Abstract
Active ingredients in residential and agricultural insecticides have changed over time, due in part to regulatory restrictions. Few studies have evaluated how changes in active ingredients have impacted insecticide levels measured in homes. We measured concentrations of insecticides in one carpet dust sample from each of 434 homes in California from 2001 to 2006. Analytes included four insecticides sold for indoor home use during our study period (carbaryl, cypermethrin, permethrin and propoxur), and four no longer sold for indoor use including dichlorodiphenyltrichloroethylene (DDT, removed from the market in 1972), chlordane (1988), chlorpyrifos (2001), and diazinon (2004). We considered other potential determinants of concentrations of insecticides in carpet dust, such as home and garden use, occupational exposure, and nearby agricultural applications. We calculated percentage change in concentration of each insecticide per year, adjusting for significant determinants. In adjusted models, concentrations of insecticides in carpet dust decreased for three of four insecticides no longer sold for residential use: chlordane (−15%/year), chlorpyrifos (−31%), diazinon (−48%), and propoxur (−34%), which is currently sold for residential use but with increased restrictions since 1997. Concentrations of other insecticides sold for indoor use (carbaryl, cypermethrin, permethrin), and DDT, did not change over time in our study population.
Keywords: insecticides, dust, pesticides, regulatory policies, temporal trends
INTRODUCTION
Active ingredients in insecticide products used in residential and agricultural environments have changed over time due in part to regulations implemented to protect human health and the environment. Organochlorine (OC) insecticides such as dichlorodiphenyltrichloroethane (DDT) were introduced in the 1940’s and used to treat termites and other insects in homes. DDT was banned from all use in the United States in 1972 due to concerns about ecological and human health.1 Chlordane, another OC insecticide, replaced DDT until it, too, was banned for residential use in 1988 due to concerns about potential carcinogenicity and developmental health effects.2 Organophosphate (OP) insecticides, including chlorpyrifos and diazinon, largely replaced OC insecticides for residential use beginning in the 1970’s. As a result of the Food Quality Protection Act of 1996 and in advance of a required phase out in 2005 due to concerns about exposure to children, sales of chlorpyrifos and diazinon for indoor home use were voluntarily ended by the manufacturers in 2001 and 2004, respectively.3–4 Carbamate insecticides, including carbaryl and propoxur, were widely used for residential indoor applications in the 1980’s and 1990’s but many registered residential uses have been cancelled, including all indoor applications of carbaryl in 2008,5 due to concerns about neurodevelopmental effects of carbamates and carcinogenic effects of propoxur,6–7 Synthetic pyrethroids, such as permethrin and cypermethrin, were developed as a less toxic alternative to OP and OC insecticides,8 and have largely replaced OPs and carbamates insecticides for residential uses.9
Carpet dust has been used as an indicator of potential long-term exposure in the residential environment for non-volatile and semi-volatile compounds because the indoor environment is protected from light, weather and microbes that degrade compounds outdoors.10 Carpet dust is an especially good environmental medium for assessing exposure to children because they spend more time on the floor and have more hand-to-mouth activity than adults,11 resulting in greater estimated intake of chemicals from non-dietary ingestion.12 Based on exposure models for children three to five years of age, estimated non-dietary ingestion was the primary route of exposure (61%) to permethrin in U.S. homes with self-reported use of the insecticide,13 and to chlorpyrifos (59%) and diazinon (76%) among farmworkers’ children from an agricultural community in California.12 Sources of insecticides inside the home include indoor and outdoor residential applications,14–15 occupational take-home,16 and drift from nearby agricultural applications.17–19
In this study, we collected carpet dust from children’s homes between 2001 and 2006 and measured the concentrations of eight insecticides. This study provided an opportunity to evaluate changes in concentrations of insecticides in carpet dust over time, while adjusting for important covariates, such as home insect treatments and nearby agricultural use. The results of our analyses provide information on the impact of regulatory changes on indoor levels of these insecticides.
MATERIALS AND METHODS
Study population.
Our analysis included 413 residences from the Northern California Childhood Leukemia Study (NCCLS), a population-based case-control study in 17 counties in the San Francisco Bay area and 18 counties in the Central Valley.20 A single carpet dust sample was collected from a subset of NCCLS homes between October 2001 and April2006. Homes were eligible for carpet dust sample collection if the child was < 8 years old at diagnosis (reference date for controls) and lived in the same home since he/she was diagnosed with leukemia (reference date for controls) and had a carpet or area rug that was at least one square meter in area.
Our analysis also included 21 residences from the Agricultural Pesticide Study (APS) in Fresno, California, which was designed to evaluate temporal variability in levels and determinants of exposure to agricultural pesticides.21 Eligibility criteria for APS residences included having at least 25% of land within 500 m of the home in agricultural production and no residents working in pesticide-related jobs during the previous six months. All APS homes had at least 2.2 m2 of carpet or rugs that had been in the residence for at least one year and each home was visited from one to seven times (median=3 visits) between April 2003 and November 2005. Both study protocols were approved by the Institutional Review Boards of participating institutions and all participants gave written informed consent prior to participation.
Dust sample collection.
A dust sample was collected from each residence at the home visit, as described previously,22 using a High Volume Small Surface Sampler (HVS3, Envirometrics, Inc., Seattle, WA). For NCCLS participants, the dust sample was taken from a carpet or rug in the room (other than the child’s bedroom) where the child spent the most time while awake in the year prior to diagnosis/reference date to provide the best estimate of exposure since the bedroom would include time spent sleeping when the child would not be in contact with the carpet. For APS participants, a sample was collected from a carpet or rug in the room located on the side of the home facing agricultural fields at each home visit. Interview staff collected approximately 10 mL of dust. The median area sampled was 2.3 m2 and the inter-quartile range was 2.2 – 3.5 m2. We obtained latitude and longitude coordinates at the front door of each home using a global positioning system device. We defined the season of dust sample collection as winter (January-March), spring (April-June), summer (July-September), or fall (October-December). We collected 16, 112, 91, 117, 69 and 29 samples each year from 2001 – 2006, respectively (Supplemental Table 1).
Pesticide selection and laboratory analysis.
Among the 50 pesticides measured in the dust samples,22 we selected eight insecticides for this analysis (Table 1) that were: (1) used historically or currently for residential treatment of insects in California; (2) frequently detected in carpet dust samples from the study populations (> 40% of residences); and (3) representative of different chemical classes of insecticides. Insecticides included in this analysis and their chemical classes are: chlordane and DDT (organochlorines), chlorpyrifos and diazinon (organophosphates), carbaryl and propoxur (carbamates), and permethrin and cypermethrin (pyrethroids).
Table 1.
Characteristics of insecticides measured in carpet dust.
| Insecticide | Field T1/2a (Days) |
Salesb (kg) |
Ag. Usec (%) of Sales |
Last Year Sold for Indoor Used |
|---|---|---|---|---|
| Organochlorines | ||||
| Chlordanee | 365 | 0 | - | 1988 |
| DDT | 7,200 | 0 | - | 1972 |
| Organophosphates | ||||
| Chlorpyrifos | 43 | 986,181 | 82% | 2001 |
| Diazinon | 7 | 402,701 | 73% | 2004 |
| Carbamates | ||||
| Carbaryl | 17 | 190,938 | 55% | 2008f |
| Propoxur | 28 | 9,263 | 6% | Current |
| Pyrethroids | ||||
| Cypermethrine | 27 | 71,591 | 15% | Current |
| Permethrine | 30 | 455,296 | 92% | Current |
Field dissipation half-life.37
Average annual statewide sales in California from 2000 – 2006.24
Percentage of statewide sales reported as agricultural in CPUR from 2000 – 2006.36
Year removed from market for indoor uses in California.27
Sum of isomers.
Removed from market after our carpet dust samples were collected.
Dust samples were stored at −20 ºC prior to laboratory analysis in 2005–2008. Detailed laboratory methods have been published elsewhere.22 Briefly, carpet dust samples were sieved (< 150 µm) and approximately 0.5 g of dust extracted in a hexane:acetone solvent mixture, centrifuged, and concentrated. We quantified concentrations of insecticides using gas chromatography/mass spectrometry. In each batch, quality control samples included duplicates spiked with 250 ng of each analyte and a solvent method blank. We spiked carbon-13 labeled surrogate recovery standards (SRS) into all samples prior to extraction as a check on method performance. The limit of detection (LOD) for the eight insecticides ranged from 1 – 5 ng/g of dust. Duplicate samples had average relative percent differences of 10 – 30%. Mean recoveries of target analytes in spiked samples ranged from 85% - 118% and SRS recoveries averaged between 82 – 111% in quality control samples. SRS-corrected and uncorrected concentrations were highly correlated (rp > 0.95); therefore, we report results for uncorrected concentrations.
Interview data.
At the time of dust collection, we asked participants about treatments for pests in the home and garden during the previous 12 months. Specifically, we asked about insecticide treatments for ants and cockroaches; bees and wasps; fleas and ticks; flies and mosquitoes; carpenter ants and termites; any other indoor insects; and professional pesticide treatments inside and outside the home. Since several insecticides included in our analyses were also used in agriculture, we assessed the potential for pesticides to be transported into the home from the workplace by asking participants in the NCCLS whether anyone in the household worked during the previous 12 months in the following occupations: farm or ranch worker; gardener, landscaper, groundskeeper or nursery worker; agricultural packer; or pesticide applicator handling, formulating or mixing pesticides. We evaluated other potentially important factors including the year in which the residence was built and the age of the sampled carpet.
Geographic-based estimates of agricultural insecticide use.
We used the latitude and longitude coordinates of each home to assign the county and 2000 U.S. census tract. To account for potential regional differences in agricultural insecticide use, we grouped counties into three well-defined geographic regions of California including the urban San Francisco Bay Area, the agricultural Central Valley, and other (Central Coast, North Coast, Sierra Foothills). To account for potential neighborhood differences in the intensity of home insecticide use that might occur in more densely populated compared to less densely populated areas, we calculated the population density (persons/km2) for each census tract by dividing the total population in each tract by the land area of the tract in square kilometers (US Census Bureau 2003). For participants that were not asked (APS) or did not know the year their residence was built (n=63, 15%), we used the median year of homes built in their census tract (US Census Bureau 2003). Five of the insecticides had agricultural use during our study period (chlorpyrifos, diazinon, carbaryl, permethrin and cypermethrin), and we calculated the density of agricultural use near each residence using methods described elsewhere.18, 23 Briefly, we used the California Department of Pesticide Use Reporting database from 2000 to 200624 to estimate use around the home in kilograms per square kilometer (kg/km2) based on reported use within two distances (500 and 1,250 m radius) for 365 days prior to collection of the dust sample, which coincided with the self-reported information on home and garden use.
Statistical analysis.
In our primary analyses, we included the 413 samples from NCCLS homes and the 21 samples collected during the first visit to APS homes. We summed the concentrations of all measured isomers of a compound (alpha + gamma chlordane, cis + trans permethrin and cypermethrin I + II + III + IV) for analyses. Individual insecticide concentrations in carpet dust samples were log-normally distributed. We evaluated bivariate relationships between concentrations of insecticides and potential determinants using non-parametric methods. We used the Kruskal-Wallis test to compare concentrations of insecticides in residences based on categorical variables and Spearman correlation coefficients for the continuous agricultural pesticide use variables. Potential determinants included self-reported home and garden use of insecticides by pest treated (use in the last 12 months, yes or no), possible occupational insecticide exposure (anyone in the household employed during the last 12 months for each occupation, yes or no), agricultural insecticide use near the home (kg applied in the last 12 months within 500m and 1,250m), year the residence was built (≤1949, 1950–1959, 1960–1969, 1970–1979, 1980–1989, ≥1990), population density (continuous variable in persons/km2), age of sampled carpet (continuous variable in months), season sample collected (winter, spring, summer and fall) and region (San Francisco Bay Area, Central Valley, other).
We used the nonparametric Mann-Kendall trend test25 to identify significant temporal trends of insecticide concentrations (p<0.05) based on a single carpet dust sample from all 434 homes. We used Tobit regression to create separate models for each insecticide with the natural log-transformed concentration as the dependent variable and time in fractional years (measured from the start of dust collection) as the independent variable to estimate the average percentage change in concentrations of insecticides over time.25 Tobit regression offers an unbiased approach for analyzing measurement data with observations below the LOD, where values below the LOD are treated as censored values that between zero and the LOD that fit the overall distribution (i.e. log-normal) of the measured data.26 We included covariates that were associated with insecticide concentrations (p<0.1) in our bivariate analysis in the multivariable model for each insecticide, and retained the covariates that remained significant (p<0.1) in the final model. We estimated the average annual change (%) in concentrations of each insecticide, adjusted for significant covariates, using Equation 1.
| [Equation 1] |
For comparison, we also calculated the average percent annual change of insecticide concentrations in dust using linear regression models adjusted for the same covariates included in the Tobit models, but using the LOD/2 for concentrations below the LOD instead of a normally distributed value between zero and the LOD. We also ran the final models stratified by the two regions (San Francisco Bay Area and Central Valley) with the greatest number of samples (average of 28 – 32 samples per year) to evaluate differences in temporal trends of insecticide concentrations between urban and agricultural areas.
To evaluate temporal trends of insecticide concentrations in carpet dust within households, we used data from the 15 APS homes in Fresno where repeated dust samples were collected. We used mixed-effects models to estimate the change in insecticide concentrations over time while accounting for the correlation between measurements from the same household (no covariates were included). We assumed mutually independent and normally distributed random intercepts for each residence. We imputed values below the LOD for APS homes with repeated measurements using a maximum likelihood procedure that assumed a lognormal distribution, which has been reported to yield unbiased estimates of regression parameters for variables that have at least 50% of measurements above the LOD.26
RESULTS
Individual insecticides were detected in 49% (cypermethrin) to 99% (permethrin) of carpet dust samples (Table 2). The geometric mean concentrations were between 14 ng/g (propoxur) and 34 ng/g (chlorpyrifos) except for permethrin, which had a geometric mean concentration two orders of magnitude higher at 1,300 ng/g. Geometric mean concentrations of insecticides in carpet dust were significantly different (p<0.05) by region for five of the eight insecticides (Table 3). Concentrations of OC insecticides DDT and chlordane were highest in the more urban San Francisco Bay Area, whereas concentrations of OP insecticides chlorpyrifos and diazinon were highest in the agricultural Central Valley. Chlordane, DDT, chlorpyrifos and propoxur concentrations were higher (p<0.001) in older homes than homes built more recently, while concentrations of cypermethrin were higher in newer homes. Permethrin concentrations were higher (p<0.05) in samples collected from older carpets. Concentrations of carbaryl were higher in homes with self-reported use of flea and tick products. Concentrations of both permethrin and cypermethrin were higher in homes that had used products to control ants and roaches, fleas and ticks, and flies and mosquitoes. Concentrations of permethrin were also higher in homes that reported using products to control bees and wasps, while concentrations of cypermethrin were higher in homes that reported outdoor treatment by professional pest controllers. Higher levels of chlorpyrifos (p<0.1) and propoxur were reported in homes with a farmworker.
Table 2.
Distributions of concentrations of insecticides (ng/g) in carpet dust samples from a single home visit (n=434).
| Insecticide | LOD | NDetect (%) | 25th | 50th | 75th | 90th | GM(GSD) |
|---|---|---|---|---|---|---|---|
| Chlordane | 5 | 418 (96%) | 9 | 20 | 55 | 228 | 25 (5) |
| DDT | 5 | 252 (58%) | <LOD | 16 | 89 | 212 | 23 (5) |
| Chlorpyrifos | 3 | 388 (89%) | 14 | 33 | 93 | 220 | 34 (5) |
| Diazinon | 1 | 376 (87%) | 4 | 15 | 46 | 152 | 16 (7) |
| Carbaryl | 1 | 342 (79%) | 5 | 18 | 50 | 233 | 17 (8) |
| Propoxur | 1 | 344 (79%) | 3 | 15 | 39 | 104 | 14 (5) |
| Cypermethrin | 1 | 211 (49%) | <LOD | <LOD | 570 | 1,986 | 24 (31) |
| Permethrin | 1 | 427 (99%) | 370 | 920 | 3,400 | 11,621 | 1,300 (6) |
LOD=Limit of Detection; GM=geometric mean; GSD=geometric standard deviation.
Table 3.
Geometric mean (geometric standard deviation) of insecticide concentrations (ng/g) in single carpet dust samples from 434 homes by potential determinants.
| CHARACTERISTIC | N | Chlordane | DDT | Chlorpyrifos | Diazinon | Carbaryl | Propoxur | Cypermethrin | Permethrin |
|---|---|---|---|---|---|---|---|---|---|
| Region | * | ** | ** | ** | ** | ||||
| Central Valley | 169 | 24 (5) | 17 (5) | 47 (4) | 26 (6) | 16 (7) | 14 (5) | 65 (29) | 1150 (4) |
| San Francisco Bay Area | 189 | 30 (5) | 31 (5) | 27 (5) | 11 (6) | 17 (8) | 16 (5) | 13 (28) | 1320 (6) |
| Othera | 76 | 17 (4) | 19 (5) | 26 (8) | 13 (7) | 17 (8) | 13 (5) | 12 (28) | 1390 (5) |
| Year House Built | ** | ** | ** | + | ** | * | |||
| 1990 or later | 113 | 8 (3) | 8 (3) | 24 (5) | 16 (7) | 13 (8) | 7 (4) | 27 (29) | 1010 (5) |
| 1980 – 1989 | 60 | 12 (3) | 11 (4) | 44 (5) | 22 (6) | 12 (7) | 17 (5) | 70 (28) | 1120 (4) |
| 1970 – 1979 | 77 | 27 (5) | 13 (3) | 34 (4) | 14 (5) | 15 (7) | 18 (5) | 29 (33) | 1720 (6) |
| 1960 – 1969 | 69 | 51 (5) | 55 (5) | 44 (4) | 17 (9) | 29 (9) | 21 (5) | 18 (35) | 1550 (6) |
| 1950 – 1959 | 58 | 83 (4) | 72 (5) | 52 (4) | 18 (6) | 20 (6) | 24 (5) | 13 (33) | 1200 (5) |
| 1949 or earlier | 57 | 47 (4) | 71 (5) | 22 (4) | 9 (9) | 20 (8) | 13 (4) | 10 (24) | 1210 (5) |
| Age of carpet (months) | + | * | |||||||
| 12 – 36 | 130 | 22 (5) | 22 (5) | 29 (5) | 13 (6) | 14 (7) | 13 (5) | 19 (26) | 900 (6) |
| 37 – 60 | 122 | 26 (5) | 24 (5) | 32 | 17 (6) | 15 (8) | 13 (4) | 28 (31) | 1260 (5) |
| 61 – 84 | 77 | 21 (4) | 21 (5) | 34 (5) | 15 (7) | 14 (7) | 15 (5) | 28 (38) | 1560 (5) |
| 85+ | 105 | 31 (6) | 23 (5) | 41 (5) | 17 (7) | 26 (7) | 17 (5) | 15 (36) | 1560 (6) |
| Self-reported treatment during year prior to dust sample collection | |||||||||
| For ants/roaches | * | * | * | * | |||||
| Yes | 276 | 21 (5) | 20 (5) | 32 (4) | 16 (6) | 15 (7) | 13 (5) | 33 (33) | 1460 (5) |
| No | 158 | 34 (5) | 28 (5) | 26 (5) | 15 (7) | 20 (9) | 17 (5) | 13 (27) | 970 (6) |
| For bees/wasps | * | ||||||||
| Yes | 64 | 25 (4) | 20 (5) | 30 (5) | 12 (4) | 20 (5) | 11 (4) | 21 (32) | 1960 (6) |
| No | 370 | 24 (5) | 23 (5) | 34 (5) | 16 (7) | 16 (8) | 15 (5) | 24 (29) | 1170 (5) |
| For fleas/ticks | * | * | * | ||||||
| Yes | 49 | 26 (4) | 25 (5) | 35 (5) | 22 (9) | 32 (9) | 20 (5) | 76 (48) | 2610 (6) |
| No | 385 | 25 (5) | 22 (5) | 33 (5) | 15 (6) | 15 (7) | 14 (6) | 20 (29) | 1150 (5) |
| For flies/mosquitoes | * | + | |||||||
| Yes | 58 | 30 (5) | 25 (5) | 39 (4) | 16 (6) | 20 (8) | 16 (4) | 60 (34) | 1920 (6) |
| No | 376 | 24 (5) | 22 (5) | 34 (5) | 15 (7) | 16 (8) | 14 (5) | 20 (30) | 1180 (5) |
| Exterior by professional | + | * | * | ||||||
| Yes | 93 | 21 (5) | 19 (5) | 31 (5) | 20 (8) | 19 (5) | 10 (4) | 59 (31) | 1420 (5) |
| No | 340 | 26 (5) | 24 (5) | 34 (5) | 14 (6) | 16 (8) | 16 (5) | 18 (30) | 1210 (6) |
| Farmworker in home during year prior to dust sample collection | |||||||||
| + | * | ||||||||
| Yes | 26 | 23 (5) | 21 (5) | 55 (5) | 25 (4) | 19 (9) | 27 (6) | 25 (30) | 1120 (3) |
| No | 408 | 25 (5) | 23 (5) | 32 (5) | 15 (7) | 17 (8) | 14 (5) | 23 (31) | 1280 (6) |
Other regions include Central Coast, North Coast and Sierra Foothills.
p < 0.1;
p < 0.05;
p<0.001 from Kruskal-Wallis test.
Agricultural use accounted for a large proportion of chlorpyrifos (82%) but not propoxur (6%) sales during our study period (Table 1).24 Concentrations of insecticides in carpet dust were not significantly higher in homes with pesticide-exposed workers and did not differ by the season in which the sample was collected (data not shown). The Spearman rank correlation coefficients between carpet dust concentrations and agricultural use near the residence during the 12-months prior to sample collection were stronger for a 1,250 m radius buffer than for a 500 m radius, and the correlation was significant (p<0.01) for three of the five study insecticides currently used in agriculture: chlorpyrifos (rs=0.16), diazinon (rs=0.18) and carbaryl (rs=0.12).
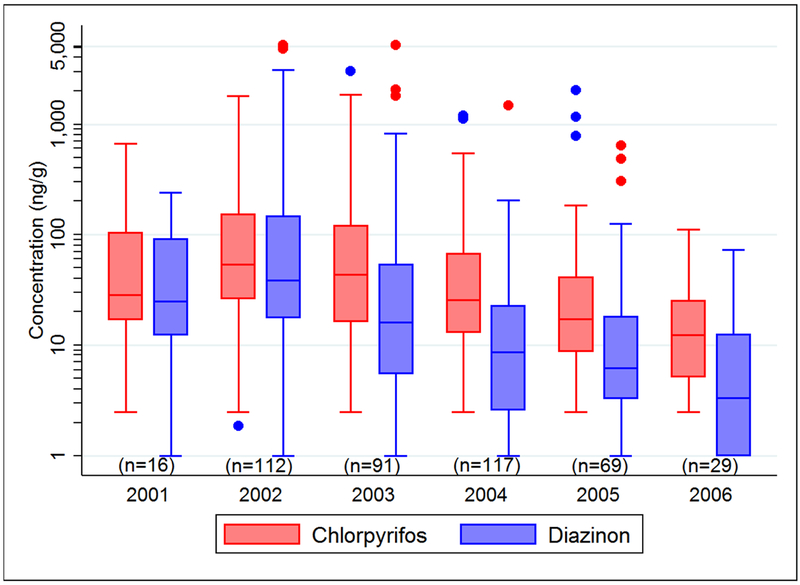
After adjusting for significant covariates in Tobit regression models, we observed that concentrations of three of four insecticides no longer sold for indoor use (chlordane, chlorpyrifos and diazinon) decreased significantly (p<0.05) across individual carpet dust samples from 434 homes during our study period of 2001–2006, while DDT concentrations did not change (Table 4). Chlorpyrifos concentrations decreased an average of 31% per year (95% confidence interval (CI): −38%, −22%), and diazinon 48% per year (95% CI: −54%, −40%). These decreases were highly significant (p<0.0001). In homes located in the Central Valley, the percentage changes for chlorpyrifos and diazinon, were not as large (−27% and −42% respectively), albeit still highly significant (data not shown). Chlordane levels decreased by 15% per year (95% CI: −23%, −6%) during the study period across our entire study area and this decrease was greater in the San Francisco Bay Area (−24%; 95% CI: −35%, −11%). Among insecticides sold for indoor use during our study period,27 only propoxur concentrations in carpet dust decreased significantly (p<0.0001). Concentrations of carbaryl, cypermethrin and permethrin did not change significantly during our study period or differ by region. Propoxur concentrations in carpet dust decreased 34% per year (95% CI: −42%, −25%) and the estimated decrease was similar by region. For all insecticides evaluated, results from linear regression models were similar to those from Tobit models (Table 4). Using the non-parametric Mann-Kendall trend test, we observed similar results with highly significant (p<0.0001) decreasing trends in carpet dust concentrations for chlorpyrifos, diazinon and propoxur, and also decreasing concentrations of chlordane (p=0.02) during our study period 2001–2006 (Table 4). Results were nearly identical when models were restricted to controls (data not shown).
Table 4.
Average percentage change in concentrations of insecticides in carpet dust per year from adjusted models for a single home visit (n=434) from 2001 – 2006.
| Insecticide | N | Tobit Regression % Changea in Concentration Per Year (95% CI) |
Linear Regression % Changea in Concentration Per Year (95% CI) |
Time Trendb |
|---|---|---|---|---|
| Chlordane c | 433 | −15 (−23, −6)* | −14 (−22, −6)* | p=0.02 |
| DDT d | 434 | 0 (−15, 18) | −1 (−11, 9) | p=0.80 |
| Chlorpyrifose | 434 | −31 (−38, −22)** | −29 (−36, −22)** | p<0.0001 |
| Diazinon f | 434 | −48 (−54, −40)** | −44 (−51, −37)** | p<0.0001 |
| Carbaryl g | 434 | 5 (−21, 14) | −4 (−17, 11) | p=0.40 |
| Propoxur h | 433 | −34 (−42, −25)** | −30 (−37, −22)** | p<0.0001 |
| Cypermethrin i | 432 | 20 (−21, 82) | 11 (−13, 41) | p=0.18 |
| Permethrin j | 428 | 4 (−8, 17) | 4 (−8, 17) | p=0.78 |
p < 0.05;
p < 0.001;
Percentage change = [Exp (β)-1]*100.
Mann-Kendall test for trend over time.
Adjusted for year residence built and census tract population density.
Adjusted for year residence built.
Adjusted for year residence built and location in Central Valley.
Adjusted for location in Central Valley and agricultural diazinon use within 1,250 m of residence during the year prior to dust sample collection.
Adjusted for age of carpet.
Adjusted for year residence built, census tract population density and farm worker in home during year prior to dust sample collection.
Adjusted for year residence built, location in Central Valley, home treatment for ants/roaches, fleas/ticks, flies/mosquitoes and outdoors by a professional during the year prior to dust sample collection.
Adjusted for age of carpet and home treatment for ants/roaches, bees/wasps, fleas/ticks during the year prior to dust sample collection.
Similar to results for a single dust sample from each home, we observed significant decreases in chlordane (p=0.01), diazinon (p<0.001) and propoxur (p=0.001) concentrations in repeated carpet dust samples collected from each of 15 residences in Fresno County from 2003 – 2005 (Table 5). The calculated percentage decreases per year were very similar to the results based on models using dust concentrations from a single dust sample collected at each of the 434 study residences (Table 4). The only differences in results produced by models using the repeated samples from 15 residences in Fresno County versus models using a single sample from all 434 residences were that concentrations of carbaryl decreased significantly in Fresno repeat homes (p<0.001) instead of remaining constant, and that chlorpyrifos concentrations remained relatively constant in Fresno homes instead of decreasing.
Table 5.
Average percentage change in concentrations of insecticides in carpet dust per year from unadjusted models for repeated visits (N=68) to the same residences (n=15) from 2003 – 2005.
| Insecticide | NDetect (%) |
% Changea in Concentration Per Year (95% CI) |
|---|---|---|
| Chlordane | 68 (100%) | −18 (−29, −5)* |
| DDT | 57 (84%) | 1 (−25, 31) |
| Chlorpyrifos | 68 (100%) | 7 (−10, 35) |
| Diazinon | 64 (94%) | −43 (−58, −24)** |
| Carbaryl | 65 (96%) | −48 (−65, −24)* |
| Propoxur | 54 (79%) | −37 (−52, −19)** |
| Cypermethrin | 54 (79%) | 18 (−5, 47) |
| Permethrin | 68 (100%) | −23 (−43, 4) |
p < 0.05;
p < 0.001;
Percentage change = [Exp (β)-1]*100.
DISCUSSION
We observed significantly decreasing trends in carpet dust concentrations of the insecticides chlorpyrifos, diazinon, propoxur, and chlordane over the period 2001–2006 across 434 homes located in both urban and agricultural areas of California. The timing of this reduction coincided with the discontinuation of sales for residential use of chlorpyrifos in 2001 and diazinon in 2004. Restrictions on household use of propoxur were initiated by the USEPA in 1997, which could explain its similar annual rate of decline (33%) as for chlorpyrifos and diazinon (31% and 48% per year, respectively).6 The persistence of chlordane and the time lapse between the chlordane ban (1988) and our study period likely explains the relatively lower annual rate of decline in concentration (15%) we observed. In contrast, we did not observe a decreasing trend for DDT, perhaps because it was banned from all use in the U.S. much earlier than chlordane, in 1972.1 However, we did record a relatively high detection frequency for DDT (58% of study homes) and a geometric mean concentration similar to chlordane (23 vs 25 ng/g). It is noteworthy that these two OC insecticides were still at detectable levels in carpet dust during the time period our samples were collected. The fact that these OC insecticides were detected in some homes built after these insecticides were removed from the market for indoor use suggests that an additional source of these compounds may be contaminated soil transported into the home. As a further indication that regulations are effective in reducing pesticide levels in household environments, we found no significant change in carpet dust concentrations of carbaryl, cypermethrin or permethrin, which were still approved for residential use at the time of our study. Results from repeated samples collected from a subset of 15 residences further support our findings that concentrations of chlordane, diazinon and propoxur in carpet dust decreased over time.
Our findings are consistent with previous studies that also reported decreasing levels of chlorpyrifos and diazinon after the residential phase-out of these insecticides. A study of 15 farmworker homes in Salinas, California found that chlorpyrifos and diazinon concentrations in dust collected in 2006 were 40–80% lower than dust concentrations from 197 farmworker homes in Salinas collected 2000 – 2002.28 A study of 50 homes in North Carolina that collected air, dust, soil, hand wipes, urine and diet samples once per year from 2003 – 2005 found that estimated doses of chlorpyrifos and diazinon from these media decreased for children over time.29 Concentrations of chlorpyrifos and diazinon in maternal air samples and chlorpyrifos levels in cord blood decreased between 1999 and 2002 in New York City.30 In the same cohort, biomarkers of prenatal chlorpyrifos exposure showed significant decreases in detection frequencies for subjects enrolled in 2003 – 2004 compared to those enrolled in 2001 – 2002.31 Average urinary metabolite levels of chlorpyrifos and diazinon decreased approximately 50% in the United States National Health and Nutrition Examination Survey (NHANES) between 1988 – 1994 and 2003 – 2004.32 Taken together, these studies provide strong evidence that regulatory changes in residential use of chlorpyrifos and diazinon as a result of the Food Quality Protection Act resulted in decreased indoor environmental levels and decreased human exposure. Concentrations of propoxur also decreased in maternal personal air samples and cord blood samples collected from 1999 – 2002 in New York City.30 Decreases in environmental and biological levels of propoxur may have been related to changes in the formulation of propoxur products during this time from ready-to-use sprays to pet collars that release less insecticide into the environment.6
We also found that levels of chlordane, DDT, chlorpyrifos and propoxur were higher in older residences, and that DDT and chlordane were higher in the San Francisco Bay Area, where development occurred earlier than other areas of Northern and Central California and historical indoor residential use of these compounds was more likely. A previous U.S. study observed higher DDT dust concentrations in homes built before 1940 and higher chlordane dust concentrations in residences built from 1940 – 1959 compared to homes built more recently.33 Levels of lead, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons are also higher in dust from older NCCLS homes compared to newer homes, suggesting that people living in older homes are exposed to higher levels of multiple persistent chemicals.34
In an earlier analysis of a subset of 89 of the study homes that were located near agricultural fields, we found that agricultural pesticide use near residences was a significant determinant of pesticide concentrations in carpet dust, including chlorpyrifos and four other pesticides not analyzed in this study, but not diazinon or carbaryl.18 However, in the current study of 434 homes, we found a significant correlation (p<0.01) between carpet dust concentrations and agricultural use near the residence during the 12 months prior to sample collection for three of five of the study insecticides currently used in agriculture: chlorpyrifos, diazinon and carbaryl. Chlorpyrifos and diazinon are still used widely in agriculture,35 especially in the Central Valley of California.36 We observed levels of these two pesticides in carpet dust of residences in this region to be two to three times higher than in residences located in the urban San Francisco Bay Area (Table 3). In APS homes located near agricultural land in the Central Valley county of Fresno, we did not observe a within-home decrease in chlorpyrifos concentrations in repeat dust samples collected over time (2003 – 2005). This may indicate that agricultural use of chlorpyrifos near APS homes, which remained fairly constant during our study period, may have obscured the effect of regulatory changes in residential chlorpyrifos use. Previous studies have observed higher chlorpyrifos concentrations in carpet dust of residences with nearby agricultural use and agricultural workers living in the home.17–18 The significant temporal decline in carbaryl concentrations observed in repeated samples from APS homes (−48%/year), but not in the first sample collected from all 434 homes (−4%/year), may have been the result of declining agricultural use near APS homes in Fresno County from 64,589 pounds in 2002 to 15,813 pounds in 2005.24
In our study, permethrin concentrations in carpet dust were almost two orders of magnitude higher than the other insecticides and were relatively stable over time. Synthetic pyrethroids such as permethrin were designed to be photo stable, have a low vapor pressure and persist on surfaces for crop protection purposes.8 High dust concentrations of permethrin in residences are likely related to the low vapor pressure of permethrin37 and increasing use of permethrin for treating insects in and around homes.35 In previous studies, permethrin concentrations in house dust were also at least an order of magnitude higher than other insecticides in agricultural Salinas, California,17 in North Carolina,38 and in low-income homes in Boston, Massachusetts.39 Detection frequencies of permethrin in personal air samples among pregnant women in New York City increased significantly from 2000 to 2006.9 A study of 90 families in Northern California found higher pyrethroid metabolite levels and lower chlorpyrifos metabolite levels in urine samples collected in 2007–2009 than the general U.S. population in 2001–2002.40 In NHANES, the geometric mean (GM) urinary concentration of 3-phenoxybenzoic acid, a metabolite of cypermethrin and permethrin, increased from 0.32 µg/g-Creatinine in 2001 – 2002 to 0.43 µg/g-Creatinine in 2007 – 2008.41 Further studies of exposure to pyrethroid insecticides from residential use and potential health effects are warranted given the high concentrations observed in carpet dust samples in this study and increasing urinary metabolite concentrations observed in other studies.40–41
Our study has several limitations. We only collected one carpet dust sample from most homes. Although pesticide measurements were conducted in the same laboratory and similar questionnaires were used to assess occupational and home insecticide use, we combined data on insecticide concentrations in carpet dust from two studies with different objectives and designs, including the eligibility criteria for APS residences having at least 25% of land within 500 m of the home in agricultural production. We only had information on home insecticide use during the 12 months prior to sample collection and did not ask about product formulation. The California Pesticide Use Report data does not include location information on neighborhood insecticide use for pest control. Our sampling period from 2001 – 2006 likely underestimated the impact of FQPA on residential levels of chlorpyrifos and diazinon after removal of these insecticides from the residential market in 2001 and 2004, due to the long indoor half-life and potential to store these products for long periods of time prior to use.
This study also has several strengths including a large number of carpet dust samples collected over a long period of time from a diverse study population and wide geographic area. We collected repeat house dust samples from a subset of homes (n=15) and the temporal trends were similar to those observed across all homes in our study (n=434) for most insecticides. We were able to adjust for potentially important confounders including home, occupational and nearby agricultural insecticide use as well as residential and carpet age.
Our findings suggest that regulations limiting residential use of OP insecticides resulted in lower concentrations of these compounds in the residential environment, but these declines may be attenuated by contamination of homes due to fugitive emissions (e.g. primary and/or secondary drift) from nearby agricultural use and insecticides tracked-in by residents. Our results indicate that the persistence of OC insecticides indoors results in the potential for continued exposure, particularly in older residences. Permethrin concentrations were an order of magnitude higher than other insecticides, warranting further study. Our findings combined with biomonitoring studies provide strong evidence that the Food Quality Protection Act resulted in decreased indoor environmental levels and human exposure to chlorpyrifos and diazinon.
Supplementary Material
Figure 1.
Distributions of chlorpyrifos and diazinon concentrations (ng/g) in carpet dust by year.
Definitions: Box represents interquartile range (25th – 75th percentiles); line dividing box represents median, whiskers represent minimum and maximum values excluding outliers, dots represent outliers greater than 1.5 interquartile ranges above the upper quartile or less than 1.5 interquartile ranges below the lower quartile.
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Environmental Health Sciences, grants R01ES09137 (P. Buffler, UCB, PI) and P42-ES04705 (P. Buffler, UCB, PI), the National Cancer Institute intramural research program (M. Ward, PI), and grant numbers 5R01CA092683 (J.R. Nuckols, CSU, PI) and R01CA92674 (P. Reynolds, CPIC, PI). This research could not have been conducted without the strong support from our clinical collaborators, participating hospitals, and the families of study participants. The opinions given by the authors are not necessarily those of the funding agencies.
LIST OF ABBREVIATIONS
- APS
Agricultural Pesticide Study
- DDT
p, p’-dichlorodiphenyltrichloroethylene
- LOD
Limit of detection
- NCCLS
Northern California Childhood Leukemia Study
- NHANES
National Health and Nutrition Examination Survey
- OC
organochlorine
- OP
organophosphate
Footnotes
Notes
Nicole Deziel discloses that her spouse is employed by Dow Chemical, which manufactures pesticides (among thousands of other chemicals and products). None of the other authors declares any actual or potential competing financial interests.
REFERENCES
- 1.United States Environmental Protection Agency (USEPA) DDT Ban Takes Effect. EPA Press; Release. http://www2.epa.gov/aboutepa/ddt-ban-takes-effect (accessed October 7, 2015). [Google Scholar]
- 2.Agency for Toxic Substance and Disease Registry (ATSDR) Toxicological Profile for Chlordane. ; Washington, D.C., 1994. [PubMed] [Google Scholar]
- 3.United States Environmental Protection Agency (USEPA) Chlorpyrifos Revised Risk Assessment and Agreement with Registrants. . http://www.ibiblio.org/london/NAFEX/message-archives/old/pdf00000.pdf (accessed October 7, 2015).
- 4.United States Environmental Protection Agency (USEPA). Diazinon Revised Risk Assessment and Agreement with Registrants. . http://www.ok.gov/~okag/forms/cps/epaagree.pdf (accessed October 7, 2015).
- 5.United States Environmental Protection Agency (USEPA) Carbaryl; Notice of Receipt of Requests to Voluntarily Cancel or to Terminate Uses of Certain Pesticide Registrations. https://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2008-0525-0015.
- 6.United States Environmental Protection Agency (USEPA). Reregistration Eligibility Decision for Propoxur. . http://archive.epa.gov/pesticides/reregistration/web/pdf/2555red.pdf (accessed October 7, 2015).
- 7.United States Environmental Protection Agency (USEPA). Amended Reregistration Eligibility Decision for Carbaryl. . http://archive.epa.gov/pesticides/reregistration/web/pdf/carbaryl-red-amended.pdf (accessed October 7, 2015).
- 8.Casida JE, Pyrethrum flowers and pyrethroid insecticides. Environ Health Perspect 1980, 34, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams MK; Rundle A; Holmes D; Reyes M; Hoepner LA; Barr DB; Camann DE; Perera FP; Whyatt RM, Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ Health Perspect 2008, 116 (12), 1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JW; Wallace LA; Camann DE; Dickey P; Gilbert SG; Lewis RG; Takaro TK, Monitoring and reducing exposure of infants to pollutants in house dust. Rev Environ Contam Toxicol 2009, 201, 1–39. [DOI] [PubMed] [Google Scholar]
- 11.Xue J; Zartarian V; Tulve N; Moya J; Freeman N; Auyeung W; Beamer P, A meta-analysis of children’s object-to-mouth frequency data for estimating non-dietary ingestion exposure. J Expo Sci Environ Epidemiol 2010, 20 (6), 536–45. [DOI] [PubMed] [Google Scholar]
- 12.Beamer PI; Canales RA; Ferguson AC; Leckie JO; Bradman A, Relative pesticide and exposure route contribution to aggregate and cumulative dose in young farmworker children. Int J Environ Res Public Health 2012, 9 (1), 73–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zartarian V; Xue J; Glen G; Smith L; Tulve N; Tornero-Velez R, Quantifying children’s aggregate (dietary and residential) exposure and dose to permethrin: application and evaluation of EPA’s probabilistic SHEDS-Multimedia model. J Expo Sci Environ Epidemiol 2012, 22 (3), 267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colt JS; Lubin J; Camann D; Davis S; Cerhan J; Severson RK; Cozen W; Hartge P, Comparison of pesticide levels in carpet dust and self-reported pest treatment practices in four US sites. J Expo Anal Environ Epidemiol 2004, 14 (1), 74–83. [DOI] [PubMed] [Google Scholar]
- 15.Deziel NC; Colt JS; Kent EE; Gunier RB; Reynolds P; Booth B; Metayer C; Ward MH, Associations between self-reported pest treatments and pesticide concentrations in carpet dust. Environ Health 2015, 14, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradman A; Whitaker D; Quiros L; Castorina R; Claus Henn B; Nishioka M; Morgan J; Barr DB; Harnly M; Brisbin JA; Sheldon LS; McKone TE; Eskenazi B, Pesticides and their metabolites in the homes and urine of farmworker children living in the Salinas Valley, CA. J Expo Sci Environ Epidemiol 2007, 17 (4), 331–49. [DOI] [PubMed] [Google Scholar]
- 17.Harnly ME; Bradman A; Nishioka M; McKone TE; Smith D; McLaughlin R; Kavanagh-Baird G; Castorina R; Eskenazi B, Pesticides in dust from homes in an agricultural area. Environ Sci Technol 2009, 43 (23), 8767–74. [DOI] [PubMed] [Google Scholar]
- 18.Gunier RB; Ward MH; Airola M; Bell EM; Colt J; Nishioka M; Buffler PA; Reynolds P; Rull RP; Hertz A; Metayer C; Nuckols JR, Determinants of agricultural pesticide concentrations in carpet dust. Environ Health Perspect 2011, 119 (7), 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward MH; Lubin J; Giglierano J; Colt JS; Wolter C; Bekiroglu N; Camann D; Hartge P; Nuckols JR, Proximity to crops and residential exposure to agricultural herbicides in iowa. Environ Health Perspect 2006, 114 (6), 893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartley K; Metayer C; Selvin S; Ducore J; Buffler P, Diagnostic X-rays and risk of childhood leukaemia. International journal of epidemiology 2010, 39 (6), 1628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deziel NC; Ward MH; Bell EM; Whitehead TP; Gunier RB; Friesen MC; Nuckols JR, Temporal variability of pesticide concentrations in homes and implications for attenuation bias in epidemiologic studies. Environ Health Perspect 2013, 121 (5), 565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colt JS; Gunier RB; Metayer C; Nishioka MG; Bell EM; Reynolds P; Buffler PA; Ward MH, Household vacuum cleaners vs. the high-volume surface sampler for collection of carpet dust samples in epidemiologic studies of children. Environ Health 2008, 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuckols JR; Gunier RB; Riggs P; Miller R; Reynolds P; Ward MH, Linkage of the California Pesticide Use Reporting Database with spatial land use data for exposure assessment. Environ Health Perspect 2007, 115 (5), 684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.California Department of Pesticide Regulation (CDPR). Reports of Pesticides Sold in California 2000 – 2006. . http://www.cdpr.ca.gov/docs/mill/nopdsold.htm (accessed October 7, 2015).
- 25.Helsel DR a. H., D.M., Statistical Methods in Water Research. Trend Analysis and Mehtods for Data below the Reporting Limit. . United States Geological Survey: Washington, D.C., 2002. [Google Scholar]
- 26.Lubin JH; Colt JS; Camann D; Davis S; Cerhan JR; Severson RK; Bernstein L; Hartge P, Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 2004, 112 (17), 1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.California Department of Pesticide Regulation (CDPR). Actively Registered Active Ingredients by Common Name. http://www.cdpr.ca.gov/docs/label/actai.htm (accessed October 7, 2015).
- 28.Quiros-Alcala L; Bradman A; Nishioka M; Harnly ME; Hubbard A; McKone TE; Ferber J; Eskenazi B, Pesticides in house dust from urban and farmworker households in California: an observational measurement study. Environ Health 2011, 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson NK; Strauss WJ; Iroz-Elardo N; Chuang JC, Exposures of preschool children to chlorpyrifos, diazinon, pentachlorophenol, and 2,4-dichlorophenoxyacetic acid over 3 years from 2003 to 2005: A longitudinal model. J Expo Sci Environ Epidemiol 2010, 20 (6), 546–58. [DOI] [PubMed] [Google Scholar]
- 30.Whyatt RM; Camann D; Perera FP; Rauh VA; Tang D; Kinney PL; Garfinkel R; Andrews H; Hoepner L; Barr DB, Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol 2005, 206 (2), 246–54. [DOI] [PubMed] [Google Scholar]
- 31.Whyatt RM; Garfinkel R; Hoepner LA; Andrews H; Holmes D; Williams MK; Reyes A; Diaz D; Perera FP; Camann DE; Barr DB, A biomarker validation study of prenatal chlorpyrifos exposure within an inner-city cohort during pregnancy. Environ Health Perspect 2009, 117 (4), 559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clune AL; Ryan PB; Barr DB, Have regulatory efforts to reduce organophosphorus insecticide exposures been effective? Environ Health Perspect 2012, 120 (4), 521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colt JS; Severson RK; Lubin J; Rothman N; Camann D; Davis S; Cerhan JR; Cozen W; Hartge P, Organochlorines in carpet dust and non-Hodgkin lymphoma. Epidemiology 2005, 16 (4), 516–25. [DOI] [PubMed] [Google Scholar]
- 34.Whitehead TP; Metayer C; Ward MH; Colt JS; Gunier RB; Deziel NC; Rappaport SM; Buffler PA, Persistent organic pollutants in dust from older homes: learning from lead. American journal of public health 2014, 104 (7), 1320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.United States Environmental Protection Agency (USEPA) Pesticide Industry Sales and Usage, 2006 and 2007 Market Estimates. Available: http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf. (accessed April 29, 2015).
- 36.California Department of Pesticide Regulation (CDPR) Pesticide Use Reporting Data Homepage. http://www.cdpr.ca.gov/docs/pur/purmain.htm (accessed October 7, 2015).
- 37.United States Department of Agriculture (USDA) Pesticide Properties Database. http://www.ars.usda.gov/Services/docs.htm?docid=14147 (accessed October 7, 2015).
- 38.Morgan MK; Wilson NK; Chuang JC, Exposures of 129 preschool children to organochlorines, organophosphates, pyrethroids, and acid herbicides at their homes and daycares in North Carolina. Int J Environ Res Public Health 2014, 11 (4), 3743–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wason SC; Julien R; Perry MJ; Smith TJ; Levy JI, Modeling exposures to organophosphates and pyrethroids for children living in an urban low-income environment. Environ Res 2013, 124, 13–22. [DOI] [PubMed] [Google Scholar]
- 40.Trunnelle KJ; Bennett DH; Tulve NS; Clifton MS; Davis MD; Calafat AM; Moran R; Tancredi DJ; Hertz-Picciotto I, Urinary pyrethroid and chlorpyrifos metabolite concentrations in Northern California families and their relationship to indoor residential insecticide levels, part of the Study of Use of Products and Exposure Related Behavior (SUPERB). Environ Sci Technol 2014, 48 (3), 1931–9. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals; Atlanta, GA, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.