Abstract
Background
Patients with chronic kidney disease (CKD) are at high risk of myocardial infarction. Cardiac troponins are the biomarkers of choice for the diagnosis of acute myocardial infarction (AMI) without ST‐segment elevation (NSTE). In patients with CKD, troponin levels are often chronically elevated, which reduces their diagnostic utility when NSTE‐AMI is suspected. The aim of this study was to derive a diagnostic algorithm for serial troponin measurements in patients with CKD and suspected NSTE‐AMI.
Methods and Results
Two cohorts, 1494 patients from a prospective cohort study with high‐sensitivity troponin I (hs‐cTnI) measurements and 7059 cases from a clinical registry with high‐sensitivity troponin T (hs‐cTnT ) measurements, were analyzed. The prospective cohort comprised 280 CKD patients (estimated glomerular filtration rate <60 mL/min/1.73 m2). The registry data set contained 1581 CKD patients. In both cohorts, CKD patients were more likely to have adjudicated NSTE‐AMI than non‐CKD patients. The specificities of hs‐cTnI and hs‐cTnT to detect NSTE‐AMI were reduced with CKD (0.82 versus 0.91 for hs‐cTnI and 0.26 versus 0.73 for hs‐cTnT) but could be restored by applying optimized cutoffs to either the first or a second measurement after 3 hours. The best diagnostic performance was achieved with an algorithm that incorporates serial measurements and rules in or out AMI in 69% (hs‐cTnI) and 55% (hs‐cTnT) of CKD patients.
Conclusions
The diagnostic performance of high‐sensitivity cardiac troponins in patients with CKD with suspected NSTE‐AMI is improved by use of an algorithm based on admission troponin and dynamic changes in troponin concentration.
Keywords: biomarker, chronic kidney disease, cohort study, decision aids, non‐ST‐segment elevation acute coronary syndrome
Subject Categories: Biomarkers, Diagnostic Testing, Acute Coronary Syndromes, Nephrology and Kidney
Clinical Perspective
What Is New?
The current study proposes algorithms to help make the diagnosis of non–ST‐elevation myocardial infarction in patients with impaired renal function on the basis of changes in high‐sensitivity cardiac troponin levels.
What Are the Clinical Implications?
The algorithms may assist clinical decision making in the emergency department, when a decision has to be made whether or not to prescribe antiplatelet medication or to perform percutaneous intervention in these high‐risk patients.
Introduction
Patients with chronic kidney disease (CKD) are at high risk for myocardial infarction presenting with atypical symptoms and without ST‐segment elevation (NSTE‐AMI) in the ECG. When the diagnosis cannot be based on pathognomonic changes in the ECG, it heavily depends on cardiac biomarkers. Cardiac troponins I (cTnI) and T (cTnT) are the biomarkers of choice for acute myocardial infarction (AMI)1 but are often nonspecifically increased in CKD patients.2
Attempts have been made to increase the diagnostic utility of cardiac troponin (cTn) in CKD. The loss of specificity in CKD patients can be compensated for by increasing the diagnostic threshold.3, 4 However, this always comes at the cost of decreased sensitivity, that is, a greater risk of missing the diagnosis. Given the progressive nature of myocardial damage in acute infarction, dynamic changes in cTn levels may be more informative, specifically in patients with potential chronic cTn elevation. Serial cTn testing is indeed recommended in evaluation of suspected NSTE‐AMI, but dedicated algorithms for CKD patients are lacking.1
In the current study we hypothesized that the performance of hs‐cTnI and hs‐cTnT to diagnose NSTE‐AMI is reduced when CKD is present and that dynamic changes may outperform static cutoffs for the diagnosis of NSTE‐AMI in CKD patients. We propose an algorithm incorporating changes in hs‐cTn levels that increases the diagnostic confidence in CKD patients.
Methods
Two cohorts with a total of more than 8500 patients were analyzed in this study. The first cohort came from a prospective multicenter biomarker study, stenoCardia (study data set NCT03227159).5 The second data set was a retrospective cohort of patients with potential AMI in a university hospital (clinical data set). The first and corresponding authors had full access to all the data in the study and take responsibility for its integrity and data analysis. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Data Set
Details of the prospective study data set have been published previously.5, 6 Briefly, between January 2007 and December 2008, the stenoCardia study enrolled adult patients who presented with acute chest pain to 1 of 3 German study centers. The study was approved by institutional review boards, and all patients provided informed consent. Primary exclusion criteria included major surgery or trauma in the previous 4 weeks, pregnancy, intravenous drug abuse, and anemia (hemoglobin <10 g/dL). Dialysis patients were not eligible to participate. A total of 1818 patients with suspected AMI were eligible for the current analysis. Estimated glomerular filtration rate (eGFR) could not be assessed in 9 patients, and 211 patients did not have hs‐cTnI measured; 104 patients with ST‐segment elevation were excluded because we were interested in those cases with suspected NSTE‐AMI (Figure 1A). The final diagnosis of AMI was adjudicated by 2 independent cardiologists, based on a complete chart review as described earlier.5, 6 Myocardial infarction was assumed when there was at least 1 abnormal conventional troponin measurement in conjunction with a rise or fall of 20% or more in the first 6 hours, together with clinical symptoms of ischemia or ECG changes consistent with new ischemia (new ST‐segment or T‐wave changes or new left bundle‐branch block) or imaging evidence of new loss of viable myocardium or detection of a culprit lesion on coronary angiography.
Figure 1.
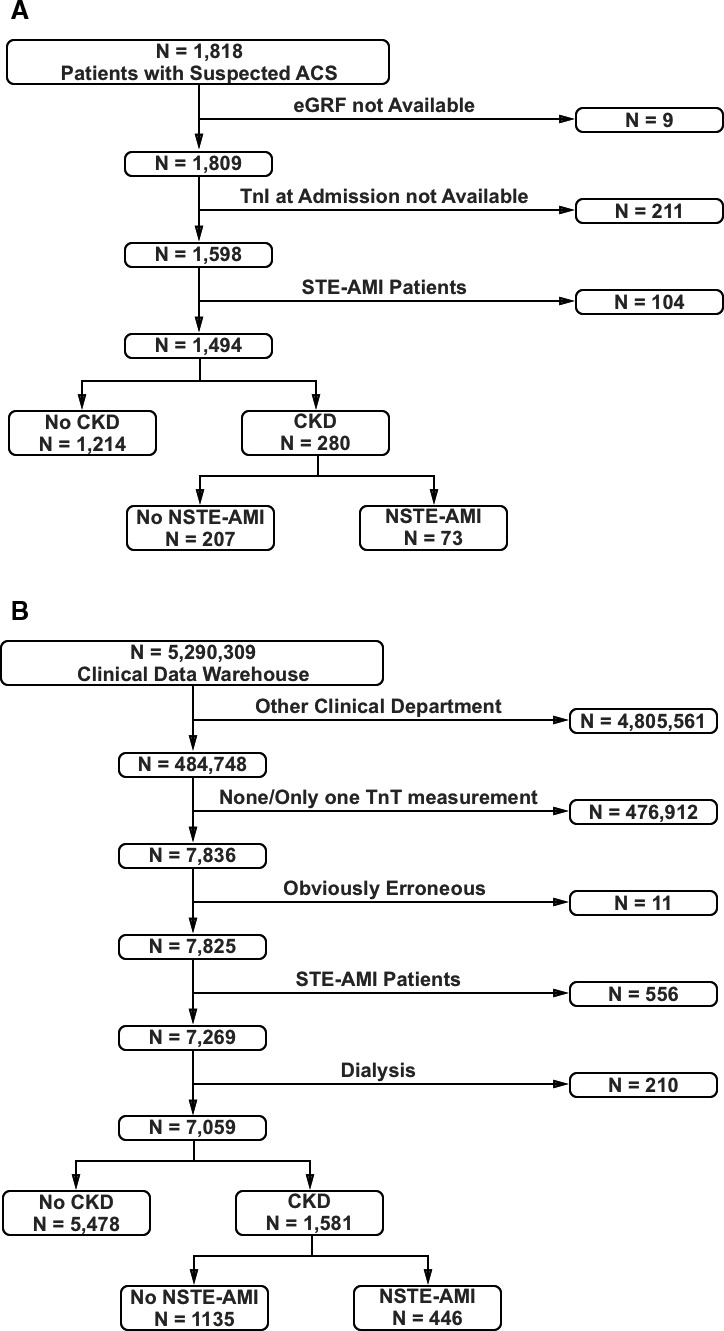
Flow charts for the inclusion of study subjects. Flow charts for subjects from (A) the prospective study cohort and (B) the clinical registry. ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; CKD, chronic kidney disease; NSTE, non–ST‐segment elevation; STE, ST‐segment elevation; TnI, troponin I; TnT, troponin T.
Clinical Data Set
The clinical data set was obtained retrospectively from the University of Würzburg's Clinical Data Warehouse, which collects all inpatient and outpatient cases in a deidentified fashion.7 The Data Warehouse continuously acquires all patient‐related data for all inpatient and outpatient cases and provides tools to retrospectively query the data, including algorithms that employ artificial intelligence to extract information from texts such as reports and discharge letters.7 The Data Warehouse is approved by the Ethics Committee of Würzburg University, and patients have given written informed consent.
The Data Warehouse was queried for all cases in the 1st Medical Department (Cardiology Department) with at least 2 measurements of hs‐cTnT between May 2011 and January 2018. Per local policy, hs‐cTnT is measured only if there is a clinically suspected AMI.
Out of 7836 cases in the initial query, 11 were disregarded due to obviously erroneous data, 556 patients due to ST‐segment elevation AMI, and we excluded 210 dialysis patients (Figure 1B). Adjudication of the clinical diagnosis of AMI was based on the final judgments of the attending physicians as stated in the patient discharge letter, which incorporated all available data over the course of the patient's treatment and overall assessment, and which determines subsequent medical care. Comorbidities such as hypertension and diabetes mellitus were based on the final discharge letters.
Blood Sampling and Laboratory Methods
In the study data set, blood was drawn directly on presentation and 3 hours after admission. The investigational hs‐cTnI was determined in thawed samples using a troponin I assay (ARCHITECT STAT hs Troponin I, Abbott Diagnostics, Abbott Park, IL) with an assay range of 0 to 50 000 ng/L, a limit of detection of 1.9 ng/L, and a 99th‐percentile diagnostic cutoff of 30 ng/L.6
The hs‐cTnT assay (Elecsys, Roche Diagnostics, Risch‐Rotkreuz, Switzerland) that was used in the clinical data set has an assay range of 3 to 10 000 ng/L and a 99th‐percentile diagnostic cutoff of 14 ng/L.
Creatinine in both cohorts was measured enzymatically in the respective central laboratories. The eGFR was computed with the CKD Epidemiology Collaboration equation.
Statistical Analyses
Variables with normal distribution were characterized by arithmetic mean and SD; skewed variables were described by median and interquartile range.
Changes in serial cTn measurements were analyzed in 2 ways, either by selecting only those cases with an increase or by using the absolute (unsigned) difference. The former reflects clinical practice, where AMI in acutely symptomatic patients is suspected only if there is a concomitant increase in cTn levels; the latter satisfies the criterion of a rise or fall that is proposed in the guidelines to accommodate situations in which the patient presents some time after the acute event.1
Sensitivity, specificity, positive (PPV) and negative predictive values, and positive and negative likelihood ratios were computed from a 2×2 table in the usual way with 95% confidence limits for binomially distributed variables using the epiR library in R. The statistical significance of the difference between diagnostic tests was determined using the McNemar test for paired samples (DTComPair package for R), and 2×2 contingency tables with chi‐squared tests for the sensitivities and specificities of unpaired samples.
To investigate the diagnostic performance of cTn in patients with CKD, the area under the receiver‐operator characteristic curve was derived in patients with and without CKD. Confidence intervals were computed from estimated respective covariance matrices.8 In the study data set, optimized cutoffs for CKD patients were defined so that their specificity was the same specificity as the 99th‐percentile cutoffs in the non‐CKD population. In the clinical data set, an upper gray‐zone limit of the assay of 50 ng/mL9 was used as optimized cutoff. Specificity‐optimized change thresholds were computed based on the Youden index.
For subsequent analyses we selected the subgroups of patients from both cohorts who had an increase in hs‐cTn levels, and we performed additional analyses with the absolute (unsigned) changes, which include both increases and decreases in troponin levels.
Youden‐optimized change thresholds were computed for the absolute and for the relative increases of the serial troponin measurements in CKD patients. After optimization, we divided our data sets into patients with initial troponin below or above the 99th percentile.
To develop diagnostic algorithms that incorporate dynamic changes in hs‐cTn levels, we computed sensitivities and specificities for optimized hs‐cTn cutoffs at baseline alone as well as for the combination of normal or abnormal baseline and second measurements. The overall diagnostic performance of these algorithms is expressed as the percentage of patients for whom AMI can be ruled in or out if these algorithms are applied.
P values less than 0.05 were considered significant. All analyses were carried out using R, versions 3.1.1 or 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Data Set Characteristics
In the study data set, 19% of patients had impaired renal function (eGFR <60 mL/min/1.73 m2). AMI was diagnosed in 15% of non‐CKD patients and in 26% of CKD patients (P<0.001). Causes of noncoronary chest pain included pulmonary embolism, acute decompensated heart failure, myocarditis, aortic dissection, and aortic valve stenosis. A second hs‐cTnI measurement was available in 1385 patients (1139 without and 246 with CKD).
In the clinical data set, 22% of patients had impaired renal function (eGFR <60 mL/min/1.73 m2). In both data sets the majority of CKD patients presented with CKD stage 3 (88% in the study data set and 84% in the clinical data set [Tables S1 and S2]).
The mean eGFR in CKD patients was 46 mL/min/1.73 m2 in the study dataset and 44 mL/min/1.73 m2 in the clinical data set. CKD patients were older than non‐CKD patients, had a higher prevalence of hypertension and diabetes mellitus and a higher risk of NSTE‐AMI (P<0.001 for all) (Tables 1 and 2). CKD patients were less likely to receive percutaneous coronary interventions, even when NSTE‐AMI was diagnosed (Tables S3 and S4).
Table 1.
Study Data Set Characteristics
| Characteristics | All Patients | Patients Without CKD | Patients With CKD | P Value |
|---|---|---|---|---|
| N | 1494 | 1214 (81.3%) | 280 (18.7%) | |
| Age (y), mean (SD) | 61 (14) | 59 (13) | 72 (9) | <0.001 |
| Female sex, x/n (%) | 499/1494 (33%) | 380/1214 (31%) | 119/280 (42%) | <0.001 |
| NSTE‐AMI, x/n (%) | 251/1494 (17%) | 178/1214 (15%) | 73/280 (26%) | <0.001 |
| Laboratory results | ||||
| eGFR (mL/[min·1.73 m²]), mean (SD) | 78 (21) | 85 (14) | 46 (12) | <0.001 |
| Initial hs‐cTnI (ng/L), median (IQR) | 6.5 (3.3, 25.6) | 5.6 (3, 18.2) | 14.7 (6.5, 76.9) | <0.001 |
| Second hs‐cTnI (ng/L), median (IQR) | 9.2 (5.6, 32) | 8.2 (5.5, 24.7) | 17.3 (8.6, 100.1) | <0.001 |
| Cardiovascular risk factor | ||||
| Hypertension, x/n (%) | 1103/1494 (74%) | 833/1214 (69%) | 270/280 (96%) | <0.001 |
| Diabetes mellitus, x/n (%) | 291/1494 (19%) | 197/1214 (16%) | 94/280 (34%) | <0.001 |
| Active smoker, x/n (%) | 349/1493 (23%) | 323/1214 (27%) | 26/279 (9%) | <0.001 |
| Former smoker, x/n (%) | 471/1432 (33%) | 365/1156 (32%) | 106/276 (38%) | 0.036 |
| Hyperlipidemia, x/n (%) | 1101/1494 (74%) | 870/1214 (72%) | 231/280 (82%) | <0.001 |
| Known CAD, x/n (%) | 573/1493 (38%) | 406/1213 (33%) | 167/280 (60%) | <0.001 |
| History of myocardial infarction, x/n (%) | 361/1491 (24%) | 254/1213 (21%) | 107/278 (38%) | <0.001 |
| Family history of CAD, x/n (%) | 533/1492 (36%) | 455/1213 (38%) | 78/279 (28%) | 0.003 |
| Obesity, x/n (%) | 394/1384 (28%) | 319/1124 (28%) | 75/260 (29%) | 0.9413 |
Baseline characteristics of the study data set. Data are presented as cases/number (percentage), mean (SD), or median (IQR), as indicated. CAD indicates coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; hs‐cTnI, high‐sensitive cardiac troponin I; IQR, interquartile range; NSTE‐AMI, non–ST‐segment elevation myocardial infarction.
Table 2.
Clinical Data Set Characteristics
| Characteristics | All Patients | Patients Without CKD | Patients With CKD | P Value |
|---|---|---|---|---|
| N | 7059 | 5478 (78%) | 1581 (22%) | |
| Age (y), mean (SD) | 66.3 (15.7) | 63.1 (15.7) | 77.3 (9.7) | <0.001 |
| Female sex, x/n (%) | 2580 (37%) | 1884 (34%) | 696 (44%) | <0.001 |
| NSTE‐AMI, x/n (%) | 1375/7059 (19%) | 929/5478 (17%) | 446/1581 (28%) | <0.001 |
| Laboratory results | ||||
| eGFR (mL/[min·1.73 m2]), mean (SD) | 67.5 (26.6) | 73.5 (25.2) | 46.4 (19.4) | <0.001 |
| Initial hs‐cTnT (ng/L), median (IQR) | 12.4 (5.3, 32.9) | 9.2 (5.0, 23.5) | 29.7 (16.1, 66.6) | <0.001 |
| Second hs‐cTnT (ng/L), median (IQR) | 13.3 (5.2, 43.6) | 9.4 (5.0, 28.0) | 36.4 (17.3, 106.6) | <0.001 |
| Cardiovascular risk factor | ||||
| Hypertension, x/n (%) | 4675/7059 (66%) | 3298/5478 (60%) | 1377/1581 (87%) | <0.001 |
| Diabetes mellitus, x/n (%) | 1490/7059 (21%) | 889/5478 (16%) | 601/1581 (38%) | <0.001 |
Baseline characteristics of the clinical data set. Data presented as cases/number (percentage), mean (SD), or median (IQR), as indicated. CKD indicates chronic kidney disease; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; IQR, interquartile range; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Diagnostic Performance of a Single Troponin Measurement
Initial cTn levels were higher in CKD patients than in non‐CKD patients, and they correlated inversely with eGFR except in the subset of patients with hs‐cTnI measurements and a final diagnosis of NSTE‐AMI (Tables 1 and 2, Figure S1).
The area under the receiver‐operator characteristic curve for the initial hs‐cTn level was slightly lower in CKD patients than in non‐CKD patients when the hs‐cTnI assay was used and considerably lower when the hs‐cTnT assay was used (Figure 2). Accordingly, the 99th‐percentile cutoffs that serve to clinically rule in or rule out NSTE‐AMI were less specific in CKD patients with both assays but especially with the hs‐cTnT assay (Table 3). Increasing the diagnostic cutoff of a single troponin measurement so that its specificity matches that of the 99th‐percentile cutoff in non‐CKD patients entails a loss in sensitivity (Table 3, Figure S2), which will result in missing patients with NSTE‐AMI.
Figure 2.
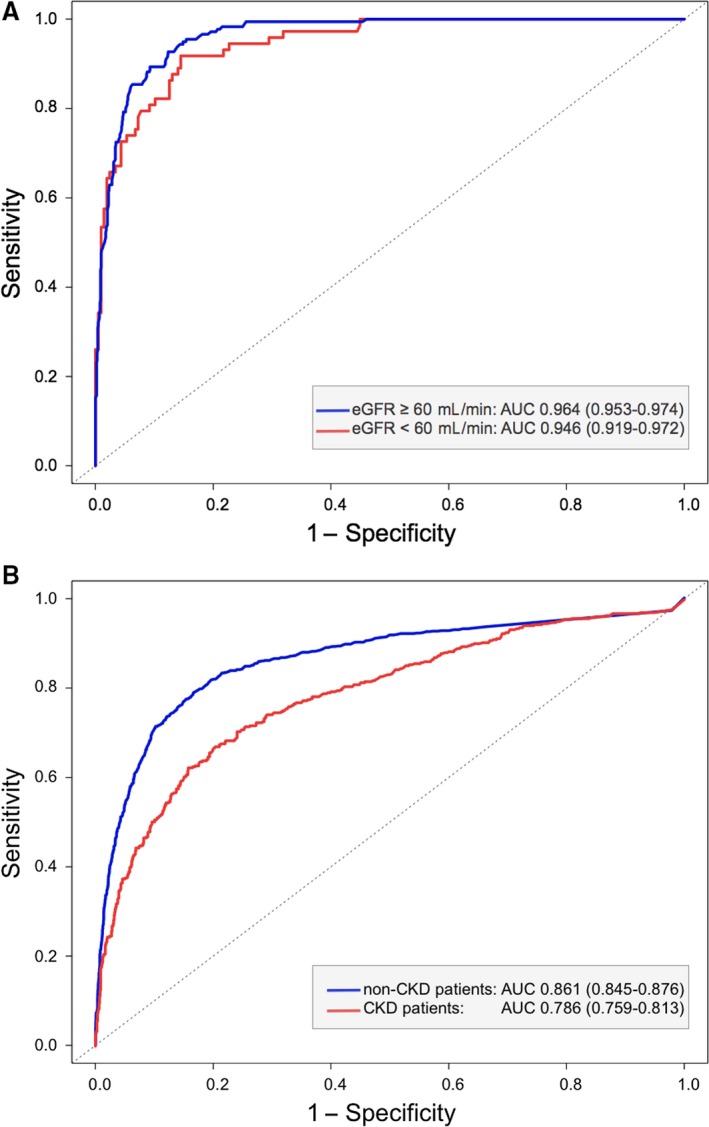
Receiver operator characteristics of initial troponin measurements for the diagnosis of NSTE‐AMI in patients with or without CKD. Receiver operating characteristic analysis in patients with acute chest pain for identification of an acute myocardial infarction by (A) hs‐cTnI and (B) hs‐cTnT levels on admission. AUC indicates area under the concentration‐time curve; CKD, chronic kidney disease; cTnI, cardiac troponin I; cTnT, cardiac troponin T; eGFR, estimated glomerular filtration rate; hs, high‐sensitivity; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Table 3.
Diagnostic Performances of Conventional and Optimized Cutoffs for Initial Troponin Measurements
| Cutoff | Sensitivity | Specificity | LR+ | LR− | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| hs‐cTnI | |||||||
| No CKD | 30 ng/L | 0.88 (0.82, 0.92) | 0.91 (0.89, 0.93) | 10.09 (8.22, 12.38) | 0.14 (0.09, 0.20) | 0.63 (0.57, 0.69) | 0.98 (0.97, 0.99) |
| CKD | 30 ng/L | 0.92 (0.83, 0.97) | 0.82 (0.76, 0.87) | 5.00 (3.72, 6.72) | 0.10 (0.05, 0.22) | 0.64 (0.54, 0.73) | 0.97 (0.93, 0.99) |
| 54.0 ng/L | 0.82 (0.71, 0.9) | 0.9a (0.85, 0.94) | 8.10 (5.33, 12.32) | 0.20 (0.12, 0.33) | 0.74 (0.63, 0.83) | 0.93 (0.89, 0.96) | |
| hs‐cTnT | |||||||
| No CKD | 14 ng/L | 0.85 (0.83, 0.88) | 0.73 (0.71, 0.74) | 3.11 (2.95, 3.29) | 0.2 (0.17, 0.24) | 0.39 (0.37, 0.41) | 0.96 (0.95, 0.97) |
| CKD | 14 ng/L | 0.94 (0.92, 0.96) | 0.26 (0.23, 0.29) | 1.27 (1.22, 1.33) | 0.22 (0.15, 0.33) | 0.33 (0.31, 0.36) | 0.92 (0.88, 0.95) |
| 50 ng/L | 0.66b (0.61, 0.7) | 0.8b (0.78, 0.83) | 3.34 (2.92, 3.82) | 0.42 (0.37, 0.48) | 0.57 (0.52, 0.61) | 0.82 (0.8, 0.84) | |
Study data set: n=1494 patients with suspected NSTE‐AMI; NSTE‐AMI in n=251 patients. Clinical data set: n=7059 patients with suspected NSTE‐AMI; NSTE‐AMI in n=1375 patients. CKD indicates chronic kidney disease; hs‐cTnI, high‐sensitivity troponin I; hs‐cTnT, high‐sensitivity troponin T; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction; PPV, positive predictive value.
This was the condition for which the optimized threshold was derived.
P<0.001 compared with the 99th‐percentile cutoff.
Troponin levels are usually stable in a given individual without acute (myocardial) disease, even with impaired renal function.10 Therefore, with acute NSTE‐AMI and rising cTn levels, a second measurement may discriminate better between infarction and other causes of chest discomfort. However, in CKD patients, neither the conventional nor the optimized cutoffs were more specific when applied to the second measurement as opposed to the first measurement (Table S5, Figure S3).
The European Society of Cardiology recently proposed raising the diagnostic cutoff to 5‐fold above the 99th percentiles (hs‐cTnI, 150 ng/L; hs‐cTnT, 70 ng/L).1 With the sensitivity of the 99th percentile in the non‐CKD population taken as a benchmark, this very high cutoff does not compromise the sensitivity of the hs‐cTnI assay (Table 3, Table S5). However, the sensitivity of the hs‐cTnT assay using the 70 ng/L cutoff was considerably lower (Table S5). Thus, applying a 5 times 99th percentile cutoff to a second measurement improved the diagnostic performance of the hs‐cTnI assay but not of the hs‐cTnT assay in the CKD population.
Diagnostic Performance of Changes in Troponin Levels
We derived optimized cutoffs for absolute changes (differences) and relative changes (ratios) that were incorporated into diagnostic algorithms for hs‐cTnI and hs‐cTnT (Figure 3, Table 4, Tables S6 through S8, Figures S4 and S5):
With a normal cTn level at presentation that does not increase more than 2.8‐fold (hs‐cTnI) or 2.5‐fold (hs‐cTnT), acute infarction in a CKD patient with chest discomfort can be ruled out safely. The negative predictive value is 0.98 for both hs‐cTnI and hs‐cTnT (Table 4). With a higher increase after 3 hours, the specificity and PPV are 0.90 and 0.20 for hs‐cTnI and 0.84 and 0.42 for hs‐cTnT. In this situation the patient should be observed.
If the initial hs‐cTn level is abnormal (above the 99th percentile) and increases more than 2.8‐fold (hs‐cTnI) or 2.5‐fold (hs‐cTnT) after 3 hours, AMI may be ruled in (PPVs 0.78 for hs‐cTnI and 0.8 for hs‐cTnT) (Table 4). Of note, these are considerably higher than the PPVs of the conventional 99th percentiles in the non‐CKD population (0.63 for hs‐cTnI and 0.39 for hs‐cTnT; Table 3), which are commonly accepted as high enough to warrant angiography. If the increases after 3 hours do not exceed the fold‐change cutoffs, the patient should be observed.
If the initial hs‐cTn level is very high (above the optimized static cutoffs [54 ng/L for hs‐cTnI and 50 ng/L for hs‐cTnT]), the PPVs are 0.74 (hs‐cTnI) and 0.57 (hs‐cTnT). Again, these are considerably higher than the PPVs for the 99th percentiles in the non‐CKD population. Thus, protocol might dictate not waiting for a second troponin measurement in these situations.
Figure 3.
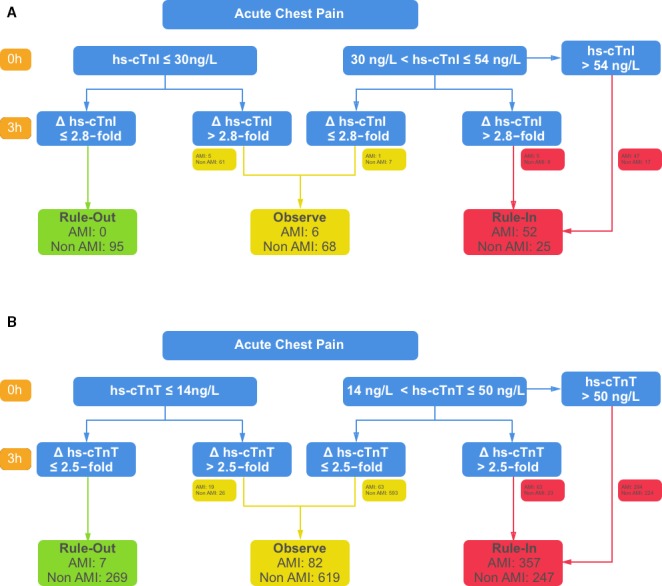
Algorithms for the diagnosis of NSTE‐AMI in patients with CKD. Diagnostic algorithms for patients with CKD, suspected myocardial infarction, and nonspecific ECGs with the (A) hs‐cTnI and (B) hs‐cTnT assays. Numbers indicate how many patients were affected in the corresponding cohorts. AMI indicates acute myocardial infarction; CKD, chronic kidney disease; Δ, change; ECG, electrocardiogram; hs‐cTnI and hs‐cTnT, high‐sensitivity cardiac troponin I and T, respectively; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Table 4.
Diagnostic Performance of Changes in Troponin Levels in CKD Patients
| Change Cutoff | Sensitivity | Specificity | LR+ | LR− | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| hs‐cTnI | |||||||
| Baseline ≤30 ng/L | 14.5 ng/L | 0.60 (0.15, 0.95) | 0.94 (0.89, 0.98) | 10.8 (3.91, 29.8) | 0.42 (0.14, 1.24) | 0.3 (0.07, 0.65) | 0.98 (0.94, 1) |
| 2.8‐fold | 0.60 (0.15, 0.95) | 0.90 (0.84, 0.95) | 6.3 (2.57, 15.43) | 0.44 (0.15, 1.3) | 0.2 (0.04, 0.48) | 0.98 (0.94, 1) | |
| Baseline >30 ng/L | 14.5 ng/L | 0.95 (0.83, 0.99) | 0.50 (0.25, 0.75) | 1.9 (1.16, 3.11) | 0.1 (0.02, 0.43) | 0.82 (0.68, 0.92) | 0.8 (0.44, 0.97) |
| 2.8‐fold | 0.41 (0.26, 0.58) | 0.88 (0.62, 0.98) | 3.28 (0.85, 12.66) | 0.67 (0.49, 0.93) | 0.89 (0.65, 0.99) | 0.38 (0.22, 0.55) | |
| hs‐cTnT | |||||||
| Baseline ≤14 ng/L | 36.0 ng/L | 0.55 (0.32, 0.76) | 0.92 (0.87, 0.96) | 7.27 (3.74, 14.14) | 0.49 (0.31, 0.78) | 0.5 (0.29, 0.71) | 0.94 (0.89, 0.97) |
| 2.5‐fold | 0.86 (0.65, 0.97) | 0.84 (0.77, 0.89) | 5.31 (3.6, 7.84) | 0.16 (0.06, 0.47) | 0.42 (0.28, 0.58) | 0.98 (0.94, 1.0) | |
| Baseline >14 ng/L | 36.0 ng/L | 0.7 (0.64, 0.75) | 0.89 (0.86, 0.92) | 6.37 (4.82, 8.43) | 0.34 (0.29, 0.4) | 0.82 (0.77, 0.87) | 0.8 (0.76, 0.84) |
| 2.5‐fold | 0.41 (0.36, 0.47) | 0.92 (0.89, 0.95) | 5.37 (3.77, 7.64) | 0.64 (0.58, 0.7) | 0.8 (0.73, 0.86) | 0.68 (0.64, 0.72) | |
Study data set: n=186 patients with CKD and increase in hs‐cTnI; NSTE‐AMI in n=44 patients. Clinical data set: n=926 patients with CKD and increase in hs‐cTnT; NSTE‐AMI in n=337 patients. CKD indicates chronic kidney disease; hs‐cTnI, high‐sensitivity troponin I; hs‐cTnT, high‐sensitivity troponin T; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction; PPV, positive predictive value.
Performance parameters for both algorithms, excluding the “observation” groups, are given in Table 5.
Table 5.
Diagnostic Performance of the Proposed Algorithms
| Algorithm | hs‐cTnI | hs‐cTnT | ||
|---|---|---|---|---|
| Rule In | Rule Out | Rule In | Rule Out | |
| Sensitivity | 0.90a (0.79, 0.96) | 1.00a (0.94, 1.00) | 0.80a (0.76, 0.84) | 0.98a (0.97, 0.99) |
| Specificity | 0.87a (0.81, 0.91) | 0.51a (0.43, 0.58) | 0.78a (0.76, 0.81) | 0.24a (0.21, 0.26) |
| PPV | 0.68 (0.56, 0.78) | 0.38 (0.31, 0.47) | 0.59 (0.55, 0.63) | 0.34 (0.31, 0.36) |
| NPV | 0.96 (0.92, 0.99) | 1.00 (0.96, 1.00) | 0.91 (0.89, 0.93) | 0.97 (0.95, 0.99) |
| LR+ | 6.74 (4.63, 9.81) | 2.02 (1.75, 2.34) | 3.68 (3.26, 4.15) | 1.29 (1.25, 1.34) |
| LR− | 0.12 (0.06, 0.26) | 0.00 (0.00, NaN) | 0.26 (0.21, 0.31) | 0.07 (0.03, 0.14) |
For the rule‐in approach, patients in the “observe” category were counted as having a negative test result; for the rule‐out approach, patients in the “observe” category were counted as having a positive test result (cf. Figure 3). Values in parentheses indicate the 95% confidence intervals. Study data set: n=172 patients with CKD and suspected NSTE‐AMI; NSTE‐AMI in n=52 patients. Clinical data set: n=880 patients with CKD and suspected NSTE‐AMI; NSTE‐AMI in n=364 patients. CKD indicates chronic kidney disease; hs‐cTnI, high‐sensitivity troponin I; hs‐cTnT, high‐sensitivity troponin T; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NaN, not a number; NPV, negative predictive value; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction; PPV, positive predictive value.
P<0.001 by McNemar test compared with optimized static cutoff (54 ng/L for hs‐cTnI, 50 ng/L for hs‐cTnT); McNemar test asserts significant differences between sensitivities and specificities only.
Discussion
Patients with CKD are at high risk for AMI with atypical symptoms and without ST‐segment elevation. Diagnosis of NSTE‐AMI in these patients strongly depends on cTn levels,1 which are often chronically elevated even in the absence of acute infarction.11 For fear of potentially causing harm when NSTE‐AMI is misdiagnosed as a false positive, CKD patients often do not receive optimal care for AMI including antithrombotic agents and coronary angiography.2 Making the correct diagnosis is especially important in CKD patients. Diagnostic cutoffs that are derived from healthy reference populations are not suitable for clinical decision making in CKD patients. Here we show that changes in serial troponin measurements make it possible to reach the correct diagnosis in the majority of CKD patients.
Our proposed algorithms outperform the conventional 99th‐percentile cutoffs when applied to CKD patients. The hs‐cTnT algorithm yields higher positive as well as negative predictive values than the static cutoff, and the hs‐cTnI algorithm yields a sensitivity of 100%.
The idea to use changes in troponin levels to make the diagnosis in patients with CKD is not new.12, 13 However, the published recommendations for change‐based cutoffs for patients with CKD are not based on evidence.1, 14 Here we propose a data‐driven decision‐making algorithm for serial measurements in patients with CKD (Figure 3). This approach defines 3 patient groups: rule in, rule out, and observe. In our study data set, this algorithm leads to a high diagnostic accuracy in 69% (39% rule in and 30% rule out) of patients if hs‐cTnI is used. Using the hs‐cTnT assay in the clinical data set is also associated with high diagnostic accuracy in 55% (17% rule in and 39% rule out) of patients. In the remaining 35% and 41%, further observation and diagnostic workup are warranted. In addition to clinical assessment, this may include imaging techniques that are not nephrotoxic, such as echocardiography or single‐photon emission computed tomography and possibly coronary angiography after renoprotective treatment. Despite our improved algorithm, patients with CKD and suspected NSTE‐AMI represent high‐risk, complex patients, and a decision for or against treating an NSTE‐AMI in such a patient must carefully balance the individual risk and benefit.
Previous attempts to optimize the diagnostic performance of troponins in patients with CKD have proposed adjusted higher static cutoffs at the cost of decreased sensitivity,3, 4 have analyzed small numbers of CKD patients,4, 15, 16 or have used non–high‐sensitivity assays16 and elected not to provide performance estimates.15 One recent retrospective study proposed a graded cutoff system for CKD stages G3 to G5. This study also included patients with STE‐AMI in whom the decision for immediate invasive imaging is primarily based on the pathognomonic ECG changes.17
Our study incorporates a large number of CKD patients from 2 cohorts with high‐sensitivity cTn measurements and arrives at comparable results for both cohorts despite the use of different hs‐cTn assays. Nonetheless, our study has several limitations.
A critical issue is the adjudication of the final diagnosis. In the prospective study cohort, 2 independent cardiologists adjudicated the final diagnosis based on a complete review of clinical, laboratory, and imaging findings.5 In the retrospective data set from the Clinical Data Warehouse, the final diagnosis was made by the physician who discharged the patient. This final clinical diagnosis was based on the entire course of the treatment and includes laboratory, other diagnostic examinations, and the overall impression and informs subsequent medical care. Still, CKD patients with NSTE‐AMI for whom the diagnosis was ruled out despite elevated troponin levels might be misclassified in the retrospective data set. However, it should be noted that the proportion of patients with CKD who were diagnosed with NSTE‐AMI is almost the same in the prospective study cohort as in the much larger retrospective registry cohort, lending credence to the validity of the latter. One might argue that the selection criteria for the registry cohort—2 measurements of troponin—may include patients with suspected diagnoses other than acute infarction. However, it is local policy to perform 2 measurements only when AMI is suspected, and the difference of 3.2 hours between the 2 hs‐cTnT measurements is very close to the guideline recommendations for suspected AMI that were applicable in the data collection period.18
The relative change cutoffs that we derived from our data sets, 280% for hs‐cTnI and 250% for hs‐cTnT, differ greatly from the 20% proposed earlier.12 However, this recommendation was not based on evidence.14, 19 Change cutoffs have been incorporated into diagnostic algorithms in the latest guideline of the European Society for Cardiology,1 but they lack specific recommendations for patients with CKD.
Change cutoffs have previously been optimized in the study cohort for the overall patient population irrespective of CKD.6 Maximum PPV was obtained with a change cutoff of 2.7‐fold,6 which is comparable to the 2.8‐fold derived for the CKD subgroup. It should be noted, however, that a much lower optimized change cutoff of 1.3‐fold within 6 hours using a non–high‐sensitive assay has also been proposed.20
For the hs‐cTnT assay and patients free of CKD, an optimized change cutoff of 1.17‐fold within 3 hours has been reported.21 This is considerably lower than the 2.5‐fold cutoff that we derived for the CKD patients in the registry cohort. The difference may be explained by the greater intraindividual variability of troponin levels in patients with impaired renal function.
Overall, the hs‐cTnI assay outperforms the hs‐cTnT assay in patients with CKD. The poor performance of the hs‐cTnT assay with impaired renal function has been noted previously.3, 22
In our study, initial creatinine was used to compute eGFR. However, initial eGFR is not the same as chronic, stable eGFR. Acute renal failure is not uncommon in acute cardiovascular disease and may be triggered by acute heart failure in the context of AMI. Not much is known if and how acute kidney injury affects hs‐cTn levels and what the time course might be. Still, nearly all published studies in the context of AMI used initial creatinine values to estimate eGFR and therefore are prone to a potential bias. Further, our results might not be transferable to dialysis patients.
Finally, our diagnostic algorithm was applied in the same cohorts that were used for derivation. To prove its applicability, safety, and robustness, it needs to be validated prospectively in independent cohorts.
Conclusions
To summarize, the diagnostic performances of hs‐cTnI and hs‐cTnT for the detection of AMI are compromised in patients with impaired renal function. This can be compensated for by increasing the diagnostic cutoff levels, but it comes at the cost of decreased sensitivity, that is, missing potential cases of AMI. Given the intraindividual stability of troponin levels even if CKD is present, changes in hs‐cTn concentrations can improve diagnostic information. We propose a diagnostic algorithm that incorporates absolute hs‐cTn levels and changes in serial measurements. This algorithm yields high diagnostic certainty in a large proportion of patients with CKD and suspected AMI. In our opinion this is a significant step forward for both clinicians and patients asking themselves: Is this myocardial infarction?
Sources of Funding
The current study did not receive dedicated funding. The initial stenoCardia study was supported by unrestricted grants of Johannes Gutenberg University of Mainz research programs “Wissen schafft Zukunft” and “Schwerpunkt Vaskuläre Prävention” as well as the BRAHMS AG Germany. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
Lackner has a modest relationship with Abbott Diagnostics (honoraria recipient, member of the Advisory Board). The other authors have nothing to disclose.
Supporting information
Table S1. Characteristics of the Study Data Set, Stratified by CKD Stage
Table S2. Characteristics of the Clinical Data Set, Stratified by CKD Stage
Table S3. Number of Patients Undergoing Percutaneous Intervention or Coronary Bypass Grafting in the Study Data Set
Table S4. Number of Patients Undergoing Percutaneous Intervention or Coronary Bypass Grafting in the Clinical Data Set
Table S5. Diagnostic Performances of Conventional and Optimized Cutoffs for Second Troponin Measurements in Patients With CKD
Table S6. Changes in Serial Troponin Levels
Table S7. Absolute (Unsigned) Changes in Troponin Levels
Table S8. Diagnostic Performance of Absolute (Unsigned) Changes in Troponin Levels in Patients With CKD
Figure S1. Initial troponin levels.
Figure S2. Sensitivity and specificity of initial troponins for the diagnosis of NSTE‐AMI. NSTE‐AMI indicates non–ST‐segment elevation acute myocardial infarction.
Figure S3. Second troponin measurements in patients with or without CKD and with or without NSTE‐AMI. CKD indicates chronic kidney disease; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Figure S4. Serial differences in troponin levels in patients with or without CKD and with or without NSTE‐AMI. CKD indicates chronic kidney disease; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Figure S5. Serial changes in troponin levels in patients with or without CKD and with or without NSTE‐AMI. CKD indicates chronic kidney disease; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Acknowledgments
The authors gratefully acknowledge Bettina J. Kraus, MD for her competent proof reading.
(J Am Heart Assoc. 2018;7:e008032 DOI: 10.1161/JAHA.117.008032.)
References
- 1. Roffi M, Patrono C, Collet J‐P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GYH, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J;. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 2. Wong JA, Goodman SG, Yan RT, Wald R, Bagnall AJ, Welsh RC, Wong GC, Kornder J, Eagle KA, Steg PG, Yan AT; Canadian Acute Coronary Syndromes I and II, and Canadian Global Registry of Acute Coronary Events (GRACE/GRACE) Investigators . Temporal management patterns and outcomes of non‐ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J. 2009;30:549–557. [DOI] [PubMed] [Google Scholar]
- 3. Twerenbold R, Wildi K, Jaeger C, Gimenez MR, Reiter M, Reichlin T, Walukiewicz A, Gugala M, Krivoshei L, Marti N, Moreno Weidmann Z, Hillinger P, Puelacher C, Rentsch K, Honegger U, Schumacher C, Zurbriggen F, Freese M, Stelzig C, Campodarve I, Bassetti S, Osswald S, Mueller C. Optimal cutoff levels of more sensitive cardiac troponin assays for the early diagnosis of myocardial infarction in patients with renal dysfunction. Circulation. 2015;131:2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chenevier‐Gobeaux C, Meune C, Freund Y, Wahbi K, Claessens Y‐E, Doumenc B, Zuily S, Riou B, Ray P. Influence of age and renal function on high‐sensitivity cardiac troponin T diagnostic accuracy for the diagnosis of acute myocardial infarction. Am J Cardiol. 2013;111:1701–1707. [DOI] [PubMed] [Google Scholar]
- 5. Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth‐Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–877. [DOI] [PubMed] [Google Scholar]
- 6. Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth‐Zotz S, Warnholtz A, Giannitsis E, Möckel M, Bickel C, Peetz D, Lackner K, Baldus S, Münzel T, Blankenberg S. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. [DOI] [PubMed] [Google Scholar]
- 7. Fette G, Ertl M, Wörner A, Klügl P, Störk S, Puppe F. Information extraction from unstructured electronic health records and integration into a data warehouse In: Goltz U, Magnor MA, Appelrath H‐J, Matthies HK, Balke W‐T, Wolf LC, eds. Informatik 2012. GI: Braunschweig, Germany; 2012:1237–1251. Available at: https://subs.emis.de/LNI/Proceedings/Proceedings208/P-208.pdf. [Google Scholar]
- 8. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 9. Stengaard C, Sørensen JT, Ladefoged SA, Christensen EF, Lassen JF, Bøtker HE, Terkelsen CJ, Thygesen K. Quantitative point‐of‐care troponin T measurement for diagnosis and prognosis in patients with a suspected acute myocardial infarction. Am J Cardiol. 2013;112:1361–1366. [DOI] [PubMed] [Google Scholar]
- 10. Jacobs LH, van de Kerkhof J, Mingels AM, Kleijnen VW, van der Sande FM, Wodzig WK, Kooman JP, van Dieijen‐Visser MP. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem. 2009;46:283–290. [DOI] [PubMed] [Google Scholar]
- 11. Jaffe AS. Chasing troponin: how low can you go if you can see the rise? J Am Coll Cardiol. 2006;48:1763–1764. [DOI] [PubMed] [Google Scholar]
- 12. NACB Writing Group , Wu AHB, Jaffe AS, Apple FS, Jesse RL, Francis GL, Morrow DA, Newby LK, Ravkilde J, Tang WHW, Christenson RH; NACB Committee , Cannon CP, Storrow AB. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B‐type natriuretic peptide or N‐terminal proB‐type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086–2096. [DOI] [PubMed] [Google Scholar]
- 13. Kanderian AS, Francis GS. Cardiac troponins and chronic kidney disease. Kidney Int. 2006;69:1112–1114. [DOI] [PubMed] [Google Scholar]
- 14. Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high‐sensitivity cardiac troponin T assay. Clin Chem. 2010;56:1086–1090. [DOI] [PubMed] [Google Scholar]
- 15. Vasudevan A, Singer AJ, DeFilippi C, Headden G, Schussler JM, Daniels LB, Reed M, Than MP, Birkhahn R, Smith SW, Barrett TW, Arnold W, Peacock WF, McCullough PA. Renal function and scaled troponin in patients presenting to the emergency department with symptoms of myocardial infarction. Am J Nephrol. 2017;45:304–309. [DOI] [PubMed] [Google Scholar]
- 16. Han JH, Lindsell CJ, Ryan RJ, Gibler WB. Changes in cardiac troponin T measurements are associated with adverse cardiac events in patients with chronic kidney disease. Am J Emerg Med. 2005;23:468–473. [DOI] [PubMed] [Google Scholar]
- 17. Yang H, Liu J, Luo H, Zeng X, Tang X, Ma L, Mai H, Gou S, Liu F, Fu P. Improving the diagnostic accuracy of acute myocardial infarction with the use of high‐sensitive cardiac troponin T in different chronic kidney disease stages. Sci Rep. 2017;7:41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamm CW, Bassand J‐P, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D; ESC Committee for Practice Guidelines . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 19. Aakre KM, Sandberg S. Can changes in troponin results be useful in diagnosing myocardial infarction? Clin Chem. 2010;56:1047–1049. [DOI] [PubMed] [Google Scholar]
- 20. Apple FS, Pearce LA, Smith SW, Kaczmarek JM, Murakami MM. Role of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse events. Clin Chem. 2009;55:930–937. [DOI] [PubMed] [Google Scholar]
- 21. Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High‐sensitivity cardiac troponin T for early prediction of evolving non‐ST‐segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem. 2010;56:642–650. [DOI] [PubMed] [Google Scholar]
- 22. Pfortmueller CA, Funk G‐C, Marti G, Leichtle AB, Fiedler GM, Schwarz C, Exadaktylos AK, Lindner G. Diagnostic performance of high‐sensitive troponin T in patients with renal insufficiency. Am J Cardiol. 2013;112:1968–1972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the Study Data Set, Stratified by CKD Stage
Table S2. Characteristics of the Clinical Data Set, Stratified by CKD Stage
Table S3. Number of Patients Undergoing Percutaneous Intervention or Coronary Bypass Grafting in the Study Data Set
Table S4. Number of Patients Undergoing Percutaneous Intervention or Coronary Bypass Grafting in the Clinical Data Set
Table S5. Diagnostic Performances of Conventional and Optimized Cutoffs for Second Troponin Measurements in Patients With CKD
Table S6. Changes in Serial Troponin Levels
Table S7. Absolute (Unsigned) Changes in Troponin Levels
Table S8. Diagnostic Performance of Absolute (Unsigned) Changes in Troponin Levels in Patients With CKD
Figure S1. Initial troponin levels.
Figure S2. Sensitivity and specificity of initial troponins for the diagnosis of NSTE‐AMI. NSTE‐AMI indicates non–ST‐segment elevation acute myocardial infarction.
Figure S3. Second troponin measurements in patients with or without CKD and with or without NSTE‐AMI. CKD indicates chronic kidney disease; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Figure S4. Serial differences in troponin levels in patients with or without CKD and with or without NSTE‐AMI. CKD indicates chronic kidney disease; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.
Figure S5. Serial changes in troponin levels in patients with or without CKD and with or without NSTE‐AMI. CKD indicates chronic kidney disease; NSTE‐AMI, non–ST‐segment elevation acute myocardial infarction.


