Abstract
Background
Cardiovascular risk factors can track from mother to child by several pathways: pregnancy complications, genetic inheritance, and shared environmental risk factors after pregnancy. The degree of tracking, and to which extent this is influenced by these pathways, is unknown. We hypothesized that cardiovascular risk factors track from mother to child regardless of pregnancy complications and environmental risk factors. We determined the degree of tracking between maternal and offspring micro‐ and macrovascular cardiovascular risk factors after pregnancy and the extent to which this is influenced by pregnancy complications and shared environmental risk factors.
Methods and Results
We included 5624 mother‐offspring pairs from The Generation R Study, an ongoing prospective, population‐based birth cohort. Information on pregnancy complications (preeclampsia, small for gestational age, and preterm birth) was obtained through hospital charts. Mother‐offspring associations were assessed 6 years after pregnancy (central retinal arteriolar and venular calibers, body mass index, blood pressure, left atrial diameter, aortic root diameter, left ventricular mass, fractional shortening, and pulse wave velocity) and 9 years after pregnancy (body mass index and blood pressure). We observed that worse cardiovascular parameters in mothers were associated with worse cardiovascular parameters in their offspring 6 and 9 years after pregnancy (P<0.001). Results were similar when mother‐offspring pairs with a previous pregnancy complication were excluded.
Conclusions
Six and 9 years after pregnancy, an adverse cardiovascular profile in mothers is strongly associated with an adverse cardiovascular profile in their offspring. Results were not attenuated by environmental exposures or a previous pregnancy complication. This supports the hypothesis that cardiovascular risk factors (micro‐ and macrovascular) track from mother to child, regardless of the course of pregnancy.
Keywords: blood pressure, child, echocardiography, heredity, microcirculation, mothers, pediatrics, preeclampsia/pregnancy, pregnancy complications, risk factors
Subject Categories: Hemodynamics, Mechanisms, Epidemiology, Pediatrics, Risk Factors
Clinical Perspective
What Is New?
Cardiovascular risk factors can track from mother to child by several pathways: intrauterine programming, genetic inheritance, and shared environmental risk factors after pregnancy.
The degree of tracking, and to which extent this is influenced by these pathways, is unknown.
Our results are novel given that tracking of the microvasculature, left atrial diameter, aortic root diameter, and fractional shortening from mother to offspring have not been studied previously.
What Are the Clinical Implications?
This study shows that cardiovascular risk factors (micro‐ and macrovascular) track from mother to child, regardless of the course of pregnancy.
Therefore, healthcare providers should not only focus on cardiovascular risk prevention in children born from complicated pregnancies, but also in those children whose mothers have a suboptimal cardiovascular risk profile 6 and 9 years after pregnancy.
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality worldwide.1 Prevalence of cardiovascular risk factors (eg obesity, hypertension, and an atherogenic lipid profile) is not only rising among adults, but also among children. Cardiovascular risk factors are most likely transmitted from parents to their offspring, which can be explained through 3 potential mechanisms: pregnancy complications, genetic inheritance, and shared environmental risk factors after pregnancy. The degree to which cardiovascular risk factors track from mother to child, and the extent to which this is influenced by pregnancy complications and shared environmental risk factors after pregnancy, is still unknown. Unraveling these mechanisms may assist us in identifying at an early stage children at risk for cardiovascular disease later in life.
The first mechanism involves pregnancy complications, such as preeclampsia, which require adaptation in order for the fetus to survive to a stressful environment. This process can affect perinatal health given that it is associated with a low birth weight and preterm birth, which themselves are risk factors for the development of cardiovascular risk factors during adolescence such as obesity and hypertension.2 Second, environmental exposures (eg diet, exposure to smoking, and physical activity) may be involved. These are often similar between parents and their offspring until they enter adulthood. Previous studies show that parents’ diet and energy intake, smoking behavior, and physical activity levels are positively associated with those of their offspring.3, 4, 5, 6, 7 Third, heritability studies have identified genetic factors as a determinant for certain cardiovascular risk factors such as body mass index (BMI) level.8, 9 Obtaining better insight in the degree of tracking and the most important mechanisms involved will help to predict and reduce future cardiovascular disease in offspring. In this study, we hypothesized that cardiovascular risk factors track from mother to child regardless of pregnancy complications and environmental risk factors. We determined the degree of tracking between maternal and offspring microvascular (central retinal and arteriolar calibers) and macrovascular (blood pressure, left atrial diameter, aortic root diameter, left ventricular mass, fractional shortening, and pulse wave velocity [PWV]) cardiovascular risk factors after pregnancy and the extent to which this is influenced by pregnancy complications and shared environmental risk factors.
Materials and Methods
Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Design and Study Population
This study was embedded in The Generation R Study, a multiethnic, population‐based, prospective cohort study from early pregnancy onward in Rotterdam, The Netherlands.10, 11 Mothers (N=8976) enrolled during pregnancy between April 2002 and January 2006. Response rate at baseline was 61% and 85% 6 years after pregnancy. The study has been approved by the Medical Ethics Committee of Erasmus Medical Center, Rotterdam, The Netherlands (MEC 198.782/2001/31), and the procedures followed were in accord with institutional guidelines.12 Written informed consent was obtained from all participants. For the present study, we included mother‐offspring pairs with at least 1 measurement of interest available 6 or 9 years after pregnancy (N=5624; Figure). Figure S1 presents an overview of our exposures, outcomes, and covariates.
Figure 1.
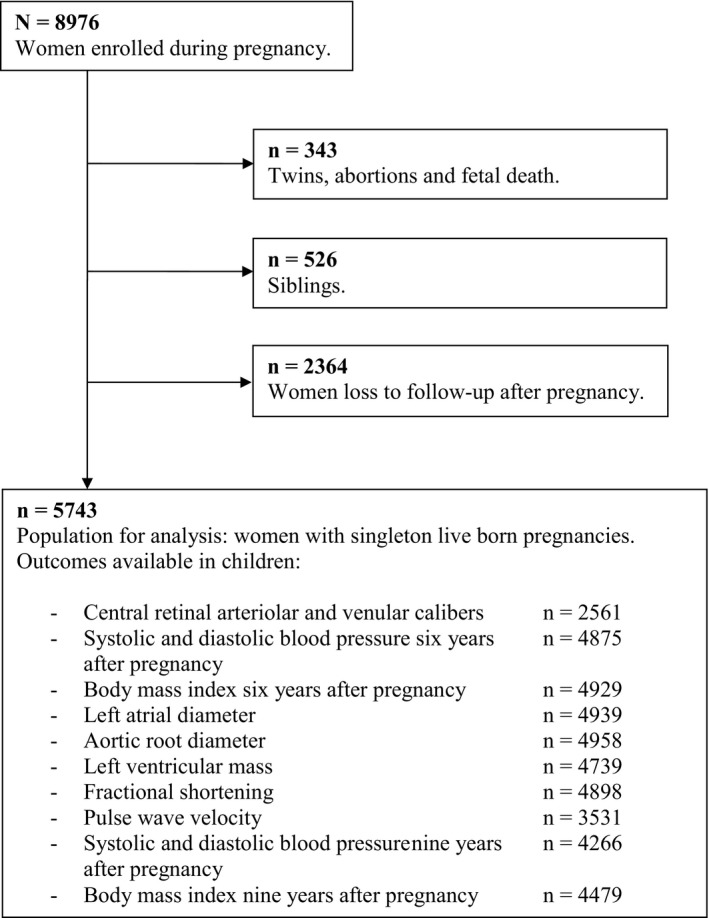
Flow chart.
Pregnancy Complications
Presence of a pregnancy complication (preeclampsia, a small for gestational age child, or spontaneous preterm birth) was determined from the original hospital charts. Preeclampsia was defined as a systolic blood pressure [SBP] ≥140 mm Hg or a diastolic blood pressure [DBP] ≥90 mm Hg after 20 weeks of gestation in previously normotensive women with concurrent new‐onset proteinuria in a random urine sample and no evidence of a urinary tract infection.13, 14 Small for gestational age was defined as a birth weight <10th percentile (based on our own cohort) and spontaneous preterm birth as the spontaneous onset of labor before 37 weeks of gestation.15
Environmental Exposures and Covariates
At study enrollment, maternal height (cm) and weight (kg) without shoes were measured, after which BMI (kg/m2) was calculated. Identical measurements were obtained during follow‐up 6 and 9 years after pregnancy for both mother and child. Pre‐pregnancy BMI was established at enrollment through a questionnaire. Pre‐pregnancy BMI was strongly correlated with BMI measured in early pregnancy (Pearson's correlation coefficient, r=0.95; P<0.001).16
Questionnaires were repeatedly applied during pregnancy to obtain information on maternal age, pre‐pregnancy weight, gravidity, ethnicity, educational level, smoking, and folic acid intake. Information on gestational age at birth, birth weight, and child's sex was obtained from medical records.15, 17 Six years after index pregnancy, we obtained information on gravidity at follow‐up and smoking through questionnaires.
Cardiovascular Measurements 6 Years After Pregnancy
Central retinal arteriolar and venular calibers were assessed in mothers and offspring by taking digital retinal photographs 6 years after index pregnancy. Details of this procedure are described previously.18, 19, 20
Research assistants in nonmedical clothing (ie, no white coat) measured blood pressure during study enrollment (median, 13.2 weeks of gestation; 90% range, 10.6, 17.0) and 6 years after pregnancy (90% range, 5.7–7.4) with the validated Omron 907 automated digital oscillometric sphygmomanometer (OMRON Healthcare Europe B.V., Hoofddorp, The Netherlands).21 Blood pressure was measured in the right upper arm and in a standardized supine position to prevent differences attributed to position. The mean value of 2 systolic and diastolic blood pressure readings (SBP and DBP) was documented for each participant.
Carotid‐femoral pulse wave velocity (PWV) was measured with an automatic noninvasive, validated device (Complior; Artech Medical, Pantin, France) to assess arterial wall stiffness. The device measures the distance between recording sites at the carotid (proximal) and femoral (distal) artery. Echocardiographic measurements were performed in 2‐dimensional M‐mode using the ATL‐Philips Model HDI 5000 (ATL‐Philips, Seattle, WA) or the Logiq E9 (GE Medical Systems, Wauwatosa, WI) devices. Fractional shortening, aortic root diameter, and left atrial diameter were measured. Left ventricular mass was calculated with the Devereux equation.22 Intra‐ and interobserver intraclass correlation coefficients were described previously and demonstrated good repeatability and reproducibility.23
Blood Pressure 9 Years After Pregnancy
The validated automatic sphygmomanometer, Datascope Accutorr Plus (Datascope, Paramus, NJ), was used to measure blood pressure in mothers and children 9.8 years after pregnancy (90% range, 9.5, 10.3).24 The average of the last 3 (of 4) blood pressure measurements was used for further analyses.
Statistical Analyses
Baseline characteristics are presented in Table 1. Mean±SD is presented for data with a normal distribution and the median with 90% confidence interval for data with a skewed distribution. In the total population for analysis, 17.7% had missing information on pre‐pregnancy BMI, 6.9% on education, 11.0% on smoking during pregnancy, 24.1% on folic acid intake during pregnancy, 1.0% on maternal SBP at study intake, 2.5% on child's ethnicity, 13.3% and 9.0% on child's SBP and DBP 6 years after pregnancy, and 5.4% on child's BMI after pregnancy. To reduce potential bias associated with missing data, we imputed missing values in variables of interest through multiple imputation.25 Complete case analysis showed similar results to those presented in Tables 2 and 3 (data not shown). Multivariate linear regression analysis was used to examine the association between mother‐offspring cardiovascular risk factors after pregnancy (Tables 2 and 3) while adjusting for confounders. Regression models adjusted for confounding were: basic model (including maternal age at enrollment and child's age during follow‐up) and 4 confounder models (including pre‐pregnancy BMI, maternal age at enrollment, maternal SBP at study enrollment, educational level mother, smoking during pregnancy, folic acid intake during pregnancy, child's sex, child's ethnicity, child's age [6 and 9 years after pregnancy], child's weight and height [6 and 9 years after pregnancy], and mean SBP and DBP 6 years after pregnancy). These confounders were selected based on their associations with exposures and outcomes of interest and based on previous studies. Effect estimates in Tables 2 and 3 are unstandardized beta‐coefficients and represent an increase in the outcome measure for each unit increase in the exposure. We applied a Benjamin–Hochberg procedure controlling for false discovery rate at 0.05 level.26 We tested whether there was effect modification in all mother‐offspring associations by pregnancy complications through inclusion of the interaction term (exposure×pregnancy complication yes) in each regression model. To estimate the proportion of total variance in offspring attributable to variation in genotypes, we calculated unadjusted heritability (h2, expressed as percentage) through regression of offspring phenotype on the maternal phenotype.27 Estimates are presented in the Results section. Last, we examined baseline characteristics between women included and excluded from this study (Table S1). Statistical analyses were performed with SPSS software (version 21.0 for Windows; SPSS, Inc, Chicago, IL).
Table 1.
Baseline Characteristics (n=5743)
| Pregnancy | |
| Age at enrollment (y), mean (SD) | 30.1 (5.1) |
| Non‐European ethnicity, % | 42.1 |
| No education/primary school, % | 11.7 |
| Pre‐pregnancy BMI (kg/m2), median (5th, 95th percentile) | 22.7 (18.7, 32.3) |
| Lean and normal, % | 71.2 |
| Overweight, % | 20.3 |
| Obese and morbidly obese, % | 8.5 |
| SBP at enrollment (mm Hg), mean (SD) | 115.6 (12.1) |
| DBP at enrollment (mm Hg), mean (SD) | 68.1 (9.5) |
| Smoking, % | 27.1 |
| Nulliparous, % | 60.1 |
| No folic acid intake, % | 28.3 |
| Diabetes mellitus, % | 0.4 |
| Gestational diabetes mellitus, % | 1.1 |
| Pregnancy outcomes | |
| Spontaneous preterm birth, % | 3.6 |
| Small for gestational age (<p10), % | 9.9 |
| Preeclampsia, % | 2.1 |
| Gestational hypertension, % | 4.2 |
| Boys, % | 49.6 |
| Non‐European ethnicity child, % | 39.0 |
| Maternal outcomes 6 years after pregnancy | |
| Follow‐up interval (years), median (5th, 95th percentile) | 6.0 (5.7, 7.4) |
| BMI (kg/m2), median (5th, 95th percentile) | 24.8 (19.7, 36.0) |
| SBP (mm Hg), mean (SD) | 119.3 (13.0) |
| DBP (mm Hg), mean (SD) | 71.0 (10.1) |
| Cardiovascular medication, % | 2.1 |
| Central retinal arteriolar caliber (μm), mean (SD) | 145.3 (16.9) |
| Central retinal venular caliber (μm), mean (SD) | 206.8 (22.5) |
| Smoking, % | 20.0 |
| Primigravid, %a | 8.5 |
| Child outcomes 6 years after pregnancy | |
| BMI (kg/m2), median (5th, 95th percentile) | 15.9 (13.9, 19.8) |
| SBP (mm Hg), mean (SD) | 103.0 (8.3) |
| DBP (mm Hg), mean (SD) | 62.0 (8.6) |
| Central retinal arteriolar caliber (μm), mean (SD) | 158.8 (14.9) |
| Central retinal venular caliber (μm), mean (SD) | 218.8 (19.9) |
| Outcomes 9 years after pregnancy | |
| BMI mother (kg/m2), median (5th, 95th percentile) | 24.8 (20.0, 36.2) |
| SBP mother (mm Hg), mean (SD) | 114.6 (12.8) |
| DBP mother (mm Hg), mean (SD) | 68.6 (8.2) |
| Cardiovascular medication, % | 2.4 |
| SBP child (mm Hg), mean (SD) | 103.2 (8.0) |
| DBP child (mm Hg), mean (SD) | 58.6 (6.4) |
| BMI child (kg/m2), median (5th, 95th percentile) | 17.0 (14.4, 23.3) |
Values are percentages for categorical variables, means (SD) for continuous variables with a normal distribution, or medians (5th, 95th percentile) for continuous variables with a skewed distribution. BMI indicates body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Up until follow‐up visit pregnancy had occurred no more than once.
Table 2.
Association Between Mother‐Offspring Cardiovascular Risk Factors 6 and 9 Years After Pregnancy N=5743
| Outcomes Child | Exposures Mother | Basic Model | P Value | Confounder Model | P Value |
|---|---|---|---|---|---|
| Beta (95% CI) | Beta (95% CI) | ||||
| Six years after pregnancy | |||||
| Microvasculature | |||||
| Central retinal arteriolar caliber (SDS) | Central retinal arteriolar caliber (n=2561) | 0.12 (0.08, 0.16) | <0.001 | 0.11 (0.07, 0.14)a | <0.001 |
| Central retinal venular caliber (SDS) | Central retinal venular caliber (n=2561) | 0.18 (0.14, 0.22) | <0.001 | 0.19 (0.15, 0.22)a | <0.001 |
| BMI, kg/m2 | BMI (n=4929) | 0.11 (0.10, 0.12) | <0.001 | 0.10 (0.09, 0.11)b | <0.001 |
| Blood pressure | |||||
| SBP, mm Hg | SBP (n=4875) | 0.11 (0.09, 0.13) | <0.001 | 0.08 (0.06, 0.10)b | <0.001 |
| DBP, mm Hg | DBP (n=4875) | 0.10 (0.08, 0.12) | <0.001 | 0.08 (0.06, 0.10)b | <0.001 |
| Cardiac measurements | |||||
| Left atrial diameter, mm | Left atrial diameter (n=4939) | 0.14 (0.12, 0.16) | <0.001 | 0.13 (0.11, 0.15)c | <0.001 |
| Aortic root diameter, mm | Aortic root diameter (n=4958) | 0.16 (0.14, 0.17) | <0.001 | 0.13 (0.12, 0.15)c | <0.001 |
| Left ventricular mass, g | Left ventricular mass (n=4739) | 0.12 (0.11, 0.13) | <0.001 | 0.10 (0.09, 0.11)c | <0.001 |
| Fractional shortening, % | Fractional shortening (n=4898) | 0.14 (0.12, 0.17) | <0.001 | 0.14 (0.12, 0.17)c | <0.001 |
| PWV, m/s | PWV (n=3531) | 0.18 (0.15, 0.21) | <0.001 | 0.17 (0.14, 0.20)c | <0.001 |
| Nine years after pregnancy | |||||
| BMI, kg/m2 | BMI (n=4479) | 0.19 (0.18, 0.21) | <0.001 | 0.16 (0.14, 0.17)d | <0.001 |
| SBP, mm Hg | SBP (n=4266) | 0.11 (0.09, 0.13) | <0.001 | 0.06 (0.04, 0.08)d | <0.001 |
| DBP, mm Hg | DBP (n=4266) | 0.12 (0.10, 0.14) | <0.001 | 0.10 (0.07, 0.12)d | <0.001 |
Values are betas with corresponding 95% CI and represent the mean difference in children per unit increase in maternal parameter. Basic model: Age mother at intake and age child during follow‐up. BMI indicates body mass index; CI, confidence interval; DBP, diastolic blood pressure; PWV, pulse wave velocity; SBP, systolic blood pressure; SDS, standard deviation score.
Confounder model: in addition to the basic model: educational level mother at intake, ethnicity child, smoking during pregnancy, mean systolic blood pressure child 6 years after pregnancy, and maternal systolic blood pressure during study intake.
Confounder model: in addition to the basic model: educational level mother at intake, ethnicity child, maternal systolic blood pressure during study intake, child's sex, and child's weight and height 6 years after pregnancy.
Confounder model: in addition to the basic model: educational level mother at intake, ethnicity child, maternal smoking during pregnancy, folic acid intake during pregnancy, pre‐pregnancy BMI, and child's weight and height and diastolic blood pressure 6 years after pregnancy.
Confounder model: in addition to the basic model: educational level mother at intake, ethnicity child, maternal systolic blood pressure during study intake, child's sex, and child's weight and height 9 years after pregnancy.
Table 3.
Association Between Mother‐Offspring Cardiovascular Risk Factors 6 and 9 Years After Pregnancy in Women Without a Pregnancy Complication During the Index Pregnancy n=4536
| Outcomes Child | Exposures Mother | Basic Model | P Value | Confounder Model | P Value |
|---|---|---|---|---|---|
| Beta (95% CI) | Beta (95% CI) | ||||
| Six years after pregnancy | |||||
| Microvasculature | |||||
| Central retinal arteriolar caliber (SDS) | Central retinal arteriolar caliber (n=2044) | 0.12 (0.08, 0.16) | <0.001 | 0.11 (0.07, 0.16)a | <0.001 |
| Central retinal venular caliber (SDS) | Central retinal venular caliber (n=2044) | 0.18 (0.14, 0.23) | <0.001 | 0.19 (0.14, 0.23)a | <0.001 |
| BMI, kg/m2 | BMI (n=3899) | 0.12 (0.10, 0.13) | <0.001 | 0.10 (0.09, 0.11)a | <0.001 |
| Blood pressure | |||||
| SBP, mm Hg | SBP (n=3865) | 0.12 (0.09, 0.14) | <0.001 | 0.09 (0.07, 0.11)a | <0.001 |
| DBP, mm Hg | DBP (n=3865) | 0.10 (0.08, 0.12) | <0.001 | 0.09 (0.06, 0.11)a | <0.001 |
| Cardiac measurements | |||||
| Left atrial diameter, mm | Left atrial diameter (n=3898) | 0.13 (0.11, 0.16) | <0.001 | 0.12 (0.10, 0.15)a | <0.001 |
| Aortic root diameter, mm | Aortic root diameter (n=3917) | 0.15 (0.13, 0.17) | <0.001 | 0.13 (0.11, 0.15)a | <0.001 |
| Left ventricular mass, g | Left ventricular mass (n=3753) | 0.12 (0.10, 0.13) | <0.001 | 0.10 (0.09, 0.11)a | <0.001 |
| Fractional shortening, % | Fractional shortening (n=3869) | 0.14 (0.12, 0.17) | <0.001 | 0.14 (0.12, 0.17)a | <0.001 |
| PWV, m/s | PWV (n=2821) | 0.19 (0.16, 0.22) | <0.001 | 0.18 (0.15, 0.22)a | <0.001 |
| Nine years after pregnancy | |||||
| BMI, kg/m2 | BMI (n=3549) | 0.19 (0.18, 0.21) | <0.001 | 0.16 (0.14, 0.18) | <0.001 |
| SBP, mm Hg | SBP (n=3382) | 0.11 (0.09, 0.14) | <0.001 | 0.06 (0.04, 0.08) | <0.001 |
| DBP, mm Hg | DBP (n=3382) | 0.12 (0.10, 0.15) | <0.001 | 0.10 (0.08, 0.13) | <0.001 |
Values are betas with corresponding 95% CI and represent the mean difference in children per unit increase in maternal parameter. BMI indicates body mass index; CI, confidence interval; DBP, diastolic blood pressure; PWV, pulse wave velocity; SBP, systolic blood pressure; SDS, standard deviation score.
Regression models were identical to those presented under Table 2.
Results
This study included 5624 mother‐offspring pairs (Figur). Mothers were, on average, 30.3 (SD, 5.1) years of age at the start of pregnancy (Table 1). They were mostly European, highly educated, and nulliparous. In total, 15.3% of pregnancies were affected by a pregnancy complication.
Tables 2 and 3 show the association between mother‐offspring cardiovascular risk factors 6 and 9 years after pregnancy for the total population and for mother‐offspring pairs without a pregnancy complication during the index pregnancy. Maternal microvasculature (central retinal arteriolar and venular calibers), BMI, blood pressure (SBP and DBP), cardiac measurements (left atrial diameter, aortic root diameter, left ventricular mass, and fractional shortening) and PWV are all positively associated with the corresponding measurements in offspring. Results did not change after environmental factors (confounders) were taken into consideration nor after excluding mother‐offspring pairs affected by a pregnancy complication (Table 3; Figure S2) or when we corrected for multiple testing. Also, effect modification analysis showed no significant interaction in all mother‐offspring associations by pregnancy complications (data not shown).
Heritability varied between 9% and 20% (central retinal arteriolar caliber [12%], central retinal venular caliber [19%], BMI, SBP, and DBP at the age of 6 [12%, 11%, and 9%], left atrial diameter [15%], aortic root diameter [15%], left ventricular mass [12%], fractional shortening [14%], PWV [16%], and BMI, SBP, and DBP at the age of 9 [20%, 11%, and 11%]).
Table S1 shows baseline characteristics of women included and excluded from this study. Excluded women were, on average, younger during study enrollment, more often non‐European, lower educated, smoked more often in pregnancy, and had more often children born small for gestational age.
Discussion
This large, prospective study shows that adverse maternal cardiovascular risk factors (central retinal arteriolar and venular calibers, BMI, blood pressure, left atrial diameter, aortic root diameter, left ventricular mass, fractional shortening, and PWV) are strongly associated with corresponding adverse cardiovascular risk factors in offspring at the age of 6 years, and for BMI and blood pressure also at the age of 9 years, independent of pregnancy complications and environmental factors. This indicates that cardiovascular risk factors most likely track from mother to child by genetic and epigenetic pathways. Possibly, central retinal arteriolar and venular calibers, cardiac ultrasound measurements, and PWV also track up to 9 years after pregnancy. However, these measurements were not available 9 years after pregnancy.
The association between mother‐offspring retinal arteriolar and venular calibers has not been studied previously. Our study shows that the offspring retinal microcirculation is a representation of the maternal microcirculation and possibly also of the paternal microcirculation, but we did not examine the latter. In adults, smaller retinal arteriolar calibers and larger retinal venular calibers are independent risk factors for cardiac heart disease and stroke death.28
Several studies have examined traits between mother and offspring, such as BMI and blood pressure.29, 30, 31 Results indicate that tracking of these cardiovascular risk factors from mother to offspring likely continues into adult life. A large, cross‐sectional study in Norwegian parent‐offspring pairs showed that a higher BMI in parents was associated with a higher BMI in their adult offspring (mean age, 38–41 years).30 A similar tracking pattern was also observed for blood pressure. Compared with our study, effect estimates were larger for BMI, but similar for blood pressure. A smaller study in mother‐offspring pairs from Ohio also showed a positive association between mother‐offspring BMI throughout childhood and adolescence. The tracking pattern was stronger from mother to daughter than from mother to son, which we did not observe in our study.29 A similar study to ours, conducted in French school children (age, 5–13 years), also showed a stronger association between maternal‐offspring BMI than paternal‐offspring BMI.32 This might be attributed to pregnancy complications, which the researchers did not correct for. Genetic and epigenetic mechanisms might also be involved in the mother‐offspring associations we examined in this study; previous studies have shown a transgenerational epigenetic inheritance of cardiovascular disease.33
Increased left atrial diameter is a risk factor for atrial fibrillation, thrombus formation, and, consequently, stroke.34, 35 BMI and blood pressure are 2 major determinants of left atrial diameter. Adjusting for these confounders did not attenuate our results significantly, indicating that other factors might be more important, such as pregnancy complications, heritability, and epigenetics. Removing those pregnancies affected by a pregnancy complication from our analyses did not affect our results. We observed relatively small heritability (15%), which was also small in previous studies examining parents and their teenagers or adult offspring (2–7%).36, 37
Aortic root diameter increases with age and BMI and is a risk factor for aortic root dissection.38, 39 The association between mother‐offspring aortic root diameter has not been studied previously. Therefore, we cannot compare our results with other studies.
Previous population and twin studies that examined the association between parent‐offspring cardiovascular risk factors have shown a strong heritability component for cardiac features, such as left ventricular mass.40, 41 Left ventricular mass is a predictor of cardiac‐ and cerebrovascular‐related morbidity and mortality.42 Family‐based studies showed a wide variation in left ventricular mass heritability estimates between different ethnicities: 12% to 41% for whites, 22% to 44% for blacks, 15% for Chinese, and 43% to 61% for Japanese.43 Our heritability estimate of 12% is comparable to that found in previous studies examining whites. Besides genetic variance, variation in heritability estimates between studies might be attributed to several other factors, including differences in study design, statistical modeling, and characteristics of study participants. Left ventricular mass is strongly related to efficacy of glucose and fatty acid oxidation by the mitochondria.44 Mitochondrial DNA is inherited through the mother, which might explain why previous studies presented stronger mother‐offspring left ventricular mass associations than father‐offspring associations.45
This is the first study to examine the association between parent‐offspring fractional shortening. Reduced fractional shortening is associated with left ventricular systolic dysfunction.
To our knowledge, PWV in children has been examined in 1 previous study of 291 American mother‐offspring pairs (child's mean age, 12 years).46 Results showed a higher heritability estimate for PWV compared with our study (26% versus 16%).46 Similar to our results, findings were not affected by environmental factors such as age and BMI.
All our results were not driven by children born from pregnancies affected by a pregnancy complication. Therefore, the impact of intrauterine growth and development on cardiovascular risk factors in offspring seemed small.
Strengths and Limitations
Some limitations need to be addressed. Retinal vascular imaging 6 years after pregnancy was introduced into the Generation R Study after recruitment of study subjects had already started. Therefore, retinal vascular images were not available for 52.6% of mothers and children who attended follow‐up visit 6 years after pregnancy. This was independent of any subject characteristics, which makes it unlikely that selection bias occurred. Compared with women included in this study, those excluded were, on average, younger during study enrollment, more often non‐European, lower educated, smoked more often in pregnancy, and had more often children born small for gestational age. This may have led to some degree of selection bias given that the included women were relatively healthy and may have led to an underestimation of our associations. Nevertheless, we still observed significant associations that support tracking from mother to child of cardiovascular traits. Data on fathers were not available. A large, population‐based study, examining cardiovascular risk factors (BMI, blood pressure, lipid profile, and waist circumference), showed that offspring risk factors were positively associated with those in both parents.31 This finding argues against a strong impact of pregnancy complications and is more in favor of a genetic or epigenetic predisposition of cardiovascular risk factors in offspring. Reported heritability estimates might be confounded by pregnancy complications and environmental risk factors given that we did not adjust for these factors. Strengths of this study are the large sample size, prospective data collection, and standardized procedures that were used for data collection, and this is the first study to examine the mother‐offspring microvasculature. Future studies should also examine tracking of the microvasculature and cardiac ultrasound measurements from father to offspring.
Conclusion
Six and 9 years after pregnancy, an adverse cardiovascular profile in mothers is strongly associated with an adverse cardiovascular profile in their offspring. Results were not attenuated by environmental exposures or a previous pregnancy complication. This supports the hypothesis that cardiovascular risk factors (micro‐ and macrovascular) track from mother to child, regardless off the course of pregnancy. Besides focusing on children born from complicated pregnancies, we should also focus our efforts on children whose mothers have a worse cardiovascular risk profile.
Author Contributions
Benschop analyzed the data and wrote the article. Schalekamp‐Timmermans, Ikram, Roeters van Lennep, Steegers, and Jaddoe contributed to the design of the study, writing of the article, interpretation of the data, and revisions and gave input at all stages. All authors have approved the final version of the article.
Sources of Funding
The Generation R Study was made possible by financial support from the Erasmus Medical Center, Erasmus University Rotterdam, and the Netherlands Organization for Health Research and Development, the Netherlands Organization for Scientific Research, the Ministry of Health, Welfare and Sport, and the Ministry of Youth and Families. Professor Jaddoe received additional grants from the Netherlands Organization for Health Research and Development (Grant Nos. 90700303, 916.10159, and VIDI 016.136.361) and a Consolidator Grant from the European Research Council (ERC‐2014‐CoG‐64916). Dr Ikram received funding from the Netherlands Organization for Health Research and Development (ZonMW; VENI project number: 91612163). This study was made possible by additional funding of the Dutch Heart Foundation (Grant No.: 2013T083).
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of Women Included and Excluded in This Study (n=8107)
Figure S1. Timeline of exposures, outcomes, and confounders measured in mother and child.
Figure S2. Overview of results in complicated and uncomplicated pregnancies.
Acknowledgments
The Generation R Study is being conducted by the Erasmus Medical Center in close collaboration with the School of Law and the Faculty of Social Sciences of the Erasmus University, Rotterdam; the Municipal Health Service, Rotterdam area; the Rotterdam Homecare Foundation; and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond, Rotterdam, The Netherlands. We gratefully acknowledge the contributions of the general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
(J Am Heart Assoc. 2018;7:e009536 DOI: 10.1161/JAHA.118.009536.)
References
- 1. Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600–609. [DOI] [PubMed] [Google Scholar]
- 2. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robson SM, Couch SC, Peugh JL, Glanz K, Zhou C, Sallis JF, Saelens BE. Parent diet quality and energy intake are related to child diet quality and energy intake. J Acad Nutr Diet. 2016;116:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vuolo M, Staff J. Parent and child cigarette use: a longitudinal, multigenerational study. Pediatrics. 2013;132:e568–e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wellman RJ, Dugas EN, Dutczak H, O'Loughlin EK, Datta GD, Lauzon B, O'Loughlin J. Predictors of the onset of cigarette smoking: a systematic review of longitudinal population‐based studies in youth. Am J Prev Med. 2016;51:767–778. [DOI] [PubMed] [Google Scholar]
- 6. Kaseva K, Hintsa T, Lipsanen J, Pulkki‐Raback L, Hintsanen M, Yang X, Hirvensalo M, Hutri‐Kahonen N, Raitakari O, Keltikangas‐Jarvinen L, Tammelin T. Parental physical activity associates with offspring's physical activity until middle age: a 30‐year study. J Phys Act Health. 2017;14:520–531. [DOI] [PubMed] [Google Scholar]
- 7. Jacobi D, Caille A, Borys JM, Lommez A, Couet C, Charles MA, Oppert JM; FLVS Study Group . Parent‐offspring correlations in pedometer‐assessed physical activity. PLoS One. 2011;6:e29195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87:398–404. [DOI] [PubMed] [Google Scholar]
- 9. Pate RR, O'Neill JR, Liese AD, Janz KF, Granberg EM, Colabianchi N, Harsha DW, Condrasky MM, O'Neil PM, Lau EY, Taverno Ross SE. Factors associated with development of excessive fatness in children and adolescents: a review of prospective studies. Obes Rev. 2013;14:645–658. [DOI] [PubMed] [Google Scholar]
- 10. Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, van der Lugt A, Mackenbach JP, Moll HA, Raat H, Rivadeneira F, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27:739–756. [DOI] [PubMed] [Google Scholar]
- 11. Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CC, Mackenbach JP, Moll HA, Raat H, Rings EH, Rivadeneira F, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Wolvius EB, Hofman A, Jaddoe VW. The Generation R Study: biobank update 2015. Eur J Epidemiol. 2014;29:911–927. [DOI] [PubMed] [Google Scholar]
- 12. World Medical Association Inc . Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009;107:403–405. [PubMed] [Google Scholar]
- 13. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX–XIV. [DOI] [PubMed] [Google Scholar]
- 14. Silva LM, Coolman M, Steegers EA, Jaddoe VW, Moll HA, Hofman A, Mackenbach JP, Raat H. Low socioeconomic status is a risk factor for preeclampsia: the Generation R Study. J Hypertens. 2008;26:1200–1208. [DOI] [PubMed] [Google Scholar]
- 15. Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW, Witteman JC. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population‐based cohort study. Ultrasound Obstet Gynecol. 2008;31:388–396. [DOI] [PubMed] [Google Scholar]
- 16. Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring). 2013;21:1046–1055. [DOI] [PubMed] [Google Scholar]
- 17. Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr Scand. 1991;80:756–762. [DOI] [PubMed] [Google Scholar]
- 18. Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 19. Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. [DOI] [PubMed] [Google Scholar]
- 20. Benschop L, Schalekamp‐Timmermans S, Roeters van Lennep JE, Jaddoe VWV, Wong TY, Cheung CY, Steegers EA, Ikram MK. Gestational hypertensive disorders and retinal microvasculature: the Generation R Study. BMC Med. 2017;15:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM‐907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241. [DOI] [PubMed] [Google Scholar]
- 22. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 23. Geelhoed MJ, Snijders SP, Kleyburg‐Linkers VE, Steegers EA, van Osch‐Gevers L, Jaddoe VW. Reliability of echocardiographic measurements of left cardiac structures in healthy children. Cardiol Young. 2009;19:494–500. [DOI] [PubMed] [Google Scholar]
- 24. Khawaja RA, Qureshi R, Mansure AH, Yahya ME. Validation of Datascope Accutorr Plus using British Hypertension Society (BHS) and Association for the Advancement of Medical Instrumentation (AAMI) protocol guidelines. J Saudi Heart Assoc. 2010;22:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benjamini Yoav HY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 27. Visscher PM, Hill WG, Wray NR. Heritability in the genomics era—concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. [DOI] [PubMed] [Google Scholar]
- 28. Wang JJ, Liew G, Klein R, Rochtchina E, Knudtson MD, Klein BE, Wong TY, Burlutsky G, Mitchell P. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28:1984–1992. [DOI] [PubMed] [Google Scholar]
- 29. Swanton S, Choh AC, Lee M, Laubach LL, Linderman JK, Czerwinski SA, Peterson MJ. Body mass index associations between mother and offspring from birth to age 18: the Fels Longitudinal Study. Obes Sci Pract. 2017;3:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vik KL, Romundstad P, Nilsen TI. Tracking of cardiovascular risk factors across generations: family linkage within the population‐based HUNT Study, Norway. J Epidemiol Community Health. 2013;67:564–570. [DOI] [PubMed] [Google Scholar]
- 31. Vik KL, Romundstad P, Carslake D, Smith GD, Nilsen TI. Comparison of father‐offspring and mother‐offspring associations of cardiovascular risk factors: family linkage within the population‐based HUNT Study, Norway. Int J Epidemiol. 2014;43:760–771. [DOI] [PubMed] [Google Scholar]
- 32. Heude B, Kettaneh A, Rakotovao R, Bresson JL, Borys JM, Ducimetiere P, Charles MA. Anthropometric relationships between parents and children throughout childhood: the Fleurbaix‐Laventie Ville Sante Study. Int J Obes (Lond). 2005;29:1222–1229. [DOI] [PubMed] [Google Scholar]
- 33. Sun C, Burgner DP, Ponsonby AL, Saffery R, Huang RC, Vuillermin PJ, Cheung M, Craig JM. Effects of early‐life environment and epigenetics on cardiovascular disease risk in children: highlighting the role of twin studies. Pediatr Res. 2013;73:523–530. [DOI] [PubMed] [Google Scholar]
- 34. Mancusi C, Canciello G, Izzo R, Damiano S, Grimaldi MG, de Luca N, de Simone G, Trimarco B, Losi MA. Left atrial dilatation: a target organ damage in young to middle‐age hypertensive patients. The Campania Salute Network. Int J Cardiol. 2018;265:229–233. [DOI] [PubMed] [Google Scholar]
- 35. Yaghi S, Moon YP, Mora‐McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, Homma S, Kamel H, Sacco RL, Elkind MS. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palatini P, Amerena J, Nesbitt S, Valentini M, Majahalme S, Krause L, Tikhonoff V, Julius S. Heritability of left atrial size in the Tecumseh population. Eur J Clin Invest. 2002;32:467–471. [DOI] [PubMed] [Google Scholar]
- 37. Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: the Framingham Heart Study. Hypertension. 1997;30:1025–1028. [DOI] [PubMed] [Google Scholar]
- 38. Kervancioglu P, Kervancioglu M, Tuncer CM. Echocardiographic study of aortic root diameter in healthy children. Saudi Med J. 2006;27:27–30. [PubMed] [Google Scholar]
- 39. Saeyeldin A, Zafar MA, Velasquez CA, Ip K, Gryaznov A, Brownstein AJ, Li Y, Rizzo JA, Erben Y, Ziganshin BA, Elefteriades JA. Natural history of aortic root aneurysms in Marfan syndrome. Ann Cardiothorac Surg. 2017;6:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lam CS, Liu X, Yang Q, Larson MG, Pencina MJ, Aragam J, Redfield MM, Benjamin EJ, Vasan RS. Familial aggregation of left ventricular geometry and association with parental heart failure: the Framingham Heart Study. Circ Cardiovasc Genet. 2010;3:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verhaaren HA, Schieken RM, Mosteller M, Hewitt JK, Eaves LJ, Nance WE. Bivariate genetic analysis of left ventricular mass and weight in pubertal twins (the Medical College of Virginia twin study). Am J Cardiol. 1991;68:661–668. [DOI] [PubMed] [Google Scholar]
- 42. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 43. Bella JN, Goring HH. Genetic epidemiology of left ventricular hypertrophy. Am J Cardiovasc Dis. 2012;2:267–278. [PMC free article] [PubMed] [Google Scholar]
- 44. Wu QS, He Q, He JQ, Chao J, Wang WY, Zhou Y, Lou JZ, Kong W, Chen JF. The role of mitofilin in left ventricular hypertrophy in hemodialysis patients. Ren Fail. 2018;40:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuznetsova T, Staessen JA, Olszanecka A, Ryabikov A, Stolarz K, Malyutina S, Fagard R, Kawecka‐Jaszcz K, Nikitin Y; European Project On Genes in Hypertension (EPOGH) Investigators . Maternal and paternal influences on left ventricular mass of offspring. Hypertension. 2003;41:69–74. [DOI] [PubMed] [Google Scholar]
- 46. Ryder JR, Pankratz ND, Dengel DR, Pankow JS, Jacobs DR Jr, Sinaiko AR, Gooty V, Steinberger J. Heritability of vascular structure and function: a parent‐child study. J Am Heart Assoc. 2017;6:e004757 DOI: 10.1161/JAHA.116.004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Women Included and Excluded in This Study (n=8107)
Figure S1. Timeline of exposures, outcomes, and confounders measured in mother and child.
Figure S2. Overview of results in complicated and uncomplicated pregnancies.


