients with a decreased RVGLS value (46.1±15.2 versus 44.2±15.1 mm Hg). In the patients with elevated pulmonary arterial pressure, reduced LV strain rather than RV strain was the major determinant of all‐cause mortality; however, all‐cause mortality between the patients with reduced RV and LV strains in normal pulmonary arterial pressure was similar. In the patients with a normal pulmonary arterial pressure, the decreased RVGLS value may have resulted from intrinsic RV muscular dysfunction rather than passive transmission of increased pulmonary arterial pressure. Thus, patients with a decreased RVGLS value might have an intrinsic myocardial dysfunction, which can influence prognosis.
Our results are different from those of the study by Ghio et al,25 who showed that the assessment of RV function did not improve the prognostic stratification of patients with HF and normal pulmonary arterial pressure. This difference might have been observed because of the different study populations and methods of measuring RV systolic function. They included patients with chronic HF and measured the RV ejection fraction via right heart catheterization. Conversely, we studied patients with acute HF and measured GLS using 2DSTE. The pathophysiology of RV dysfunction in acute HF is different from that in chronic HF. In acute HF, pulmonary artery pressure increased by congestion might worsen RV function. Moreover, RVGLS could represent an intrinsic myocardial property that could not be measured using volumetric methods.
Limitations
The study has several limitations. First, this study was retrospective, without a standardized protocol that used only 1 echocardiographic machine or acquired a focused RV view in the echocardiographic examinations. Moreover, the treatment pattern for HF might differ among physicians and hospitals; however, the enrolled patients were treated and followed up at an HF clinic with standard treatment guidelines for acute HF, and data on all‐cause deaths were collected from the National Insurance data or National Death Records. We gathered all echocardiographic images using standardized imaging protocols. Second, there was vendor dependency in the strain measurement. We used a vendor‐independent strain algorithm for the measurement of LVGLS and RVGLS. Because there can be different strain values using other algorithms, the cutoff values obtained in this study should be used with caution in other study populations in which other strain algorithms are used. Third, we measured RVGLS from the RV free wall and interventricular septum together because of the technical difficulty of RV strain measurement with this feature‐tracking algorithm. If we were to use total RVGLS along with the RVGLS value from the RV free wall separately, the result might be more interesting and informative in the prediction of clinical outcomes. Fourth, this study might have potential selection bias. Although the RV strain was measured in the randomly selected patients, the study patients had higher NT‐proBNP levels and worse LV systolic and diastolic parameters as well as a higher incidence of all‐cause death than did those excluded from the STRATS‐AHF registry. Finally, although the strain values are currently the best echocardiographic markers reflecting myocardial systolic function, using them has not yet been regarded as the gold standard method.5, 26 Further prospective studies with standardized protocols are needed to determine the clinical significance of these values.
Conclusions
In patients with acute HF, RVGLS was significantly associated with all‐cause mortality regardless of LVGLS, and those who had decreased biventricular GLS showed the worst prognosis. The predictive power of the RV strain was more prominent in the absence of pulmonary hypertension.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e009331 DOI: 10.1161/JAHA.118.009331.)
References
- 1. de Groote P, Millaire A, Foucher‐Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. [DOI] [PubMed] [Google Scholar]
- 2. Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. [DOI] [PubMed] [Google Scholar]
- 3. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community‐based study. Circulation. 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kovacs A, Olah A, Lux A, Matyas C, Nemeth BT, Kellermayer D, Ruppert M, Torok M, Szabo L, Meltzer A, Assabiny A, Birtalan E, Merkely B, Radovits T. Strain and strain rate by speckle‐tracking echocardiography correlate with pressure‐volume loop‐derived contractility indices in a rat model of athlete's heart. Am J Physiol Heart Circ Physiol. 2015;308:H743–H748. [DOI] [PubMed] [Google Scholar]
- 6. Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2‐dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54:618–624. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 8. Park SJ, Park JH, Lee HS, Kim MS, Park YK, Park Y, Kim YJ, Lee JH, Choi SW, Jeong JO, Kwon IS, Seong IW. Impaired RV global longitudinal strain is associated with poor long‐term clinical outcomes in patients with acute inferior STEMI. JACC Cardiovasc Imaging. 2015;8:161–169. [DOI] [PubMed] [Google Scholar]
- 9. Fukuda Y, Tanaka H, Sugiyama D, Ryo K, Onishi T, Fukuya H, Nogami M, Ohno Y, Emoto N, Kawai H, Hirata K. Utility of right ventricular free wall speckle‐tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. J Am Soc Echocardiogr. 2011;24:1101–1108. [DOI] [PubMed] [Google Scholar]
- 10. Park JH, Park MM, Farha S, Sharp J, Lundgrin E, Comhair S, Tang WH, Erzurum SC, Thomas JD. Impaired global right ventricular longitudinal strain predicts long‐term adverse outcomes in patients with pulmonary arterial hypertension. J Cardiovasc Ultrasound. 2015;23:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–1957. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 13. Motoki H, Borowski AG, Shrestha K, Hu B, Kusunose K, Troughton RW, Tang WH, Klein AL. Right ventricular global longitudinal strain provides prognostic value incremental to left ventricular ejection fraction in patients with heart failure. J Am Soc Echocardiogr. 2014;27:726–732. [DOI] [PubMed] [Google Scholar]
- 14. Lakatos E. Designing complex group sequential survival trials. Stat Med. 2002;21:1969–1989. [DOI] [PubMed] [Google Scholar]
- 15. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD; PARAMOUNT Investigators . Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nahum J, Bensaid A, Dussault C, Macron L, Clemence D, Bouhemad B, Monin JL, Rande JL, Gueret P, Lim P. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3:249–256. [DOI] [PubMed] [Google Scholar]
- 17. Anavekar NS, Skali H, Bourgoun M, Ghali JK, Kober L, Maggioni AP, McMurray JJ, Velazquez E, Califf R, Pfeffer MA, Solomon SD. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study). Am J Cardiol. 2008;101:607–612. [DOI] [PubMed] [Google Scholar]
- 18. Meris A, Faletra F, Conca C, Klersy C, Regoli F, Klimusina J, Penco M, Pasotti E, Pedrazzini GB, Moccetti T, Auricchio A. Timing and magnitude of regional right ventricular function: a speckle tracking‐derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr. 2010;23:823–831. [DOI] [PubMed] [Google Scholar]
- 19. Verhaert D, Mullens W, Borowski A, Popovic ZB, Curtin RJ, Thomas JD, Tang WH. Right ventricular response to intensive medical therapy in advanced decompensated heart failure. Circ Heart Fail. 2010;3:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cameli M, Lisi M, Righini FM, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Ballo P, Galderisi M, Mondillo S. Right ventricular longitudinal strain correlates well with right ventricular stroke work index in patients with advanced heart failure referred for heart transplantation. J Card Fail. 2012;18:208–215. [DOI] [PubMed] [Google Scholar]
- 21. Santamore WP, Dell'Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40:289–308. [DOI] [PubMed] [Google Scholar]
- 22. Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH. Right ventricular dysfunction in left‐sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. [DOI] [PubMed] [Google Scholar]
- 23. Konstam MA, Salem DN, Isner JM, Zile MR, Kahn PC, Bonin JD, Cohen SR, Levine HJ. Vasodilator effect on right ventricular function in congestive heart failure and pulmonary hypertension: end‐systolic pressure—volume relation. Am J Cardiol. 1984;54:132–136. [DOI] [PubMed] [Google Scholar]
- 24. Morrison D, Goldman S, Wright AL, Henry R, Sorenson S, Caldwell J, Ritchie J. The effect of pulmonary hypertension on systolic function of the right ventricle. Chest. 1983;84:250–257. [DOI] [PubMed] [Google Scholar]
- 25. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. [DOI] [PubMed] [Google Scholar]
- 26. Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, Veulemans P, Pellens M, La Gerche A, Rademakers F, Herijgers P, D'Hooge J. The relative value of strain and strain rate for defining intrinsic myocardial function. Am J Physiol Heart Circ Physiol. 2012;302:H188–H195. [DOI] [PubMed] [Google Scholar]


