Abstract
Background
During treatment with direct oral anticoagulants (DOAC), coagulation assessment is required before thrombolysis, surgery, and if anticoagulation reversal is evaluated. Limited data support the accuracy of DOAC‐specific coagulation assays around the current safe‐for‐treatment threshold of 30 ng/mL.
Methods and Results
In 481 samples obtained from 96 patients enrolled at a single center, DOAC concentrations were measured using Hemoclot direct thrombin inhibitor assay, Biophen direct thrombin inhibitor assay or ecarin clotting time for dabigatran, chromogenic anti‐Xa assay (AXA) for factor Xa inhibitors (rivaroxaban, apixaban) and ultraperformance liquid chromatography–tandem mass spectrometry as reference. All dabigatran‐specific assays had high sensitivity to concentrations >30 ng/mL, but specificity was lower for Hemoclot direct thrombin inhibitor assay (78.2%) than for Biophen direct thrombin inhibitor assay (98.9%) and ecarin clotting time (94.6%). AXA provided high sensitivity and specificity for rivaroxaban, but low sensitivity for apixaban (73.8%; concentrations up to 82 ng/mL were misclassified as <30 ng/mL). If no DOAC‐specific calibration for AXA is available, results 2‐fold above the upper limit of normal indicate relevant rivaroxaban concentrations. For apixaban, all elevated results should raise suspicion of relevant anticoagulation.
Conclusions
DOAC‐specific tests differ considerably in diagnostic performance for concentrations close to the currently accepted safe‐for‐treatment threshold. Compared with Biophen direct thrombin inhibitor assay and ecarin clotting time, limited specificity of Hemoclot direct thrombin inhibitor assay poses a high risk of unnecessary anticoagulation reversal or treatment delays in patients on dabigatran. While AXA accurately detected rivaroxaban, the impact of low apixaban levels on the assay was weak. Hence, AXA results need to be interpreted with extreme caution when used to assess hemostatic function in patients on apixaban.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifiers: NCT02371044, NCT02371070.
Keywords: anticoagulation reversal, coagulation testing, direct oral anticoagulants, emergency surgery, thrombolysis
Subject Categories: Diagnostic Testing, Anticoagulants, Embolism, Intracranial Hemorrhage, Ischemic Stroke
Clinical Perspective
What Is New?
Direct oral anticoagulant‐specific coagulation tests differ considerably in diagnostic performance when analyzing samples with low plasma concentrations.
False (low) test results might be critically misleading when selecting patients for surgical procedures, thrombolysis in acute ischemic stroke, or reversal therapy in case of bleeding.
What Are the Clinical Implications?
Emergency physicians are advised to know the direct oral anticoagulant–specific coagulation test that is used in their hospital's laboratory and its measuring accuracy around the then‐current safe‐for‐treatment threshold.
Introduction
Direct oral anticoagulants (DOAC) are an effective and easy‐to‐use treatment option for conditions that require long‐term anticoagulation. Although the fixed‐dose scheme omits the requirement of routine coagulation assessment, testing may be needed under certain clinical conditions, such as before surgery or invasive procedures, in case of trauma or bleeding, or if thrombolysis for acute ischemic stroke is evaluated.1, 2
Ultraperformance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS) remains the criterion standard for exact quantification of DOAC concentrations, but limited availability and slow turnaround times prevent routine clinical implementation of this method.3 Hence, if coagulation testing is deemed necessary, current clinical practice guidelines advise to use DOAC‐specific coagulation assays, ie, diluted thrombin time or ecarin clotting time (ECT) for dabigatran and chromogenic anti‐Xa assays (AXA) for factor Xa (FXa) inhibitors (rivaroxaban, apixaban, and edoxaban).4, 5, 6, 7 For all of these assays, quantitative assessment of DOAC concentrations can be achieved with the help of substance‐specific calibrators.7
A special situation arises for the FXa inhibitors: AXA are set up in many institutions but calibrations are mostly limited to the traditional indication of therapeutic drug monitoring during low‐molecular‐weight heparin therapy.8 If DOAC‐specific calibration is not available, different cut‐offs for AXA have been proposed, below which unimpaired coagulation can be expected (see Methods for details).1, 5, 9, 10, 11
Results of all DOAC‐specific assays provide a strong correlation to drug levels across a wide range of therapeutic and supratherapeutic plasma concentrations.7 However, little data support the validity of these assays for low DOAC concentrations around 30 ng/mL, the currently accepted safe‐for‐treatment threshold for invasive procedures, thrombolysis, and anticoagulation reversal.5, 12, 13, 14 Acknowledging this limitation, the need for a head‐to‐head evaluation of DOAC‐specific assays in real‐life patients using liquid chromatography–tandem mass spectrometry as reference has been voiced.4
Our study aimed to clarify the diagnostic accuracy of different DOAC‐specific coagulation assays for the discrimination of samples below and above the recommended safe‐for‐treatment threshold of 30 ng/mL using real‐life samples of patients treated with dabigatran, rivaroxaban, and apixaban.
Methods
Study Design
We studied samples obtained in 2 prospective observational trials with blinded outcome assessment (Clinical Trial Registration Information unique identifiers: NCT02371044 and NCT02371070).15, 16 Institutional Review Board approval was obtained from the ethics committee of Tübingen University Hospital (protocol‐no 259/2013BO1 and 270/2015B01). The studies were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all patients before enrollment. ME and SP had full access to all the data in the study and take responsibility for its integrity and the data analysis. The authors declare that all supporting data are available within the article and its online data supplements.
Setting and Eligibility Criteria
The studies were conducted at the Department of Neurology & Stroke and the Department of Cardiology and Cardiovascular Medicine of Tübingen University Hospital, a tertiary care facility between July 2013 and November 2015 and enrolled patients receiving first doses of apixaban, rivaroxaban, or dabigatran. Exclusion criteria were known coagulopathy, abnormal coagulation at baseline (Quick <70% or aPTT >37 seconds), intake of vitamin K antagonist or NOAC within 14 days, low‐molecular‐weight heparins within 24 hours or unfractionated heparin within 12 hours before DOAC intake. Use of antiplatelet agents was permitted. Predominantly low dabigatran concentrations in these samples required inclusion of additional samples from patients on maintenance therapy with dabigatran. The same exclusion criteria as above applied, except that abnormal coagulation at baseline (because of dabigatran intake) was allowed.
In total, we analyzed 481 samples acquired from 96 patients (dabigatran: N=34, 178 samples; rivaroxaban: N=32, 146 samples; apixaban: N=30, 157 samples).
Sample Collection and Coagulation Testing
A total of 6 blood samples were collected from each subject via a venous catheter or direct venipuncture at prespecified time points: before drug intake, 30 minutes, 1, 2, and 8 hours after intake, and at trough (12 hours for dabigatran and apixaban, 24 hours for rivaroxaban). Samples were collected in 3.2% sodium‐citrate tubes (Sarstedt, Nümbrecht, Germany), and instantly centrifuged to acquire plasma. We used Chromogenix COAMATIC Heparin AXA (Instrumentation Laboratory, Kirchheim, Germany) under routine conditions at the central laboratory, and TECHNOVIEW calibrators (Technoclone, Vienna, Austria) to determine FXa inhibitor concentrations. Dabigatran concentrations were determined from frozen (−80°C) and re‐thawed citrated plasma samples, using the Hemoclot direct thrombin inhibitor assay (HTI) and the chromogenic anti‐IIa Biophen direct thrombin inhibitor assay (BDTI) (both: Hyphen BioMed, Neuville‐sur‐Oise) on a Sysmex CS5100 (Siemens Healthcare Diagnostics, Eschborn, Germany) and ECT (Ecarin Clotting Assays; Stago, Asnières sur Seine, France) on a BCS XP (Siemens Healthcare Diagnostics, Eschborn, Germany).
As the criterion standard, DOAC concentrations were measured via UPLC‐MS/MS for all collected samples using frozen (−80°C) and rethawed citrated plasma as recently described.17 All tests were performed according to the respective manufacturers’ instructions by thoroughly trained investigators and technicians.
Blinding
All coagulation tests (AXA, HTI, anti‐IIa BDTI, and ECT) were conducted and interpreted by technicians blinded to UPLC‐MS/MS results. Technicians performing coagulation tests using frozen samples (HTI, BDTI, ECT) were additionally blinded for the time point samples were drawn and whether different samples belonged to the same patient. Fully automated measurements of AXA were conducted during routine operation at our central laboratory without further blinding.
Definition of Safe‐for‐Treatment Concentration Thresholds
The study is based on a safe‐for‐treatment concentration threshold of 30 ng/mL that has been recommended by international guidelines12, 14 and expert recommendations5, 13 for surgical procedures and for thrombolysis in acute ischemic stroke and to trigger reversal therapy in patients with intracerebral hemorrhage for all 3 investigated DOAC. A higher anticoagulation reversal threshold of 50 ng/mL was proposed for less critical bleeding.14
Furthermore, we evaluated 3 different safe‐for‐treatment AXA cut‐offs that have been recommended if no DOAC‐specific calibration is available: below the upper limit of normal (ULN, in our case 0.09 U/mL)1, 9, less than 2 times the ULN,10, 11 or below 0.3 U/mL.5
Statistical Analysis
For test performance analyses, results of real‐life patient samples were pooled in order to obtain a broad concentration spectrum. SPSS v23 (IBM, Armonk, NY) was used for statistics. Diagnostic accuracy was expressed in terms of sensitivity, specificity, positive and negative predictive value, and likelihood ratio. Agreement between coagulation tests and UPLC‐MS/MS (criterion standard) are visualized with Bland‐Altman plots modified following suggestions made by Krouwer.18
Sensitivity was defined as percentage of samples with DOAC concentrations above the respective safe‐for‐treatment threshold, which were correctly identified by the test as not eligible for treatment without prior anticoagulation reversal. Correspondingly, specificity was defined as percentage of samples with DOAC concentrations below the corresponding threshold, which were correctly identified as eligible for treatment or not requiring reversal therapy. These definitions differ from our prior publications that focused on unspecific global tests in emergency scenarios.15, 16, 19 A sensitivity >95% was predefined as sufficiently safe for clinical application. Positive likelihood ratio was defined as sensitivity divided by (1‐specificity). Negative likelihood ratio was defined as (1‐sensitivity) divided by specificity.
Sensitivity and specificity are given with 2‐sided 95% confidence intervals (CIs). CIs for sensitivity/specificity, predictive values, and likelihood ratios were calculated according to the efficient‐score method using the free online VassarStats Clinical Calculator 1.20 All DOAC concentrations are reported as median and interquartile range. Differences between quantitative results of different coagulation assays are reported as mean and SD with the corresponding 95% CIs. This study was performed in accordance with the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines for studies on diagnostic tests.21
Results
Characteristics of Study Samples
Complete test results were available for all study samples. A median DOAC concentration of 28 (interquartile range 10–67) ng/mL dabigatran, 102 (interquartile range 34–189) ng/mL rivaroxaban, and 54 (interquartile range 35–95) ng/mL apixaban were detected using UPLC‐MS/MS. Concentrations below the 30 ng/mL safe‐for‐treatment threshold were detected in 51.7% (92/178) of dabigatran, 23.3% (34/146) of rivaroxaban, and 19.7% (31/157) of apixaban samples. Data on patient characteristics and baseline laboratory values are provided in Tables 1 and 2.
Table 1.
Patient Characteristics in the 3 DOAC Groups
| Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|
| Dose* | 110 mg: 10 (39%) | 15 mg: 2 (7%) | 2.5 mg: 13 (41%) |
| 150 mg: 16 (62%) | 20 mg: 28 (93%) | 5 mg: 19 (59%) | |
| Sex, female* | 13 (50%) | 13 (43%) | 15 (47%) |
| Age, y† | 74±14 | 69±15 | 75±13 |
| Body weight, kg† | 80±23 | 80±19 | 72 ±15 |
| Body mass index† | 27±6 | 27±6 | 25±4 |
| Risk factors | |||
| Arterial hypertension* | 20 (77%) | 19 (63%) | 27 (84%) |
| Diabetes mellitus* | 6 (23%) | 5 (17%) | 8 (25%) |
| Hyperlipidemia* | 15 (58%) | 7 (23%) | 17 (53%) |
| Smoking* | 2 (8%) | 4 (13%) | 3 (9%) |
| Concomitant antiplatelet agents (last dose <7 d) | |||
| Acetylsalicylic acid* | 7 (27%) | 15 (50%) | 15 (47%) |
| Others* | 0 (0%) | 1 (3%) | 1 (3%) |
| Prophylactic dose of heparins at any time during admission | |||
| Heparin* | 19 (73%) | 20 (67%) | 23 (72%) |
| Enoxaparin* | 6 (23%) | 14 (47%) | 12 (38%) |
| Indication for oral anticoagulation | |||
| Atrial fibrillation* | 24 (92%) | 21 (70%) | 15 (47%) |
| Patent foramen ovale* | 2 (8%) | 9 (30%) | 0 (0%) |
| ESUS* | 0 (0%) | 0 (0%) | 17 (53%) |
DOAC indicates direct oral anticoagulants; ESUS, embolic stroke of undetermined source.
* Number (%), †mean±SD.
Table 2.
Baseline Laboratory Results of Patients in the 3 DOAC Groups
| Dabigatran | Rivaroxaban | Apixaban | Normal Range | |
|---|---|---|---|---|
| WBC, /μL | 8297±2889 | 7147±2013 | 6969±1581 | 3800–10 300 |
| RBC, 106/μL | 4.5±0.5 | 4.4±0.6 | 4.2±0.7 | 4.2–6.2 |
| Hematocrit | 0.4±0.04 | 0.4±0.05 | 0.38±0.05 | 0.42–0.52 |
| Hemoglobin, mmol/L | 8.4±1 | 8.4±1.1 | 7.88±1.3 | 8.7–11.2 |
| Platelet count, 103/μL | 226±79 | 236±57 | 239±70 | 150–450 |
| Quick, % | 92±12 | 99±11 | 96±13 | 70–120 |
| INR | 1.1±0.1 | 1.0±0. | 1.0±0.1 | 0.9–1.2 |
| aPTT, s | 27±4 | 26±6 | 27±5 | <40 |
| Anti‐Xa, IE‐aXa/mL | <0.09 | <0.09 | <0.09 | <0.09 |
| Fibrinogen, μmol/L | 9.8±2.3 | 10.1±2.4 | 9.8±2.1 | 5–12.1 |
| D‐dimer, nmol/L | 3.8±4.3 | 4.9±7.7 | 2.7±3.3 | <2.7 |
| Creatinine, μmol/L | 68.6±15.3 | 68.6±15.3 | 76.3±38.1 | 45.8–83.9 |
| GFR, mL/min/kg | 75±17 | 81±19 | 78±31 | >60 |
| Protein total, g/L | 68±7 | 70±7 | 68±7 | 65–85 |
| Albumin, g/L | 40±4 | 41±4 | 39±5 | 34–48 |
| CRP, nmol/L | 142.9±123.8 | 285.7±438.1 | 219.1±342.9 | <47.6 |
| Procalcitonin, μg/L | 0.09±0.04 | 0.10±0.08 | 0.11±0.09 | ≤0.10 |
| AST, U/L | 0.58±0.3 | 0.55±0.2 | 0.65±0.42 | ≤0.83 |
| ALT, U/L | 0.52±0.33 | 0.53±0.3 | 0.6±0.53 | ≤0.83 |
| GGT, U/L | 1.05±1.15 | 0.78±0.6 | 1.15±1.49 | ≤1.00 |
Results are presented in mean±SD. ALT indicates alanine transaminase; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; CRP, C‐reactive protein; DOAC, direct oral anticoagulants; GFR, glomerular filtration rate; GGT, γ‐glutamyl transferase; INR, international normalized ratio; RBC, red blood count; WBC, white blood count.
Diagnostic Accuracy of Calibrated Tests for Dabigatran
As depicted in Figure 1 A through C, dabigatran concentrations were overestimated by HTI and ECT (delta‐mean 8.2±32.1 ng/mL, 95% CI [3.5–12.9] and 6.9±17.8 ng/mL, 95% CI [4.3, 9.5], respectively), while BDTI more closely matched UPLC‐MS/MS results (delta‐mean −1.4±15.8 ng/mL, 95% CI [−3.7 to 0.9]). Interestingly, the HTI assay had a much higher tendency for overestimation at low concentrations <60 ng/mL (delta‐mean 12.3±25.2 ng/mL, 95% CI [8.0, 16.6]), compared with concentrations >60 ng/mL (delta‐mean −2.6±44.1 ng/mL, 95% CI [−14.9, 9.7]; Table 3). Dabigatran concentrations in all false‐negative samples were close to the safe‐for‐treatment threshold, ranging from >30 to 42 ng/mL.
Figure 1.
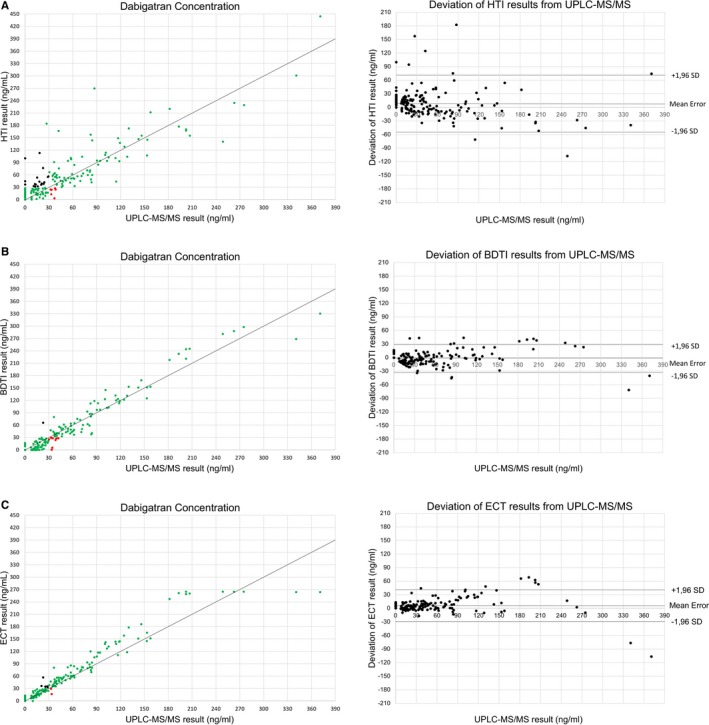
Diagnostic accuracy of dabigatran‐specific coagulation tests. Left: Scatter plots showing the correlation of dabigatran concentrations determined by ultraperformance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS, criterion standard) and dabigatran‐specific coagulation tests: Hemoclot direct thrombin inhibitor assay (HTI, A), Biophen direct thrombin inhibitor assay (BDTI, B) and calibrated ecarin clotting time (ECT, C). Green dots represent samples correctly identified as below or above the safe‐for‐treatment threshold of 30 ng/mL (true‐negative and true‐positive results). Red dots represent samples incorrectly identified as below the safe‐for‐treatment threshold (false negative). Orange dots represent samples incorrectly identified as above the safe‐for‐treatment threshold (false positive). Right: Bland‐Altman blot of UPLC‐MS/MS and coagulation test results showing the mean measurement error and SD of test results.
Table 3.
Mean Deviation in Test Results in Samples With Low and High DOAC Concentrations
| Samples <60 ng/mL | Samples ≥60 ng/mL | All Samples | |
|---|---|---|---|
| Dabigatran | |||
| HTI | 12.3±25.2 ng/mL | −2.6±44.1 ng/mL | 8.2±32.0 ng/mL |
| 95% CI [8.0, 16.6] | 95% CI [−14.9, 9.7] | 95% CI [3.5, 12.9] | |
| BDTI | −3.1±10.2 ng/mL | −2.9±24.9 ng/mL | −1.4±15.8 ng/mL |
| 95% CI [−4.9, −1.3] | 95% CI [−9.9, 4.1] | 95% CI [−3.7, 0.9] | |
| ECT | 4.3±7.8 ng/mL | 13.6±30.6 ng/mL | 6.9±17.8 ng/mL |
| 95% CI [3.0, 5.6] | 95% CI [5.0, 22.2] | 95% CI [4.3, 9.5] | |
| Rivaroxaban | |||
| Calibrated AXA | −2.9±18.5 ng/mL | −10.2±32.9 ng/mL | −7.7±29.0 ng/mL |
| 95% CI [−8.0, 2.2] | 95% CI [−16.8, −3.6] | 95% CI [−12.4, −3.0] | |
| Apixaban | |||
| Calibrated AXA | −12.3±14.7 ng/mL | −7.7±28.8 ng/mL | −10.2±22.2 ng/mL |
| 95% CI [−15.4, −9.2] | 95% CI [−14.4, −1.0] | 95% CI [−13.7, −6.7] | |
Samples=178 (dabigatran), 146 (rivaroxaban), 157 (apixaban). Values±SD. AXA indicates anti‐Xa activity; BDTI, Biophen direct thrombin inhibitor assay; CI, confidence interval; DOAC, direct oral anticoagulants; ECT, ecarin clotting time; HTI, Hemoclot direct thrombin inhibitor assay.
All 3 tests reached high sensitivity for the detection of dabigatran concentrations above 30 ng/mL, but only ECT satisfied our 95% sensitivity target (97.6%, Table 4). Specificity differed between assays and was lower for HTI (78.2%) than for BDTI (98.9%) and ECT (94.6%).
Table 4.
Diagnostic Accuracy of DOAC‐Specific Tests to Drug Concentrations <30 ng/mL
| Substance | Coagulation Assay | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR | NLR |
|---|---|---|---|---|---|---|---|
| Dabigatran | HTI | 93.0 (84.5–97.1) | 78.2 (68.2–85.9) | 80.0 (70.6–87.1) | 92.3 (83.4–96.8) | 4.3 (2.8–6.3) | 0.1 (0.0–0.2) |
| BDTI | 90.7 (82.0–95.6) | 98.9 (93.2–99.9) | 98.7 (92.2–99.9) | 91.9 (84.2–96.2) | 83.4 (11.9–586.8) | 0.1 (0.0–0.2) | |
| ECT | 97.7 (91.1–99.6) | 94.3 (86.5–97.9) | 94.4 (86.8–97.9) | 97.6 (90.9–99.6) | 17.0 (7.3–39.8) | 0.0 (0.0–0.1) | |
| Rivaroxaban | Calibrated AXA | 98.2 (93.1–99.7) | 97.1 (82.9–99.8) | 99.1 (94.4–100.0) | 94.3 (79.4–99.0) | 33.4 (4.8–230.3) | 0.0 (0.0–0.1) |
| Uncalibrated AXA <ULN | 98.2 (93.0–99.7) | 64.7 (46.4–79.7) | 90.1 (82.3–94.5) | 91.7 (71.5–98.5) | 2.8 (1.8–4.4) | 0.0 (0.0–0.1) | |
| Uncalibrated AXA <2ULN | 97.3 (91.7–99.3) | 82.3 (64.8–92.6) | 94.7 (88.4–97.8) | 90.3 (73.0–97.5) | 5.5 (2.7–11.4) | 0.0 (0.0–0.1) | |
| Uncalibrated AXA <0.3 U/mL | 92.0 (84.9–96.0) | 94.1 (78.9–99.0) | 98.1 (92.6–99.7) | 78.0 (62.0–88.9) | 15.6 (4.1–60.0) | 0.1 (0.0–0.2) | |
| Apixaban | Calibrated AXA | 73.8 (65.1–81.0) | 96.8 (81.5–99.8) | 98.9 (93.3–99.9) | 47.6 (35.0–60.5) | 22.9 (3.3–157.8) | 0.3 (0.2–0.4) |
| Uncalibrated AXA <ULN | 93.7 (87.5–97.0) | 90.3 (73.1–97.5) | 97.5 (92.4–99.4) | 77.8 (60.4–89.2) | 9.7 (3.3–28.4) | 0.1 (0.0–0.1) | |
| Uncalibrated AXA <2ULN | 81.0 (72.8–87.2) | 96.8 (81.5–99.8) | 99.0 (93.9–99.9) | 55.6 (41.5–68.8) | 25.1 (3.6–172.9) | 0.2 (0.1–0.3) | |
| Uncalibrated AXA <0.3 U/mL | 68.3 (59.3–76.1) | 100 (86.3–100) | 100 (94.7–100) | 43.7 (32.1–55.9) | 0 (n.c.) | 0.3 (0.2–0.4) |
Samples=178 (dabigatran), 146 (rivaroxaban), 157 (apixaban). Test accuracy calculations are provided with 95% confidence intervals. The ULN for anti‐Xa activity as determined by our laboratory is 0.09 U/mL. AXA indicates anti‐Xa activity; BDTI, Biophen direct thrombin inhibitor assay; DOAC, direct oral anticoagulants; ECT, ecarin clotting time; HTI, Hemoclot direct thrombin inhibitor assay; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; ULN, upper limit of normal.
Diagnostic Accuracy of Calibrated AXA for FXa Inhibitors
Calibrated AXA underestimated rivaroxaban concentrations by a mean of −7.7±29.0 ng/mL, 95% CI [−12.4, −3.0] (Figure 2 A), and 2 samples (containing 34 and 43 ng/mL) were misclassified as <30 ng/mL. One of 146 samples was incorrectly classified as above the 30 ng/mL threshold. Test accuracy calculations (Table 4) showed high sensitivity (98.2%) and specificity (97.1%).
Figure 2.
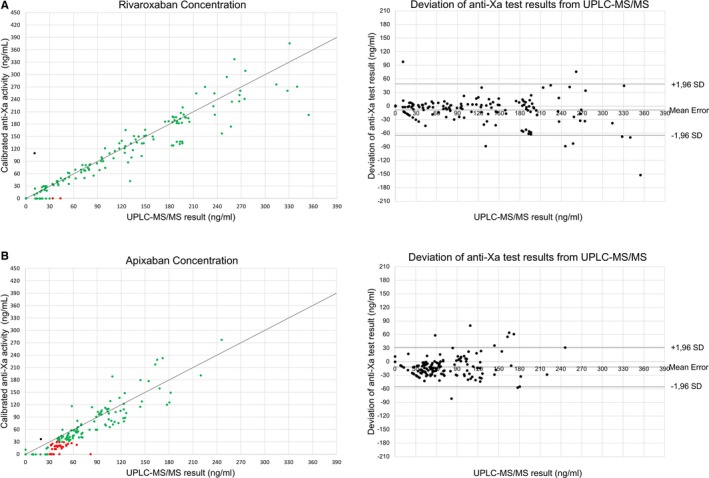
Diagnostic accuracy of anti‐Xa activity testing. Left: Scatter plots showing the correlation of FXa inhibitor concentrations (A, rivaroxaban; B, apixaban) determined by UPLC‐MS/MS, criterion standard and calibrated anti‐Xa activity testing. Green dots represent samples correctly identified as below or above the safe‐for‐treatment threshold of 30 ng/mL (true‐negative and true‐positive results). Red dots represent samples incorrectly identified as below the safe‐for‐treatment threshold (false negative). Orange dots represent samples incorrectly identified as above the safe‐for‐treatment threshold (false positive). Right: Bland‐Altman blot of UPLC‐MS/MS and anti‐Xa activity results showing the mean measurement error and SD of test results. UPLC‐MS/MS indicates ultraperformance liquid chromatography–tandem mass spectrometry.
Less accurate results were found for apixaban (Figure 2 B). Calibrated AXA underestimated real apixaban concentrations by a mean of −10.2±22.2 ng/mL, 95% CI [−13.7, −6.7]. Thirty‐three of 126 samples containing apixaban concentrations above the safe‐for‐treatment threshold were misclassified by the test as <30 ng/mL. In these samples, apixaban concentrations reached up to 82 ng/mL. One sample was misclassified as >30 ng/mL. Test results provided 73.8% sensitivity and 96.8% specificity. Hence, the predefined 95% sensitivity target was not met for apixaban.
Diagnostic Accuracy of Uncalibrated AXA for FXa Inhibitors
We assessed the diagnostic accuracy of 3 different uncalibrated AXA cut‐offs, which have been suggested to rule out relevant concentrations of FXa inhibitors: below the ULN, below 2 times the ULN, and below 0.3 U/mL (Table 4). For rivaroxaban, the 3 cut‐offs provided 98.2%/97.3%/92.0% sensitivity and 64.7%/82.3%/94.1% specificity, respectively. For apixaban we found 93.7%/81.0%/68.3% sensitivity and 90.3%/96.8%/100% specificity, respectively.
Discussion
Our study evaluated the performance of DOAC‐specific coagulation assays in real‐life patient samples. Different from prior reports,22, 23, 24 our analyses focused on the diagnostic accuracy of the assays in the low concentration spectrum and their ability to discriminate between concentrations above and below the currently accepted safe‐for‐treatment threshold of 30 ng/mL.5, 12, 13, 14
Previous publications by our group provided evidence that unspecific point‐of‐care tests15, 16 and laboratory‐based assays19 can help to identify intact hemostatic function in patients presumably on DOAC treatment. However, diagnostic performance of these unspecific tests is far from ideal, as only a limited fraction of patients with DOAC concentrations below 30 ng/mL could be detected. Our present analysis investigated guideline‐recommended DOAC‐specific tests that should theoretically provide better diagnostic accuracy. In clinical practice, the question whether it is safe to proceed with treatments that require intact hemostasis (eg, surgery with high bleeding risk or thrombolysis for acute ischemic stroke) is frequently encountered. Similar concerns arise when reversal therapy is evaluated in DOAC‐associated bleeding. Unnecessary reversal therapy comes at considerable financial cost in the case of dabigatran, as idarucizumab is a safe yet expensive therapeutic option.25 The situation is even more complicated for FXa inhibitors, where prothrombin complex concentrate remains the mainstay of reversal therapy.7, 12 Prothrombin complex concentrate increases the risk of thromboembolic complications, and unnecessary application might expose patients to unwarranted risks.26
Safe‐for‐Treatment Thresholds
While firm evidence supports the efficacy of DOACs for long‐term anticoagulation,5 reliable data on DOAC concentration thresholds that result in a clinically significant coagulation impairment are lacking. Although we acknowledge this ambiguity, the safe‐for‐treatment threshold of 30 ng/mL, which has been proposed for invasive procedures or thrombolysis in a number of recent guidelines12, 14 and expert recommendations,5, 13 seems to have been chosen with a focus on patient safety and is lower than previous recommendations.10 In case of severe but less critical bleeding, a threshold of 50 ng/mL has been proposed to warrant antidote adminstration.14 We have included test accuracy calculations for this higher threshold in Table 5.
Table 5.
Diagnostic Accuracy of DOAC Specific Tests to Drug Concentrations <50 ng/mL
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR | NLR | |
|---|---|---|---|---|---|---|
| Dabigatran | ||||||
| HTI | 86.2 (74.1–93.4) | 83.3 (75.2–89.3) | 71.4 (59.2–81.2) | 95.6 (85.5–96.5) | 5.1 (3.4–7.8) | 0.2 (0.1‐0.3) |
| BDTI | 81.0 (68.2–89.7) | 95.0 (89.0–98.0) | 88.7 (76.3–95.3) | 91.2 (84.4–95.3) | 16.2 (7.4–35.7) | 0.2 (0.1‐0.3) |
| ECT | 100 (92.2–100) | 90.8 (83.8–95.1) | 84.1 (72.8–91.4) | 100 (95.7–100) | 10.9 | 0 (n.c.) |
| Rivaroxaban | ||||||
| Calibrated AXA | 96.0 (89.5–98.7) | 97.8 (87.0–99.9) | 99.0 (93.6–99.9) | 91.8 (79.5–97.4) | 44.2 (6.4–307.0) | 0.0 (0.0‐0.1) |
| Uncalibrated AXA <ULN | 100 (95.4–100) | 53.3 (38.0–68.1) | 82.6 (74.5–88.7) | 100 (82.8–100) | 2.1 (1.6–2.9) | 0 (n.c.) |
| Uncalibrated AXA <2ULN | 100 (95.4–100) | 68.9 (53.2–81.4) | 87.7 (80.0–92.9) | 100 (86.3–100) | 3.2 (2.1–5.0) | 0 (n.c.) |
| Uncalibrated AXA <0.3 U/mL | 99.0 (93.8–99.9) | 87.0 (73.0–94.6) | 94.3 (87.5–97.7) | 97.6 (85.6–99.9) | 7.6 (3.6–16.0) | 0.0 (0.0–0.1) |
| Apixaban | ||||||
| Calibrated AXA | 74.2 (63.6–82.6) | 98.5 (91.0–99.9) | 98.5 (90.9–99.9) | 74.4 (64.0–82.8) | 50.4 (7.2–354.2) | 0.3 (0.2–0.4) |
| Uncalibrated AXA <ULN | 98.9 (93.0–99.9) | 51.5 (39.1–63.6) | 72.7 (63.7–80.2) | 97.2 (83.8–99.6) | 2.1 (1.6–2.6) | 0.0 (0.0–0.2) |
| Uncalibrated AXA <2ULN | 97.8 (91.4–99.6) | 76.5 (64.4–85.6) | 84.5 (75.7–90.6) | 96.3 (86.2–99.4) | 4.2 (2.7–6.3) | 0.0 (0.0–0.1) |
| Uncalibrated AXA <0.3 U/mL | 88.8 (79.9–94.2) | 89.7 (79.3–95.4) | 91.9 (83.4–96.4) | 85.9 (75.2–92.7) | 8.6 (4.3–17.5) | 0.1 (0.1–0.2) |
Samples=178 (dabigatran), 146 (rivaroxaban), 157 (apixaban). Test accuracy calculations are provided with 95% confidence intervals. The ULN for AXA as determined by our laboratory is 0.09 U/mL. AXA indicates anti‐Xa activity; BDTI, Biophen direct thrombin inhibitor assay; DOAC, direct oral anticoagulants; ECT, ecarin clotting time; HTI, Hemoclot direct thrombin inhibitor assay; n.c., not calculable ; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; ULN, upper limit of normal.
It should be noted that these threshold recommendations are founded on an extrapolation based on retrospective analyses of existing trial data.13 Supporting these thresholds with better quality clinical data is of paramount importance, because the number of DOAC‐treated patients is steadily increasing.27 To accomplish this, concentration measurements using DOAC‐specific assays in all patients who develop ischemic or hemorrhagic events seem advisable. Furthermore, wide availability of DOAC‐specific coagulation assays is very important in this context, both to guide further research and for treatment of these patients.
Given the weak evidence for both thresholds, it seems likely that our understanding of what constitutes a relevant DOAC concentration will develop as our clinical experience with DOAC increases, similar to the finding that thrombolysis is safe in ischemic stroke patients despite an elevated international normalized ratio (ie, up to an international normalized ratio of 1.7) during warfarin treatment.28 First indications that DOAC might not impact bleeding risk after thrombolysis as much as currently believed stem from experimental animal data29 and a clinical study investigating an intravenous thrombin inhibitor (with a similar mode of action as dabigatran) as an adjunct to thrombolysis.30
When interpreting coagulation test results, it is critical to consider the time of last DOAC intake: Different from the relatively stable anticoagulation during vitamin K antagonist treatment, concentrations of all DOAC increase rapidly after intake and peak after about 3 hours. Hence, test results should be interpreted cautiously if obtained within 4 hours after intake.7
Dabigatran‐Specific Coagulation Tests
Significant differences in diagnostic accuracy were found between dabigatran‐specific assays. All evaluated assays (HTI, BDTI, and ECT) had a high sensitivity for dabigatran and the 30 ng/mL threshold but only ECT satisfied our 95% sensitivity target for safe clinical use. However, in case of false‐negative HTI or BDTI results, real dabigatran concentrations (as determined by the criterion standard UPLC‐MS/MS) did not exceed 42 ng/mL. Yet, specificity differed considerably between assays: Consistent with prior reports,31, 32 we observed a high tendency of the HTI assay for overestimation in the low concentration spectrum, which resulted in a specificity of only 78% for dabigatran concentrations >30 ng/mL. This means that almost 25% of patients with plasma concentrations <30 ng/mL would have been exposed to unwarranted treatment with idarucizumab or unnecessary treatment delays. BDTI and ECT performed considerably better and provided specificity results well above 90%.
Therefore, use of HTI, the assay with the lowest specificity, seems less advantageous than ECT and BDTI. As the use of ECT‐based dabigatran assays is hampered by a lack of Conformité Européenne (CE)‐labeled quality‐controlled diagnostic reagents,6 our results suggest that BDTI currently might be the most preferable alternative for clinical implementation.
FXa Inhibitor‐Specific Coagulation Tests
Calibrated AXA
Rivaroxaban and apixaban showed striking differences in their impact on AXA. For rivaroxaban, calibrated AXA provided excellent diagnostic accuracy with high sensitivity and specificity for concentrations around the 30 ng/mL safe‐for‐treatment threshold. Apixaban had less impact on AXA, and samples with concentrations almost 3‐fold above the threshold were classified as <30 ng/mL. Hence, our results suggest that calibrated AXA does not provide sufficient sensitivity for the reliable detection of apixaban concentrations >30 ng/mL. Hints for imprecision of AXA in the low apixaban concentration range can also be derived from an earlier report, which highlighted the strong overall test correlation.33
This finding puts the current recommendation for apixaban into question to use calibrated AXA to select suitable patients for surgery or thrombolysis.5, 7
Uncalibrated AXA
If only uncalibrated AXA is available, our results for rivaroxaban suggest that a cut‐off of less than 2 times the ULN can provide the most advantageous balance between sensitivity and specificity: A more restrictive cut‐off (below ULN) did not significantly enhance sensitivity, but lowered specificity considerably. The more liberal <0.3 U/mL cut‐off produced a higher number of false‐negative results leading to incorrect selection of samples with rivaroxaban concentrations up to 54 ng/mL as eligible for invasive treatment. Similar to the calibrated assays, uncalibrated AXA is of limited value in apixaban‐treated patients. If used, our results suggest that the most restrictive cut‐off (ie, below ULN) should be used to maximize sensitivity and thus patient safety. However, it needs to be acknowledged that even at this cut‐off, 22% of test results below ULN were false negatives (8/36) and our 95% sensitivity target was not met.
Strength and Limitations
Our study provides a comprehensive overview and head‐to‐head comparison of the diagnostic performance of guideline‐recommended DOAC‐specific coagulation tests for the detection of clinically relevant DOAC concentrations. This distinguishes our report from prior studies, which investigated the performance of coagulation test for the quantification across the whole range of therapeutic drug concentrations but fail to inform about the clinically most important aspect: the tests’ diagnostic accuracy for the detection of patients with DOAC concentrations that are low enough to allow emergency surgery, thrombolysis, or that do not warrant anticoagulation reversal in case of bleeding. Different from previous reports (eg, 23, 34, 35), we used real‐life patient samples and the current criterion standard for DOAC concentration measurement (UPLC‐MS/MS) as reference. We included 3 different coagulation assays for dabigatran measurement and provide a comparison of the impact of the FXa inhibitors rivaroxaban and apixaban on calibrated as well as uncalibrated AXA.
Although our study includes a variety of DOAC‐specific assays, we only used a single reagent for each type of test and thus cannot provide data that compare different test reagents. This applies especially to AXA, where a variety of reagents are available. However, reports comparing our reagent (Chromogenix COAMATIC Heparin Test) to other reagents for rivaroxaban36 and apixaban37 measurement found no difference in diagnostic performance. Our study does not include the FXa inhibitor edoxaban, as this DOAC was not approved for clinical use in Europe when the present study was initiated. Our test accuracy calculations were limited by the number of samples included in this study, and the provided CIs need to be taken into account when interpreting the results of all analyses. However, even the lower CIs suggest a sensitivity above 0.90 for all tests that satisfied the 0.95 sensitivity target. Sequential samples were acquired from each patient. Hence, a bias because of repeated measurements cannot be excluded, but this approach allowed us to cover a wide spectrum of DOAC concentrations.
Furthermore, generalizability of our results is limited by the single‐center nature of the study. We therefore suggest confirming the observed deviations in a multicenter trial.
Conclusion
Despite their strong overall correlation with DOAC concentrations, DOAC‐specific coagulation tests differ considerably in their diagnostic performance when used in the low concentration spectrum close to the currently accepted safe‐for‐treatment threshold of 30 ng/mL. All dabigatran‐specific assays reliably detected samples >30 ng/mL, but the limited specificity of the HTI poses a high risk of unwarranted anticoagulation reversal or unnecessary treatment delays. BDTI and ECT provided better diagnostic performance for this indication. For rivaroxaban, AXA yielded excellent results and provided high sensitivity as well as specificity. Apixaban, on the other hand, had a much less pronounced impact on this assay. Based on the results of our study, we recommend extreme caution if AXA is used to assess hemostatic function in apixaban‐treated patients.
Disclosures
SP received speaker's honoraria and consulting honoraria from Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb/Pfizer, Daiichi Sankyo and Werfen, reimbursement for congress traveling and accommodation from Bayer and Boehringer‐Ingelheim, and research support from Bristol‐Myers Squibb/Pfizer (significant), Boehringer‐Ingelheim, Daiichi Sankyo (significant), and Helena Laboratories (all other contributions: modest). ME and FH received reimbursement for congress traveling and accommodation from Bayer (contributions: modest). IB received speaker's honoraria from Bristol‐Myers Squibb/Pfizer, and CSL Behring and reimbursement for congress traveling and accommodation from Baxalta, Bayer, and CSL Behring and performed contract research for Siemens Healthcare (all contributions: modest). UZ has received personal fees from Biogen Idec GmbH, Bayer Vital GmbH, Bristol‐Myers Squibb/Pfizer, CorTec GmbH, Medtronic GmbH, and grants from Biogen Idec GmbH, Servier, and Janssen Pharmaceuticals NV, outside of the submitted work (all contributions: modest). The remaining authors have no disclosures to report.
Acknowledgments
We acknowledge support from Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tübingen. This work did not receive financial support.
(J Am Heart Assoc. 2018;7:e009807 DOI: 10.1161/JAHA.118.009807.)
References
- 1. Reiffel JA, Weitz JI, Reilly P, Kaminskas E, Sarich T, Sager P, Seltzer J; Cardiac Safety Research Consortium p, participants . NOAC monitoring, reversal agents, and post‐approval safety and effectiveness evaluation: a cardiac safety research consortium think tank. Am Heart J. 2016;177:74–86. [DOI] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie‐Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke C . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 3. Douxfils J, Mani H, Minet V, Devalet B, Chatelain B, Dogne JM, Mullier F. Non‐VKA oral anticoagulants: accurate measurement of plasma drug concentrations. Biomed Res Int. 2015;2015:345138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Douxfils J, Tamigniau A, Chatelain B, Goffinet C, Dogne JM, Mullier F. Measurement of non‐VKA oral anticoagulants versus classic ones: the appropriate use of hemostasis assays. Thromb J. 2014;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drouet L, Bal Dit Sollier C, Steiner T, Purrucker J. Measuring non‐vitamin K antagonist oral anticoagulant levels: when is it appropriate and which methods should be used? Int J Stroke. 2016;11:748–758. [DOI] [PubMed] [Google Scholar]
- 6. Poli S, Hartig F, Spencer C, Ebner M, Birschmann I, Kuhn J, Faix S, Ziemann U, Haring HU, Lehmann R, Peter A, Horber S. Diagnostic accuracy of a novel chromogenic direct thrombin inhibitor assay: clinical experiences for dabigatran monitoring. Thromb Haemost. 2017;117:2369–2375. [DOI] [PubMed] [Google Scholar]
- 7. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. Updated European Heart Rhythm Association practical guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467–1507. [DOI] [PubMed] [Google Scholar]
- 8. Purrucker JC, Haas K, Rizos T, Khan S, Poli S, Kraft P, Kleinschnitz C, Dziewas R, Binder A, Palm F, Jander S, Soda H, Heuschmann PU, Veltkamp R; Investigators R . Coagulation testing in acute ischemic stroke patients taking non‐vitamin K antagonist oral anticoagulants. Stroke. 2017;48:152–158. [DOI] [PubMed] [Google Scholar]
- 9. Godier A, Martin AC, Leblanc I, Mazoyer E, Horellou MH, Ibrahim F, Flaujac C, Golmard JL, Rosencher N, Gouin‐Thibault I. Peri‐procedural management of dabigatran and rivaroxaban: duration of anticoagulant discontinuation and drug concentrations. Thromb Res. 2015;136:763–768. [DOI] [PubMed] [Google Scholar]
- 10. Steiner T, Bohm M, Dichgans M, Diener HC, Ell C, Endres M, Epple C, Grond M, Laufs U, Nickenig G, Riess H, Rother J, Schellinger PD, Spannagl M, Veltkamp R. Recommendations for the emergency management of complications associated with the new direct oral anticoagulants (DOACS), apixaban, dabigatran and rivaroxaban. Clin Res Cardiol. 2013;102:399–412. [DOI] [PubMed] [Google Scholar]
- 11. Diener H‐C, Foerch C, Riess H, Röther J, Schroth G, Weber R. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti‐thrombotic treatment. Lancet Neurol. 2013;12:677–688. [DOI] [PubMed] [Google Scholar]
- 12. Ahmed N, Steiner T, Caso V, Wahlgren N. Recommendations from the eso‐karolinska stroke update conference, Stockholm 13–15 November 2016. Eur Stroke J. 2017;2:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pernod G, Albaladejo P, Godier A, Samama CM, Susen S, Gruel Y, Blais N, Fontana P, Cohen A, Llau JV, Rosencher N, Schved JF, de Maistre E, Samama MM, Mismetti P, Sie P; Working Group on Perioperative Haemostasis . Management of major bleeding complications and emergency surgery in patients on long‐term treatment with direct oral anticoagulants, thrombin or factor‐xa inhibitors: proposals of the working group on perioperative haemostasis (GIHP)—March 2013. Arch Cardiovasc Dis. 2013;106:382–393. [DOI] [PubMed] [Google Scholar]
- 14. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI; Subcommittee on Control of Anticoagulation . When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:623–627. [DOI] [PubMed] [Google Scholar]
- 15. Ebner M, Peter A, Spencer C, Hartig F, Birschmann I, Kuhn J, Wolf M, Winter N, Russo F, Zuern CS, Blumenstock G, Ziemann U, Poli S. Point‐of‐care testing of coagulation in patients treated with non‐vitamin K antagonist oral anticoagulants. Stroke. 2015;46:2741–2747. [DOI] [PubMed] [Google Scholar]
- 16. Ebner M, Birschmann I, Peter A, Spencer C, Hartig F, Kuhn J, Blumenstock G, Zuern CS, Ziemann U, Poli S. Point‐of‐care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants. Crit Care. 2017;21:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuhn J, Gripp T, Flieder T, Dittrich M, Hendig D, Busse J, Knabbe C, Birschmann I. UPLC‐MRM Mass Spectrometry method for measurement of the coagulation inhibitors dabigatran and rivaroxaban in human plasma and its comparison with functional assays. PLoS One. 2015;10:e0145478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krouwer JS. Why bland‐altman plots should use x, not (y+x)/2 when x is a reference method. Stat Med. 2008;27:778–780. [DOI] [PubMed] [Google Scholar]
- 19. Ebner M, Birschmann I, Peter A, Hartig F, Spencer C, Kuhn J, Blumenstock G, Zuern CS, Ziemann U, Poli S. Emergency coagulation assessment during treatment with direct oral anticoagulants: limitations and solutions. Stroke. 2017;48:2457–2463. [DOI] [PubMed] [Google Scholar]
- 20. Vassarstats: website for statistical computation. Available at: http://vassarstats.net/clin1.html. Accessed February 5, 2018.
- 21. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF; STARD Group . STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stangier J, Feuring M. Using the hemoclot direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23:138–143. [DOI] [PubMed] [Google Scholar]
- 23. Gouin‐Thibault I, Flaujac C, Delavenne X, Quenet S, Horellou MH, Laporte S, Siguret V, Lecompte T. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti‐Xa assays. A multicentre French GEHT study. Thromb Haemost. 2014;111:240–248. [DOI] [PubMed] [Google Scholar]
- 24. Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, Le Flem L, Rohde G, Martinoli JL; Rivaroxaban Anti‐Factor Xa Chromogenic Assay Field Trial Laboratories . Evaluation of the anti‐factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107:379–387. [DOI] [PubMed] [Google Scholar]
- 25. Pollack CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–520. [DOI] [PubMed] [Google Scholar]
- 26. Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACS) in VTE treatment. J Thromb Thrombolysis. 2016;41:206–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–1305.e1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xian Y, Liang L, Smith EE, Schwamm LH, Reeves MJ, Olson DM, Hernandez AF, Fonarow GC, Peterson ED. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. JAMA. 2012;307:2600–2608. [DOI] [PubMed] [Google Scholar]
- 29. Ploen R, Sun L, Zhou W, Heitmeier S, Zorn M, Jenetzky E, Veltkamp R. Rivaroxaban does not increase hemorrhage after thrombolysis in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barreto AD, Ford GA, Shen L, Pedroza C, Tyson J, Cai C, Rahbar MH, Grotta JC; ARTSS‐2 Investigators . Randomized, multicenter trial of ARTSS‐2 (Argatroban With Recombinant Tissue Plasminogen Activator for Acute Stroke). Stroke. 2017;48:1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hawes EM, Deal AM, Funk‐Adcock D, Gosselin R, Jeanneret C, Cook AM, Taylor JM, Whinna HC, Winkler AM, Moll S. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross‐sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. 2013;11:1493–1502. [DOI] [PubMed] [Google Scholar]
- 32. Antovic JP, Skeppholm M, Eintrei J, Boija EE, Soderblom L, Norberg EM, Onelov L, Ronquist‐Nii Y, Pohanka A, Beck O, Hjemdahl P, Malmstrom RE. Evaluation of coagulation assays versus LC‐MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol. 2013;69:1875–1881. [DOI] [PubMed] [Google Scholar]
- 33. Becker RC, Yang H, Barrett Y, Mohan P, Wang J, Wallentin L, Alexander JH. Chromogenic laboratory assays to measure the factor Xa‐inhibiting properties of apixaban—an oral, direct and selective factor Xa inhibitor. J Thromb Thrombolysis. 2011;32:183–187. [DOI] [PubMed] [Google Scholar]
- 34. Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–997. [DOI] [PubMed] [Google Scholar]
- 35. Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu‐Bureau G, Depasse F, Perzborn E. Assessment of laboratory assays to measure rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–825. [DOI] [PubMed] [Google Scholar]
- 36. Francart SJ, Hawes EM, Deal AM, Adcock DM, Gosselin R, Jeanneret C, Friedman KD, Moll S. Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. A cross‐sectional pharmacodynamic study based on peak and trough plasma levels. Thromb Haemost. 2014;111:1133–1140. [DOI] [PubMed] [Google Scholar]
- 37. Hillarp A, Gustafsson KM, Faxalv L, Strandberg K, Baghaei F, Fagerberg Blixter I, Berndtsson M, Lindahl TL. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti‐FXa assays. J Thromb Haemost. 2014;12:1545–1553. [DOI] [PubMed] [Google Scholar]


