Abstract
Microchannels made of fluoropolymers show potential merits due to their excellent solvent resistance, but such channels have not been widely used because of the complexity to fabricate them. This communication describes a method to prototype microfluidic devices using fluoropolymer films. The fabrication requires only two steps; cutting fluoropolymer films with a desktop cutting plotter and applying heat and pressure to laminate them. The method is rapid, simple, and low-cost. The conditions for heat press were identified for two common fluoropolymers: polytetrafluoroethylene and fluorinated ethylene propylene. The laminated films were confirmed to remain sealed with an internal pressure of at least 300 kPa. The fabricated devices were tested for the resistance to a set of organic solvents that would not be compatible with typical devices fabricated in polydimethylsiloxane. To highlight the potential of the fluoropolymer devices fabricated in this method, generation of droplets in a continuous stream of organic solvent using a T-junction channel was demonstrated. Our method offers a simple avenue to prototype microfluidic devices to conduct experiments involving organic solvents such as organic chemistry and in-channel synthesis of microparticles.
INTRODUCTION
This paper describes a method to prototype solvent-resistant microfluidic devices by laminating fluoropolymer films. In the method, stencils of fluoropolymer films are fabricated with a desktop cutting plotter. The stencils are then aligned and laminated with the same type of films via heat pressing to form microchannels. While heat press is a common method to seal polymer films, the processing parameters to seal fluoropolymer films to form microchannels have never been studied. This is the first report to identify them for two types of commercially available fluoropolymer films: polytetrafluoroethylene (PTFE) and fluorinated ethylene propylene (FEP). The entire fabrication can be completed on a laboratory benchtop within 1 h, and the process does not require intricate technologies and processes in a clean room. Rapid fabrication of such channels should offer a convenient platform to explore and perform experiments requiring solvent-resistant and non-adhering surfaces that fluoropolymers offer.
Since the inception of soft lithography,1,2 polydimethylsiloxane (PDMS) has been a gold standard in the fabrication of microfluidic devices. Many microfluidic devices have been developed using soft lithography and PDMS in academic laboratories owing to the simplicity of the fabrication and well-characterized properties of PDMS. A wide range of applications has been demonstrated using PDMS devices, including material syntheses, separation and sorting, diagnostics, and bioanalysis.3–9 Materials other than PDMS have been used for fabrication of fluidic devices such as epoxy, polyurethane, and thiol-ene.10,11–15 These alternative structural materials offer improved chemical and physical properties (e.g., hydrophobicity, resistance, and stability upon exposure to various solvents and different elasticities), while many of them are still not compatible with strong organic solvents including dichloromethane (DCM) and chloroform. Fluoropolymers are a general class of synthetic materials that contain fluorine atoms replacing hydrogen atoms in olefins. PTFE and FEP are two representative fluoropolymers that are widely available in various forms of commercial products—films, blocks, and tubes. Fluoropolymers exhibit good resistance to organic solvents and display chemical inertness.16,17 To this end, fluoropolymers are attractive alternative materials for microchannels when the applications require increased stability of microchannels.
Several research groups have demonstrated the fabrication of microchannels using fluoropolymers. In one approach, the inner lumens of microchannels were coated with a synthetic precursor of fluoropolymers to achieve desired surface properties.18 This type of post-coating changes dimensions of channels and also requires preparing channels using other materials in advance. Fluoropolymer channels (i.e., channels that entirely consist of fluoropolymers) have been prepared using microfabrication methods—hot embossing19 and molding.20 In either method, complementary patterns of the molds were prepared, and the patterns were transferred to fluoropolymers to create concave microchannels. Alternatively, fluoropolymer microchannels have been crafted directly using micromachining tools.21 When these approaches are employed, bonding of the patterned substrate to other flat surfaces is necessary to obtain closed channels. Bonding or sealing was achieved by heat press,19 polymer curing,20 and mechanical press.21 Despite these successful demonstrations, prototyping methods of fluoropolymer microchannels have not been widely used. This is largely because of the complexity of the fabrication; the process still requires operations in a clean room and/or synthesized precursors.19,20 These requirements have hindered convenient access to fluoropolymer microchannels in spite of their potential merits.
To alleviate these technical limitations, we developed a rapid and facile prototyping method for the fluoropolymer microchannels with minimal fabrication requirements. Rapid prototyping of microchannels has been demonstrated using xurography (i.e., a process to cut films with a razor blade to create stencils), followed by bonding of films and stencils to form closed channels.22–24 Using commercially available polymeric films and a digitally-controlled desktop cutting plotter, xurography is rapid, simple, and low-cost. To date, there has been no demonstration to apply xurography for fabricating microchannels in fluoropolymers. The objective of this study was to develop a method to prototype fluoropolymer microchannels using xurography. One of the challenges was that fluoropolymers were generally chemically non-reactive. As adhesive layers were not readily available for such fluoropolymers, we chose to bond the films using heat press. To this end, we identified optimal conditions for heat pressing for two types of commercially available fluoropolymers—PTFE and FEP. Finally, we validated that the fluoropolymer microchannels we fabricated exhibited resistance to representative organic solvents (dichloromethane, hexane, and toluene) that would quickly swell PDMS.
RESULTS AND DISCUSSION
Fabrication
The objective of this study was to develop a simple method to fabricate microchannels using commercially available films of fluoropolymers. Specifically, we aimed to develop a method performed in an ordinary laboratory with readily available starting materials. We selected xurography as a mean of fabrication for its simplicity. PTFE and FEP were materials for channels because of their low cost (0.4 USD for 100 × 100 mm2) and high commercial availability.
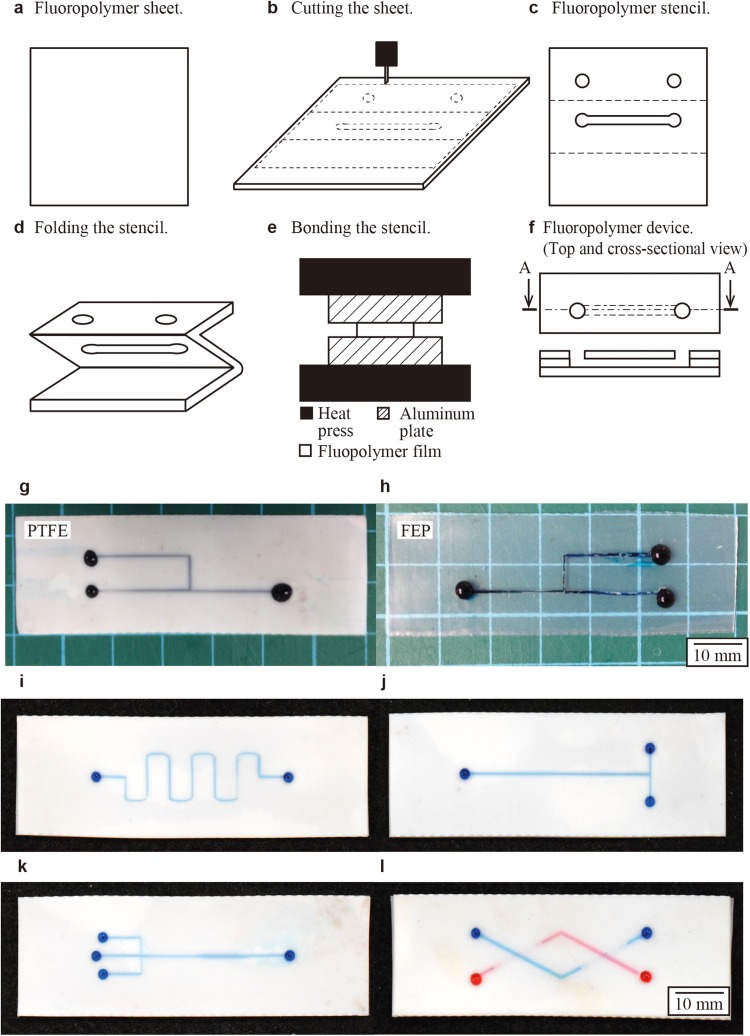
The fabrication consisted of three major steps: (1) patterning, (2) aligning, and (3) bonding. In the first step, patterns of microchannels were prepared as a computer-aided design (CAD) file. A desktop digital cutting plotter was used to make incisions on fluoropolymer films to create stencils [Fig. 1(a)]. In the simplest case, the stencil had three segments, each of which was 23 mm in width and 69 mm in length. Three layers served as the top, middle, and bottom layers of the device, respectively. The top layer provided the inlets and outlets of fluids; the middle layer had the patterns of microchannels; the bottom layer was without any incision and provided the bottom surface of the channel. To facilitate aligning different layers, we designed all three segments of the device in a single piece of the film; the adjacent segments were connected by dotted incisions (designed as dotted lines in the CAD file). These dotted incisions aided to fold the films and aligned the fluid openings with the channel. We folded the stencil in zig zag pattern [Fig. 1(b)]. The arrangement in zig zag pattern was to avoid the misalignment of different layers due to the thickness of the film. Subsequently, heat and pressure were applied to the folded construct to bond the surfaces that were in contact [Fig. 1(c)]. After heat press, the device was ready for use. A schematic illustration of the cross-sectional view of the assembled device is given in Fig. 1(d). Assembly of the microchannels in PTFE and FEP was confirmed using blue ink [Figs. 1(e) and 1(f)]. Optical micrographs showed that the device was free of leakage. Using this method, we fabricated the microchannels with representative geometries such as a T-junction and a flow-focusing nozzle [Figs. 1(i)–1(l)]. We note that this method can be readily extended for the fabrication of 3D microchannels using additional layers of the fluoropolymer film [Fig. 1(l)]. Microchannels with multiple inlets, branches, and intersections can be fabricated in this method, which would be difficult to achieve by simply combining Teflon tubes.
FIG. 1.
Fabrication of a fluoropolymer device using films. (a) and (b) A film of fluoropolymers (PTFE and FEP) was cut and patterned using a desktop cutting plotter. (c) A stencil of fluoropolymer was created. In this example, the stencil consisted of three (top, middle, and bottom) layers connected by dotted incisions. (d) The stencil was folded to create a device. (e) Heat and pressure were applied to seal the adjacent films in contact. (f) Schematic illustrations (top-down and cross-sectional) of the bonded films and [(g) and (h)] optical micrographs of the film devices entirely consisting of PTFE and FEP are shown. (i)–(l) Representative geometries of microchannels fabricated in PTFE. The channels are filled with water containing aqueous dyes.
Optimization of the conditions for heat press
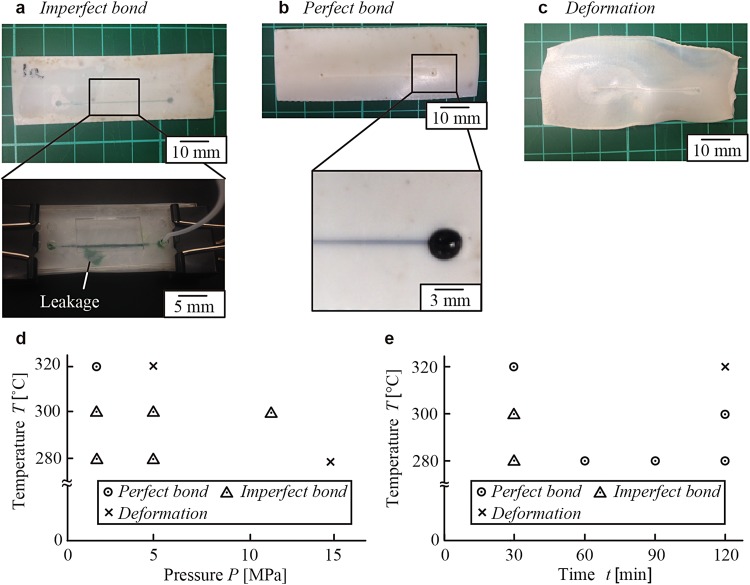
Bonding of fluoropolymer films by heat press was the essential part of the fabrication process. In preliminary experiments, we observed that both PTFE and FEP made adhesive bonding to the same film below their melting temperature (Tmp) (327 °C for PTFE and 275 °C for FEP).25 Care must be taken to perform heat press properly. We observed that PTFE and FEP films shrank at high temperature, and the films would easily deform (i.e., curl up and winkle) at and above Tmp. Deformation of films compromises proper contact between adjacent films and led to the failure of the fabrication. Therefore, the application of appropriate pressure is essential to achieve proper bonding. It is also worth noting that deformation and adhesion of the films can occur simultaneously at and above Tmp. The process needed to be carefully studied to identify the proper working conditions. We grouped the results of heat pressing as one of the following: (1) No bond: the films remained separate, which was observed when the applied temperature was too low, or when the duration of heating was too short. (2) Imperfect bond: the films did not adhere perfectly. In the perfusion test, fluids could permeate in the space between the films [Condition A, Fig. 2(a)]. (3) Perfect bond: the films adhered properly without any leakage of fluids and without any apparent deformation of the channels [Condition B, Fig. 2(b)]. (4) Deformation: the films deformed and no longer maintained the original shape. In this condition, the channels were likely to be collapsed [Condition C, Fig. 2(c)]. Herein, the goal was to identify parameters to perform the proper sealing of the films (Condition B).
FIG. 2.
Representative outcome of the bonding of fluoropolymer films. (a)–(c) The outcomes of bonding were grouped into three categories: Imperfect bond (Condition A), Perfect bond (Condition B), and Deformation (Condition C). Optimization of the condition for the heat press: (d) Initially, the heat press was performed for a constant duration of time (30 min) with a variation of pressure (P) and temperature (T). This study suggested that the outcome was sensitive to the change in pressure. (e) Under the fixed pressure (P), the heat press was tested with a variation of time (t) and temperature (T). Overall, the condition of (P, T, t) = (1.7 MPa, 320 °C, 30 min) for the bonding of PTFE films.
We considered three variables to conduct heat press of fluoropolymer films: (1) temperature, (2) pressure, and (3) duration of heating. Finding the right parameters for each variable was necessary to perform the fabrication successfully. To find the right condition for the sealing of PTFE, we initially fixed the duration of heating as 30 min and varied temperature and pressure. We observed the condition of films after the heat press [Fig. 2(d)]. Our observation suggested when the pressure was 5 MPa (or greater), the outcome was sensitive to small changes in the temperature. Indeed, we observed that the outcome changed from imperfect bond to deformation when the temperature was changed from 300 °C to 320 °C. It is rational to consider that there are optimal conditions between these temperatures, but it was not practical to identify these conditions within such a small operating window. In practice, there was little control over the outcome of bonding at this pressure (5 MPa or above), and it would be challenging to consistently achieve perfect bond. When the applied pressure was decreased to 1.7 MPa, the outcome varied gradually along with the increase in the temperature; at 320 °C, we achieved perfect bond of the PTFE films. Our result suggested that low pressure (1.7 MPa) was necessary to perform bonding of PTFE films by heat press with sufficient window of working conditions. In light of this outcome, we fixed the pressure at 1.7 MPa and conducted heat press by changing temperature and duration of heating. Our observation suggested that controlling the outcome of heat press was relatively easy when the pressure was 1.7 MPa; at this pressure, the duration of heating served as another parameter (in addition to temperature) to control the outcome state from imperfect bond to perfect bond for PTFE. For the identification of ideal bonding parameters, it was necessary to identify the condition of one parameter with which the system can vary gradually (i.e., P = 1.7 MPa). For adhesive bonding of PTFE films, our observation suggested that lowering the operating pressure was essential to understand and control the outcome of the bonding. We investigated the operation parameters for the bonding of FEP films in similar manners by fixing the applied pressure. Heat press performed at 1.7 MPa, at 240 °C, and for 10 min resulted in perfect bond of FEP films to fabricate microfluidic channels without leakage. We demonstrated that both fluoropolymers were applicable for the fabrication of microchannels using the heat press. Both materials exhibit the resistance to organic solvents. FEP is optically transparent and suitable to create microchannels that require observations under an optical microscope.
Delamination of the films
Appropriate bonding between the fluoropolymer films is a key requirement for the fabrication of microchannels. We performed a delamination test to ensure the strength of the bonding. The setup of the experiment is illustrated in the supplementary material (Fig. S1). Briefly, we applied a variable pressure to the closed microchannel fabricated using this method. We increased the applied pressure from 0 kPa at the increment of 50 kPa. We did not observe the delamination of the films at 300 kPa (∼3 atm). Further increase in the pressure caused the leakage of the fluid at the inlet of the channel (i.e., the interface between the syringe and the film). We note that the presented method of film bonding was performed on the laboratory benchtop, not in the clean room. In such conditions, uncontrolled encapsulation of small dust particles could occur. The fabricated device nevertheless remained sealed at least at 300 kPa. Because 300 kPa would be sufficiently high to deliver fluids with moderate viscosity (such as water, hydrocarbons, and fluorocarbons) through the channel on the order of 100 μm, we concluded that the proposed method of bonding provided appropriate sealing for our intended applications.
Dimension of the fabricated channels
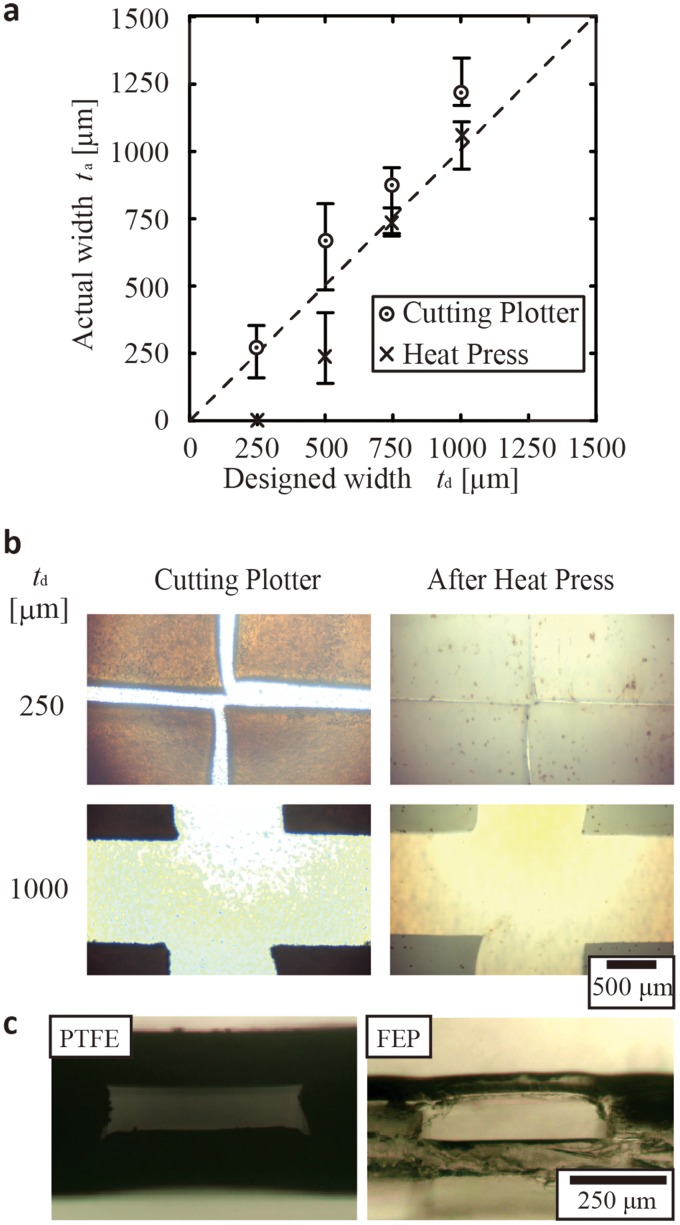
Adhesive bonding by heat press inevitably results in changes in channel dimensions. We chose the thickness of films as 200 μm for PTFE and 150 μm for FEP to ensure the opening of the channel without collapse after heat press and measured the widths of crossing microchannels before and after heat press. Optical micrographs of the PTFE stencils and channels, which correspond to patterns before and after heat press, respectively, are shown [Fig. 3(b)]. The bonding was performed at 1.7 MPa and 320 °C for 30 min.
FIG. 3.
(a) A plot showing the relationship between the designed width (td) and the actual width (ta) of the straight segment of the PTFE channel (film thickness = 200 μm). (b) Representative optical micrographs showing the changes in the dimension of the channels by heat press. The opening in a stencil was fully collapsed after the heat press for the channels with td = 250 μm, while the change was not as prominent for the channels with td = 1000 μm. (c) Optical micrographs showing the cross sections of the channels formed using this method of fabrication for PTFE (film thickness = 200 μm) and FEP films (film thickness = 150 μm). Each device was created by sealing three layers of the same films by heat press.
We observed variations in the channel widths that were inherent to the desktop cutting plotter used in the experiment [Fig. 3(a)]. The cutting plotter could create incisions with an average width of 250 μm. After heat press, the width of the resulting channels became smaller than the original width by 200 to 300 μm. When the original width was 250 μm, the channels were collapsed [Fig. 3(b), top]. The change in the dimension of the channel was more pronounced for narrow incisions (250 μm) than for wide incisions (1000 μm). This difference can be attributed to the different aspect ratio of the microchannels. In the current experiment, the thickness of the PTFE films was fixed as 200 μm, which was the primary factor to determine the height of the channel. The aspect ratio (defined as width divided by height) of the channel (before heat press) increased along with the increase in the channel width. We believe that the smaller aspect ratio of the narrower channels would lead to the larger deformation in horizontal dimensions because the pressure was applied from the vertical directions. It is also worth mentioning that the decrease in the dimensions was isotropic in all horizontal direction by heat press, suggested by the optical micrographs of the micropatterns [Fig. 3(b)].
In the related work by Wu et al., bonding two Teflon surfaces was achieved by exploiting volume expansion of Teflon at high temperature.19 The change in the lateral dimension of channels was not reported in that work. The difference is likely to be explained by the required bonding pressure. As the preceding work used replica molding on an SU-8 patterned silicon wafer to transfer patterns to Teflon, the resulting surface should be smoother than the films used in our current study. The lower surface roughness would require the lower bonding pressure by heat press. Our current work used commercially available fluoropolymer films as purchased; the roughness of the film was not controlled, which may result in the requirement of a certain pressure to achieve bonding the films.
Solvent resistance of fluorinated channels
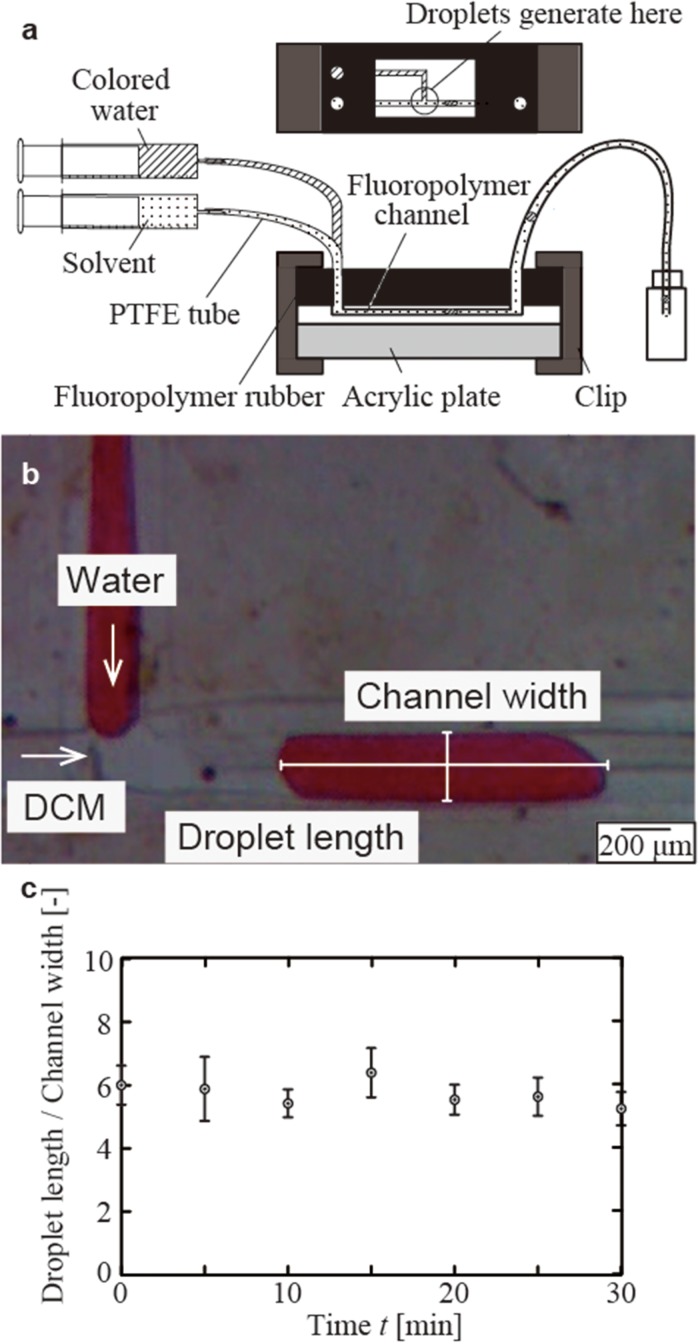
Fluoropolymers are known to exhibit resistance to solvents; they do not swell and dissolve when they are exposed to a wide range of solvents that would not be compatible with PDMS.18,21 We experimentally validated solvent resistance of microchannels made of FEP and PTFE. To demonstrate the solvent resistance of FEP, T-junction microchannels consisting of FEP films were tested for continuous generation of aqueous droplets in dichloromethane (DCM). DCM was used as a continuous phase directly in contact with the main channel. If the DCM was not compatible with the device, either the device would not maintain its original structure (by dissolving or swelling) or the performance of droplet formation would be compromised. For example, swelling of the channel would alter the apparent size of plug-shaped droplets in the channel. The setup of the experiment is illustrated [Fig. 4(a)]; glass syringes, PTFE tubes, and PTFE rubber blocks were used to deliver the fluid to the device. The tubing was held in the holes created in a PTFE rubber (with a diameter of 1 mm). To avoid leakage of the fluids, the pieces of PTFE rubber were manually forced to stay in conformal contact with the inlets and outlets of fluids on the film. We generated droplets of distilled water in a channel filled with DCM; the flow rates of distilled water and DCM were 150 μl/min and 50 μl/min, respectively. We observed the generation of droplets continuously for 30 min, and the in-channel length of the droplet was measured every 5 min [Figs. 4 (b) and 4(c)]. The length of the formed droplets (along the direction of the flow, scaled by the width of the channel) remained constant within their standard deviations. This observation suggested that the device entirely consisting of FEP is resistant to DCM.
FIG. 4.
Continuous generation of aqueous droplets in the stream of dichloromethane (DCM) in a T-junction of the FEP device. (a) Schematic illustration of the arrangement of the device. A block of fluoropolymer rubber with cylindrical apertures was used to ensure the connection of the tubes with the inlets and the outlets. (b) A representative optical micrograph showing the formation of aqueous droplets in DCM. (c) A plot showing the lengths of droplets (characterized as their non-dimensional lengths, divided by the width of the channel) over 30 min.
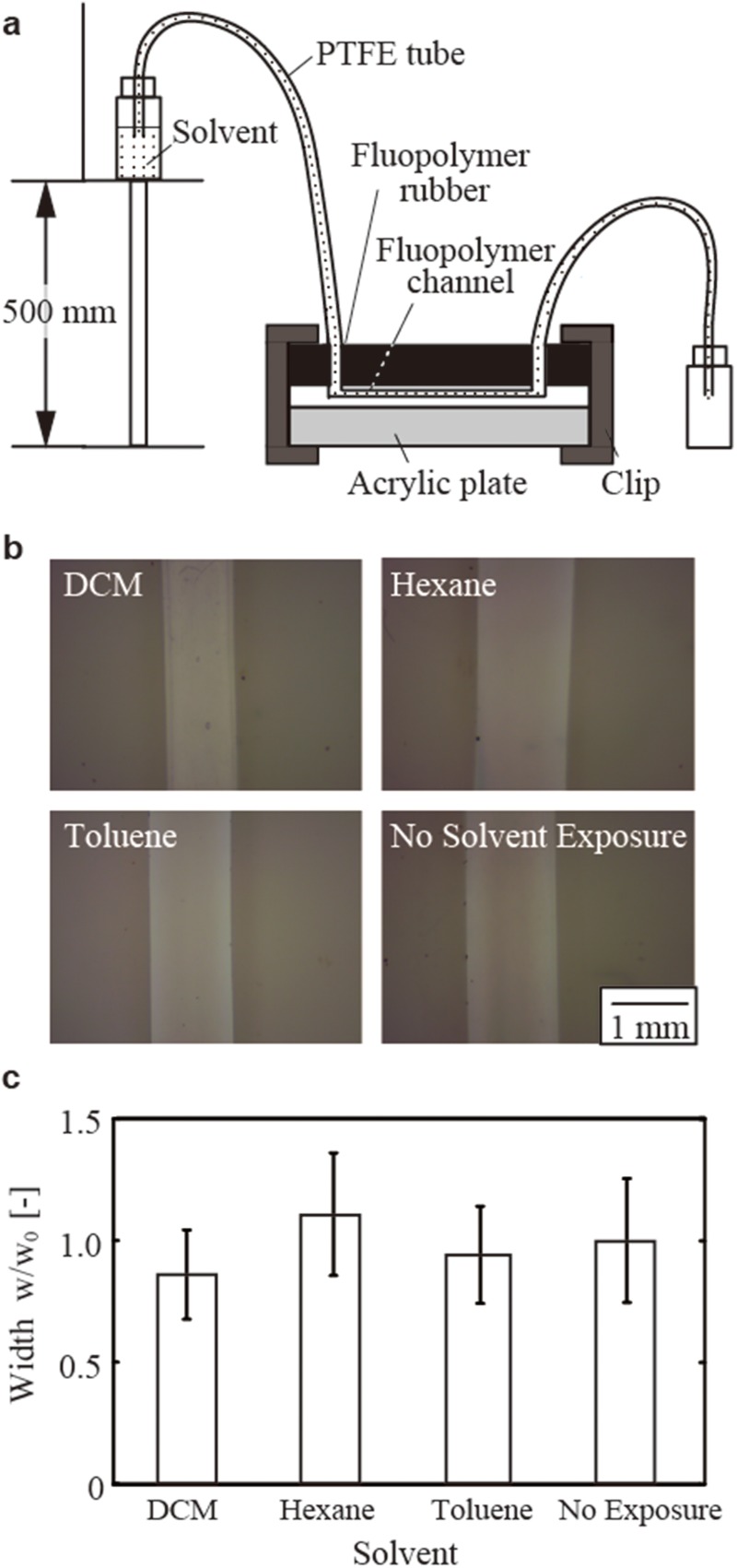
PTFE is an opaque material. While solutions with dark colors can be observed through it, the measurement of the droplet sizes by optical images was not reliable through PTFE. To validate the solvent resistance of PTFE channels, the width of the channels was measured after the exposure to solvents, and it was compared with the width of the channels of the same design without exposure to the solvents. We perfused three solvents (i.e., DCM, hexane, and toluene) through the channels for at least 6 h [Fig. 5(a)]. After the perfusion, the device was forced to delaminate, and the resulting width of the channels was measured [Fig. 5(b)]. The width of the channels (where the width was designed to be 750 μm in the CAD file) remained unchanged after the exposure to all three organic solvents [Fig. 5(c)]. Observed variations of the channel width were attributed to the precision of manufacturing by the cutting plotter we used. Within these errors (defined as the standard deviation, n = 4), we concluded that there was no change in the width of channels by the exposure to the three organic solvents that were tested. While we tested a set of three solvents for two types of fluoropolymers, chemical compatibility of fluoropolymers has been well-studied.21 We concluded that the solvent compatibility conferred by the property of fluoropolymers was retained after sealed microchannels were fabricated.
FIG. 5.
Continuous exposure of organic solvents to the PTFE channels. (a) Schematic illustration of the arrangement of the device. The solvents were delivered by elevating the container of the fluid above the ground level. (b) Optical micrographs showing the width of the channels. The films were delaminated to visualize the channels and measure their widths. (c) A graph showing the widths of the channel after the exposure to the solvents (characterized as their non-dimensional widths).
CONCLUSION
We developed a method for rapid prototyping of microchannels consisting of fluoropolymers using xurography and heat press. A cutting plotter was used to create stencils of fluoropolymer films, and heat press was used to laminate them to form microfluidic devices entirely consisting of fluoropolymer. We identified the conditions for heat press for two common fluoropolymers, polytetrafluoroethylene (PTFE) and fluorinated ethylene propylene (FEP), and the fabricated devices were shown to exhibit good compatibilities to selected organic solvents including dichloromethane with which thermoplastics and photocurable polymers were largely incompatible.
Microchannels consisting of PDMS can be readily prototyped by soft lithography, but they can be problematic for certain applications using organic solvents. On the other hand, fluoropolymers exhibit great solvent compatibility, but to date rapid and facile prototyping methods have not been developed. This work is the first demonstration to bridge those gaps by applying xurography and heat press. The developed method is simple, rapid, and low-cost. The entire fabrication can be completed in less than 1 h using commercially available fluoropolymer films and a desktop cutting plotter. Microchannels consisting of fluoropolymers can be useful in performing organic syntheses of materials and drugs26,27 as well as regulating adhesion of biological molecules, cells, and bacteria.28,29 We believe that xurography and heat press of PTFE and FEP films offer a new avenue to prototype microchannels for various applications that require the inert and non-reactive properties of the channels.
EXPERIMENTAL SECTION
Fabrication of microfluidic devices
Films of polytetrafluoroethylene (PTFE) (200-μm thick, Monotaro, Amagasaki, Japan) and fluorinated ethylene propylene (FEP) (150-μm thick, Shenzhen Forbest Technology Co. Ltd., China) were patterned using a desktop cutting plotter (Silhouette CAMEO 2, Silhouette America, Inc., UT, USA). An adhesive cutting mat (CAMEO Cutting Mat, Silhouette America, Inc., UT, USA) was used to hold the fluorinated films during cutting. Adhesive materials were transferred from the cutting mat to the fluoropolymer films. The fluorinated film was cleaned with Scotch Magic Tape (3M, MN, USA) and washed with running water for 30 s. The stencil was folded along the fold lines as designed. The fold stencil was placed between two 150 × 100 mm2 aluminium plates. Heat and pressure were applied with a heat press instrument (4386, CARVER, IN, USA). The successful bonding was achieved for FEP (at 1.7 MPa and 240 °C for 10 min) and PTFE (at 1.7 MPa and 320 °C for 30 min).
Delamination of bonded fluoropolymer films
A setup to study the delamination of the bonded fluoropolymer films is shown [Fig. S1(a) in the supplementary material]. A pressure controlled, fluid dispenser (MS-1, Musashi Engineering, Inc., Tokyo, Japan) was used to apply the pressure to the closed microchannel filled with colored water. The discharge pressure of the colored water was measured using a pressure sensor (PSAN-L1CV, Autonics Corp., Busan, Korea) connected in parallel to the dispensing syringe. For both PTFE and FEP, the device was fabricated as described. The pattern of the channel was straight (width 500 μm, length 20 mm) with one inlet and no outlet to study the delamination of the films upon the applied pressure [Fig. S1(c) in the supplementary material]. The channel was fully filled with the colored water before applying the pressure. Introduction of colored water was carried out by placing a droplet of the colored water on the inlet of the fluoropolymer channel using a syringe and degassed with a desiccator (0.2 MPa, 10 min). After the channel was fully filled with the colored water, the syringe needle integrated with the rubber seal was placed above the inlet of the fluoropolymer channel. The digitally controlled dispenser applied the pressure (0, 50, 100, 150, 200, 250, and 300 kPa) to the syringe connected to the inlet of the device for 10 s. After applying each pressure, the fluoropolymer channel was optically examined for delamination and photographed with a single lens reflex camera (D-5300, Nikon Corp., Tokyo, Japan).
Imaging
The fabricated devices and fluidic experiments were imaged and observed using a microscope (MU500, AmScope, CA, USA).
Fluidic experiments
The sealed microfluidic device was placed between two fluoropolymer rubber blocks (3-mm thick, Monotaro, Amagasaki, Japan) with manually punctured cylindrical apertures (1-mm diameter) for connection to tubes. The fluoropolymer rubber blocks were clipped to the film device to avoid leakage of the solvents at the inlet. The device was connected to 10-ml glass syringes (Sigma-Aldrich, Hamilton Syringes, Singapore) with PTFE tubes (EW-06417-21, OD = 1.06 mm, Cole-Parmer, IL, USA). Syringe pumps (NE-4000, New Era Pump Systems, Inc., NY, USA) were used to deliver fluids for the experiments in droplet generation in the FEP devices. Elevation of the fluid containers (500 mm above the height of the device) was used to provide the pressure difference to deliver the fluids for continuous flow experiments in the PTFE devices. All solvents (dichloromethane, hexane, and toluene) were used as purchased (Sigma-Aldrich, Singapore). Water was coloured with food dye (Liquid Artificial Apple Green Colour, Tesco PLC, Star Brand, Welwyn Garden City, UK) for visualization.
SUPPLEMENTARY MATERIAL
An additional figure was provided as a supplementary material to illustrate the details of the experiments.
ACKNOWLEDGMENTS
T.H. and E.I. thank the Top Global University Project at Waseda University, supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan. H.M. thanks Start-up Research Grant (No. SREP14088) at Singapore University of Technology and Design for the financial support. P.P. thanks the SUTD-MIT postdoctoral fellowship program.
References
- 1.Xia Y. and Whitesides G. M., Annu. Rev. Mater. Sci. 28, 153–184 (1998). 10.1146/annurev.matsci.28.1.153 [DOI] [Google Scholar]
- 2.Duffy D. C., McDonald J. C., Schueller O. J., and Whitesides G. M., Anal. Chem. 70, 4974–4984 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- 3.Téllez L., J. Mater. Sci. 38, 1773–1780 (2003). 10.1023/A:1023240129477 [DOI] [Google Scholar]
- 4.Xu Q., Hashimoto M., Dang T. T., Hoare T., Kohane D. S., Whitesides G. M., Langer R., and Anderson D. G., Small 5, 1575–1581 (2009). 10.1002/smll.200801855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh D., Torisawa Y. S., Hamilton G. A., Kim H. J., and Ingber D. E., Lab Chip 12, 2156–2164 (2012). 10.1039/c2lc40089h [DOI] [PubMed] [Google Scholar]
- 6.Mazutis L., Gilbert J., Ung W. L., Weitz D. A., Griffiths A. D., and Heyman J. A., Nat. Protoc. 8, 870–891 (2013). 10.1038/nprot.2013.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valencia P. M., Pridgen E. M., Rhee M., Langer R., Farokhzad O. C., and Karnik R., ACS Nano 7, 10671–10680 (2013). 10.1021/nn403370e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J. J., Jeong K. J., Hashimoto M., Kwon A. H., Rwei A., Shankarappa S. A., Tsui J. H., and Kohane D. S., Nano Lett. 14, 1–5 (2014). 10.1021/nl3047305 [DOI] [PubMed] [Google Scholar]
- 9.Shields C. W., Reyes C. D., and Lopez G. P., Lab Chip 15, 1230–1249 (2015). 10.1039/C4LC01246A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay R., Anal. Chem. 79, 3248–3253 (2007). 10.1021/ac071903e [DOI] [PubMed] [Google Scholar]
- 11.Jackman R. J., Floyd T. M., Ghodssi R., Schmidt M. A., and Jensen K. F., J. Micromech. Microeng. 11, 263–269 (2001). 10.1088/0960-1317/11/3/316 [DOI] [Google Scholar]
- 12.Piccin E., Coltro W. K., Fracassi da Silva J. A., Neto S. C., Mazo L. H., and Carrilho E., J. Chromatogr. A 1173, 151–158 (2007). 10.1016/j.chroma.2007.09.081 [DOI] [PubMed] [Google Scholar]
- 13.Bartolo D., Degre G., Nghe P., and Studer V., Lab Chip 8, 274–279 (2008). 10.1039/B712368J [DOI] [PubMed] [Google Scholar]
- 14.Carlborg C. F., Haraldsson T., Oberg K., Malkoch M., and van der Wijngaart W., Lab Chip 11, 3136–3147 (2011). 10.1039/c1lc20388f [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M., Langer R., and Kohane D. S., Lab Chip 13, 252–259 (2013). 10.1039/C2LC40888K [DOI] [PubMed] [Google Scholar]
- 16.Arcella V., Ghielmi A., and Tommasi G., Ann. N.Y. Acad. Sci. 984, 226–244 (2003). 10.1111/j.1749-6632.2003.tb06002.x [DOI] [PubMed] [Google Scholar]
- 17.Dolbier W. R., J. Fluor. Chem. 126, 157–163 (2005). 10.1016/j.jfluchem.2004.09.033 [DOI] [Google Scholar]
- 18.Riche C. T., Zhang C., Gupta M., and Malmstadt N., Lab Chip 14, 1834–1841 (2014). 10.1039/C4LC00087K [DOI] [PubMed] [Google Scholar]
- 19.Ren K., Dai W., Zhou J., Su J., and Wu H., Proc. Natl. Acad. Sci. U.S.A. 108, 8162–8166 (2011). 10.1073/pnas.1100356108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolland J. P., Van Dam R. M., Schorzman D. A., Quake S. R., and DeSimone J. M., J. Am. Chem. Soc. 126, 2322–2323 (2004). 10.1021/ja031657y [DOI] [PubMed] [Google Scholar]
- 21.Cybulski O., Jakiela S., and Garstecki P., Lab Chip 16, 2198–2210 (2016). 10.1039/C6LC00375C [DOI] [PubMed] [Google Scholar]
- 22.Bartholomeusz D. A., Boutte R. W., and Andrade J. D., J. Microelectromech. Syst. 14, 1364–1374 (2005). 10.1109/JMEMS.2005.859087 [DOI] [Google Scholar]
- 23.Renaud L., Selloum D., and Tingry S., Microfluid. Nanofluid. 18, 1407–1416 (2015). 10.1007/s10404-014-1539-z [DOI] [Google Scholar]
- 24.Neuville A., Renaud L., Luu T. T., Minde M. W., Jettestuen E., Vinningland J. L., Hiorth A., and Dysthe D. K., Lab Chip 17, 293–303 (2017). 10.1039/C6LC01253A [DOI] [PubMed] [Google Scholar]
- 25.Taberham A., Kraft M., Mowlem M., and Morgan H., J. Micromech. Microeng. 18, 064011 (2008). 10.1088/0960-1317/18/6/064011 [DOI] [Google Scholar]
- 26.Fletcher P. D. I., Haswell S. J., Pombo-Villar E., Warrington B. H., Watts P., Wong S. Y. F., and Zhang X., Tetrahedron 58, 4735–4757 (2002). 10.1016/S0040-4020(02)00432-5 [DOI] [Google Scholar]
- 27.Watts P. and Haswell S. J., Chem. Soc. Rev. 34, 235–246 (2005). 10.1039/b313866f [DOI] [PubMed] [Google Scholar]
- 28.Lu H., Koo L. Y., Wang W. M., Lauffenburger D. A., Griffith L. G., and Jensen K. F., Anal. Chem. 76, 5257–5264 (2004). 10.1021/ac049837t [DOI] [PubMed] [Google Scholar]
- 29.Young E. W., Wheeler A. R., and Simmons C. A., Lab Chip 7, 1759–1766 (2007). 10.1039/b712486d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An additional figure was provided as a supplementary material to illustrate the details of the experiments.