Abstract
Background
Clopidogrel was thought to be superior to aspirin for secondary prevention of vascular diseases in clinical trials. In this study we assessed the safety and efficacy of clopidogrel versus aspirin in real‐world practice by using the Taiwan Stroke Registry.
Methods and Results
Patients with ischemic stroke (2006–2016) on aspirin or clopidogrel for secondary stroke prevention were identified in the Taiwan Stroke Registry. Stroke recurrence and mortality rates in patients receiving aspirin (N=34 679) were compared with those receiving clopidogrel (N=7611) during a 12‐month follow‐up period. Propensity score matching and conditional Cox proportional hazards regression model were applied to control confounding factors with 6443 patients in each group. After propensity score matching, stroke recurrence rates were comparable between groups, with 223 patients in the aspirin (3.46%) and 244 in the clopidogrel group (3.79%) (hazard ratio=1.13, 95% confidence interval=0.89–1.43, P=0.311). However, the mortality rate was significantly higher in the clopidogrel group (362 patients, 5.62%) than in the aspirin group (302 patients, 4.69%) (hazard ratio=1.30, 95% confidence interval=1.07–1.58, P=0.008). Results were consistent before and after propensity score matching.
Conclusions
Clopidogrel was as effective as aspirin for prevention of recurrent stroke in real‐world practice. However, the mortality rate was significantly higher in the clopidogrel than in the aspirin group.
Keywords: aspirin, clopidogrel, prevention, stroke
Subject Categories: Ischemic Stroke, Mortality/Survival, Secondary Prevention
Clinical Perspective
What Is New?
In this 1‐year retrospective study of real‐world data comparing the safety and efficacy between aspirin and clopidogrel in the secondary prevention of ischemic stroke, stroke recurrence rates were comparable between aspirin and clopidogrel, but the mortality rate was higher in the clopidogrel group than in the aspirin group.
What Are the Clinical Implications?
In patients with ischemic stroke, clopidogrel was not superior to aspirin for preventing either mortality or recurrent stroke according to real‐world data, even though clinical trials have suggested that clopidogrel users have fewer major bleeding events than aspirin users.
Introduction
Patients with ischemic stroke or transient ischemic attack carry a substantially higher risk of developing recurrent stroke and death than those without a previous stroke or transient ischemic attack.1, 2, 3, 4, 5, 6 Results of prospective clinical trials and subsequent systematic reviews have established well‐accepted guidelines that antiplatelet agents are effective for secondary stroke prevention at both acute and chronic stages.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Aspirin is the most widely prescribed antiplatelet agent as the mainstay for secondary stroke prevention.21, 22 However, there have been several studies comparing the efficacy and safety of aspirin with other antiplatelet agents.7, 8, 23 Clopidogrel is another guideline‐recommended antiplatelet agent21, 22 and has been shown to be superior to aspirin in preventing composite vascular events and reducing hemorrhagic complications in a randomized controlled trial, CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events).7 However, whether clopidogrel is superior to aspirin in stroke prevention is unclear, and current guidelines do not recommend using clopidogrel instead of aspirin as the first‐line antiplatelet agent for secondary stroke prevention. In addition, clopidogrel was proven to be as effective as aspirin plus dipyridamole in secondary stroke prevention with the advantage of fewer hemorrhagic complications in another randomized controlled trial, the PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes) trial.14 Although the 2 large clinical trials reported clopidogrel to have similar efficacy and lower hemorrhagic complication rates compared with aspirin in secondary stroke prevention, there has been no prior study comparing the safety and efficacy between these 2 major antiplatelet agents based on real‐world practice outcomes. Thus, extrapolation from clinical trial results on aspirin versus clopidogrel in secondary stroke prevention to real‐world practice outcomes remains to be established. It has been increasingly recognized that real‐world practice outcomes or real‐world evidence may not be consistent with results derived from randomized clinical trials.24 Other studies have also raised concerns on extending clinical trial results of secondary stroke prevention to real‐world practice.25 Patients with older age and complex comorbidities are usually excluded from clinical trials, and up to 75% of the stroke patient population could be excluded based on preset exclusion criteria in clinical trials on stroke prevention.26 Clopidogrel is more expensive than aspirin, and most cost effectiveness studies of clopidogrel monotherapy in the prevention of vascular events were based on the data of the CAPRIE trial.27, 28, 29, 30 The cost effectiveness of clopidogrel versus aspirin in real‐world practice is unclear. The objective of the present study was to compare the safety and efficacy of clopidogrel with aspirin in secondary stroke prevention based on real‐world practice outcome using the TSR (Taiwan Stroke Registry) with more than 130 000 stroke admissions. TSR data reflecting quality of care within its network hospitals have been reported earlier.31
Methods
Taiwan Stroke Registry
Data for the present study were obtained from the TSR for the period from May 1, 2006 to February 29, 2016. The TSR program, which was launched in 2006, is a government‐funded project with approval from Institutional Review Boards of 59 academic and community hospitals in Taiwan (Appendix S1). Informed consent from the participants in this study was waived. Registration of stroke patients who constitute the study population in the present study has been previously reported in detail.31 TSR data have also been noted to be representative of the national stroke population in the National Health Insurance database.31 The severity of stroke was assessed using the National Institutes of Health Stroke Scale,30 and the outcome was determined using the modified Rankin Scale.32, 33 Each stroke patient was followed up by the case managers of each hospital through their medical record and/or telephone visit every 3 months for at least 12 months after discharge. Only the individuals for whom we confirmed survival status were included in this study. At each follow‐up visit, data relevant to secondary stroke prevention, including data on stroke recurrence, other vascular event or death, were systematically collected. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patients and Study Design
Patients continually receiving either aspirin or clopidogrel after the diagnosis of ischemic stroke but not transient ischemic attack were selected and analyzed. The observation period was 12 months. Inclusion and exclusion criteria are listed in Figure 1. The primary outcomes were stroke recurrence and death within 12 months after ischemic stroke under preventive measure using aspirin or clopidogrel. The exclusions were patients who received a combination of aspirin and clopidogrel (N=1443), received other medicine including aggrenox, ticlopidine, cilostazol, or warfarin (N=1995), died during hospitalization for acute ischemic stroke (N=101), with missing data for length of stay (N=9286), TOAST (Trial of Org 10172 in Acute Stroke Treatment) type (N=2556), modified Rankin Scale records (N=8), died at discharge (N=291), and with recurrent stroke before discharge (N=69) (Figure 1).
Figure 1.
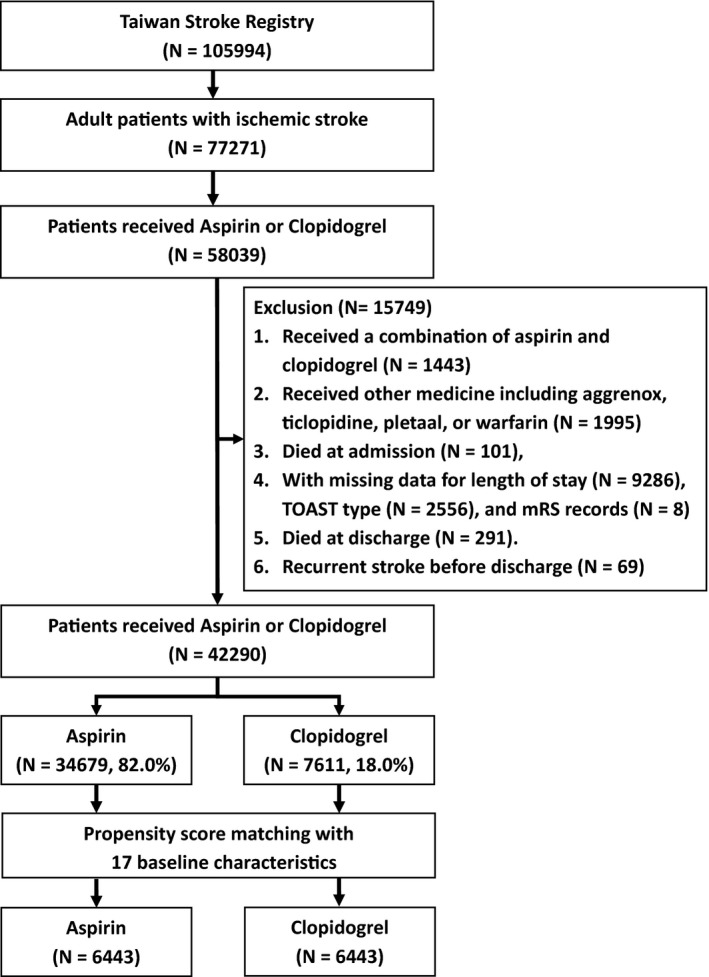
Flowchart of patient recruitment. TOAST indicates Trial of Org 10172 in Acute Stroke Treatment.
Statistical Analysis
To reduce baseline disparities anticipated in a retrospective study, propensity score matching (PSM) analysis was conducted to balance the distribution of variables between groups (Table 1).34 The PSM applied logistic regression analysis with 17 variables, namely, age, body mass index, hospitalization length (in days), sex, stroke subtypes based on TOAST,35 hypertension, diabetes mellitus, dyslipidemia, previous cerebrovascular event or transient ischemic attack, atrial fibrillation, ischemic heart disease including acute myocardial infarction, congestive heart failure, smoking, alcohol consumption, upper gastrointestinal bleeding, and National Institutes of Health Stroke Scale scores on admission and modified Rankin Scale upon discharge. We detected the collinearity of the variables using the Cox model before PSM. The result revealed that the correlations between the parameters were all lower than 0.01, and suggested that there was no collinearity of variables (Tables S1 and S2). Furthermore, patients in the aspirin group were matched with those in the clopidogrel group using the nearest logit of the propensity score.36 The PSM ratio was 1:1. The distribution of confounders between patients with aspirin and clopidogrel was balanced between the group with PSM (propensity scores were 0.23±0.14 and 0.23±0.14, respectively, t test, P=0.973).
Table 1.
Baseline Characteristics Before and After PS Matchinga
| Characteristics | Before PS Matching | After PS Matchinga | ||||
|---|---|---|---|---|---|---|
| Aspirin (N=34 679) | Clopidogrel (N=7611) | P Value | Aspirin (N=6443) | Clopidogrel (N=6443) | P Value | |
| Age (y), mean±SD | 67.4±13.7 | 71.8±12.9 | <0.001 | 71.8±16.3 | 71.4±13.2 | 0.140 |
| BMI, mean±SDb | 24.8±3.89 | 24.2±3.91 | <0.001 | 24.1±3.73 | 24.2±3.92 | 0.210 |
| Hospitalization length (d), median (interquartile range) | 7 (7) | 11 (16) | <0.001 | 9 (14) | 10 (15) | 0.340 |
| Male, n (%) | 21 159 (61.0) | 4539 (59.6) | 0.026 | 3878 (60.2) | 3898 (60.5) | 0.719 |
| Obesity, n (%)c | 7974 (23.0) | 1492 (19.6) | <0.001 | 1328 (20.6) | 1448 (22.5) | 0.010 |
| TOAST subtype, n (%) | ||||||
| LAA | 9921 (28.6) | 2580 (33.9) | <0.001 | 2204 (34.2) | 2147 (33.3) | 0.514 |
| SVD | 17 113 (49.4) | 2960 (38.9) | 2510 (39.0) | 2593 (40.3) | ||
| Cardioembolism | 1816 (5.24) | 731 (9.60) | 635 (9.86) | 598 (9.28) | ||
| Specific cause | 375 (1.08) | 85 (1.12) | 68 (1.06) | 68 (1.06) | ||
| Undetermined cause | 5454 (15.7) | 1255 (16.5) | 1026 (15.9) | 1037 (16.1) | ||
| Risk factors, n (%) | ||||||
| Hypertension | 25 980 (74.9) | 5966 (78.4) | <0.001 | 5100 (79.2) | 5068 (78.6) | 0.425 |
| Diabetes mellitus | 13 832 (39.9) | 3202 (42.1) | <0.001 | 2724 (42.3) | 2713 (42.1) | 0.844 |
| Dyslipidemia | 17 100 (49.3) | 3446 (45.3) | <0.001 | 2902 (45.0) | 2956 (45.9) | 0.339 |
| Previous CVA/TIA | 7607 (21.9) | 2534 (33.3) | <0.001 | 2230 (34.6) | 2106 (32.7) | 0.021 |
| Heart disease | 7249 (20.9) | 2598 (34.1) | <0.001 | 2129 (33.0) | 2219 (34.4) | 0.094 |
| Atrial fibrillation | 896 (2.58) | 357 (4.69) | <0.001 | 279 (4.33) | 277 (4.30) | 0.931 |
| Ischemic heart disease | 3034 (8.75) | 1274 (16.7) | <0.001 | 1155 (17.9) | 1104 (17.1) | 0.237 |
| Congestive heart failure | 411 (1.19) | 181 (2.38) | <0.001 | 142 (2.20) | 142 (2.20) | 1.000 |
| Acute MI | 31 (0.09) | 27 (0.35) | <0.001 | 16 (0.25) | 22 (0.34) | 0.330 |
| Smoking | 12 834 (37.0) | 2531 (35.3) | <0.001 | 2148 (33.3) | 2213 (34.4) | 0.226 |
| Alcohol use | 4720 (13.6) | 764 (10.0) | <0.001 | 600 (9.31) | 657 (10.2) | 0.091 |
| UGI bleeding | 141 (0.41) | 442 (5.81) | <0.001 | 119 (1.85) | 173 (2.69) | 0.001 |
| mRS scores at discharge, n (%)d | ||||||
| 0 | 2417 (6.97) | 400 (5.24) | <0.001 | 343 (5.32) | 372 (5.77) | 0.022 |
| 1 | 8949 (25.8) | 1164 (15.3) | 1211 (18.8) | 1087 (16.9) | ||
| 2 | 7273 (21.0) | 1208 (15.9) | 1127 (17.5) | 1111 (17.2) | ||
| 3–5 | 16 040 (46.3) | 4839 (63.6) | 3762 (58.4) | 3873 (60.1) | ||
| NIHSS scores at discharge, n (%)e | ||||||
| <5 | 24 079 (69.4) | 4058 (53.3) | <0.001 | 3733 (57.9) | 3630 (56.3) | 0.001 |
| 5–19 | 9522 (27.5) | 2906 (38.2) | 2309 (35.8) | 2309 (35.8) | ||
| ≥20 | 1078 (3.11) | 647 (8.50) | 401 (6.22) | 504 (7.82) | ||
BMI indicates body mass index; CVA, cerebrovascular attack; LAA, large artery atherosclerosis; MI, myocardial infarction; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PS, propensity score; SVD, small vessel disease; TIA, transient ischemic attack; TOAST, Trial of Org 10172 in Acute Stroke Treatment; UGI, upper gastrointestinal.
Before PS matching, the baseline characteristics of the 2 groups were significantly different (P<0.05) because of potential sampling bias (population ratio: aspirin/clopidogrel=5.05/1). After PS matching, there were no significant differences between the 2 groups for any variables.
The BMI is the weight in kilograms divided by the square of the height in meters.
Obesity was defined as a BMI of 27 or more.
Scores on the mRS ranged from 0 to 5, and higher scores indicated greater disability.
Higher scores on the NIHSS indicated greater stroke severity.
Baseline features of the matched patients were compared statistically between groups using independent t test and χ2 test for means and percentages, respectively. The differences were considered significant when P values were <0.05. Kaplan–Meier analysis was used to estimate the survival curves for the stroke‐ and death‐free probability.37 The cumulative risk of recurrent stroke and death was calculated using the Cox proportional hazards regression model and expressed as hazard ratios (HRs).38 Finally, the propensity score was used as a covariate to adjust the HR in the Cox models stratifying on matched pairs.39 The precision of the HR estimates was described as 95% confidence intervals (95% CIs). The data were analyzed using the Statistical Analysis System (SAS System for Windows, Version 9.1, Cary, NC) and Statistical Package for the Social Sciences software (Version 18.0, SPSS Inc, Chicago, IL).
Results
Baseline Characteristics
In the TSR, 34 679 stroke patients on aspirin and 7611 stroke patients on clopidogrel were identified (Figure 1). Before the PSM, the baseline characteristics including confounding factors between groups were not comparable (Table 1). Compared with the aspirin group, the clopidogrel group had a higher proportion of upper gastrointestinal bleeding, certain stroke risk factors (except dyslipidemia, smoking, and alcohol use), and modified Rankin Scale in the range of 3 to 5 but a lower proportion of patients with small vessel disease, and National Institutes of Health Stroke Scale score <5 upon discharge (Table 1). To reduce biases caused by the uneven distribution of baseline features between groups, PSM was applied for further analysis.34, 40 No significant differences in baseline characteristics between groups were noted after PSM. The total number of patients in each study group was 6443.
Antiplatelet Therapies on Recurrent Stroke
There were 1421 patients with recurrent stroke within 12 months of the previous stroke in the study population of 42 290 patients (Table 2); 1141 (3.29%) and 280 (3.68%) patients developed recurrent stroke during a 12‐month follow‐up period in the aspirin and clopidogrel groups, respectively, with no significant difference between groups (HR=1.12, 95% CI=0.98–1.27, P=0.094). In the propensity‐score‐matched pairs (N=6443, in each group), 223 patients (3.46%) in the aspirin group and 244 patients (3.79%) in the clopidogrel group developed recurrent stroke. The adjusted HR did not differ between the groups (HR=1.13; 95% CI=0.89–1.43, P=0.311, Table 2). During the 12‐month follow‐up period, the stroke‐free curves show no significant difference between groups (Figure 2A).
Table 2.
Primary Outcomes, Recurrent Stroke, and Death, Before and After PS Matchinga
| Outcome | Before PS Matching | After PS Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Aspirin (N=34 679) | Clopidogrel (N=7611) | HR (95% CI)b | P Value | Aspirin (N=6443) | Clopidogrel (N=6443) | HR (95% CI)c | P Value | |
| N (%) | N (%) | |||||||
| Recurrent stroke | ||||||||
| 12 mo | 1141 (3.29) | 280 (3.68) | 1.12 (0.98–1.27) | 0.094 | 223 (3.46) | 244 (3.79) | 1.13 (0.89–1.43) | 0.311 |
| Death | ||||||||
| 3 mo | 385 (1.11) | 183 (2.40) | 2.11 (1.77–2.51) | <0.001 | 116 (1.80) | 144 (2.23) | 1.24 (0.94–1.63) | 0.137 |
| 4–12 mo | 582 (1.68) | 266 (3.49) | 2.16 (1.89–2.49) | <0.001 | 186 (2.89) | 218 (3.38) | 1.36 (1.04–1.78) | 0.026 |
| 12 mo | 967 (2.79) | 449 (5.90) | 2.14 (1.91–2.39) | <0.001 | 302 (4.69) | 362 (5.62) | 1.30 (1.07–1.58) | 0.008 |
CI indicates confidence interval; HR, hazard ratio; PS, propensity score.
Death significantly differed between the 2 groups (P<0.001) during 0–360 d and 91–360 d.
Unadjusted HR.
Propensity score–adjusted HR.
Figure 2.
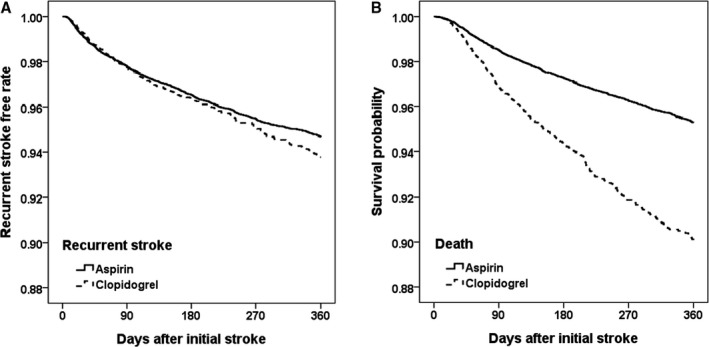
Survival probability in ischemic stroke patients receiving aspirin or clopidogrel: (A) recurrent stroke and (B) death after propensity score matching. Kaplan–Meier analysis was used to estimate the survival curves for the risk of recurrent stroke (A) during 12 months in the ischemic stroke patients in the aspirin group (__) and clopidogrel group (—). During the 12‐month study period, 223 patients (3.46%) in the aspirin group and 244 patients (3.79%) in the clopidogrel group had recurrent stroke, respectively. The differences between the 2 study groups in recurrent stroke were not significant (HR=1.13, 95% CI=0.89–1.43, P=0.311). However, the survival curves showed a significant difference in the cumulative risk of death within 12 months after the prior stroke and the initiation of antiplatelet therapeutics (B). More ischemic stroke patients in the clopidogrel group (N=362, 5.62%) died during the 12‐month follow‐up period compared with those in the aspirin group (N=302, 4.69%). The propensity‐adjusted cumulative risk of death in the aspirin group was significantly different from that in the clopidogrel group (HR=1.30, 95% CI=1.07–1.58, P=0.008). CI indicates confidence interval; HR, hazard ratio.
Antiplatelet Therapies on All‐Cause Death
Before the PSM, a total of 1416 patients, 967 in the aspirin group and 449 in the clopidogrel group, died during the 12‐month follow‐up period (Table 2). The difference for HR of death was significant between groups in favor of aspirin (clopidogrel to aspirin HR=2.14, 95% CI=1.91–2.39, P<0.001). The difference between groups remains significant after adjustments based on PSM with the Cox proportional hazards regression model. The 12‐month mortality in the clopidogrel group (5.62%) was 1.3‐fold higher than that in the aspirin group (4.69%) even after the data were adjusted by reducing potential biases associated with 17 common confounding factors (HR=1.30, 95% CI=1.07–1.58, P=0.008, Table 2 and Figure 2B). The mortality rates during the first 90 days were not different between groups, but significantly higher mortality in the clopidogrel group compared with the aspirin group was observed during the 4 to 12 months (adjusted clopidogrel to aspirin HR=1.36, P=0.026) (Table 2). The higher mortality rate in the clopidogrel group could not be ascribed to recurrent stroke rate, which is not different between groups.
Discussion
The data of patients with ischemic stroke included in the present study were collected from 59 hospitals in Taiwan. Stroke patients enrolled in TSR are representative of the population of all stroke patients in Taiwan and therefore reflect the stroke population receiving interventions for secondary stroke prevention in current practice in Taiwan.37 The results from this retrospective study based on real‐world practice outcomes show comparable risk of developing recurrent stroke within 12 months between groups. The adjusted recurrence rates were 3.46% and 3.79% (P=0.311) during the 12‐month follow‐up period in the aspirin and clopidogrel groups, respectively. The mortality rates during the first 90 days show no significant difference between groups, which agreed with the mortality rates observed in a short‐term follow‐up trial.18 However, the adjusted 12‐month mortality in the clopidogrel group (5.62%) was 1.3‐fold higher than that in the aspirin group (4.69%) (HR=1.30, 95% CI=1.07–1.58, P=0.008).
The higher overall mortality in the clopidogrel group than in the aspirin group was unexpected, and was not observed in the CAPRIE trial. In the SPS3 trial (Secondary Prevention of Small Subcortical Strokes), an unexpected higher mortality was found in patients receiving clopidogrel plus aspirin compared with patients receiving aspirin alone, which was not attributed to fatal hemorrhage41; the mechanism was investigated but remained uncertain.42 The average age of participants was similar between CAPRIE and SPS3 trials, but the proportions of vascular comorbidities such as hypertension and diabetes mellitus were higher in the SPS3 than in the CAPRIE trial. Therefore, clopidogrel may have an unknown detrimental effect on mortality only in lacunar stroke patients with complex comorbidities. In this study, the proportions of hypertension and diabetes mellitus before and after PSM were similar to those in the SPS3 trial; therefore, our results support that the higher overall mortality in the clopidogrel plus aspirin group of SPS3 trial may be attributed to complex comorbidities rather than by chance. It is known that diabetic patients have upregulation of the P2Y12 pathway, which results in decreased therapeutic effect of clopidogrel and lower reduction in risk of overall mortality in patients with myocardial infarction.43 Therefore, a potential contributing factor for the increased mortality of patients on clopidogrel is the reduced effectiveness of clopidogrel in diabetic patients, who comprised ≈40% of the study cohort.
The results derived from clinical trials and practice may be different because the inclusion and exclusion criteria of a clinical trial usually restrict enrollment of only a selective population of patients, which may not be representative of the whole patient population in real‐world practice. Furthermore, most stroke clinical trials on preventive measures set an end point at 3 months after stroke onset. It should also be noted that patients in real‐world practice are usually older with higher frequency of cormorbidities than those in randomized controlled trials.26, 44 Similar discrepancies have been noted in other diseases such as diabetes mellitus.45, 46 Therefore, the complication and mortality rates in real‐world practice are usually higher than those in clinical trials. To understand the gap between clinical trials and real‐world practice, real‐world evidence has been increasingly recognized for its significance and impact. Real‐world practice outcomes have been recognized by the US Food and Drug Administration in assessing the safety and efficacy of preventive and therapeutic drugs after valid clinical trials with postmarketing surveillance incorporating real‐world practice outcomes. Healthcare payers including government agencies and insurance companies have also heightened their attention on real‐world practice outcomes beyond the clinical trials. Analysis of registration database is one of the strategies for collecting real‐world evidence. In the TSR, patients with carotid stenosis who were eligible for carotid endarterectomy accounted for 10.6% of ischemic stroke patients, and patients discharged in a severely handicapped status accounted for 11.2% of ischemic stroke survivors31; these patients could have a poorer outcome than those without these conditions, and they are commonly noted in the stroke patient population but excluded from clinical trials such as the CAPRIE trial according to the enrollment criteria. The patients excluded from clinical trials are more likely to develop certain side effects or complications that could confound the clinical trial results but would be under health care in real‐world practice. For example, hormone replacement therapy showed benefits in postmenopausal patients with osteoporosis during clinical trials but was later found to increase the risk of coronary artery heart diseases, stroke, thromboembolic events, breast cancer, and cholecystitis after its wide application in the general population.47, 48 In addition to antiplatelet effects, which may reduce the death rate caused by vascular events, aspirin reduces metastasis and death in cancer.49 Aspirin can also prevent free radical formation, lipid peroxidation, DNA damage, and oxidative tissue damage.50 These antioxidant effects may contribute to the pharmacological benefits of aspirin, including favorable effects in preventing further ischemic injury in the cardiovascular and cerebrovascular systems.51 Aspirin also inhibits cyclooxygenase to exert anti‐inflammatory and antipyretic actions. These additional effects of aspirin likely contribute in slowing the progression of cardiovascular and cerebrovascular diseases and, possibly, malignancy or other diseases that may affect lifespan. The TSR does not provide causes of death other than stroke, limiting the ability to elucidate the mechanism of action of aspirin in lowering mortality.
The effectiveness of clopidogrel is partially determined by genotypes of cytochrome CYP2C19 and interaction with proton pump inhibitors.52, 53 Both are involved in the formation of active metabolites of clopidogrel. CYP2C19*2 is associated with a weaker antiplatelet action of clopidogrel and results in a poorer cardiovascular outcome.52 The allele frequencies of CYP2C19*2 in Taiwanese populations are not significantly different from those of whites.54 In addition, proton pump inhibitors did not significantly affect rehospitalization rates in cardiovascular patients on clopidogrel therapy in Taiwan.55 The exact cause of a higher mortality rate in the clopidogrel group remains to be explored.
Clopidogrel is usually restricted as the second‐line drug, following aspirin, for secondary prevention of ischemic stroke by health insurance agencies, because the cost of clopidogrel is substantially higher than that of aspirin. Pharmacoeconomic analyses showed favorable cost‐effectiveness of clopidogrel compared with aspirin,27, 28, 29, 30 but this finding was derived from clinical trial data in the CAPRIE trial, but not real‐world evidence. Our results suggest that aspirin at a much lower cost than clopidogrel was as effective as clopidogrel in preventing recurrent stroke. Furthermore, stroke patients on clopidogrel had significantly higher mortality rates than those of patients on aspirin (HR=1.30; P=0.008). Based on these findings, we conclude that aspirin is not worse than clopidogrel in safety for secondary stroke prevention over a follow‐up period of 12 months.
The present study has several limitations. First, biases might still exist in this retrospective study, although most measured confounders were balanced between the 2 groups through PSM. Unmeasured confounders (history of adverse events of using aspirin, or history of cancer) might trigger the prescription of clopidogrel and affect the outcome. Second, unmeasured confounders themselves might affect the results of this study, such as the causes of death other than stroke‐related events. Further exploration of the differential mortality rates between groups in the TSR population and other patient populations in real‐world practice is warranted.
In conclusion, the present study based on TSR shows real‐world practice outcomes with aspirin and clopidogrel to be equally effective in secondary stroke prevention. However, a significantly higher mortality rate among stroke patients who were on clopidogrel than those on aspirin was noted in 12‐month follow‐up.
Sources of Funding
Hu and Hsu were supported by the Ministry of Health and Welfare Clinical Trial and Research Center of Excellence of Taiwan, (MOHW107‐TDU‐B‐212‐123004) China Medical University Hospital (DMR‐107‐192), Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM10701010021), Ministry of Science and Technology Clinical Trial Consortium for Stroke (MOST 106‐2321‐B‐039‐005), Tseng‐Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Disclosures
None.
Supporting information
Appendix S1. List of Taiwan Stroke Registry Investigators.
Table S1. Correlation Matrices of the Variables for 1‐Year Mortality by Cox Model
Table S2. Correlation Matrices of the Variables for 1‐Year Stroke Recurrence by Cox Model
Acknowledgments
The authors thank the administrators of the Taiwan Stroke Registry (IL Tsai and D Lin) for assisting in data preparation and analysis.
(J Am Heart Assoc. 2018;7:e009856 DOI: 10.1161/JAHA.118.009856)
References
- 1. Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The Framingham study. Stroke. 1982;13:290–295. [DOI] [PubMed] [Google Scholar]
- 2. Hier DB, Foulkes MA, Swiontoniowski M, Sacco RL, Gorelick PB, Mohr JP, Price TR, Wolf PA. Stroke recurrence within 2 years after ischemic infarction. Stroke. 1991;22:155–161. [DOI] [PubMed] [Google Scholar]
- 3. Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long‐term risk of recurrent stroke after a first‐ever stroke. The Oxfordshire Community Stroke Project. Stroke. 1994;25:333–337. [DOI] [PubMed] [Google Scholar]
- 4. Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, Stewart‐Wynne EG. Long‐term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29:2491–2500. [DOI] [PubMed] [Google Scholar]
- 5. Kolominsky‐Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long‐term survival in ischemic stroke subtypes: a population‐based study. Stroke. 2001;32:2735–2740. [DOI] [PubMed] [Google Scholar]
- 6. Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–646. [DOI] [PubMed] [Google Scholar]
- 7. Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. [DOI] [PubMed] [Google Scholar]
- 8. Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European stroke prevention study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. [DOI] [PubMed] [Google Scholar]
- 9. CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641–1649. [PubMed] [Google Scholar]
- 10. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 11. Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry‐Ribaudo L, Booth J, Topol EJ; CHARISMA Investigators . Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. [DOI] [PubMed] [Google Scholar]
- 12. Group ES , Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. [DOI] [PubMed] [Google Scholar]
- 13. Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, Li Z, Zhang W, Ding M, Gao X, Fan D, Zeng J, Wong K, Lu C, Xiao J, Yao C; Cilostazol versus Aspirin for Secondary Ischaemic Stroke Prevention cooperation I . Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double‐blind, pilot study. Lancet Neurol. 2008;7:494–499. [DOI] [PubMed] [Google Scholar]
- 14. Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW; Group PRS . Aspirin and extended‐release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, Kitagawa Y, Kusuoka H, Nishimaru K, Tsushima M, Koretsune Y, Sawada T, Hamada C; Group C . Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin‐controlled, double‐blind, randomised non‐inferiority trial. Lancet Neurol. 2010;9:959–968. [DOI] [PubMed] [Google Scholar]
- 16. McArthur KS, Quinn TJ, Dawson J, Walters MR. Diagnosis and management of transient ischaemic attack and ischaemic stroke in the acute phase. BMJ. 2011;342:d1938. [DOI] [PubMed] [Google Scholar]
- 17. Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen‐Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA, Alonso‐Coello P, Guyatt GH, Akl EA. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e601S–e636S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC; Investigators C . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 19. Sandercock PA, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;3:CD000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gouya G, Arrich J, Wolzt M, Huber K, Verheugt FW, Gurbel PA, Pirker‐Kees A, Siller‐Matula JM. Antiplatelet treatment for prevention of cerebrovascular events in patients with vascular diseases: a systematic review and meta‐analysis. Stroke. 2014;45:492–503. [DOI] [PubMed] [Google Scholar]
- 21. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 22. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease, Council on Clinical Cardiology . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 23. Milionis HJ, Gerotziafas G, Kostapanos MS, Vemmou A, Zis P, Spengos K, Elisaf M, Vemmos KN. Clopidogrel vs. aspirin treatment on admission improves 5‐year survival after a first‐ever acute ischemic stroke. Data from the Athens Stroke Outcome Project. Arch Med Res. 2011;42:443–450. [DOI] [PubMed] [Google Scholar]
- 24. Marietta M. Direct oral anticoagulants in atrial fibrillation: can data from randomized clinical trials be safely transferred to the general population? No. Intern Emerg Med. 2015;10:647–650. [DOI] [PubMed] [Google Scholar]
- 25. Akao M, Chun YH, Esato M, Abe M, Tsuji H, Wada H, Hasegawa K; Fushimi AFRI . Inappropriate use of oral anticoagulants for patients with atrial fibrillation. Circ J. 2014;78:2166–2172. [DOI] [PubMed] [Google Scholar]
- 26. Maasland L, van Oostenbrugge RJ, Franke CF, Scholte Op Reimer WJ, Koudstaal PJ, Dippel DW; Netherlands Stroke Survey Investigators . Patients enrolled in large randomized clinical trials of antiplatelet treatment for prevention after transient ischemic attack or ischemic stroke are not representative of patients in clinical practice: the Netherlands Stroke Survey. Stroke. 2009;40:2662–2668. [DOI] [PubMed] [Google Scholar]
- 27. Schleinitz MD, Weiss JP, Owens DK. Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost‐effectiveness analysis. Am J Med. 2004;116:797–806. [DOI] [PubMed] [Google Scholar]
- 28. Berger K, Hessel F, Kreuzer J, Smala A, Diener HC. Clopidogrel versus aspirin in patients with atherothrombosis: CAPRIE‐based calculation of cost‐effectiveness for Germany. Curr Med Res Opin. 2008;24:267–274. [DOI] [PubMed] [Google Scholar]
- 29. Logman JF, Heeg BM, Herlitz J, van Hout BA. Costs and consequences of clopidogrel versus aspirin for secondary prevention of ischaemic events in (high‐risk) atherosclerotic patients in Sweden: a lifetime model based on the CAPRIE trial and high‐risk CAPRIE subpopulations. Appl Health Econ Health Policy. 2010;8:251–265. [DOI] [PubMed] [Google Scholar]
- 30. Durand‐Zaleski I, Bertrand M. The value of clopidogrel versus aspirin in reducing atherothrombotic events: the CAPRIE study. Pharmacoeconomics. 2004;22(suppl 4):19–27. [DOI] [PubMed] [Google Scholar]
- 31. Hsieh FI, Lien LM, Chen ST, Bai CH, Sun MC, Tseng HP, Chen YW, Chen CH, Jeng JS, Tsai SY, Lin HJ, Liu CH, Lo YK, Chen HJ, Chiu HC, Lai ML, Lin RT, Sun MH, Yip BS, Chiou HY, Hsu CY; Taiwan Stroke Registry Investigators . Get with the guidelines‐stroke performance indicators: surveillance of stroke care in the Taiwan Stroke Registry: Get With the Guidelines‐Stroke in Taiwan. Circulation. 2010;122:1116–1123. [DOI] [PubMed] [Google Scholar]
- 32. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moomaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 33. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 34. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 35. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 36. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 37. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 38. Miller RG. Survival Analysis. New York: J Wiley and Sons; 1981. [Google Scholar]
- 39. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 40. Li S, Barywani S, Fu M. Prognostic power of lower pulse pressure on long‐term all‐cause mortality in octogenarians with acute coronary syndrome: a propensity‐score‐matched cohort study. J Hypertens. 2015;33:279–286. [DOI] [PubMed] [Google Scholar]
- 41. Investigators SPS , Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma M, Pearce LA, Benavente OR, Anderson DC, Connolly SJ, Palacio S, Coffey CS, Hart RG. Predictors of mortality in patients with lacunar stroke in the secondary prevention of small subcortical strokes trial. Stroke. 2014;45:2989–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andersson C, Lyngbaek S, Nguyen CD, Nielsen M, Gislason GH, Kober L, Torp‐Pedersen C. Association of clopidogrel treatment with risk of mortality and cardiovascular events following myocardial infarction in patients with and without diabetes. JAMA. 2012;308:882–889. [DOI] [PubMed] [Google Scholar]
- 44. Caro JJ, Migliaccio‐Walle K. Generalizing the results of clinical trials to actual practice: the example of clopidogrel therapy for the prevention of vascular events. CAPRA (CAPRIE Actual Practice Rates Analysis) Study Group. Clopidogrel versus aspirin in patients at risk of ischaemic events. Am J Med. 1999;107:568–572. [DOI] [PubMed] [Google Scholar]
- 45. Parkinson B, Viney R, Haas M, Goodall S, Srasuebkul P, Pearson SA. Real‐world evidence: a comparison of the Australian Herceptin Program and clinical trials of trastuzumab for HER2‐positive metastatic breast cancer. Pharmacoeconomics. 2016;34:1039–1050. [DOI] [PubMed] [Google Scholar]
- 46. Elliott L, Fidler C, Ditchfield A, Stissing T. Hypoglycemia event rates: a comparison between real‐world data and randomized controlled trial populations in insulin‐treated diabetes. Diabetes Ther. 2016;7:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, Robinson V, Henry D, O'Connell D, Cranney A; Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group . Meta‐analyses of therapies for postmenopausal osteoporosis. V. Meta‐analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr Rev. 2002;23:529–539. [DOI] [PubMed] [Google Scholar]
- 48. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. [DOI] [PubMed] [Google Scholar]
- 49. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. [DOI] [PubMed] [Google Scholar]
- 50. Andoh T, Chock PB, Chiueh CC. The roles of thioredoxin in protection against oxidative stress‐induced apoptosis in SH‐SY5Y cells. J Biol Chem. 2002;277:9655–9660. [DOI] [PubMed] [Google Scholar]
- 51. Shi X, Ding M, Dong Z, Chen F, Ye J, Wang S, Leonard SS, Castranova V, Vallyathan V. Antioxidant properties of aspirin: characterization of the ability of aspirin to inhibit silica‐induced lipid peroxidation, DNA damage, NF‐kappaB activation, and TNF‐alpha production. Mol Cell Biochem. 1999;199:93–102. [DOI] [PubMed] [Google Scholar]
- 52. Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrall JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double‐blind OCLA (Omeprazole Clopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256–260. [DOI] [PubMed] [Google Scholar]
- 54. Liou YH, Lin CT, Wu YJ, Wu LS. The high prevalence of the poor and ultrarapid metabolite alleles of CYP2D6, CYP2C9, CYP2C19, CYP3A4, and CYP3A5 in Taiwanese population. J Hum Genet. 2006;51:857–863. [DOI] [PubMed] [Google Scholar]
- 55. Lin SL, Chang HM, Liu CP, Chou LP, Chan JW. Clinical evidence of interaction between clopidogrel and proton pump inhibitors. World J Cardiol. 2011;3:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of Taiwan Stroke Registry Investigators.
Table S1. Correlation Matrices of the Variables for 1‐Year Mortality by Cox Model
Table S2. Correlation Matrices of the Variables for 1‐Year Stroke Recurrence by Cox Model


