Abstract
With the device integration of sweat stimulation, sweat becomes a stronger candidate for non-invasive continuous biochemical sensing. However, sweat stimulants are cholinergenic agents and non-selective to just the sweat glands, and so, direct placement of sweat stimulants poses additional challenges in the possibility for uncontrollable transport of the stimulant into the body and challenges in contamination of the sweat sample. Reported here is membrane isolation of repeated-use sweat stimulants for mitigating direct dermal contact, dilution of the sweat stimulant, and contamination of the sweat sample. The membrane dramatically reduces passive diffusion of the sweat stimulant carbachol by roughly two orders of magnitude, while still allowing repeated sweat stimulation by iontophoretic delivery of the carbachol through the membrane and into the skin. Both in-vivo and in-vitro validation reveal feasibility for reliable integration of sweat stimulants within a wearable device for use periods of 24 h or more. In addition, advanced topics and confounding issues such as stimulant gel design, osmotic pressure, and ionic impurities are speculatively and theoretically discussed.
I. INTRODUCTION
Eccrine sweat is arguably an attractive biofluid for wearable biosensing compared to other non-invasive alternatives.1,2 However, many advantages of sweat biosensing are largely dependent upon the subject actively sweating.2 Fortunately, there have been recent demonstrations of integrated iontophoretic sweat stimulation3–5 along with proof that stimulation can last as long as several days.6 A typical sweat stimulation device has a hydrogel which contains a surplus of dissolved stimulant (>100×) such that a reliable and repeated iontophoretic dosing can be achieved.6 The most promising sweat stimulants are those that are slowly or not-metabolized by the body (e.g., they have a long lasting effect).6 The long-lasting cholinergenic stimulants such as methacholine and carbachol,3,6 while advantageous for stimulating sweat, are non-selective to just sweat glands and could pose potential risk if the additional stimulant could enter the body through a defect in the skin or passive diffusion through the epidermis6–8 and even could be aided by advective flow because interstitial fluid is at lower pressure than atmospheric pressure.9 One could argue to simply limit the total dose available in the reservoir of stimulant, but this could preclude the ability to provided repeated dosing, which in some cases may be required for >24 h of continuous sweat access and sensing.6 Also, as the amount of stimulated area (<1 cm2) and sweat generation rate (<1 nl/min/gland) are reduced, the amount of required stimulant decreases dramatically,6 and one could further argue that the side effects from direct dermal exposure of the stimulant would be insignificant. However, even if so, a second and even greater challenge exists which could prevent the repeated use and stimulation over a 24 h or longer period. As sweat is generated beneath the stimulant gel, emerging sweat could dilute the stimulant in the gel to the point where the iontophoretic dosage is dominated, not by the sweat stimulant but, simply the iontophoretic transport of Na+, K+, and other naturally present ions in sweat. Furthermore, just as the sweat can dilute the stimulant, the volume of the gel can dilute the sweat sample and make it difficult if not impossible to obtain accurate concentration measurements of analytes in sweat.3 This confounding issue has been ignored in previous work.4 Therefore, there is a clear need for reliable methods to bring repeatable sweat stimulation to the skin surface, while properly isolating the stimulant from unintended dermal exposure and undesirable sweat dilution. This could be achieved mechanically, or by using one-use stimulant gels, but from a simplicity and ergonomics perspective, a fully integrated and non-mechanical approach is arguably a desirable option.
Reported here is a membrane isolation technique for repeated-use of sweat stimulants while mitigating both the direct dermal contact and the sweat dilution of the stimulant. The membrane reduces passive diffusion of the carbachol sweat stimulant by roughly two orders of magnitude, while still allowing repeated sweat stimulation as needed by iontophoretic delivery of the carbachol through the membrane and into the skin. Also presented are additional theoretical design considerations that further reveal the necessity of an isolation technique. For the empirical work, four potential membranes were selected and tested in-vitro (one track-etch and three nanofiltration membranes). The results show a reduction of passive carbachol diffusion from 2.17 μmol/cm2 per min for a track-etch membrane to 3.56 × 10−2 μmol/cm2 per min for a nanofiltration membrane. In-vivo validation of iontophoretic sweat stimulation was also performed over an area of 1.76 cm2, showing both proper dermal isolation of the stimulant until needed and uniform sweat stimulation. Only nanofiltration membranes moderately achieved a major goal of retaining >90% of stimulant for 24 h. Extensive in-vitro testing showed that there is a large variability in flux characteristics between membrane samples (between 71.7% and 96.5% retention of carbachol over 24 h), suggesting the need for further development of the membrane materials. In addition, advanced topics and confounding issues such as stimulant gel design, osmotic pressure, and ionic impurities are speculatively and theoretically discussed at the end of this report. This work has a significant applied value for wearable sweat biosensing technology by potentially increasing both the safety of use of sweat stimulation and the duration of use of an integrated sweat stimulation device and by mitigating dilution/contamination of the sweat sample to be measured.
II. MATERIALS AND METHODS
A. IRB protocol
Human subject testing was performed under the guidance of the University of Cincinnati's (UC) Human Research Protection Program (ID# 2016-4386 approved by the UC Institutional Review Board).
B. Reagents and materials
Carbachol 99% (CAS 51-83-2) was purchased from Professional Compounding Centers of America (PCCA, Houston, TX), 99.9% agarose (A9539) and bromophenol blue (B0126) were purchased from Sigma Aldrich (St. Louis, MO). Pure 5000 cST and 1000 cST cosmetic-grade polydimethylsiloxane (PDMS) were purchased from ClearCo Products (Willow Grove, PA). 0.25″ and 0.06″ thick acrylic was purchased from McMaster-Carr (Aurora, OH). SPI-Pore Polycarbonate track etch membrane filters (TEM) with a pore size of 0.01 μm (#E00157-MB) were purchased from SPI Supplies (West Chester, PA). Nanofiltration membranes (NFX, NFW, and NFG) were obtained from Synder Filtration (Vacaville, CA).
C. Formulation of in-vitro solutions/calibration curves
A stock solution of 1% carbachol (%w/w) was mixed together in deionized (DI) water. The stock solution of carbachol was then serially diluted in half 16 times to provide 17 different solution concentrations. Additionally, a 1.87% solution of sucrose (%w/w) in DI water was formulated to minimize osmotic pressure during passive diffusion testing (Sec. II D). All solutions were mixed for at least 30 min before testing. Each of the carbachol solution concentrations were tested for solution conductivity 3 times using a Gamry potentiostat Reference 600™ (Gamry Instruments, Warminster, PA) using the built-in single frequency EIS measuring mode with the following conditions: 2 kHz monitoring frequency, 5 mV AC voltage, 12 min, and 0.636 cm2 testing area. The measured conductance from these tests was then averaged and plotted for a calibration curve against the known concentration of carbachol. A trend line was then best-fit to the curve, which was used to determine the amount of carbachol for further testing. See supplementary material for this calibration curve and trend line function.
D. Apparatus for in-vitro Gamry potentiostat testing
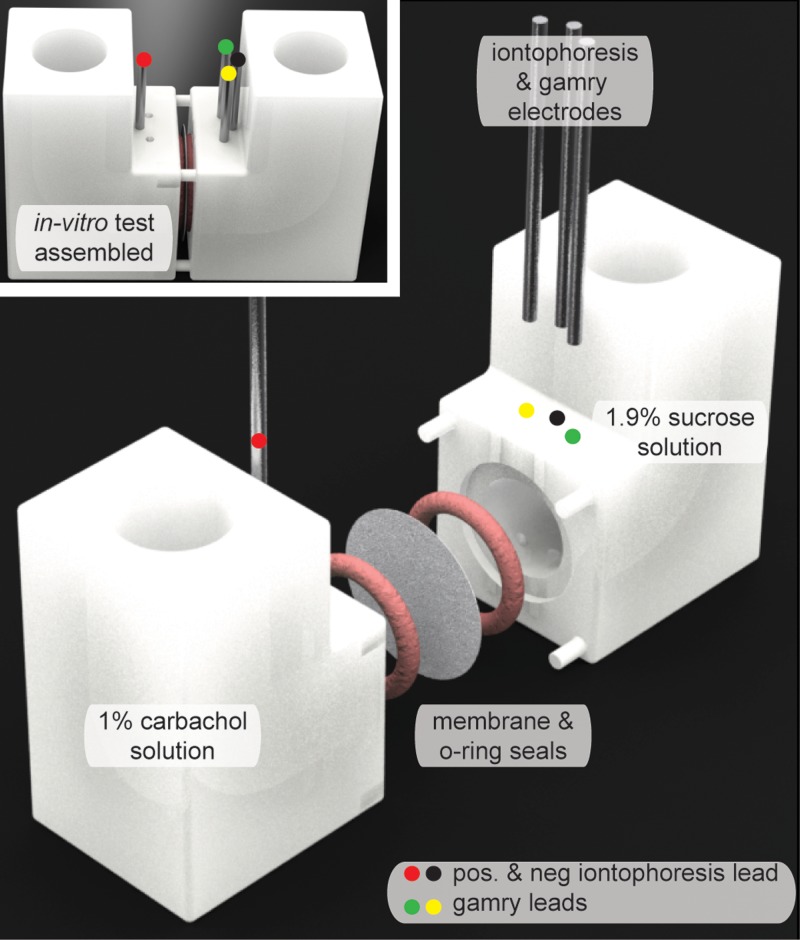
The apparatus for in-vitro testing is shown in Fig. 1, and the SolidWorks (Dassault Systemes, Waltham, MA) dimensions for this apparatus are provided as PDF files in the supplementary material for this paper. The apparatus parts were then 3-dimensionally (3-D) printed using a Form 2 printer with clear resin GPCL02 (Formlabs Inc., Somerville, MA) and the highest printing resolution (25 μm layer thickness). Silicone O-rings (ID = 1.5 cm, OD = 1.9 cm Item # 113S70, Monroe Engineering, Rochester Hills, MI) were adhered with Loctite Go2 glue (Henkel Corporation, Westlake, OH) to the apparatus to ensure that no leaking of fluid would occur. For all in-vitro tests utilizing the Gamry Potentiostat, each membrane was placed in-between the two parts of the apparatus and clamped together so that the solid seals would be made between the O-rings and the membrane. The resulting membrane area exposed to the solutions was therefore 1.76 cm2 which matches the stimulation area used later for in-vivo testing. The sensing leads for the Gamry potentiostat were inserted into parts of the apparatus across each other as seen in Fig. 1. Next, leads for an ActivaDose II (ActivaTek, Gilroy, CA) iontophoresis controller were inserted (one into each part with the positive lead being placed in the part opposite of the Gamry leads, Fig. 1). Then, the part of the apparatus with the Gamry leads was filled with 6 ml of the 1.87% sucrose solution (this ensures no osmotic water flux because the molality will be similar to the other side which has carbachol solution: 1.87 is the ratio of molecular weights 342/183). Opposite to the sucrose side solution, the cavity on the other side of the membrane was filled with 6 ml of 1% carbachol solution. The same routine mentioned above to obtain the conductivity calibration curve of the different carbachol solutions was used for all the in-vitro tests.
FIG. 1.
In-vitro test set-up for measuring both passive diffusion and iontophoresis across the membranes.
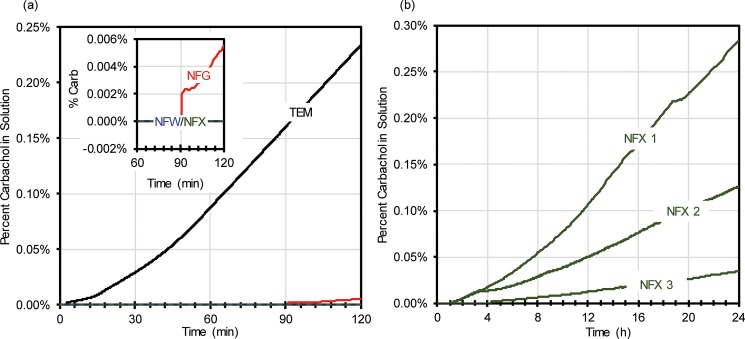
Passive diffusion tests for carbachol across the membranes were then ran for a total of 2 h for each membrane, with the results as shown in Fig. 3(a). The best membrane detailed in Fig. 3(a) was found to be the NFX membrane, which as shown in Fig. 3(b) was then tested three more times over 24 h. To further validate the best membrane which had the least passive diffusion (NFX membrane), repetitive iontophoresis testing was performed 3 times on the membrane for over 48 h with a total of 6 iontophoretic dosages, each dosage at the level used commercially in the Wescor Nanoduct iontophoresis system (0.28 mA/cm2, 42 mC/cm2). Mechanical failure testing was also performed on NFX membranes for 60 min. For both tests, a needle (18 gauge) was used to puncture a hole in the membrane, at roughly half way through the passive diffusion testing period.
FIG. 3.
(a) Percent of carbachol in solution over time for a single membrane (TEM—black, NFG—red, NFW—blue, and NFX—green) subject to passive diffusion for 2 h; (b) Percent of carbachol in solution over time for a single NFX membrane subject to passive diffusion for 24 h.
E. Gels for iontophoretic stimulation of sweat
Carbachol gel discs were fabricated using 1% carbachol and 3% agarose, by weight, in DI water. The carbachol and agar solution was first heated to 150 °C and stirred for 30 min. The aqueous solution of carbachol remains stable even when heated.10 Next, DI water lost due to evaporation during the heating/stirring process was added back to the solution, and this solution was then again heated at 80 °C for 30 min to ensure that the added water was evenly distributed. The carbachol/agarose solution was then cast in an acrylic mold which provided an array of discs with the dimensions of 0.75 mm radius and 1.5 mm thickness. The mold was then placed into a refrigerator at 8 °C where the discs were allowed to solidify. Finally, the carbachol discs were removed from the mold and stored at 8 °C in a plastic bag containing an additional 1% carbachol (%w/w) in DI water to help to prevent any drying of the disks. Additionally, control gels only containing 3% (%w/w) agarose (no carbachol) were formulated in the same way.
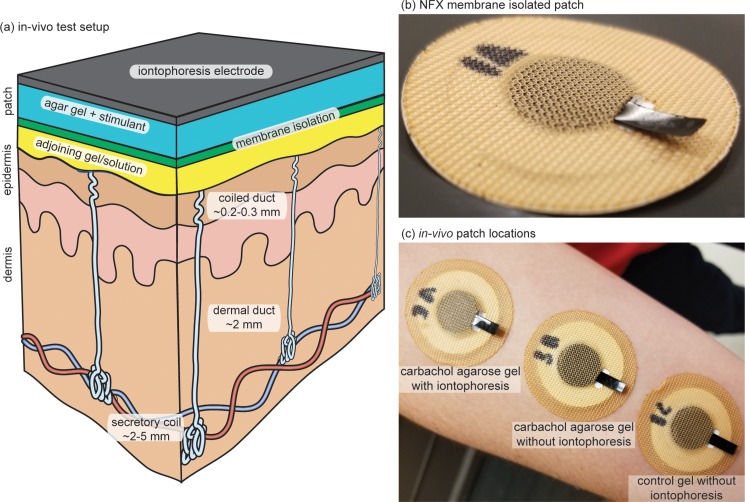
F. In-vivo patch design and assembly
The in-vivo patch was designed in AutoCAD (Autodesk San Rafael, CA). The full dimensions for these patches are provided as PDF file in the supplementary material. Various layers for the patches were then laser cut and placed together by lamination. The order of layers from the bottom of the patch (skin side) is as follows: along the periphery, only a ring of 3M 1577 double sided adhesive (3M, Maplewood, MN); for the adjoining gel/solution, a disk of Kimwipe soaked in DI water (Kimberly-Clark Professional, Roswell, GA) was placed inside the ring of 3M 1577; nanofiltration membrane (NFX); 1% carbachol gel or a control 3% agarose gel; carbon-coated Kapton film (<105 Ω/square); and 3M 9926T medical adhesive (3M, Maplewood, MN). Note that the carbon Kapton film was inserted through the 3M 9926T medical adhesive so that good electrical contact for iontophoresis could be made without allowing liquid from the gel to wick to the skin. Also, it is important to mention that a carrier layer was used to help to align all the layers during assembly. For each subject, 2 patches were assembled using the carbachol gels, while the third was assembled with the control agarose-only gel.
G. Iontophoresis stimulation
Iontophoresis was performed utilizing a commercial iontophoresis unit with current and dosage controls (ActivaDose II, ActivaTek, Gilroy, CA). Before stimulation, subjects first had the volar surface of their dominate forearm cleaned with isopropanol (IPA) and DI water to remove potential contaminants. Then, 3 patches were fixated to the clean location as shown in Fig. 2(c). A return electrode of the same gel disk was also placed on the clean surface. Iontophoresis (0.28 mA/cm2, 42 mC/cm2) was then performed, only at the carbachol agarose gel patch that received iontophoresis as shown in Fig. 2(c) which is the standard iontophoretic dose commonly used by the commercial Wescor Nanoduct sweat stimulation device.
FIG. 2.
In vivo testing of membrane technology. (a) In-vivo test setup; (b) Iontophoresis stimulation patch with NFX membrane isolation; (c) In-vivo test set-up.
H. Sweat pore imaging
Sweat pore imaging was performed to qualitatively validate the efficacy of the membrane isolation technique in-vivo. Sweat pores were imaged 30 min following testing. For each photograph, a suspension of bromophenol blue dye in cosmetic-grade silicone oil was placed on the arm for 1 min. Images were then taken, and ImageJ was used to count the number of pores. This oil/dye technique has been previously utilized and reported by our group11 and by others.12 The oil/dye mixture used here consisted of pure 5000 cSt and 100 cSt cosmetic grade PDMS oil which was mixed at a 1:3.5 ratio. For mixing, bromophenol blue dye (powder form) was added to obtain a final concentration of 7% (w/w) in the oil and dispersed with a vortex mixer and ultrasonication (the dye does not dissolve in the oil).
With the oil/dye approach, the oil prevents evaporation and the pH-sensitive dye partitions from the oil into the sweat emerging from the sweat ducts and stains the sweat blue. The result is that only locations of sweat are stained dark blue (the oil remains fairly clear). A photograph of the represented sweat pore result is shown in Fig. 6.
FIG. 6.
In-vivo bromophenol blue stain of 3 human subjects under each patch.
I. Statistical analysis
The conductance of the 17 diluted carbachol solutions (n = 3) was plotted vs their respective concentrations. From this, a form fit function was applied to the data. The best fitting function (R2 = 0.9995) was − 2 × 10−5, where x is the conductance (S) and y is the % of carbachol in solution. All further conductance data obtained in the Gamry potentiostat testing were substituted into this formula to give the respective concentration of carbachol.
The 3 human subjects were all Caucasian with ages ranging between 23 and 29. One of the subjects was female. No irritation was demonstrated in any subject following iontophoresis; however, basic iontophoresis is known to possibly cause slight irritation.6
III. RESULTS AND DISCUSSION
In this section, the data and analysis techniques are described in further detail. In terms of the discussion, we will first review the general importance of this type of study and then discuss the data such that they can be appreciated in the appropriate context. Finally, we will discuss the feasibility of possible alternate methods for incorporation of stimulation methods into a long-term wearable patch.
A. Passive diffusion membrane characterization
Passive diffusion of carbachol across the 4 different membranes is presented in Fig. 3(a) in terms of percent of carbachol in solution over time. The slope (determined from the conductivity measurements) from these 2-h tests was then used to determine what the concentration of carbachol would be following 24 h of testing. Using this method, it was determined that the TEM would reach equilibrium between the two solutions in roughly 3.3 h which is an order of magnitude shorter than all the nanofiltration membranes (NFG = ∼46.3 h, NFW = ∼88.0 h, and NFX = ∼260.5 h). As for the major goal of retaining 90% of the stimulant in solution over 24 h, these slopes can be used to determine an estimated flux. Using this method, only the NFW and NFX membranes appropriately limited the passive diffusion across the membrane by retaining 93% and 99% of the total carbachol, respectively (NFG was close, retaining 82% of carbachol). These results are expected if we consider the approximate molecular weight cutoff for each membrane (NFX = 150–300 Da, NFW = 300–500 Da, NFG = 600–800 Da, and TEM = ∼10 000 Da). Simply, the larger the molecular weight cutoff for the membrane, the larger the diffusive flux of carbachol (molecular weight of 182.696 Da).13
Initial testing shows that the nanofiltration membrane, NFX, provided the best overall chances for achieving 90% carbachol retention over 24 h (confirmed through the conductivity slope determination). However, the data from Fig. 3(a) are speculative as 24 h was not actually tested. Therefore, Fig. 3(b) details 3 separate tests on NFX membranes that were subject to 24 h of passive diffusion. From these results, it is easy to determine that there is a large variability in the carbachol flux for the NFX membrane (of the three tests, only one membrane achieved the major goal of retaining >90% of stimulant for 24 h, and the three tests retained 96.5%, 87.4%, and 71.7% of the total carbachol over 24 h). This leads to several additional implications and thoughts. First, it is possible with generic, nonspecific membranes to fulfill our major goal of retaining 90% of carbachol over 24 h. Second, additional work on quality control for NFX membranes is necessary for our particular application requirements. Third, it is likely that custom membrane design and fabrication could provide results that well exceed the performance shown herein. Fourth and lastly, it is possible to place 2 or more membranes in series to reduce the overall variability of a single layer membrane. An increase in membrane iontophoresis resistance14 would merely increase higher voltages utilized (along with proper current limiting for safety). As previously mentioned, NFX membranes have the lowest molecular weight cutoff of all membranes tested.
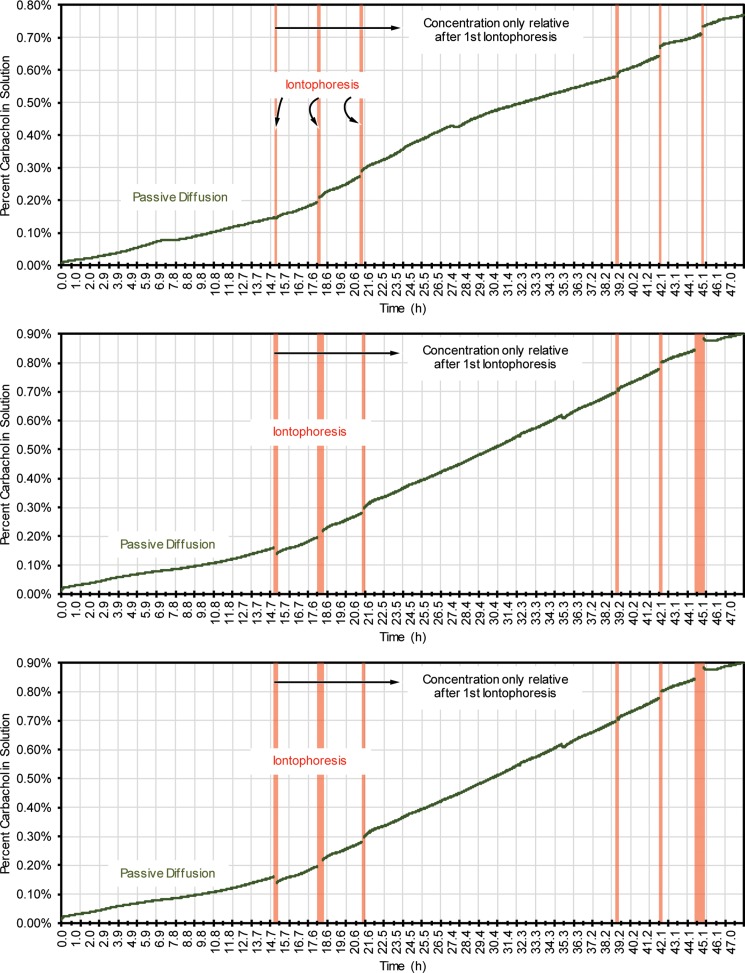
B. Repetitive iontophoresis NFX characterization
As stated in Sec. I, it is desirable to enable the potential for multiple iontophoresis stimulations for sweat stimulation.6 Additionally, the electrical stresses delivered to the skin in iontophoresis have never been previously described in membrane isolation technology. Therefore, it is critical to test the membranes against the effects of repeated iontophoresis and ensure that no degradation occurs. Fig. 4 shows 3 tests on the NFX membrane over 48 h and subject to 6 total iontophoresis stimulations, again at the dosages used for the Wescor Nanoduct system. All data during iontophoresis were removed from the graphs as they distract the data analysis (see supplementary material for the entire dataset). The data show a desirable outcome: repeated iontophoresis (19.88 μmol/cm2 h ± 16.6, 11.06 μmol/cm2 h ± 17.02, and 11.52 μmol/cm2 h ± 8.75 rate of carbachol flux due to iontophoresis for each test, respectively) does not significantly alter the rate of passive diffusion of carbachol through the membrane (3.01 μmol/cm2 h ± 0.89, 3.23 μmol/cm2 h ± 1.26, and 1.92 μmol/cm2 h ± 0.89 rate of passive carbachol flux for each test, respectively). As the results were initially analyzed, it was considered if the iontophoresis would cause hydrolysis of water, which could significantly skew the conductivity measurements in Fig. 4. With the testing conditions used, we do not believe that this is the case and postulate that the movement of carbachol across the membrane during iontophoresis is the major factor in the conductance change of the sucrose solution. However, to be safe, the reader should consider data after the initiation of iontophoresis as relative, and the concentration increase cannot be assumed to be entirely due to only carbachol diffusion.
FIG. 4.
Long duration and repeated stimulation testing with NFX 150-300 Dal membranes. Iontophoresis data were removed; see the text for more information and supplementary material for complete data.
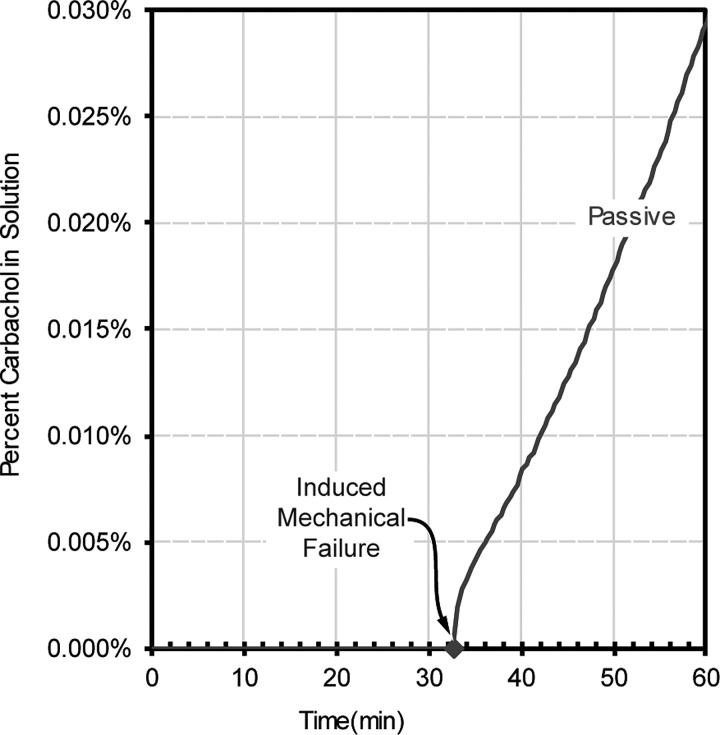
C. Induced mechanical failure NFX characterization
It is certainly possible that during manufacturing or use, the membrane could become damaged. An obvious solution would be to utilize two membranes (if the chance for a catastrophic membrane failure was one in one-thousand membranes, then two membranes in series reduce that chance to 1 in a million). We therefore performed tests to determine what a membrane failure might look like. As shown in Fig. 5, the percent of carbachol in solution following an 18-gauge needle puncture (induced mechanical failure) of the NFX membrane is shown in terms of passive diffusion. Before mechanical failure occurred, there was no measurable carbachol flux for the passive test; however, once mechanical failure occurred, the flux changed to 0.31 μmol/cm2h.
FIG. 5.
Induced mechanical failure testing of NFX 150–300 Dal membranes during passive diffusion.
D. In-vivo patch validation
Arguably, the most significant data originate from the in-vivo testing where it is confirmed that uniform sweat stimulation can be achieved by iontophoretic delivery of carbachol through the membrane and into the skin. For the visualization of the activated glands, several different patch testing conditions can be found in Fig. 6. All the patches were fabricated in the same manner but included different agarose gels, and only one had iontophoresis performed on it. From this data, ImageJ counts show that patches with carbachol gels with iontophoresis had ∼93, 67, and 94 glands/cm2 stimulated for subjects 1–3 respectively. Patches that had carbachol gels and no iontophoresis had ∼6, 19, and 35 glands/cm2 stimulated for subjects 1–3, respectively. Lastly, no glands were stimulated in patches that had pure agarose gels with no iontophoresis. This data confirms both the data obtained in-vitro and the efficacy that the patches work as designed. Still, it is evident that a small amount of passive diffusion of carbachol across the membrane and into the skin is still occurring, but as stated previously, this could be eliminated through careful optimization of the membranes themselves. The stimulation by passive diffusion is interesting, as it has not been previously demonstrated, but is of little applied value in our opinion because it is less controllable in dosage than iontophoretic delivery.
IV. THEORETICAL/DESIGN CONSIDERATIONS
This section addresses further justification for the testing conditions, theoretical design considerations, and additional rationale for incorporating a membrane isolation technique. It also delves into a more complex system that may be used for a more “perfect” membrane isolation technique that relies on other mechanisms than a membrane to ensure stimulant gel purity.
A. Design of the stimulant gel volume and percentage carbachol
To be consistent with our previous work3,6 and the work of others,15 the stimulation area was the same as the commercially utilized Wescor Nanoduct iontophoretic sweat stimulation system.16 This device utilizes pilocarpine, a short-duration sweat stimulant (∼60–90 min), and has a surface area of 1.76 cm2. Using the same device and total charge dose (∼74 mC) for delivery of carbachol, theoretically, 142 μg of carbachol stimulant should be delivered.6 If we assume over 24 h a delivery of 3 dosages of carbachol in this manner, a total of 426 μg of carbachol would be delivered. It is necessary to design these gels in a manner where the amount of carbachol will never be fully used, because if the carbachol is substantially depleted, other ions will dominate the iontophoretic dosage. Therefore, a design requirement is proposed here such that not more than 10% of the total stimulant is used in these 3 dosages, and therefore, 4.26 mg of carbachol would need to be placed in the gel. These gels are commonly 1% stimulant with 3% agarose by weight as described in Sec. II E. By using the total weight of the carbachol in the gel and knowing that this is only 1% concentration by weight, the total weight of the gel can be determined to be 0.426 g (wcarb/% of carbachol = wtotal). The gel is primarily water (96% or ∼0.409 cm3), and with our previous stimulation area requirement of 1.76 cm2, the height of the gel should therefore be ∼2.324 mm [height = volume/(π*radius2)]. This height is much less than ∼6.35 mm which is currently utilized in the Wescor Nanoduct and desirable from the perspective of designing a low profile (thin) wearable device. Finally, with reference to Fig. 3 and related discussion, we restate that loss of carbachol is not only due to iontophoresis (intended use) but also due to loss by a significant variation in the amount of passive diffusion of carbachol through the membrane. Therefore, the above calculations are dependent on implementation of improved membranes (e.g., tighter quality control, custom membranes, etc.).
B. Potential issues due to osmotic pressure across the membrane
Osmotic pressure across the membrane could cause water increase or loss in the carbachol solution and must be considered in the design requirements. Osmotic pressure can be calculated with the following equation: , with [Ci] being the molar concentration (mol/l), R the gas constant, and T the absolute temperature.17 Both the 1% carbachol gels (Sec. IV A) and the 1% carbachol in-vitro testing solutions (see Sec. II C) should theoretically have an osmolality of 55.3 mmol/kg carbachol. The mean osmolality of sweat is 117 mmol/kg for men and 134 mmol/kg for women,18 which is roughly double the osmolality of the carbachol disk. The osmolality of sweat depends heavily on the sweat generation rate3 and accounts for all the major (sodium, chloride, potassium, calcium, bicarbonate, and lactate), minor (magnesium, phosphate, sulphate, iron, copper, zinc, manganese, and iodine), and small organic solutes and proteins19 Therefore, water will at least be initially driven from the carbachol gel disk into sweat. There are several options to deal with this potential confounding issue. One option is to simply allow the gel disk to shrink (lose water). Another option would be to double the carbachol concentration, an equally viable option. A third option would be to constrain the gel with a rigid and impermeable housing on all sides except the membrane side such that osmotic pressure driven flow of water would be prevented. This third option is feasible because the resulting hydrostatic pressure on the membrane would be only 2.4 mN/cm2 (less than the pressure of 1 g/cm2). Finally, the osmotic pressure in the gel can be neglected if the gel was encapsulated in a rigid housing that was also affixed to the membrane. In this work, none of these options were implemented and were reserved for future work as they are all equally viable and simply dependent on design and use preferences.
C. Problems due to diffusion of ions between the stimulant and sweat
The passive diffusion of ions between the sweat and the stimulant reservoir is an important factor justifying the use of the isolation membrane. To keep the calculations simple in this subsection, we will make several assumptions. First, we will assume that the dominant cation is Na+ in sweat,19 and we will assume concentrations of sodium in sweat in both men and women of 36 mmol/kg and 41 mmol/kg,18 again both highly dependent on the sweat generation rate.3 Following previous discussion, the total volume of the gel would be 0.409 cm3. This would equal ∼0.015 mmol of sodium which is 38.7% of the total moles of carbachol located in the gel for men and ∼0.017 mmol/41.83% for women. In addition, previous calculations show that even in the absence of sweat, due to impurities, roughly only 30% of iontophoretic dose is carbachol during a normal stimulation.20 The potential increase in impurities due to ions in sweat would further decrease the amount of carbachol delivered and increase the total amount of iontophoresis needed which could cause increased skin irritation or damage due to the iontophoresis itself. This points again to the use of higher concentrations of carbachol in the gel.
V. SUPPLEMENTARY MATERIAL
See supplementary material for the entire iontophoresis data for repeated stimulation testing as well as mechanical failure, calibration curve, trend line function, and dimension and design files for the testing apparatuses.
ACKNOWLEDGMENTS
The authors would also like to acknowledge support from the U.S. Air Force Research Labs Award #FA8650-16-C-6760. This research was supported in part by an appointment to the Student Research Participation Program at the U.S. Air Force Research Laboratory, 711th Human Performance Wing administered by the Oak Ridge Institute for Science and Education Through an interagency agreement between the U.S. Department of Energy and USAFRL.
Dr. Jason Heikenfeld has an equity interest in Eccrine Systems, Inc., a company that may potentially benefit from the research results, and also serves on the company's board. The terms of this arrangement have been reviewed and approved by the University of Cincinnati in accordance with its conflict of interest policies.
References
- 1. Sonner Z., Wilder E., Heikenfeld J., Kasting G., Beyette F., Swaile D., Sherman F., Joyce J., Hagen J., Kelley-Loughnane N., and Naik R., Biomicrofluidics 9, 31301 (2015). 10.1063/1.4921039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heikenfeld J., Electroanalysis 28, 1242 (2016). 10.1002/elan.201600018 [DOI] [Google Scholar]
- 3. Sonner Z., Wilder E., Gaillard T., Kasting G., and Heikenfeld J., Lab Chip 17, 2550 (2017). 10.1039/C7LC00364A [DOI] [PubMed] [Google Scholar]
- 4. Kim J., Jeerapan I., Imani S., Cho T. N., Bandodkar A., Cinti S., Mercier P. P., and Wang J., Am. Chem. Soc. Sens. 1, 1011 (2016). 10.1021/acssensors.6b00356 [DOI] [Google Scholar]
- 5. Emaminejad S., Gao W., Wu E., Davies Z. A., Yin H., Nyein Y., Challa S., Ryan S. P., Fahad H. M., Chen K., Shahpar Z., Talebi S., Milla C., Javey A., and Davis R. W., Proc. Natl. Acad. Sci. U.S.A. 114, 4625 (2017). 10.1073/pnas.1701740114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmers P., Li S. K., Kasting G., and Heikenfeld J., J. Dermatol. Sci. 89, 40–51 (2017). 10.1016/j.jdermsci.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 7. Schytz H. W., Dan. Med. Bull. 57, B4223 (2010), PMID: 21122466. [PubMed] [Google Scholar]
- 8. Seeley R. R., Tate P., and Stephens T. D., Anatomy & Physiology, 8th ed ( McGraw-Hill, Dubuque, IA, 2008). [Google Scholar]
- 9. Guyton A. C. and Coleman T. G., Ann. N. Y. Acad. Sci. 150, 537 (1968). 10.1111/j.1749-6632.1968.tb14705.x [DOI] [PubMed] [Google Scholar]
- 10. O'Neil M. J., The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed. ( Royal Society of Chemistry, 2013). 10.1007/s13398-014-0173-7.2 [DOI] [Google Scholar]
- 11. Peng R., Sonner Z., Hauke A., Wilder E., Kasting J., Gaillard T., Swaille D., Sherman F., Mao X., Hagen J., Murdock R., and Heikenfeld J., Lab Chip 16, 4415–4423 (2016). 10.1039/C6LC01013J [DOI] [PubMed] [Google Scholar]
- 12. Tashiro G., Wada M., and Sakurai M., J. Invest. Dermatol. 36, 3 (1961). 10.1038/jid.1961.2 [DOI] [PubMed] [Google Scholar]
- 13.National Center for Biotechnology Information, PubChem Compound Database; (ID=5831, https://pubchem.ncbi.nlm.nih.gov/compound/5831).
- 14. Priya B., Rashmi T., and Bozena M., Expert Opin. Drug Delivery 3, 127 (2006). 10.1517/17425247.3.1.127 [DOI] [Google Scholar]
- 15. Losty H. C., Wheatley H., Doull I., Addresses A., and Losty H., Ann. Clin. Biochem. 43, 375 (2006). 10.1258/000456306778520025 [DOI] [PubMed] [Google Scholar]
- 16. Desax M.-C., Ammann R. A., Hammer J., Schoeni M. H., and Barben J., Eur. J. Pediatr. 167, 299 (2008). 10.1007/s00431-007-0485-0 [DOI] [PubMed] [Google Scholar]
- 17. Huang H.-C. and Xie R., arXiv:1201.0912 (2012).
- 18. Willing S. K. and Gamlen T. R., Clin. Chem. 33, 612 (1987), PMID: 3829402. [PubMed] [Google Scholar]
- 19. Bovell D., J. Local Glob. Health Sci. 2015, 5 (2015). 10.5339/jlghs.2015.5 [DOI] [Google Scholar]
- 20. Heikenfeld J. and Simmers P., UOC-17009WO, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See supplementary material for the entire iontophoresis data for repeated stimulation testing as well as mechanical failure, calibration curve, trend line function, and dimension and design files for the testing apparatuses.