Abstract
Aging and diabetes are associated with decreased aerobic fitness, an independent predictor of mortality. Aerobic exercise is prescribed to improve aerobic fitness; however, middle-aged/older diabetic patients often suffer from mobility limitations which restrict walking. Non-weight-bearing/low-impact exercise is recommended but the optimal exercise prescription is uncertain. The goal of this randomized controlled trial was twofold: 1) to test if high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT), implemented on a non-weight-bearing all-extremity ergometer, are feasible, well-tolerated and safe in middle-aged/older adults with type 2 diabetes; and 2) to test whether all-extremity HIIT is more effective in improving aerobic fitness than MICT. A total of 58 sedentary individuals with type 2 diabetes (46 to 78 years; 63±1) were randomized to all-extremity HIIT (n=23), MICT (n=19) or non-exercise control (CONT; n=16). All-extremity HIIT and MICT, performed 4×/week for 8 weeks under supervision, resulted in no adverse events requiring hospitalization or medical treatment. Aerobic fitness (VO2peak) improved by 10% in HIIT and 8% in MICT and maximal exercise tolerance increased by 1.8 and 1.3 min, respectively (P≤0.002 vs. baseline; P≥0.9 for HIIT vs. MICT). In conclusion, all-extremity HIIT and MICT are feasible, well-tolerated and safe and result in similar improvements in aerobic fitness in middle-aged/older individuals with type 2 diabetes. These findings have important implications for exercise prescription for diabetic patients; they indicate that all-extremity exercise is a feasible alternative to weight-bearing exercise and those who are unable or unwilling to engage in HIIT may receive similar benefits from MICT.
Keywords: All-extremity aerobic exercise, VO2peak, aerobic fitness, aging, diabetes, cardiovascular disease risk
1. Introduction
More than 30 million adults have diabetes in the United States (CDC, 2017). The percentage of adults with diabetes increases at middle age and continues to rise reaching 25% among those aged 65 years and older (CDC, 2017). Individuals with type 2 diabetes have higher risk of all-cause and cardiovascular disease (CVD) mortality compared with those without diabetes (Gregg et al., 2012). Moreover, type 2 diabetes patients with low aerobic fitness are at ~7 times higher risk of all-cause mortality and ~3 times higher risk of CVD mortality even if they are normal weight (Church et al., 2004; Church et al., 2005). Regular aerobic exercise involving major muscle groups of the legs, arms, and trunk is recommended to improve aerobic fitness (Ross et al., 2016). However, the optimal exercise prescription for improving aerobic fitness for middle-aged and older patients with type 2 diabetes remains uncertain.
In the last decade, increasing reports in patients with cardiometabolic diseases indicate that high-intensity interval training (HIIT) is more effective in improving aerobic fitness compared with moderate-intensity continuous training (MICT) of equal caloric expenditure (Mitranun et al., 2014; Schjerve et al., 2008; Tjonna et al., 2008; Wisloff et al., 2007). However, this effective HIIT protocol involves high-intensity “uphill” treadmill walking or running which may not be tolerated by middle-aged and older patients with type 2 diabetes. These patients often suffer from lower-limb mobility limitations and are at a high risk of falling (Kirkman et al., 2012; Sinclair et al., 2008), therefore, non-weight-bearing, low-impact exercise is preferable. To our knowledge, there are no randomized controlled interventions investigating HIIT on a cycle ergometer in type 2 diabetes. We have recently implemented Tjonna et al.’s HIIT and MICT isocaloric treadmill protocols (Tjonna et al., 2008), on a non-weight-bearing all-extremity air-braked ergometer to allow application in a larger portion of the aging population. We have demonstrated that all-extremity HIIT and MICT performed 4 times per week over 8 weeks are feasible, well-tolerated and safe in older adults free of diabetes and other major clinical disease (Hwang et al., 2016).
Our newly-established all-extremity HIIT and MICT exercise intervention may be ideally suited for individuals with type 2 diabetes, particularly middle-aged and older patients. Using a non-weight-bearing all-extremity air-braked ergometer offers the following advantages: 1) it eliminates weight-bearing concerns; 2) it allows compensation for muscle weakness or fatigue; 3) it uses a large amount of muscle mass because it engages the legs, arms and core muscles; and 4) it promotes heat dissipation during exercise by generating airflow as the blades of the fan-wheel rotate. This is advantageous because diabetic patients are at increased risk of heat-related illness (Schwartz, 2005) and those who are older have reduced capacity to dissipate heat during exercise (Kenny et al., 2013).
However, prior to prescribing all-extremity HIIT and MICT protocols for diabetes management, it is necessary to establish their feasibility/safety specifically in individuals with type 2 diabetes. This is essential given the known exercise intolerance (Colberg et al., 2016; Poitras et al., 2018) and increased risk of hypoglycemia in diabetes, particularly in older patients (Colberg et al., 2016; Poitras et al., 2018). Currently, there are no data on all-extremity HIIT vs. MICT in type 2 diabetes. Therefore, the goal of this randomized controlled clinical trial was twofold: 1) to test if all-extremity HIIT and MICT are feasible, well-tolerated and safe in sedentary middle-aged and older adults with type 2 diabetes; and 2) to test whether all-extremity HIIT is more effective in improving aerobic fitness than all-extremity MICT.
2. Methods
2.1. Study design
The current study used a randomized controlled parallel group design (ClinicalTrials.gov NCT01883258). Middle-aged and older adults with type 2 diabetes were recruited from endocrinology clinics and the community using flyers and advertisements on the radio/internet and in newspapers/magazines. Participants who met the study inclusion criteria were enrolled in the clinical trial by our onsite clinician. Allocation of participants to HIIT, MICT or non-exercise control (CONT) was performed by the study coordinator. A computer-generated random sequence stratified by peak oxygen consumption (VO2peak, L/min) was created by a statistician and used for group assignment. Participants were asked not to change their habitual physical activity, diet, or medications during study participation to prevent confounding, and these factors were monitored over the intervention. Measures were obtained at baseline and following the 8-week intervention by the same researchers strictly following established standard operating procedures. Maximal exercise testing was performed by the onsite clinician who was blind to individual group assignment. Adverse events, number and reasons for dropouts and non-attendance of prescribed exercise sessions were monitored to examine the feasibility, tolerability, and safety of all-extremity HIIT and MICT.
The study was conducted at the Integrative Cardiovascular Physiology Laboratory, Center for Exercise Science, at the University of Florida and was approved by the Institutional Review Board. Study procedures, potential risks and benefits were explained to the study participants and questions were addressed prior to obtaining written informed consent.
2.2. Study participants
Volunteers were screened based on medical history, physical examination, ankle brachial index, echocardiography, and blood analysis including comprehensive metabolic panel, lipid panel, insulin, complete blood count with differential, and hemoglobin A1C (HbA1C). To screen for cardiac autonomic dysfunction, ECG and blood pressure were evaluated during postural testing. To screen for the presence of myocardial ischemia or arrhythmias, ECG and blood pressure were evaluated during a clinically-supervised maximal graded exercise test.
Study inclusion criteria consisted of the following: 1) diagnosis of type 2 diabetes; 2) 30-79 years of age; and 3) being sedentary or engaging in <30 min of aerobic exercise training <3 times/week for at least 1 year prior to study enrollment. Participants were excluded from the study if they met any of the following exclusion criteria: 1) history of diabetic proliferative retinopathy or autonomic or peripheral neuropathy; 2) history of CVD; 3) history of renal impairment; 4) history of gout or hyperuricemia; 5) history of hepatic disease or infection with hepatitis B or C; 6) history of seizures, or other relevant on-going or recurrent illness or recent or recurrent hospitalizations; 7) systolic blood pressure ≥160 mmHg or diastolic ≥100 mmHg; 8) use of tobacco products; 9) pregnancy or lactation; 10) use of hormone replacement or oral contraceptives in the past 2 years; and 11) >5 % weight change in the prior 6 months.
2.3. Exercise intervention
Participants assigned to HIIT or MICT completed 4 exercise sessions/week for 8 weeks at the Integrative Cardiovascular Physiology Laboratory under the direct supervision of an exercise physiologist. Exercise sessions were scheduled on Monday, Tuesday, Thursday, and Friday, to prevent having more than two consecutive days without exercise. Unless there was a schedule conflict, participants completed all sessions at the same time on the planned days between 6:30 am to 6:30 pm.
HIIT and MICT were performed on an all-extremity non-weight-bearing air-braked ergometer (Airdyne cycle, model AD4, Schwinn) as we have previously published (Hwang et al., 2016). Our HIIT and MICT protocols were originally designed to provide equal volume of training (i.e., isocaloric expenditure) on the treadmill (Tjonna et al., 2008). Our group has confirmed using submaximal oxygen consumption measures that these HIIT and MICT protocols also result in equal caloric expenditure when adapted to an all-extremity ergometer (Hwang et al., 2016).
Both HIIT and MICT included a 10-min warm-up and a 5-min cool-down at 70% of peak heart rate (HRpeak). HIIT consisted of 4×4-min intervals at 90% of HRpeak interspersed by 3×3-min active recovery at 70% of HRpeak for a total of 25 min, while MICT consisted of 32 min at 70% HRpeak. Therefore, the total duration was 40 min for HIIT and 47 min for MICT. HRpeak was determined during a maximal graded exercise test. A telemetry system was used to monitor and record heart rate throughout all exercise sessions (Polar Team 2 Pro, version 1.4.3) as follows: A Polar chest strap transmitted the data to a computer allowing real-time heart rates to be displayed for multiple participants simultaneously on a 30-inch screen that was mounted to the wall. The displayed heart rates were color coded to allow immediate detection of deviations from target heart rate. Participants were instructed to increase the cadence in order to increase heart rate or to decrease the cadence in order to decrease heart rate.
Prior to the 8-week exercise intervention, a period of familiarization/preconditioning was completed. During the initial session of familiarization/preconditioning, the exercise duration/intensity was self-selected based on the participant’s motivation, fitness level, and capacity to perform all-extremity exercise. The initial self-selected exercise duration/intensity was 65 to 75% of HRpeak for at least 10 min for most participants and the duration was increased during each subsequent session as tolerated until 40 min of continuous all-extremity exercise could be completed at 70% of HRpeak. Once this threshold was met, participants in MICT progressed to 47 min at 70% HRpeak, while participants in HIIT gradually added as many 4-min intervals at 90% HRpeak as tolerated until they could complete all of the 4 prescribed intervals. The average number of sessions required for familiarization/preconditioning was equal for HIIT and MICT.
Foot health was monitored throughout the intervention for new onset of blisters, ulcers, or swelling. To minimize the risk for exercise-induced hypoglycemia, blood glucose was measured before and after each exercise session and a carbohydrate supplement (usually raisins or occasionally juice) was provided based on the following published guidelines: If blood glucose was <100 mg/dL prior to exercise then 30 g of carbohydrates were provided and if it was 100 to 180 mg/dL then 15 g of carbohydrates were provided (Albright, 2013). However, if blood glucose was > 250 mg/dL, the exercise session was canceled due to safety concerns (Colberg et al., 2016). At the end of exercise, if blood glucose was < 70 mg/dL, then 15 g of carbohydrates were provided and glucose was rechecked after 20 min (Albright, 2013). An additional 15 g of carbohydrates were provided if blood glucose remained < 70 mg/dL (Albright, 2013).
2.4. Study procedures
2.4.1. Aerobic fitness
VO2max, the gold standard measure of aerobic fitness, was assessed using computer-assisted open-circuit spirometry during a maximal graded exercise test. A treadmill test was selected for the following reasons: 1) walking is a familiar activity to everyone. Individuals who are unaccustomed to all-extremity exercise and are deconditioned may not be able to exert maximal effort during a maximal test on the Airdyne ergometer due to muscular fatigue; 2) a treadmill test is used extensively in clinical settings for diagnostic purposes and as a predictor of CVD morbidity and mortality; 3) a treadmill test allows translation of gains from all-extremity training to walking ability which is highly relevant for the patients’ functional independence; and 4) maximal heart rate and VO2max are not different between a maximal test consisting of combined arm and leg exercise on an Airdyne ergometer compared with treadmill exercise (Hagan et al., 1983).
An individualized ramp treadmill test was used in accordance with recent recommendations for individuals with chronic disease (Ross et al., 2016), and consisted of a constant customized speed and grade increases of 2.5% every 2 min until volitional exhaustion as we have previously described (Hwang et al., 2016). The speed was determined during a 6-min warm-up based on the participant’s walking ability and, whenever possible, corresponding to 70~80% of the age-predicted maximal heart rate to induce a test duration of 10 to 12 min. Maximal exercise tests were repeated at follow-up using each participant’s individualized baseline protocol to allow evaluation of changes in test duration, which assessed maximal exercise tolerance. VO2max was attained when at least three of the following criteria were met: a) a plateau in oxygen consumption (<100 ml) with increasing exercise intensity; b) a maximal respiratory exchange ratio of at least 1.15; c) a heart rate within 10 bpm of age-predicted max heart rate (220-age); and d) a score of at least 18 on the Borg Rating of Perceived Exertion scale. Some of the participants did not meet these criteria, as is frequently the case in clinical populations (Ross et al., 2016), therefore, the term VO2peak is used when presenting the aerobic fitness data. Ventilatory threshold was determined by the v-slope method (Beaver et al., 1986).
2.4.2. Body composition
Height, body weight and waist and hip circumferences were measured as we have previously described (Hwang et al., 2016). Fat and fat-free mass and % body fat were assessed using a whole-body scan using dual-energy X-ray absorptiometry (Lunar Prodigy Advance, GE, version 8.70.005) (Hwang et al., 2016).
2.4.3. HbA1C, blood glucose, insulin and lipids
HbA1C, fasting blood glucose, insulin and lipids were measured using spectrophotometry, an immunoassay, and immunoturbidimetry, respectively, by a clinical laboratory following standard procedures.
2.4.4. Habitual physical activity and dietary analysis
Habitual physical activity was assessed using a triaxial accelerometer (ActiGraph GT3X, software version 5.10.0) as we have previously described (Hwang et al., 2016) and dietary intake was analyzed using a food diary (ESHA Food Processor SQL version 10.7) over 3 weekdays and 1 weekend day, at baseline and following the 8-week intervention.
2.5. Statistical analyses
Statistical analyses were performed by the original assigned groups using IBM SPSS Statistics (Essentials, Version 22). Statistical significance was set at P< 0.05 and data were examined for normality and outliers. To examine baseline group differences, one-way ANOVA was used for continuous variables and χ2 was used for categorical variables. To examine the effect of the intervention on the outcomes of interest, 3 × 2 mixed ANOVA with repeated measures was used. This model included the following: 1) group as a between subject factor which consisted of 3 levels (HIIT, MICT, and CONT), 2) time as a within subject factor which consisted of 2 levels (baseline vs follow-up) and 3) the group × time interaction. When the group × time interaction was significant indicating the outcome of interest changed differently among the groups, then Bonferroni post hoc pairwise comparisons were performed to determine how the groups differed. Data were also expressed as change from pre- to post-intervention (i.e., delta) and group differences were examined using one-way ANOVA followed by Bonferroni post hoc pairwise comparisons, when indicated.
3. Results
3.1. Feasibility, tolerability and safety of all-extremity HIIT and MICT
A total of 58 participants met the inclusion criteria and were randomized to HIIT, MICT and CONT, of which 86% completed the study (Fig. 1). Approximately 22% of participants in HIIT and 16% in MICT discontinued the exercise intervention (P=0.6 for HIIT vs. MICT). Self-reported reasons for withdrawal are presented in Fig. 1. Participant characteristics for those who withdrew vs. those who completed the study are presented in Table 1.
Fig 1.
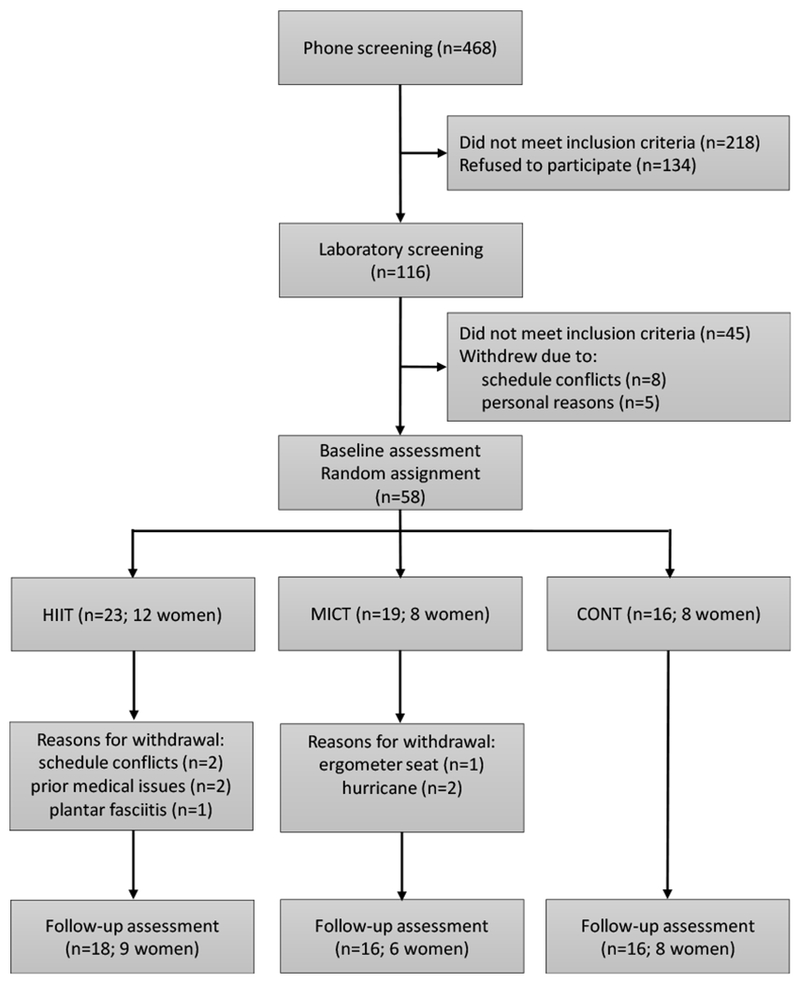
Study flowchart. Middle-aged and older adults with type 2 diabetes were recruited, screened and randomized to 8 weeks of all-extremity high-intensity interval training (HIIT), moderate-intensity continuous training (MICT) or non-exercise control (CONT).
Table 1.
Baseline characteristics of participants who withdrew vs. completed the study.
| Completed | Withdrew | P | |
|---|---|---|---|
| n | 50 | 8 | - |
| Male/female, n | 27/23 | 3/5 | 0.5 |
| Age, years | 63±1 | 60±2 | 0.3 |
| Duration of diabetes, years | 8.0±0.8 | 10.2±2.5 | 0.3 |
| Weight, kg | 92.0±2.5 | 100.3±4.0 | 0.2 |
| BMI, kg/m2 | 32.4±0.8 | 38.3±2.1 | 0.007 |
| Systolic blood pressure, mmHg | 125±2 | 129±4 | 0.5 |
| Diastolic blood pressure, mmHg | 72±1 | 71±2 | 0.6 |
| VO2peak, L/min | 2.03±0.08 | 1.77±0.22 | 0.2 |
| VO2peak, mL/kg/min | 22.0±0.6 | 17.7±1.9 | 0.02 |
Data are means±SE. BMI = body mass index; VO2peak= peak oxygen consumption.
During the initial exercise sessions, some participants had difficulty with the ergometer seat being uncomfortable and one withdrew. For the remaining participants, this issue resolved over time by wearing padded cycling garments or selecting a different seat design. The average number of sessions required for familiarization/preconditioning was 4±1 for HIIT and 4±1 for MICT. Over the 8-week intervention, the average number of sessions attended per week were 3.3±0.2 for HIIT and 3.4±0.2 for MICT (P=0.8). One participant in HIIT and one in MICT missed a scheduled session due to exercise-related fatigue. Other reasons for non-attendance were unrelated to the exercise intervention and included schedule conflicts or holidays (11%), two hurricanes (3%), pre-exercise glucose above threshold (1%), and illness (1%). Specific reasons for non-attendance are presented by group in Table 2. Overall, most of the participants reported that the exercise training was enjoyable, except a small number in MICT complained of boredom.
Table 2.
Reasons for non-attendance of scheduled HIIT and MICT sessions.
| HIIT | MICT | |
|---|---|---|
| Schedule conflicts or holidays | 12.0 | 10.4 |
| Hurricanes | 2.4 | 3.7 |
| Illness unrelated to the study | 1.4 | 1.4 |
| Pre-exercise glucose above threshold | 1.0 | 0.6 |
| Fatigue due to preceding exercise session | 0.2 | 0.2 |
Data are number of missed sessions/total number of scheduled sessions (%). HIIT = high-intensity interval training; MICT = moderate-intensity continuous training.
There were no serious adverse events requiring hospitalization or medical treatment (i.e., deaths or nonfatal events such as myocardial infarction). One participant experienced shortness of breath once during HIIT and another participant who was on insulin experienced dizziness and hypotension once following HIIT; however, neither experienced hypoglycemia. Both participants quickly recovered after resting and rehydrating with water and returned for the subsequent exercise session without any problems. No other adverse events were noted.
3.2. Participant characteristics and baseline values
Participants who completed the study ranged from 46 to 78 years of age and female participants were postmenopausal for at least 2 years (mean: 15±2 years). There were no baseline differences in age, duration of diabetes, medications or other participant characteristics among HIIT, MICT and CONT (P≥0.1; Table 3). Baseline aerobic fitness, body composition, glycemic control and blood lipids were also not different among the groups (P≥0.4; Table 4). Participants maintained their baseline habitual physical activity and dietary habits over the intervention as instructed (P≥0.2 for group×time interaction and P≥0.5 for time effect; Table 5).
Table 3.
Baseline participant characteristics.
| HIIT | MICT | CONT | P | |
|---|---|---|---|---|
| Age, years | 65±2 | 62±2 | 61±2 | 0.4 |
| Duration of diabetes, years | 7.8±1.3 | 8.3±1.5 | 8.2±1.5 | 0.96 |
| Height, cm | 170±3 | 170±3 | 164±2 | 0.2 |
| Body weight, kg | 92.0±4.7 | 92.6±4.5 | 91.5±3.9 | >0.99 |
| BMI, kg/m2 | 31.7±1.3 | 31.8±1.4 | 33.9±1.4 | 0.5 |
| HbA1C, % | 7.1±0.3 | 7.2±0.3 | 7.4±0.4 | 0.8 |
| Glucose, mg/dL | 133±9 | 140±10 | 147±16 | 0.7 |
| Systolic BP, mmHg | 124±2 | 127±5 | 126±4 | 0.9 |
| Diastolic BP, mmHg | 70±1 | 73±2 | 73±2 | 0.2 |
| Medications, n (%) | ||||
| Metformin | 10 (56) | 10 (63) | 14 (88) | 0.1 |
| SGLT2 inhibitors | 2 (11) | 2 (13) | 2 (13) | >0.99 |
| Sulfonylureas | 4 (22) | 2 (13) | 4 (25) | 0.7 |
| DPP-4 inhibitors | 2 (11) | 5 (31) | 3 (19) | 0.4 |
| GLP-1 agonists | 1 (6) | 0 (0) | 1 (6) | >0.99 |
| Thiazolidinediones | 3 (17) | 0 (0) | 1 (6) | 0.3 |
| Insulin | 4 (22) | 4 (25) | 3 (19) | >0.99 |
| Statins | 13 (72) | 13 (81) | 12 (75) | 0.9 |
| Anti-hypertensives | 12 (67) | 12 (75) | 11 (69) | 0.9 |
| Aspirin | 8 (44) | 6 (38) | 5 (31) | 0.7 |
Data are means±SE or n (%). BMI, body mass index; BP, blood pressure; CONT = non-exercise control; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; HbA1C = hemoglobin A1C; HIIT = high-intensity interval training; MICT = moderate-intensity continuous training; SGLT2, sodium-glucose co-transporter 2.
Table 4.
Responses to all-extremity HIIT and MICT.
| HIIT | MICT | CONT | P | P | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Group×time | |
| Aerobic fitness | ||||||||
| VO2peak, L/min | 2.06±0.15 | 2.25±0.17* | 1.96±0.12 | 2.11±0.13† | 1.96±0.12 | 1.92±0.12 | 0.8 | 0.006 |
| VO2peak, mL/kg/min | 22.3±1.0 | 24.6±1.3* | 21.6±1.2 | 23.3±1.2† | 21.4±1.3 | 20.9±1.2 | 0.8 | 0.002 |
| Test duration, min | 11.7±0.5 | 13.5±0.6* | 11.4±0.9 | 12.6±1.0† | 10.6±0.6 | 10.7±0.7 | 0.6 | 0.01 |
| VT, L/min | 1.41±0.10 | 1.55±0.13† | 1.33±0.08 | 1.51±0.11† | 1.36±0.10 | 1.26±0.08 | 0.8 | 0.001 |
| HRrest, beats/min | 61±2 | 59±1 | 70±3 | 68±3 | 65±3 | 67±3 | 0.053 | 0.1 |
| HRpeak, beats/min | 152±3 | 151±3 | 155±5 | 155±4 | 147±4 | 147±5 | 0.4 | 0.8 |
| Body composition | ||||||||
| Body weight, kg | 92.0±4.7 | 91.4±4.5 | 92.6±4.5 | 92.2±4.8 | 91.5±3.9 | 91.4±3.9 | >0.99 | 0.7 |
| BMI, kg/m2 | 31.7±1.3 | 31.5±1.2 | 31.8±1.4 | 31.7±1.5 | 33.9±1.4 | 33.9±1.4 | 0.5 | 0.7 |
| Body fat, % | 37.9±1.9 | 37.7±2.0 | 36.3±2.1 | 35.2±2.2‡ | 37.6±2.3 | 38.5±2.3‡ | 0.8 | 0.01 |
| Fat-free mass, kg | 56.9±3.2 | 56.7±3.2 | 58.5±2.8 | 59.1±3.0 | 55.4±2.2 | 54.6±2.2 | 0.9 | 0.09 |
| Fat mass, kg | 35.1±2.7 | 34.6±2.6 | 34.1±2.7 | 33.1±2.8 | 34.5±3.1 | 35.3±3.2 | 0.96 | 0.07 |
| Waist circ, cm | 108.6±3.3 | 107.7±3.4 | 109.0±3.7 | 108.2±4.0 | 106.3±2.6 | 106.6±2.7 | 0.95 | 0.2 |
| WHR | 0.96±0.02 | 0.95±0.02 | 0.97±0.02 | 0.97±0.02 | 0.94±0.02 | 0.96±0.02 | 0.9 | 0.4 |
| Glycemic control and lipids | ||||||||
| HbA1C, % | 7.1±0.3 | 6.8±0.2 | 7.2±0.3 | 7.0±0.2 | 7.4±0.4 | 7.5±0.4 | 0.8 | 0.1 |
| Glucose, mg/dL | 133±9 | 127±9 | 140±10 | 139±7 | 147±16 | 152±17 | 0.7 | 0.7 |
| Insulin, μU/mL | 11.1±2.4 | 9.2±1.5 | 12.0±1.9 | 11.6±2.0 | 11.7±1.5 | 11.8±1.7 | 0.95 | 0.4 |
| HOMA-IR | 3.65±0.90 | 2.75±0.46 | 4.13±0.84 | 4.08±0.78 | 3.97±0.45 | 4.39±0.70 | 0.9 | 0.3 |
| Total chol, mg/dL | 178±12 | 180±13 | 159±10 | 166±10 | 166±11 | 165±12 | 0.5 | 0.6 |
| HDL, mg/dL | 51±4 | 51±4 | 49±6 | 51±6 | 49±4 | 49±3 | 0.9 | 0.5 |
| LDL, mg/dL | 85±9 | 94±7 | 83±7 | 91±8 | 92±10 | 87±10 | 0.9 | 0.3 |
| Triglycerides, mg/dL | 160±35 | 144±27 | 142±19 | 125±13 | 146±23 | 154±24 | 0.9 | 0.4 |
| Resting blood pressure | ||||||||
| Systolic BP, mmHg | 124±2 | 122±2 | 127±5 | 126±5 | 126±4 | 125±4 | 0.9 | 0.9 |
| Diastolic BP, mmHg | 70±1 | 68±1 | 73±2 | 71±2 | 73±2 | 74±2 | 0.2 | 0.4 |
Data are means±SE. BMI = body mass index; BP = blood pressure; chol = cholesterol; circ = circumference; CONT = non-exercise control; HbA1C = hemoglobin A1C; HDL = high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; HIIT = high-intensity interval training; HRpeak = peak heart rate; HRrest = resting heart rate; LDL = low-density lipoprotein; MICT = moderate-intensity continuous training; VO2Peak= peak oxygen consumption; VT = ventilatory threshold; WHR = waist to hip ratio.
P<0.0001;
P<0.005;
P<0.05 vs. baseline.
Table 5.
Physical activity and dietary intake.
| HIIT | MICT | CONT | P | P | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Group×time | |
| Physical activity | ||||||||
| Counts/min/day | 340±16 | 345±17 | 427±24 | 422±31 | 406±45 | 433±52 | 0.06 | 0.7 |
| Steps/day | 5056±392 | 5035±339 | 6351±526 | 6047±509 | 5357±781 | 5742±899 | 0.2 | 0.5 |
| Dietary intake | ||||||||
| Energy, kcal | 1830±176 | 1984±134 | 1928±183 | 1857±139 | 1845±136 | 1690±144 | 0.9 | 0.4 |
| Protein, g | 81±7 | 94±8 | 82±9 | 75±6 | 78±6 | 81±5 | 0.9 | 0.2 |
| Fat, g | 74±9 | 79±7 | 86±10 | 79±8 | 69±7 | 64±9 | 0.5 | 0.6 |
| Carbohydrate, g | 210±23 | 235±18 | 206±19 | 212±15 | 212±17 | 182±21 | 0.98 | 0.2 |
Data are means±SE.
3.3. Responses to all-extremity HIIT and MICT
3.3.1. Aerobic fitness
In response to the intervention, VO2peak increased by 10% in HIIT and 8% in MICT (P≤0.002 vs. baseline; P>0.99 for HIIT vs. MICT), while it did not change in CONT (P=0.4; Table 4 and Fig. 2). Maximal exercise test duration increased by 1.8 min in HIIT and 1.3 min in MICT (P≤0.002 vs. baseline; P=0.9 for HIIT vs. MICT; Table 4 and Fig. 3) but remained unchanged in CONT (P=0.8). Ventilatory threshold also increased in HIIT and MICT by 11% and 14%, respectively (P≤0.004 vs. baseline; P>0.99 for HIIT vs. MICT; Table 4), while it did not change in CONT (P=0.08). HRpeak was not affected by the intervention (P=0.8; Table 4).
Fig. 2.
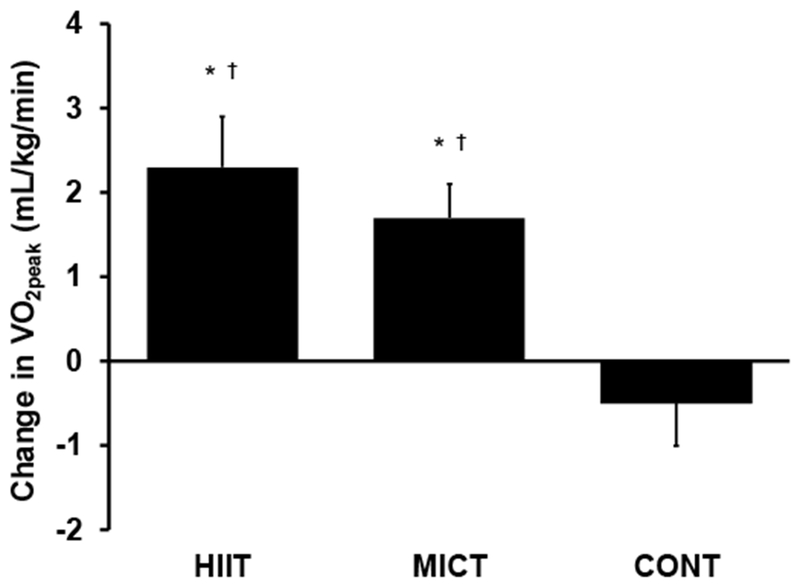
Change in peak oxygen consumption (VO2peak) in response to 8-week all-extremity high-intensity interval training (HIIT), moderate-intensity continuous training (MICT) or non-exercise control group (CONT) in middle-aged and older adults with type 2 diabetes. *P≤0.002 baseline vs. follow-up; †P≤0.02 vs. CONT.
Fig. 3.
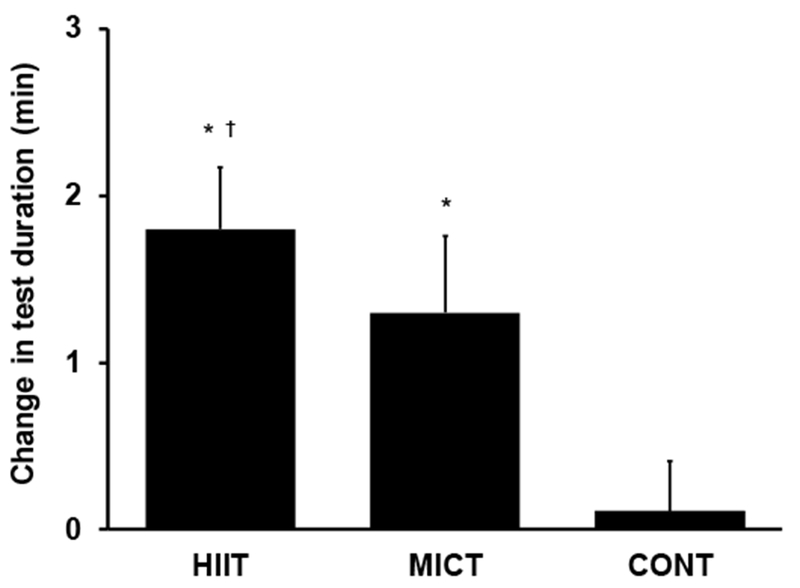
Change in maximal exercise tolerance (maximal test duration) in response to 8-week all-extremity high-intensity interval training (HIIT), moderate-intensity continuous training (MICT) or non-exercise control group (CONT) in middle-aged and older adults with type 2 diabetes. *P≤0.002 baseline vs. follow-up; †P=0.01 vs. CONT.
3.3.2. Body composition, glycemic control, and blood lipids
Body weight, body mass index, waist circumference and waist to hip ratio remained unchanged in response to the intervention (P≥0.1; Table 4). Percent body fat decreased by 1% in MICT (P=0.02) and increased by 0.9% in CONT (P=0.046) while it did not change in HIIT (P=0.6). Fat and fat-free mass were not significantly affected by the intervention (P≥0.07). In addition, glycemic control and lipids remained unchanged (P≥0.1).
4. Discussion
This is the first study to examine the feasibility, tolerability and safety of HIIT and MICT implemented on a non-weight-bearing all-extremity ergometer in middle-aged and older adults with type 2 diabetes. This randomized controlled trial demonstrated that 1) all-extremity HIIT and MICT are feasible, well-tolerated, and safe in middle-aged and older adults with type 2 diabetes; and 2) 8 weeks of all-extremity HIIT and MICT similarly increased aerobic fitness and maximal exercise tolerance.
4.1. Feasibility, tolerability and safety of all-extremity HIIT and MICT
Our findings strongly support that both all-extremity HIIT and MICT are feasible in patients with type 2 diabetes. This is based on the 81% completion rate for HIIT and MICT, which surpasses or is comparable with the completion rates in other published exercise interventions including our own study in healthy middle-aged and older adults (Hwang et al., 2016). The eight participants who withdrew from the current study had higher obesity and lower aerobic fitness, but reasons for withdrawal were mainly unrelated to these factors: four withdrew due to schedule conflicts/hurricanes, one due to the ergometer seat being uncomfortable, two due to prior medical issues, and one due to plantar fasciitis. Participants who completed HIIT and MICT had a wide range of BMI and VO2peak (20.8 to 41.4 kg/m2 and 12.3 to 29.2 ml/kg/min, respectively), which demonstrates that our all-extremity exercise protocols are feasible even for diabetic patients who are morbidly obese or severely deconditioned.
Retention of participants was not statistically different for HIIT vs. MICT (78% vs. 84%). In addition, exercise adherence, calculated as number of sessions attended divided by number of sessions prescribed, was 83±4% and 84±4%, respectively. One week was required, on average, for familiarization/preconditioning prior to being able to complete all-extremity HIIT and MICT as prescribed. This suggests that these protocols can be implemented without problems in middle-aged and older diabetic patients who are unaccustomed to exercising. There were no serious or non-serious adverse events requiring hospitalization or medical treatment in HIIT and MICT. In addition, none of the participants experienced hypoglycemia during study participation, including those on insulin or insulin secretagogues. Together our results demonstrate that both all-extremity HIIT and MICT are feasible, well-tolerated and can be implemented with minimal risk in middle-aged and older adults with type 2 diabetes who are sedentary.
4.2. Responses to all-extremity HIIT and MICT
4.2.1. Aerobic fitness
In the current study, aerobic fitness increased by 2.3 mL/kg/min in response to all-extremity HIIT and by 1.7 mL/kg/min in response to all-extremity MICT over 8 weeks. This is clinically important because an increase of 3.5 mL/kg/min has been associated with a 15% reduction in all-cause mortality and 19% reduction in CVD mortality (Lee et al., 2011). In addition, higher aerobic fitness is associated with higher quality of life in individuals with type 2 diabetes (Awotidebe et al., 2017). In our study, both HIIT and MICT also significantly improved maximal exercise tolerance as indicated by increases in maximal exercise test duration. Longer test duration is associated with lower all-cause and CVD mortality in individuals with type 2 diabetes (Lyerly et al., 2008). Collectively, our results indicate that our all-extremity exercise regimens are effective in improving aerobic fitness and maximal exercise tolerance in middle-aged and older adults with type 2 diabetes.
A recent study reported in 20- to 70-year-old patients with type 2 diabetes, that HIIT consisting of walking or running on the treadmill improved aerobic fitness by 20% over 12 weeks, whereas MICT resulted in no change (Stoa et al., 2017). In contrast to these findings, our data suggest that all-extremity MICT is an effective alternative to all-extremity HIIT in middle-aged and older patients with type 2 diabetes who may be unable or unwilling to engage in HIIT. Although multiple studies report superior effects of HIIT vs. MICT on aerobic fitness (Amundsen et al., 2008; Hollekim-Strand et al., 2014; Mitranun et al., 2014; Moholdt et al., 2012; Molmen-Hansen et al., 2012; Schjerve et al., 2008; Stoa et al., 2017; Terada et al., 2013; Tjonna et al., 2008; Wisloff et al., 2007), some studies report no difference consistent with our findings (Conraads et al., 2015; Mobius-Winkler et al., 2016; Moholdt et al., 2009).
It is not clear why in the current study HIIT and MICT resulted in similar effects on aerobic fitness. Exercise intensity was closely monitored during all exercise sessions and was maintained within the prescribed range for HIIT and MICT, therefore it cannot explain the similar results between all-extremity HIIT and MICT. In addition, we have previously demonstrated increases in aerobic fitness in response to all-extremity HIIT, but not all-extremity MICT, in healthy middle-aged/older adults indicating that the two protocols can result in different adaptations in this age group. It is possible that due to our participants’ low fitness level and physical inactivity, both all-extremity HIIT and MICT represent a dramatic increase to their activity level and may therefore be equally beneficial, at least initially. Longer than 8 weeks of training may be required for superior effects of HIIT to be become evident. Indeed, most studies showing greater effects in response to HIIT are longer than our study. Whether extending the length of our intervention would result in greater improvements in aerobic fitness in response to all-extremity HIIT compared with MICT remains to be evaluated in future studies.
We have demonstrated that all-extremity aerobic exercise results in improved maximal exercise tolerance on the treadmill which indicates that walking ability is improved. This may have important implications for enhancing activities of daily living and personal independence and extends the applicability and generalizability of our findings. We recognize that improvements in maximal exercise tolerance may have been greater than the ones observed in our study if the maximal exercise test was performed on an all-extremity Airdyne ergometer as the exercise training. However, this was not feasible because our participants were sedentary and unaccustomed to all-extremity exercise at baseline which could lead to early test termination due to muscular discomfort or fatigue. We do not anticipate that improvements in aerobic fitness in the present study were negatively influenced by use of the maximal treadmill test because VO2max has been shown not be different based on a treadmill vs. an all-extremity Airdyne test (Hagan et al., 1983).
4.2.2. Body composition
In the present study, % body fat increased by ~1% in CONT while all-extremity HIIT prevented an increase in body fat and all-extremity MICT decreased body fat by ~1%. Body weight and absolute amount of fat or fat-free mass did not significantly change in response to all-extremity HIIT and MICT. In support of our findings, two meta-analysis concluded that body weight (Boule et al., 2001) and fat and fat free mass (Grace et al., 2017) do not change in response to exercise training in patients with type 2 diabetes. Our participants were instructed to maintain their diet the same over study participation to isolate the unique effect of exercise training and this was confirmed using food diaries. More than 8 weeks of aerobic exercise training with concomitant dietary modifications may be required to induce large changes in body weight and composition in individuals with type 2 diabetes.
4.2.3. Glycemic control, and blood lipids
Our study was not specifically designed to investigate the effect of all-extremity HIIT and MICT on glycemic control, therefore medication use may have confounded our measures of insulin, fasting glucose and HOMA-IR. However, we used a randomized controlled design to ensure that participant characteristics, including medication use, were similar among the groups. Therefore, medication use should not have influenced our group comparisons. Future studies focusing on metabolic control should enroll a large sample size that would allow statistically isolating the effects of each type of medication on metabolic adaptations to exercise training. HbA1C, an indicator of long-term glycemic control, remained unchanged in our study, but this may be due to the relatively short length of our intervention. Future studies should also extend the duration of the exercise intervention and include more sensitive tests of glucose control such as a euglycemic hyperinsulinemic clamp. Blood lipids also remained unchanged in the current study, however, 77% of our participants were on statins which may have contributed to the lack of response. A recent meta-analysis reported that aerobic exercise does not induce additional lipid lowering effects compared to statins alone (Gui et al., 2017).
4.3. Study strengths and limitations
Our study has several strengths. The current exercise intervention was randomized, controlled and supervised. Comparisons between all-extremity HIIT and MICT were not confounded by differences in exercise volume because HIIT and MICT were designed to result in equal caloric expenditure. Our results can be attributed to the prescribed exercise training rather than to overall increases in habitual physical activity or changes in diet because these factors were monitored. Our intervention focused on individuals with type 2 diabetes who were middle-aged/older and sedentary and ~50% of the participants in each group were women. Due to the increased prevalence of type 2 diabetes over 45 years of age and high level of physical inactivity in diabetic patients, our findings have enhanced applicability and generalizability but in a supervised setting.
Our study also has some limitations that should be considered when applying our results. We have demonstrated that all-extremity HIIT is safe over the length of our intervention, but safety during longer-term interventions remains to be determined. In addition, in the present study risks were minimized by rigorously screening the participants and closely monitoring glucose levels before and after exercise. Future studies should investigate how to safely implement all-extremity HIIT and MICT in the community and in diabetic patients with established mobility problems or functional limitations.
5. Conclusions
Our findings have several important implications for exercise prescription in middle-aged and older diabetic patients, especially those who have difficulties participating in weight-bearing exercise. First, we have established that all-extremity HIIT and MICT are feasible, well-tolerated and safe in previously sedentary middle-aged and older adults with type 2 diabetes. Second, we have shown that all-extremity HIIT and MICT similarly improve aerobic fitness and maximal exercise tolerance. Third, our results indicate that HIIT and MICT on a non-weight-bearing all-extremity ergometer may be an effective alternative to HIIT and MICT on the treadmill. Finally, our results also suggest that middle-aged and older diabetic patients who are unable or unwilling to engage in all-extremity HIIT may receive similar benefits from all-extremity MICT.
Highlights.
High-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) were implemented in middle-aged/older diabetic patients
HIIT and MICT were implemented on a non-weight bearing all-extremity ergometer
All-extremity HIIT and MICT were feasible, well-tolerated and safe
HIIT and MICT similarly improved aerobic fitness and maximal exercise tolerance
Acknowledgments.
The authors would like to thank Vernie Wade, Astrid Gallego, Saul Ramirez, Gabrielle Tatro, Robert Cueto, Karen Mackay, Areila Maturell, Daniel Zhang, Lisa Fusco, Stephanie Lapierre, and Yasemin Sakarya for assistance with conducting this study. The authors would also like to express their gratitude to the study participants for their time and efforts.
Funding. This work was supported by the National Institutes of Health [AG 050203] to DDC. The funding source had no involvement in study design, collection, analysis and interpretation of data or writing of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests. The authors have no conflict of interest to declare.
REFERENCES
- Albright AL, 2013. Diabetes, in: Ehrman JK, Gordon PM, Visich PS, Keteyian SJ (Eds.), Clinical Exercise Physiology, Human Kinetics, Champaign, 91–112. [Google Scholar]
- Amundsen BH, Rognmo O, Hatlen-Rebhan G, Slordahl SA, 2008. High-intensity aerobic exercise improves diastolic function in coronary artery disease, Scand Cardiovasc J. 42, 110–117. [DOI] [PubMed] [Google Scholar]
- Awotidebe TO, Adedoyin RA, Oke KI, Ativie RN, Opiyo R, Ikujeyisi EO, Ikem RT, Afolabi MA, 2017. Relationship between functional capacity and health-related quality of life of patients with type-2 diabetes, Diabetes Metab Syndr. 11, 1–5. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ, 1986. A new method for detecting anaerobic threshold by gas exchange, J Appl Physiol (1985). 60, 2020–2027. [DOI] [PubMed] [Google Scholar]
- Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ, 2001. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials, JAMA. 286, 1218–1227. [DOI] [PubMed] [Google Scholar]
- CDC, 2017. National Diabetes Statistics Report, 2017, Centers for Disease Control and Prevention, US Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN, 2004. Exercise capacity and body composition as predictors of mortality among men with diabetes, Diabetes Care. 27, 83–88. [DOI] [PubMed] [Google Scholar]
- Church TS, LaMonte MJ, Barlow CE, Blair SN, 2005. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes, Arch Intern Med. 165, 2114–2120. [DOI] [PubMed] [Google Scholar]
- Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF, 2016. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association, Diabetes Care. 39, 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conraads VM, Pattyn N, De Maeyer C, Beckers PJ, Coeckelberghs E, Cornelissen VA, Denollet J, Frederix G, Goetschalckx K, Hoymans VY, Possemiers N, Schepers D, Shivalkar B, Voigt JU, Van Craenenbroeck EM, Vanhees L, 2015. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study, Int J Cardiol. 179, 203–210. [DOI] [PubMed] [Google Scholar]
- Grace A, Chan E, Giallauria F, Graham PL, Smart NA, 2017. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis, Cardiovasc Diabetol. 16, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EW, Cheng YJ, Saydah S, Cowie C, Garfield S, Geiss L, Barker L, 2012. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey, Diabetes Care. 35, 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui YJ, Liao CX, Liu Q, Guo Y, Yang T, Chen JY, Wang YT, Hu JH, Xu DY, 2017. Efficacy and safety of statins and exercise combination therapy compared to statin monotherapy in patients with dyslipidaemia: A systematic review and meta-analysis, Eur J Prev Cardiol. 24, 907–916. [DOI] [PubMed] [Google Scholar]
- Hagan RD, Gettman LR, Upton SJ, Duncan JJ, Cummings JM, 1983. Cardiorespiratory Responses to Arm, Leg, and Combined Arm and Leg Work on an Air-Braked Ergometer, J Cardiac Rehabil. 3, 689–703. [Google Scholar]
- Hollekim-Strand SM, Bjorgaas MR, Albrektsen G, Tjonna AE, Wisloff U, Ingul CB, 2014. High-intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic dysfunction: a randomized controlled trial, J Am Coll Cardiol. 64, 1758–1760. [DOI] [PubMed] [Google Scholar]
- Hwang CL, Yoo JK, Kim HK, Hwang MH, Handberg EM, Petersen JW, Christou DD, 2016. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults, Exp Gerontol. 82, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny GP, Stapleton JM, Yardley JE, Boulay P, Sigal RJ, 2013. Older adults with type 2 diabetes store more heat during exercise, Med Sci Sports Exerc. 45, 1906–1914. [DOI] [PubMed] [Google Scholar]
- Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS, 2012. Diabetes in older adults, Diabetes Care. 35, 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, Stanford FC, Kohl HW 3rd, Blair SN, 2011. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study, Circulation. 124, 2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly GW, Sui X, Church TS, Lavie CJ, Hand GA, Blair SN, 2008. Maximal exercise electrocardiography responses and coronary heart disease mortality among men with diabetes mellitus, Circulation. 117, 2734–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitranun W, Deerochanawong C, Tanaka H, Suksom D, 2014. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients, Scand J Med Sci Sports. 24, e69–76. [DOI] [PubMed] [Google Scholar]
- Mobius-Winkler S, Uhlemann M, Adams V, Sandri M, Erbs S, Lenk K, Mangner N, Mueller U, Adam J, Grunze M, Brunner S, Hilberg T, Mende M, Linke AP, Schuler G, 2016. Coronary Collateral Growth Induced by Physical Exercise: Results of the Impact of Intensive Exercise Training on Coronary Collateral Circulation in Patients With Stable Coronary Artery Disease (EXCITE) Trial, Circulation. 133, 1438–1448; discussion 1448. [DOI] [PubMed] [Google Scholar]
- Moholdt T, Aamot IL, Granoien I, Gjerde L, Myklebust G, Walderhaug L, Brattbakk L, Hole T, Graven T, Stolen TO, Amundsen BH, Molmen-Hansen HE, Stoylen A, Wisloff U, Slordahl SA, 2012. Aerobic interval training increases peak oxygen uptake more than usual care exercise training in myocardial infarction patients: a randomized controlled study, Clin Rehabil. 26, 33–44. [DOI] [PubMed] [Google Scholar]
- Moholdt TT, Amundsen BH, Rustad LA, Wahba A, Lovo KT, Gullikstad LR, Bye A, Skogvoll E, Wisloff U, Slordahl SA, 2009. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life, Am Heart J. 158, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB, Stoylen A, 2012. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients, Eur J Prev Cardiol. 19, 151–160. [DOI] [PubMed] [Google Scholar]
- Poitras VJ, Hudson RW, Tschakovsky ME, 2018. Exercise intolerance in Type 2 diabetes: is there a cardiovascular contribution?, J Appl Physiol (1985). 124, 1117–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisloff U, American Heart Association Physical Activity Committee of the Council on, L., Cardiometabolic, H., Council on Clinical, C., Council on, E., Prevention, Council on, C., Stroke, N., Council on Functional, G., Translational, B., Stroke, C., 2016. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association, Circulation. 134, e653–e699. [DOI] [PubMed] [Google Scholar]
- Schjerve IE, Tyldum GA, Tjonna AE, Stolen T, Loennechen JP, Hansen HE, Haram PM, Heinrich G, Bye A, Najjar SM, Smith GL, Slordahl SA, Kemi OJ, Wisloff U, 2008. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults, Clin Sci (Lond). 115, 283–293. [DOI] [PubMed] [Google Scholar]
- Schwartz J, 2005. Who is sensitive to extremes of temperature?: A case-only analysis, Epidemiology. 16, 67–72. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Conroy SP, Bayer AJ, 2008. Impact of diabetes on physical function in older people, Diabetes Care. 31, 233–235. [DOI] [PubMed] [Google Scholar]
- Stoa EM, Meling S, Nyhus LK, Glenn S, Mangerud KM, Helgerud J, Bratland-Sanda S, Storen O, 2017. High-intensity aerobic interval training improves aerobic fitness and HbA1c among persons diagnosed with type 2 diabetes, Eur J Appl Physiol. 117, 455–467. [DOI] [PubMed] [Google Scholar]
- Terada T, Friesen A, Chahal BS, Bell GJ, McCargar LJ, Boule NG, 2013. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes, Diabetes Res Clin Pract. 99, 120–129. [DOI] [PubMed] [Google Scholar]
- Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U, 2008. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study, Circulation. 118, 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T, 2007. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study, Circulation. 115, 3086–3094. [DOI] [PubMed] [Google Scholar]


