Abstract
Background
Remote ischemic preconditioning (RIPC) attenuates myocardial damage during elective and primary percutaneous coronary intervention. Recent studies suggest that coronary microcirculatory function is an important determinant of clinical outcome. The aim of this study was to assess the effect of RIPC on markers of microcirculatory function.
Methods and Results
Patients referred for cardiac catheterization and fractional flow reserve measurement were randomized to RIPC or sham. Operators and patients were blinded to treatment allocation. Comprehensive physiological assessments were performed before and after RIPC/sham including the index of microcirculatory resistance and coronary flow reserve after intracoronary glyceryl trinitrate and during the infusion of intravenous adenosine. Thirty patients were included (87% male; mean age: 63.1±10.0 years). RIPC and sham groups were similar with respect to baseline characteristics. RIPC decreased the calculated index of microcirculatory resistance (median, before RIPC: 22.6 [interquartile range [IQR]: 17.9–25.6]; after RIPC: 17.5 [IQR: 14.5–21.3]; P=0.007) and increased coronary flow reserve (2.6±0.9 versus 3.8±1.7, P=0.001). These RIPC‐mediated changes were associated with a reduction in hyperemic transit time (median: 0.33 [IQR: 0.26–0.40] versus 0.25 [IQR: 0.20–0.30]; P=0.010). RIPC resulted in a significant decrease in the calculated index of microcirculatory resistance compared with sham (relative change with treatment [mean±SD] was −18.1±24.8% versus +6.1±37.5; P=0.047) and a significant increase in coronary flow reserve (+41.2% [IQR: 20.0–61.7] versus −7.8% [IQR: −19.1 to 10.3]; P<0.001).
Conclusions
The index of microcirculatory resistance and coronary flow reserve are acutely improved by remote ischemic preconditioning. This raises the possibility that RIPC confers cardioprotection during percutaneous coronary intervention as a result of an improvement in coronary microcirculatory function.
Clinical Trial Registration
URL: http://www.anzctr.org.au/. Unique identifier: CTRN12616000486426.
Keywords: coronary flow reserve, coronary physiology, microcirculation, microcirculatory resistance, remote ischemic preconditioning
Subject Categories: Coronary Circulation, Physiology
Clinical Perspective
What Is New?
Remote ischemic preconditioning causes an acute improvement in coronary microcirculatory function, which is an important determinant of prognosis during elective and primary percutaneous coronary intervention.
What Are the Clinical Implications?
An improvement in microcirculatory function may help explain the benefit of remote ischemic preconditioning at the time of coronary stenting and raises the possibility that this treatment may be used to augment the microcirculation in other clinical settings.
In remote ischemic preconditioning (RIPC), brief nonharmful ischemia to a remote organ can protect the heart against ischemia reperfusion injury.1, 2 RIPC has been used before elective and primary percutaneous coronary intervention (PCI), resulting in reduced post‐PCI troponin levels and reduced myocardial infarct size.3, 4, 5, 6 In addition, patients who receive RIPC before elective and primary PCI have been found to have reduced clinical events during long‐term follow‐up.7, 8 However, large multicenter randomized trials in the setting of cardiac surgery have demonstrated no benefit of RIPC.9, 10 These discrepant results demonstrate a context‐specific benefit of RIPC.11
The human coronary microcirculation is recognized as an important determinant of patient prognosis. The coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR), a pressure–temperature sensor wire–derived index,12 have been shown to predict outcome in patients with epicardial coronary artery disease (CAD), including those undergoing primary and elective PCI.13, 14, 15, 16, 17, 18 The combination of high IMR and low CFR confers a substantially increased risk of major cardiovascular events independent of the severity of epicardial coronary stenosis.13, 16, 17, 18, 19 In addition, the coronary microcirculation has been proposed as a target of RIPC‐mediated cardiac protection.20
Clarifying the mechanism by which RIPC may exert a protective effect on the heart in the setting of PCI could help guide future studies and clinical protocols by identifying patient populations most likely to benefit. Studies demonstrating that RIPC protects against ischemia reperfusion injury–associated forearm vascular endothelial dysfunction21 and increases CFR, as assessed by indirect echocardiographic evaluation of the left anterior descending artery,22 suggest an effect of RIPC on microcirculatory function. Given the significant impact that the microcirculation has on the prognosis of patients undergoing PCI17, 18 and the demonstrated benefits of RIPC in this population, we hypothesized that RIPC may improve coronary microvascular function. To explore the mechanism behind RIPC‐mediated protection in the setting of PCI, we undertook a randomized, blinded, placebo‐controlled proof‐of‐concept study to investigate the effect of RIPC on invasively measured coronary physiological parameters, IMR and CFR, in patients with CAD.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Patient Enrollment
Clinically stable patients referred for nonurgent coronary angiography, with symptoms or noninvasive investigations suggestive of significant CAD, at a single tertiary referral center were invited to participate in the study before their planned procedure. Coronary angiography was performed, and patients were included if they required a clinically indicated fractional flow reserve (FFR) assessment of an angiographically equivocal lesion in a major epicardial coronary artery, as determined by the interventional cardiologist performing the procedure. We included only patients who required FFR measurement to justify the increased risk associated with coronary artery wiring. Consecutive patients who met these inclusion criteria were included in the study.
Exclusion criteria included the need for emergent coronary angiography, contraindication to inflation of a sphygmomanometer on the left arm (eg, arteriovenous fistula for renal dialysis, peripheral vascular disease involving the limb), and existing neuropathy or myopathy that may predispose to nerve or muscle damage from upper limb ischemia. In addition, patients with prior myocardial infarction in the target artery territory or coronary anatomy that would affect accurate coronary physiology assessment, such as left or right coronary ostial disease leading to guide pressure “damping,” were also excluded, as were patients in atrial fibrillation, because irregularity of the cardiac cycle could affect thermodilution measurements. Patients with severe asthma were not invited to participate because adenosine can exacerbate airway disease.
Once participants met the inclusion criteria, they were randomized to either RIPC or sham treatment by way of a closed‐envelope system during their procedure. Data regarding patient demographics, comorbidities, medications, and preprocedure investigations were collected.
Coronary Physiology Measurements
After informed consent, a 6F radial or femoral arterial sheath was inserted, and coronary angiography was performed by standard techniques under conscious sedation with at least 1 mg midazolam and 25 μg fentanyl administered intravenously. Quantitative coronary angiography (Artis; Siemens) was performed off‐line in 2 orthogonal views. Unfractionated heparin was administered at a dose of 70 U/kg. Coronary physiology measurements were performed immediately before and immediately after the RIPC/sham treatment protocol, as described previously.17, 18, 23 In brief, a 6F guiding catheter without side holes was used to engage either the left main coronary artery or the right coronary artery. A pressure–temperature sensor guidewire (Certus; St Jude Medical) was advanced to the tip of the guiding catheter for pressure equalization before being advanced to the distal segment of the target artery, ensuring that the sensor position was at least 30 mm distal to the lesion in question. The wire position was fluoroscopically stored and maintained throughout the study protocol. Intracoronary glyceryl trinitrate was administered at a dose of 100 μg before each coronary physiology study. Thermodilution curves were produced in triplicate to determine the mean transit time at rest (shown as TmnR) by briskly injecting 3 mL of room temperature saline down the coronary artery. In addition, the mean proximal pressure and mean distal pressure were recorded at rest.
Hyperemia was induced with an intravenous infusion of adenosine (140 μg/kg per minute) via a 4F femoral venous sheath. In a similar manner, thermodilution curves were produced to determine the mean transit time during hyperemia (shown as TmnH). Mean proximal pressure (shown as Pa) and mean distal pressure (shown as Pd) during hyperemia were also recorded. As described previously, the FFR was calculated by Pd/Pa during hyperemia and the CFR was calculated by TmnR/TmnH.23 The IMR was calculated by 2 methods. In patients where there was no hemodynamically significant epicardial stenosis, as determined by an FFR >0.80, the IMR was calculated during hyperemia by the formula Pd×TmnH.12 The IMR overestimates microcirculatory resistance in the presence of significant epicardial stenoses due to the presence of collateral flow. To avoid measuring the coronary wedge pressure to account for collateral flow, which requires coronary balloon inflation, the IMR was also calculated in all patients using the formula derived by Yong et al (calculated IMR [IMRcalc]): Pa×TmnH×(1.34×Pd/Pa−0.32).24 All measurements were recorded using the RadiAnalyzer console (St Jude Medical).
Patients then received either RIPC or sham treatment while on the catheterization laboratory table. Physiology measurements were repeated in an identical manner immediately after RIPC/sham after ensuring that the pressure–temperature sensor was in a position identical to that when pretreatment measurements were performed. The procedure then proceeded as clinically indicated. All study measurements were taken before coronary balloon inflation to avoid the effect of local ischemic preconditioning and distal embolization confounding the results.
Remote Ischemic Preconditioning
In the RIPC group, a sphygmomanometer was inflated to either 200 mm Hg or 50 mm Hg greater than systolic blood pressure (whichever was greater) for 5 minutes on the left arm, followed by deflation for 5 minutes, and this cycle was repeated 3 times using an automated sphygmomanometer (HeartGuard; Condicion).4, 25 Sham treatment involved sphygmomanometer inflation to a pressure of 10 mm Hg but was otherwise identical to the RIPC protocol. To confirm ischemia, the radial pulse was examined in each patient, ensuring that it was impalpable during RIPC and unaffected during sham treatment. Figure 1 outlines patient flow and randomization.
Figure 1.
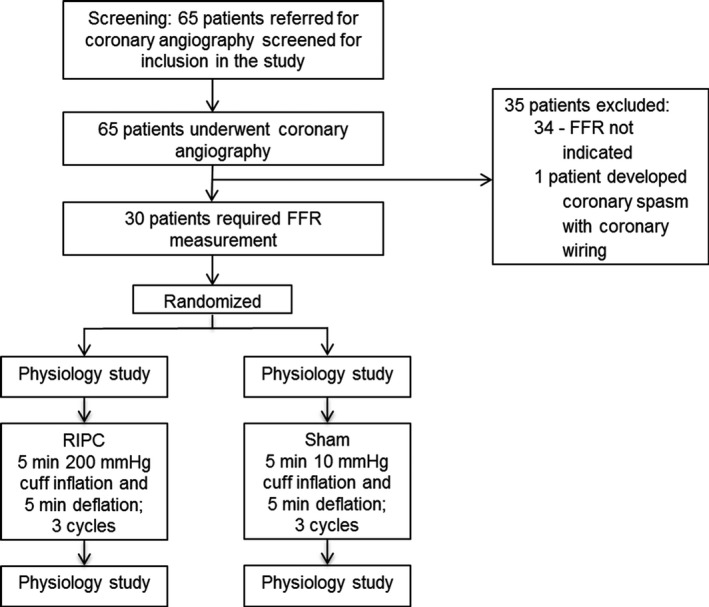
Patient recruitment and randomization. Patients were randomized to RIPC or sham treatment after coronary angiogram, and the need for FFR measurement was established. A coronary physiology study was performed before and after the allocated treatment. FFR indicates fractional flow reserve; RIPC, remote ischemic preconditioning.
Patients were not informed of their treatment allocation but were warned of possible discomfort from sphygmomanometer inflation. Patients were asked not to express discomfort unless it was intolerable, with all patients complying with this request. The operator performing physiological measurements was also blinded to the treatment allocation, with RIPC or sham treatment delivered by an assistant who did not communicate with the operator. To maintain blinding, the sphygmomanometer was obscured from view, and music was played throughout the laboratory to mask the sound of sphygmomanometer inflation.
To ensure that the RIPC protocol was effectively inducing ischemia to the treated upper limb, in a cohort of patients who did not undergo coronary physiology measurements, venous blood was drawn into tubes containing lithium heparin from the cubital fossa of the treated upper limb before and immediately after the RIPC and sham protocols for measurement of blood lactate.
Plasma Collection and Analysis
Nitric oxide is known to be a regulator of coronary microcirculatory function, with nitrite being its major metabolite.26 To determine the effect of RIPC on circulating nitrite levels, blood was collected from the femoral venous sheath after each coronary physiology study, before and after the allocated treatment, into tubes containing 7.2 mg (1.8 mg/mL) K2EDTA. The blood was centrifuged at 2500g for 15 minutes, and the plasma was stored immediately at −80°C. Because circulating nitrite levels have been shown to be elevated and important for cardiac protection induced by RIPC,27 plasma that had not previously been thawed was analyzed for nitrite concentration with a commercially available ELISA kit (R&D Systems), as per the manufacturer's instructions.
Statistical Analysis
The D'Agostino and Pearson normality test was used to determine whether data were normally distributed. Categorical variables are presented as frequency and percentage. Continuous variables are expressed as mean±SD for normally distributed data and as median (interquartile range [IQR]) for nonnormally distributed data. Categorical data were compared using the χ2 or Fisher exact test, as appropriate. Comparisons between continuous variables were performed using the paired or unpaired t test, as appropriate, for normally distributed data and the Wilcoxon signed rank test or Mann–Whitney U test, as appropriate, for nonnormally distributed data. Correlations between continuous variables were assessed with the Pearson correlation.
Sample size calculation was performed based on the primary analysis, which was amount of change in the IMR with RIPC. Based on preliminary data, an absolute decrease in IMRcalc with RIPC was expected to be 5.5±7.0. With a power of 80% and a 2‐sided α value of 0.05, it was estimated that 15 patients would need to be studied for a paired difference analysis to detect a change in IMRcalc with RIPC.
We aimed to perform a secondary analysis to determine change in IMRcalc with sham treatment to ensure that there was no artifactual change in coronary physiology indexes as a result of a prolonged catheterization procedure; therefore, we recruited 30 patients who were randomized to RIPC or sham (15 RIPC and 15 sham). Other secondary analyses included changes in IMR, CFR, mean transit time at rest, mean transit time during hyperemia, and FFR with RIPC.
In addition, the relative change in IMRcalc, IMR, and CFR was calculated as [(post−pre)/pre]×100%, where pre represents that value measured before RIPC/sham and post represents the value measured after RIPC/sham. The relative change in the markers of coronary microcirculatory function in the RIPC cohort was compared with the relative change in the sham cohort.
All analyses were performed using SPSS v22 (IBM Corp) or GraphPad Prism (GraphPad Software). A 2‐tailed probability value <0.05 was considered statistically significant.
Ethical Considerations
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Ethics approval was granted by the Sydney Local Health District Human Research Ethics Committee of Sydney, Australia (CH62/6/2014‐016). The study was registered with the Australia and New Zealand Clinical Trials Registry (ACTRN12616000486426). Each participant gave written informed consent for participation and for use of their health information. All patient data were deidentified and analyzed anonymously.
Results
A total of 65 patients underwent coronary angiography and were screened for participation in the study. As Figure 1 shows, 34 patients were excluded after coronary angiography because there was no clinical indication for FFR measurement. One additional patient was excluded because coronary wiring was associated with significant coronary spasm. Thirty patients underwent randomization and the full study protocol. An example of the coronary physiology data obtained from 1 patient are presented in Figure 2. This patient was randomized to receive RIPC on the catheterization laboratory table during the procedure. Figure 2A displays the coronary physiology indexes before RIPC, and Figure 2B displays the same indexes after RIPC. In this case, RIPC was associated with a decrease in IMR and an increase in CFR.
Figure 2.
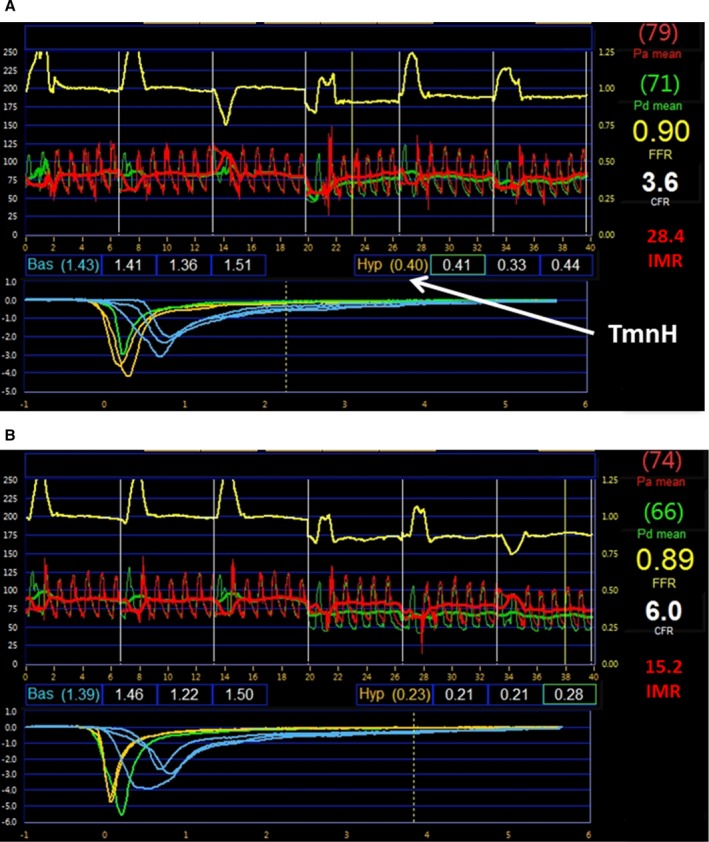
Coronary physiology measurements obtained from 1 patient who was randomized to RIPC. Data were obtained before (A) and after (B) RIPC. There was a reduction in IMR and TmnH with RIPC, whereas the CFR increased. IMR=Pd×TmnH. CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; Pa, mean proximal pressure; Pd, mean distal pressure; RIPC, remote ischemic preconditioning; TmnH, mean transit time during hyperemia.
Of the 30 patients who underwent randomization to either RIPC or sham treatment, the mean age was 63.1±10.0 years, and 26 (87%) were male. Coronary physiology measurements were performed in all patients before and after the allocated treatment. Fifteen patients were randomized to RIPC, and 15 patients were randomized to sham treatment. The allocated treatment was tolerated by all patients, and there were no complications of treatment. The baseline characteristics, lesion characteristics, and medications of the RIPC and sham groups did not differ significantly (Table 1).
Table 1.
Baseline Characteristics of Patients
| Characteristic | RIPC (n=15) | Sham (n=15) | P Value |
|---|---|---|---|
| Age, y | 64.5±8.8 | 61.7±11.2 | 0.465 |
| Male | 13 (87) | 13 (87) | 1.000 |
| Prior myocardial infarction | 1 (7) | 4 (27) | 0.142 |
| Prior PCI | 2 (13) | 6 (40) | 0.099 |
| Prior CABG | 0 (0) | 0 (0) | ··· |
| Heart failure | 0 (0) | 1 (7) | 0.309 |
| Prior stroke | 1 (7) | 2 (13) | 0.543 |
| Peripheral vascular disease | 0 (0) | 2 (13) | 0.143 |
| Hypertension | 11 (73) | 10 (67) | 0.690 |
| Diabetes mellitus | 7 (47) | 3 (20) | 0.121 |
| Dyslipidemia | 13 (87) | 9 (60) | 0.099 |
| Current smoking | 1 (7) | 3 (20) | 0.283 |
| Normal left ventricular contractility | 14 (93) | 14 (93) | 1.000 |
| Left ventricular hypertrophy | 2 (13) | 1 (7) | 0.543 |
| Medications | |||
| Aspirin (100 mg daily) | 14 (93) | 15 (100) | 0.309 |
| P2Y12 antagonist | 12 (80) | 10 (67) | 0.409 |
| Clopidogrel | 9 (60) | 9 (60) | |
| Ticagrelor | 3 (20) | 1 (7) | |
| Warfarin/NOAC | 0 (0) | 0 (0) | ··· |
| Statin | 14 (93) | 14 (93) | 1.000 |
| β‐Blocker | 5 (33) | 8 (53) | 0.269 |
| ACEI or ARB | 11 (73) | 7 (47) | 0.136 |
| Nitrate | 1 (7) | 1 (7) | 1.000 |
| Coronary assessment | |||
| LAD assessed | 12 (80) | 10 (67) | 0.409 |
| Lesion diameter stenosis, %a | 38.7±10.0 | 39.5±6.4 | 0.779 |
| Vessel reference diameter, mma | 2.9±0.5 | 2.6±0.5 | 0.051 |
| Lesion length, mma | 9.4±4.3 | 10.1±4.2 | 0.651 |
| Parameters during admission | |||
| Systolic blood pressure, mm Hg | 128.5±12.8 | 136.1±12.3 | 0.108 |
| Heart rate, beats/min | 68.4±11.0 | 67.9±10.5 | 0.893 |
| Hemoglobin concentration, g/L | 138.1±19.9 | 130.9±15.2 | 0.276 |
| eGFR, mL/min/1.73 m2 | 83.1±11.1 | 84.9±13.4 | 0.682 |
Data are shown as mean±SD or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; LAD, left anterior descending artery; NOAC, novel oral anticoagulant; PCI, percutaneous coronary intervention; RIPC, remote ischemic preconditioning.
Average value from quantitative coronary angiographic assessment of 2 views of each lesion per patient.
Effect of RIPC on Blood Lactate Measurements
Sixteen patients, who were not part of the main study cohort, underwent blood lactate measurements before and after RIPC/sham. There was a significant rise in blood lactate in response to the RIPC protocol (before versus after RIPC: 1.36±0.61 versus 1.69±0.56 mmol/L, respectively; P=0.004, n=10), whereas there was no effect after sham treatment (before versus after sham: median: 1.4 [IQR: 1.1–1.6] versus 1.5 [IQR: 1.2–1.7]; P=0.500, n=6).
Effect of RIPC on Coronary Physiology Measurements
Twenty‐two (73%) of the lesions assessed were in the left anterior descending artery, 12 in the RIPC cohort and 10 in the sham cohort (P=0.409). The mean pretreatment (RIPC/sham) FFRs in the RIPC and sham cohorts were not significantly different (0.83±0.06 versus 0.82±0.08; P=0.762). There were 3 (20%) and 6 (40%) pretreatment FFR measurements ≤0.80 in the RIPC and sham groups, respectively (P=0.232).
Primary analysis
The coronary physiology indexes recorded before and after RIPC and before and after sham treatment are displayed in Table 2. Within the RIPC cohort, there was a significant reduction in IMRcalc when the pre‐ and post‐RIPC measurements were compared (Figure 3A).
Table 2.
The Effect of RIPC on Coronary Physiology Indexes
| Marker | RIPC (n=15) | Sham (n=15) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Mean Differencea | P Valueb | Pre | Post | Mean Differencea | P Valueb | |
| IMRcalc | 22.6 (17.9–25.6) | 17.5 (14.5–21.3) | 5.1 | 0.007 | 16.0 (10.8–20.5) | 16.8 (10.8–21.2) | 1.1 | 0.847 |
| IMRc | 24.3 (18.5–26.1) | 17.7 (13.2–21.7) | 5.1 | 0.005 | 16.1 (9.3–22.8) | 11.4 (10.6–24.7) | 1.0 | 0.820 |
| CFR | 2.6±0.9 | 3.8±1.7 | 1.2 | 0.001 | 3.1±1.5 | 3.1±1.6 | 0.0 | 0.971 |
| FFR | 0.83±0.06 | 0.83±0.07 | 0.0 | 0.999 | 0.82±0.08 | 0.81±0.09 | 0.0 | 0.052 |
Data are shown as mean±SD or median (interquartile range). CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; IMRcalc, calculated index of microcirculatory resistance; RIPC, remote ischemic preconditioning.
Absolute difference in mean between pre and post within each cohort.
Comparison between pre and post values within each group was performed with the paired t test or Wilcoxon signed rank test for normally or non‐normally distributed data, respectively.
Patients with FFR >0.80: 12 in the RIPC group and 9 in the sham group.
Figure 3.
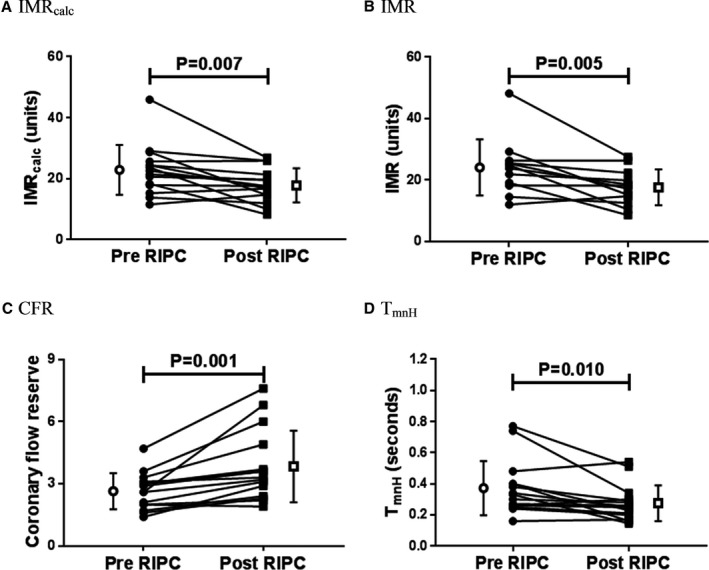
Remote ischemic preconditioning reduces the IMR and increases the CFR through an increase in hyperemic coronary flow. There was a significant reduction in IMRcalc (A) and the IMR (B) with RIPC, whereas the CFR (C) increased significantly. There was a significant reduction in TmnH (D) with RIPC, suggesting an increase in hyperemic coronary flow. IMRcalc, CFR and TmnH: n=15; IMR: n=12; Individual filled symbols represent measurements before or after RIPC in each patient joined with a line, and open symbols and bars represent mean±SD. Bas indicates baseline; CFR, coronary flow reserve; Hyp, hyperemic; IMR, index of microcirculatory resistance; IMRcalc, calculated index of microcirculatory resistance; RIPC, remote ischemic preconditioning; TmnH, mean transit time during hyperemia.
Secondary analyses
There was also a significant reduction in IMR in patients with pretreatment FFR >0.80 and a significant increase in CFR in patients who were randomized to receive RIPC (Figure 3B and 3C). There was no effect of RIPC on FFR. The predominant driver of these physiological effects was a reduction in the hyperemic transit time (median: 0.33 [IQR: 0.26–0.40] versus 0.25 [IQR: 0.20–0.30]; P=0.010; Figure 3D) with no change in mean distal pressure during hyperemia (68.5±15.4 versus 70.5±16.1 mm Hg; P=0.495) or mean proximal pressure during hyperemia (81.5±14.5 versus 84.3±14.0 mm Hg; P=0.406). There was no change in the resting transit time (0.95±0.43 versus 1.03±0.55 seconds; P=0.293) with RIPC.
There was no change in IMRcalc, IMR, CFR, or mean transit time during hyperemia with sham treatment (Figure 4).
Figure 4.
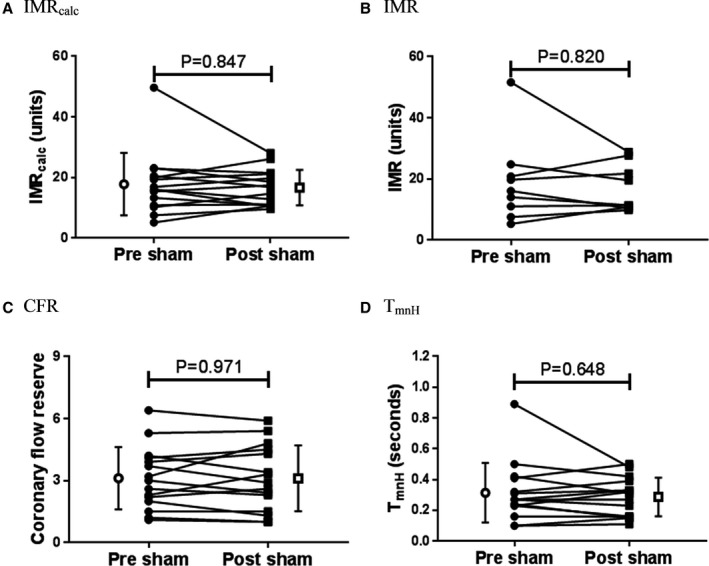
Sham had no effect on IMR, CFR, or hyperemic coronary flow. There was no effect of sham on IMRcalc (A), IMR (B), CFR (C), or TmnH (D). IMRcalc, CFR, and TmnH: n=15; IMR: n=9. Individual filled symbols represent measurements before or after sham in each patient joined with a line, and open symbols and bars represent mean±SD. CFR indicates coronary flow reserve; IMR, index of microcirculatory resistance; IMRcalc, calculated index of microcirculatory resistance; RIPC, remote ischemic preconditioning; TmnH, mean transit time during hyperemia.
A comparison of the change in markers of coronary microcirculatory function induced by RIPC and sham treatment is displayed in Table 3. Change in IMRcalc was significantly greater when comparing the RIPC and sham cohorts (Figure 5A). Change in CFR was also significantly greater when comparing the RIPC and sham cohorts (Figure 5B). There was no difference in the effect of RIPC and sham on FFR (RIPC versus sham group: median: 0.0% [IQR: −2.4 to 1.4] versus −1.5% [IQR: −3.4 to 1.2]; P=0.269).
Table 3.
Comparison of the Effect of RIPC and Sham on Coronary Physiology Indexes
| Marker | RIPC (n=15) | Sham (n=15) | P Valuea |
|---|---|---|---|
| IMRcalc | −18.1±24.8% | +6.1±37.5% | 0.047 |
| IMRb | −22.5±25.2% | +6.8±45.5% | 0.074 |
| CFR | +41.2% (20.0–61.7) | −7.8% (−19.1 to 10.3) | <0.001 |
| FFR | 0.0% (−2.4 to 1.4) | −1.5% (−3.4 to 1.2) | 0.269 |
Relative change in index with RIPC/sham is shown, with negative (−) values indicating a reduction after treatment compared with before treatment and positive (+) values indicating an increase. Data are shown as mean±SD or median (interquartile range). CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; IMRcalc, calculated index of microcirculatory resistance; RIPC, remote ischemic preconditioning.
Comparison of relative change in RIPC cohort with relative change in sham cohort with the unpaired t test or Mann–Whitney U test for normally and nonnormally distributed data, respectively.
Patients with FFR >0.80: 12 in the RIPC group and the 9 in sham group.
Figure 5.
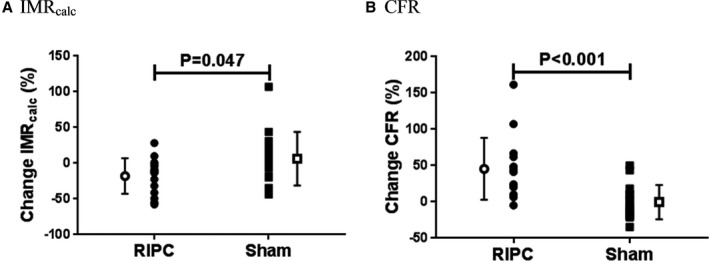
Comparison of change in markers of coronary microcirculatory function with remote ischemic preconditioning and sham. The relative change in IMRcalc (A) and CFR (B) induced by RIPC was significantly different to the change due to sham treatment. Individual filled symbols represent relative change in measurement with RIPC/sham in each patient, with negative and positive values indicative of reductions and increases with treatment, respectively. Open symbols and bars represent mean±SD. CFR indicates coronary flow reserve; IMRcalc, calculated index of microcirculatory resistance; RIPC, remote ischemic preconditioning.
No significant association or correlation was noted between the RIPC‐induced change in IMRcalc and CFR and any of the baseline demographics, comorbidities, and medications listed in Table 1. The P value was >0.1 for all these factors on univariable regression analysis; therefore, a multivariable regression analysis was not performed.
Plasma Nitrite Measurement
There was no change in plasma nitrite with RIPC (before versus after RIPC: 1.6±0.2 versus l.6±0.3 μmol/L; P=0.997; Figure S1).
Discussion
RIPC has been proposed to confer cardioprotection in patients undergoing elective and primary PCI, with reductions in post‐PCI troponin and infarct size3, 4, 5 through multiple potential but as yet uncertain mechanisms.28 We demonstrated that RIPC acutely improved coronary microcirculatory function, as assessed by validated coronary pressure–temperature sensor wire–based techniques. To the best of our knowledge, this study provides the first demonstration of these effects of RIPC on coronary microcirculatory function.
The coronary microcirculation is recognized as an important determinant of prognosis in patients with CAD. The IMR is an index that assesses resistance to flow in the coronary microvasculature, whereas the FFR is an index that is used to assess the hemodynamic significance of an epicardial coronary lesion. The CFR is an index that provides assessment of both the epicardial artery and the microcirculation.29
Multiple studies have shown the utility of the CFR to predict cardiac events, and it has been shown to be of greater importance than the degree of disease in the epicardial coronary arteries in determining prognosis.14, 16, 19, 30, 31 The IMR, which correlates with true microcirculatory resistance,12 has been shown to be predictive of post‐PCI myocardial infarction after elective PCI and the occurrence of death and heart failure hospitalization after primary PCI.17, 18 The IMR has been shown to be reproducible and independent of hemodynamic conditions and thus is a reliable method to assess the microcirculatory resistance.23
We demonstrate that RIPC leads to a rapid reduction in the IMR and an increase in CFR. This suggests that beneficial effects on the coronary microcirculation may contribute to RIPC‐mediated cardioprotection during PCI. The baseline clinical characteristics appeared to have little effect on the change in IMRcalc and CFR with RIPC. The results of this study raise the possibility that RIPC may be beneficial in other clinical settings involving microcirculatory dysfunction such as microvascular angina, congestive heart failure, and aortic stenosis.32, 33 Our results suggest that research into the use of RIPC beyond CAD is warranted.
Data support the role of the coronary microcirculation as a target of RIPC‐mediated cardioprotection.20 In a study by Kono et al,22 10 healthy volunteers and 10 patients with heart failure who received RIPC twice per day for 1 week demonstrated an increase in CFR, as assessed by echocardiographic spectral Doppler analysis of flow in the distal left anterior descending artery. In addition, RIPC has been shown to improve endothelial function, reducing vasoconstriction after acetylcholine administration during cardiac catheterization.34
Despite this, there is conflicting evidence regarding the effects of RIPC on the coronary microcirculation. Studies of RIPC in the setting of primary PCI have reported no changes in surrogate markers of microcirculatory function, such as the Thrombolysis in Myocardial Infarction (TIMI) frame count and the appearance of microvascular obstruction on magnetic resonance imaging.3, 5 Moreover, a study by Hoole et al found that RIPC had no effect on coronary microvascular resistance in 11 patients assessed by a Doppler/pressure wire–based technique requiring coronary balloon inflation during cardiac catheterization.35 The null result in this study may have been due to local preconditioning, or distal embolization, induced by the coronary balloon inflation. There was a small numerical increase in the microcirculatory resistance after coronary balloon inflation in patients who did not undergo RIPC in this study, but the study may have been underpowered to detect a statistically significant difference.
Our study demonstrated an effect on CFR, IMR, and transit time and confirmed the absence of an effect of sham treatment. It is notable that there was an outlier in both the RIPC and sham cohorts, with each of these patients demonstrating a marked reduction in IMRcalc and IMR with RIPC/sham. However, the decrease in IMRcalc and IMR with RIPC remained significant after removal of this outlier (Figure S2).
There was no change in mean transit time at rest, suggesting that RIPC does not affect resting coronary flow. Conversely, there was an increase in hyperemic flow, indicated by a reduction in mean transit time during hyperemia, in the absence of change in distal or proximal coronary pressures. This increase in hyperemic flow supports the interpretation that RIPC reduces coronary microcirculatory resistance.
Adenosine and nitric oxide/nitrite contribute to the coronary microcirculatory tone, and because they have been implicated in RIPC‐mediated cardiac protection,27, 36, 37 they are candidate mediators for the effect of RIPC on the microcirculation. However, given the supraphysiological doses of adenosine and intracoronary glyceryl trinitrate that were administered at the time of the coronary physiology study to achieve hyperemia, it is unlikely that these mediators are primarily responsible for the effects that we observed. The lack of change in plasma nitrite levels with RIPC, the major metabolite of nitric oxide, supports this conclusion with respect to nitric oxide. Although nitrite has been shown previously to be increased by RIPC in an animal model,27 the administration of glyceryl trinitrate during the procedure may have masked any effect of RIPC on nitrite in this study.
Other mediators and pathways that have been shown to be involved in cardiac preconditioning, include bradykinin, potassium ATP channels, and calcium‐activated potassium channels of the BK type.21, 38, 39 Although angiotensin‐converting enzyme inhibitors are known to reduce degradation of bradykinin, in the RIPC cohort, the relative reduction in IMRcalc and the increase in CFR were numerically smaller, but not statistically significant, in patients taking this class of drug. Because potassium ATP channels are involved in RIPC‐mediated protection against ischemia–reperfusion injury–associated endothelial dysfunction,21 they may play a role in the improved microcirculatory function that we demonstrated and warrant future investigation. Although a large body of evidence supports a circulating humoral factor mediating the physiological effects of RIPC, some studies suggest a contribution by a neural pathway. In animal models, the transection of a peripheral nerve supplying the limb undergoing RIPC or the use of a nicotinic acetyl choline ganglion blocker attenuated the protective effects of RIPC.40, 41
Limitations
We are unable to comment on the durability of the RIPC effect and cannot be certain that we identified the maximal effect on the CFR and IMR. Despite this, we demonstrated an acute enhancement of microcirculatory function with RIPC that may contribute to the protective effects of RIPC demonstrated in the literature when RIPC was delivered within 2 hours of PCI. Because of the administration of glyceryl trinitrate, additional assessments of endothelial function, such as response to acetylcholine administration, were not performed in this study.
Finally, because this was a mechanistic proof‐of‐concept study with only small numbers of patients undergoing PCI, clinical outcomes and the correlation of microcirculatory change with changes in post‐PCI troponin were not assessed. Although the inclusion of patients who required FFR measurement resulted in a cohort that predominantly did not require PCI, the results of this study herald the need for a study correlating change in coronary microcirculatory status and clinical outcomes with RIPC.
Conclusion
The IMR and CFR are acutely improved by RIPC. This suggests that RIPC confers cardioprotection during PCI as a result of improvement in coronary microcirculatory function. The application of RIPC to augment the coronary microcirculation in other settings warrants investigation.
Sources of Funding
This work was supported by a National Health and Medical Research Council/National Heart Foundation of Australia Postgraduate Scholarship (1094384 to Lau) and a National Health and Medical Research Council Program Grant (1037903 to Kritharides).
Disclosures
Dr Yong has received research support from Abbott Laboratories and Philips, and minor honoraria from Abbott Laboratories. Dr Fearon has received research support form Abbott Laboratories. The remaining authors have no disclosures to report.
Supporting information
Figure S1. No change in plasma nitrite with remote ischemic preconditioning.
Figure S2. The effect of remote ischemic preconditioning on the index of microcirculatory resistance with outliers removed.
Acknowledgments
We would like to recognize the contribution of Ms Kitty Xu, Dr Rong Bing, Dr Gabrielle J. Pennings, and Dr Caroline J. Reddel for their assistance in conducting the trial and processing the plasma samples. In addition, we would like to thank the Concord Hospital catheterization laboratory staff for their assistance and patience with this study.
(J Am Heart Assoc. 2018;7:e009058 DOI: 10.1161/JAHA.118.009058.)
References
- 1. Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–1646. [DOI] [PubMed] [Google Scholar]
- 2. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 3. Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. [DOI] [PubMed] [Google Scholar]
- 4. Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O'Sullivan M, Dutka DP. Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–827. [DOI] [PubMed] [Google Scholar]
- 5. White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–188. [DOI] [PubMed] [Google Scholar]
- 6. Heusch G, Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre‐, post‐, and remote conditioning. Circ Res. 2016;119:676–695. [DOI] [PubMed] [Google Scholar]
- 7. Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long‐term follow‐up. Circ Cardiovasc Interv. 2013;6:246–251. [DOI] [PubMed] [Google Scholar]
- 8. Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE. Improved long‐term clinical outcomes in patients with ST‐elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. [DOI] [PubMed] [Google Scholar]
- 9. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 10. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg‐Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer‐Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 11. Heusch G, Gersh BJ. ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not!. Eur Heart J. 2016;37:200–202. [DOI] [PubMed] [Google Scholar]
- 12. Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 13. Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67:1158–1169. [DOI] [PubMed] [Google Scholar]
- 15. Ahn SG, Hung OY, Lee JW, Lee JH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Lee SH, Yoon J, Kwon W, Samady H. Combination of the thermodilution‐derived index of microcirculatory resistance and coronary flow reserve is highly predictive of microvascular obstruction on cardiac magnetic resonance imaging after ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2016;9:793–801. [DOI] [PubMed] [Google Scholar]
- 16. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng MK, Yong AS, Ho M, Shah MG, Chawantanpipat C, O'Connell R, Keech A, Kritharides L, Fearon WF. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv. 2012;5:515–522. [DOI] [PubMed] [Google Scholar]
- 18. Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. 2016;118:1643–1658. [DOI] [PubMed] [Google Scholar]
- 21. Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)‐channel dependent mechanism. Circulation. 2007;116:1386–1395. [DOI] [PubMed] [Google Scholar]
- 22. Kono Y, Fukuda S, Hanatani A, Nakanishi K, Otsuka K, Taguchi H, Shimada K. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Devel Ther. 2014;8:1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. [DOI] [PubMed] [Google Scholar]
- 24. Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbourn R, Macisaac A, Kritharides L, Wilson A, Ng MK. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53–58. [DOI] [PubMed] [Google Scholar]
- 25. Luo SJ, Zhou YJ, Shi DM, Ge HL, Wang JL, Liu RF. Remote ischemic preconditioning reduces myocardial injury in patients undergoing coronary stent implantation. Can J Cardiol. 2013;29:1084–1089. [DOI] [PubMed] [Google Scholar]
- 26. Lundberg JO, Weitzberg E, Gladwin MT. The nitrate‐nitrite‐nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. [DOI] [PubMed] [Google Scholar]
- 27. Rassaf T, Totzeck M, Hendgen‐Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. [DOI] [PubMed] [Google Scholar]
- 28. Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2017;469:159–181. [DOI] [PubMed] [Google Scholar]
- 29. Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA; American Heart Association Committee on D, Interventional Cardiac Catheterization CoCC . Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. [DOI] [PubMed] [Google Scholar]
- 30. Majmudar MD, Murthy VL, Shah RV, Kolli S, Mousavi N, Foster CR, Hainer J, Blankstein R, Dorbala S, Sitek A, Stevenson LW, Mehra MR, Di Carli MF. Quantification of coronary flow reserve in patients with ischaemic and non‐ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging. 2015;16:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naya M, Tamaki N, Tsutsui H. Coronary flow reserve estimated by positron emission tomography to diagnose significant coronary artery disease and predict cardiac events. Circ J. 2014;79:15–23. [DOI] [PubMed] [Google Scholar]
- 32. Lee JF, Barrett‐O'Keefe Z, Garten RS, Nelson AD, Ryan JJ, Nativi JN, Richardson RS, Wray DW. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart. 2016;102:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahn JH, Kim SM, Park SJ, Jeong DS, Woo MA, Jung SH, Lee SC, Park SW, Choe YH, Park PW, Oh JK. Coronary microvascular dysfunction as a mechanism of angina in severe AS: prospective adenosine‐stress CMR study. J Am Coll Cardiol. 2016;67:1412–1422. [DOI] [PubMed] [Google Scholar]
- 34. Corcoran D, Young R, Cialdella P, McCartney P, Bajrangee A, Hennigan B, Collison D, Carrick D, Shaukat A, Good R, Watkins S, McEntegart M, Watt J, Welsh P, Sattar N, McConnachie A, Oldroyd KG, Berry C. The effects of remote ischaemic preconditioning on coronary artery function in patients with stable coronary artery disease. Int J Cardiol. 2018;252:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoole SP, Heck PM, White PA, Khan SN, O'Sullivan M, Clarke SC, Dutka DP. Remote ischemic preconditioning stimulus does not reduce microvascular resistance or improve myocardial blood flow in patients undergoing elective percutaneous coronary intervention. Angiology. 2009;60:403–411. [DOI] [PubMed] [Google Scholar]
- 36. Surendra H, Diaz RJ, Harvey K, Tropak M, Callahan J, Hinek A, Hossain T, Redington A, Wilson GJ. Interaction of delta and kappa opioid receptors with adenosine A1 receptors mediates cardioprotection by remote ischemic preconditioning. J Mol Cell Cardiol. 2013;60:142–150. [DOI] [PubMed] [Google Scholar]
- 37. Leung CH, Wang L, Nielsen JM, Tropak MB, Fu YY, Kato H, Callahan J, Redington AN, Caldarone CA. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther. 2014;28:7–17. [DOI] [PubMed] [Google Scholar]
- 38. Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000;278:H1571–H1576. [DOI] [PubMed] [Google Scholar]
- 39. Frankenreiter S, Bednarczyk P, Kniess A, Bork N, Straubinger J, Koprowski P, Wrzosek A, Mohr E, Logan A, Murphy MP, Gawaz M, Krieg T, Szewczyk A, Nikolaev VO, Ruth P, Lukowski R. cGMP‐elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte‐specific BK channels. Circulation. 2017;136:2337–2355. [DOI] [PubMed] [Google Scholar]
- 40. Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra‐arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–H1603. [DOI] [PubMed] [Google Scholar]
- 41. Wong GT, Lu Y, Mei B, Xia Z, Irwin MG. Cardioprotection from remote preconditioning involves spinal opioid receptor activation. Life Sci. 2012;91:860–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. No change in plasma nitrite with remote ischemic preconditioning.
Figure S2. The effect of remote ischemic preconditioning on the index of microcirculatory resistance with outliers removed.


