Abstract
Background
Cardiorenal syndrome type 1 (CRS1) as a complication of acute myocardial infarction can lead to adverse outcomes, and a method for early detection is needed. This study investigated the individual and integrated effectiveness of amino‐terminal pro–brain natriuretic peptide (Pro‐BNP), estimated glomerular filtration rate (eGFR), and high‐sensitivity C‐reactive protein (CRP) as predictive factors for CRS1 in patients with acute myocardial infarction.
Methods and Results
In a retrospective analysis of 2094 patients with acute myocardial infarction, risk factors for CRS1 were analyzed by logistic regression. Receiver operating characteristic curves were constructed to determine the predictive ability of the biomarkers individually and in combination. Overall, 177 patients (8.45%) developed CRS1 during hospitalization. On multivariable analysis, all 3 biomarkers were independent predictors of CRS1 with odds radios and 95% confidence intervals for a 1‐SD change of 1.792 (1.311‐2.450) for log(amino‐terminal pro–brain natriuretic peptide, 0.424 (0.310‐0.576) for estimated glomerular filtration rate, and 1.429 (1.180‐1.747) for high‐sensitivity C‐reactive peptide. After propensity score matching, the biomarkers individually and together significantly predicted CRS1 with areas under the curve of 0.719 for amino‐terminal pro–brain natriuretic peptide, 0.843 for estimated glomerular filtration rate, 0.656 for high‐sensitivity C‐reactive peptide, and 0.863 for the 3‐marker panel (all P<0.001). Also, the integrated 3‐marker panel performed better than the individual markers (P<0.05). CRS1 risk correlated with the number of biomarkers showing abnormal levels. Abnormal measurements for at least 2 biomarkers indicated a greater risk of CRS1 (odds ratio 36.19, 95% confidence interval 8.534‐153.455, P<0.001).
Conclusions
The combination of amino‐terminal pro–brain natriuretic peptide, estimated glomerular filtration rate, and high‐sensitivity C‐reactive peptide at presentation may assist in the prediction of CRS1 and corresponding risk stratification in patients with acute myocardial infarction.
Keywords: cardiorenal syndrome, high‐sensitivity C‐reactive protein, amino‐terminal pro–brain natriuretic peptide, prediction
Subject Categories: Cardiorenal Syndrome, Myocardial Infarction, Heart Failure, Risk Factors, Biomarkers
Clinical Perspective
What Is New?
Amino‐terminal pro–brain natriuretic peptide, estimated glomerular filtration rate, and high‐sensitivity C‐reactive protein at admission significantly predicted the development of cardiorenal syndrome type 1 and showed good discriminative ability.
The combination of the 3 biomarkers showed better predictive capability than any of the biomarkers individually.
Abnormal levels of 2 or more of these markers according to the identified cutoff values were associated with an elevated risk of cardiorenal syndrome type 1.
What Are the Clinical Implications?
Use of amino‐terminal pro–brain natriuretic peptide, estimated glomerular filtration rate, and high‐sensitivity C‐reactive protein in combination may assist in the prediction of cardiorenal syndrome type 1 and corresponding risk stratification in patients with acute myocardial infarction.
Introduction
Cardiorenal syndrome (CRS) is defined as “a complex pathophysiologic disorder of the heart and kidneys where acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ.”1, 2 Five subtypes of CRS have been defined according to the primary organ, an acute versus chronic time frame, and whether cardiac and renal codysfunction occur secondary to systemic disease. CRS type 1 (CRS1) is characterized by acute worsening of heart function leading to acute kidney injury (AKI). The reported incidence of CRS1 ranges from 25% to 33% in patients admitted with acute decompensated heart failure,3 and the reason for this variation may be the differences in the definitions of kidney dysfunction and the heterogeneity of populations. The pathophysiology of CRS1 is multifaceted and involves both hemodynamic and nonhemodynamic mechanisms that remain largely unknown.4 However, it has been shown that passive central venous congestion and inflammatory activation play vital roles in the mechanisms leading to CRS1 in patients with acute heart failure.5, 6, 7
AKI is 1 of the most frequent in‐hospital complications in patients with acute myocardial infarction (AMI) and is associated with adverse short‐term and long‐term outcomes.8, 9 At the same time, AMI is a common antecedent event that predisposes patients to acute heart failure. Patients who experience both acute heart failure and AKI have worse outcomes than those who experience only 1 of these conditions.10 Therefore, there is an urgent need to understand the risk factors for CRS1 and to establish an ideal biomarker for its prediction in AMI patients. Over the past decade researchers have evaluated many traditional and novel biomarkers, such as serum creatinine, cystatin C, the urea albumin creatinine ratio, neutrophil gelatinase‐associated lipocalin, and others. However, whether these biomarkers possess adequate prognostic accuracy for early detection of CRS1 remains to be determined. Moreover, a multimarker panel may provide a more effective model for CSR1 risk prediction.
Toward the development of such a biomarker panel, we selected amino‐terminal pro–brain natriuretic peptide (NT‐proBNP), the estimated glomerular filtration rate (eGFR), and high‐sensitivity C‐reactive protein (hs‐CRP) as promising markers of heart failure, renal injury, and inflammation, respectively, and investigated the predictive value of these biomarkers individually and in combination for CRS1 in AMI patients.
Methods
The data, analytic methods, and study materials for this study will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The materials will be made available by the corresponding author on reasonable request.
Study Population
The study population for this retrospective, single‐center observational study was identified from the AMI patient database of the Cardiovascular Center of Beijing Friendship Hospital, which includes patients treated from 2012 onward. From December 2012 to February 2017, 2712 patients were included in the database according to the following criteria: age of more than 18 years, confirmed diagnosis of AMI presenting with ST‐segment elevation myocardial infarction or non–ST‐elevation myocardial infarction and treated within 48 hours after the onset of symptoms. All medical data and study end points were collected by trained study coordinators. AMI was defined according to published guidelines.11, 12 The study protocol was performed in accordance with the principles of the Declaration of Helsinki and was approved by the local institutional ethics committee with a waiver for informed consent (No. 2017‐P2‐123‐01), and permission was granted to use data for analysis. For the present analysis, exclusion criteria included (1) chronic renal failure and/or need for regular hemodialysis (n=22) or peritoneal dialysis (n=6); (2) serum creatinine ≥442 μmol/L at first admission (n=9); (3) prior treatment with renal transplantation (n=1); (4) presence of autoimmune disease and sepsis that might result in worsening renal function (n=6); and (5) absence of either the initial or peak creatinine values and admission data (n=574). According to these criteria, this study population consisted of the remaining 2094 patients (Figure 1).
Figure 1.
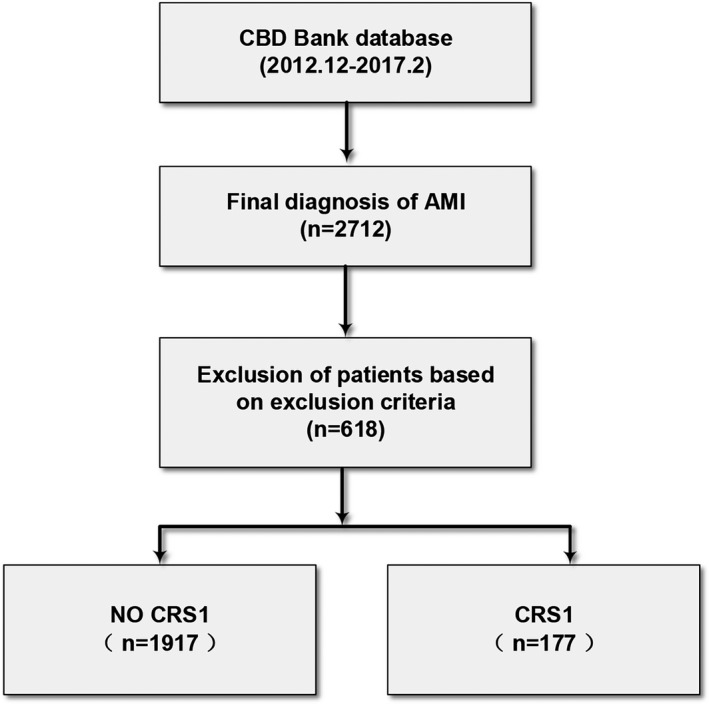
Selection of patient population. AMI indicates acute myocardial infarction; CBD, Cardiovascular Center of Beijing Friendship Hospital Database; CRS1, cardiorenal syndrome type 1.
Data Collection and Biomarker Assays
On the basis of renal function and cardiac function, patients were assigned to either the CRS1 or no‐CRS1 group. The collected clinical data included (1) basic information including age, sex, duration of hospitalization (days), classification of AMI, blood pressure, heart rate at admission, and body mass index at admission; (2) a medical history including coronary artery disease, percutaneous revascularization, coronary artery bypass grafting, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, peripheral arterial disease, and stroke; (3) treatment and prognosis involving an intra‐aortic balloon pump, percutaneous revascularization, drug intervention (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker [ACEI/ARB], diuretic, antiplatelet agent, β‐blocker, statins), and clinical outcomes; (4) laboratory data and auxiliary examinations at admission, such as levels of NT‐proBNP, creatinine, hs‐CRP, hemoglobin, hematocrit, albumin, glucose, and glycated hemoglobin. NT‐proBNP levels were assays based on a chemiluminescence enzyme immune assay and MAGTRATION methodology, and measurements were taken by a PATHFAST NT‐proBNP analyzer (Mitsubishi Kagaku Iatron, Tokyo, Japan). Serum creatinine concentrations were measured using a picric acid method and Beckman Coulter analyzers (Beckman Coulter Inc, Brea, CA). The hs‐CRP was measured by an ultrasensitive method based on particle‐enhanced immunoturbidimetry (DiaSys Diagnostic System, Holzheim, Germany). Troponin I was measured every 3 to 6 hours, and the peak value for each case was recorded. All the patients underwent echocardiography examination on admission, and the left ventricular ejection fraction was acquired via the modified Simpson method.
Heart Function and Kidney Function: CRS Definition
Heart function was evaluated using the Killip‐Kimball classification during the episode, and heart failure was identified if the patient was considered class II or higher. Patients' eGFRs were calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.13 AKI was determined using the Acute Kidney Injury Network criteria14 and defined as an increase in serum creatinine of ≥0.3 mg/dL or 1.5‐fold higher than normal. The baseline serum creatinine was obtained on admission. For some patients, the serum creatinine concentration at discharge was lower than that at admission, and the serum creatinine concentration was considered to be the basal concentration. CRS1 was defined by the sum of these 2 components.15, 16 Acute heart failure was followed by AKI; that is, with a Killip–Kimball score ≥II and AKI. The primary end point of this study was the development of CRS1 during hospitalization.
Statistical Analysis
Continuous variables appeared to have non‐Gaussian distributions, and therefore, the data are presented as median values with interquartile ranges. Comparisons between the study groups were performed by nonparametric rank test (Mann‐Whitney U‐test). Categorical variables are presented as numbers and percentages, and the chi‐squared test was used to compare variables between the study groups. Because the distribution of NT‐proBNP was highly skewed, log transformation of the data was carried out.
Logistic regression analysis was used to obtain odds ratios (ORs) and 95% confidence intervals (CIs) for the development of CRS1. To determine the factors that could independently predict CRS1, variables that were significant in the univariate logistic regression analysis were incorporated into the multivariate logistic regression analysis. Multivariable analysis was performed to evaluate the effects of the number of abnormal biomarker levels on the risk of developing CRS1.
The receiver operating characteristic (ROC) curve analysis was used to evaluate the discriminatory capability of the biomarkers for CRS1. To control for confounding factors, covariates were included in the ROC analysis, and propensity score matching analysis was performed. The cutoff value was defined for the maximum Youden index. Statistical analyses were performed with using SPSS version 19.0 (SPSS, Chicago, IL) and Med‐calc software version 15.8 (MedCalc Software bvba, Mariakerke, Belgium). A 2‐sided P value of less than 0.05 was considered to indicate a statistically significant difference.
Propensity Score Matching Analysis
Propensity score matching is used to reduce selection bias in observational studies. The matching process was conducted with a minimum‐distance scoring method and a 1‐to‐1 match between the CRS1 group and the no‐CRS1 group. In this study, propensity scores were calculated through a binary logistic regression model, including covariates of age, sex, hemoglobin, hematocrit, albumin, glucose, previous coronary artery disease, previous hypertension, previous diabetes mellitus, previous chronic kidney disease, angiography, ACEI and/or ARB use after admission, and diuretic use after admission. Ultimately, 120 CRS1 patients were individually matched to 120 no‐CRS1 controls using nearest available score matching with SPSS version 22.0. The ROC curve analysis from the data set after matching was used to further evaluate the discriminatory capability of the biomarkers for CRS1.
Results
Patient Characteristics and the Prevalence of CRS1
A total of 2094 patients presenting with AMI from December 2012 to February 2017 were included in this study. Among the affected inpatients, 656 (31.33%) developed acute heart failure, and 177 (8.45%) developed CRS1. A total of 63 (3%) patients died during hospitalization, and all‐cause mortality was higher among those with CRS1 than among those without (17.5% versus 1.7%; P<0.001).
The demographic, clinical, and laboratory characteristics of the patients with and without CRS1 are provided in Table 1. There were no significant differences between the groups in the incidence of ST‐elevation myocardial infarction, systolic blood pressure, diastolic blood pressure, prior percutaneous revascularization, dyslipidemia, or peak serum levels of troponin I and glycated hemoglobin. Compared with the patients without CRS1, patients who developed CRS1 were older, more often female, had a longer hospital stay, and more frequently presented with a medical history of coronary artery disease, coronary artery bypass grafting, hypertension, diabetes mellitus, chronic kidney disease, peripheral arterial disease, or stroke. The CRS1 group also had higher mean levels of NT‐proBNP (5324.0 versus 626.5 pg/mL; P<0.001), creatinine (113.0 versus 77.0 μmol/L; P<0.001), hs‐CRP (16.5 versus 5.9 mg/L; P<0.001), and glucose (8.5 versus 7.8 mmol/L; P=0.043), as well as lower levels of hemoglobin (124 versus 135 g/L; P<0.001), hematocrit (37.4% versus 40.5%; P<0.001), and albumin (36.4 versus 39.0 g/dL; P<0.001) and a lower eGFR (50.5 versus 85.6 mL/[min·1.73 m2]; P<0.001). It should be noted that the treatment after admission was significantly different. A higher percentage of patients with CRS1 were treated with an intra‐aortic balloon pump and diuretics (P<0.001), whereas treatments involving an ACEI and/or ARB, antiplatelet agent, β‐blocker, statins, angiography, and percutaneous revascularization were used more often in patients without CRS1 (all P<0.001).
Table 1.
Distribution of Variables in Patients With AMI According to the Development of CRS1
| Characteristic | Total (N=2094) | NO CRS1 (n=1917) | CRS1 (n=177) | P Value |
|---|---|---|---|---|
| Age, y (median, interquartile range) | 65 (57, 77) | 64 (56, 76) | 77 (67, 82) | <0.001 |
| Female (n, %) | 612 (29.2) | 541 (28.2) | 71 (40.1) | 0.001 |
| Hospital stay, d (median, interquartile range) | 8 (6, 10) | 7 (6, 9) | 10 (7, 14) | <0.001 |
| STEMI (n, %) | 1003 (47.9) | 916 (47.8) | 87 (49.2) | 0.727 |
| Measurements (median, interquartile range) | ||||
| Systolic blood pressure at admission, mm Hg | 130 (114, 143) | 130 (115, 143) | 125 (111, 148) | 0.509 |
| Diastolic blood pressure at admission, mm Hg | 73 (65, 81) | 73 (65, 81) | 71 (64, 80) | 0.142 |
| Heart rate at admission, bpm | 74 (65, 82) | 74 (65, 84) | 83 (69, 97) | <0.001 |
| Body mass index, kg/m2 | 25.4 (23.0, 27.7) | 25.4 (23.1, 27.7) | 24.3 (21.0, 27.6) | 0.009 |
| Left ventricular ejection fraction, % | 62 (53, 67) | 62 (55, 67) | 50 (35, 64) | <0.001 |
| Laboratory values (median, interquartile range) | ||||
| Hemoglobin at admission, g/L | 134 (121, 148) | 135 (122, 148) | 124 (104.8136.0) | <0.001 |
| Hematocrit at admission, % | 40.2 (36.3, 44.0) | 40.5 (36.6, 44.3) | 37.4 (32.2, 41.1) | <0.001 |
| Albumin at admission, g/dL | 38.9 (35.8, 41.7) | 39.0 (36.0, 41.8) | 36.4 (33.4, 39.7) | <0.001 |
| NT‐proBNP at admission, pg/mL | 738.0 (207.5, 2817.5) | 626.5 (193.0, 2236.8) | 5324.0 (2 357.5, 17 730.5) | <0.001 |
| Creatinine at admission, μmol/L | 78.0 (68.0, 96.3) | 77.0 (67.5, 92.3) | 113.0 (89.0147.5) | <0.001 |
| eGFR at admission, mL/(min/1.73 m2) | 83.6 (64.3100.3) | 85.6 (67.6101.5) | 50.5 (32.9, 68.1) | <0.001 |
| Peak troponin I, ng/mL | 4.9 (1.1, 16.1) | 3.9 (0.8, 12.4) | 5.5 (1.3, 18.9) | 0.081 |
| Glucose at admission, mmol/L | 7.9 (6.4, 10.8) | 7.8 (6.4, 10.7) | 8.5 (6.6, 11.8) | 0.043 |
| hs‐CRP at admission, mg/L | 6.6 (2.3, 17.3) | 5.9 (2.2, 15.7) | 16.5 (8.1, 30.1) | <0.001 |
| Glycated hemoglobin at admission, % | 6.0 (5.5, 7.1) | 6.0 (5.5, 7.0) | 6.2 (5.7, 7.4) | 0.055 |
| Medical history (n, %) | ||||
| Coronary artery disease | 828 (39.5) | 737 (38.4) | 91 (51.4) | 0.001 |
| Percutaneous revascularization | 326 (15.6) | 290 (15.1) | 36 (20.3) | 0.067 |
| Coronary artery bypass grafting | 45 (2.1) | 34 (1.8) | 11 (6.2) | <0.001 |
| Hypertension | 1383 (66.0) | 1244 (64.9) | 139 (78.5) | <0.001 |
| Diabetes mellitus | 710 (33.9) | 633 (33.0) | 77 (43.5) | 0.005 |
| Dyslipidemia | 934 (44.6) | 849 (44.3) | 85 (48.0) | 0.339 |
| Chronic kidney disease | 118 (5.6) | 81 (4.2) | 37 (20.9) | <0.001 |
| Peripheral arterial disease | 124 (5.9) | 99 (5.2) | 25 (14.1) | <0.001 |
| Stroke | 356 (17.0) | 316 (16.5) | 40 (22.6) | 0.038 |
| Treatment after admission (n, %) | ||||
| IABP | 41 (2.0) | 30 (1.6) | 11 (6.2) | <0.001 |
| Angiography | 1669 (79.7) | 1587 (82.8) | 82 (46.3) | <0.001 |
| Percutaneous revascularization | 1482 (70.8) | 1417 (73.9) | 65 (36.7) | <0.001 |
| ACEI and/or ARB | 1426 (68.1) | 1339 (69.8) | 87 (49.2) | <0.001 |
| Diuretics | 309 (14.8) | 239 (12.5) | 70 (39.5) | <0.001 |
| Antiplatelet agent | 1942 (92.7) | 1810 (94.4) | 132 (74.6) | <0.001 |
| β‐blocker | 1512 (72.2) | 1410 (73.6) | 102 (57.6) | <0.001 |
| Statins | 1802 (86.1) | 1686 (87.9) | 116 (65.5) | <0.001 |
| Clinical end points (n, %) | ||||
| All‐cause mortality | 63 (3.0) | 32 (1.7) | 31 (17.5) | <0.001 |
| Cardiac mortality | 56 (2.7) | 27 (1.4) | 29 (16.4) | <0.001 |
ACEI indicates angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; bpm, beats/min; CRS1, cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; IABP, intra‐aortic balloon pump; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide; STEMI, ST‐segment–elevation myocardial infarction.
Predictors of CRS1
Table 2 shows the results of the univariate and multivariate logistic regression analyses. On univariate analysis, increased log(NT‐proBNP) and hs‐CRP levels and decreased eGFR at admission were significantly associated with CRS1, as were advanced age, female sex, history of coronary artery disease, hypertension, diabetes mellitus, chronic kidney disease, decreased hemoglobin, hematocrit, and albumin, angiography use, ACEI and/or ARB use, increased glucose, and diuretic use. After multivariable adjustment, increased log(NT‐proBNP) (OR 2.136; 95% CI 1.422‐3.209; P<0.001), glucose (OR 1.003; 95% CI 1.000‐1.006; P=0.03), and hs‐CRP (OR 1.031; 95% CI 1.014‐1.048; P<0.001) as well as diuretic use (OR 1.811; 95% CI 1.130‐2.902; P=0.014), decreased eGFR (OR 0.967; 95% CI 0.956‐0.979; P<0.001), and ACEI and/or ARB use (OR 0.509; 95% CI 0.328‐0.789; P=0.003) were determined to be independent predictors of CRS1 in AMI patients.
Table 2.
Univariate and Multivariate Logistic Regression Analysis of CRS1 Occurrence in Patients With AMI
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Age, y | 1.073 (1.057‐1.089) | <0.001 | 1.021 (0.999‐1.045) | 0.065 |
| Female, % | 1.704 (1.241‐2.338) | 0.001 | 0.801 (0.503‐1.276) | 0.350 |
| Hemoglobin at admission, g/dL | 0.969 (0.962‐0.977) | <0.001 | 1.016 (0.974‐1.060) | 0.458 |
| Hematocrit at admission, % | 0.897 (0.874‐0.921) | <0.001 | 0.978 (0.848‐1.128) | 0.763 |
| Albumin at admission, g/dL | 0.892 (0.862‐0.923) | <0.001 | 1.041 (0.986‐1.099) | 0.146 |
| log(NT‐proBNP) at admission | 6.005 (4.524‐7.971) | <0.001 | 2.136 (1.422‐3.209)a | <0.001 |
| eGFR at admission, mL/(min·1.73 m2) | 0.95 (0.943‐0.956) | <0.001 | 0.967 (0.956‐0.979)b | <0.001 |
| Glucose at admission, mmol/L | 1.003 (1.001‐1.005) | 0.002 | 1.003 (1.000‐1.006) | 0.03 |
| hs‐CRP at admission, mg/L | 1.046 (1.035‐1.059) | <0.001 | 1.031 (1.014‐1.048)c | <0.001 |
| Previous coronary artery disease | 1.694 (1.244‐2.307) | 0.001 | 1.067 (0.691‐1.646) | 0.770 |
| Previous hypertension | 1.979 (1.366‐2.867) | <0.001 | 1.378 (0.813‐2.334) | 0.234 |
| Previous diabetes mellitus | 1.562 (1.143‐2.137) | 0.005 | 0.666 (0.397‐1.116) | 0.123 |
| Previous chronic kidney disease | 5.99 (3.916‐9.165) | <0.001 | 1.771 (0.924‐3.396) | 0.085 |
| Angiography after admission | 0.179 (0.131‐0.247) | <0.001 | 0.744 (0.460‐1.202) | 0.227 |
| ACEI and/or ARB after admission | 0.417 (0.306‐0.569) | <0.001 | 0.509 (0.328‐0.789) | 0.003 |
| Diuretics after admission | 4.593 (3.301‐6.391) | <0.001 | 1.811 (1.130‐2.902) | 0.014 |
ACEI indicates angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CI, confidence interval; CRS1, cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide; OR, odds ratio.
The odds ratio and 95%CI for 1 standard deviation change in the logNT‐proBNP were 1.792 (1.311–2.450).
The odds ratio and 95%CI for 1 standard deviation change in the eGFR was 0.424 (0.310–0.576).
The odds ratio and 95%CI for 1 standard deviation change in the hs‐CRP was 1.429 (1.180–1.747).
ROC Curve Analysis of the Value of NT‐proBNP, eGFR, and hs‐CRP, as Biomarkers
The results of the ROC analysis detailed in Table 3 revealed that all 3 biomarkers significantly predicted the development of CRS1 (area under the ROC curve [AUC]: NT‐proBNP 0.813, eGFR 0.828, and hs‐CRP 0.693; all P<0.01). According to the maximum Youden indexes, the cutoff values for eGFR, NT‐proBNP, and hs‐CRP were 71.29 mL/[min·1.73 m2], 2573 pg/mL, and 8.03 mg/L, respectively. Notably, the specificity of hs‐CRP (57.04%) was less than those of eGFR (71.31%) and NT‐proBNP (77.82%). The AUC for the combination of eGFR, NT‐proBNP, and hs‐CRP was 0.856 (P<0.01), indicating very good discriminative ability for the prediction of CRS1. Table 4 shows the results of the covariate adjusted model analysis. After including the covariates, the biomarkers individually and together still significantly predicted CRS1 with AUC values of 0.858 for log(NT‐proBNP), 0.866 for eGFR, 0.849 for hs‐CRP, and 0.882 for the combination of these 3 markers (all P<0.001). The differences in the AUC values between the individual biomarkers and their combinations (Figure 2) were statistically significant (P<0.05), which indicated that the predictive capability of the 3‐biomarker panel is better than each individual marker.
Table 3.
Prematching Receiver Operating Characteristic Curve Analysis of NT‐proBNP, eGFR, and hs‐CRP for the Prediction of CRS1
| Cutoff Value | Abnormality (n, %)a | AUC | P Value | 95% CI | Sensitivity | Specificity | Youden Index | ||
|---|---|---|---|---|---|---|---|---|---|
| No‐CRS1 | CRS1 | ||||||||
| eGFR at admission, mL/(min·1.73 m2) | 71.29 | 550 (28.7) | 142 (80.2) | 0.828 | <0.001 | 0.811 to 0.844 | 0.8079 | 0.7131 | 0.521 |
| NT‐proBNP at admission, pg/mL | 2573 | 385 (22.2) | 120 (74.5) | 0.813 | <0.001 | 0.777 to 0.849 | 0.7453 | 0.7782 | 0.524 |
| hs‐CRP at admission, mg/L | 8.03 | 806 (43.0) | 125 (76.2) | 0.693 | <0.001 | 0.653 to 0.733 | 0.7622 | 0.5704 | 0.333 |
| NT‐proBNP +eGFR+ hs‐CRP | ··· | ··· | ··· | 0.856 | <0.001 | 0.825 to 0.886 | ··· | ··· | ··· |
AUC indicates area under the receiver operating characteristic curve; CI, confidence interval; CRS1, cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide.
Abnormal biomarkers levels were defined as eGFR≤71.29 mL/(min·1.73 m2), NT‐proBNP≥2573 pg/mL, and hs‐CRP≥8.03 mg/L individually.
Table 4.
Prematching Receiver Operating Characteristic Curve Analysis of NT‐proBNP, eGFR, and hs‐CRP Adjusted by Covariates for the Prediction of CRS1
| AUC | P Value | 95% CI | |
|---|---|---|---|
| Covariates | 0.837 | <0.001 | 0.804 to 0.870 |
| eGFR+ covariates | 0.866 | <0.001 | 0.850 to 0.881 |
| Log(NT‐proBNP)+covariates | 0.858 | <0.001 | 0.824 to 0.892 |
| hs‐CRP+ covariates | 0.849 | <0.001 | 0.832 to 0.865 |
| Log(NT‐proBNP)+eGFR+hs‐CRP+covariates | 0.882 | <0.001 | 0.850 to 0.913 |
The covariates included age, sex, hemoglobin, hematocrit, albumin, glucose, previous coronary artery disease, previous hypertension, previous diabetes mellitus, previous chronic kidney disease, angiography, ACEI and/or ARB use after admission, and diuretic use after admission. The differences in the AUC values adjusted by covariates between the individual biomarkers and their combinations were statistically significant, with P values of 0.0330 (eGFR+covariates vs Log[NT‐proBNP]+eGFR+hs‐CRP+covariates), 0.0070 (Log[NT‐proBNP]+covariates vs Log[NT‐proBNP]+eGFR+hs‐CRP+covariates), 0.0062 (hs‐CRP+covariates vs Log[NT‐proBNP]+eGFR+hs‐CRP+covariates), and 0.0008 (covariates vs Log[NT‐proBNP]+eGFR+hs‐CRP+covariates). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AUC, area under the receiver operating characteristic curve; CI, confidence interval; CRS1, cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide.
Figure 2.
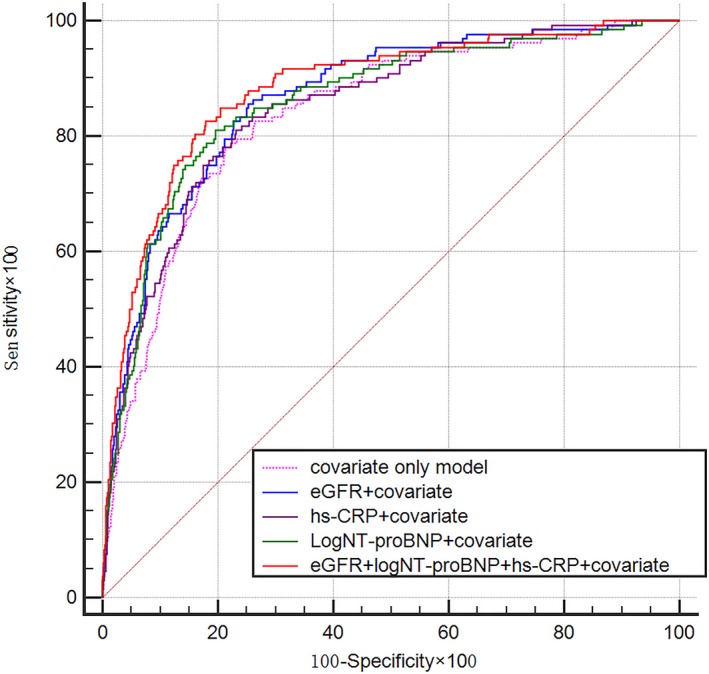
Prematching receiver operating characteristic curve analysis including covariates for the prediction of CRS1 by eGFR, NT‐proBNP, and hs‐CRP. CRS1 indicates cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide.
After propensity score matching, the baseline age and sex along with hemoglobin, hematocrit, albumin, glucose, history of coronary artery disease, hypertension, diabetes mellitus, chronic kidney disease, angiography use, ACEI and/or ARB use, and diuretic use were not statistically different between the CRS1 and no‐CRS1 groups (Table 5). The results of ROC analysis (Table 6, Figure 3) still showed that the discriminatory capability of the 3‐biomarker panel was good (AUC 0.863, 95% CI 0.816‐0.910) and stronger than that of the individual biomarkers, with AUC values of 0.719 for NT‐proBNP, 0.843 for eGFR, and 0.656 for hs‐CRP (all P<0.001).
Table 5.
Pre‐ and Postmatching Distribution of Covariates in Patients With AMI According to the Development of CRS1
| Covariate | Prematching | Postmatching | ||||
|---|---|---|---|---|---|---|
| No‐CRS1 (n=1917) | CRS1 (n=177) | P Value | No‐CRS1 (n=120) | CRS1 (n=120) | P Value | |
| Age, y (median, IQR) | 64 (56, 76) | 77 (67, 82) | <0.001 | 74 (61, 81) | 76.5 (67, 82) | 0.091 |
| Min/max | 25/99 | 42/98 | ··· | 29/99 | 50/91 | ··· |
| ≤60 (n, %) | 741 (38.7) | 22 (12.4) | <0.001 | 27 (22.5) | 16 (13.3) | 0.122 |
| 61 to 70 (n, %) | 513 (26.8) | 34 (19.2) | 24 (20.0) | 22 (18.3) | ||
| 71 to 80 (n, %) | 405 (21.1) | 59 (33.3) | 32 (26.7) | 47 (39.2) | ||
| ≥81 (n, %) | 258 (13.5) | 62 (35.0) | 37 (30.8) | 35 (29.2) | ||
| Female (n, %) | 541 (28.2) | 71 (40.1) | 0.001 | 36 (30) | 48 (40) | 0.104 |
| Hemoglobin at admission, g/L (median, IQR) | 135 (122, 148) | 124 (104.8136.0) | <0.001 | 129 (117.3142.8) | 126 (110, 137.8) | 0.171 |
| Hematocrit at admission, % (median, IQR) | 40.5 (36.6, 44.3) | 37.4 (32.2, 41.1) | <0.001 | 38.55 (35.4, 43.0) | 38.35 (33.5, 42.0) | 0.195 |
| Albumin at admission, g/dL (median, IQR) | 39.0 (36.0, 41.8) | 36.4 (33.4, 39.7) | <0.001 | 37.9 (33.9, 40.7) | 37.45 (34.5, 39.9) | 0.677 |
| Glucose at admission, mmol/L (median, IQR) | 7.8 (6.4, 10.7) | 8.5 (6.6, 11.8) | 0.043 | 8.8 (6.7, 13.9) | 8.6 (6.5, 12.1) | 0.366 |
| Previous coronary artery disease (n, %) | 737 (38.4) | 91 (51.4) | 0.001 | 55 (45.8) | 60 (50) | 0.518 |
| Previous hypertension (n, %) | 1244 (64.9) | 139 (78.5) | <0.001 | 89 (74.2) | 96 (80) | 0.282 |
| Previous diabetes mellitus (n, %) | 633 (33.0) | 77 (43.5) | 0.005 | 41 (34.2) | 49 (40.8) | 0.286 |
| Previous chronic kidney disease (n, %) | 81 (4.2) | 37 (20.9) | <0.001 | 9 (7.5) | 18 (15) | 0.066 |
| Angiography after admission (n, %) | 1587 (82.8) | 82 (46.3) | <0.001 | 61 (50.8) | 63 (52.5) | 0.796 |
| ACEI and/or ARB after admission (n, %) | 1339 (69.8) | 87 (49.2) | <0.001 | 57 (47.5) | 67 (55.8) | 0.196 |
| Diuretics after admission (n, %) | 239 (12.5) | 70 (39.5) | <0.001 | 55 (45.8) | 42 (35) | 0.087 |
ACEI indicates angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CRS1, cardiorenal syndrome type 1; IQR, interquartile range.
Table 6.
Postmatching Receiver Operating Characteristic Curve Analysis of NT‐proBNP, eGFR, and hs‐CRP for the Prediction of CRS1
| AUC | P Value | 95% CI | |
|---|---|---|---|
| eGFR at admission, mL/(min·1.73 m2) | 0.843 | <0.001 | 0.792 to 0.894 |
| NT‐proBNP at admission, pg/mL | 0.719 | <0.001 | 0.654 to 0.784 |
| hs‐CRP at admission, mg/L | 0.656 | <0.001 | 0.587 to 0.725 |
| NT‐proBNP+eGFR+hs‐CRP | 0.863 | <0.001 | 0.816 to 0.910 |
The differences in the AUC values between the individual biomarkers and their combinations were statistically significant, with P values of 0.0372 (eGFR vs NT‐proBNP+eGFR+hs‐CRP), <0.0001 (NT‐proBNP vs NT‐proBNP+eGFR+hs‐CRP), and <0.0001 (hs‐CRP vs NT‐proBNP+eGFR+hs‐CRP). AUC indicates area under the receiver operating characteristic curve; CI, confidence interval; CRS1, cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide.
Figure 3.
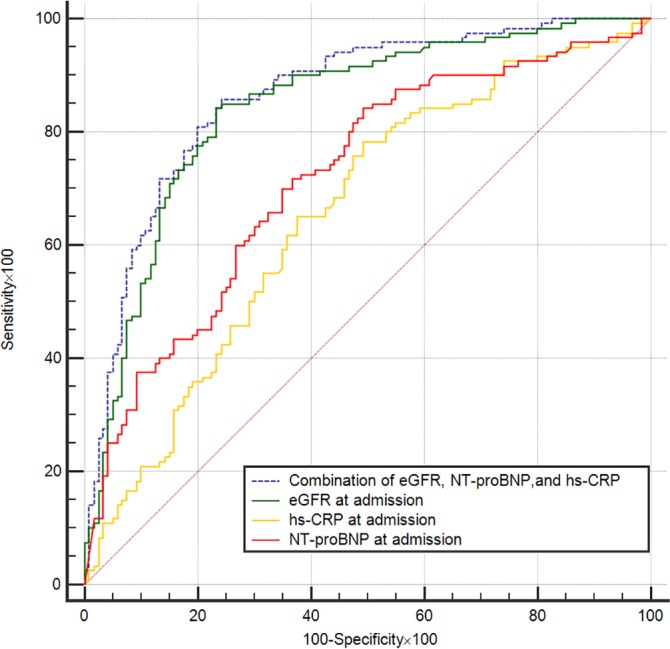
Postmatching receiver operating characteristic curve analysis for the prediction of CRS1 by eGFR, NT‐proBNP, and hs‐CRP. CRS1 indicates cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide.
Association Between Number of Abnormal Biomarker Levels and CRS1
The data presented in Figure 4 illustrate the association between the number of abnormal biomarker levels and the risk of CRS1. Using the cutoff values derived from the ROC analysis to define biomarker levels as normal or abnormal, the risk of CRS1 increased significantly with an increasing number of abnormal biomarker levels. In a multivariate adjusted logistic regression model, the odds of CRS1 were increased by 35‐fold if patients presented with abnormal levels of 2 or more biomarkers compared with no abnormal levels (Table 7). This finding implies that abnormal levels of the biomarkers at presentation may facilitate better CRS1 risk stratification.
Figure 4.
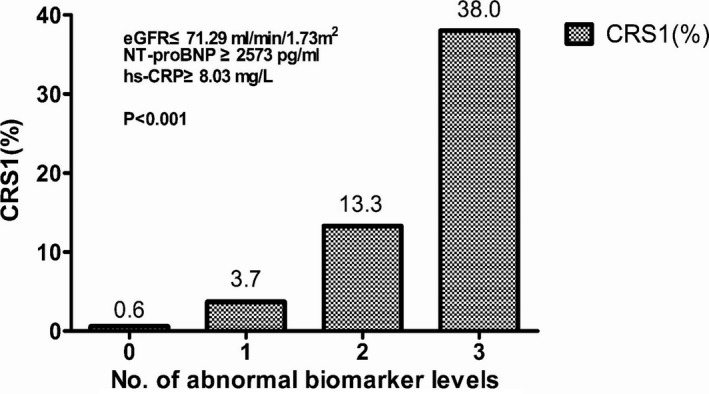
Association of the number of abnormal biomarker levels based on the identified cutoff values and the risk of CRS1. The risk of developing CRS1 increased significantly with an increase in the number of biomarkers showing abnormal levels according to the identified cutoff values. CRS1 indicates cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide.
Table 7.
Multivariable Logistic Regression Analysis of Number of Abnormal Biomarker Levels and the Odds of Developing CRS1
| No. of Abnormal Biomarkersa | No‐CRS1 (n, %) | CRS1 (n, %) | Adjusted OR (95% CI)b | P Value |
|---|---|---|---|---|
| 0 | 657 (99.4) | 4 (0.6) | 1 | ··· |
| 1 | 620 (96.3) | 24 (3.7) | 8.907 (2.065‐38.409) | 0.003 |
| ≥2 | 426 (77.5) | 124 (22.5) | 36.188 (8.534‐153.455) | <0.001 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CRS1, cardiorenal syndrome type 1; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, amino‐terminal pro–brain natriuretic peptide; OR, odds ratio.
Cutoff values for abnormal biomarker levels were eGFR≤71.29 mL/(min·1.73 m2), NT‐proBNP≥2573 pg/mL, and hs‐CRP≥8.03 mg/L.
Adjusted by age, sex, hemoglobin at admission, hematocrit at admission, albumin at admission, glucose at admission, previous coronary artery disease, previous hypertension, previous diabetes mellitus, previous chronic kidney disease, angiography, ACEI and/or ARB use after admission, and diuretic use after admission.
Discussion
To our knowledge, this is the first study to examine the predictive ability of a combination of 3 traditional biomarkers of heart failure: (NT‐proBNP), renal function (eGFR), and inflammation (hs‐CRP) for CSR1. The major findings of this study were these: (1) abnormal levels of NT‐proBNP, eGFR, and hs‐CRP at admission were independent risk factors for in‐hospital CRS1; (2) the sensitivity of NT‐proBNP, eGFR, and hs‐CRP were relatively high, but the specificity of hs‐CRP was relatively poor; (3) the discriminatory capability of the 3‐biomarker panel was stronger than those of the individual biomarkers; and (4) abnormal levels of 2 or more biomarkers based on the identified cutoff values were associated with an elevated risk of CRS1.
AMI is 1 of the critical conditions that can lead to CRS1. The majority of existing reports noted that CRS1 in patients with acute coronary syndrome was associated with longer hospital stay and higher in‐hospital mortality.17 Recently, in patients who were hospitalized for acute coronary syndrome, the risk of in‐hospital mortality associated with CRS1 was greater than the sum of the risks associated with acute heart failure and AKI.10 CRS1 also was responsible for more than half of all cases of in‐hospital mortality. In contrast to acute coronary syndrome, there are few reports in the literature on CRS1 in the setting of only AMI. Our study provides evidence that CRS1 may adversely affect clinical outcomes in AMI patients. In our cohort the all‐cause mortality and cardiovascular mortality in the CRS1 group were 9 times higher than those among the group of patients who did not develop CRS1. Therefore, it is important to understand the risk factors and develop methods for early prevention of the development of CRS1.
A large number of studies have evaluated the associations of various predictors with the occurrence of AKI in AMI patients and found that advanced age, hypertension, low body mass index, initial hemodynamic instability, extent of vessel disease, severe Killip class, abnormal heart rate, reduced GFR at presentation, longer door‐to‐needle time, increased spot urine albumin‐to‐creatinine ratio, hyperglycemia at admission, history of chronic kidney disease, and decreased hemoglobin levels are associated with worsening renal function (or AKI).17, 18, 19, 20, 21, 22 In the present study we found that baseline cardiorenal dysfunction and inflammation were independent predictors of CRS1 in patients with AMI, which is consistent with previous results. Furthermore, we found that NT‐proBNP, eGFR, and hs‐CRP had good discriminative ability for the prediction of CRS1. The reason may be based on the correlation of these markers with the pathophysiology mechanisms of CRS1.
Many studies have emerged in recent years trying to explain the pathophysiology of CRS1, and they have primarily focused on the hemodynamic and nonhemodynamic mechanisms. The traditional theory is that hypoperfusion of the kidneys followed by “forward failure” might lead to acute tubular necrosis, which is regarded as the key underlying mechanism contributing to renal dysfunction in the acute clinical setting.23, 24 However, a growing body of research indicates that elevations in right atrial pressure, which correlate with central venous pressure rather than a decline in cardiac output and/or cardiac index, are associated with declining renal function.5, 25, 26, 27, 28 NT‐proBNP has already been established as a diagnostic and prognostic marker in chronic as well as acute heart failure, and this protein is rapidly released from cardiomyocytes after stretching. Moreover, recent studies have reported that NT‐proBNP is a strong independent predictor of worsening renal function within 18 months in patients with systolic heart failure29 and a biochemical marker of integrated cardiorenal function in the chronic phase after myocardial infarction.30 In the present study we hypothesized that NT‐proBNP is an indirect indicator of elevations in central venous pressure and/or right atrial pressure during the pathophysiology of CRS1. Accordingly, we found that NT‐proBNP had good discriminatory ability and could serve as an indirect marker of CRS1 in patients with AMI.
In addition to hemodynamic pathways inflammation plays a pivotal role in the nonhemodynamic mechanisms of CRS1 pathophysiology. In a study by Virzi et al, serum levels of the proinflammatory cytokines tumor necrosis factor‐α and interleukin‐6 were significantly elevated in CRS1 patients compared with healthy controls.31 Another recent study found that elevated levels of inflammatory factors such as interleukin‐1β, endothelin‐1, interleukin‐10, and resolvin‐D1 were positively correlated with worsening renal function in patients with ACS.7 Among the various cytokines and mediators, hs‐CRP has received significant attention due to its association with atherosclerosis and cardiac disease as well as its prognostic value for heart failure and long‐term mortality.32, 33, 34 The results of the current study indicate that hs‐CRP is a powerful and independent predictor of CRS1. Despite its poor specificity, hs‐CRP showed relatively good sensitivity at a cutoff value of 8.03 mg/L. Our results are consistent with those of a previous report indicating that hs‐CRP >9 mg/L at admission is an independent predictor of AKI in patients with ST‐elevation myocardial infarction following primary percutaneous coronary intervention.35 Interestingly, elevation of hs‐CRP not only is a marker of AKI but also plays a pathogenic role in AKI by inhibiting tubular epithelium cell regeneration and altering macrophage polarization.36, 37 Therefore, anti‐inflammatory treatment targeting the hs‐CRP pathway may offer a new therapeutic approach to preventing or treating CRS1.
So far, the ideal marker for early detection of CRS1 has remained elusive. Several novel biomarkers for early detection of AKI have been investigated in patients with heart failure, including cystatin C, neutrophil gelatinase‐associated lipocalin, N‐acetyl‐β‐D‐glucosaminidase, kidney injury molecule‐1, interleukin‐18, and tissue inhibitor of metalloproteinase‐2.38, 39 These indicators have different clinical significance and features. However, no single marker satisfied the conditions of high organ specificity, high sensitivity for diagnosis, and being reflective of the disease course. Therefore, a multimarker model has been proposed for risk prediction. The current analysis represents the first examination of the combination of NT‐proBNP, eGFR, and hs‐CRP for predicting CRS1 in patients with AMI. This combination of biomarkers may be used to predict CRS1 and to facilitate risk stratification of AMI patients.
Study Limitations
We acknowledge several limitations in our study. First, this was a single‐center study, and our findings may not apply to other samples of patients in whom CRS1 was defined according to different criteria. Second, we did not investigate the contribution of nephrotoxic drugs to CRS1 development. Although we performed adjustment for pharmacological treatments and the catheter laboratory procedure, the amount of contrast agent was unknown. Third, as discussed in other studies on CRS1, the measurement of serum creatinine for evaluation of dynamic changes in renal function was performed at variable time intervals in our study. Fourth, some baseline clinical variables may have been missed. Therefore, it is possible that unmeasured or residual confounding factors may explain some of our results. Last, it should be noted that our study was an observational study and not an interventional study. The characteristics and clinical course of CRS1 may have been influenced by different treatments, causing our results to differ slightly from those of later trials. Further prospective multicenter studies are needed to validate our findings and identify even better multimarker models including other existing biomarkers and parameters.
In summary, we assessed the performance of 3 traditional markers (NT‐proBNP, eGFR, and hs‐CRP) for detecting the development of CRS1 in a cohort of 2094 patients with AMI. These 3 simple markers are easy to measure and apply in clinical practice. Because of their relation to the pathophysiology of CRS1, NT‐proBNP, eGFR, and hs‐CRP at admission were found to be independent risk factors for in‐hospital CRS1 and to show good discriminative ability. The combination of the 3 biomarkers showed better predictive capability than any of the biomarkers individually. Abnormal levels of 2 or more of these markers according to the identified cutoff values were associated with an elevated risk of CRS1. Therefore, the multimarker panel of NT‐proBNP, eGFR, and hs‐CRP may assist in the prediction of CRS1 and the corresponding risk stratification of patients with AMI.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e009162 DOI: 10.1161/JAHA.118.009162.)
References
- 1. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P; Acute Dialysis Quality Initiative consensus . Cardio‐renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ronco C. The cardiorenal syndrome: basis and common ground for a multidisciplinary patient‐oriented therapy. Cardiorenal Med. 2011;1:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, Mahon NG, Murray PT. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med. 2013;3:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Virzi GM, Clementi A, Brocca A, de Cal M, Vescovo G, Granata A, Ronco C. The hemodynamic and nonhemodynamic crosstalk in cardiorenal syndrome type 1. Cardiorenal Med. 2014;4:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho E, Kim M, Ko YS, Lee HY, Song M, Kim MG, Kim HK, Cho WY, Jo SK. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transpl. 2013;28:2766–2778. [DOI] [PubMed] [Google Scholar]
- 7. Ortega‐Hernandez J, Springall R, Sanchez‐Munoz F, Arana‐Martinez JC, Gonzalez‐Pacheco H, Bojalil R. Acute coronary syndrome and acute kidney injury: role of inflammation in worsening renal function. BMC Cardiovasc Disord. 2017;17:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD. Short‐term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the National Cardiovascular Data Registry. Circulation. 2012;125:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, Masoudi FA. The prognostic importance of worsening renal function during an acute myocardial infarction on long‐term mortality. Am Heart J. 2010;160:1065–1071. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez RP, Comba PC, Esteban MR, Sanchez JJA, Afonso JH, Perez MDR, Rodriguez IM, Diaz BB, Elosua R, de Leon AC. Incidence, mortality and positive predictive value of type 1 cardiorenal syndrome in acute coronary syndrome. PLoS One. 2016;11:e0167166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 12. Steg PG, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van‘t Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 16. McCullough PA, Kellum JA, Haase M, Muller C, Damman K, Murray PT, Cruz D, House AA, Schmidt‐Otti KM, Vescovo G, Bagshaw SM, Hoste EA, Briguori C, Braam B, Chawla LS, Costanzo MR, Tumlin JA, Herzog CA, Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C. Pathophysiology of the cardiorenal syndromes: executive summary from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:82–98.23689657 [Google Scholar]
- 17. Eren Z, Ozveren O, Buyukoner E, Kaspar E, Degertekin M, Kantarci G. A single‐centre study of acute cardiorenal syndrome: incidence, risk factors and consequences. Cardiorenal Med. 2012;2:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choe JC, Cha KS, Ahn J, Park JS, Lee HW, Oh J‐H, Kim JS, Choi JH, Park YH, Lee HC, Kim JH, Chun KJ, Hong TJ, Ahn Y, Jeong MH. Persistent renal dysfunction after percutaneous coronary intervention in patients with acute myocardial infarction: incidence, predictors, and impact on prognosis. Angiology. 2017;68:159–167. [DOI] [PubMed] [Google Scholar]
- 19. Vavalle JP, van Diepen S, Clare RM, Hochman JS, Weaver WD, Mehta RH, Pieper KS, Patel MR, Patel UD, Armstrong PW, Granger CB, Lopes RD. Renal failure in patients with ST‐segment elevation acute myocardial infarction treated with primary percutaneous coronary intervention: predictors, clinical and angiographic features, and outcomes. Am Heart J. 2016;173:57–66. [DOI] [PubMed] [Google Scholar]
- 20. Tziakas D, Chalikias G, Kareli D, Tsigalou C, Risgits A, Kikas P, Makrygiannis D, Chatzikyriakou S, Kampouromiti G, Symeonidis D, Voudris V, Konstantinides S. Spot urine albumin to creatinine ratio outperforms novel acute kidney injury biomarkers in patients with acute myocardial infarction. Int J Cardiol. 2015;197:48–55. [DOI] [PubMed] [Google Scholar]
- 21. Moriyama N, Ishihara M, Noguchi T, Nakanishi M, Arakawa T, Asaumi Y, Kumasaka L, Kanaya T, Miyagi T, Nagai T, Yamane T, Fujino M, Honda S, Fujiwara R, Anzai T, Kusano K, Goto Y, Yasuda S, Ogawa H. Admission hyperglycemia is an independent predictor of acute kidney injury in patients with acute myocardial infarction. Circ J. 2014;78:1475–1480. [DOI] [PubMed] [Google Scholar]
- 22. Queiroz RE, de Oliveira LS, de Albuquerque CA, Santana C de A, Brasil PM, Carneiro LL, Liborio AB. Acute kidney injury risk in patients with ST‐segment elevation myocardial infarction at presentation to the ED. Am J Emerg Med. 2012;30:1921–1927. [DOI] [PubMed] [Google Scholar]
- 23. Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1. J Am Coll Cardiol. 2012;60:1031–1042. [DOI] [PubMed] [Google Scholar]
- 24. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12:610–623. [DOI] [PubMed] [Google Scholar]
- 25. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the escape trial. J Am Coll Cardiol. 2008;51:1268–1274. [DOI] [PubMed] [Google Scholar]
- 26. Gnanaraj JF, von Haehling S, Anker SD, Raj DS, Radhakrishnan J. The relevance of congestion in the cardio‐renal syndrome. Kidney Int. 2013;83:384–391. [DOI] [PubMed] [Google Scholar]
- 27. Chen KP, Cavender S, Lee J, Feng M, Mark RG, Celi LA, Mukamal KJ, Danziger J. Peripheral edema, central venous pressure, and risk of AKI in critical illness. Clin J Am Soc Nephrol. 2016;11:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 29. Pfister R, Muller‐Ehmsen J, Hagemeister J, Hellmich M, Erdmann E, Schneider CA. NT‐pro‐BNP predicts worsening renal function in patients with chronic systolic heart failure. Intern Med J. 2011;41:467–472. [DOI] [PubMed] [Google Scholar]
- 30. Luchner A, Hengstenberg C, Lowel H, Trawinski J, Baumann M, Riegger GA, Schunkert H, Holmer S. N‐terminal pro‐brain natriuretic peptide after myocardial infarction: a marker of cardio‐renal function. Hypertension. 2002;39:99–104. [DOI] [PubMed] [Google Scholar]
- 31. Virzi GM, Torregrossa R, Cruz DN, Chionh CY, de Cal M, Soni SS, Dominici M, Vescovo G, Rosner MH, Ronco C. Cardiorenal syndrome type 1 may be immunologically mediated: a pilot evaluation of monocyte apoptosis. Cardiorenal Med. 2012;2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Araujo JP, Lourenco P, Azevedo A, Frioes F, Rocha‐Goncalves F, Ferreira A, Bettencourt P. Prognostic value of high‐sensitivity C‐reactive protein in heart failure: a systematic review. J Card Fail. 2009;15:256–266. [DOI] [PubMed] [Google Scholar]
- 33. Kozdag G, Ertas G, Kilic T, Acar E, Agir A, Sahin T, Cetin M, Bildirici U, Ural D. Elevated level of high‐sensitivity C‐reactive protein is important in determining prognosis in chronic heart failure. Med Sci Monit. 2010;16:Cr156–Cr161. [PubMed] [Google Scholar]
- 34. Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D. Early inflammation and risk of long‐term development of heart failure and mortality in survivors of acute myocardial infarction: predictive role of C‐reactive protein. J Am Coll Cardiol. 2006;47:962–968. [DOI] [PubMed] [Google Scholar]
- 35. Shacham Y, Leshem‐Rubinow E, Steinvil A, Keren G, Roth A, Arbel Y. High sensitive C‐reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. Clin Exp Nephrol. 2015;19:838–843. [DOI] [PubMed] [Google Scholar]
- 36. Tang Y, Huang XR, Lv J, Chung AC, Zhang Y, Chen JZ, Szalai AJ, Xu A, Lan HY. C‐reactive protein promotes acute kidney injury by impairing g1/s‐dependent tubular epithelium cell regeneration. Clin Sci (Lond). 2014;126:645–659. [DOI] [PubMed] [Google Scholar]
- 37. Pegues MA, McCrory MA, Zarjou A, Szalai AJ. C‐reactive protein exacerbates renal ischemia‐reperfusion injury. Am J Physiol Renal Physiol. 2013;304:F1358–F1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCullough PA, Jefferies JL. Novel markers and therapies for patients with acute heart failure and renal dysfunction. Am J Med. 2015;128:312.e1–312.e22. [DOI] [PubMed] [Google Scholar]
- 39. Palazzuoli A, McCullough PA, Ronco C, Nuti R. Kidney disease in heart failure: the importance of novel biomarkers for type 1 cardio‐renal syndrome detection. Intern Emerg Med. 2015;10:543–554. [DOI] [PubMed] [Google Scholar]


