Abstract
Background:
A subset of cannabis users develop some degree of Cannabis Use Disorder (CUD). Although behavioral therapy has some success in treating CUD, many users relapse, often citing altered sleep, mood, and irritability. Preclinical animal models of cannabinoid withdrawal focus primarily on somatic-related behaviors precipitated by a cannabinoid receptor antagonist. The goal of the present study was to develop novel cannabinoid withdrawal models that are either antagonist-precipitated or spontaneously induced by abstinence.
Methods:
C57BL/6J mice were repeatedly administered the phytocannabinoid Δ9-tetrahydrocannabinol (THC; 1, 10 or 50 mg/kg, s.c.), the synthetic cannabinoid receptor agonist JWH-018 (1 mg/kg, s.c.), or vehicle (1:1:18 parts ethanol:Kolliphor EL:saline, s.c.) for 6 days. Withdrawal was precipitated with the cannabinoid receptor inverse agonist rimonabant (3 mg/kg, i.p.) or elicited via abstinence (i.e., spontaneous withdrawal), and behavior was scored. Classic somatic signs of cannabinoid withdrawal were also quantified.
Results:
Precipitated THC withdrawal significantly increased plasma corticosterone. Precipitated withdrawal from either THC or JWH-018 suppressed marble burying, increased struggling in the tail suspension test, and elicited somatic withdrawal behaviors. The monoacylglycerol lipase inhibitor JZL184 attenuated somatic precipitated withdrawal, but had no effect on marble burying or struggling. Spontaneous THC or JWH-018 withdrawal induced paw tremors, head twitches, and struggling in the tail suspension test after 24–48 h abstinence. JZL184 or THC attenuated these spontaneous withdrawal-induced behaviors.
Conclusion:
Outcomes from tail suspension and marble burying tests reveal that THC withdrawal is multifaceted, eliciting and suppressing these behaviors in addition to well-documented somatic signs of withdrawal.
Keywords: Cannabinoid withdrawal, marijuana, drug dependence, cannabis use disorder, stress
1. Introduction
Cannabis accounts for over 75% of all illicit drug use in the United States alone (Ramesh and Haney, 2015). As legal restrictions ease and access to cannabis increases, many view cannabis as a low-risk alternative to other drugs (Hall and Kozlowski, 2017). Regardless, 2–6% of cannabis users in the United States will develop some level of dependence (Hasin et al., 2016), as defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as Cannabis Use Disorder (CUD). Individuals experiencing withdrawal from cannabis often report a constellation of negative symptoms including sleep disturbances, changes in appetite, depression, anxiety, nausea, and increased irritability (American Psychiatric Association 2013). The negative side effects of withdrawal after attempted cessation increase the likelihood of relapse (Budney et al., 2008; Haney et al., 2013a).
There are currently no suitable pharmacological treatments specifically for cannabis dependence (Allsop et al., 2015; Mason et al., 2016). Several psychosocial treatment models are in use, including cognitive-behavioral therapy, motivational-enhancement therapy, contingency management, and family-based therapies, all of which yield modest success rates (Brezing and Levin, 2017; Sherman and McRae-Clark, 2016). Approximately 70% of individuals treated with psychosocial therapies relapse into cannabis use (Ramesh and Haney, 2015). With the recent policy changes surrounding cannabis and its social acceptability, misuse and dependence will likely increase. The endocannabinoid system offers multiple potential targets for developing pharmaceutical therapies for cannabis dependence (Haney et al., 2013b; Schlosburg et al., 2009).
Cannabinoid dependence and withdrawal has been modeled in non-human animals (Lichtman et al., 2001; Lichtman et al., 1998). For example, somatic signs of cannabinoid withdrawal typically include paw tremors (i.e., a clapping motion of the forepaws) and head shakes (i.e., quick rotation of the head from side to side) and are commonly reported in rodents. Although these are reliable measures, somatic withdrawal signs do not model the primary symptoms of cannabinoid withdrawal in humans, such as altered stress, emotion, and cognition. Moreover, their reliance on antagonist administration limits the external validity of precipitated THC withdrawal models.
Several studies have reported conflicting findings on the effects of THC and synthetic cannabinoid withdrawal on behavioral tasks evaluating aspects of attentional filtering, sensorimotor-gating, and preattentional processes (Bortolato et al., 2005; Marusich et al., 2014; Schneider and Koch, 2005). Conversely, preclinical studies on emotionality-related changes during THC withdrawal are scant, with a few notable exceptions. For instance, in male adolescent rats, spontaneous THC withdrawal increased open arm time in the elevated plus maze, but decreased open arm time for female rats (Harte-Hargrove and Dow-Edwards, 2012). In adult male mice, rimonabant-precipitated THC withdrawal reduced open arm time (Huang et al., 2010). The disparate findings of these studies may be due to age, sex or species differences as well as myriad methodological differences that affect performance in the plus maze (Hogg, 1996). Regardless, these data indicate that, in addition to the well characterized somatic withdrawal, emotional circuits are activated during THC withdrawal.
In chronic cannabis users, withdrawal induces stress and anxiety (Haney et al., 1999). Similarly, withdrawal from synthetic cannabinoids increases plasma concentrations of the glucocorticoid stress hormone corticosterone in rats (Rodriguez De Fonseca et al., 1997) and mice (Oliva et al., 2003). Withdrawal from the synthetic cannabinoid agonist CP55,940 suppresses time in the lit portion of the light/dark box test and time in the open arms of the elevated plus maze (Oliva et al., 2003). Repeated administration of THC desensitizes CB1 receptors (Sim-Selley, 2003), and genetic deletion of Cnr1 (which encodes the CB1 receptor) decreases struggling in the tail suspension test (Aso et al., 2008). In addition to their utility as drug screens, these behavioral models of emotionality are also stressors because each induces corticosterone release (Aso et al., 2008; Chotiwat and Harris, 2006).
In our present studies, separate groups of mice were exposed repeatedly to THC or the synthetic cannabinoid JWH-018, a prototypical component of first generation synthetics, colloquially known as “Spice,”sold in the US and Europe (Atwood et al., 2011). Withdrawal was precipitated with the CB1 receptor selective inverse agonist rimonabant, and behaviors were measured in marble burying test, novel object test, light-dark box, tail suspension test, prepulse inhibition, or social interaction test, in addition to somatic signs of withdrawal. THC withdrawal behaviors were challenged with a MAGL or FAAH inhibitor, or THC. Finally, mice were exposed to THC for 6 days, and behavior was quantified at multiple time points during abstinence to establish spontaneous THC withdrawal models.
2.1. Methods
2.2. Animals
Adult male and female C57BL/6J mice (Jackson Laboratory; Bar Harbor, ME) were housed in the same room in Polysulfone plastic cages (4–5 per cage) with food and water available ad libitum. All mice were 8–14 weeks old at time of testing. The mean body mass of males was 27 g and the mean body mass of females was 19 g. Mice were housed in a single, temperature (20–22 °C) and humidity (50 ± 5%) controlled room. Mice were kept on a 12:12 h light/dark cycle, with lights on at 5:00 AM, and were randomly assigned to each treatment group such that each cage contained mice from at least two different treatment groups (e.g., no cage contained only THC-treated mice). All experiments were carried out by trained technicians who were blinded to treatment conditions. The Animal Care and Use Committee at West Virginia University approved all experimental protocols.
2.3. Drugs
Δ9-tetrahydrocannabinol (THC), JWH-018 (also known as AM-678), and rimonabant (SR141716A) were generously provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). In a pilot experiment, 5–6 days of twice-daily injections of JWH-018 (10 mg/kg, s.c.) (Brents et al., 2013) induced convulsions resulting in death of 11 of 16 mice, either during dosing (3) or after rimonabant administration (8). Thus, 1 mg/kg JWH-018 was used in the present study. JZL184, PF-3845, and mifepristone (RU-486) were purchased from Cayman Chemical (Ann Arbor, MI). Propranolol was purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in a vehicle composed of 5% ethanol, 5% Kolliphor EL (Sigma-Aldrich, St. Louis, MO), and 90% saline (Kinsey and Cole, 2013), with the exception of mifepristone, which was dissolved in a vehicle of 10% DMSO (Sigma-Aldrich), 10% TWEEN 80, and 80% saline. Doses and pretreatment times were chosen based on previous experience and literature (Al-tubuly et al., 2008; Chester et al., 2014; Kinsey et al., 2011; Lichtman et al., 2001). JZL184 (8 mg/kg, i.p.), PF-3845 (10 mg/kg, i.p.), and mifepristone (25 mg/kg, i.p.) were administered 150 minutes before the final THC injection (Padgett et al., 1998; Ahn et al., 2009; Long et al., 2009). Propranolol (1 or 10 mg/kg, i.p.) was administered 50 min (Lin et al., 2014) before the final THC injection. All solutions were warmed to room temperature before injection at a volume of 10 μl/g body mass.
2.3a. Precipitated withdrawal paradigm:
Mice were weighed daily and injected subcutaneously (s.c.) with THC (10 or 50 mg/kg) (Falenski et al., 2010; Lichtman et al., 2001), JWH-018 (1 mg/kg) (Wiley et al., 2012), or vehicle every 12 h for 6 days (Falenski et al., 2010; Schlosburg et al., 2009). Injections occurred between 9:00 and 10:00 each morning and 21:00 and 22:00 each night. On the sixth day, all mice received a final injection of THC, JWH-018, or vehicle. After 30 min, mice received an intraperitoneal (i.p.) injection of rimonabant (3 mg/kg) (Lichtman et al., 2001) to precipitate withdrawal. Control mice received a vehicle injection. Mice were assessed in one or (maximally) two behavioral tests, as detailed below, by an experimenter who was blinded to drug treatment conditions.
2.3b. Spontaneous withdrawal paradigm:
Mice were weighed daily and injected with either THC (10 or 50 mg/kg, s.c.), JWH-018 (1 mg/kg, s.c.) or vehicle every 12 h for 6 days (Falenski et al., 2010; Schlosburg et al., 2009; Wiley et al., 2012). Injections occurred between 9:00 and 10:00 each morning and 21:00 and 22:00 each night. Behavioral assessments were conducted repeatedly following the final THC or vehicle injection. For the somatic and tail suspension tests, mice were tested at 0, 6, 12, 24, 36, and 48 h after the final THC injection. For the marble burying test, mice were tested at 0, 2, 4, 8, 12, 24, 36, and 48 h after the final THC injection. Mice tested at 12 and 36 h post-injection had injection schedules offset by 12 h to allow for testing during the light phase.
2.4. Corticosterone Quantification
Corticosterone was measured in the plasma of mice treated for 6 days with either vehicle or THC (10 mg/kg). Blood was drawn on day 6 of treatment, via cardiac puncture, into syringes coated with 100 μL of 20 mM EDTA in distilled water (Zhang et al., 2015) 30 min after rimonabant or vehicle injection. Samples were placed on ice, then centrifuged at 2000 × g and 4˚C for 20 min within 30 min of collection. Plasma was aspirated and frozen at −80˚C until assay. Samples were diluted 10-fold and assayed in duplicate by sandwich ELISA (R&D Systems, Inc, Minneapolis, MN) according to manufacturer’s instruction. The plates were read on a VMax Kinetic ELISA Microplate Reader (Molecular Devices) at 450 nm with a 560 nm correction and fitted to a standard curve to determine concentrations as ng/mL.
2.5. Behavioral Assessments
In order to reduce animal numbers, mice were individually tested in one or two of the following assays. Tests were run simultaneously in groups of four mice, with the exception of prepulse inhibition, in which two mice were run simultaneously. If multiple tests were run, the test batteries were arranged in ascending order of presumed aversion (i.e., ranked in order of increased probability of activating the stress response), as follows: (1) marble burying or somatic signs, then tail suspension; or (2) novel object, then light/dark box. In precipitated withdrawal experiments, somatic signs, social interaction, and prepulse inhibition assays were the only tests conducted on test day. In instances where mice were tested in two assays, testing occurred consecutively, with approximately 10 min between tests.
2.5a. Somatic signs of withdrawal:
Somatic signs of withdrawal were measured by placing each mouse into an empty, plastic test chamber (20 cm W × 20 cm L × 15 cm H) inside a sound-attenuating chamber outfitted with a fan and white LED lighting. The box had three clear sides and one mirrored side that faced a camera to allow for observation of behavior when the mouse faced away from the camera.
Mice were habituated to the test apparatus following final THC or vehicle injection for 30 min and were then removed and injected with rimonabant or vehicle, as previously reported (Schlosburg et al., 2009). The boxes were cleaned using a paper towel moistened with distilled water. The mice were then placed back into the boxes and video recorded for 60 min. Mice used to evaluate spontaneous withdrawal were tested repeatedly, and testing began immediately following the final THC injection.
Each video was renamed to remove any identifying information and scored by a trained observer. A subset of nineteen videos was scored by a second observer and these scores were compared by correlation (r = 0.92) and paired t-test (p = 0.701) to ensure inter-rater reliability. The dependent variables were incidences of paw tremors (clapping of the forepaws), and head twitches (twisting of the head). These dependent variables were chosen because they have been identified consistently in mice undergoing THC withdrawal (Falenski et al., 2010; Lichtman et al., 2001; Marusich et al., 2014; Schlosburg et al., 2014). An incidence was scored for ‘paw tremor’ when the behavior was observed, not for each individual motion. Incidences were considered separate when either (1) another behavior occurred between the incidences, or (2) there was a time span of at least 1 s between incidences (Schlosburg et al., 2009).
2.5b. Tail Suspension Test:
The tail suspension test was run as previously described (Kinsey et al., 2007; Steru et al., 1985). Mice were suspended by their tails with adhesive tape from a horizontal bar placed approximately 40 cm above the counter and video recorded for 6 min. The amount of time the mice actively struggled was scored using ODLog software (Macropod Software, Yarraville, VIC, Australia). Active struggling was defined as one or more legs kicking repeatedly within one second or arching of spine, but not head movement. A subset of five videos was scored by a second, trained observer to ensure inter-rater reliability (r = 0.98).
2.5c. Marble Burying Test:
Marble burying was measured as previously described (Broekkamp et al., 1986; Kinsey et al., 2011), with minor changes. Plastic test chambers (30 cm W × 40 cm L × 16 cm H) filled with Teklad Aspen Sani-Chip (7090A; Envigo, Indianapolis, IN) wood bedding (5 cm deep) were placed inside a sound-attenuating chamber outfitted with a fan and LED lighting. A 5 × 5 array of 25 clear glass marbles was laid gently across the top of the leveled bedding. Each mouse was placed individually into the chamber and allowed to freely explore for 20 min. At the end of the test, each mouse was quickly and carefully removed and the number of unburied marbles (≥1/3 of the surface showing) was recorded then subtracted from the 25 total marbles. Marbles were counted by one or more trained individuals (inter-rater reliability r = 0.99). Locomotor activity was simultaneously recorded for the duration of the test by a camera mounted on the top of the test chamber. The video data were analyzed in real time using ANY-maze (Stoetling, Wool Dale, IL) video tracking software. Dependent variables included: total time immobile and number of marbles buried. A subset of marble burying videos was hand scored for somatic and other behaviors including digging, grooming, scratching, rearing and retropulsion.
2.5d. Light/Dark Box:
The light/dark box test was conducted as described previously (Crawley and Goodwin, 1980). The apparatus consisted of two connected Plexiglas chambers, with a small passage hole at floor level. The larger chamber (30 cm W × 40 cm L × 30 cm H) was open and brightly lit by an overhead lamp, and the smaller chamber (30 cm W × 20 cm L × 30 cm H) was covered and constructed using dark red Plexiglas. An infrared LED array (IR3, C&M Vision Technologies Inc, Houston, TX), along with a video camera (Logitech HD Pro Webcam C920) with the infrared filer removed, was used to visualize the mice while they were in the dark chamber. Each mouse was placed in the brightly lit area of the apparatus and allowed to freely explore for 5 min. Locomotor activity was analyzed in real time using ANY-maze software (Stoetling, Wool Dale, IL) for total time spent in the dark area and time spent immobile.
2.5e. Novel Object Test:
The novel object test was conducted as described previously (Bailey et al., 2009; van Gaalen and Steckler, 2000). Clean, plastic test chambers (30 cm W × 40 cm L × 16 cm H) were placed in individual sound attenuating test chambers, as described above. A novel plastic object (LEGO Group, Billund, Denmark) was positioned in the center of one side of the cage. Each mouse was placed in the opposite side of the test chamber and recorded for 10 min. The distance from the new object to the mouse’s head was measured in real time using ANY-maze software (Stoelting, Wool Dale, IL).
2.5f. Social Interaction:
The social interaction test assesses sociability by measuring the amount of time an experimental mouse spends near a novel, caged “target” mouse, as compared with an empty chamber and a chamber with a cage but no “target” mouse, and was conducted based on published methods (Crawley, 2007). The apparatus consisted of three clear Plexiglas chambers (30 cm W × 13 cm L × 16 cm H), each connected by passage holes that were covered by a clear Plexiglas door. A wire mesh cage (8 cm diameter × 11 cm H) was placed in each of the side chambers. The apparatus was placed inside a sound-attenuating chamber, equipped with a fan, LED lighting, and an overhead camera. Each experimental mouse was habituated in the empty middle chamber for 10 min, followed by habituation to the entire chamber for 10 min (Vargish et al., 2017). After the habituation, a novel same-sex “target” mouse was placed in one of the cages in the side chamber (counterbalanced between subjects), while the other wire cage was left empty. The Plexiglas doors were remotely opened for the 10 min test. The total time spent in each chamber (target mouse, middle, or empty chamber) and time spent immobile were each quantified using ANY-maze software (Stoetling, Wool Dale, IL).
2.5g. Prepulse Inhibition:
Prepulse inhibition was adapted from previously described methods (Geyer and Dulawa, 2003). Mice were habituated to restrainer and chamber (SR-Lab, San Diego Instruments, San Diego, CA) with background noise for 2 days prior to testing. Mice acclimated to the startle response chambers for one day in two five-min sessions. Once acclimated, mice were placed in a small cylinder mounted to a piezoelectric accelerometer that transduces motoric force output (i.e., startle) from the animal to a voltage; these voltages thus correspond to startle response magnitude. Startle pulse stimuli consisted of broad-spectrum white noise bursts at an intensity of 120 dB and a duration of 40 ms. Background noise intensity was set at 65 dB. During the PPI testing session, startle stimuli were preceded by a 20-ms prepulse 100 ms prior to the onset of the startle pulse, constituting one trial. Prepulse intensities were 4, 8, 12, 16, and 20 dB above background. Inter-trial intervals (ITI) were randomized between 8 and 23 seconds, such that the average ITI was ~15 seconds in order to minimize habituation to stimuli.
2.6. Statistical analyses
Data from all studies were analyzed using ANOVA, followed by Bonferroni or Dunnett’s post hoc tests, as appropriate, with the exception of Experiment 2.1, in which a Student’s t-test was used to compare the two groups (THC vs. vehicle) and the correlation between marble burying and digging. Prepulse inhibition and social interaction data were analyzed via 2×2 repeated-measures ANOVA. For Experiment 3, a 2×2 between-subjects ANOVA was used (THC vs. vehicle, and JZL184 vs. vehicle). For Experiments 4.1 and 4.2, a mixed design ANOVA was used (THC vs. vehicle as between subjects, ‘time’ as within-subjects independent variable). All data are presented as mean ± S.E.M. Differences were considered statistically significant if p < 0.05.
3.1. Results
3.2. Experiment 1: Precipitated THC withdrawal increased plasma corticosterone
Dependence was established using the precipitated withdrawal protocol detailed above. On day 6 of repeated THC (10 mg/kg, s.c.) or vehicle administration, male mice were administered the CB1 receptor selective antagonist rimonabant (3 mg/kg, i.p.) to precipitate withdrawal. Blood was drawn via cardiac puncture 30 min after rimonabant administration. Precipitated THC withdrawal significantly increased plasma corticosterone levels [F(1,25)= 4.1; p = 0.05; Fig 1]. Post hoc analyses revealed that this effect was driven by the repeated THC/rimonabant treated group.
Figure 1.
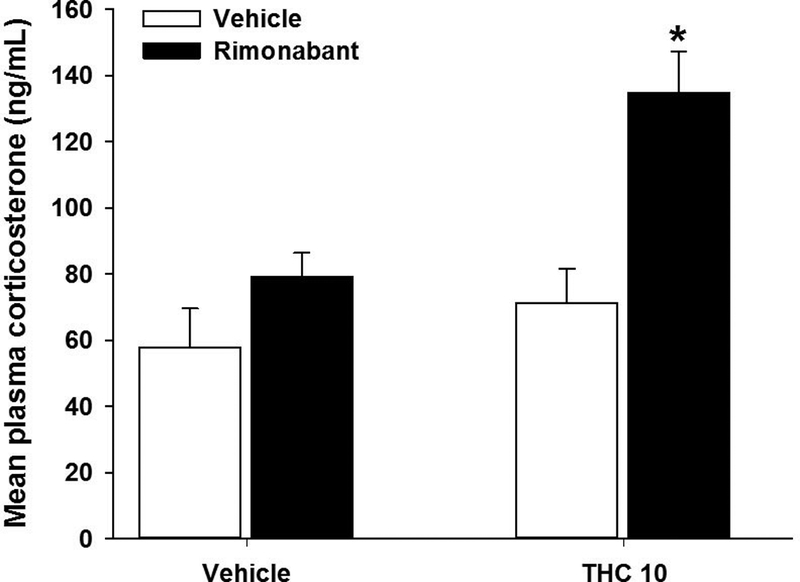
Precipitated THC withdrawal increases plasma corticosterone. Male mice were administered THC (10 mg/kg) for 6 days and withdrawal was induced with the CB1 selective antagonist rimonabant (3 mg/kg) 30 min prior to testing. THC treatment caused a significant increase in plasma corticosterone. Data represent mean ± SEM (n = 6–8/group). *p < 0.05 vs. vehicle control.
3.3. Experiment 2.1: Precipitated THC withdrawal induces somatic signs, alters marble burying and struggling behaviors
Male mice were subjected to rimonabant-precipitated THC (1, 10, or 50 mg/kg) or JWH-018 (1 mg/kg) withdrawal. In order to determine whether these behavioral effects of THC withdrawal were specific to the high dose of THC chosen, multiple tests were repeated using relatively lower THC doses. As reported previously, rimonabant-precipitated THC (10 or 50 mg/kg) withdrawal significantly increased paw tremors [F(3,28)=23.7, p<0.05; Fig 2A]. THC (50 mg/kg) significantly increased head twitches [F(3,28)= 8.0, p<0.05]. THC withdrawal did not affect hind leg scratching [p =0.12] and THC (1 mg/kg) withdrawal did not increase somatic signs of withdrawal.
Figure 2.
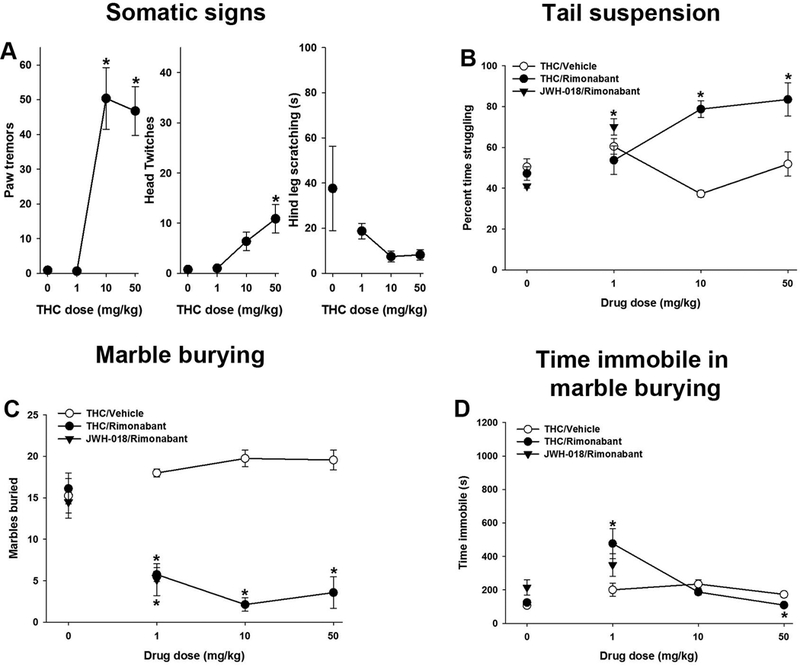
Precipitated THC withdrawal affects emotionality and induces somatic alterations. Male mice were administered THC (1, 10 or 50 mg/kg) or JWH-018 (1 mg/kg) for 6 days and withdrawal was induced with the CB1 selective antagonist rimonabant (3 mg/kg) 30 min prior to testing. THC (10 or 50 mg/kg) withdrawal increased paw tremors and head twitches (A), THC (10 or 50 mg/kg) or JWH-018 (1 mg/kg) increased struggling in the tail suspension test (B), and THC (1, 10 or 50 mg/kg) or JWH-018 (1mg/kg) decreased marbles buried (C). THC (1 mg/kg) increased time immobile in marble burying, while THC (50 mg/kg) withdrawal decreased time spent immobile (D). Data represent mean ± SEM (n = 7–12/group). *p < 0.05 vs. corresponding non-rimonabant control.
In the tail suspension test, precipitated THC (10 or 50 mg/kg) or JWH-018 (1 mg/kg) withdrawal significantly increased time struggling, as compared to control mice [F(4,69)=12.5, p<0.05; Fig 2B]. Mice in THC (1, 10 or 50 mg/kg) or JWH-018 (1 mg/kg) withdrawal buried significantly fewer marbles during precipitated withdrawal than control mice [F(4,68)=6.8, p<0.05; Fig 2C]. Time spent immobile in marble burying was increased by THC (1 mg/kg) withdrawal and decreased by THC (50 mg/kg) withdrawal [F(4,67)=4.8,p<0.05; Fig 2D]. Rimonabant, per se, had no effect in somatic signs, tail suspension, or marble burying. Similar effects of JWH-018 were seen in females. JWH-018 (1mg/kg) increased struggling [F(2, 26)=8.0, p<0.05; Fig S1] and decreased marbles buried in females [F(2,26)=8.3,p<0.05]. Unlike males, JWH0–018 withdrawal increased time immobile in during marble burying [F(2,25)=4.8, p<0.05].
Neither 10 mg/kg [p = 0.14] nor 50 mg/kg [p = 0.40] THC withdrawal affected time spent in the dark. Precipitated THC (50 mg/kg) withdrawal had no effect in either the social interaction test [p = 0.72; Fig S2] or novel object test [p = 0.22]. Precipitated THC (10 mg/kg) withdrawal did not affect startle response [p = 0.45] nor prepulse inhibition of acoustic startle [p = 0.71]. To test the hypothesis that THC withdrawal-suppressed marble burying may be caused by interference from somatic withdrawal behaviors, a separate group of mice was subjected to precipitated THC withdrawal, and recorded in the marble burying test. These videos were hand scored for digging, grooming, retropulsion, head twitches, rearing, and hind leg scratching. Again, THC withdrawal suppressed marble burying [t(14) = 8.0; p< 0.05; Fig S3], which positively correlated (r = 0.97) with decreased digging time [t(14) = 6.8; p< 0.05]. Head twitches (r = −0.52), grooming (r = 0.70), scratching (r = 0.50) immobility (r = −0.25), and rearing (r = −0.29) did not have strong correlations with marble burying. THC withdrawal significantly increased head twitches [t(14) = −2.2; p < 0.05], decreased grooming time [t(14) = 3.7; p < 0.05] and scratching time [t(14)=3.8, p<.05], and had no effect on immobility [p = 0.15] or rearing [p = 0.28]; no retropulsion was observed.
3.4. Experiment 3: MAGL inhibition attenuates somatic signs, but not marble burying nor struggling, during THC withdrawal
Several different pathways were pharmacologically challenged in attempts to reverse altered marble burying and struggling following repeated THC exposure. On day 6 of repeated THC (50 mg/kg, s.c.) or vehicle, male mice were administered the MAGL inhibitor JZL184 (40 mg/kg, i.p.) 120 min prior to the final THC injection (Long et al., 2009). Rimonabant (3 mg/kg, i.p.) was administered to all mice 30 min later. As reported previously (Schlosburg et al., 2009), THC withdrawal-induced paw tremors [F(1,24) =6.0, p<0.05; Fig 3A] and head twitches [F(1,24) =5.9, p<0.05; Fig 3B] were both attenuated by JZL184. No withdrawal effect was observed in hind leg scratching [p = 0.30], but a main effect of JZL184 was observed [F(1,20)=6.9, p<0.05; Fig 3C]. Conversely, there was no significant THC × JZL184 interaction in tail suspension [p = 0.39; Fig 4D]. There was, however, a significant main effect of THC [F(1,28)= 12.9; p<.05]. JZL184 did not affect struggling in the tail suspension test. Similarly, JZL184 did not normalize THC withdrawal-induced decreases in marble burying [F(1,26) =19.6; p<0.05; Fig 3E]. Though the THC × JZL interaction is significant, post hoc analyses revealed this effect was driven by JZL184-induced decrease in marble burying in the JZL184/vehicle treated group, not the JZL184/THC group. As reported previously, JZL184 increased time immobile in marble burying [F(1,24)=8.4, p<0.05; Fig 3F].
Figure 3.
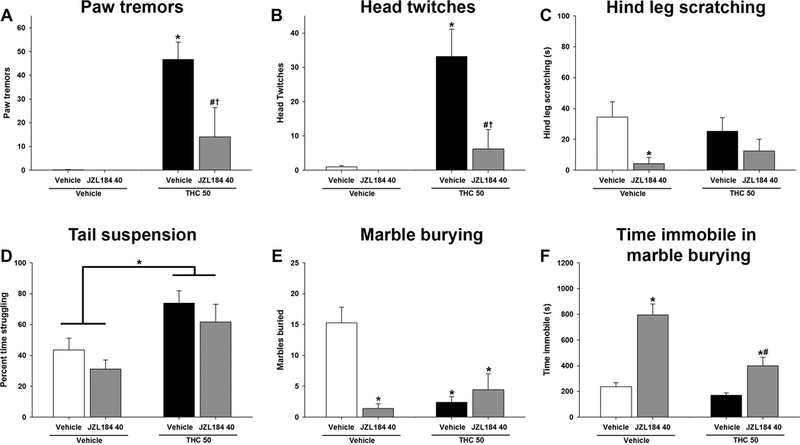
MAGL inhibition attenuates somatic signs of precipitated THC withdrawal. Male mice were administered THC (50 mg/kg) for 6 days and withdrawal was induced with the CB1 selective antagonist rimonabant (3 mg/kg) 30 min prior to testing. JZL184 (40 mg/kg) was administered 2 h prior to testing. JZL184 reduced withdrawal-induced paw tremors (A) and head twitches (B), and decreased hind leg scratching (C). JZL184 did not decrease time struggling in the tail suspension test (D) or increase marbles buried (E), but did increase time immobile in marble burying (F). Data represent mean ± SEM (n = 6–8/group). *p < 0.05 vs. vehicle/vehicle control; # p < 0.05 vs. vehicle/JZL184 control; † p < 0.05 vs. THC/vehicle control.
Figure 4.
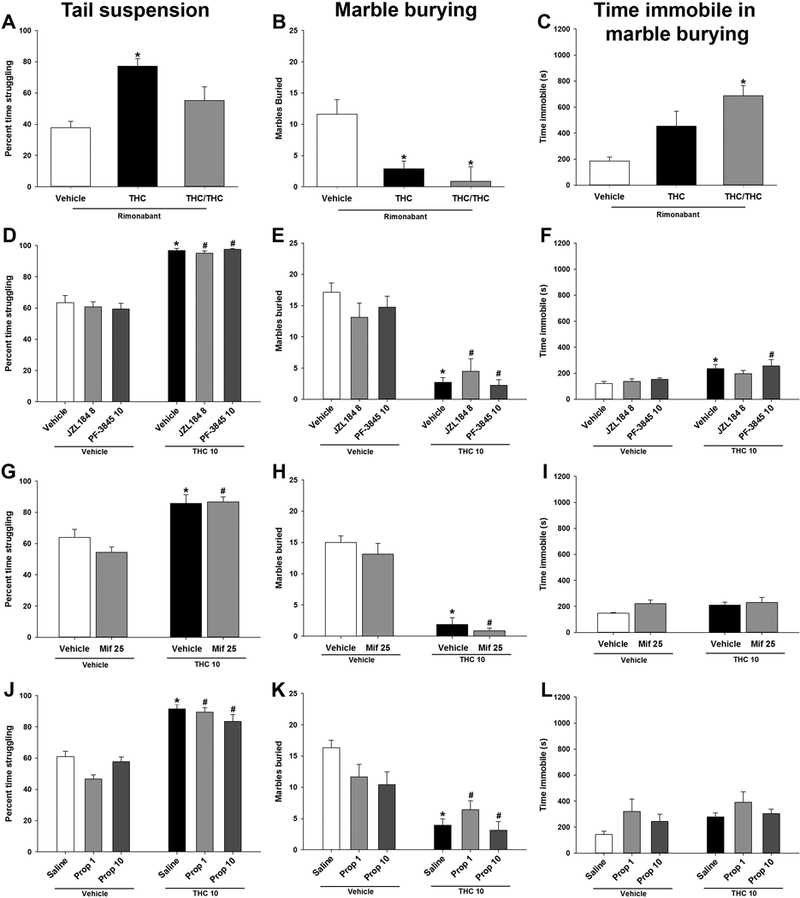
Rimonabant-precipitated THC withdrawal effects on behavior persist despite cannabinoid and β-adrenergic receptor manipulation. Male mice were administered THC (10 mg/kg) for 6 days and withdrawal was induced with the CB1 selective antagonist rimonabant (3 mg/kg) 30 min prior to testing. Acute administration of THC (50 mg/kg) normalized struggling in the tail suspension test (A), did not reverse withdrawal induced marble burying (B), and increased time immobile in marble burying (C). The MAGL inhibitor JZL184 (8 mg/kg), or the FAAH inhibitor PF-3845 (10 mg/kg), did not attenuate struggling (D), or marble burying (E), and PF-3845 increased time immobile in marble burying (F). The glucocorticoid receptor antagonist mifepristone (25 mg/kg, G, H, I) or the β-adrenergic receptor antagonist propranolol (1, 10 mg/kg, J, K, L) did not attenuate increased struggling in the tail suspension test or withdrawal-induced decreases in marble burying but did not affect time immobile in marble burying. Data represent mean ± SEM (n = 7–8/group). *p < 0.05 vs. vehicle control; # p < 0.05 vs. corresponding non-THC control.
Acute THC (50 mg/kg) was administered to attenuate THC (10 mg/kg) withdrawal-induced alterations in marble burying and struggling. In a separate experiment, on day 6, the endocannabinoid system was targeted with the MAGL inhibitor JZL184 (8 mg/kg, i.p.) or the FAAH inhibitor PF-3845 (10 mg/kg, i.p.). To test the hypothesis that withdrawal-induced activation of the stress response suppresses marble burying and increases struggling, the β-adrenergic receptor antagonist propranolol (1 or 10 mg/kg, i.p.) or the glucocorticoid antagonist mifepristone (25 mg/kg, i.p.) was administered. Drugs were administered 180 min (JZL184, PF-3845 or mifepristone) (Ahn et al., 2009; Long et al., 2009; Padgett et al., 1998) or 50 min (propranolol) (Lin et al., 2014) before testing. Rimonabant (3 mg/kg, i.p.) was administered to all mice. Acute administration of THC (50 mg/kg) following repeated THC (10 mg/kg) administration normalized time struggling in the tail suspension test [F(2,21)=10.2,p<0.05; Fig 4A] but did not affect marble burying [F(2,21)=13.8, p<0.05; Fig 4B]. Acute THC (50 mg/kg) increased time immobile in marble burying [F(2,20)=20.0, p<0.05; Fig 4C]. Neither JZL184, nor PF-3845 normalized withdrawal-induced struggling in the tail suspension test [F(1,41)=218.8, p<0.05; Fig 4D] or marble burying [F(1,41)=75.9, p<0.05; Fig 4E], but only PF-3845 increased time immobile in marble burying [F(1,41)=15.7,p<0.05; Fig 4F]. Mifepristone also did not normalize struggling in the tail suspension test [p = 0.24; Fig 4G] or marble burying [p = 0.55; Fig 4H], and additionally did not increase time immobile in marble burying [p = 0.32; Fig 4I]. Propranolol did not normalize time struggling in the tail suspension test [F(2,62)=3.8; p<0.05; Fig 4I] or marble burying [p = 0.06; Fig 4J], and additionally did not increase time immobile in marble burying [p = 0.81; Fig 4L] .
3.5. Experiment 4.1: Mice exhibit spontaneous cannabinoid withdrawal
In order to evaluate whether THC withdrawal can be observed spontaneously, (i.e., in the absence of antagonist administration), male and female mice were injected with either THC (10 or 50 mg/kg, s.c.), the synthetic cannabinoid agonist JWH-018 (1 mg/kg, s.c.) or vehicle for 6 days. On day 6, mice were injected with THC, JWH-018, or vehicle and immediately tested. Tests were repeated at 6, 12, 24, 36, and 48 h for observation of somatic signs. Abstinence from 10 or 50 mg/kg THC or JWH-018 significantly increased paw tremors in male [F (15,80)= 4.3; p<0.05; Fig. 5A] and female mice [F (15,110)= 3.4; p<0.05; Fig. 5D], and head twitches in male [F(15,80)= 10.3; p<0.05; Fig. 5B] and female mice [F(3,15)= 2.193; p<0.05; Fig. 5E] at 36 h post-injection. There was a treatment × time interaction in hind leg scratching in males [F(15,90)=3.3, p<0.05; Fig 5C] and a significant main effect of treatment in hind leg scratching in females [F(3,110)=12.6,p<0.05; Fig 5F]. At 36 h post-injection, there are significant main effects of sex [F(1,54)= 7.8; p<0.05] and treatment [F(3,54)= 9.9; p<0.05] in paw tremors, but no significant interaction [p = 0.31]. There is a small but statistically significant treatment × sex interaction in head twitches at 36 h [F(3,54)=4.7; p<0.05], indicating spontaneous cannabinoid withdrawal induces increased head twitches in males compared to females.
Figure 5.
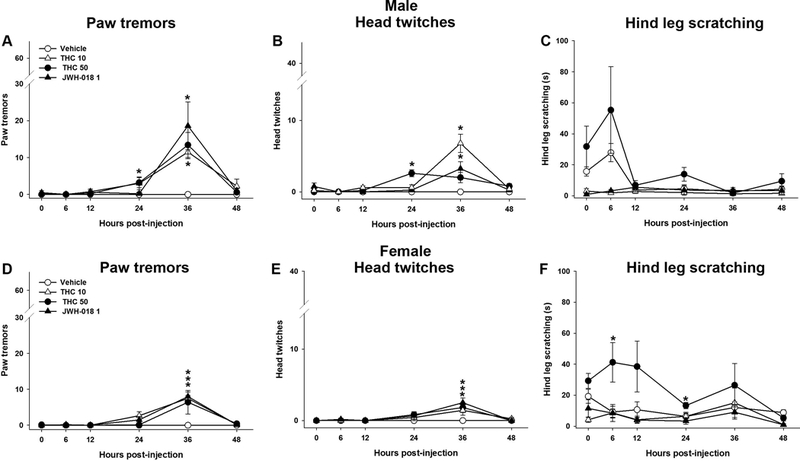
Spontaneous THC withdrawal increases somatic signs of withdrawal. Male and female mice were administered THC (10 or 50 mg/kg) or JWH-018 (1 mg/kg) for 6 days. Spontaneous withdrawal was evaluated at various time points after the final THC injection. At 24 h post-injection, males treated with THC (50 mg/kg) had increased paw tremors (A) and head twitches (B). At 36 h post-injection, males treated with THC (10 mg/kg) or JWH-018 exhibited increased paw tremors and at 24 and 36 h post-injection head twitches increased. No changes were observed in male hind leg scratching (C). Similarly, females treated with THC (10 or 50 mg/kg) or JWH-018 displayed increased paw tremors (D) and head twitches (E) at 36 h. Females treated with THC (50 mg/kg) showed increased hind leg scratching at 6 and 24h post-injection (F). Data represent mean ± SEM (n = 7–8/group) *p < 0.05 vs. vehicle control.
3.6. Experiment 4.2: Spontaneous THC withdrawal attenuated by acute cannabinoid intervention
To determine whether spontaneous withdrawal is reversible, cannabinoid interventions were attempted at 36 h abstinence, based on the Exp 4.1 time course. After the final repeated injection of THC (50 mg/kg, s.c.) or vehicle on day 6, male mice were injected with vehicle, JZL184 (40 mg/kg, i.p.) or THC (50 mg/kg) at 34 h abstinence (i.e., 2 h prior to testing). JZL184 or THC significantly attenuated paw tremors [F(3,25) 15.4; p<0.05; Fig. 6A]. Similarly, head twitches were significantly increased after 36 h THC abstinence [F(3,25)= 11.5; p<0.05; Fig 6B]. There was no effect of withdrawal or drug treatment on hind leg scratching [p = 0.66; Fig 6C]. JZL184 or THC pretreatment blocked the THC withdrawal-induced head twitches.
Figure 6.
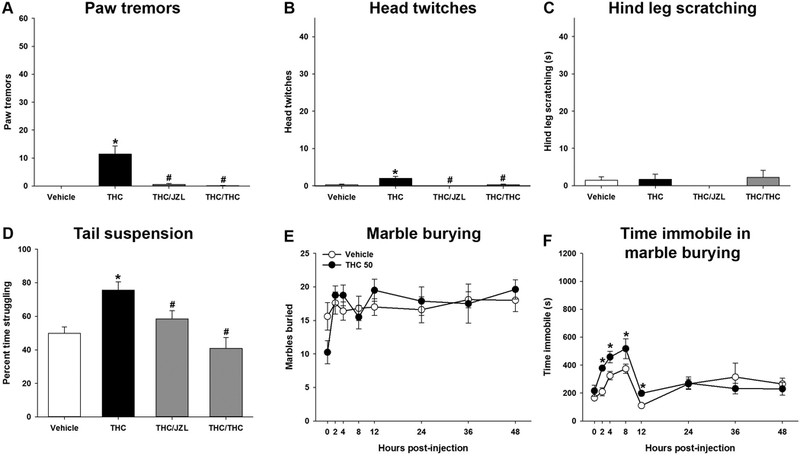
Spontaneous THC withdrawal is attenuated by either acute THC or MAGL inhibition. Male mice were administered THC (50 mg/kg) for 6 days. Spontaneous withdrawal was evaluated at 36 h following a 2 h pretreatment with either JZL184 (40 mg/kg) or acute THC (50 mg/kg). Either acute JZL184 or acute THC decreased paw tremors (A), head twitches (B), and there was no change in hind leg scratching (C). Time spent struggling in the tail suspension test was attenuated by JZL184 or acute THC (D). Spontaneous withdrawal was not observed in marble burying (E), but an increase in immobility was observed at 2, 4, 8, and 12 h post-injection (F). Data represent mean ± SEM (n = 7–12/group). *p < 0.05 vs. vehicle control. # p < 0.05 vs. THC.
Mice were observed in the tail suspension test at 37 h post-THC abstinence. Withdrawal-induced increases in struggling were attenuated by either JZL184 or acute THC administration [F(3,37)= 8.6, p<0.05; Fig.6D].
Marble burying was assessed at 0, 2, 4, 8, 12, 24, 36 h THC (50 mg/kg) abstinence. In contrast to precipitated THC withdrawal, THC abstinence did not affect marble burying [p=.14; Fig. 6E]. Immobility during marble burying increased at 2, 4, 8, and 12 h post-injection [F(7,112)= 2.3, p<0.05; Fig 6F].
4. Discussion
The current study aimed to develop novel models of cannabinoid withdrawal, including a spontaneous model of THC withdrawal. Mice were repeatedly exposed to THC or JWH-018 for 6 days and withdrawal precipitated using the CB1 selective antagonist rimonabant. In addition to behaviors associated with somatic aspects of cannabinoid withdrawal, i.e., paw tremors and head twitches, precipitated cannabinoid withdrawal also decreased marble burying and increased struggling in the tail suspension test. The decrease in marble burying was not due to decreased locomotor activity nor the presence of somatic signs of withdrawal, suggesting that the difference observed was related to a change in behavior and not sedation/ataxia, per se. Similarly, withdrawal was precipitated in mice exposed to the prototypical synthetic cannabinoid agonist JWH-018.
We noted that mice subjected to THC or JWH-018 withdrawal were hyperreflexive and susceptible to convulsions. Somatic withdrawal signs and increased struggling in the tail suspension test were evident at ≥10 mg/kg THC, whereas JWH-018 withdrawal was evident at ≥1 mg/kg. Although full potency curves were not attempted here, the relatively higher potency of JWH-018 reflects that JWH-018 is a full agonist at CB1, whereas THC is a partial CB1 agonist (Atwood et al., 2010; Wiley et al., 2012). No sex differences were observed.
Corticosterone was increased in mice subjected to precipitated THC withdrawal, suggesting that the observed changes in marble burying and tail suspension may be related to an increased stress response. However, neither mifepristone nor propranolol reversed THC withdrawal-induced decreases in marble burying or increases in struggling in the tail suspension test. A plausible explanation for why non-cannabinoid drugs (i.e., mifepristone and propranolol) were ineffective is that the doses tested were simply insufficient to significantly affect withdrawal-induced behavior. However, dosing was based on published studies that demonstrated that these doses are sufficient to block the neuroendocrine stress response in mice (Al-tubuly et al., 2008; Chester et al., 2014; Kinsey et al., 2011; Lichtman et al., 2001). Thus, we speculate that THC withdrawal is a stressor that modulates the THC withdrawal-induced behaviors reported here, albeit not via catecholamine nor glucocorticoid receptors. Ongoing studies are investigating the possible contribution of corticotrophin-releasing factor in the expression of THC withdrawal.
The marble burying test is generally used to screen anxiolytic drugs. One interpretation of the present data is that THC withdrawal is anxiolytic in the marble burying test. Long considered a test of generalized anxiety and even obsessive-compulsive disorder, the marble burying test is a proxy of digging behavior that inconsistently correlates with other measures of anxiety-like behavior (Thomas et al., 2009). In mice, marble burying is decreased by the anxiolytic benzodiazepine diazepam (Jimenez-gomez et al., 2011; Kinsey et al., 2011). But, marble burying is also decreased by the anxiogenic benzodiazepine inverse agonist β-carboline-3-carboxylate, as well as the dopamine agonist pramipexole or psychostimulant D-amphetamine, regardless of changes in locomotor activity (Jimenez-gomez et al., 2011). Similarly, struggling in the tail suspension test is decreased by antidepressants and increased by D-amphetamine and atropine (Oliva et al., 2004; Steru et al., 1985). Given that THC withdrawal increased both corticosterone level (Oliva et al., 2003) and struggling in the tail suspension test, we propose that cannabinoid withdrawal-induced suppression of marble burying indicates increased stress and/or agitation.
A common critique of animal models of drug dependence is that withdrawal is precipitated with a receptor selective antagonist. In humans, drug withdrawal typically occurs in the absence of a precipitating agent, somewhat limiting the external validity of precipitated withdrawal models. Spontaneous withdrawal from potent synthetic cannabinoids has been reported. For example the synthetic cannabinoid, WIN55,212–2 induced wet dog shakes and facial rubs in male rats (Aceto et al., 2001). Similarly, repeated CP55,940 administration induces somatic withdrawal signs and increases plasma corticosterone (Oliva et al., 2004, 2003), and also reduces time in the dark side of the light/dark box in male mice (Aracil-Fernández et al., 2011). In adolescent rats exposed to repeated THC, males spent more time in the open arms of the elevated plus maze and females spent more time in the closed arms during abstinence following repeated THC exposure (Harte-Hargrove and Dow-Edwards, 2012).
In the present study, we detected spontaneous THC withdrawal peaks at 24 to 36 h in both male and female mice. These behaviors were generally of a lower magnitude than rimonabant-precipitated withdrawal, suggesting that spontaneous withdrawal is less intense than precipitated withdrawal. For example, head twitches and paw tremors are five-fold higher in the precipitated withdrawal model, as compared with the spontaneous model. An exception is struggling in the tail suspension test. Although struggling was similarly increased in both precipitated and spontaneous withdrawal models, MAGL inhibition reversed struggling only in the spontaneous model, again suggesting that spontaneous withdrawal is of a relatively lower intensity than precipitated withdrawal.
There is mixed literature on sex differences in acute cannabinoid administration as well as cannabinoid withdrawal. In rats, acute sex differences vary in magnitude and as a function of which tests are used (Craft et al., 2017). Marked differences have been reported between adolescent male and female rats during spontaneous withdrawal (Harte-Hargrove and Dow-Edwards, 2012). Others report limited sex differences in rats during precipitated THC withdrawal (Marusich et al., 2014). The present study found a statistically significant increase in spontaneous JWH-018 withdrawal in male vs. female mice, but the small magnitude of the signal precludes interpretation of biological relevance of this finding.
Whereas either MAGL inhibition or additional THC dosing reversed THC withdrawal-induced struggling and somatic signs, neither drug was effective at blocking withdrawal-suppressed marble burying. It is plausible that withdrawal-suppressed behaviors, much like pain-suppressed behaviors (Richardson et al., 2008), are more difficult to reverse than withdrawal-induced behaviors. For example, the MAGL inhibitor JZL184 blocked somatic signs and spontaneous (but not precipitated) withdrawal-induced struggling. These data support the idea that JZL184-induced increases in brain 2-AG levels functionally ended THC abstinence, but 2-AG displacement of rimonabant is insufficient to prevent precipitated withdrawal across all models.
The present study has several limitations. The findings presented in this study are specific to adult C57BL/6J mice. Although efforts were made to incorporate female mice into many of the studies, withdrawal can vary across age and strain. (Damaj et al., 2003; Harte-Hargrove and Dow-Edwards, 2012), and a more thorough examination of THC withdrawal using large samples of both males and females may yield sex differences. Similarly, it is plausible that a broader set of dependent variables, for example for somatic signs of withdrawal, would capture additional effects. Whereas we chose to focus on head twitches and paw tremors as common somatic signs of cannabinoid withdrawal (Falenski et al., 2010; Lichtman et al., 2001; Marusich et al., 2014; Schlosburg et al., 2014), others quantify ptosis, piloerection, grooming/face rubbing, rearing, scratching, retropulsion, and mastication, among others (Maldonado, 2002). Future studies will also examine gastric effects of cannabinoid withdrawal, as a possible somatic sign. Finally, as previously mentioned, the doses and pretreatment times chosen for compounds challenging withdrawal may be inadequate in a withdrawal state.
5. Conclusion
Cannabinoid withdrawal affects stress-related behaviors, in addition to well-characterized somatic signs of withdrawal. In addition to altering behavior, THC withdrawal is a stressor that activates the hypothalamic-pituitary-adrenal axis, resulting in increased corticosterone release. Withdrawal was evident after exposure to the plant-derived phytocannabinoid Δ9-THC, as well as the potent synthetic cannabinoid JWH-018. Further, we report novel methods for detecting spontaneous cannabinoid withdrawal in mice. These models may be useful in designing new pharmacological interventions for cannabinoid withdrawal, a central component of Cannabis Use Disorder.
Supplementary Material
Highlights.
THC withdrawal increases plasma corticosterone levels.
THC or JWH-018 withdrawal increases struggling in the tail suspension test.
THC or JWH-018 withdrawal suppresses marble burying.
Monoacylglycerol lipase (MAGL) inhibition attenuates THC withdrawal.
THC withdrawal is induced spontaneously, during abstinence.
Acknowledgements
We thank Kristin Gabella, M. Kate Cardullo, and Elliott Chiartas for technical assistance.
Author Disclosures
Role of Funding Source
This project was supported by The National Institutes of Health [AR066806, DA038714, DA039335, DA043331, and GM081741]. Sponsors had no role in the design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication.
Nonstandard abbreviations
- 2-AG
2-arachidonoylglycerol
- CB1
Cannabinoid receptor subtype 1
- CB2
Cannabinoid receptor subtype 2
- FAAH
Fatty acid amide hydrolase
- JZL184
4-nitrophenyl-4-[bis(1,3-benzodioxol-5-yl)(hydroxy)methyl]piperidine-1-carboxylate
- JWH-018
Naphthalen-1-yl-(1-pentylindol-3-yl)methanone
- MAGL
Monoacylglycerol lipase
- Rimonabant
SR141716A; 5-(4-Chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- THC
Δ9-tetrahydrocannabinol
Footnotes
Conflict of Interest
No conflict declared.
Contributor Information
Kristen R. Trexler, Department of Psychology, West Virginia University
Sara R. Nass, Department of Psychology, West Virginia University; Department of Pharmacology & Toxicology, Virginia Commonwealth University
Molly S. Crowe, Department of Psychology, West Virginia University; Department of Physiology & Biophysics, Virginia Commonwealth University
Joshua D. Gross, Department of Physiology, Pharmacology and Neuroscience, West Virginia University
Margaret S. Jones, Department of Psychology, West Virginia University
Austin W. McKitrick, Department of Psychology, West Virginia University
David P. Siderovski, Department of Physiology, Pharmacology and Neuroscience, West Virginia University
Steven G. Kinsey, Department of Psychology, West Virginia University
References
- Aceto M, Scates S, Martin B, 2001. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212–2. Eur. J. Pharmacol 416, 75–81. 10.1016/S0014-2999(01)00873-1 [DOI] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, Mckinney K, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Becelaere K Van, Stevens RC, Cravatt BF, 2009. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem. Biol 16, 411–420. 10.1016/j.chembiol.2009.02.013.Discovery [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-tubuly R, Aburawi S, Alghzewi E, Gorash Z, Errwami S, 2008. The Effect of Sympathetic Antagonists on the Antidepressant Action of Alprazolam Libyan J Med, AOP : 080101. Libyan J Med 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Lintzeris N, Copeland J, Dunlop A, McGregor IS, 2015. Cannabinoid Replacement Therapy (CRT): Nabiximols (Sativex) as a novel treatment for cannabis withdrawal. Clin Pharmacol Ther 97, 571–574. 10.1002/cpt.109 [DOI] [PubMed] [Google Scholar]
- Aracil-Fernández A, Almela P, Manzanares J, 2011. Pregabalin and topiramate regulate behavioural and brain gene transcription changes induced by spontaneous cannabinoid withdrawal in mice. Addict. Biol 18, 252–262. 10.1111/j.1369-1600.2011.00406.x [DOI] [PubMed] [Google Scholar]
- Aso E, Ozaita A, Valdizán EM, Ledent C, Pazos Á, Maldonado R, Valverde O, 2008. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem 105, 565–572. 10.1111/j.1471-4159.2007.05149.x [DOI] [PubMed] [Google Scholar]
- Atwood BK, Huffman J, Straiker A, MacKie K, 2010. JWH018, a common constituent of “Spice” herbal blends, is a potent and efficacious cannabinoid CB1 receptor agonist. Br. J. Pharmacol 160, 585–593. 10.1111/j.1476-5381.2009.00582.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K, 2011. CP47,497-C8 and JWH073, commonly found in “Spice” herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol 659, 139–145. 10.1016/j.ejphar.2011.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B, 2009. Social stress enhances IL-1β and TNF-α production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol. Behav 98, 351–358. 10.1016/j.physbeh.2009.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Aru GN, Frau R, Orrù M, Luckey GC, Boi G, Gessa GL, 2005. The CB receptor agonist WIN 55,212–2 fails to elicit disruption of prepulse inhibition of the startle in Sprague-Dawley rats. Psychopharmacology (Berl). 177, 264–271. 10.1007/s00213-004-1941-4 [DOI] [PubMed] [Google Scholar]
- Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE, 2013. Differential drug-drug interactions of the synthetic Cannabinoids JWH-018 and JWH-073: implications for drug abuse liability and pain therapy. J Pharmacol Exp Ther 346, 350–361. 10.1124/jpet.113.206003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezing CA, Levin FR, 2017. The Current State of Pharmacological Treatments for Cannabis Use Disorder and Withdrawal. Neuropsychopharmacology 1–71. 10.1038/npp.2017.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-gelouin D, Lloyd KL, 1986. Major tranquillizers can be distinguished from minor tranquillizerson the basis of effects on marble burying and swim-induced grooming in mice. Eur. J. Pharmacol 126, 223–229. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z, 2008. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. J. Subst. Abuse Treat 35, 362–368. 10.1016/j.jsat.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Kirchhoff AM, Barrenha GD, 2014. Relation between corticosterone and fear-related behavior in mice selectively bred for high or low alcohol preference. Addict. Biol 19, 663–675. 10.1111/adb.12034.Relation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiwat C, Harris RBS, 2006. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm. Behav 50, 489–495. 10.1016/j.yhbeh.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Craft RM, Haas AE, Wiley JL, Yu Z, Clowers BH, 2017. Gonadal hormone modulation of Δ 9 -tetrahydrocannabinol-induced antinociception and metabolism in female versus male rats. Pharmacol. Biochem. Behav. J 152, 36–43. 10.1016/j.pbb.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK, 1980. Preliminary Report of a Simple Animal Behavior Model for the Anxiolytic Effects of Benzodiazepines 13, 167–170. [DOI] [PubMed] [Google Scholar]
- Crawley JN, 2007. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 17, 448–459. 10.1111/j.1750-3639.2007.00096.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR, 2003. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J. Pharmacol. Exp. Ther 307, 526–534. 10.1124/jpet.103.054908\njpet.103.054908 [pii] [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ, 2010. FAAH−/− Mice Display Differential Tolerance, Dependence, and Cannabinoid Receptor Adaptation After Δ9-Tetrahydrocannabinol and Anandamide Administration. Neuropsychopharmacology 35, 1775–1787. 10.1038/npp.2010.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M, Dulawa S, 2003. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr. Protoc. Neurosci 8. [DOI] [PubMed] [Google Scholar]
- Hall W, Kozlowski LT, 2017. The diverging trajectories of cannabis and tobacco policies in the United States : reasons and possible implications. Addiction 1–7. 10.1111/add.13845 [DOI] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, Foltin RW, 2013a. Predictors of marijuana relapse in the human laboratory: Robust impact of tobacco cigarette smoking status. Biol. Psychiatry 73, 242–248. 10.1016/j.biopsych.2012.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW, 2013b. Nabilone Decreases Marijuana Withdrawal and a Laboratory Measure of Marijuana Relapse. Neuropsychopharmacology 38, 1557–1565. 10.1038/npp.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW, 1999. Abstinence symptoms following smoke marijuana in humans. Psychopharmacology (Berl). 141, 395–404. 10.1007/s002130050848 [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL, 2012. Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav Brain Res 231, 48–59. https://doi.org/S0166-4328(12)00177-5 [pii]\r10.1016/j.bbr.2012.02.048 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, Jung J, Zhang H, Grant BF, 2016. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: Findings from the national epidemiologic survey on alcohol and related conditions-III. Am. J. Psychiatry 173, 588–599. 10.1176/appi.ajp.2015.15070907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S, 1996. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav 54, 21–30. 10.1016/0091-3057(95)02126-4 [DOI] [PubMed] [Google Scholar]
- Huang P, Liu-Chen L-Y, Kirby LG, 2010. Anxiety-like effects of SR141716-precipitated delta9-tetrahydrocannabinol withdrawal in mice in the elevated plus-maze. Neurosci. Lett 475, 165–8. 10.1016/j.neulet.2010.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-gomez C, Osentoski A, Woods JH, 2011. As an Animal Model of Compulsion and / or Anxiety. Behav. Brain Res 22, 711–713. 10.1097/FBP.0b013e32834afebe.Pharmacological [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R, 2007. Repeated Social Defeat Causes Increased Anxiety-Like Behavior and Alters Splenocyte Functino in C57BL/6 and CD-1 Mice. Brain Behav. Immunol 21, 458–466. 10.1038/nmeth.2250.Digestion [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Cole EC, 2013. Acute Δ9-tetrahydrocannabinol blocks gastric hemorrhages induced by the nonsteroidal anti-inflammatory drug diclofenac sodium in mice. Eur. J. Pharmacol 715, 111–116. 10.1016/j.ejphar.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH, 2011. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol. Biochem. Behav 98, 21–27. 10.1016/j.pbb.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR, 2001. Precipitated cannabinoid withdrawal is reversed by D 9 -tetrahydrocannabinol or clonidine 69, 181–188. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR, 2001. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol. Biochem. Behav 69, 181–8. https://doi.org/S0091-3057(01)00514-7 [pii] [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Wiley JL, Lavecchia KL, Neviaser ST, Arthur DB, Wilson DM, Martin BR, 1998. Effects of SR 141716A after acute or chronic cannabinoid administration in dogs. Eur. J. Pharmacol 357, 139–148. [DOI] [PubMed] [Google Scholar]
- Lin P, Wang C, Xu B, Gao S, Guo J, Zhao X, Huang H, Zhang J, Chen X, Wang Q, Zhou W, 2014. The VGF-derived peptide TLQP62 produces antidepressant-like effects in mice via the BDNF / TrkB / CREB signaling pathway. Pharmacol. Biochem. Behav 120, 140–148. 10.1016/j.pbb.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF, 2009. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol 5, 37–44. 10.1038/nchembio.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, 2002. Study of cannabinoid dependence in animals. Pharmacol. Ther 95, 153–164. 10.1016/S0163-7258(02)00254-1 [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL, 2014. Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend. 137, 20–28. 10.1016/j.drugalcdep.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BL, Mustafa A, Filbey F, Brown ES, Mason BL, 2016. Novel Pharmacotherapeutic Interventions for Cannabis Use Disorder. Curr. Addict. Reports 10.1007/s40429-016-0094-y [DOI] [Google Scholar]
- Oliva JM, Ortiz S, Palomo T, Manzanares J, 2003. Behavioural and gene transcription alterations induced by spontaneous cannabinoid withdrawal in mice. J. Neurochem 85, 94–104. 10.1046/j.1471-4159.2003.01627.x [DOI] [PubMed] [Google Scholar]
- Oliva JM, Servicio SO, Palomo T, Manzanares J, 2004. Spontaneous cannabinoid withdrawal produces a differential time-related responsiveness in cannabinoid CB1 receptor gene expression in the mouse brain José. J. Psychopharmacol 18, 59–65. 10.1177/0192513X12437708 [DOI] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, Sheridan JF, 1998. Restraint Stress Slows Cutaneous Wound Healing in Mice. Brain. Behav. Immun 73, 64–73. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Haney M, 2015. Textbook of Addiction Treatment: International Perspectives. Textb. Addiciont Treat. Int. Prespectives 367–380. 10.1007/978-88-470-5322-9 [DOI] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF, 2008. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol. Biochem. Behav 88, 497–510. 10.1016/j.pbb.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Carrera MRA, Navarro M, Koob GF, Weiss F, 1997. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science (80-.). 276, 2050–2054. 10.1126/science.276.5321.2050 [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson B.L. a, Ramesh D, Abdullah R. a, Long JZ, Cravatt BF, Lichtman AH, 2009. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 11, 342–352. 10.1208/s12248-009-9110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Ignatowska-jankowska B, Ramesh D, Abdullah RA, Tao Q, Booker L, Long JZ, Selley DE, Cravatt BF, Lichtman AH, 2014. Prolonged Monoacylglycerol Lipase Blockade Causes Equivalent Cannabinoid Receptor Type 1 Receptor – Mediated Adaptations in Fatty Acid Amide Hydrolase Wild-Type and Knockout Mice 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Koch M, 2005. Deficient Social and Play Behavior in Juvenile and Adult Rats after Neonatal Cortical Lesion : Effects of Chronic Pubertal Cannabinoid Treatment 944–957. 10.1038/sj.npp.1300634 [DOI] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, 2016. Treatment of Cannabis Use Disorder: Current Science and Future Outlook. Pharmacother. J. Hum. Pharmacol. Drug Ther 10.1002/phar.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, 2003. Regulation of Cannabinoid CB1 Receptors in the Central Nervous System by Chronic Cannabinoids. Crit. Rev. Neurobiol 15, 91–119. 10.1177/0192513X12437708 [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Siman P, 1985. The tail suspension test: A new method for screening antidepressant in mice. Psychopharmacology (Berl). 85, 367. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R, 2009. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl). 204, 361–373. 10.1007/s00213-009-1466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Steckler T, 2000. Behavioural analysis of four mouse strains in an anxiety test battery. Behav. Brain Res 115, 95–106. [DOI] [PubMed] [Google Scholar]
- Vargish GA, Pelkey KA, Yuan X, Chittajallu R, Collins D, McBain C, 2017. Persistent inhibitory circuit defects and disrupted social behavior following in utero exogenous cannabinoid exposure Geoffrey. Mol. Psychiatry 22, 56–67. 10.1001/jamasurg.2014.1086.Feasibility [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Martin BR, Huffman JW, 2012. 1-Pentyl-3-Phenylacetylindoles and JWH-018 Share In Vivo Cannabinoid Profiles in Mice. Drug Alcohol Depend. 123, 148–153. 10.1016/j.drugalcdep.2011.11.001.1-Pentyl-3-Phenylacetylindoles [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WQ, Smolik CM, Barba-escobedo PA, Gamez M, Sanchez JJ, Javors MA, Daws LC, Gould GG, 2015. Neuropharmacology Acute dietary tryptophan manipulation differentially alters social behavior , brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacology 90, 1–8. 10.1016/j.neuropharm.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


