Abstract
Cells exhibit biologically heterogeneous phenotypes, particularly in pathogenic states. To study cell behavior at the single cell level, a variety of micropatterning techniques have been proposed that allow the spatial organization of cells with great control over cell volume, morphology, and intercellular interactions. Among these strategies, microstencil patterning has traditionally been eschewed due to fragility of membranes and lack of control over cell configurations within patterns. Here, we present a simple and reproducible strategy to create robust microstencils and achieve consistent and efficient cell patterns requiring less than 4 μl of cell solution. Polydimethylsiloxane microstencils fabricated with this technique can be used dozens of times over the course of several months with minimal wear or degradation. Characterization of pattern size, cell suspension density, and droplet volume allows on-demand configurations of singlets, doublets, triplets, or multiple cells per individual space. In addition, a novel technique to suppress evaporative convection provides precise and repeatable results, with a twofold increase in patterning efficacy. Selective dual surface modification to create hydrophilic islands on a hydrophobic substrate facilitates a significantly longer and healthier lifespan of cells without crossover of pattern boundaries. The ability to pattern individual cells with or without an extracellular matrix substrate and to control the magnitude of cell-cell contact as well as spread area provides a powerful approach to monitoring cell functions such as proliferation and intercellular signaling.
I. INTRODUCTION
Cells are known to exhibit biologically heterogeneous phenotypes.1 These differences may arise as a result of intrinsic noise, or due to intercellular communications, and dynamically influence the stochastic behavior of cells at the population level.2 Once cell cultures have been sustained for longer than a 24-hour period, it is incredibly difficult to study single cell activity in a colony.3–7 To better understand cell and disease functions, there is a growing interest toward analyses of cell behavior at the single cell level. However, handling and manipulation of individual cells remain challenging, and a majority of the in vitro studies are still performed at the bulk level.8–16 Such population-based studies only provide ensemble-averaged results obscuring the minute cell-cell variability.
Cell volume, intercellular interactions, cell spreading, and morphology are some of the critical factors regulating cell growth and signaling behaviors.10,17–19 For in vitro analysis of individual cell characteristics or interactions, a variety of physical, chemical, or combination techniques have been developed to create orderly patterns of mammalian or bacterial cells.20 Physical approaches include inkjet cell printing21,22 and cell localization using optical,23,24 laser-based,25,26 magnetic,27 acoustic,28,29 or dielectrophoretic30 forces. While these techniques physically trap/propel cells at desired coordinates and offer unique features for specific applications, their level of complexities limits their wide-scale adaptation for cell micropatterning. On the other hand, chemical techniques are used to manipulate surface characteristics, such as the electrostatic charge, extracellular matrix (ECM) deposition, etc., making the surface amenable to mammalian or bacterial cell attachment.31,32 To immobilize individual or clusters of adherent cells that readily attach to surfaces through ECM binding, a combination of physical and chemical micropatterning techniques are commonly utilized to control multiple parameters such as spatial arrangement, underlying ECM substrate, cell spread area, cell-cell contact, and morphology of cells.33–35
Among all the available techniques, currently there are two strategies commonly utilized to achieve cell/ECM patterning: (a) microcontact printing (μCP) and (b) microstencil patterning (μSP). Microcontact printing is an established single cell analysis (SCA) technique used to create a selective extracellular matrix (ECM) environment for cells to adhere and grow.36 Proteins are “stamped” onto the culture surfaces, which are subsequently treated again to be selectively hospitable to cell adhesion.37 Many different substrates and proteins can be used as “ink” for micro-contact stamps, and once created, stamps can be cleaned, reused, and maintained over time.38,39 Alternatively, the microstencil printing is accomplished through a stencil membrane containing through-holes of the desired pattern geometry.40–42 The membrane is placed in close contact with an untreated substrate (e.g., polystyrene Petri dish), and the open surface (through membrane perforations) is chemically or ionically modified to support cell adhesion. Though, the activated surfaces last for a relatively shorter duration and membrane stencils do not have a similar lifespan as microcontact printing stamps.43 While both methods have merits for specific applications, the microstencil approach has been eschewed in favor of μCP primarily due to the delicacy of stencils and the complexity of processes involved in fabricating them.
Following surface modification, multiple factors limit the production of clean and reproducible cellular patterns for high-throughput single cell analyses including (1) requirement of a large number of cells even though the pattern area is relatively small, (2) lack of control over cell clustering within individual patterns, (3) inability to customize spatial distribution of cells to obtain specific cell density within each pattern, and (4) inability to pattern cells without requiring an underlying protein substrate. Although cell clumping can be minimized by passing the cells through a filtering sieve to obtain a homogeneous single cell suspension, the addition of cells several orders of magnitude in excess than the number of available adhesive patterns exacerbates cell clustering. The requirement of excess cells results as the existing procedures involve filling of the entire patterned Petri dish with culture media to obtain uniform patterns. Attempts have been made to circumvent this need by placing a small droplet of cell suspension specifically on the patterned region.9 However, when placed inside the CO2 incubator for attachment, the droplet experiences evaporation induced convective flows, a phenomenon well known and characterized for the formation of coffee stains.44,45 In case of saline droplets, these flows are governed by the forces generated due to temperature, concentration, and density gradients as a result of uneven evaporation. Additional factors such as aspect ratio, wettability, substrate temperature, humidity, and surface roughness also influence intra-droplet convective currents.46 Consequently, convection induced intradroplet movement of cells introduces additional constraints to the cell-substrate interactions and subsequent efficacy of existing micropatterning techniques.44–47 Additionally, the widely used μCP technique only allows production of patterns with pre-deposited proteins which may be undesirable where endogenous production of ECM plays a participating role. Thus, analyses of cell functions at the single cell level have been impeded, in part, due to various technical challenges and primarily due to lack of characterized strategies to obtain cellular patterns in a desired configuration, i.e., single cells within each pattern with no contact with neighboring cells, two cells with individual contact between the cells, or multiple contacting cells, which may be critical in studies of cell growth, motility, or communication through contact.
Here, we present a simple and reliable technique to fabricate structurally robust microstencils and create stable patterns with desired cell distribution within each spot. By selecting for pattern dimension and cell seeding density, patterns with specific cell configurations as singlets, doublets, or triplets can be consistently generated. The presented approach is rapidly reproducible, creates long lasting experimental platforms for single cell analysis, and offers high throughput requiring small sample volumes. The microstencils can be reused multiple times (>50) without degrading or requiring intense sonication or chemical cleaning procedures. Most importantly, our unique floating coverslip approach suppresses the convective flow effects that are responsible for inconsistencies in patterning efficiency. Further extrapolation of the observed patterns using a Poisson's probability distribution provides a characteristic model, which can be used to design experimental protocols for a wide variety of single cell applications.
II. MATERIALS AND METHODS
A. Fabrication of microstencils for cell patterning
Microstencil membranes with through holes were created by flowing polydimethylsiloxane (PDMS) (Dow Corning, MI) between SU8 pillar structures. A silicon wafer containing cylindrical SU8 pillars of 57 μm height and 20, 30, 40, 50, and 100 μm diameters was initially fabricated utilizing standard soft lithography process. This pillar height was used to maintain feasible aspect ratios of SU8 which, however, can be customized. Pillars were spaced 50 μm edge-to-edge for each diameter. The stencil membranes were created by spin-coating a small amount (∼1 g) of polydimethylsiloxane at a 10:1 ratio with a curing agent.48,49 Due to varied surface areas of different diameter pillars and hence their surface tension mediated resistance to the flow of PDMS, the pillar dimensions required different spinning parameters (Table I) to form a uniform PDMS coating.
TABLE I.
PDMS spinning parameters for fabrication of micro-stencils with different perforation dimensions.
| Pillar diameter | Step 1 RPM/time (s) | Step 2 RPM/Time (s) | Healing time (min) |
|---|---|---|---|
| 20 μm | 800/30 | 2600/40 | 15 |
| 30, 40, 50 μm | 800/30 | 2800/40 | 5 |
| 80, 100 μm | 800/30 | 2800/80 | 5 |
These parameters were characterized to obtain ∼40 μm-thick membranes for sufficient structural integrity, yet keeping the thickness lower than pillar heights in order to achieve through-holes. After spin-coating PDMS, the wafer was placed on a leveled surface at room temperature for 5 min allowing PDMS to uniformly settle and heal. Subsequently, PDMS was cured on a hotplate at 150 °C for 2 min followed by gradual cooling to room temperature.
A major challenge that has traditionally limited the utilization of microstencils is the membrane fragility. To prevent membrane tendency to break while peeling off from the wafer, a subsequent layer of PDMS was spread with a spatula around the patterned portion to create a thicker border above the cured PDMS membrane. An area of ∼2 × 2 cm was carefully painted leaving a circular region of about 5 mm in the middle to retain the perforated membrane region. Several stencils of each pattern dimension were developed individually using this technique, cured at 150 °C for 5 min, cut, and carefully lifted off the wafer to obtain a membrane stencil as shown in Fig. 1. Membranes were analyzed for the presence of through holes utilizing a phase contrast microscope.
FIG. 1.
(a) Sketch of the silicon wafer containing SU-8 pillars of different dimensions. Four replicas of each dimension (20, 30, 40, and 50 μm diameter) were fabricated. Pillars were spaced at 50 μm edge-edge in each design. (b) A thin PDMS layer (represented in light blue color) was spin coated on SU-8 features such that the membrane thickness is slightly lower than the pillar height. The dark blue region represents the space where uncured PDMS (∼1 mm thick) was manually deposited to provide structural robustness to the membrane. (c) demonstrates the perforated stencil being cut and gently peeled off the wafer. (d) left: placement of the stencil within an untreated polystyrene Petri dish. The borders of the stencil and the perforated region were marked on the back side of Petri dish for convenient location of patterns after plasma exposure and subsequent removal of the membrane. Right: micrograph of a perforated membrane surrounded by a thicker border.
For proper maintenance of membranes, all stencils were stored inside covered Petri dishes and cleaned of debris and dust when necessary by brief sonication in 70% isopropanol for 20 s followed by a rinse in deionized water. We were able to use each microstencil at least 50 times after which usage monitoring was discontinued.
B. Surface modification for creating hydrophilic pattern islands
For creating hydrophilic micropatterned regions, non-tissue culture treated 6 well plates were utilized for all experiments. After transferring PDMS membranes within the wells, corners of the membrane were lifted with tweezers to escape trapped air bubbles until the membrane was uniformly placed against the surface and properly centered. The dish and membrane assembly was oxygen plasma (Plasma Etch, Carson City, NV) treated for 1 min. This resulted in a pattern of hydrophilic islands wherever the hydrophobic Petri dish surface was exposed corresponding to the holes in the membrane film. The outline of the stencil as well as the membrane region was traced onto the bottom surface of the well with a lab marker before removing the stencil. The 6-well plate was subsequently exposed to ultraviolet radiation for 5 min to sterilize the device. The surface was then treated with 10 μl of 1% non-fouling solution of tripolymer Pluronics F-127 for 15 min.49,50 This enhances the disparity between hydrophilic and hydrophobic regions suppressing the non-specific adhesion of cells on hydrophobic regions (Fig. 2).10 F-127 was then removed by aspiration, and the dish was washed twice with sterilized deionized water and thoroughly dried.
FIG. 2.
(a) A 30 μm membrane imaged at 20× in phase contrast with an inverted microscope. Holes can be ascertained through refraction of light through PDMS. (b) Validation of hydrophilic micropatterns after plasma exposure using Rhodamine-B. (c) MDA-MB-231 cells patterned without Pluronic F-127 treatment exhibit projections across islands, and (d) cells patterned after the Pluronic surface modification remain contained within pattern spaces. Scale bar = 100 μm.
C. Preparation of parafilm coverslips and cell solutions
The MDA-MB-231 breast cancer cell line was used for all experiments (ATCC, Manassas, VA). Cells were cultured to confluence in DME (Dulbecco's Modified Eagle Medium) containing 10% Fetal Bovine Serum and 1% Penicillin-Streptomycin followed by resuspension to a final concentration of 6.0 × 106 cells/ml in warm media. Before diluting to the desired cell concentration, cells were filtered through a 40 μm filter tube (P/N 352235 Corning Inc., Corning, NY) to remove cell aggregates and obtain a homogeneous suspension of single cells.
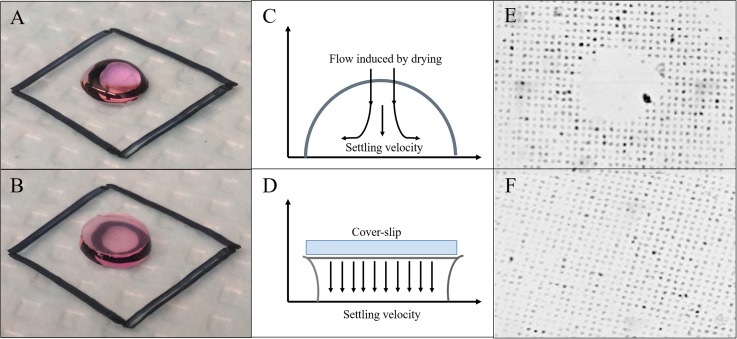
We utilized small amounts of cell solutions and created uniform arrays of desired cell distributions using only a 2-4 μl droplet of cell suspension spread across the patterned region. The number of individual pattern spaces per stencil was enumerated (by analyzing the membrane image with ImageJ), and cells were then diluted to a range of corresponding seeding concentrations in a series of microcentrifuge tubes. Cells were added in varying droplet volumes to patterns in seeding concentrations from 50% to 300%, where 50% corresponds to half as many cells as individual pattern spaces available. Depending on the droplet aspect ratio, temperature gradients and evaporation can cause circular convective flows within the droplets. Since these flows can interfere with the gravitational settling of cells and inhibit their attachment, cell suspension droplets were either covered with a buoyant circular coverslip created by hole-punching through parafilm to suppress convective flows or left uncovered [Figs. 3(a) and 3(b), respectively]. In addition to the coverslip, adding 1 ml of PBS (Phosphate-Buffered Saline) to the outside of the pattern near the well edges prevented cells from drying while cells were allowed to adhere and incubated for 3 h at 37 °C. Patterns were subsequently washed by adding 1 ml of PBS gently on the outside of the pattern area and allowing the entire pattern to be immersed. The PBS was then aspirated without allowing the surface to dry. DMEM was gradually added in 200 μl increments to keep the patterned surface hydrated at all times.
FIG. 3.
(a) An uncovered cell suspension droplet placed on the patterned surface. (b) Droplets covered with a 6 mm diameter circular parafilm coverslip to suppress convective flows. (c) Convective flow pattern generated within uncovered droplets as a result of droplet dimension and drying behavior. (d) Sketch of suppressed convection within the droplet covered with a suspended parafilm coverslip. (e) Non-uniform cell patterning due to the creation of a convective zone in the middle of the droplet where cells are in motion and fail to adhere. (f) An even and efficient cell patterning under covered droplet conditions.
D. Imaging
Fluorescence as well as bright field microscopy was achieved using the EVOS FL Auto microscope. The patterned region was defined across opposing vertices and scanned at regular intervals to obtain a time-lapsed representation of the complete pattern (supplementary material: micropatterningvideo.mov). An onstage incubator was used to maintain cells under appropriate culture conditions. All image processing and data measurements were performed using ImageJ (NIH, Bethesda, MD) and GraphPad Prism software (GraphPad Software, La Jolla, CA).
Immunofluorescence was used to validate cell configuration. This was accomplished after washing the patterned surfaces with PBS thrice, followed by fixing cells with 4% paraformaldehyde, and permeabilization with 0.01% Triton X-100. After blocking with 1% BSA (bovine serum albumin) in PBS with 0.05% Tween-20 to prevent nonspecific binding of antibodies, cells were counterstained with a 1 μM concentration DAPI (Life Technologies, Grand Island, NY). Phalloidin-Cy5 conjugate (Life Technologies) was added only to the pattern area in the center of the dish and was allowed to incubate in dark for 15 min.
III. RESULTS
The fragility of perforated membranes has remained challenging, inhibiting widespread utilization of microstencil based patterning of single cells. We have developed a novel and simple technique to address this limitation and created structurally robust microstencils illustrating practical feasibility of the stencil-based approach. In addition, multiple factors that affect cell patterning efficiency including cell seeding density, size of the patterned islands, size and shape of the cell droplet, and the desired spatial configuration of cells on individual islands were analyzed to generate characterization plots for optimized pattern uniformity and throughput.
A. Stencil creation, surface modification, and cell patterning
Uncured PDMS at 10:1 ratio is highly viscous, and thus pillar diameter and spacing have a significant effect on its flow characteristics due to surface tension. Both spinning speed and time affect membrane thickness, and these parameters were characterized to reliably produce perforated membranes for different diameter patterns. Lower than optimum speeds resulted in membranes too thick to achieve through holes, while non-uniform or broken membranes were obtained at higher speeds. Consequently, larger diameter pillars required higher speeds or longer durations as compared to lower diameters. Parameters listed in Table I were the result of different spins (data not shown) characterized to obtain a membrane thickness of ∼40 μm and reliably produced through holes using masters with 57 μm tall pillars. The addition of a secondary PDMS layer around the central perforated film resulted in significantly robust (∼1 mm thick) stencils (Fig. 1), allowing each stencil to be used for extended periods (>50 times). Among other microscopy techniques, the presence of holes was easily verified by visually inspecting the wafer for a leftover layer of PDMS film on top of pillars that rendered the surface slightly less reflective.
The hydrophilic islands on a polystyrene substrate, corresponding to stencil holes, were created by exposure to oxygen plasma. The presence of hydrophilic islands was validated by placing a drop of Rhodamine-B solution on the pattern region and visualizing the preferential deposition of the fluorescent dye on the exposed surface as shown in Fig. 2(b). Exposure duration had little effect on pattern formation; however, a one minute exposure consistently provided uniform patterns and was used for all subsequent experiments. The hydrophilicity of oxygen plasma treated substrates is short-term (∼2-3 h) and requires further surface modifications to avoid cell attachment in the unexposed regions. Therefore, cells were seeded immediately after plasma exposure and sterilization steps. Patterning efficacy was verified by filling the Petri dish with a cell solution containing MDA-MB-231 cells in excess (∼300 000 cells for about 3000 available patterned spaces). Although cells mostly adhered to the hydrophilic islands, they occasionally adhered onto the non-specific hydrophobic regions as well [Fig. 2(c)]. Treatment with 1% Pluronic F-127 resulted in a highly non-adhesive surface for the unexposed (or hydrophobic) regions of the polystyrene substrate while not affecting the hydrophilic regions. We observed that cell adhesion on non-patterned regions was significantly suppressed and cells attached only on the circular modified surfaces [Fig. 2(d)]. While higher concentrations of F-127 (5% and 10%) also demonstrated similar effects, 1% solution required fewer washing steps and provided comparable results. Additionally, cells remained restricted to the patterned regions even after 4 days of culture in contrast to the F127-untreated patterns where cells began to spread beyond the pattern boundaries. We believe this unconfined expansion is mediated either by the gradual deposition of serum proteins on the non-patterned space or due to the production of exogenous ECM by patterned cells. In conclusion, we have demonstrated a simple technique to rapidly fabricate robust microstencils and generate clean cell patterns on ECM free substrates.
B. Effect of intra-droplet convective flows on cell patterning
To obtain clean spatial patterns and avoid the requirement of excess cells to fill the entire Petri dish, a small droplet of cell suspension was placed over the patterned area and incubated for 3-4 h. This is the time normally required by MDA-MB-231 cells to attach on the tissue culture treated substrates. With this approach, we observed that cells either adhered on the peripheral islands [Figs. 3(a), 3(c), and 3(e)] or only in the middle region (data not shown) of the pattern array. Allowing longer incubation times did not improve the patterning efficiency. Instead, the patterning performance was diminished as the droplet size reduced due to evaporation, desiccating the peripheral patterned cells. In contrast, cells patterned uniformly when the entire well was filled with cell solution. Although, doing so also deposited cells outside the stencil area that was exposed with oxygen plasma, which was another limitation in addition to the requirement of excessive cells when filling the entire Petri dish. To further elucidate this behavior, we explored the possibility of insufficient cell-substrate interactions causing region-specific patterning. When observed through time-lapse microscopy, cells demonstrated intra-droplet movement confirming that the cell-surface interaction was transient and inconsistent. The cell suspension droplet experiences several factors such as evaporative drying and cell settling that govern their surface interactions and subsequent attachment. As the droplet dries at the contact edge, fluid is pulled outward from the center of the droplet due to the concentration gradient created across the droplet.37
Numerical analysis of evaporation induced intra-droplet convection is well established in the literature.44–47 However, for the first time, we empirically observed these effects in the context of cell patterning as the cells failed to uniformly adhere and only patterned forming a ring along the periphery of the droplet or only in the center of the array. The larger the drop, the higher the droplet aspect ratio, and therefore the stagnation zone (area with no flow where cells do not move relative to original placement) remained in the central region of the droplet. With smaller droplets, the lower aspect ratio induced stagnation around the periphery of the droplet due to relatively small contact angle, allowing cells to remain adhered while cells in the center of the droplet continuously moved out toward the periphery.
To investigate the effects of convective flows, experiments were initially conducted using different droplet sizes ranging from 2 μl to 20 μl volume. The overall diameter of the region containing an array of patterns was ∼5 mm, and the 2 μl droplet was not enough to cover the entire patterned area but provided relatively better pattern uniformity. Increasing droplet volume provided sufficient coverage but also diminished patterning efficacy due to the increased aspect ratio. Therefore, to suppress intra-droplet flows, we developed a simple yet effective method by introducing a buoyant coverslip over the cell suspension droplet [Fig. 3(b)]. Placement of a 6 mm diameter parafilm coverslip (conveniently and consistently prepared using a hole punch) not only prevented evaporation and consequent convection but also maintained a very low aspect ratio of fluid regardless of the droplet volume [Figs. 3(d) and 3(f)]. Additionally, the fluid coverage area was dictated by the diameter of the coverslip and remained constant throughout the incubation period independent of the droplet volume. Utilizing different droplet volumes as before, the height of fluid meniscus varied from 40 to 180 μm for 4-20 μl droplets, respectively. With this approach, we reliably obtained >80% patterning efficacy in terms of the occupied versus available pattern spaces. A 4 μl droplet was used in all subsequent experiments to obtain a monolayer of cells underneath the coverslip without any physical contact with cells. For comparison, the microcontact approach provides almost full coverage, while inkjet cell printing has been reported to provide 87% coverage.21 However, the complexities of these processes as well as the requirement of an initial number of cells were significantly higher and offer little control over the configuration of cells within individual patterns.
To further compare the advantages of the coverslip technique, patterns from uncovered and covered droplets were analyzed for two different parameters: (1) effect of pattern dimension and (2) droplet volume. Seeding density of cells was kept constant at 300% of the available spaces for each dimension. Resulting micropatterns were scanned to assess the effects of convective flows on pattern efficacy. A two-tailed t-test was performed to derive significance of efficacy between covered and uncovered approaches. Across all four pattern sizes (20, 30, 40, and 50 μm diameter), the uncovered droplets showed significant variability and inconsistency in the patterned surface as compared to the covered droplets (p < 0.05) [Fig. 4(a)]. In addition, the coverslip approach provided almost a twofold increase in the average number of occupied pattern spaces with >80% coverage across all dimensions.
FIG. 4.
Pattern efficacy characterized for pattern dimension and droplet volume between covered and uncovered droplets. (a) The effect of pattern diameter on patterning efficacy. Patterning diameter had little effect on efficacy with an exception of 20 μm where coverage was ∼50% due to insufficient adhesion area available to cells. The patterning efficacy for uncovered droplets was inconsistent compared to covered droplets (n = 6) with >80% for covered droplets. (b) The effect of droplet volume on patterning efficacy. For droplets greater than 10 μl volume, coverage was significantly improved for covered droplets. Error bar = SD and * represents p < 0.05.
Intra-droplet convection largely depends on the droplet aspect ratio which in turn depends on the droplet volume particularly in case of uncovered drops placed on a tissue culture plastic substrate. Cell suspension droplets of 2, 6, 10, 15, and 20 μl volumes were placed on the patterned area (40 μm diameter) and evaluated for patterning efficacy with and without a coverslip. Due to greater intra-droplet convection, a larger droplet volume contributed to a greater drift of cells outward from the center of the pattern and up toward the apex of the droplet. This flow behavior contributed to a significantly lower patterning efficacy and distribution between different configurations of micro-cultures for uncovered droplets of volumes 10 μl or greater [Fig. 4(b)]. However, the pattern coverage was similar for 2 and 6 μl volumes for both conditions while demonstrating greater inconsistency for uncovered droplets. We found that placement of parafilm coverslips greatly inhibited intra-droplet convection for larger droplets (≥10 μl volume) and produced precise micro-culture distributions that inhabited a greater percentage of the patterned area (p < 0.05) [Fig. 4(b)].
The uncovered droplets, in addition to selectively created patterns in the center of the modified surface, also exhibited drying and influenced the configurations of cells by presenting a higher incidence of clusters as further discussed in Sec. III C. In contrast, the results obtained by the covered droplets were consistent across all repetitions. Thus, when large sample sizes, smaller pattern spaces, or customized cell configuration within each pattern are desirable, the methodology described herein allows quick customization and reliable results.
C. Effect of pattern dimension and seeding density on cell configuration
Micropatterned cells appeared in five different configurations; singlets occupying the entire pattern area, singlets partially occupying the patterned area, and doublets, triplets, and clusters of greater than three cells. For various applications, it may be necessary to control the incidence or percentage of pattern spaces occupied by a specific configuration. The configuration of cells within a pattern is relevant to the amount of contact cells will experience, how cells will spread across the growth space, and the potential for rearrangement. For instance, a study involving cell-cell contact may require a field of two or three isolated cells (doublets or triplets, respectively), whereas a study involving motility or cell-cell signaling may require a lower density of cells in a larger spread area. With existing techniques, there is little to no control over how cells adhere to a pattern. With the control of intra-droplet flows utilizing the coverslip technique, we were able to characterize and reproduce desired pattern configurations. We tested all relevant combinations of cell seeding densities (50%-300% cells with respect to available pattern spaces) and cell spread areas to establish a correlation between the pattern creation and resulting cell configurations. Results were compared with probabilistic Poisson distribution and characteristic plots were created that can allow selection of customized parameters for any given study design.
We found that the distribution of cells in these five different configurations is directly correlated to the dimension of the pattern area as well as cell seeding density. The characteristics of four pattern dimensions used in our study are summarized in Table II. In these experiments, we randomly chose patterns featuring a circular geometry; however, other geometries can also be achieved using the stencil formation method described in this manuscript. All patterns featured a 50 μm edge-to-edge distance between adjacent hydrophilic islands for consistency.
TABLE II.
Observed cell behaviors and utility of different dimensioned micro-stencils.
| Pattern diameter | Spread area | Predominant configuration | Application | ||
|---|---|---|---|---|---|
| Cell motility | Cell-cell communication | Cell growth | |||
| 20 μm | ∼300 μm2 | Singlets | No | No | No |
| 30 μm | ∼700 μm2 | Doublets | No | Yesa | Yesa |
| 40 μm | ∼1250 μm2 | Triplets | Yes | Yes | Yesa |
| 50 μm | ∼2000 μm2 | Clusters | Yes | Yes | Yesa |
These dimensions can be used for the respective applications when coupled with appropriate seeding densities. The combination of these factors allows for control over resulting cell configurations.
Cell suspensions were serially diluted to 50% through 300% seeding densities, such that 50% represents 1 cell per 2 pattern spaces and likewise. Patterns of 20, 30, 40, and 50 μm diameters were seeded with cell dilutions after enumerating the number of holes present in each specific stencil. Since each membrane was created individually, the number of holes varied between stencils due to manual painting of the thicker PDMS border. Allowing sufficient time for cells to adhere (3-5 h) after cell seeding, scans of each pattern space were annotated for each configuration type across the micropattern. The distribution of configurations on each pattern was tabulated and used to characterize the effect of seeding densities. As an illustration, Figs. 5(a) and 5(b) show micrographs of a 40 μm pattern seeded at 300% cell density. A pattern region was randomly picked [Fig. 5(b)] where cells were patterned in all possible configurations of singlets, doublets, triplets, and multiples within the same frame. This further demonstrates the applicability of characterized parameters to minimize such random occurrences and obtain a majority of the desired configurations. Figure 5(c) shows normalized ratios of cells constituting each configuration at different pattern diameters and seeding densities. The 20 μm diameter pattern resulted in mostly singlets due to lack of enough adhesion area for more than one cell to attach. The increase in pattern diameter resulted in a greater number of cells/pattern island. Similarly, the number of cells per pattern demonstrated an upward trend with increasing cell densities for each pattern size. Thus, the characterization plot in Fig. 5 provides a quick and convenient way to choose the appropriate seeding densities for any desired cell configuration for a given pattern diameter depending on some of the applications summarized in Table II. For the purposes of this experiment, all droplets (4 μl volume) were covered with the parafilm coverslip to promote greatest pattern efficacy.
FIG. 5.
(a) A 40 μm micropattern of MDA-MB-231 cells imaged at 10× in bright field seeded at 300% cell density. Cells can be seen clearly occupying the pattern spaces and remaining within the circular confines of each island, however in different configurations. (b) Image of the same pattern as in (a) stained with Phalloidin for actin filaments (pink) and DAPI for the nuclei (blue). Image shows the distribution of four different configurations (singlet, doublet, triplet, and cluster) within a randomly selected single frame reflecting heterogeneity of patterning configuration when seeded at a random cell density. (c) Effect of seeding density on configuration distribution across different pattern sizes. For covered droplets (n = 6) across six seeding densities, the configuration shifts toward a great number of cells per island as the cell seeding density and the pattern size increases under greater seeding concentration conditions. This provides a characterization plot to choose appropriate parameters for obtaining a desired pattern configuration. Scale bar = 100 μm. Error bar = SEM.
The 50 μm stencil offered the most successful pattern for larger micro-cultures with the greatest pattern efficacy as well as configuration diversity at all seeding densities. Individual cells occasionally spread across the entire circular pattern, while multi-celled islands primarily shared the patterned space. Through time-lapse imaging, continued cell motility was observed in the form of rotational movement and rearrangement within the restricted area. As the patterned cells divided over the course of five days, cells competitively occupied the patterned space. This replicates results from colony studies, which indicate cell size and available area as critical factors in cell growth.51 The overall distribution of cell configurations varied greatly depending on the combination of pattern size and seeding density.
Utilizing the resultant empirical data in conjunction with Poisson's probability distribution, characteristic curves were derived, which can be used to decide optimal seeding densities for specific applications. Using Poisson's distribution, the probability of producing a desired incidence of a particular configuration can be calculated. Poisson's distribution is a limiting form of the binomial distribution and can be used to predict the number of independent outcomes in a given time interval or region of space based on average observations.52 Using the observed experimental outcome, the probability of producing the same outcome can be projected. The probability of a given outcome is defined by
| (1) |
where μ is the average observed outcomes in a given region and x is the incidence of interest. Treating each of the configurations as a dominant independent occurrence, the probabilities for the incidence of singlets, doublets, and triplets are described in Fig. 6.
FIG. 6.
Probability distributions for singlets, doublets, and triplets across different seeding densities and pattern diameters. Only the three most relevant seeding densities are shown for each pattern size.
Thus, Poisson's distribution provides a correlation between the dimensions of the stencil, the seeding densities, and the droplet size to the end pattern. For instance, to achieve a 50% incidence of singlets using a 50 μm microstencil, cells must be seeded at 150% seeding density, since these parameters yield the highest probability of achieving the desired pattern configuration. Similarly, to achieve the highest number of doublets, a 40 μm microstencil with 200% seeding must be used. In short, these plots can be used as a reference to customize the cell adherence behaviors for any given application, by modifying the size of the stencil used and the seeding density. While we specifically used the human breast cancer cell line MDA-MB-231 to characterize the cell configuration, these probability distributions can be used for any adherent cell type for single cell analyses or colony studies.
IV. DISCUSSION
In addition to physical characteristics of cells, a number of signaling and genetic pathways are responsible for normal and aberrant cell growth, based on their involvement in cell motility, cell division, and cell adhesion.53–55 Current studies assess cell size, cell area, cell density, and cell interactions in a culture environment rather than with a Single Cell Analysis (SCA) approach. While these studies are relevant to a greater understanding of the physical cellular growth mechanisms and their physiological counterparts, they neglect individual cell behaviors. For instance, the concurrence of cellular processes in a colony masks the hallmarks of cancer development,55,56 whereas recent literature promotes the idea that a specific subset of individual cancer cells may be responsible for metastatic growth. During normal development of epithelial tissues, metastatic transformations occur which are characterized by loss of mobility, mitotic arrest, and acquisition of morphology. Contact between cells is not sufficient for inhibition of mitosis; rather, the mechanical restrictions are further required to cease the subsequent divisions producing cells with smaller cell area. Also, once the cell division cycle becomes longer than 24 h due to cell density and contact inhibition, it is difficult to study single cell activity within a colony.57,58 SCA using micropatterning allows a greater level of control both on experimental design, as well as cellular growth and observation capabilities. Due to the relatively small number of cells (hundreds to a few thousand) in the patterned culture, nutrient consumption was minimal. To retain the secreted factors within the culture, part of the old media was carefully removed and replenished on the third day of the five-day study. Depending on the pattern size and occupancy, cells divide in some islands but not in others. Typically, on a 40 or 50 μm island, cells tend to divide more often until there are 2-4 cells/island. Subsequently, either the division slows down or the daughter cells are created in the suspension due to unavailability of adhesion surface while the parent cell stays attached. We did not observe any detachment of cells due to the competition from daughter cells or during media replenishment. This further offers interesting insights into cancer progression under restrained microenvironments and provides a robust in vitro strategy to investigate conditions that may lead the generation of circulating tumor cells.
Micropatterning also creates the possibilities of greater control over the mechanical environment of patterned cells. Traditionally, micro-contact printing has been used due to the delicate nature of micropatterning films. However, in our approach of painting a thicker border of PDMS around the patterning film, the resulting microstencil patterning (μSP) membranes are robust and withstand aging and damage. We have used these μSP membranes over the course of four months (>50 times) without loss in integrity. The process of creating these membranes is also far more rapid compared to existing techniques and is on the order of minutes rather than hours. Owing to the characterization of spin speeds and times, the membranes can reliably be created each time, minimizing the need for time-consuming tests on films to ensure hole creation. The microstencil patterning may also facilitate convenient deposition of ECM islands without the incubation and treatment time needed in micro-contact printing. Whereas protein inks must be created and back-treated in order to be hospitable to cell adhesion in micro-contact printing, ECM substrates could be directly applied in significantly smaller amounts before peeling off the stencil from the Petri dish to obtain well defined ECM patterns. The reusability is not an issue when hydrophilic micropatterns are created with oxygen plasma alone without any ECM deposition. However, as in the case of microcontact printing, application of ECM proteins through microstencils may result in protein adsorption on PDMS risking cross-contamination when reused with different proteins. To avoid this, microstencils can be heated at 150 °C for a few minutes after each use to denature proteins, or each stencil can be dedicated for use with a specific ECM. Additionally, exposure to high energy oxygen plasma (one of the patterning steps) will also denature any adsorbed proteins on the microstencil membrane preventing the risk of cross-contamination between experiments.
The addition of Pluronics deposition to the μSP protocol allows significant control and precision in the resulting cell field. Without enhancing the hydrophobic regions surrounding each hydrophilic pattern space, cells were prone to projecting into neighboring regions. To increase the integrity of cell interactions within patterns, a 1% Pluronics F-127 solution was allowed to deposit on the space for 15 min. While greater concentrations, such as 5% and 10%, may also be used to the same effect, we observed that the 1% solution is least viscous and demands the simplest washing procedure.
Confounding factors such as convective evaporative flows were also optimized to improve pattern efficacy. The forces governing convective flows are relatively stronger than gravity mediated cell settling and sufficiently counterbalance the cell-surface interaction necessary for cell adhesion. These flows are usually inferior when the evaporative flux is high, or when the solution has a flat and relatively uniform surface tension. Thus, with the utilization of a buoyant coverslip, the influence of convective flows on μSP is negligible when the entire patterned surface is covered such that cell density dominates the buoyancy forces. Our novel technique of covering droplets also reduces drying, controls droplet contact area, normalizes surface tension across the droplet, and allows for reproducible, non-random pattern generation.
We have created precise and rapidly producible coverslips with a single hole-punch to cut the parafilm. Parafilm provides an appropriate weight and hydrophobicity to alter the droplet dimensions such that there is a uniform concentration gradient even if the droplet gradually dries under the coverslip. All covered droplets exhibited significantly greater pattern efficacy and reduced incubation with 3-4 h as an optimal incubation duration as compared to uncovered droplets.
A major challenge with existing micropatterning techniques is their integration within microfluidic devices.59 Most PDMS based microfluidic devices are bonded onto the glass or other PDMS surfaces through oxygen plasma treatment, which can be detrimental to the patterned surfaces. However, in the microstencil approach presented here, we already expose our microstencil with oxygen plasma in order to expose the open hole regions, as part of the patterning process. This could serve a dual purpose and allow the subsequent bonding of another separately prepared PDMS device to bond to the patterned surface. Although this functionality was not validated as part of this study, the potential to integrate a variety of micropatterns (cells, proteins, DNA, etc.) within microchannels can offer enormous potential for applications such as combinatorial drug screening, response to shear stresses, and biosensors.
A greater level of precision is possible with micropatterning; the μSP protocol herein satisfies the criteria of longevity, robustness, precision, and customization potential. Factors allowing superior precision in single cell analysis open the possibilities of studying crucial growth mechanisms in cancer development and metastasis.60,61 Quantification of individual cell characteristics such as stress can also be accurately measured using this μSP protocol.62–64 The seeding density, dual-step surface modification, and convective drying of droplets all contribute to the precision and longevity of micropatterned cultures. Cultures can be maintained long-term without loss of growth, mobility, or contact. The micropattern array can still be treated as a culture study; chemokines and growth factors can be added to the cell line while identifying and enumerating individual responses.
V. CONCLUSIONS
The analysis of cell and disease functions at the single cell level is critical to account for cell-cell variability. We have presented a simple and rapid strategy to create robust microstencils for practical feasibility, which can be used dozens of times to create patterns of cells or any desired substrate. We have also developed a unique floating coverslip approach to conveniently suppress intra-droplet convective flows that interfere with cell-surface interaction and reduce micropatterning efficacy. Finally, we have characterized various parameters such as cell seeding density, pattern size, and droplet volume and created characteristic plots for obtaining the desired configuration of cells within micropatterning as appropriate for specific applications. The results presented herein provide a significantly simpler and reliable technique to deposit cells and substrates for applications such as the mechanical anisotropy, cell-cell communication, and contact inhibition.
SUPPLEMENTARY MATERIAL
See supplementary material for the time-lapse animation of randomly distributed cells self-assembling at locations of hydrophilic islands forming a micropatterned cell array.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (NSF) (Award No. 1550976).
References
- 1.Snijder B. and Pelkmans L., Nat. Rev. Mol. Cell Biol. 12(2), 119–125 (2011). 10.1038/nrm3044 [DOI] [PubMed] [Google Scholar]
- 2.Basan M., Elgeti J., Hannezo E., Rappel W. J., and Levine H., Proc. Natl. Acad. Sci. U.S.A. 110(7), 2452–2459 (2013). 10.1073/pnas.1219937110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puliafito A., Hufnagel L., Neveu P., Streichan S., Sigal A., Fygenson D. K., and Shraiman B. I., Proc. Natl. Acad. Sci. U.S.A. 109(3), 739–744 (2012). 10.1073/pnas.1007809109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee R. M., Kelley D. H., Nordstrom K. N., Ouellette N. T., and Losert W., New J. Phys. 15(2), 025036 (2013). 10.1088/1367-2630/15/2/025036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marel A. K., Rappl S., Alberola A. P., and Radler J. O., Macromol. Biosci. 13(5), 595–602 (2013). 10.1002/mabi.201200400 [DOI] [PubMed] [Google Scholar]
- 6.Nnetu K. D., Knorr M., Pawlizak S., Fuhs T., and Kas J. A., Soft Matter 9(39), 9335–9341 (2013). 10.1039/c3sm50806d [DOI] [Google Scholar]
- 7.Wilk G., Iwasa M., Fuller P. E., Kandere-Grzybowska K., and Grzybowski B. A., Phys. Rev. Lett. 112(13), 138104 (2014). 10.1103/PhysRevLett.112.138104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streichan S. J., Hoerner C. R., Schneidt T., Holzer D., and Hufnagel L., Proc. Natl. Acad. Sci. U.S.A. 111(15), 5586–5591 (2014). 10.1073/pnas.1323016111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukoreshtliev N. V., Haase K., and Pelling A. E., Cell Tissue Res. 352(1), 77–94 (2013). 10.1007/s00441-012-1531-4 [DOI] [PubMed] [Google Scholar]
- 10.Blagovic K., Gong E. S., Milano D. F., Natividad R. J., and Asthagiri A. R., Curr. Opin. Biotech. 24(5), 940–947 (2013). 10.1016/j.copbio.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campas O., Mammoto T., Hasso S., Sperling R. A., O’Connell D., Bischof A. G., Maas R., Weitz D. A., Mahadevan L., and Ingber D. E., Nat. Methods 11(3), 349–349 (2014). 10.1038/nmeth0314-349d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canavan H. E., Cheng X. H., Graham D. J., Ratner B. D., and Castner D. G., J. Biomed. Mater. Res. A 75a(1), 1–13 (2005). 10.1002/jbm.a.30297 [DOI] [PubMed] [Google Scholar]
- 13.Cochet-Escartin O., Ranft J., Silberzan P., and Marcq P., Biophys. J. 106(1), 65–73 (2014). 10.1016/j.bpj.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillot C. and Lecuit T., Science 340(6137), 1185–1189 (2013). 10.1126/science.1235249 [DOI] [PubMed] [Google Scholar]
- 15.Li J. F. and Lowengrub J., J. Theor. Biol. 343, 79–91 (2014). 10.1016/j.jtbi.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L., Hartley R., Reiss B., Sun Y. H., Pu J., Wu D., Lin F., Hoang T., Yamada S., Jiang J. X., and Zhao M., Cell Mol. Life Sci. 69(16), 2779–2789 (2012). 10.1007/s00018-012-0951-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz S. A. and Chen C. S., Soft Matter 3(2), 168–177 (2007). 10.1039/B613349E [DOI] [PubMed] [Google Scholar]
- 18.Theveneau E. and Mayor R., Cell Mol. Life Sci. 70(19), 3481–3492 (2013). 10.1007/s00018-012-1251-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler A. R., Throndset W. R., Whelan R. J., Leach A. M., Zare R. N., Liao Y. H., Farrell K., Manger I. D., and Daridon A., Anal. Chem. 75(14), 3581–3586 (2003). 10.1021/ac0340758 [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Rivas A., González-Quijano G., Proa-Coronado S., Séverac C., and Dague E., Micromachines 8(12), 347 (2017). 10.3390/mi8120347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yusof A., Keegan H., Spillane C. D., Sheils O. M., Martin C. M., O’Leary J. J., Zengerle R., and Koltay P., Lab Chip 11(14), 2447–2454 (2011). 10.1039/c1lc20176j [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., Krishnamoorthy S., Song H., Zhang Z., and Xu C., J. Appl. Phys. 121(12), 124904 (2017). 10.1063/1.4978744 [DOI] [Google Scholar]
- 23.Ozkan M., Pisanic T., Scheel J., Barlow C., Esener S., and Bhatia S. N., Langmuir 19(5), 1532–1538 (2003). 10.1021/la0261848 [DOI] [Google Scholar]
- 24.Kim J. J., Bong K. W., Reategui E., Irimia D., and Doyle P. S., Nat. Mater. 16(1), 139–146 (2017). 10.1038/nmat4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phamduy T. B., Sweat R. S., Azimi M. S., Burow M. E., Murfee W. L., and Chrisey D. B., Integr. Biol. 7(9), 1068–1078 (2015). 10.1039/C5IB00151J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahmias Y. and Odde D. J., Nat. Protoc. 1(5), 2288–2296 (2006). 10.1038/nprot.2006.386 [DOI] [PubMed] [Google Scholar]
- 27.Ino K., Ito A., and Honda H., Biotechnol. Bioeng. 97(5), 1309–1317 (2007). 10.1002/bit.21322 [DOI] [PubMed] [Google Scholar]
- 28.Collins D. J., Devendran C., Ma Z., Ng J. W., Neild A., and Ai Y., Sci. Adv. 2(7), e1600089 (2016). 10.1126/sciadv.1600089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding X., Lin S. C., Kiraly B., Yue H., Li S., Chiang I. K., Shi J., Benkovic S. J., and Huang T. J., Proc. Natl. Acad. Sci. U.S.A. 109(28), 11105–11109 (2012). 10.1073/pnas.1209288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho C. T., Lin R. Z., Chang W. Y., Chang H. Y., and Liu C. H., Lab Chip 6(6), 724–734 (2006). 10.1039/b602036d [DOI] [PubMed] [Google Scholar]
- 31.Thery M., Racine V., Piel M., Pepin A., Dimitrov A., Chen Y., Sibarita J. B., and Bornens M., Proc. Natl. Acad. Sci. U.S.A. 103(52), 19771–19776 (2006). 10.1073/pnas.0609267103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerf A., Cau J. C., Vieu C., and Dague E., Langmuir 25(10), 5731–5736 (2009). 10.1021/la9004642 [DOI] [PubMed] [Google Scholar]
- 33.Guckenberger D. J., de Groot T. E., Wan A. M. D., Beebe D. J., and Young E. W. K., Lab Chip 15(11), 2364–2378 (2015). 10.1039/C5LC00234F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui E. E. and Bhatia S. N., Proc. Natl. Acad. Sci. U.S.A. 104(14), 5722–5726 (2007). 10.1073/pnas.0608660104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurth F., Eyer K., Franco-Obregon A., and Dittrich P. S., Curr. Opin. Chem. Biol. 16(3–4), 400–408 (2012). 10.1016/j.cbpa.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 36.Russell M. T., Pingree L. S. C., Hersam M. C., and Marks T. J., Langmuir 22(15), 6712–6718 (2006). 10.1021/la060319i [DOI] [PubMed] [Google Scholar]
- 37.Thery M. and Piel M., Cold Spring Harbor Protoc. 2009(7), pdb.prot5255 (2009). 10.1101/pdb.prot5255 [DOI] [PubMed] [Google Scholar]
- 38.Johnson D. M., LaFranzo N. A., and Maurer J. A., J. Vis. Exp. (55), e3164 (2011). 10.3791/3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen K., Qi J., and Kam L. C., J. Vis. Exp. (22), 1065 (2008). 10.3791/1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azioune A., Storch M., Bornens M., Thery M., and Piel M., Lab Chip 9(11), 1640–1642 (2009). 10.1039/b821581m [DOI] [PubMed] [Google Scholar]
- 41.Folch A., Jo B. H., Hurtado O., Beebe D. J., and Toner M., J. Biomed. Mater. Res. 52(2), 346–353 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Tan J. L., Liu W., Nelson C. M., Raghavan S., and Chen C. S., Tissue Eng. 10(5–6), 865–872 (2004). 10.1089/1076327041348365 [DOI] [PubMed] [Google Scholar]
- 43.Qin D., Xia Y., and Whitesides G. M., Nat. Protoc. 5(3), 491–502 (2010). 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- 44.Hu H. and Larson R. G., J. Phys. Chem. B 110(14), 7090–7094 (2006). 10.1021/jp0609232 [DOI] [PubMed] [Google Scholar]
- 45.Majumder M., Rendall C. S., Eukel J. A., Wang J. Y. L., Behabtu N., Pint C. L., Liu T.-Y., Orbaek A. W., Mirri F., Nam J., Barron A. R., Hauge R. H., Schmidt H. K., and Pasquali M., J. Phys. Chem. B 116(22), 6536–6542 (2012). 10.1021/jp3009628 [DOI] [PubMed] [Google Scholar]
- 46.Kang K. H., Lim H. C., Lee H. W., and Lee S. J., Phys. Fluids 25(4), 042001 (2013). 10.1063/1.4797497 [DOI] [Google Scholar]
- 47.Pline A. D., “Infrared surface temperature measurements for the surface tension driven convection experiment.” NASA Technical Memorandum 101353 (1989).
- 48.Cheng Q., Li S., and Komvopoulos K., Biomaterials 30(25), 4203–4210 (2009). 10.1016/j.biomaterials.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 49.Cheng Q. A., Komvopoulos K., and Li S., J Biomed. Mater. Res. A 96a(3), 507–512 (2011). 10.1002/jbm.a.32992 [DOI] [PubMed] [Google Scholar]
- 50.Decker J. T., Sheats J. T., and Brennan A. B., Langmuir 30(50), 15212–15218 (2014). 10.1021/la504215b [DOI] [PubMed] [Google Scholar]
- 51.Cerruti B., Puliafito A., Shewan A. M., Yu W., Combes A. N., Little M. H., Chianale F., Primo L., Serini G., Mostov K. E., Celani A., and Gamba A., J. Cell Biol. 20(2), 359–372 (2013). 10.1083/jcb.201305044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain R., The Art of Computer Systems Performance Analysis: Techniques for Experimental Design, Measurement, Simulation, and Modeling (Wiley, 1991). [Google Scholar]
- 53.Deforet M., Hakim V., Yevick H. G., Duclos G., and Silberzan P., Nat. Commun. 5, 3747 (2014). 10.1038/ncomms4747 [DOI] [PubMed] [Google Scholar]
- 54.Hirashima T., Hosokawa Y., Iino T., and Nagayama M., Biol. Open. 2(7), 660–666 (2013). 10.1242/bio.20134523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim N. G., Koh E., Chen X., and Gumbiner B. M., Proc. Natl. Acad. Sci. U.S.A. 108(29), 11930–11935 (2011). 10.1073/pnas.1103345108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kempf H., Hatzikirou H., Bleicher M., and Meyer-Hermann M., PLoS Comput. Biol. 9(11), e1003295 (2013). 10.1371/journal.pcbi.1003295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gagliardi P. A., Puliafito A., di Blasio L., Chianale F., Somale D., Seano G., Bussolino F., and Primo L., Sci. Rep. 5, 10206 (2015). 10.1038/srep10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatzikirou H., Bottger K., and Deutsch A., Math. Model. Nat. Phenom. 10(1), 94–107 (2015). 10.1051/mmnp/201510105 [DOI] [Google Scholar]
- 59.Rhee S. W., Taylor A. M., Tu C. H., Cribbs D. H., Cotman C. W., and Jeon N. L., Lab Chip 5(1), 102–107 (2005). 10.1039/b403091e [DOI] [PubMed] [Google Scholar]
- 60.Chiou K. K., Hufnagel L., and Shraiman B. I., PLoS Comput. Biol. 8(5), e1002512 (2012). 10.1371/journal.pcbi.1002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McClatchey A. I. and Yap A. S., Curr. Opin. Cell Biol. 24(5), 685–694 (2012). 10.1016/j.ceb.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 62.Deguchi S., Ohashi T., and Sato M., J. Biomech. 39(14), 2603–2610 (2006). 10.1016/j.jbiomech.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 63.Delarue M., Monte F., Vignjevic D., Prost J., Joanny J. F., and Cappello G., Biophys. J. 107(8), 1821–1828 (2014). 10.1016/j.bpj.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagayama K. and Matsumoto T., J. Biomech. 43(8), 1443–1449 (2010). 10.1016/j.jbiomech.2010.02.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See supplementary material for the time-lapse animation of randomly distributed cells self-assembling at locations of hydrophilic islands forming a micropatterned cell array.