Abstract
Background
Type 2 diabetes mellitus is closely associated with metabolic risk factors that all contribute to impairment of the left ventricle. The implications of having type 2 diabetes mellitus with well‐controlled metabolic risk factors compared to an increasing burden of uncontrolled metabolic risk factors on left ventricular structure and function are not known.
Methods and Results
We compared patients with type 2 diabetes mellitus (n=751) with different degrees of uncontrolled metabolic risk factors present with a control group of individuals without present uncontrolled metabolic risk factors as recommended by the World Health Organization (n=80). In patients with well‐controlled metabolic risk factors, only diastolic but neither structural nor systolic measures were impaired compared to the control group: the (early diastolic mitral inflow velocity)/(atrial diastolic mitral inflow velocity) ratio (median 0.94 [interquartile range 0.80, 1.08] versus 1.11 [0.85, 1.38], P<0.001), lateral early diastolic myocardial velocity at the level of the mitral annulus (mean 9.6 m/s [SD 2.5] versus 10.8 [3.5], P<0.001) and lateral (early diastolic mitral inflow velocity)/(early diastolic myocardial velocity at the level of the mitral annulus) (7.7 [6.5, 10.2] versus 6.3 [4.9, 7.8], P<0.001). With an increasing burden of uncontrolled metabolic risk factors, there were increased left ventricular mass index and wall thicknesses and impaired systolic function measured as global longitudinal strain: control group −15.9 (2.0); 0 uncontrolled risk factors −15.3 (2.4); 1 to 2 −14.6 (2.8); and ≥3 −14.0 (2.8), P<0.001. Within the diabetes mellitus group, there were uni‐ and multivariable associations of left ventricular measures and systolic blood pressure, body mass index, hemoglobin A1c, and HDL‐cholesterol.
Conclusions
In patients with type 2 diabetes mellitus, having well‐controlled metabolic risk factors was associated with only left ventricular diastolic impairment but not with either structural or even subtle measures of systolic function. Increasing burden of uncontrolled metabolic risk factors was associated with structural and functional impairments.
Keywords: echocardiography, metabolic syndrome, remodeling, type 2 diabetes mellitus
Subject Categories: Echocardiography; Diabetes, Type 2; Metabolic Syndrome
Clinical Perspective
What Is New?
Even with well‐controlled metabolic risk factors there is evidence of diastolic but not systolic or structural cardiac impairment in patients with type 2 diabetes mellitus.
With increasing burden of metabolic risk factors, the risk of further cardiac impairment increases in patients with type 2 diabetes mellitus.
What Are the Clinical Implications?
Patients with type 2 diabetes mellitus should be considered for echocardiography even when the metabolic risk factors are well controlled.
In assessing patients with type 2 diabetes mellitus, the risk of cardiac impairment is higher in patients with concomitant uncontrolled metabolic risk factors.
Introduction
A dominant feature of type 2 diabetes mellitus (T2D) is its association with the metabolic syndrome, which in the World Health Organization definition includes, beyond insulin resistance, the metabolic risk factors obesity, hypertension, increased triglycerides and high‐density lipoprotein (HDL)‐cholesterol, and albuminuria. Although there is firm evidence of an increased risk of cardiovascular disease in patients with metabolic syndrome,1, 2, 3 there has been a strong debate about whether this is beyond the sum of the contributions of each of the metabolic risk factors that constitute the syndrome.4 There is a clear association between the metabolic risk factors diabetes mellitus,5, 6, 7 obesity,8 hypertension9, 10 and albuminuria11 and the risk of developing heart failure (HF). However, whether dyslipidemia is associated with HF is less well elucidated. Nevertheless, in a recent study including 113 554 individuals from the general population, increasing nonfasting triglyceride was associated with a stepwise increase in the risk of developing HF.12 These findings suggest a relationship between dyslipidemia and the risk of HF. Hence, evidence indicates that all of the metabolic risk factors in the World Health Organization definition of the metabolic syndrome are associated with an increased risk of developing HF.
For patients with T2D, a distinct effect of diabetes mellitus on the myocardium, the diabetic cardiomyopathy, has been suggested and characterized by a number of previous studies. They have demonstrated that patients with diabetes mellitus have increased left ventricular wall thicknesses, left ventricular hypertrophy,13, 14, 15 and both decreased systolic and diastolic function.16, 17, 18, 19, 20, 21 We have previously shown that these changes were amplified with increasing duration of T2D, supporting the suggested causal relationship between T2D and left ventricular (LV) remodeling and functional measures.22
However, because of the marked association between metabolic risk factors and diabetes mellitus, and because both hypertension and obesity have been closely associated with changes in cardiac mechanics, the direct effect of diabetes mellitus on the myocardium is difficult to discern from the effect of the coexisting metabolic risk factors. The aim of the present study was to examine the association of the burden of uncontrolled metabolic risk factors with LV structure and function in patients with T2D receiving multifactorial treatment compared to a nondiabetic control group without or with well‐controlled metabolic risk factors. Previously, we demonstrated an association between triglyceride level23 and microalbuminuria24 and LV remodeling and function in this population. Therefore, our aim here was in addition to examine this association with the remainder of the metabolic risk factors: obesity, hypertension, hemoglobin A1c (HbA1c), and HDL‐cholesterol levels.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. However, researchers may request specific data and study materials for the purpose of reproducing the results by contacting the corresponding author.
Study Population
The Thousand&2 study recruited patients with T2D from 2 large, secondary care centers in Copenhagen, the Capital Region, Denmark: Steno Diabetes Center and Center for Diabetes Research, Gentofte Hospital. Details on study inclusion and study visit have been published previously.24, 25 In brief, a total of 2158 patients were invited and 1030 participated in the study. Before attending the examination the patients filled out a questionnaire with information on current medication, previous heart disease (myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, congestive heart failure, and atrial fibrillation), previous stroke and peripheral artery disease, family history of coronary heart disease, smoking habits, height, and weight. The questionnaire was reviewed with the patient at the study visit by P.G.J. Blood pressure was measured in the supine position after at least 15 minutes of rest. Body mass index (BMI) was calculated (weight [kg]/height [m]2) based on self‐reported measurements.
Patients with atrial fibrillation during the echocardiographic examination, more than moderate valve disease, and/or previous heart valve surgery were excluded (n=96). Also, for the analyses of numbers of metabolic risk factors and relation to LV structure and function, patients with incomplete information on BMI, systolic blood pressure, HbA1c, HDL‐cholesterol, triglyceride levels, or albuminuria status were excluded (n=183).
The study was conducted in accordance with the Declaration of Helsinki, approved by The Danish National Committee on Biomedical Research Ethics, amendment to protocol no. H‐3‐2009‐139.26 All participants gave written informed consent.
The control group consisted of a sample of people from the Copenhagen City Heart Study, a prospective cohort study of cardiovascular risk factors in patients from the general population. Details on the sampling have been published previously.22 In brief, patients from the Thousand&2 study were randomly matched 4:1 on age, sex, and systolic blood pressure with people from the Copenhagen City Heart Study without diabetes mellitus (n=252). The metabolic risk factors were measured in all patients with the exception of the presence of albuminuria. From the control group, people with known heart disease, atrial fibrillation at the time of the echocardiographic exam, or presence of any of the metabolic risk factors as defined above were excluded (n=172). Hence, the control group consisted of 80 people without diabetes mellitus, known heart disease, or any of the metabolic risk factors. A flow chart of the derivation of the study population is provided in Figure 1.
Figure 1.
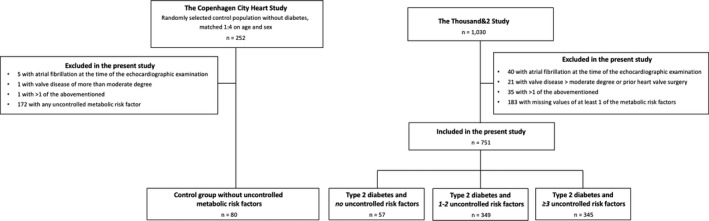
Flowchart of the derivation of the study sample.
Metabolic Risk Factors
According to the World Health Orgnization definition of the metabolic syndrome,27 the metabolic risk factors constitute T2D or impaired fasting glucose, impaired glucose tolerance, increased systolic or diastolic blood pressure or antihypertensive treatment, elevated plasma triglycerides, HDL levels, increased BMI or waist:hip ratio, and presence of albuminuria. Accordingly, we regarded metabolic risk factors as uncontrolled when systolic blood pressure was >140 mm Hg, body mass index was >30 kg/m2, HbA1c was >48 mmol/L, HDL‐cholesterol was <1.0 mmol/L for women and <0.9 mmol/L for men, triglyceride was >1.7 mmol/L, and in the presence of micro‐ or macroalbuminuria.
Biochemistry
Lipid levels, HbA1c, and creatinine were obtained from routine blood tests performed at either Steno Diabetes Center or the Center for Diabetes Research, Herlev and Gentofte Hospital. Urine albumin/creatinine ratio and/or 24‐hour urine albumin excretion rate are evaluated at least annually at both centers. Microalbuminuria was defined as a urine albumin/creatinine ratio between 30 and 300 mg/g or urine albumin excretion rate between 30 and 300 mg/day, and macroalbuminuria as a urine albumin/creatinine ratio above 300 mg/g or urine albumin excretion rate above 300 mg/day on 2 consecutive measurements.
Echocardiography
Details on the echocardiographic examinations have been published previously.22, 23, 24, 25 In brief, chamber quantification was done in accordance with the recommendations of the European Association of Echocardiography and the American Society of Echocardiography.28 LV mass was indexed according to height,27 which was chosen in line with our previous studies. Sphericity index was calculated in end‐diastole as LV length in an apical 4‐chamber view/left ventricular internal diameter in a parasternal long‐axis view. LV ejection fraction was measured with the Simpson biplane method, and reduced ejection fraction was defined as an LV ejection fraction <50%. 2‐Dimensional speckle tracking was performed using GE EchoPAC software, BT13. Midmyocardial global strain provided by the software algorithm was used for both longitudinal and circumferential strain. Global longitudinal strain (GLS) was the mean value of the GLS from all 3 standard projections. Global circumferential strain (GCS) was measured at the level of the papillary muscle. Strain rate was the rate of these deformations in either longitudinal or circumferential directions and measured in seconds–1.
People from the Copenhagen City Heart study were examined with the same echocardiographic protocol as patients in the Thousand&2 study using Vivid E9 (GE Vingmed Ultrasound, Horten, Norway).
Statistical Analysis
Continuous variables are presented as mean (SD)/median (interquartile range) and compared using Welsh t tests or 1‐way analysis of variance/Mann‐Whitney U tests or Kruskall‐Wallis tests where appropriate. Categorical values are presented as number (percentage) and compared using chi‐squared tests. Systolic blood pressure, BMI, HbA1c, and HDL‐cholesterol were nonnormally distributed and transformed using the binary logarithm (log2), after which they were normally distributed. Association of these and echocardiographic measures were examined using linear regression models. P‐values less than 0.05 on 2‐sided tests were considered significant. Statistics were performed using R for Mac, version 3.4.2 (R Project for Statistical Computing, Vienna University of Economics and Business Administration, Vienna, Austria).
Results
We identified 751 patients from the diabetes mellitus cohort with all metabolic risk factors measured. The distribution of uncontrolled metabolic risk factors among the patients with T2D is found in Table 1. We identified 57 patients without any or all well‐controlled metabolic risk factors after aggressive multifactorial treatment. Of these, 14% received β‐blockers, 21% angiotensin‐converting enzyme inhibitors, 32% angiotensin II receptor blockers, and 28% calcium antagonists. Also, 72% received metformin, 10% to 21% received other antidiabetic treatment, and 86% received statins.
Table 1.
Distribution of the Metabolic Risk Factors Present Among Patients With Type 2 Diabetes Mellitus
| Number of Uncontrolled Metabolic Risk Factors Present | |||||||
|---|---|---|---|---|---|---|---|
| 0 (n=57) | 1 (n=137) | 2 (n=212) | 3 (n=170) | 4 (n=120) | 5 (n=43) | 6 (n=12) | |
| Elevated systolic blood pressure, n (%) | 0 (0) | 8 (6) | 46 (22) | 63 (37) | 61 (51) | 31 (72) | 12 (100) |
| Elevated BMI, n (%) | 0 (0) | 17 (12) | 75 (35) | 99 (58) | 91 (76) | 40 (93) | 12 (100) |
| Elevated HDL‐cholesterol, n (%) | 0 (0) | 7 (5) | 13 (6) | 34 (20) | 44 (37) | 30 (70) | 12 (100) |
| Elevated triglyceride, n (%) | 0 (0) | 24 (18) | 87 (41) | 122 (72) | 102 (85) | 43 (100) | 12 (100) |
| Albuminuria, n (%) | 0 (0) | 7 (5) | 35 (17) | 47 (28) | 66 (55) | 30 (70) | 12 (100) |
| Elevated hemoglobin A1c, n (%) | 0 (0) | 74 (54) | 168 (79) | 145 (85) | 116 (97) | 41 (95) | 12 (100) |
Criteria for metabolic risk factors: hemoglobin A1c >48 mmol/L, systolic blood pressure >140 mm Hg, body mass index >30 kg/m2, HDL‐cholesterol <1.0 mmol/L for women and <0.9 mmol/L for men, triglycerides >1.7 mmol/L, and presence of micro‐ or macroalbuminuria. BMI indicates body mass index; HDL, high‐density lipoprotein.
Patients from the diabetes mellitus group were further divided into categories with, respectively, 0 (n=57), 1 to 2 (n=349), and ≥3 (n=345) uncontrolled metabolic risk factors and compared with the control group (n=80). The population demographics after this subdivision are shown in Table 2. Compared to the control group, patients with T2D were of similar age and sex regardless of the presence of uncontrolled metabolic risk factors. Blood pressure, both systolic and diastolic, was insignificantly higher in patients with T2D compared to the control group but was significantly higher in patients with T2D with any uncontrolled metabolic risk factor present. Similar results were seen with for triglyceride levels, but there were significantly higher BMI and lower LDL‐ and HDL‐cholesterol in all patients with T2D compared with the control group. Patients with T2D were more often treated with antihypertensive drugs, diuretics, and statins regardless of the presence of uncontrolled metabolic risk factors compared with the control group.
Table 2.
Population Demographics
| Control Group | Type 2 Diabetes Mellitus and | P Value (Across All Groups) | P Value (Control vs 0 Risk Factors) | P Value (Control vs 1 to 2 Risk Factors) | P Value (Control vs ≥3 Risk Factors) | |||
|---|---|---|---|---|---|---|---|---|
| 0 Uncontrolled Risk Factors | 1 to 2 Uncontrolled Risk Factors | ≥3 Uncontrolled Risk Factors | ||||||
| n=80 | n=57 | n=349 | n=345 | |||||
| Clincal | ||||||||
| Age, y | 63 [57, 68] | 65 [59, 72] | 65 [59, 71] | 65 [57, 70] | 0.03 | 0.17 | 0.005a | 0.11 |
| Male sex, % | 52 (65.0) | 32 (56.1) | 233 (66.8) | 231 (67.0) | 0.44 | 0.38 | 0.87 | 0.84 |
| Diabetes mellitus duration, y | 7 [2, 15] | 11 [5, 17] | 13 [8, 20] | |||||
| Body mass index, kg/m2 | 24.1 [22. 8, 26.1] | 26.0 [23.5, 27.8] | 27.7 [25.3, 30.4] | 32.3 [29.0, 35.5] | <0.001a | 0.004a | <0.001a | <0.001a |
| Systolic blood pressure, mm Hg | 123 (9) | 126 (10) | 132 (16) | 142 (18) | <0.001a | 0.09 | <0.001a | <0.001a |
| Diastolic blood pressure, mm Hg | 76 (10) | 77 (8) | 78 (10) | 82 (11) | <0.001a | 0.37 | 0.05a | <0.001a |
| Coronary heart disease, % | 10 (17.5) | 58 (16.6) | 63 (18.3) | |||||
| Laboratory values | ||||||||
| LDL‐cholesterol, mmol/L | 3.1 [2.6, 3.5] | 1.9 [1.6, 2.3] | 2.0 [1.6, 2.6] | 2.0 [1.5, 2.6] | <0.001a | <0.001a | <0.001a | <0.001a |
| HDL‐cholesterol, mmol/L | 1.7 [1.5, 2.0] | 1.5 [1.2, 1.8] | 1.2 [1.0, 1.5] | 1.0 [0.9, 1.3] | <0.001a | 0.006 | <0.001a | <0.001a |
| Triglyceride, mmol/L | 1.0 [0.8, 1.3] | 1.1 [0.8, 1.3] | 1.4 [1.0, 2.0] | 2.3 [1.8, 3.1] | <0.001a | 0.45 | <0.001a | <0.001a |
| Albuminuria, % | 42 (12.0) | 155 (44.9) | ||||||
| Microalbuminuria, % | 37 (10.6) | 100 (29.0) | ||||||
| Macroalbuminuria, % | 5 (1.4) | 55 (15.9) | ||||||
| Hemoglobin A1c, mmol/mol | 43 [40, 46] | 54 [46, 62] | 60 [53, 73] | |||||
| Hemoglobin A1c, % | 6.1 (0.4) | 7.3 (1.3) | 8.0 (1.5) | |||||
| Creatinine, μmol/L | 78 [71, 87] | 72 [63, 84] | 77 [66, 92] | 82 [65, 103] | 0.01 | 0.06 | 0.72 | 0.19 |
| Medication | ||||||||
| Metformin, % | 41 (71.9) | 251 (71.9) | 252 (73.0) | |||||
| DPP4 inhibitors, % | 7 (12.3) | 30 (8.6) | 35 (10.1) | |||||
| Sulfonylurea, % | 6 (10.5) | 58 (16.6) | 51 (14.8) | |||||
| Glucagon‐like peptide 1‐receptor agonist, % | 11 (19.3) | 63 (18.1) | 103 (29.9) | |||||
| Insulin, % | 12 (21.1) | 159 (45.6) | 201 (58.3) | |||||
| β‐Blockers, % | 8 (10.0) | 8 (14.0) | 80 (22.9) | 94 (27.2) | <0.001a | 0.002a | <0.001a | <0.001a |
| Angiotensin‐converting enzyme inhibitors, % | 5 (6.2) | 12 (21.1) | 144 (41.3) | 128 (37.1) | <0.001a | 0.12 | <0.001a | <0.001a |
| Angiotensin II receptor blockers, % | 8 (10.0) | 18 (31.6) | 125 (35.8) | 163 (47.2) | <0.001a | <0.001a | <0.001a | <0.001a |
| Calcium antagonists, % | 8 (10.0) | 16 (28.1) | 111 (31.8) | 120 (34.8) | <0.001a | 0.01a | <0.001a | <0.001a |
| Diuretics, % | 8 (10.0) | 23 (40.4) | 155 (44.4) | 204 (59.1) | <0.001a | <0.001a | <0.001a | <0.001a |
| Statins, % | 8 (10.0) | 49 (86.0) | 278 (79.7) | 263 (76.2) | <0.001a | <0.001a | <0.001a | <0.001a |
Criteria for uncontrolled metabolic risk factors include hemoglobin A1c >48 mmol/L, systolic blood pressure >140 mm Hg, body mass index >30 kg/m2, HDL‐cholesterol <1.0 mmol/L for women and <0.9 mmol/L for men, triglycerides >1.7 mmol/L, and presence of micro‐ or macroalbuminuria. Continuous traits are reported as mean (SD) or median [interquartile range] in case of nonnormal distribution. DPP4 indicates dipeptidyl peptidase‐4; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
P<0.05.
Association of Burden of Uncontrolled Metabolic Risk Factors and Echocardiographic Findings
The associations of numbers of uncontrolled metabolic risk factors and echocardiographic findings are found in Table 3. There was a highly significant difference between the control group, patients with 0, 1 to 2, and ≥3 uncontrolled metabolic risk factors, and all the structural and diastolic findings except LV end‐diastolic diameter and left atrial volume index. The association was less pronounced in the systolic measures because this was only the case for the proportion with LV ejection fraction <50% and GLS. Thus, GLS rate, GCS, and GCS rate were not affected by an increasing number of uncontrolled metabolic risk factors present.
Table 3.
Association of Number of Uncontrolled Metabolic Risk Factors and Echocardiographic Findings
| Control Group | Type 2 Diabetes Mellitus and | P Value (Across All Groups) | P Value (Control vs 0 Risk Factors) | P Value (Control vs 1 to 2 Risk Factors) | P Value (Control vs ≥3 Risk Factors) | |||
|---|---|---|---|---|---|---|---|---|
| 0 Uncontrolled Risk Factors | 1 to 2 Uncontrolled Risk Factors | ≥3 Uncontrolled Risk Factors | ||||||
| n=80 | n=57 | n=349 | n=345 | |||||
| Structural measures | ||||||||
| Left ventricular mass index, g/m2.7 | 35.5 (8.0) | 35.5 (9.6) | 37.1 (11.7) | 41.1 (11.1) | <0.001a | 0.99 | 0.26 | <0.001a |
| Interventricular septum thickness, mm | 10.0 (1.3) | 9.9 (1.4) | 10.4 (1.8) | 11.2 (1.7) | <0.001a | 0.87 | 0.06 | <0.001a |
| End‐diastolic internal diameter, mm | 46.8 (4.8) | 46.0 (6.8) | 45.6 (6.3) | 45.9 (6.0) | 0.51 | 0.43 | 0.13 | 0.21 |
| Posterior wall thickness, mm | 9.4 (1.3) | 9.5 (1.2) | 10.1 (1.5) | 10.8 (1.6) | <0.001a | 0.76 | <0.001a | <0.001a |
| Relative wall thickness | 0.41 (0.07) | 0.42 (0.07) | 0.45 (0.09) | 0.48 (0.10) | <0.001a | 0.26 | <0.001a | <0.001a |
| Sphericity index | 0.17 (0.02) | 0.17 (0.02) | 0.18 (0.03) | 0.18 (0.03) | 0.17 | 0.60 | 0.11 | 0.05a |
| Left atrial end‐systolic volume index, mL/m2 | 26 [22, 32] | 26 [21, 31] | 25 [20, 32] | 25 [20, 31] | 0.81 | 0.82 | 0.79 | 0.56 |
| Diastolic measures | ||||||||
| Peak E velocity, m/s | 0.67 [0.55, 0.78] | 0.74 [0.63, 0.87] | 0.75 [0.63, 0.87] | 0.76 [0.65, 0.88] | <0.001a | 0.01a | <0.001a | <0.001a |
| Peak A velocity, m/s | 0.60 (0.16) | 0.79 (0.19) | 0.85 (0.19) | 0.86 (0.20) | <0.001a | <0.001a | <0.001a | <0.001a |
| E/A ratio | 1.11 [0.85, 1.38] | 0.94 [0.80, 1.08] | 0.86 [0.75, 1.07] | 0.87 [0.75, 1.06] | <0.001a | 0.005a | <0.001a | <0.001a |
| E deceleration time, ms | 191 [164, 219] | 209 [168, 247] | 220 [183, 263] | 220 [188, 269] | <0.001a | 0.14 | <0.001a | <0.001a |
| Lateral e′ (per second) | 10.8 (3.5) | 9.6 (2.5) | 8.7 (2.6) | 8.5 (2.5) | <0.001a | 0.03a | <0.001a | <0.001a |
| Septal e′, m/s | 8.4 (2.7) | 7.2 (1.8) | 6.8 (1.9) | 6.5 (1.8) | <0.001a | 0.002a | <0.001a | <0.001a |
| E/e′lateral | 6.3 [4.9, 7.8] | 7.7 [6.5, 10.2] | 8.6 [6.9, 10.6] | 9.0 [7.2, 12.1] | <0.001a | <0.001a | <0.001a | <0.001a |
| E/e′septal | 7.7 [6.6, 9.9] | 10.5 [8.6, 12.4] | 10.9 [9.2, 13.3] | 11.6 [9.6, 14.9] | <0.001a | <0.001a | <0.001a | <0.001a |
| Systolic measures | ||||||||
| Ejection fraction, % | 61 [56, 65] | 62 [57, 66] | 61 [57, 65] | 60 [55, 65] | 0.14 | 0.52 | 0.96 | 0.24 |
| Reduced ejection fraction (<50%), % | 5 (6.5) | 2 (3.6) | 34 (9.9) | 48 (14.4) | 0.03a | 0.73 | 0.48 | 0.09 |
| Global longitudinal systolic strain, % | −15.9 (2.0) | −15.3 (2.4) | −14.6 (2.8) | −14.0 (2.8) | <0.001a | 0.13 | <0.001a | <0.001a |
| Global longitudinal systolic strain rate (per second) | −0.81 (0.11) | −0.80 (0.14) | −0.79 (0.17) | −0.77 (0.18) | 0.09 | 0.53 | 0.37 | 0.04a |
| Global circumferential systolic strain, % | −18.1 (4.1) | −18.7 (4.8) | −18.0 (5.2) | −17.4 (5.3) | 0.34 | 0.49 | 0.87 | 0.35 |
| Global circumferential systolic strain rate (per second) | −0.97 (0.22) | −0.99 (0.28) | −1.05 (0.33) | −1.03 (0.34) | 0.27 | 0.71 | 0.07 | 0.19 |
Criteria for uncontrolled metabolic risk factors include hemoglobin A1c >48 mmol/L, systolic blood pressure >140 mm Hg, body mass index >30 kg/m2, HDL‐cholesterol <1.0 mmol/L for women and <0.9 mmol/L for men, triglyceride >1.7 mmol/L, and presence of micro‐ or macroalbuminuria. Continuous traits are reported as mean (SD) or median [interquartile range] in case of nonnormal distribution. A indicates atrial diastolic mitral inflow velocity; E, early diastolic mitral inflow velocity; e′, early diastolic myocardial velocity at the level of the mitral annulus.
P<0.05.
When the control group is compared with the patients with T2D without any uncontrolled metabolic risk factor present, there were no significant differences in any structural or systolic measure, but there was clear evidence of reduced diastolic function expressed as decreased lateral and septal early diastolic myocardial velocity at the level of the mitral annulus and (early diastolic mitral inflow velocity)/(early diastolic myocardial velocity at the level of the mitral annulus), (early diastolic mitral inflow velocity)/(atrial diastolic mitral inflow velocity) ratio, and increased peak early and atrial diastolic mitral inflow velocities. With increasing numbers of uncontrolled metabolic risk factors present, there was increasing evidence of both structural remodeling with increasing LV wall thicknesses and relative wall thicknesses but no changes in LV end‐diastolic diameter or left atrial volume index. Regarding diastolic measures, there was an increase in diastolic dysfunction with increasing number of uncontrolled metabolic risk factors present. With respect to the systolic measures, GLS was decreased when ≥1 uncontrolled risk factor was present and more so when ≥3 were present, and GLS rate was not affected unless ≥3 uncontrolled risk factors were present. There were no differences in LV ejection fraction, GCS, or GCS rate with increasing number of uncontrolled metabolic risk factors present.
Figure 2 shows how LV structural, diastolic, and systolic measures were affected with increasing numbers of uncontrolled metabolic risk factors present. Here, these patterns are repeated: there was diastolic but not structural or systolic impairment without concomitant metabolic risk factors. Structural and systolic impairment emerged as numbers of uncontrolled metabolic risk factors increased.
Figure 2.
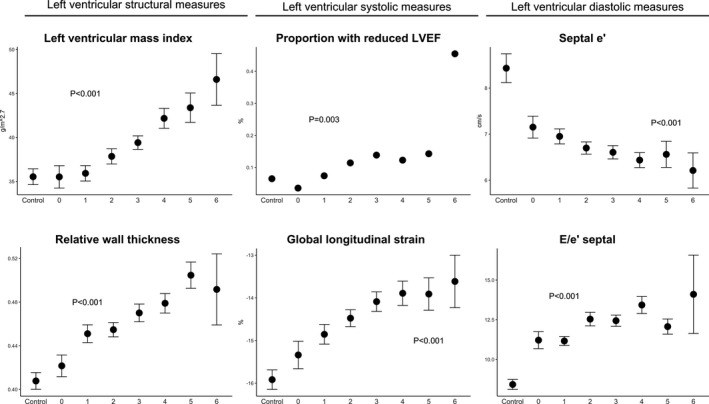
Association of number of uncontrolled metabolic risk factors and measures of left ventricular structural and systolic and diastolic measures. e′, early diastolic myocardial velocity; E/e′, ratio of early mitral inflow velocity and early diastolic myocardial velocity; LVEF, left ventricular ejection fraction.
Association of Systolic Blood Pressure, BMI, HbA1c, and HDL‐Cholesterol With Echocardiographic Findings in Patients With T2D
Univariable and multivariable associations of systolic blood pressure, BMI, HbA1c, HDL‐cholesterol, and echocardiographic findings are found in Tables 4 and 5. In general, there was an overall association with LV structural measures (LV mass index [not for HbA1c], LV wall thicknesses, and relative wall thickness [not for HDL‐cholesterol]). This, however, was attenuated overall for HbA1c and HDL‐cholesterol but persisted for systolic blood pressure and BMI after adjustment for the other metabolic risk factors. Regarding diastolic measures, they were consistently associated with systolic blood pressure and BMI, in particular evidence of elevated filling pressures expressed as (early diastolic mitral inflow velocity)/(early diastolic myocardial velocity at the level of the mitral annulus) and decreased LV relaxation expressed as lateral early diastolic myocardial velocity at the level of the mitral annulus. On the other hand, there was no consistent pattern of association between either HbA1c or HDL‐cholesterol and diastolic measures. For the systolic measures, all metabolic risk factors were univariably associated with GLS, but after adjustment for the other metabolic risk factors, there was a significant association only with BMI. Interestingly, after adjusting, where BMI was associated with both GLS and GCS and not their corresponding rates, the opposite was the case for systolic blood pressure. Other than that, there was no clear pattern of associations between either and the systolic LV measures except LV ejection fraction, which increased with HDL‐cholesterol in both uni‐ and multivariable analyses.
Table 4.
Univariable Association of Systolic Blood Pressure, BMI, HbA1c, HDL‐Cholesterol, and Echocardiographic Findings in Patients With T2D
| Log2 (Systolic Blood Pressure) | Log2 (BMI) | Log2 (HbA1c) | Log2 (HDL‐Cholesterol) | |||||
|---|---|---|---|---|---|---|---|---|
| β‐Coefficient (SE) | P Value | β‐coefficient (SE) | P Value | β‐Coefficient (SE) | P Value | β‐Coefficient (SE) | P Value | |
| Structural measures | ||||||||
| Left ventricular mass index, g/m2.7 | 11.5 (2.3) | <0.001a | 15.3 (1.5) | <0.001a | −0.02 (1.2) | 0.98 | −3.8 (1.0) | <0.001a |
| Interventricular septum diameter, mm | 1.3 (0.4) | <0.001a | 1.6 (0.2) | <0.001a | 0.5 (0.2) | 0.003a | −0.7 (0.2) | <0.001a |
| End‐diastolic internal diameter, mm | 1.1 (1.3) | 0.41 | 4.3 (0.9) | <0.001a | −1.6 (0.6) | 0.01a | −2.1 (0.5) | <0.001a |
| Posterior wall diameter (mm) | 1.3 (0.3) | <0.001a | 1.6 (0.2) | <0.001a | 0.3 (0.2) | 0.07 | −0.7 (0.1) | <0.001a |
| Relative wall thickness | 0.05 (0.02) | 0.01a | 0.03 (0.01) | 0.04a | 0.03 (0.01) | 0.002a | −0.01 (0.01) | 0.27 |
| Sphericity index (per 0.01 increase) | −0.24 (0.55) | 0.66 | −0.58 (0.38) | 0.13 | 0.46 (0.27) | 0.08 | −0.08 (0.23) | 0.72 |
| Left atrial end‐systolic volume index, mL/m2 | 4.4 (1.7) | 0.01a | −1.0 (1.2) | 0.38 | −1.7 (0.8) | 0.04a | 0.2 (0.7) | 0.78 |
| Diastolic measures | ||||||||
| Peak E velocity (cm/s) | 0.18 (0.04) | <0.001a | 0.11 (0.03) | 0.0001a | 0.00 (0.02) | 0.99 | 0.001 (0.02) | 0.97 |
| Peak A velocity, cm/s | 0.29 (0.04) | <0.001a | 0.07 (0.03) | 0.009a | 0.03 (0.02) | 0.12 | 0.03 (0.02) | 0.08 |
| E/A ratio | −0.10 (0.06) | 0.09 | 0.05 (0.04) | 0.25 | −0.03 (0.03) | 0.32 | −0.03 (0.02) | 0.19 |
| E deceleration time, ms | 4.0 (15.5) | 0.80 | 10.2 (10.5) | 0.33 | 16.0 (7.3) | 0.03a | 3.4 (6.5) | 0.60 |
| Lateral e′, cm/s | −2.4 (0.5) | <0.001a | −0.7 (0.4) | 0.04a | −0.5 (0.3) | 0.03a | 0.15 (0.2) | 0.52 |
| Septal e′, cm/s | −1.1 (0.4) | 0.004a | −0.2 (0.3) | 0.40 | −0.4 (0.2) | 0.05 | 0.1 (0.2) | 0.40 |
| E/e′lateral | 6.0 (0.9) | <0.001a | 2.1 (0.6) | 0.002a | 0.6 (0.5) | 0.22 | −0.3 (0.4) | 0.46 |
| E/e′septal | 5.4 (1.1) | <0.001a | 2.1 (0.7) | 0.003a | 1.0 (0.5) | 0.05a | −0.2 (0.5) | 0.68 |
| Systolic measures | ||||||||
| Ejection fraction, % | −0.8 (1.7) | 0.65 | −2.9 (1.2) | 0.02a | −0.8 (0.9) | 0.35 | 3.1 (0.7) | <0.001a |
| Global longitudinal systolic strain, % | 1.2 (0.6) | 0.05a | 1.8 (0.4) | <0.001a | 0.6 (0.3) | 0.03a | −1.0 (0.2) | <0.001a |
| Global longitudinal systolic strain rate (per second) | 0.11 (0.04) | 0.003a | 0.05 (0.03) | 0.04a | 0.02 (0.02) | 0.37 | −0.03 (0.02) | 0.09 |
| Global circumferential systolic strain, % | 1.3 (1.2) | 0.28 | 2.6 (0.9) | 0.003a | 0.3 (0.6) | 0.61 | −0.7 (0.5) | 0.19 |
| Global circumferential systolic strain rate (s−1) | 0.2 (0.08) | 0.01a | −0.01 (0.05) | 0.79 | −0.01 (0.04) | 0.75 | 0.01 (0.03) | 0.82 |
A indicates atrial diastolic mitral inflow velocity; BMI, body mass index; E, early diastolic mitral inflow velocity; e′, early diastolic myocardial velocity at the level of the mitral annulus; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; SE, standard error; T2D, type 2 diabetes mellitus.
P<0.05.
Table 5.
Multivariable Association of Systolic Blood Pressure, Body Mass Index, HbA1c, HDL‐Cholesterol, and Echocardiographic Findings in Patients With T2D
| Log2 (Systolic Blood Pressure) | Log2 (BMI) | Log2 (Hemoglobin A1c) | Log2 (HDL‐Cholesterol) | |||||
|---|---|---|---|---|---|---|---|---|
| β‐Coefficient (SE) | P Value | β‐Coefficient (SE) | P Value | β‐Coefficient (SE) | P Value | β‐Coefficient (SE) | P Value | |
| Structural measures | ||||||||
| Left ventricular mass index, g/m2.7 | 6.9 (2.2) | 0.002a | 15.2 (1.6) | <0.001a | −1.8 (1.1) | 0.09 | −2.6 (1.1) | 0.02a |
| Interventricular septum diameter, mm | 0.8 (0.3) | 0.01a | 1.5 (0.2) | <0.00a1 | 0.3 (0.2) | 0.09 | −0.1 (0.2) | 0.50 |
| End‐diastolic internal diameter, mm | 1.6 (1.3) | 0.20 | 4.2 (0.9) | <0.001a | −1.8 (0.6) | 0.004a | −1.2 (0.6) | 0.06 |
| Posterior wall diameter, mm | 0.9 (0.3) | 0.004a | 1.6 (0.2) | <0.001a | 0.03 (0.1) | 0.84 | −0.1 (0.1) | 0.32 |
| Relative wall thickness | 0.02 (0.02) | 0.29 | 0.03 (0.01) | 0.03a | 0.02 (0.01) | 0.02a | 0.01 (0.01) | 0.46 |
| Sphericity index (per 0.01 increase) | 0.55 (1.09) | 0.61 | −0.79 (0.41) | 0.05 | 0.42 (0.29) | 0.15 | 0.31 (0.28) | 0.26 |
| Left atrial end‐systolic volume index, mL/m2 | 3.8 (1.7) | 0.03a | 0.4 (1.2) | 0.77 | −1.6 (0.8) | 0.06 | −0.5 (0.8) | 0.56 |
| Diastolic measures | ||||||||
| Peak E velocity, cm/s | 0.15 (0.04) | <0.001a | 0.10 (0.03) | <0.001a | −0.01 (0.02) | 0.58 | −0.02 (0.02) | 0.38 |
| Peak A velocity, cm/s | 0.21 (0.04) | <0.001a | 0.08 (0.03) | 0.003a | 0.03 (0.02) | 0.09 | −0.01 (0.02) | 0.42 |
| E/A ratio | −0.04 (0.06) | 0.52 | 0.02 (0.04) | 0.70 | −0.04 (0.03) | 0.12 | 0.00 (0.03) | 0.98 |
| E deceleration time, ms | −19.3 (15.6) | 0.22 | 21.0 (11.0) | 0.06 | 17.2 (7.4) | 0.02a | 2.8 (7.5) | 0.71 |
| Lateral e′, cm/s | −1.1 (0.5) | 0.02a | −1.1 (0.3) | 0.002a | −0.4 (0.2) | 0.05 | 0.4 (0.2) | 0.12 |
| Septal e′, cm/s | −0.2 (0.4) | 0.53 | −0.5 (0.3) | 0.05 | −0.3 (0.2) | 0.06 | 0.4 (0.2) | 0.02a |
| E/e′lateral | 4.1 (0.9) | <0.001a | 2.0 (0.6) | 0.002a | 0.2 (0.4) | 0.60 | −1.1 (0.4) | 0.02a |
| E/e′septal | 3.3 (1.0) | 0.001a | 2.3 (0.7) | 0.002a | 0.4 (0.5) | 0.13 | −1.0 (0.5) | 0.04a |
| Systolic measures | ||||||||
| Ejection fraction, % | −0.5 (1.8) | 0.77 | −2.2 (1.3) | 0.09 | −0.3 (0.9) | 0.72 | 2.3 (0.9) | 0.01a |
| Global longitudinal systolic strain, % | 0.6 (0.6) | 0.29 | 1.7 (0.4) | <0.001a | 0.4 (0.3) | 0.17 | −0.6 (0.3) | 0.05 |
| Global longitudinal systolic strain rate, s–1 | 0.08 (0.04) | 0.03a | 0.05 (0.03) | 0.07 | 0.01 (0.02) | 0.58 | −0.04 (0.02) | 0.04a |
| Global circumferential systolic strain, % | 0.9 (1.3) | 0.46 | 2.3 (0.9) | 0.01a | 0.07 (0.6) | 0.91 | 0.1 (0.6) | 0.82 |
| Global circumferential systolic strain rate, s–1 | 0.17 (0.08) | 0.04a | −0.03 (0.06) | 0.59 | −0.02 (0.04) | 0.63 | −0.05 (0.04) | 0.25 |
Multivariable model adjusted for age, sex, hemoglobin A1c, body mass index, systolic blood pressure, HDL‐cholesterol, triglyceride level, and albuminuria. A indicates atrial diastolic mitral inflow velocity; BMI, body mass index; E, early diastolic mitral inflow velocity; e′, early diastolic myocardial velocity at the level of the mitral annulus; HDL, high‐density lipoprotein; SE, standard error.
P<0.05.
Discussion
The primary findings in this study were that in patients with T2D with well‐controlled metabolic risk factors, there was evidence of cardiac impairment characterized only by diastolic dysfunction with neither structural changes nor systolic dysfunction. Furthermore, with an increasing number of uncontrolled metabolic risk factors present, there was also a progressive impairment of LV structure and LV systolic (longitudinal) function. Hence, despite reaching treatment goals in patients with T2D and eliminating the contribution of the metabolic risk factors, we were still able to identify an effect on the myocardium characterized only by impaired diastolic functional measures.
Metabolic Risk Factors and LV Structure and Function in T2D
We found that in patients with well‐controlled metabolic risk factors there was no evidence of structural or systolic impairment of the LV. This is in contrast to previous studies that have demonstrated the presence of both structural and systolic in addition to diastolic impairment in patients with T2D. The Strong Heart Study demonstrated increased LV wall thicknesses, LV mass index, and decreased fractional shortening in a population of American Indians with a high prevalence of T2D.14 The same tendency was found in hypertensive patients in the HyperGEN study, in which T2D was associated with increased LV mass and wall thicknesses,15 and in the ARIC study, in which increasing levels of HbA1c were associated with LV mass, wall thicknesses, GLS, and diastolic measures including septal and lateral early diastolic myocardial velocity at the level of the mitral annulus and (early diastolic mitral inflow velocity)/(early diastolic myocardial velocity at the level of the mitral annulus).29 Additionally, Ernande et al compared 144 patients with T2D without cardiac disease with 88 healthy controls without T2D, hypertension, low levels of total and LDL‐cholesterol, high levels of HDL‐cholesterol, and normal renal function and found that T2D was associated with decreased systolic function expressed as radial and longitudinal strain and strain rate.18 The same group also concluded in a different analysis that the deformation changes were closely associated with increased LV wall thicknesses associated with T2D.19 Common among these studies is that there were differences between the compared groups regarding BMI (Strong, ARIC, HyperGEN, and Ernande), systolic blood pressure (Strong, HyperGEN, and Ernande), and lipid levels (ARIC, HyperGEN, and Ernande), and although adjusted models were constructed, the complex interaction of obesity, blood pressure, and lipid levels is difficult to examine fully in any of these cohorts. Hence, our study indicates that the presence of other metabolic risk factors in T2D accounts for the structural changes found in T2D and possibly therefore for the changes in systolic function as suggested in the abovementioned study by Ernande et al.19 Thus, our findings suggest that the previously found effect of diabetes mellitus on LV structural and systolic function may have been caused by the presence of confounding, concomitant metabolic risk factors. Recently, this complex interaction was addressed in a study that suggested cardiac phenotypes in patients with T2D. This was based on cluster analysis and found that obesity and hypertension were particularly associated with worse prognosis in women, whereas in the case of men this was seen with LV hypertrophy and systolic dysfunction.30 Surprisingly, there was no association of left atrial size and increasing burden of uncontrolled metabolic risk factors. This is contradictory to what we would expect because of the strong association of the burden of uncontrolled metabolic risk factors and diastolic dysfunction. Our results suggest that left atrial size was influenced by other unmeasured confounding factors in this population.
Metabolic Syndrome and LV Mechanics
In this study we confirmed the association of systolic blood pressure, BMI, and HbA1c with LV structure and function. Also, we found an undescribed but also rather inconsistent association of HDL‐cholesterol and LV structure and function. Previous studies have established a close relation between hypertension, obesity, and HbA1c and LV structure and function. The association of hypertension and LV hypertrophy is 1 of the earliest described in cardiology and is caused by pressure overload of the LV.9 When present, LV hypertrophy is closely related to prognosis whether detected by electrocardiography,31 echocardiography,32 or magnetic resonance imaging,33 and regression of LV hypertrophy in serial ECGs has also been linked to improved prognosis.34, 35 In obesity, there is a strong association of both diastolic and systolic dysfunction that seems to be related to obesity severity,36 and regarding dysglycemia, a close relationship of HbA1c with LV mechanics exists even in elderly patients without overt diabetes mellitus.29 The same is the case for low‐grade states of albuminuria.37 Thus, we have previously described a close association of LV structure and function with both microalbuminuria and increasing levels of triglycerides in this cohort,23, 24 and there is convincing evidence that all components of the metabolic syndrome have an impact on the myocardium.
Strengths and Limitations
The strength of this study is the size of the cohort, which enables stratification of patients in groups with increasing burden of uncontrolled metabolic risk factors present (except that only 12 patients had all metabolic risk factors uncontrolled). In addition, all patients and the control group underwent comprehensive echocardiography. Some limitations of this study must be acknowledged. A hallmark of the metabolic syndrome is increased waist circumference, which was not measured in this study, and thus, BMI was used as the only measure of obesity. Albuminuria was not assessed in the control group, and we were not able to exclude people with this metabolic risk factor from the control group. Another limitation is that only patients from specialized diabetes mellitus clinics were included in the study, thus limiting the interpretability to T2D patients followed in primary care. Although the presented diastolic measures are the most commonly used, other diastolic measurements, including strain rate during isovolumetric relaxation and ratio of early diastolic mitral inflow velocity and strain rate during isovolumetric relaxation,38 may be more sensitive markers of diastolic dysfunction and were not measured in this cohort. In addition, we were not able to evaluate the association of biomarkers of cardiac function, in particular NT‐pro–brain natriuretic peptide. Also, HDL functionality, eg, macrophage cholesterol efflux, which is considered to be the main determinant of the beneficial effect of HDL‐cholesterol, was not measured.39 Finally, because this is an observational study, no inferences on causality can be drawn from the data.
Conclusion
In this study we have shown that patients with T2D and well‐controlled metabolic risk factors had impaired diastolic function but preserved LV structure and systolic function. This was in contrast to previous studies that had not excluded patients with present metabolic risk factors. Because the burden of uncontrolled metabolic risk factors increased, increasing LV structural and systolic changes emerged, further indicating that these changes are predominantly caused by an effect of concomitant metabolic risk factors.
Disclosures
Rossing reports grants from and shares in Novo Nordisk; advisory board service at MSD, Astra Zeneca, Boehringer, Eli Lilly, Janssen, Novo Nordisk, Astellas, and AbbVie Inc (all fees to institution); lecture fees to institution from Bayer. Vilsbøll has received lecture fees and/or unrestricted research grants from, participated in advisory boards of, and/or consulted for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Novo Nordisk, and Sanofi. All of the above‐mentioned are classified as less than modest. The remaining authors have no disclosures to report
Acknowledgments
The authors would like to thank the Copenhagen City Heart Study for making it possible to include a control group. We would also like to thank GE Healthcare for making an ultrasound machine available to the Copenhagen City Heart Study.
(J Am Heart Assoc. 2018;7:e008856 DOI: 10.1161/JAHA.118.008856.)
References
- 1. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. [DOI] [PubMed] [Google Scholar]
- 2. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta‐analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. [DOI] [PubMed] [Google Scholar]
- 3. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 4. Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. [DOI] [PubMed] [Google Scholar]
- 6. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow‐up study. Arch Intern Med. 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 7. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. [DOI] [PubMed] [Google Scholar]
- 8. Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 9. Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad‐Tarazi F, Horan MJ, Marcus M, Massie B, Pfeffer MA, Re RN, Roccella EJ, Savage D, Shub C. The heart in hypertension. N Engl J Med. 1992;327:998–1008. [DOI] [PubMed] [Google Scholar]
- 10. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 11. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S; HOPE Study Investigators . Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 12. Varbo A, Nordestgaard BG. Nonfasting triglycerides, low‐density lipoprotein cholesterol, and heart failure risk: two cohort studies of 113 554 individuals. Arterioscler Thromb Vasc Biol. 2018;38:464–472. [DOI] [PubMed] [Google Scholar]
- 13. Kuperstein R, Hanly P, Niroumand M, Sasson Z. The importance of age and obesity on the relation between diabetes and left ventricular mass. J Am Coll Cardiol. 2001;37:1957–1962. [DOI] [PubMed] [Google Scholar]
- 14. Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the Strong Heart Study. Circulation. 2000;101:2271–2276. [DOI] [PubMed] [Google Scholar]
- 15. Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck M‐Y, Kitzman DW, Hopkins PN, Morgan D, Rao DC, Devereux RB. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) Study. Circulation. 2001;103:102–107. [DOI] [PubMed] [Google Scholar]
- 16. Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41:611–617. [DOI] [PubMed] [Google Scholar]
- 17. Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Schnohr P, Jensen JS. Tissue Doppler echocardiography in persons with hypertension, diabetes, or ischaemic heart disease: the Copenhagen City Heart Study. Eur Heart J. 2009;30:731–739. [DOI] [PubMed] [Google Scholar]
- 18. Ernande L, Rietzschel ER, Bergerot C, De Buyzere ML, Schnell F, Groisne L, Ovize M, Croisille P, Moulin P, Gillebert TC, Derumeaux G. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle‐tracking imaging study. J Am Soc Echocardiogr. 2010;23:1266–1272. [DOI] [PubMed] [Google Scholar]
- 19. Ernande L, Bergerot C, Girerd N, Thibault H, Davidsen ES, Gautier Pignon‐Blanc P, Amaz C, Croisille P, De Buyzere ML, Rietzschel ER, Gillebert TC, Moulin P, Altman M, Derumeaux G. Longitudinal myocardial strain alteration is associated with left ventricular remodeling in asymptomatic patients with type 2 diabetes mellitus. J Am Soc Echocardiogr. 2014;27:479–488. [DOI] [PubMed] [Google Scholar]
- 20. Boonman‐de Winter LJM, Rutten FH, Cramer MJM, Landman MJ, Liem AH, Rutten GEHM, Hoes AW. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55:2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–875. [DOI] [PubMed] [Google Scholar]
- 22. Jørgensen PG, Jensen MT, Mogelvang R, Fritz‐Hansen T, Galatius S, Biering‐Sørensen T, Storgaard H, Vilsbøll T, Rossing P, Jensen JS. Impact of type 2 diabetes and duration of type 2 diabetes on cardiac structure and function. Int J Cardiol. 2016;221:114–121. [DOI] [PubMed] [Google Scholar]
- 23. Jørgensen PG, Jensen MT, Biering‐Sørensen T, Mogelvang R, Galatius S, Fritz‐Hansen T, Rossing P, Vilsbøll T, Jensen JS. Cholesterol remnants and triglycerides are associated with decreased myocardial function in patients with type 2 diabetes. Cardiovasc Diabetol. 2016;15:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jørgensen PG, Biering‐Sørensen T, Mogelvang R, Fritz‐Hansen T, Vilsbøll T, Rossing P, Jensen JS. Presence of micro‐ and macroalbuminuria and the association with cardiac mechanics in patients with type 2 diabetes. Eur Heart J Cardiovasc Imaging. 2018;19:1034–1041. [DOI] [PubMed] [Google Scholar]
- 25. Jørgensen PG, Jensen MT, Mogelvang R, von Scholten BJ, Bech J, Fritz‐Hansen T, Galatius S, Biering‐Sørensen T, Andersen HU, Vilsbøll T, Rossing P, Jensen JS. Abnormal echocardiography in patients with type 2 diabetes and relation to symptoms and clinical characteristics. Diab Vasc Dis Res. 2016;13:321–330. [DOI] [PubMed] [Google Scholar]
- 26. Jensen MT, Sogaard P, Andersen HU, Bech J, Hansen TF, Galatius S, Jørgensen PG, Biering‐Sørensen T, Møgelvang R, Rossing P, Jensen JS. Prevalence of systolic and diastolic dysfunction in patients with type 1 diabetes without known heart disease: the Thousand & 1 Study. Diabetologia. 2014;57:672–680. [DOI] [PubMed] [Google Scholar]
- 27. Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C; American Heart Association, National Heart, Lung, and Blood Institute . Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 28. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J‐U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. [DOI] [PubMed] [Google Scholar]
- 29. Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, Bello N, Aguilar D, Vardeny O, Matsushita K, Selvin E, Solomon S. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk in the Community study. Circ Heart Fail. 2015;8:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ernande L, Audureau E, Jellis CL, Bergerot C, Henegar C, Sawaki D, Czibik G, Volpi C, Canoui‐Poitrine F, Thibault H, Ternacle J, Moulin P, Marwick TH, Derumeaux G. Clinical implications of echocardiographic phenotypes of patients with diabetes mellitus. J Am Coll Cardiol. 2017;70:1704–1716. [DOI] [PubMed] [Google Scholar]
- 31. Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;71:89–105. [DOI] [PubMed] [Google Scholar]
- 32. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 33. Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. [DOI] [PubMed] [Google Scholar]
- 35. Bang CN, Devereux RB, Okin PM. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction—a LIFE review. J Electrocardiol. 2014;47:630–635. [DOI] [PubMed] [Google Scholar]
- 36. Wong CY, O'Moore‐Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. [DOI] [PubMed] [Google Scholar]
- 37. Katz DH, Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim K‐YA, Peng J, Sha J, Irvin MR, Eckfeldt JH, Turner ST, Freedman BI, Arnett DK, Shah SJ. Association of low‐grade albuminuria with adverse cardiac mechanics: clinical perspective. Circulation. 2014;129:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, Khoury DS, Thohan V, Torre‐Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383. [DOI] [PubMed] [Google Scholar]
- 39. Santos‐Gallego CG, Badimon JJ, Rosenson RS. Beginning to understand high‐density lipoproteins. Endocrinol Metab Clin North Am. 2014;43:913–947. [DOI] [PubMed] [Google Scholar]


